
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 30 June 2022
Sec. Ophthalmology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.870808
This article is part of the Research Topic Ocular Ultrasonography and Optical Coherence Tomography in the Optic Nerve Disease View all 9 articles
 Livio Vitiello1
Livio Vitiello1 Giulio Salerno1
Giulio Salerno1 Maddalena De Bernardo1*
Maddalena De Bernardo1* Olga D'Aniello1
Olga D'Aniello1 Luigi Capasso2
Luigi Capasso2 Giuseppe Marotta3
Giuseppe Marotta3 Nicola Rosa1
Nicola Rosa1In recent years, the measurement of optic nerve sheath diameter with ultrasound to detect the presence of increased intracranial pressure has widely spread. It can be qualitatively and effectively used to identify intracranial hypertension. Intracranial pressure can rise due to acute injury, cerebral bleeding, hydrocephalus, brain tumors and other space-occupying abnormalities, and it is linked to a high death rate. The purpose of this review is to give a general overview of the most relevant scientific publications on ultrasonographic evaluation of the optic nerve in case of brain injuries published in the last 30 years, as well as to analyze the limits of the most extensively used B-scan approach. Fifty-two papers chosen from the PubMed medical database were analyzed in this review. Our findings revealed that ocular ultrasound is an useful diagnostic tool in the management of intracranial hypertension when it exceeds a certain value or after head trauma. As a result, an ultrasound of the optic nerve can be extremely helpful in guiding diagnosis and treatment. The blooming effect is one of the most critical restrictions to consider when using B-scan ultrasonography. Since amplitude-scan ultrasound, also known as A-scan, does not have this limit, these two diagnostic techniques should always be used together for a more full, accurate, and trustworthy ultrasound examination, ensuring more data objectivity.
Post-traumatic damage and other space-occupying lesions, such as cerebral hemorrhage, brain abscesses and subdural or epidural hematomas, can produce increased intracranial pressure (ICP) (1). Intracranial hypertension can also develop in the context of venous blood flow obstacles, such as hypoxia-ischemia and venous sinus thrombosis, increased cerebral volume, infections and inflammatory processes, and hepatic or hypertensive encephalopathy. Moreover, ICP is sometimes caused by an unknown cause (1).
Intraventricular catheterization and intraparenchymal probes are the gold standard procedures for measuring ICP, together with lumbar puncture. They entail various dangers, including infection and bleeding and are therefore contraindicated in individuals who require long-term monitoring or who are inclined to coagulopathy or platelet diseases (2).
Moreover, lumbar puncture is contraindicated in case of brain tumors (3).
Over the years, different non-invasive methods for evaluating ICP have been developed (4). Because of its feasibility, repeatability, safety, and lack of radiation hazard or known side effects, ultrasonographic measurement of optic nerve sheath diameter (ONSD) has come to be accepted (5).
ICP rise will result in increase in ONSD in the retrobulbar compartment because the sheath encircling the optic nerve is an outgrowth of the dura-mater and is filled with cerebrospinal fluid (CSF) (6). Ultrasound is a well-established tool for evaluating optic nerve sheath dilatation, which aids clinicians in the management of intracranial hypertension, when it exceeds a particular value (7, 8). Moreover, increased ICP following a head injury must be recognized quickly in order to receive effective treatment (1). Bedside ultrasonography, which is available in most trauma centers, should be practical and rapid to use in this clinical condition (9, 10), or could even be used in a prehospital setting (11).
Moreover, it has been shown to be effective in monitoring the changes of ICP in case of idiopathic intracranial hypertension (12). In addition, to detect the correlation between ONSD and ICP and to find a cut off value is mandatory to establish the role of US in detecting the presence of increased ICP.
Considering all the aforesaid reasons, the purpose of this paper is to review the effectiveness and reliability of ocular ultrasonography in monitoring ICP in case of traumatic brain injury (TBI) and space-occupying lesions, through the analysis of the published scientific literature in the last 30 years.
We searched within the PubMed medical database and we entered search strings including terms related to “traumatic brain injury,” “ocular ultrasonography,” and “raised intracranial pressure.” The text terms were taken from current literature and/or associated bibliographies. Additional entries were found by manually searching bibliographies from the original searches.
The current study included only original researches, case reports, and case series on optic nerve ultrasonography examination for diagnosing intracranial hypertension and traumatic brain injury. The earliest date of publication was chosen for January 1990, and the search was completed in August 2021.
