
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 08 July 2022
Sec. Nephrology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.871767
Background: Current evidence on the relationship between carotenoids and chronic kidney disease (CKD) patients are limited and controversial.
Methods: Data were obtained from the Nutrition and Health Examination Survey (NHANES) database and the NHANES Linked Mortality File, both from a nationally representative sample. Dietary intake was assessed through 24-h dietary recall, and information was available both on dietary and serum α-carotene, β-carotene, β-cryptoxanthin, lycopene, and lutein/zeaxanthin (combined) through the NHANES cycles used. We used multivariable Cox proportional hazards regression models to estimate the risk for all-cause mortality associated with carotene intakes and serum levels, adjusting for potential confounding factors.
Results: Of the 6,095 CKD participants, 1,924 subjects died (mean follow-up time, 8.1 years). After eliminating all the confounding factors, we found that high levels of total carotene (HR = 0.85, 95% CI, 0.75-0.97, P = 0.011) intakes at baseline were significantly associated with a lower risk of death. And the serum concentrations of carotenoid were also showing that a-carotene (HR = 0.77, 95%CI, 0.65–0.92, P = 0.002), beta-cryptoxanthin (HR = 0.83, 95%CI, 0.70–0.98, P = 0.019), lycopene (HR = 0.77, 95% CI, 0.65–0.91, P = 0.002), and lutein + zeaxanthin (HR = 0.82, 95% CI, 0.70–0.96, P = 0.002) was significantly associated with decreased all-cause mortality of CKD patients. The associations remained similar in the sensitivity analyses.
Conclusion: Findings suggest that high-level carotene dietary intake and the serum concentration were associated with a lower risk of mortality in the CKD population.
Chronic kidney disease (CKD) is a major problem that threatens global public health with an increasing incidence and prevalence, while 697.5 million cases of CKD globally when the prevalence of CKD was estimated as 9.1% in 2017 (1–3). And the CKD patients, which can progress to end-stage renal disease (ESRD) and become a major contributor to cardiovascular death, has been reported that the mortality rate is much higher than people without CKD (1–4). However, there are still not many effective strategies that can slow the progression of CKD. To improve the survival in CKD, a great many studies conducted and nutritional supplementation has been considered as a direction of treatment (5).
It is known to all that CKD is a chronic inflammatory disease that involves oxidative stress, which is thought to be the key factor in the progression of CKD (6). Though the precise mechanisms have not been elucidated yet, oxidative stress characterized by an imbalance between the accumulation of reactive oxygen species (ROS) and the natural ability of anti-oxidant in the cell occurs frequently in CKD (7–9). Furthermore, oxidative stress also plays a vital role in the conditions of cancer, cardiovascular disease, diabetes, hypertension, and infection, which are known to be strongly associated with mortality in patients with CKD (7, 8).
As powerful antioxidants, carotenoids can remove ROS and enhance the cell's ability to prevent oxidative stress to delay the progression of the disease, which is considered as an emerging therapeutic direction in CKD patients (10). It can be classified into two major types: carotenes and xanthophylls. Carotenes, which include β-carotene, α-carotene, and lycopene as well as other less-studied species, are unoxygenated terpenes, whereas xanthophylls, which include lutein, zeaxanthin, and β-cryptoxanthin, are oxygenated (11). All these carotenoids have been verified that have their unique antioxidant properties (12) and can scavenge radicals in three steps such as electron transfer, hydrogen abstraction, and addition, to act as the main scavenger of the ROS (12). Previous studies have shown that carotenoids are associated with decreased all-cause mortality and lung cancer mortality in US adults (13, 14). Moreover, researchers also found that serum carotenoids were associated with estimated glomerular filtration rate (eGFR), both in an aged cohort and preserved kidney function patients (15, 16). And there are animal studies to support that the β-carotene possesses a nephroprotective effect through bromobenzene-administered rats (17, 18). However, whether the carotenoid intake was associated with the mortality of CKD patients hasn't been found yet.
Therefore, this study aims to characterize whether carotenoid intake was associated with the mortality risk of CKD patients, while data was obtained from the Nutrition and Health Examination Survey (NHANES) database and the NHANES Linked Mortality File.
Data were screened out of the NHANES database from 2001 to 2014, which is a periodic survey conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) (19). And the NHANES is a complex, multistage probability sampling design using a nationally representative sample of the non-institutionalized civilian population of the USA. All participants were selected randomly through a complex statistical process each year. They complete personal structured interviews at home and then undergo a physical examination at a mobile examination central, including height, weight, laboratory measurements, and a computer-assisted 24-h dietary recall (20). Both ethics approval and data collection for NHANES were obtained from the NCHS Research Ethics Review Board and written informed consent was provided by every participant. More details are available on the NHANES website (19).
Eligibility criteria included CKD participants with age ≥ 20 years old and nonpregnant. CKD was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 (using the Chronic Kidney Disease Epidemiology Collaboration equation) and/or urinary albumin: creatinine ratio >30 mg/g (1). To assess the quality and completeness of a survey participant's response to the dietary recall section, we used the dietary recall status evaluated by the interviewer and only included the ones that were classified as ‘reliable and met the minimum criteria. Finally, 6,313 participants with complete data were analyzed (Figure 1).
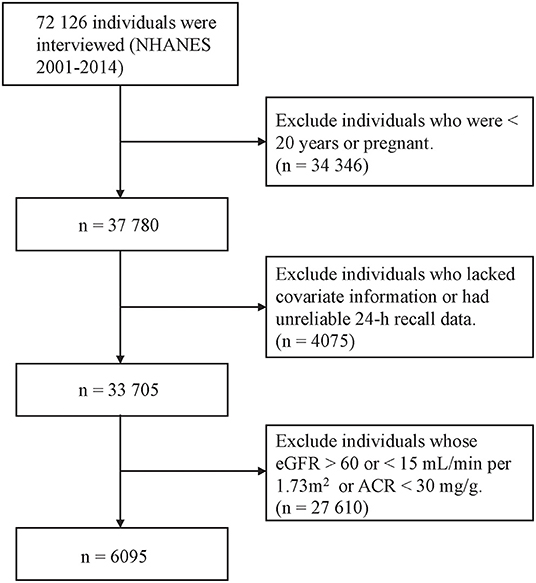
Figure 1. Flow diagram of the selection of eligible participants, National Health and Nutrition Examination Survey 2001–2014.
