
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med., 17 May 2022
Sec. Intensive Care Medicine and Anesthesiology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.870637
This article is part of the Research TopicMicronutrients and Critically Ill PatientsView all 5 articles
Introduction: Serum phosphate level is often deranged during critical illness. Hyperphosphatemia, as a marker of disease severity, attracts more and more attention. This study aimed to evaluate the impact of hyperphosphatemia on clinical outcomes in critically ill patients.
Methods: We searched for relevant studies in PubMed, EMBASE, and the Cochrane database up to Jan 10, 2022. Two authors independently screened studies, extracted data, and assessed the study quality. Meta-analyses were performed to determine hyperphosphatemia prevalence and evaluate its relationship with prognosis and important clinical outcomes. We also conducted subgroup analysis and sensitivity analyses to explore the sources of heterogeneity.
Results: Ten studies with 60,358 patients met the inclusion criteria. These studies were moderate to high quality. The median prevalence of hyperphosphatemia was 30% (range from 5.6 to 45%). Patients with hyperphosphatemia had a significantly higher risk of all-cause mortality than those without (OR 2.85; 95% CI, 2.35 to 3.38, P < 0.0001). Subgroup analyses, sensitivity analyses, and regression analyses further confirmed these results. In addition, patients with hyperphosphatemia required more CRRT (OR 4.96; 95% CI, 2.43 to 10.2, P < 0.0001) but not significantly increased duration of mechanical ventilation (mean difference, MD 0.13, 95% CI −0.04 to 0.30; P = 0.138), length of stay in intensive care unit (ICU) (SMD 0.164 day, 95% CI −0.007 to 0.335; P = 0.06), and length of stay in hospital (SMD 0.005 day, 95% CI −0.74 to 0.75; P = 0.99).
Conclusions: Our results indicated that hyperphosphatemia was associated with all-cause mortality in critically ill patients. However, due to the retrospective design of the included studies, more prospective, well-designed research is required in the future.
Systematic Review Registration: [https://doi.org/10.37766/inplasy2021.12.0130], identifier [INPLASY2021120130].
Serum phosphorus disorders, including hypophosphatemia and hyperphosphatemia, are common findings in critical illness (1, 2). The incidence of acute blood phosphorus abnormalities admitted to the intensive care unit (ICU) can be as high as 45% (3). Many endogenous and exogenous factors can lead to serum phosphorus dyshomeostasis, such as intestinal loss, sepsis, trauma, impaired renal clearance, catabolic processes, diabetic ketoacidosis, acid-base disorders, and many medications (1, 4, 5). In total, 30% of serum phosphate exists in inorganic form and constitutes the clinically measured component of blood phosphorus with a normal reference range of 0.7–1.5 mmoL/L (6). Despite low levels in the body, serum phosphorus plays a vital role in many physiological produces such as energy metabolism, bone metabolism, and cell signaling and affects almost all organ systems (7). Therefore, it is crucial to maintain normal blood phosphorus levels.
Compare to hypophosphatemia that has been attracted more clinical attention (1), acute hyperphosphatemia in the ICU setting seems to be often overlooked, which might relate to its usually mild symptoms, mainly limited to concomitant hypocalcemia (e.g., muscle weakness, hand and foot twitching, cardiac arrhythmias, and hypotension). Meanwhile, treatment of hyperphosphatemia requires identification and correction of the underlying cause, with the therapy goals of normalizing serum phosphorus concentrations (2.7–4.5 mg/dL), avoiding or resolving symptoms of hyperphosphatemia, and maintaining serum (calcium × phosphorus) at less than 55–60 mg2/dL2 (8). On the other hand, the importance of hyperphosphatemia has been largely and consistently emphasized in patients with chronic kidney disease (CKD) and chronic cardiovascular disease (9, 10); however, studies on its prognosis in critically ill patients have come to different conclusions. Thus, it remains unclear whether hyperphosphatemia correlates with prognosis or is merely a marker of severe disease.
Several studies on hyperphosphatemia in critical ill patients have been published recently, though some have a modest sample size (4, 9, 11, 12). Therefore, with the power of meta-analysis, we aimed to perform a systematic review and meta-analysis to explore the prognostic value of hyperphosphatemia in such a patient population.
We followed the PRISMA guidance to prepare the present systematic review and meta-analysis (13) (Additional File 1). The protocol for this study has been registered on the International Platform of Registered Systematic Review and Meta-analysis Protocols database (INPLASY2021120130) and is available in full on inplasy.com (https://inplasy.com/inplasy-2021-12-0130/).
Two authors (W-HZ and YY) independently systemically searched PubMed, EMBASE, and Cochrane Library from inception through Jan 10, 2022 (the last search date). Search terms included “hypophosphatemia,” AND “critical care,” “critically ill,” “intensive care,” using a combination of the Medical Subject Headings and keywords. Details of the complete search strategy are attached in Additional File 2. The search included all study designs with no language restriction. We imported the studies into Endnote to exclude duplicate studies and then screened literature (titles, abstracts, and full texts) for relevant articles. We also reviewed the reference lists of included full-text articles for more potential studies. Disagreements were solved by discussions between the two authors or decided by a third author (H-BH).
We considered including the studies if they met the following PICOS criteria: (1) Patients: adult (>18 years old) critically ill patients; (2) Exposure: hyperphosphatemia (defined by the authors); (3) Comparator: normal phosphate level; and (4) Outcomes: mortality and other clinical outcomes; (5) Study design: cohort, case-control or randomized controlled design. We excluded the studies that did not report clear hyperphosphatemia definitions, provide prognostic outcomes, or studies recruited children or pregnant women. Studies available only in comment, abstract or meeting reports were also excluded. We contacted the authors if the data on predefined outcomes from their studies were required.