We found 125 items in our initial search, of which 13 were excluded because they are not directly related to the discussed topic. In addition, 15 papers dealing with increased ICP due to idiopathic intracranial hypertension and 45 papers related to surgical maneuvers were also excluded from the study.
Finally, 52 publications were included, 30 for intracranial hypertension due to space-occupying lesions and 22 for traumatic brain injury.
To get reproducible and reliable measurements of the ONSD is a very important topic. For such a purpose in the literature, several methods have been described.
The ONSD was measured for the first time by KC Ossoinig utilizing the so called Standardized A scan (7, 8).
Later on, several authors tried to measure the ONSD utilizing the B scan technique with transversal or coronal scans, as it is shown in Table 1.
Most of the authors utilized the probe over the closed lids, measuring the distance between the borders of an hyporeflective area surrounding the optic nerve, 3 mm behind the optic nerve insertion. Not all the authors utilized this approach.
Chen et al. placed the B scan probe over the open eyes, on the corneal temporal limbus, after topical anesthesia (23).
Topcuoglu et al. (24) suggested to measure both the outermost hypoechogenic space, which they claimed to correspond to the subarachnoid space and the diameter of the hyperechogenic space around the nerve. This way to measure the optic nerve was taken up in other studies (25, 26).
Ossoinig et al. were the first to suggest to measure the ONSD with ultrasound. They utilized the so called standardized A-scan probe that was performed placing the probe at the temporal limbus over an anesthetized eye (7, 8). This technique utilizes an 8 MHz non-focused probe, with a special S shaped amplification, which showed easily discerned high-reflective spikes at the interface between arachnoid and subarachnoid fluid (7, 8).
This technique was later utilized by Saenz et al. (27), who also utilized the so called “30 degrees test” introduced by KC Ossoinig (7, 8), and concluded that A-scan could assist differentiation of mild papilledema from pseudopapilledema (27).
Unfortunately, in some papers, the utilized technique was not specified (28, 29).
The use of ultrasound to evaluate the optic nerve sheath dilatation is a well-established method that help clinicians in the management of intracranial hypertension when exceeding a certain value. In fact, Karl Ossoinig had already proposed this diagnostic tool in the 1970's for the evaluation of intracranial pressure changes in several pathological conditions (7, 8).
In 1997, Hansen et al. (30) also understood the importance of the optic nerve sheath elasticity that allowed a detectable dilation in response to intracranial hypertension. They investigated the optic nerve sheath response to pressure during CSF absorption using repetitive optic nerve ultrasound B-mode scans on 12 patients.
During the years, many other authors have correlated the ONSD increase with ICP elevation and they have tried to find a cut-off that could be reliable in case of intracranial hypertension related to space-occupying lesions (Table 1).
Many studies have continued to emphasize the importance of optic nerve ultrasound, especially in Emergency Department (ED), where rapid decision-making can be crucial.
Albert et al. (31) found that an ONSD of more than 5.25 mm and an ONSD/ETD (eyeball transverse diameter) ratio of more than 0.232 on initial CT may identify malignant middle cerebral artery (MCA) stroke patients at high risk of developing malignant MCA syndrome, helping to schedule a decompressive craniectomy. These findings were also supported by the study of Güzeldag et al. (32), who claimed the predictive importance of optic nerve ultrasound in the management and follow-up of MCA infarction.
The possibility of associating changes in ONSD assessable by ultrasound with brain death (BD) was evaluated by Topcuoglu et al. (24) and Toscano et al. (33). In fact, they found that ONSD is significantly greater in subjects with BD. However, quantification of ONSD cannot discriminate BD subjects from comatose ones with raised ICP with a 100% certainty.
In addition, hyperacute stage of intracerebral hemorrhage (34–36), acutely fluctuating intracranial pressure (37), effect of osmotherapy (mannitol administration) (38) and use of Mesalazine (39, 40) are also all factors to concern when measuring the ONSD due to the possibility of rapid change in the obtained values. This demonstrated the optic nerve sheath sensibility to rapid ICP increase or decrease (41).
Kitano et al. (42) analyzed the ONSD enlargement due to vasogenic edema in a case of hypertensive encephalopathy either with no intracranial hypertension, and they demonstrated its lowering after reduction of patient's blood pressure.
Increased ICP after TBI requires rapid recognition to allow for appropriate treatments. Bedside ultrasound, which is available in most trauma units, should be feasible and quickly performed. For this reason, ultrasound optic nerve examination may provide useful information to guide diagnosis and therapy.