In all cycles of the NHANES 2001–2014, dietary intakes were estimated using data from twice 24-h dietary recalls (2003–2014), or only one (2001–2002). As for the primary dietary interview, one was conducted in person at the mobile examination center, and the other was conducted by telephone after 3–10 days. All interviews were carried out by trained investigators according to the US Department of Agriculture Automated Multiple-Pass Method for the 24-h recall (21, 22). According to previous analyses, we used the nutritional information from foods and beverages collected in the single 24-h dietary recall to calculate the carotenes, energy, and nutrient intakes of participants in the 2001–2002 NHANES. And for participants in the 2003–14 NHANES, we used the mean of the nutritional information from both recalls.
As for the measurements of serum carotenoids, the information of part participants was available on α-carotene, β-carotene, β-cryptoxanthin, lycopene, and lutein/zeaxanthin (combined). In these cycles, participants aged more than 6 years provided serum samples for measurement of six carotenoids (α-carotene, trans-β-carotene, cis β-carotene, β-cryptoxanthin, combined lutein/zeaxanthin, trans-lycopene, and total lycopene) using high-performance liquid chromatography (HPLC) (23). And we evaluated serum levels of carotenoids to CKD outcomes as a sub-analysis.
Based on the existing literature, the following variables were selected as confounding factors measured in the baseline survey. The demographic characteristics included age (years), sex (men or women), race/ethnicity, education level, and marital status (24). Lifestyle-related behaviors included leisure-time physical activity, smoking status, and alcohol consumption status (25). The details of the data collection and definition have been described in detail elsewhere.
Blood specimen collections and measurements of blood pressure, body weight, and height were conducted during mobile physical examinations. Body mass index (BMI) was calculated as body weight (kg) divided by the square of height (m2). Three consecutive blood pressure readings were obtained after resting quietly in a sitting position for 5 min, and the means of readings were calculated (19). Moreover, the fasting plasma glucose, glycosylated hemoglobin, blood total cholesterol, HDL level, urine albumin, urine creatinine (Cr), serum creatinine, serum phosphorus, and hemoglobin were measured in the same laboratory, where detail was available on the NHANES website (19). More particularly, the serum Cr was measured using a recalibration equations for 1999–2000 and 2005–2006 NHANES surveys: standard serum Cr (mg/dL) = 0.147 + 1.013 × uncalibrated serum Cr (mg/dL) and standard serum Cr (mg/dL) = −0.016 + 0.978 × uncalibrated serum Cr (mg/dL) (26). And Serum Cr-based eGFR was estimated using the CKD Epidemiology Collaboration equation.
Diabetes was defined that reported: (1) self-reported diabetes; (2) the use of anti-hyperglycemic agents; (3)measured fasting plasma glucose ≥ 126 mg/dL; (4) 2-h plasma glucose ≥ 200 mg/dL during a 75-g oral glucose tolerance test; (5) glycol-hemoglobin ≥ 6.5% (27). And hypertension was defined as people who: (1) self-reported hypertension; (2) reported use of antihypertensive agents; (3) measured systolic blood pressure ≥ 140 mmHg; (4)diastolic blood pressure ≥ 90 mmHg (27). High cholesterol levels were defined as the total-to-HDL cholesterol ratio was more than 5.9 (27). And participants were considered to have a history of cardiovascular disease if they reported ever being told they had any of the following conditions by a health care provider: congestive heart failure, coronary heart disease, angina pectoris, heart attack, or stroke (28).
The study's endpoint was the follow-up to December 31, 2015, or death. If the patient had died before December 31, 2015, the time of death would be the end time of follow-up. The National Center for Health Statistics (NCHS) has linked data collected from NHANES with death certificate records from the National Death Index (NDI) with a probabilistic matching algorithm and social security number, name, father's surname, birthday, state of birth, state of residence, sex, race, and marital status were used to match NHANES records with National Death Index records. The International Statistical Classification of Diseases, 10th Revision was used to identify the causes of deaths, like previous studies (29–31).
Baseline characteristics were described across the groups, according to intakes of carotenes, and between-group differences were tested by analysis of variance for continuous variables and Rao-Scott χ2 test for categorical variables. Multivariable Cox proportional hazards regression models were used to estimating the risk for all-cause mortality associated with carotene intakes, adjusting for potential confounding factors including demographic factors, lifestyle behaviors, history of chronic health conditions, and dietary factors. We used the Schoenfeld residual plots to examine the proportional hazards assumption, and no violations were noted. We adjusted for age (years, continuous), sex, family income-poverty ratio (≤ 1, > 1 to <4, and ≥ 4), self-reported race (non-Hispanic white, non-Hispanic black, Mexican American, and others), an education level (less than high school graduates, high school graduates or equivalent, and college or above), marital status (married and not married), alcohol consumption (none, ≤ 2 drinks/d for men or ≤ 1 drink/d for women, 2–5 drinks/d for men or 1–4 drink/d for women, > 5 drinks/d for men or >4 drink/d for women), cigarette smoking (never, former, current), and vigorous/moderate recreational activities for at least 10 min continuously per week (%) in model 1, log-transformed total energy intake (kilocalorie, continuous), HEI2015, baseline eGFR, log-transformed urinary ACR, BMI (kg/m2, continuous), high cholesterol levels, serum phosphorus, hemoglobin, hypertension, and diabetes, and history of CVD and cancer were additionally adjusted in model 2. Restricted cubic splines (RCSs) were applied to examine the possible nonlinear relationship between intakes of carotenes (as continuous variables) and mortality, using the median intakes of each group as knots (32).