Two reviewers (W-HZ and YY) independently extracted data from the included studies, such as study design, country, publication year, sample size, setting, follow up and severity of illness, outcomes of interest, hyperphosphatemia criteria and prevalence, and methodological quality.
The above two independent reviewers (W-HZ and YY) evaluated the quality of each included study using the Newcastle-Ottawa Scale (NOS) for cohort studies (14). The NOS has three domains based on the cohort’s selection, the groups’ comparability, and the outcomes’ quality. A study was given a maximum of one point in each item within the Selection and Outcome domains and a maximum of two points for the Comparability domain. The scale scores ranged from 0 to 9 points, with 8 or 9 classified as high quality, 6 or 7 as moderate quality, and five or below as low quality. Discrepancies were identified and resolved through discussion.
The primary outcome was the short-term mortality rate (ICU or hospital mortality or mortality within a 90-day follow-up after admission, the longest observation period was preferred). Secondary outcomes were the duration of mechanical ventilation (MV), length of stay in the hospital (LOS), and CRRT requirement.
We combined the results from all relevant studies to estimate the pooled odds ratio (OR) and associated 95% confidence intervals (CIs) for dichotomous outcomes and to estimate mean differences (MD) and 95% CI as the effective results for continuous outcomes. Before data analysis, we estimated the mean from median and standard deviations (SD) from IQR using the previous study’s methods, if require (15). According to the different reporting forms of mortality provided by the included studies, we separately conducted two types of meta-analyses for the risk estimation between hyperphosphatemia and all-cause mortality as follows: (1) For studies reporting mortality rates (crude data) between patients with or without hyperphosphatemia, we calculated OR and associated 95% CI as the primary outcome. (2) For studies utilizing regression analyses to adjust for effects of confounding factors on mortality, we combine the mortality estimates with corresponding standard errors by the generic inverse variance method in the sensitivity analysis. Thus, the OR and hazard ratio (HR) reported in these studies required natural logarithmic transformations before merging. If not stated otherwise, we preferred adjusted analysis results.
We used the I2 statistic to examine the heterogeneity across these trials. An I2 > 50% indicates significant heterogeneity. We chose a fixed-effect model for I2 < 50% and a random-effect model for I2 ≥ 50%. We assessed publication bias by visually exploring funnel plots for asymmetry, Egger’s test, and Begg’s test. In all analyses, we used STATA version 14.0 (STATA Corporation, College Station, TX, United States).
To explore the potential influence factors for the primary outcome, we performed subgroup analyses by pooling studies with the following properties: (1) Geographic location: Asian or not Asian countries; (2) Sample size:>200 or ≤200; (3) Measured unit: >4.5 mg/dL or >1.5 mmol/L; (4) Follow-up duration: long-term (mortality at least 6 months follow-up after hospital discharge) or short-term; (5) Using initial serum phosphate level or not, and (6) Mortality prevalence: mortality rate <30%, between 30 and 45%, or >45%. Additionally, we conducted sensitivity analyses by excluding one study at a time to explore whether an individual study’s particular result drove the results. We also conducted sensitivity analyses by pooling studies that only reported 30-, 60-, and 90-day, hospital discharge, ICU mortality, or utilizing regression analyses to investigate the relationship between hyperphosphatemia and mortality.
Our search identified 817 records from defined databases. After screening the titles and abstracts, 18 were qualified for full-text review. Based on the full-text evaluation, we excluded 8 studies summarized in Additional File 3 (Supplementary Table 3) with exclusion reasons based on the full-text evaluation. Thus, the remaining 10 studies (4, 9, 11, 12, 16–21) were included in the final meta-analysis (Figure 1).
The main characteristics of the included studies concerning 60,358 adult patients are summarized in Table 1, and the predefined outcome details are provided in Table 2. These studies were retrospective designs and were published between 2013 and 2021 from 7 countries (Austria n = 3, China n = 3, Korea n = 1, United States n = 1, Sweden n = 1, and Saudi Arabia n = 1). Included studies focused on patients from ICU (4, 9, 11, 12, 17, 18, 20, 22), trauma centers (21), or severe burn (19), with sample sizes ranging from 197 to 32,333. On average, the mean age of patients was 59.5 years, and 60% of them were male. The mortality endpoints varied among the included studies. The 8 of the 10 included studies reported the prevalence of hyperphosphatemia, with the mean prevalence ranging from 5.6–45%. When calculated, the mean prevalence was 21% (11,397/54,012). Nine studies provided mortality rates between hyperphosphatemia and normal serum phosphorus, we investigated mortality between groups using OR and associated 95% CI as the primary outcome. Meanwhile, nine studies utilized regression analyses to adjust for effects of confounding factors on mortality was pooled and investigated using sensitivity analysis. In addition, a total of nine included studies (4, 9, 11, 12, 16, 17, 19–21) provided data on hypophosphatemia in the prognosis and important clinical outcomes, and the main conclusions were summarized in Additional File 5. Overall, almost all the included studies showed no association between mortality and baseline hypophosphatemia.
The quality assessment is available in Additional File 6 and shows the study quality ranging from moderate to high quality, with scores ranging from 7 to 9 on the NOS scale. Nine studies were considered high quality, and the other two studies were of moderate quality.