Most of the examined papers concerning brain injury, found a good correlation between ONSD and ICP (43–46).
Good sensitivity and specificity in the detection of increased ICP was found, even if the cut-off values where very different among the papers. Most of the papers agreed in determining the cut-off for the presence of increased ICP when ONSD was more than 5.0 mm (46–49), whereas other suggested different values, such as 4.1 mm (50), 5.205 mm (51), 5.7 mm (52), 5.86 mm (53), 5.95 mm (54), 6.6 mm (55), and 7 mm (56).
Moreover, Cardim et al. (57) found ONSD cut-off values should not be adjusted for sex or age for the ICP assessment in TBI patients.
On the contrary, Strumwasser et al. (58), correlating ONSD and ICP, found a weak connection between ONSD and ICP, suggesting that sonographic ONSD measurements were not reliable as a surrogate for elevated ICP in the absence of invasive monitoring. Similar results were obtained by Martin et al. (59) that, attempting to evaluate the reliability of ultrasound ONSD, and pulsatility index calculation with transcranial Doppler found that both tools did not allow clinicians to rigorously ascertain early ICP increase.
Some others suggested to use ONSD increase together with other parameters to increase the usefulness of non-invasive diagnostic tool.
Singer et al. (60) suggested to combine ONSD measurement with pupillometry and transcranial ultrasonographic Doppler to estimate and follow ICP in patients with severe TBI.
Robba et al. (61) recommended to combine ONSD ultrasonography and transcranial venous straight sinus Doppler to identify critical patients with increased ICP. In another paper, the same authors suggested to utilize the so called “diastolic arterial formula” (62), which include the ONSD measurement, with a sensitivity and specificity of 85%.
The same research group (63) proposed to utilize ICP values, ultrasound ONSD and the pressure reactivity index to obtain information about patients at risk of developing intracranial hypertension and impaired self-regulation.
Du et al. (64) evaluated 52 adults undergoing craniotomy for TBI. The ONSD and ETD of each eyeball were measured by ultrasound and CT within 24 h after an optical probe was placed in the lateral ventricle, finding ONSD/ETD as a good and reliable parameter for predicting increased intracranial pressure in patients with TBI. Concerning ONSD ultrasound measurements, the authors found a mean ONSD of 5.7 mm in TBI patients.
The importance of ocular ultrasound for the assessment, progression, and management of intracranial hypertension in ICUs has been widely discussed (65–67). The ONSD measurement 3 mm behind the eyeball has shown different cut-offs between normality and ICP increase.
This explains why several authors suggest, both in cerebral hypertension due to organic causes and in TBI, to combine ONSD measurements with other parameters, such as ONSDE, ONSDI and ONSD/ETD ratio.
The combination of very different cut-offs and the suggestion to combine ONSD measurements with other parameters, clearly show not only that B-scan technique is not very reliable, but also that ONSD measurements cannot be performed by inexperienced operators, needing a physics and anatomical knowledge of the structures to be examined (68–71).
Among B-scan ultrasonography limitations, there is the presence of the so-called “blooming effect” (72, 73).
It is related to the absence of a standard sensitivity setting, which means that changing the gain during the examination can affect the result. For example, some lesions may appear larger than they really are when the gain is turned down and smaller when the gain is turned up (74).
Conversely, A-scan ultrasonography does not have this limitation (75). It is not only suitable for detecting and diagnosing ocular and orbital lesions but also much more reliable in making the measurements. Above all, it is not affected by the blooming effect. This makes it much more suitable for measuring the ONSD because this technique shows easily discernible high reflective spikes from the interface between the arachnoid and subarachnoid fluid (Figure 1). Unfortunately, in almost all the studies considered in this review, B-scan ultrasonography was used, and only Saenz et al. used the standardized A-scan (27), which allows to perform the “30-degree test.”
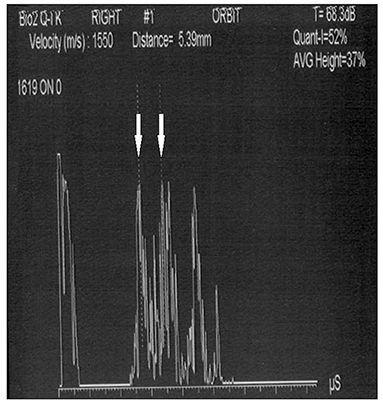
Figure 1. Standardized A-scan image of the optic nerve, showing the two high spikes (white arrows) used to perform ONSD measurement (5.39 mm).