To assess whether the association of SSB with all-cause mortality was different because of the demographic characteristics and life behavioral habits of CKD patients, stratified analyses was conducted using a Wald test according to age (<60 vs. ≥ 60 years), sex, self-reported race (non-Hispanic white vs. minority ethnic groups), BMI (<25 vs. ≥ 25 kg/ m2), history of hypertension, diabetes, CVD, or cancer, CKD stage, serum phosphorus and hemoglobin level. A cross-product term between dietary intakes and each grouping variable was correspondingly added to the model, the likelihood ratio test was used to evaluate if there was a statistically significant interaction. And several sensitivity analyses were conducted. First, we repeated the main analysis by replacing the HEI-2015 with intakes of major food groups (i.e., vegetable, fruit, whole grain, red meat, and processed meat) or macronutrients. Second, we repeated the main analysis by adjusting for waist circumference instead of BMI. Third, concerning previous studies, we excluded adults who died within 1 year after the survey to minimize potential reverse causation. Simple correlation analyses were performed using Pearson correlation to assess associations between carotene intakes and its correspondence serum level. Hazards ratios (HRs) and 95% confidence intervals (CIs) were reported (33). A 2-sided p < 0.05 was considered statistically significant. Multiple imputations by chained equations were used for dealing with missing data regarding covariates (34). All data management and analyses were performed in RStudio statistical software (version 1.1.423).
Table 1 compares the characteristics of the study participants which are divided into four quartiles. Of 6,095 CKD participants, 1,924 subjects died (mean follow-up time, 8.1 years). Compared with the lower quartiles, the upper quartiles group of carotenoid concentrations was more likely to be patients who were non-Hispanic whites, married, higher educated, higher family income-poverty ratio level, moderate drinker, never smoking, more active, higher dietary acid load, and cancer. And it also has the characteristics of more plurality prevalent cases of non-drinkers, current-smokers, diabetes, hypertension, and history of CVD in the group of lower serum carotenoid. Furthermore, the people with higher serum carotenoid concentration seem to have lower BMI, total energy intake, HEI-2015, dietary acid load, and eGFR.
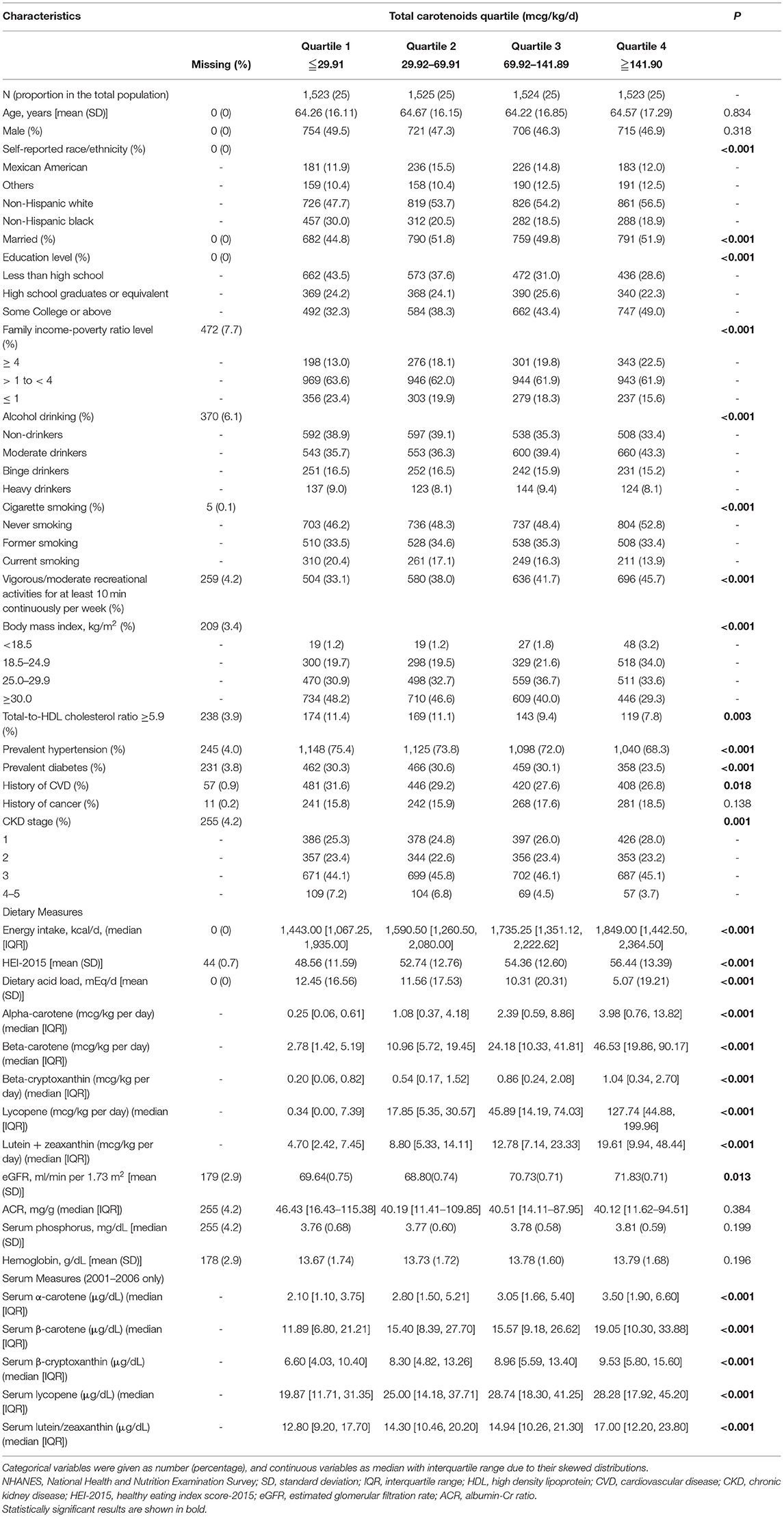
Table 1. Baseline characteristics of participants by quartiles of total carotenoids, NHANES, 2001–2014.