All included studies reported the outcome of all-cause mortality. Nine studies with 47,570 patients compared mortality rates between patients with or without hyperphosphatemia (4, 11, 12, 16–21). Among these patients, 11,504 had hyperphosphatemia, and 3,469 died (30.2%) compared to 36,173 patients of normal serum phosphorus level with 5,250 deaths (14.5%) observed. Hyperphosphatemia was associated with a higher risk of mortality (OR 2.82, 95% CI 2.35 to 3.38, random effect model) with the heterogeneity of 86.5% observed (Figure 2). We found no evidence of publication bias with Begg’s test or Egger’s test (P = 0.443), and the funnel plots did not suggest asymmetry (Additional File 7).
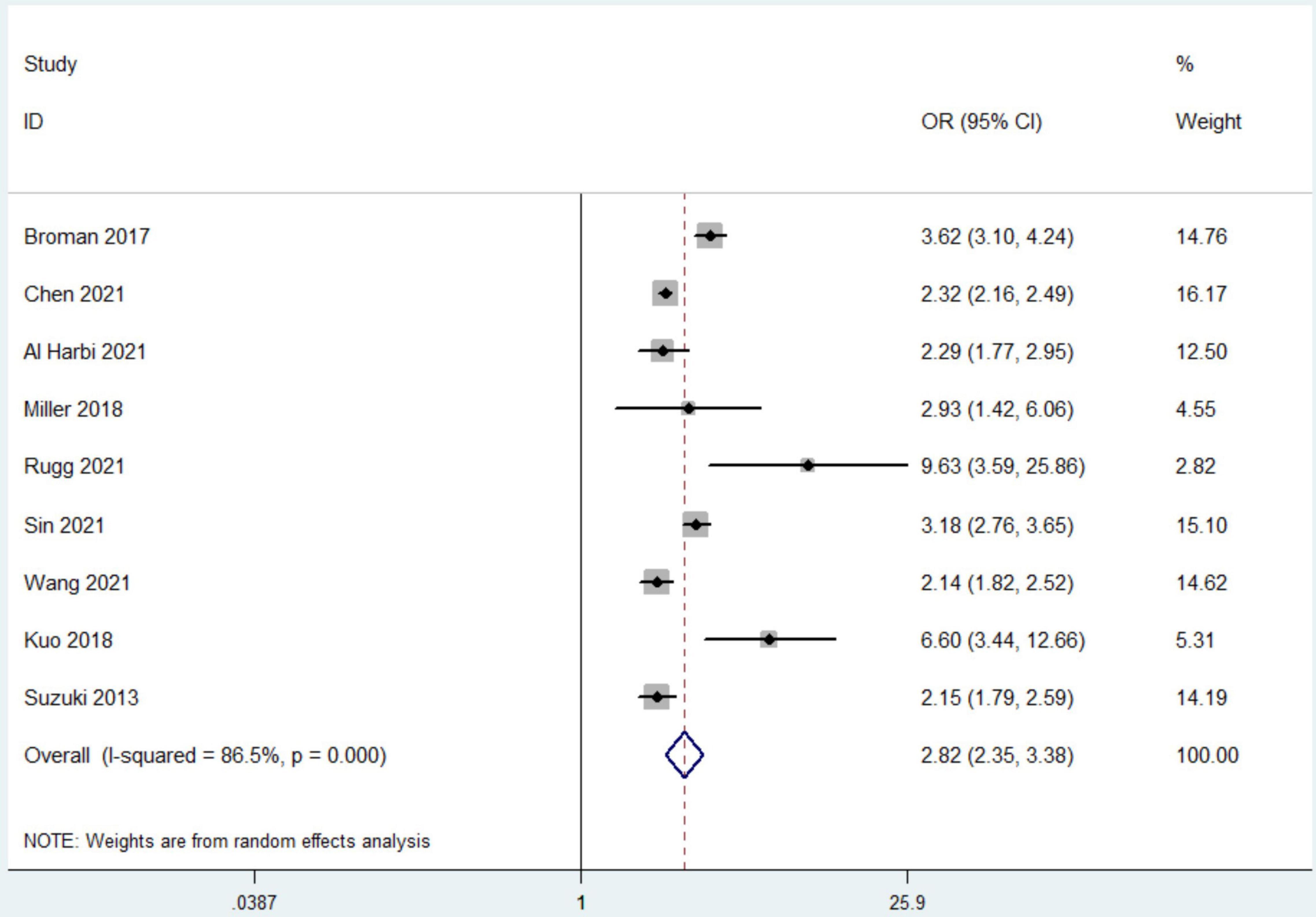
Figure 2. The forest plot in assessing the impact of hyperphosphatemia on mortality. CI, confidence interval; OR, odds ratio. Pooled analyses from crude data provided by associated included studies.
Subsequently, we conducted subgroup analyses to explore potential heterogeneity sources. In terms of between groups mortality analyses, hyperphosphatemia was associated with a higher risk of mortality in all the subgroups, including sample size, geographic location, using initial serum phosphate level or not, measured unit, duration of follow-up, or mortality prevalence (all P-values < 0.0001 with I2 ranging from 0 to 90.9%) (Table 3).