This dynamic test measures optic nerve thickness in the primary gaze position and after shifting gaze 30 degrees temporally from the primary position (76).
Moreover, this method allows to differentiate whether the enlargement of the optic nerve head is due to intracranial hypertension or to solid lesions or papillitis (77). In fact, in the case of intracranial hypertension, after the “30-degree test,” the stretching of the optic nerve and sheath causes a drastic reduction in the ONSD thickness.
In conclusion, to increase the accuracy and precision, we recommend combining the use of the B-scan method with the standardized A-scan technique. This method requires some training, but is the only way to have precise, repeatable and affordable results.
LV, GS, OD'A, and LC analyzed the literature and wrote the original draft. MD, GM, and NR conceived the article and reviewed the manuscript. All authors read and approved the final manuscript.
The research was funded with the FARB grant from the University of Salerno.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Canac N, Jalaleddini K, Thorpe SG, Thibeault CM, Hamilton RB. Review: pathophysiology of intracranial hypertension and noninvasive intracranial pressure monitoring. Fluids Barriers CNS. (2020) 17:40. doi: 10.1186/s12987-020-00201-8
2. Tavakoli S, Peitz G, Ares W, Hafeez S, Grandhi R. Complications of invasive intracranial pressure monitoring devices in neurocritical care. Neurosurg Focus. (2017) 43:E6. doi: 10.3171/2017.8.FOCUS17450
3. Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med. (1999) 159:2681–5. doi: 10.1001/archinte.159.22.2681
4. Vitiello L, De Bernardo M, Capasso L, Cornetta P, Rosa N. Optic nerve ultrasound evaluation in animals and normal subjects. Front Med. (2022) 8:797018. doi: 10.3389/fmed.2021.797018
5. Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intens Care Med. (2011) 37:1059–68. doi: 10.1007/s00134-011-2224-2
6. Blaivas M, Theodoro D, Sierzenski PR. Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med. (2003) 10:376–81. doi: 10.1111/j.1553-2712.2003.tb01352.x
7. Ossoinig KC, Cennamo G, Frazier-Byrne S. Echographic differential diagnosis of optic nerve lesions. In Thijssen JM, Verbeek AM, editors. Documenta Ophthalmologica Proceedings Series vol 29, Ultrasonography in Ophthalmology. Hague: Springer Netherlands (1981). doi: 10.1007/978-94-009-8659-6_51
8. Ossoinig KC. Standardized echography of the optic nerve. In Till P, editor. Documenta Ophthalmologica Proceedings Series. Vol. 55. Dordrecht: Springer (1990). doi: 10.1007/978-94-011-1846-0_1
9. De Bernardo M, Rosa N. Comment on “optic nerve sheath diameter ultrasound evaluation in intensive care unit: possible role and clinical aspects in neurological critical patients' daily monitoring”. Biomed Res Int. (2018) 2018:6154357. doi: 10.1155/2018/6154357
10. Vitiello L, De Bernardo M, Guercio Nuzio S, Mandato C, Rosa N, Vajro P. Pediatric liver diseases and ocular changes: What hepatologists and ophthalmologists should know and share with each other. Dig Liver Dis. (2020) 52:1–8. doi: 10.1016/j.dld.2019.11.009
11. Houzé-Cerfon CH, Bounes V, Guemon J, Le Gourrierec T, Geeraerts T. Quality and feasibility of sonographic measurement of the optic nerve sheath diameter to estimate the risk of raised intracranial pressure after traumatic brain injury in prehospital setting. Prehosp Emerg Care. (2019) 23:277–83. doi: 10.1080/10903127.2018.1501444
12. De Bernardo M, Vitiello L, Rosa N. Optic nerve evaluation in idiopathic intracranial hypertension. AJNR Am J Neuroradiol. (2019) 40:E36. doi: 10.3174/ajnr.A6091
13. Kimberly HH, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. (2008) 15:201–4. doi: 10.1111/j.1553-2712.2007.00031.x