Table 2 shows the baseline carotenoid intake levels of the total and lycopene were associated with the low mortality in CKD patients. When adjustments were made for model 1 (age, sex, family income-poverty ratio level, race, education level, marital status, alcohol consumption, smoking, and leisure-time physical activity), the risk of death was remain reduced in participants with higher total carotene (HR = 0.82, 95% CI, 0.72-0.93, P = 0.003), β-carotene intake (HR = 0.82, 95%CI, 0.73–0.94, P = 0.005) and lycopene intake (HR = 0.84, 95%CI, 0.74–0.95, P=0.01)levels at baseline than in those with the lowest levels. And when additionally adjusted for model 2 (model 1 plus log-transformed total energy intake, HEI-2015, baseline eGFR, log-transformed urinary ACR, body mass index, total-to-HDL cholesterol ratio, serum phosphorus, hemoglobin, hypertension, diabetes, and history of cardiovascular disease and cancer), the significant differences of the total carotene(HR = 0.85, 95% CI, 0.75-0.97, P = 0.011), β-carotene (HR = 0.86, 95%CI, 0.76–0.99, P = 0.007) as well as lycopene intake (HR = 0.85, 95% CI, 0.75–0.97, P = 0.028) are still existing in CKD persons. The dose-response analysis of the associations of daily intakes of carotene with mortality shows the same results, and a non-linear relationship was not indicated in all kinds of carotenoids (Supplementary Figure 1). However, results were substantially changed when we further adjusted for substituting HEI-2015 by food groups, substituting HEI-2015 by macronutrients, waist circumference instead of BMI, or excluding those who died within 1 year (Table 3). The intake of total carotene always has a significant association with the death of CKD patients while the β-carotene and lycopene were not.
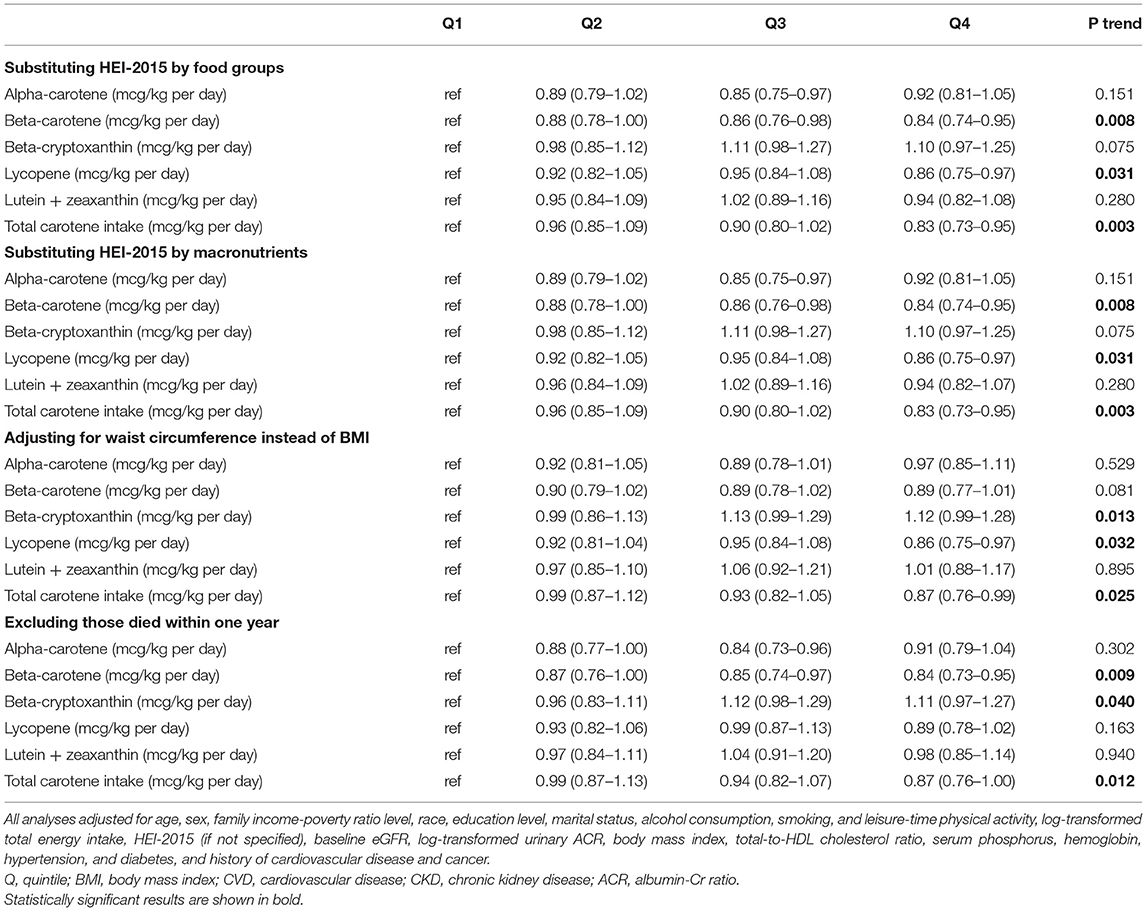
Table 3. Results of sensitivity analyses of the associations between carotene intakes and mortality.
We conducted subgroup analyses of the associations between total carotene intakes and mortality (Table 4). And the relationship seems to be more obvious and significant in people who were higher educated, fatter, drinkers, and without diabetes than others. In addition, subgroup analyses of the associations between other kinds of carotenoid intakes and mortality also have been conducted (Supplementary Tables 1–5).