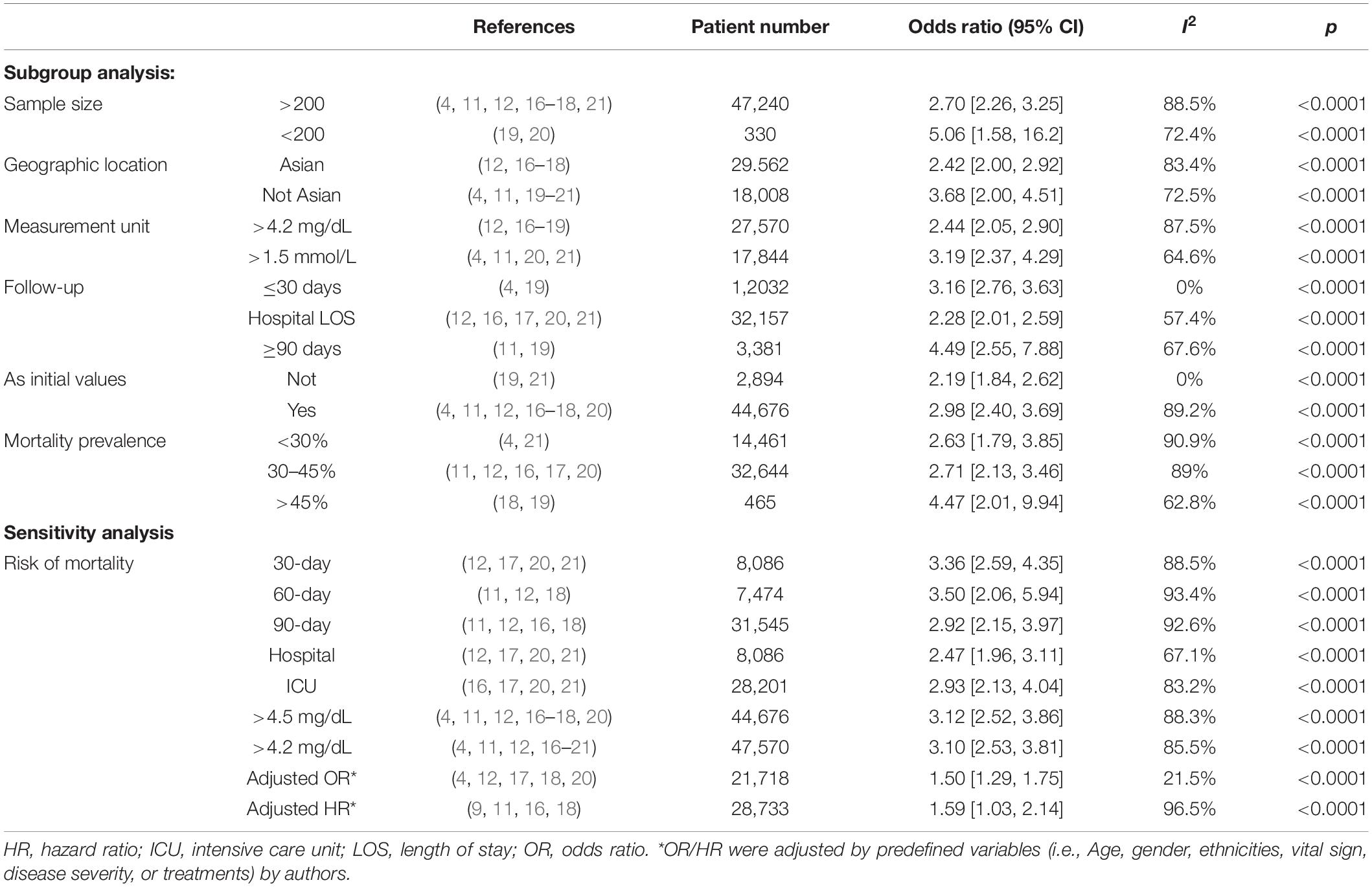
Table 3. Subgroup analysis and sensitivity analyses on risk of mortality in patients with hyperphosphatemia.
In the sensitivity analyses, hyperphosphatemia were associated with significantly increased mortality when compared with normal serum phosphorus at hospital discharge, ICU duration, 30-, 60-, and 90-day after recruitment, with the OR of 2.47 (95% CI 1.96, 3.11), 2.93 (95% CI 2.13, 4.04), 3.36 (95% CI 2.59, 4.35), 3.50 (95% CI 2.06, 5.94), and 2.92 (95% CI 2.15, 3.97), respectively. As to the nine studies utilizing regression analyses, five with multivariate logistic regression analyses (4, 12, 17, 18, 20) and four with Cox proportional hazard regression analyses (9, 11, 16, 18) were pooled for assessing the risk for mortality. Pooled data showed hyperphosphatemia was associated with an increased risk of mortality (adjusted OR 1.50, 95% CI 1.29–1.75, P < 0.0001, and adjusted HR 1.59, 95% CI 1.03–2.14, P < 0.00001), respectively (Table 3).
Data from 7 studies indicated that hyperphosphatemia was not associated with decreased ICU LOS (n = 44,052; SMD 0.16 day, 95% CI −0.01 to 0.33; I2 = 97.4%; P = 0.06) (4, 12, 16, 17, 19–21) (Figure 3). Five studies reported hospital LOS as an interest (12, 17, 19–21). Pooled the analysis showed that hyperphosphatemia did not significantly increase hospital LOS compared to normal serum phosphorus (n = 8,250; SMD 0.005 day, 95% CI −0.74 to 0.75; I2 = 99.4%; P = 0.99) (Figure 4). CRRT requirement during the follow-up was available from 6 studies (4, 16–19, 21) and indicated significantly increase in the hyperphosphatemia group (n = 40,291; OR 4.96, 98% CI 2.43 to 10.12; I2 = 94.9%; P < 0.0001) (Figure 5). In addition, the duration of MV was reported by 3 studies and was comparable between the two groups with or without hyperphosphatemia (n = 4,128; SMD 0.13, 98% CI−0.04 to 0.30; I2 = 76.1%; P = 0.138) (17, 19, 21) (Figure 6).