14. Aduayi OS, Asaleye CM, Adetiloye VA, Komolafe EO, Aduayi VA. Optic nerve sonography: A noninvasive means of detecting raised intracranial pressure in a resource-limited setting. J Neurosci Rural Pract. (2015) 6:563–7. doi: 10.4103/0976-3147.165347
15. Lee SU, Jeon JP, Lee H, Han JH, Seo M, Byoun HS, et al. Optic nerve sheath diameter threshold by ocular ultrasonography for detection of increased intracranial pressure in Korean adult patients with brain lesions. Medicine. (2016) 95:e5061. doi: 10.1097/MD.0000000000005061
16. Bolesch S, von Wegner F, Senft C, Lorenz MW. Transcranial ultrasound to detect elevated intracranial pressure: comparison of septum pellucidum undulations and optic nerve sheath diameter. Ultrasound Med Biol. (2015) 41:1233–40. doi: 10.1016/j.ultrasmedbio.2014.12.023
17. Salahuddin N, Mohamed A, Alharbi N, Ansari H, Zaza KJ, Marashly Q, et al. The incidence of increased ICP in ICU patients with non-traumatic coma as diagnosed by ONSD and CT: a prospective cohort study. BMC Anesthesiol. (2016) 16:106. doi: 10.1186/s12871-016-0267-1
18. Wang L, Feng L, Yao Y, Wang Y, Chen Y, Feng J, et al. Optimal optic nerve sheath diameter threshold for the identification of elevated opening pressure on lumbar puncture in a Chinese population. PLoS ONE. (2015) 10:e0117939. doi: 10.1371/journal.pone.0117939
19. Komut E, Kozaci N, Sönmez BM, Yilmaz F, Komut S, Yildirim ZN, et al. Bedside sonographic measurement of optic nerve sheath diameter as a predictor of intracranial pressure in ED. Am J Emerg Med. (2016) 34:963–7. doi: 10.1016/j.ajem.2016.02.012
20. Moretti R, Pizzi B, Cassini F, Vivaldi N. Reliability of optic nerve ultrasound for the evaluation of patients with spontaneous intracranial hemorrhage. Neurocrit Care. (2009) 11:406–10. doi: 10.1007/s12028-009-9250-8
21. Moretti R, Pizzi B. Optic nerve ultrasound for detection of intracranial hypertension in intracranial hemorrhage patients: confirmation of previous findings in a different patient population. J Neurosurg Anesthesiol. (2009) 21:16–20. doi: 10.1097/ANA.0b013e318185996a
22. Major R, Girling S, Boyle A. Ultrasound measurement of optic nerve sheath diameter in patients with a clinical suspicion of raised intracranial pressure. Emerg Med J. (2011) 28:679–81. doi: 10.1136/emj.2009.087353
23. Chen Q, Chen W, Wang M, Sun X, Sha Y, Li Z, et al. High-resolution transbulbar ultrasonography helping differentiate intracranial hypertension in bilateral optic disc oedema patients. Acta Ophthalmol. (2017) 95:e481–5. doi: 10.1111/aos.13473
24. Topcuoglu MA, Arsava EM, Bas DF, Kozak HH. Transorbital ultrasonographic measurement of optic nerve sheath diameter in brain death. J Neuroimaging. (2015) 25:906–9. doi: 10.1111/jon.12233
25. Naldi A, Pivetta E, Coppo L, Cantello R, Comi C, Stecco A, et al. Ultrasonography monitoring of optic nerve sheath diameter and retinal vessels in patients with cerebral hemorrhage. J Neuroimaging. (2019) 29:394–9. doi: 10.1111/jon.12604
26. Pansell J, Bell M, Rudberg P, Friman O, Cooray C. Optic nerve sheath diameter measurement by ultrasound: Evaluation of a standardized protocol. J Neuroimaging. (2022) 32:104–10. doi: 10.1111/jon.12936
27. Saenz R, Cheng H, Prager TC, Frishman LJ, Tang RA. Use of a-scan ultrasound and optical coherence tomography to differentiate papilledema from pseudopapilledema. Optom Vis Sci. (2017) 94:1081–89. doi: 10.1097/OPX.0000000000001148
28. Liu D, Li Z, Zhang X, Zhao L, Jia J, Sun F, et al. Assessment of intracranial pressure with ultrasonographic retrobulbar optic nerve sheath diameter measurement. BMC Neurol. (2017) 17:188. doi: 10.1186/s12883-017-0964-5
29. Gökcen E, Caltekin I, Savrun A, Korkmaz H, Savrun ST, Yildirim G. Alterations in optic nerve sheath diameter according to cerebrovascular disease sub-groups. Am J Emerg Med. (2017) 35:1607–11. doi: 10.1016/j.ajem.2017.04.073
30. Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg. (1997) 87:34–40. doi: 10.3171/jns.1997.87.1.0034
31. Albert AF, Kirkman MA. Clinical and radiological predictors of malignant middle cerebral artery infarction development and outcomes. J Stroke Cerebrovasc Dis. (2017) 26:2671–79. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.041
32. Güzeldag S, Yilmaz G, Tuna M, Altuntaş M, Özdemir M. Measuring the optic nerve sheath diameter with ultrasound in acute middle cerebral artery stroke patients. J Stroke Cerebrovasc Dis. (2021) 30:105523. doi: 10.1016/j.jstrokecerebrovasdis.2020.105523
33. Toscano M, Spadetta G, Pulitano P, Rocco M, Di Piero V, Mecarelli O, et al. Optic nerve sheath diameter ultrasound evaluation in intensive care unit: Possible role and clinical aspects in neurological critical patients' daily monitoring. Biomed Res Int. (2017) 2017:1621428. doi: 10.1155/2017/1621428
34. Skoloudík D, Herzig R, Fadrná T, Bar M, Hradílek P, Roubec M, et al. Distal enlargement of the optic nerve sheath in the hyperacute stage of intracerebral haemorrhage. Br J Ophthalmol. (2011) 95:217–21. doi: 10.1136/bjo.2009.172890
35. Koo HW. Real-time change of optic nerve sheath diameter after rebleeding of ruptured intracranial dissecting aneurysm. J Cerebrovasc Endovasc Neurosurg. (2020) 22:287–93. doi: 10.7461/jcen.2020.E2020.11.003
36. Bender M, Lakicevic S, Pravdic N, Schreiber S, Malojcic B. Optic nerve sheath diameter sonography during the acute stage of intracerebral hemorrhage: a potential role in monitoring neurocritical patients. Ultrasound J. (2020) 12:47. doi: 10.1186/s13089-020-00196-1
37. Rajajee V, Fletcher JJ, Rochlen LR, Jacobs TL. Comparison of accuracy of optic nerve ultrasound for the detection of intracranial hypertension in the setting of acutely fluctuating vs. stable intracranial pressure: post-hoc analysis of data from a prospective, blinded single center study. Crit Care. (2012) 16:R79. doi: 10.1186/CC11336
38. Launey Y, Nesseler N, Le Maguet P, Mallédant Y, Seguin P. Effect of osmotherapy on optic nerve sheath diameter in patients with increased intracranial pressure. J Neurotrauma. (2014) 31:984–8. doi: 10.1089/neu.2012.2829
39. Rottembourg D, Labarthe F, Arsene S, Jonville-Béra AP, Maurage C, Rolland JC. Headache during mesalamine therapy: a case report of mesalamine-induced pseudotumor cerebri. J Pediatr Gastroenterol Nutr. (2001) 33:337–8. doi: 10.1097/00005176-200109000-00021
40. Rosa N, Giamundo A, Jura A, Iaccarino G, Romano A. Mesalazine-associated benign intracranial hypertension in a patient with ulcerative colitis. Am J Ophthalmol. (2003) 136:212–3. doi: 10.1016/S0002-9394(02)02275-4
41. Williams P. Optic nerve sheath diameter as a bedside assessment for elevated intracranial pressure. Case Rep Crit Care. (2017) 2017:3978934. doi: 10.1155/2017/3978934
42. Kitano T, Nezu T, Mukai T, Uemura J, Wada Y, Yagita Y. A case of hypertensive encephalopathy with enlarged optic nerve sheath measured by transorbital sonography. J Stroke Cerebrovasc Dis. (2017) 26:e20–1. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.014
43. Geeraerts T, Launey Y, Martin L, Pottecher J, Vigué B, Duranteau J, et al. Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intensive Care Med. (2007) 33:1704–11. doi: 10.1007/s00134-007-0797-6
44. Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. (2007) 49:508–14. doi: 10.1016/j.annemergmed.2006.06.040
45. Geeraerts T, Merceron S, Benhamou D, Vigué B, Duranteau J. Non-invasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Intensive Care Med. (2008) 34:2062–7. doi: 10.1007/s00134-008-1149-x
46. Goel RS, Goyal NK, Dharap SB, Kumar M, Gore MA. Utility of optic nerve ultrasonography in head injury. Injury. (2008) 39:519–24. doi: 10.1016/j.injury.2007.09.029