Table 5 shows the associations of the quartiles of the serum carotenoid levels relative to Quartile 1 with mortality among the total participants both in the unadjusted and adjusted model. In addition to the α-carotene, the rest of the four baseline serum carotenoid levels were associated with the decline of death in CKD patients. When adjustments were made for model 1, the risk of death was reduced in CKD participants with these five higher serum carotenoids levels (P < 0.05). And this result still persisted in α-carotene (HR = 0.77, 95%CI, 0.65-0.92, P = 0.002), β-cryptoxanthin (HR = 0.83, 95%CI, 0.70–0.98, P = 0.019), Lycopene (HR = 0.77, 95%CI, 0.65–0.91, P = 0.002), and Lutein + zeaxanthin (HR = 0.82, 95%CI, 0.70–0.96, P = 0.002) after adjusting for Model 2. However, it becomes less pronounced in Model 2 of serum β-carotene (HR = 0.80, 95%CI, 0.67–0.95, P = 0.079). Results were not substantially changed when we further adjusted for substituting HEI-2015 by food groups, substituting HEI-2015 by macronutrients, waist circumference instead of BMI, or excluding those who died within 1 year (Table 6). The dose-response analysis of the associations of serum carotene with mortality shows the same result and finds that a non-linear relationship was not indicated in β-carotene, β-cryptoxanthin, and lycopene (Supplementary Figure 2). And it's worth mentioning that the result of serum β-cryptoxanthin (HR = 0.83, 95%CI, 0.70-0.98, P = 0.019) was completely different from that of dietary (HR = 1.15, 95%CI, 1.00–1.31, P = 0.013) In addition, the dietary intake of α-carotene, β-carotene, β-cryptoxanthin, lycopene, as well as lutein + zeaxanthin were weakly linear correlated to the correspondence serum contents (Supplementary Table 6). Subgroup analyses of the associations between serum carotenoid levels and mortality have been conducted (Supplementary Tables 7–11).
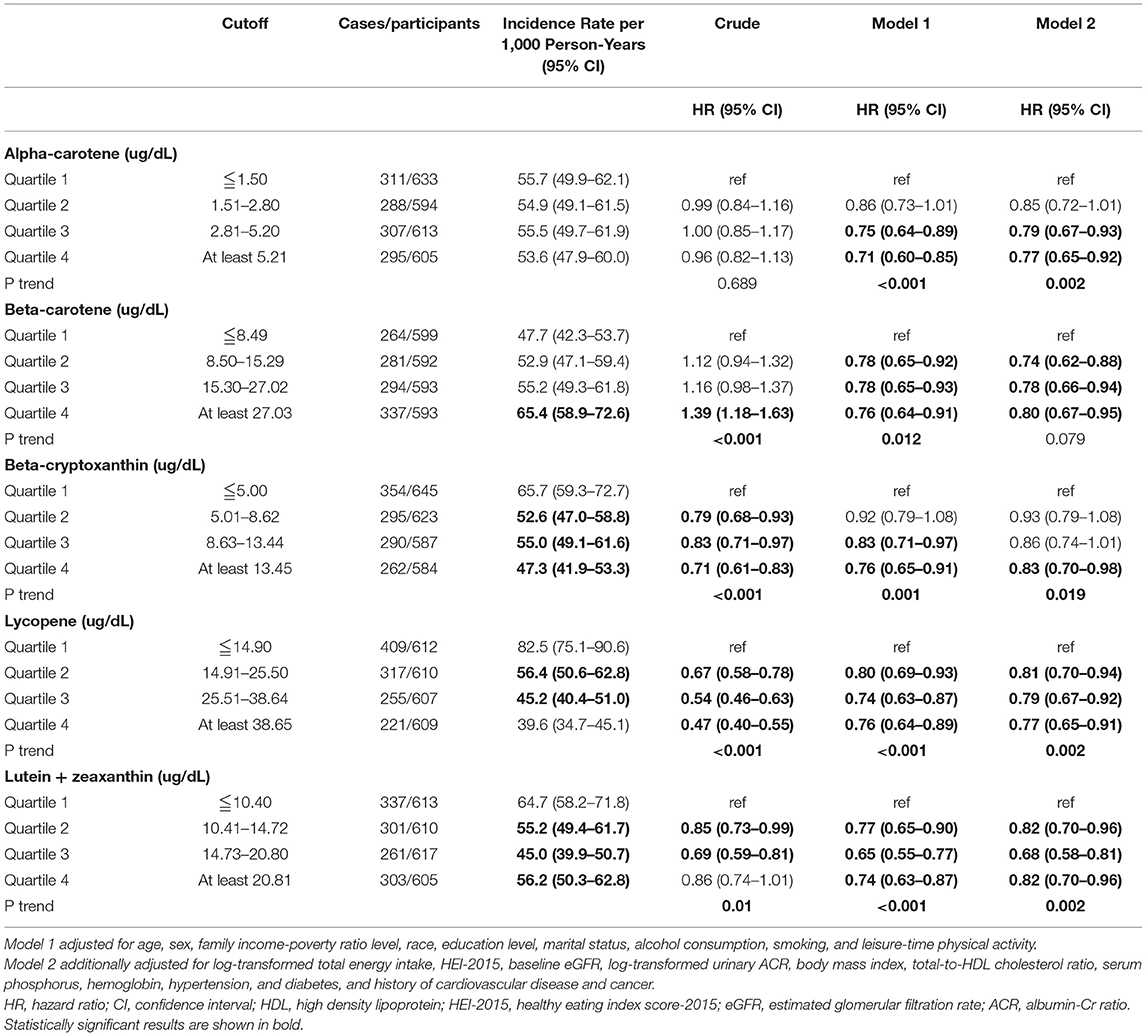
Table 5. The associations of the quartile of serum carotenoids, relative to Quartile 1 with mortality.