Figure 3. The forest plot in assessing the impact of hyperphosphatemia on length of stay in ICU. CI, confidence interval, SMD, standardized mean difference. Pooled analyses from crude data provided by associated included studies.
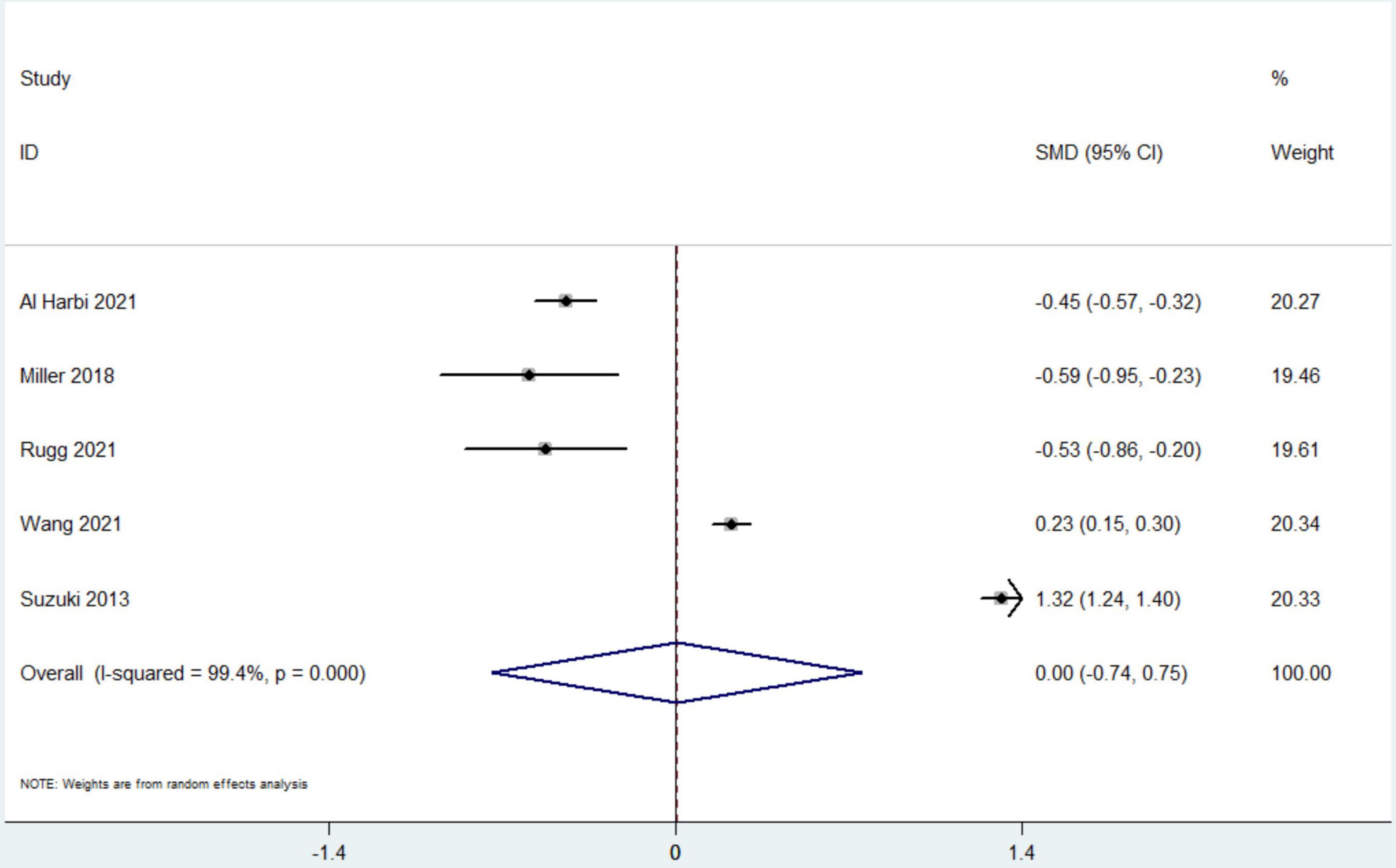
Figure 4. The forest plot in assessing the impact of hyperphosphatemia on length of stay in hospital. CI, confidence interval; SMD, standardized mean difference. P Pooled analyses from crude data provided by associated included studies.