47. Maissan IM, Dirven PJ, Haitsma IK, Hoeks SE, Gommers D, Stolker RJ. Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J Neurosurg. (2015) 123:743–7. doi: 10.3171/2014.10.JNS141197
48. Kaur A, Gautam PL, Sharma S, Singh VP, Sharma S. Bedside ultrasonographic assessment of optic nerve sheath diameter as a means of detecting raised intracranial pressure in neuro-trauma patients: A cross-sectional study. Ann Indian Acad Neurol. (2021) 24:63–8. doi: 10.4103/aian.AIAN_51_20
49. Chang T, Yan X, Zhao C, Zhang Y, Wang B, Gao L. Noninvasive evaluation of intracranial pressure in patients with traumatic brain injury by transcranial Doppler ultrasound. Brain Behav. (2021) 11:e2396. doi: 10.1002/brb3.2396
50. Thotakura AK, Marabathina NR, Danaboyina AR, Mareddy RR. Role of serial ultrasonic optic nerve sheath diameter monitoring in head injury. Neurochirurgie. (2017) 63:444–8. doi: 10.1016/j.neuchi.2017.06.001
51. Raffiz M, Abdullah JM. Optic nerve sheath diameter measurement: a means of detecting raised ICP in adult traumatic and non-traumatic neurosurgical patients. Am J Emerg Med. (2017) 35:150–3. doi: 10.1016/j.ajem.2016.09.044
52. Soldatos T, Karakitsos D, Chatzimichail K, Papathanasiou M, Gouliamos A, Karabinis A. Optic nerve sonography in the diagnostic evaluation of adult brain injury. Crit Care. (2008) 12:R67. doi: 10.1186/cc6897
53. Lovrenčić-Huzjan A, Bosnar-Puretić M, Hustić I, Kobasić I, Budišić M, Corić L, et al. Optic nerve sheath sonography is a promising tool for assessment of raised intracranial pressure in patients admitted to neurological intensive care unit. Acta Clin Croat. (2020) 59:50–4. doi: 10.20471/acc.2020.59.01.06
54. Dhanda A, Singh GP, Bindra A. Correlation between invasive and noninvasive technique of intracranial pressure measurement in children with traumatic brain injury: An observational study. J Neurosurg Anesthesiol. (2020) 34:221–6. doi: 10.1097/ANA.0000000000000751
55. Çelik K, Demiryurek BE. The association between intracranial pressure and optic nerve sheath diameter on patients with head trauma. Arq Neuropsiquiatr. (2021) 79:879–85. doi: 10.1590/0004-282x-anp-2020-0478
56. Cammarata G, Ristagno G, Cammarata A, Mannanici G, Denaro C, Gullo A. Ocular ultrasound to detect intracranial hypertension in trauma patients. J Trauma. (2011) 71:779–81. doi: 10.1097/TA.0b013e3182220673
57. Cardim D, Czosnyka M, Chandrapatham K, Badenes R, Bertuccio A, Noto AD, et al. Effects of age and sex on optic nerve sheath diameter in healthy volunteers and patients with traumatic brain injury. Front Neurol. (2020) 11:764. doi: 10.3389/fneur.2020.00764
58. Strumwasser A, Kwan RO, Yeung L, Miraflor E, Ereso A, Castro-Moure F, et al. Sonographic optic nerve sheath diameter as an estimate of intracranial pressure in adult trauma. J Surg Res. (2011) 170:265–71. doi: 10.1016/j.jss.2011.03.009
59. Martin M, Lobo D, Bitot V, Couffin S, Escalard S, Mounier R, et al. Prediction of early intracranial hypertension after severe traumatic brain injury: a prospective study. World Neurosurg. (2019) 127:e1242–48. doi: 10.1016/j.wneu.2019.04.121
60. Singer KE, Wallen TE, Jalbert T, Wakefield D, Spuzzillo A, Sharma S, et al. Efficacy of noninvasive technologies in triaging traumatic brain injury and correlating with intracranial pressure: A prospective study. J Surg Res. (2021) 262:27–37. doi: 10.1016/j.jss.2020.12.042
61. Robba C, Cardim D, Tajsic T, Pietersen J, Bulman M, Donnelly J, et al. Ultrasound non-invasive measurement of intracranial pressure in neurointensive care: A prospective observational study. PLoS Med. (2017) 14:e1002356. doi: 10.1371/journal.pmed.1002356