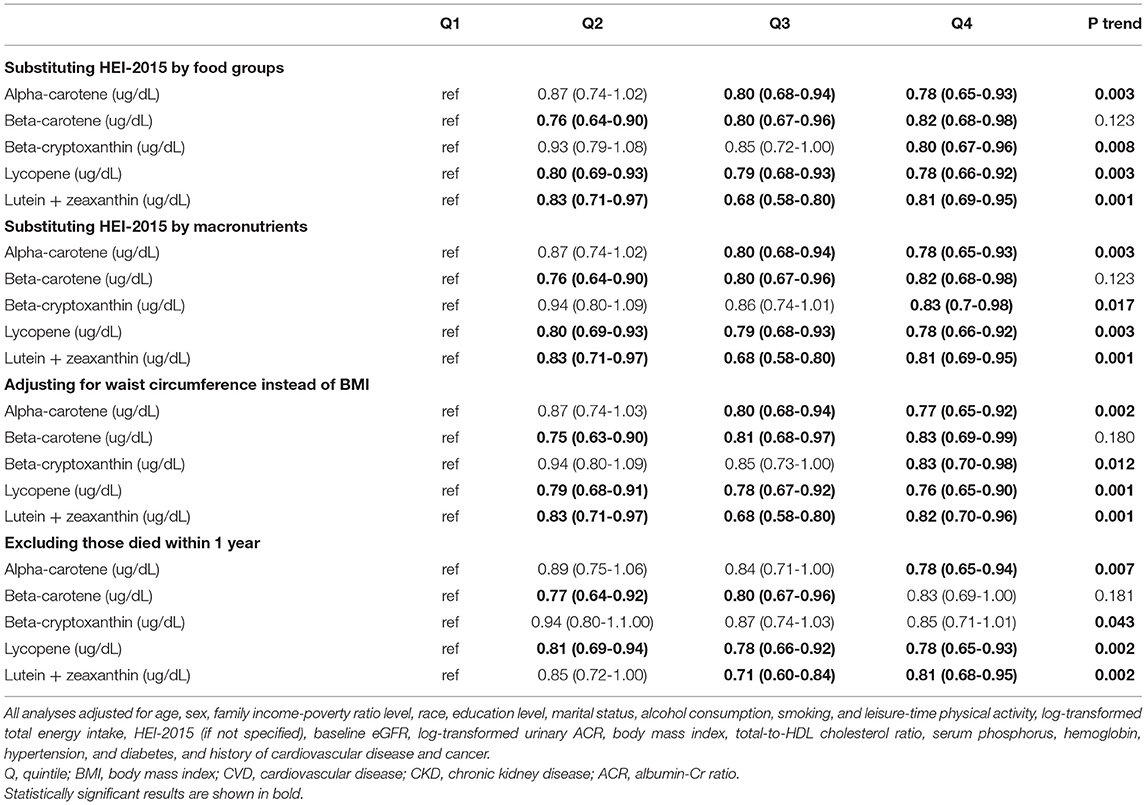
Table 6. Results of sensitivity analyses of the associations between serum carotenoids and mortality.
In this population-based retrospective cohort study, higher intake of total carotene was inversely associated with the mortality of CKD patients after adjustment for potential confounders. And the serum concentration of carotenoid is also showing that the high level of α-carotene, β-cryptoxanthin, lycopene, as well as lutein + zeaxanthin was significantly associated with decreased all-cause mortality in CKD patients. The dietary modification to increase carotenoid intake represents a possible route for the amelioration prognosis of CKD patients.
Our research substantiated that a higher intake of total carotenoids was inversely associated with mortality of CKD patients after adjustment for potential confounders. We consider that more carotenoids can be intake by CKD patients to improve the prognosis of the disease. Although the mechanism is not clear yet, considerable research has indicated that carotenoids may improve the prognosis of CKD patients through its antioxidant properties (9). Oxidative stress is a contributor to many diseases, and shown kidney disease is associated with permanent inflammation accompanied by oxidative stress (9). The disturbance of the redox balance is associated with an increase in ROS production and a decrease in antioxidant capacity. As the CKD progresses, a gradual elevation of oxidative damage with a reduction in endogenous antioxidant defenses (35). Furthermore, over-production of the ROS may lead to renal fibrosis, inflammation, and endothelial dysfunction (12, 36). These are known risk factors for the onset of serious systemic complications, cardiovascular disease, anemia, and mineral disorders, which are closely related to death in CKD patients. Therefore, carotenoids, which beneficial effects are mainly derived from their antioxidant properties as the main scavenger of ROS such as singlet molecular oxygen (O2), can indeed improve the prognosis to some extent. However, each carotenoid has its unique antioxidant properties, and which kind influences the mortality of CKD most has not been clear yet.
Moreover, both the cox regression analysis and dose-response analysis recognized that the β-carotene and lycopene intake do reduce the mortality of CKD patients while other carotenoids do not. These differences may be due to the following reasons. First, different carotenoids have different antioxidant activities according to their structure and environmental polarity. For instance, the efficiency scavengers of free radicals are different in polar carotenoids (lutein + zeaxanthin and β-cryptoxanthin) and non-polar hydrocarbon carotenoids (α-carotene, β-carotene, and lycopene) (12). Each carotenoid's antioxidant properties depend on its unique functional group and magnitude of conjugated double-bonds (12). Secondly, the potential beneficial effects of a carotenoid may depend on the concentrations of another (14). Different carotenoids interact with each other, contributing to the different impacts on CKD patients. Lastly, partial carotenoids can act as prooxidants at high carotenoid concentrations or high oxygen concentrations (12, 37). Indeed, this property was proposed by Burton as early as 1984 that when under a higher partial pressure of oxygen and higher concentration of carotenoids (> 500 μM), there will be a transition from antioxidant behavior to pro-oxidative behavior (38). And the subsequent experiments in vivo and in vitro also confirmed this. All in all, the study on the antioxidant activity of carotenoids was not complete yet (37, 38). And it seems to be more beneficial to intake β-carotene and lycopene rather than other carotenoids for patients with CKD. However, results were substantially changed when we further adjusted for substituting waist circumference instead of BMI or excluding those who died within 1 year, while high-level of β-carotene and lycopene intake was not correlated with the decreased mortality of CKD patients. More research into the effects of β-carotene and lycopene intake is required.