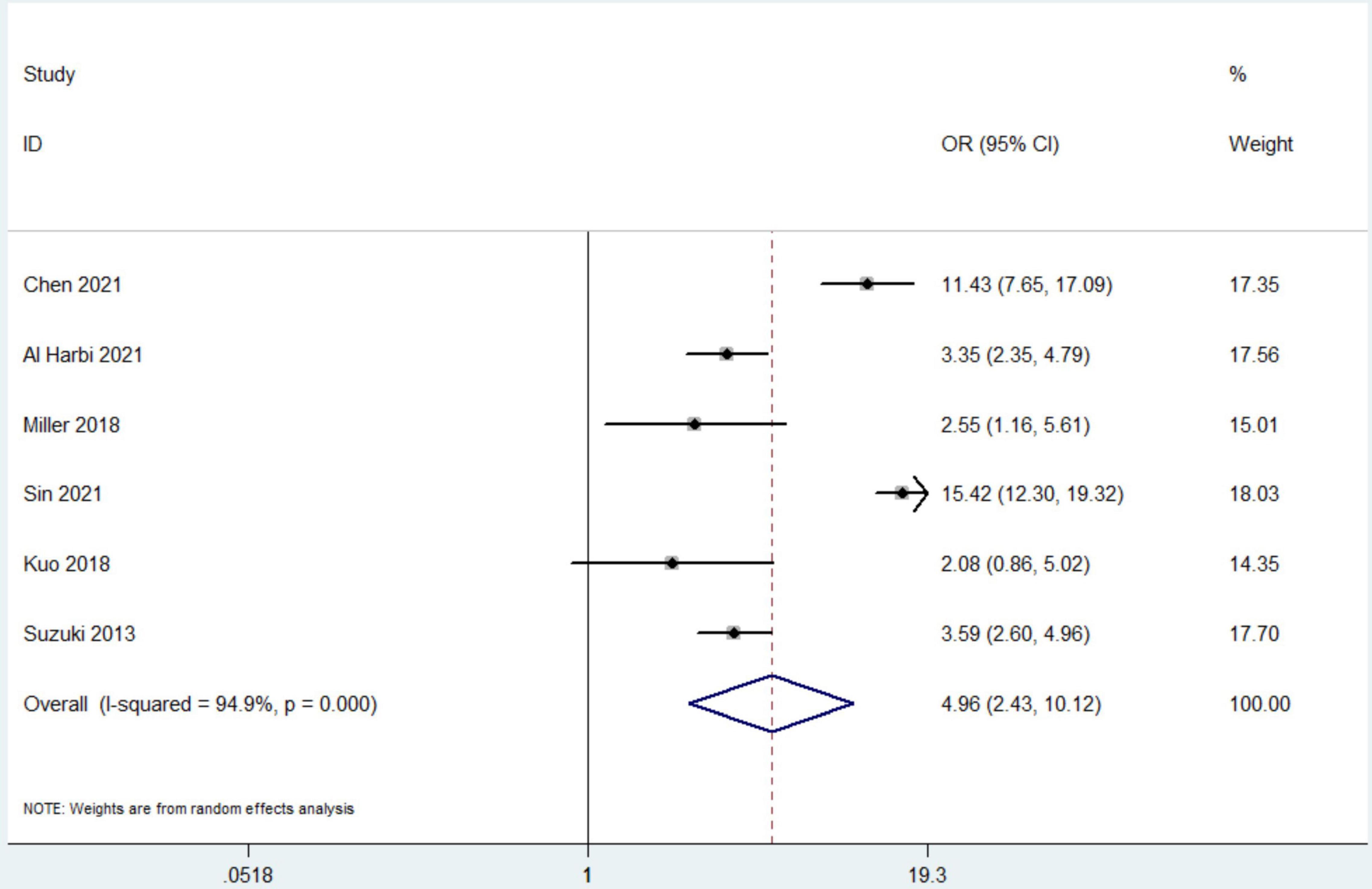
Figure 5. The forest plot in assessing the impact of hyperphosphatemia on risk of receiving continuous renal replacement therapy. CI, confidence interval, OR, odds ratio. Pooled analyses from crude data provided by associated included studies.
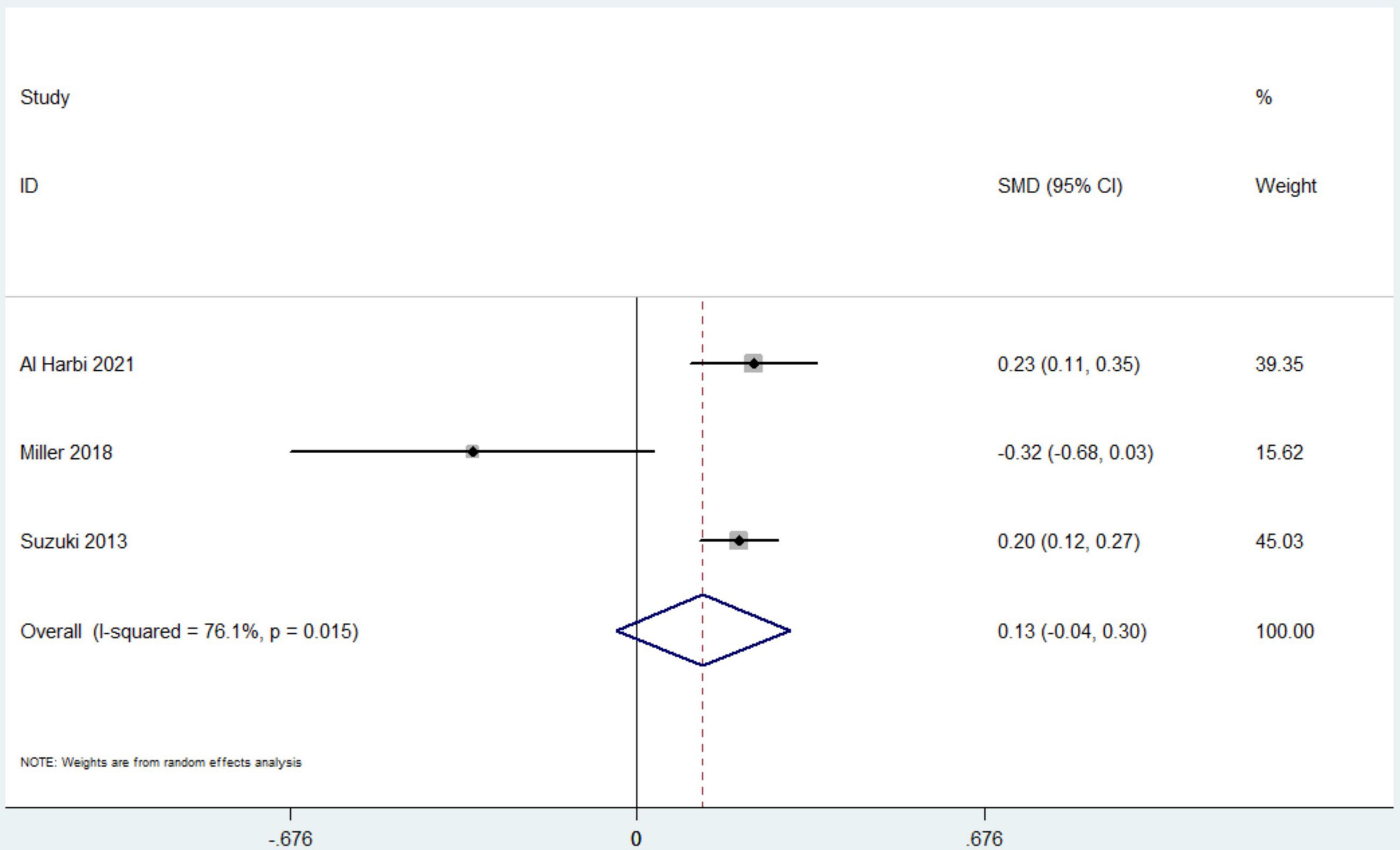
Figure 6. The forest plot in assessing the impact of hyperphosphatemia on duration of mechanical ventilation. CI, confidence interval; SMD, standardized mean difference. Pooled analyses from crude data provided by associated included studies.
To the best of our knowledge, the current study is the first systematic review and meta-analysis investigating the impact of hyperphosphatemia on clinical outcomes. Our results showed that critically ill patients usually suffer hyperphosphatemia, with the prevalence ranging from 5.6 to 67.9% (4, 9, 11, 12, 16–21). Hyperphosphatemia was an independent risk factor of short-term mortality by a nearly twofold increase. Further subgroup analyses and sensitivity analyses confirmed this finding. Moreover, hyperphosphatemia was associated with a significantly increased CRRT requirement, but not the duration of MV, ICU and hospital LOS.
Our study has several strengths. The current meta-analysis provided robust evidence to fill the gaps in previous guidelines (22); that is, hyperphosphatemia can be evaluated to assess prognosis in critically ill patients. Second, our findings are consistent with the previous guideline of other patient populations, including CKD, cardiovascular disease with normal renal function, and tumor lysis syndrome, highlighting adverse clinical outcomes with hyperphosphatemia (10, 23). As a result, our study adds a new population of evidence. Third, we have thoroughly evaluated mortality risk from several general ICU and trauma/burn center cohorts, including mortality between hyperphosphatemia with normal levels and linear relationships between hyperphosphatemia and mortality. In addition, we included a large sample size of more than 60,000 cases, with sufficient statistical power to allow for subgroup analyses and sensitivity analyses based on different potential influencing factors. The results were reliable and further supported the robustness of our main results.