62. Robba C, Cardim D, Tajsic T, Pietersen J, Bulman M, Rasulo F, et al. Non-invasive intracranial pressure assessment in brain injured patients using ultrasound-based methods. Acta Neurochir Suppl. (2018) 126:69–73. doi: 10.1007/978-3-319-65798-1_15
63. Robba C, Donnelly J, Cardim D, Tajsic T, Cabeleira M, Citerio G, et al. Optic nerve sheath diameter ultrasonography at admission as a predictor of intracranial hypertension in traumatic brain injured patients: a prospective observational study. J Neurosurg. (2019) 132:1279–85. doi: 10.3171/2018.11.JNS182077
64. Du J, Deng Y, Li H, Qiao S, Yu M, Xu Q, et al. Ratio of optic nerve sheath diameter to eyeball transverse diameter by ultrasound can predict intracranial hypertension in traumatic brain injury patients: a prospective study. Neurocrit Care. (2020) 32:478–85. doi: 10.1007/s12028-019-00762-z
65. Cornetta P, Marotta G, De Bernardo M, Vitiello L, Rosa N. Ultrasound and optic neuritis. Am J Emerg Med. (2019) 37:1598. doi: 10.1016/j.ajem.2019.02.001
66. De Bernardo M, Vitiello L, Rosa N. Optic nerve ultrasonography for evaluating increased intracranial pressure in severe preeclampsia. Int J Obstet Anesth. (2019) 38:147. doi: 10.1016/j.ijoa.2018.12.010
67. De Bernardo M, Vitiello L, Cornetta P, Rosa N. Ocular ultrasound evaluation of optic nerve sheath diameter in military environments. Mil Med Res. (2019) 6:16. doi: 10.1186/s40779-019-0207-8
68. De Bernardo M, Rosa N. Optic nerve sheath diameter measurement in patients with idiopathic normal-pressure hydrocephalus. Eur J Neurol. (2018) 25:e24. doi: 10.1111/ene.13530
69. Tenuta M, De Bernardo M, Rosa N. Comments on “neuromuscular ultrasonography of cranial nerves.” J Clin Neurol. (2017) 13:212–3. doi: 10.3988/jcn.2017.13.2.212
70. De Bernardo M, Vitiello L, Rosa N. Ocular ultrasound assessment to estimate the risk of increased intracranial pressure after traumatic brain injury in prehospital setting. Prehosp Emerg Care. (2019) 23:746–7. doi: 10.1080/10903127.2019.1568652
71. Marotta G, De Bernardo M, Vitiello L, Rosa N. Ocular ultrasonography to detect intracranial hypertension in subarachnoid hemorrhage patients. Neurocrit Care. (2020) 33:855–6. doi: 10.1007/s12028-020-00999-z
72. Vitiello L, De Bernardo M, Rosa N. Ocular ultrasonography in patients with subarachnoid hemorrhage and Terson syndrome. Can J Anaesth. (2020) 67:1072–3. doi: 10.1007/s12630-020-01600-z
73. De Bernardo M, Marotta G, Rosa N. Sonography of the optic nerve sheath diameter. J Ultrasound Med. (2018) 37:1845. doi: 10.1002/jum.14486
74. De Bernardo M, Rosa N. Transbulbar B-mode sonography in multiple sclerosis: clinical and biological relevance. Ultrasound Med Biol. (2018) 44:508. doi: 10.1016/j.ultrasmedbio.2017.10.002
75. Capasso L, De Bernardo M, Vitiello L, Rosa N. Ultrasound options for measuring optic nerve sheath diameter in children. Pediatr Crit Care Med. (2021) 22:e329–30. doi: 10.1097/PCC.0000000000002676
76. De Bernardo M, Vitiello L, Rosa N. Ultrasound optic nerve sheath diameter evaluation in patients undergoing robot-assisted laparoscopic pelvic surgery. J Robot Surg. (2019) 13:709–10. doi: 10.1007/s11701-019-00966-7
Keywords: head trauma, intracranial pressure, optic nerve, optic nerve sheath diameter, ultrasonography
Citation: Vitiello L, Salerno G, De Bernardo M, D'Aniello O, Capasso L, Marotta G and Rosa N (2022) Ultrasound Detection of Intracranial Hypertension in Brain Injuries. Front. Med. 9:870808. doi: 10.3389/fmed.2022.870808
Received: 07 February 2022; Accepted: 13 June 2022;
Published: 30 June 2022.
Edited by:
Alessandro Meduri, University of Messina, ItalyCopyright © 2022 Vitiello, Salerno, De Bernardo, D'Aniello, Capasso, Marotta and Rosa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maddalena De Bernardo, bWRlYmVybmFyZG9AdW5pc2EuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.