In addition, we found that the carotenoid may be more useful in specific groups of people such as the high-educated, non-Hispanic white, fatter, drinker, and without diabetes through the subgroup analysis of total carotenoid intake. This might partly be due to the difference in the CKD population, which has been verified that can affect the prognosis of patients yet, such as diabetes and obesity. Furthermore, the patients' redox status can be affected by many factors like CKD stage and age, which are attributed to the metabolism status of carotenoids in the body (39). And the bioavailability and absorption of each carotenoid may be effect by multi-factors, such as the carotenoid type, isomeric forms, interaction with fat and fiber, aging, and nutritional status (40). Finally, the different genetic backgrounds and lifestyles between non-Hispanic whites and others may attribute to the diversity. And despite that, the causes of these factors' associations with carotenoid intake effect in CKD patients are not yet fully understood. This conclusion provides more reference for the choice of clinicians. And more prospective studies and fundamental research should be done nearly.
Interestingly, the results between dietary intake and serum concentration of other carotenoids are not exactly coincidental though most of the serum carotenoids show a negative correlation with the mortality of CKD patients. This may be caused by two reasons. On one hand, the bioavailability of each carotenoid is not consistent at all. And the absorption, distribution, and metabolism of every kind of carotenoid are not exactly consistent, too. The increase of oral carotenoids is not necessarily followed by an increase in carotenoid concentration. On the other hand, the limitations in estimation methods of the oral carotenoids contribute to the instability of our results. These differences remind us that we should put more attention to the sub carotenoid research, and the improvements in methodology are crucial. In any case, the high-level of total carotenoid dietary intake does negatively correlated with the all-cause mortality of CKD patients, and more reliable prospective research should be conducted.
To the best of our knowledge, this research was the first retrospective cohort study that assessed the relation of dietary intake of carotene and serum carotene with the mortality of CKD patients of the NHANES database. And our study had several strengths including the large sample size and the well-characterized study population enabling appropriate adjustment for confounding effects. We conducted several analyses such as dose-response analysis and sensitivity analyses to demonstrate our results to ensure reliability.
Some important limitations deserve mention. First, our retrospective study design has inherent limitations consistent with that of other retrospective studies. Second, several variables were dependent on self-reported data and may be subject to recall bias. Third, although we have adjusted for lots of confounding factors, there may still be some unrecognized confounding factors which we couldn't control. Finally, the study population is limited to US adults, which makes it difficult to generalized our findings to the broader community of patients with CKD.
Overall, in this retrospective cohort study of the US CKD population, we found that higher intake of total carotene was significantly associated with a lower risk of death. In addition to β-carotene, other kinds of serum carotenoids were also significantly associated with the mortality of CKD patients. These findings are promising given the further therapeutic options for treating CKD.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.cdc.gov/nchs/nhanes/participant.htm.
The studies involving human participants were reviewed and approved by NCHS Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
Conceptualization and data curation: YH and XC. Methodology: XC and HG. Project administration: NZ, SG, and YY. Software: NZ. Supervision and writing—review and editing: SG and YY. Validation: YL and YM. Writing—original draft: YH and HG. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.871767/full#supplementary-material
1. Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2020) 395:709–33. doi: 10.1016/S0140-6736(20)30045-3
2. El Nahas AM, Bello AK. Chronic kidney disease: the global challenge. Lancet. (2005) 365:331–40. doi: 10.1111/j.1523-1755.2005.00774.x
3. Stevens PE, Levin A. Evaluation and management of chronic kidney disease_ synopsis of the kidney disease_ improving global outcomes 2012 clinical practice guideline. Ann Intern Med. (2013). doi: 10.7326/0003-4819-158-11-201306040-00007
4. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJL, Mann JF, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. (2013) 382:339–52. doi: 10.1016/S0140-6736(13)60595-4
5. Kalantar-Zadeh K, Fouque D. Nutritional management of chronic kidney disease. N Engl J Med. (2017) 377:1765–76. doi: 10.1056/NEJMra1700312
6. Ilori TO, Sun Ro Y, Kong SY, Gutierrez OM, Ojo AO, Judd SE, et al. Oxidative balance score and chronic kidney disease. Am J Nephrol. (2015) 42:320–7. doi: 10.1159/000441623
7. Go. AS, M. G, Chertow MDM, Dongjie Fan MS. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. (2012) 351:1296–305. doi: 10.1056/NEJMoa041031
8. de Jager DJ, Vervloet MG, Dekker FW. Noncardiovascular mortality in CKD: an epidemiological perspective. Nature reviews. Nephrology. (2014) 10:208–14. doi: 10.1038/nrneph.2014.8
9. Rapa SF, Di Iorio BR, Campiglia P, Heidland A, Marzocco S. Inflammation and oxidative stress in chronic kidney disease-potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int J Mol Sci. (2019) 21. doi: 10.3390/ijms21010263
10. Zuluaga Tamayo M, Choudat L, Aid-Launais R, Thibaudeau O, Louedec L, Letourneur D, et al. Astaxanthin complexes to attenuate muscle damage after in vivo femoral ischemia-reperfusion. Marine Drugs. (2019) 17. doi: 10.3390/md17060354
11. Widomska J, Zareba M, Subczynski WK. Can xanthophyll-membrane interactions explain their selective presence in the retina and brain? Foods. (2016) 5. doi: 10.3390/foods5010007
12. El-Agamey A, Lowe GM, McGarvey DJ, Mortensen A, Phillip DM, Truscott TG, et al. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys. (2004) 430:37–48. doi: 10.1016/j.abb.2004.03.007
13. Min KB, Min JY. Serum carotenoid levels and risk of lung cancer death in US adults. Cancer Sci. (2014) 105:736–43. doi: 10.1111/cas.12405
14. Shardell MD, Alley DE, Hicks GE, El-Kamary SS, Miller RR, Semba RD, et al. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: the Third National Health and Nutrition Examination Survey. Nutrition research. (2011) 31:178–89. doi: 10.1016/j.nutres.2011.03.003
15. Hirahatake KM, Jacobs DR, Gross MD, Bibbins-Domingo KB, Shlipak MG, Mattix-Kramer H, et al. The association of serum carotenoids, tocopherols, and ascorbic acid with rapid kidney function decline: The Coronary Artery Risk Development in Young Adults (CARDIA) study. J Ren Nutr. (2019) 29:65–73. doi: 10.1053/j.jrn.2018.05.008
16. Lin J, Hu FB, Curhan GC. Associations of diet with albuminuria and kidney function decline. Clin J Am Soc Nephrol. (2010) 5:836–43. doi: 10.2215/CJN.08001109
17. Akkara PJ, Sabina EP. Pre-treatment with beta carotene gives protection against nephrotoxicity induced by bromobenzene via modulation of antioxidant system, pro-inflammatory cytokines and pro-apoptotic factors. Appl Biochem Biotechnol. (2020) 190:616–33. doi: 10.1007/s12010-019-03111-0
18. Li J, Yang Y, Wei S, Chen L, Xue L, Tian H, et al. Bixin protects against kidney interstitial fibrosis through promoting STAT6 degradation. Front Cell Dev Biol. (2020) 8:576988. doi: 10.3389/fcell.2020.576988
19. Centers for Disease Control Prevention/National Center for Health Statistics. About the National Health and Nutrition Examination Survey. (2017). Available online at: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm#data (accessed April 27, 2021).
20. Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol. (2013) 8:1389–95. doi: 10.2215/CJN.11661112
21. Kant AK, Graubard BI, Atchison EA. Intakes of plain water, moisture in foods and beverages, and total water in the adult US population–nutritional, meal pattern, and body weight correlates: National Health and Nutrition Examination Surveys 1999-2006. Am J Clin Nutr. (2009) 90:655–63. doi: 10.3945/ajcn.2009.27749
22. Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T. The US department of agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am J Clin Nutr. (2008). doi: 10.1093/ajcn/88.2.324
23. Lauretani F, Semba RD, Dayhoff-Brannigan M, Corsi AM, Di Iorio A, Buiatti E, et al. Low total plasma carotenoids are independent predictors of mortality among older persons: the InCHIANTI study. Eur J Nutr. (2008) 47:335–40. doi: 10.1007/s00394-008-0732-9
24. Centers for Disease Control Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Available online at: https://www.cdc.gov/nchs/nhanes/index.htm (accessed April 27, 2021).
25. U.S. Department of Health and Human Services and U.S. (2021). Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th Edition. Available online at: https://www.dietaryguidelines.gov/ (accessed April 27, 2021).
26. Leve AS, Stevens LA. A New equation to estimate glomerular filtration rate. Ann Intern Med. (2009). doi: 10.7326/0003-4819-150-9-200905050-00006
27. He S, Ryan KA, Streeten EA, McArdle PF, Daue M, Trubiano D, et al. Prevalence, control, and treatment of diabetes, hypertension, and high cholesterol in the Amish. BMJ Open Diabetes Res Care. (2020) 8. doi: 10.1136/bmjdrc-2019-000912
28. Saydah SH, Siegel KR, Imperatore G, Mercado C, Gregg EW. The cardiometabolic risk profile of young adults with diabetes in the U. S. Diabetes care. (2019) 42:1895–902. doi: 10.2337/dc19-0707
29. Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. (2013) 368:341–50. doi: 10.1056/NEJMsa1211128
30. Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat. (2013) 2:1–24.
31. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat. (2013) 1–37.
32. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. (2010) 29:1037–57. doi: 10.1002/sim.3841
33. Shan Z, Guo Y, Hu FB, Liu L, Qi Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern Med. (2020) 180:513–23. doi: 10.1001/jamainternmed.2019.6980
34. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. (2011) 30:377–99. doi: 10.1002/sim.4067
35. Poulianiti KP, Kaltsatou A, Mitrou GI, Jamurtas AZ, Koutedakis Y, Maridaki M, et al. Systemic redox imbalance in chronic kidney disease: a systematic review. Oxid Med Cell Longev. (2016) 2016:8598253. doi: 10.1155/2016/8598253
36. Gwozdzinski K, Pieniazek A, Gwozdzinski L. Reactive oxygen species and their involvement in red blood cell damage in chronic kidney disease. Oxid Med Cell Longev. (2021) 2021:6639199. doi: 10.1155/2021/6639199
37. Jomova K, Valko M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur J Med Chem. (2013) 70:102–10. doi: 10.1016/j.ejmech.2013.09.054
38. Burton GW, Ingold KU. a-Carotene: an unusual type of lipid antioxidant. Science. (1984) 224:569–73. doi: 10.1126/science.6710156
39. Rubin LP, Ross AC, Stephensen CB, Bohn T, Tanumihardjo SA. Metabolic effects of inflammation on vitamin a and carotenoids in humans and animal models. Adv Nutr. (2017) 8:197–212. doi: 10.3945/an.116.014167
Keywords: chronic kidney disease, carotenoid, mortality, dietary intake, NHANES
Citation: Hu Y, Cai X, Zhang N, Li Y, Mao Y, Ge S, Yao Y and Gao H (2022) Relation Between Dietary Carotenoid Intake, Serum Concentration, and Mortality Risk of CKD Patients Among US Adults: National Health and Nutrition Examination Survey 2001–2014. Front. Med. 9:871767. doi: 10.3389/fmed.2022.871767
Received: 08 February 2022; Accepted: 18 May 2022;
Published: 08 July 2022.
Edited by:
Ying-Yong Zhao, Northwest University, ChinaReviewed by:
Jinwei Wang, Peking University, ChinaCopyright © 2022 Hu, Cai, Zhang, Li, Mao, Ge, Yao and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuwang Ge, Z2VzaHV3YW5nQHRqaC50am11LmVkdS5jbg==; Ying Yao, eWFveWluZ2trQDEyNi5jb20=; Hui Gao, Z2h1aTE5ODhAdGpoLnRqbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.