Our results showed that the incidence of hyperphosphatemia in the included studies was approximately 21% (11,397/54,012, 9 studies), but the incidence varied widely between studies (17–45%). This might be related to the different ICU populations selected by the included studies. As in several studies recruiting non-selected ICU patients, the hyperphosphatemia incidence ranged from 18.5 to 26.3% (4, 11, 16, 19, 21). In one study focusing only on patients requiring CRRT, hyperphosphatemia was found in 721 (67.5%) patients before starting CRRT and remained in 399 (40.6%) at 24 h after CRRT initiation (9). For polytrauma patients, the incidence was about 33.7% (21). However, it is note that all these studies retrospectively included only patients with serum phosphate levels measured, so these reported incidence rates may be underestimated.
Compared with a bulk of literature focusing on the origins and consequences of hyperphosphatemia in chronic renal and cardiovascular disease in non-ICU patients (10), the causes of hyperphosphatemia in ICU patients are much more complex. Besides decreased renal clearance or too large an intake (e.g., phosphate-containing laxatives), ischemic tissue injury, such as intestinal or critical limb ischemia, coronary heart disease, cardiac arrest, lactic acidosis and diabetic ketoacidosis may cause hyperphosphatemia (24–29). However, we could not further evaluate the association between the causes and mortality in the present meta-analysis because specific data from included studies were not available. Also, patients may have multiple origins of hyperphosphatemia and have undergone different therapeutic management to restore normal blood phosphorus concentrations (4, 9, 11, 12, 16–21).
Our results showed a lower survival rate in patients with hyperphosphatemia, with a mean mortality rate of approximately 30.4% (15–58.5%) (4, 9, 11, 12, 16–21). However, the relevance of hyperphosphatemia to prognosis needs further discussion. First, most included studies assessed baseline blood phosphorus concentrations at ICU admission, rather than described overall phosphate exposure to describe the effect of hyperphosphatemia on ICU patients. Only one included study added to this gap (19). In this retrospective cohort study of 197 mechanically ventilated patients with severe sepsis or septic shock, Miller and colleagues found that time-weighted hypophosphite patients were more likely to die within 28 days of admission to the ICU (19). More studies are needed in the future to focus on patients’ phosphate levels throughout their ICU stay, which may better assess the impact of phosphate status on the prognosis. Second, hyperphosphatemia appears to be associated with mortality in a dose-dependent manner. In a study focusing on sepsis patients with abnormal initial phosphate concentration, the authors suggested that moderate to severe hyperphosphatemia, but not mild hyperphosphatemia, was an independent prognostic factor of mortality (30). This appears to partly explain the variations in mortality rates among the ICU hyperphosphatemic patients.
Third, the prognosis of hyperphosphatemia might be related to its treatment responsiveness. In the study by Kuo et al. (18), the authors reported that patients with severe burns and initial hyperphosphatemia had lower 90-day survival. Furthermore, they found the worst survival among patients with persistently high phosphate levels among patients who survived longer than 3 days. Similar results were also found in the study by Jung and colleagues (9), who demonstrated that patients with increased phosphate levels during 24 h were at a higher risk of death than those with stable (P = 0.001) or decreased phosphate levels (P < 0.001). These results suggested that timely correction of hyperphosphatemia may be a necessary therapeutic measure. On the other hand, mortality rates were higher in patients without improvement after treatment of hyperphosphatemia, again suggesting that residual hyperphosphatemia still had prognostic value (9).
Interestingly, most included studies (9/10) concluded that hypophosphatemia did not predict prognosis (4, 9, 11, 12, 16, 17, 19–21 (Appendix File 4). However, as another phosphorus disorder, hyperphosphatemia is an independent prognostic predictor, not just a marker of severity. Thus, it should deserve more clinical attention. In addition, in several studies reporting a higher CRRT requirement in the hyperphosphatemic group (4, 12, 16–18, 20, 21), there was clear evidence of more renal injury in the hyperphosphatemic group, including more renal failure patients (12, 16–18) or higher creatinine levels (4, 21). This evidence supports the idea that renal failure is a significant cause of hyperphosphatemia. Phosphate homeostasis is strongly dependent on adequate renal excretion. Thus, from this perspective, more CRRT requirements is likely to reflect renal failure as the cause of hyperphosphatemia rather than hyperphosphatemia per se. Also, all the included studies did not provide indications for the use of CRRT in hyperphosphatemia and its therapeutic effects. Therefore, we could not draw an association between phosphate levels and CRRT requirements based on the results of the current meta-analysis.
Several limitations must be considered in our meta-analysis. First, the observational design of all included studies excluded any causal inference. Meanwhile, only patients who underwent serum phosphorus testing were included in retrospective studies, which is prone to selection bias. Second, most studies assessed only baseline serum phosphate levels at ICU admission, ignoring assessments of serum phosphate levels over time. Third, most studies lack data on levels of parathyroid hormone, FGF23, and 1,25 -dihydroxyvitamin D3. Fourth, in addition to traumatic populations, most studies focus on unselected critically ill patients, and the uneven distribution of different underlying diseases in these studies may also exert different prognostic values. Fifth, in the subgroup and sensitivity analyses, we could not have considered all the confounding factors that might play a role in linking phosphate levels to ICU mortality, such as the effects of CRRT intensity, phosphatidic hormone levels, nutritional status, and artificial nutrition. Sixth, only a few studies suggest the severity of phosphate abnormalities and their prognostic impact, but the lack of a grading scale prevents further studies. Finally, whether correction of hyperphosphatemia could reduce mortality in ICU patients was still required further large-sample, prospective studies to confirm.
The hyperphosphatemia is commonly seen and varied in ICU patients. Hyperphosphatemia is a reliable factor for overall mortality in such a patient population. These findings were further confirmed in subgroup and sensitivity analysis. However, due to the retrospective design of the included studies, more prospective, well-designed research is required in the future.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
H-BH contributed to the conception of the study, analysis and drafting of the manuscript, and were responsible for the integrity of the work as a whole and from inception to publication of the manuscript. W-HZ, YY, and HZ contributed to data collection and analysis. YX contributed to design and revisions of this manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Yi-Bing Zhu, MD, for her assistance in searching the literature and reviewing statistics section.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.870637/full#supplementary-material
1. Sin JCK, King L, Ballard E, Llewellyn S, Laupland KB, Tabah A. Hypophosphatemia and outcomes in ICU: a systematic review and meta-analysis. J Intensive Care Med. (2021) 36:1025–35. doi: 10.1177/0885066620940274
2. Lee JW. Fluid and electrolyte disturbances in critically ill patients. Electrolyte Blood Press. (2010) 8:72–81. doi: 10.5049/EBP.2010.8.2.72
3. Gaasbeek A, Meinders AE. Hypophosphatemia: an update on its etiology and treatment. Am J Med. (2005) 118:1094–101. doi: 10.1016/j.amjmed.2005.02.014
4. Sin JCK, Laupland KB, Ramanan M, Tabah A. Phosphate abnormalities and outcomes among admissions to the intensive care unit: a retrospective multicentre cohort study. J Crit Care. (2021) 64:154–9. doi: 10.1016/j.jcrc.2021.03.012
5. Koumakis E, Cormier C, Roux C, Briot K. The causes of hypo-and hyperphosphatemia in humans. Calcif Tissue Int. (2021) 108:41–73. doi: 10.1007/s00223-020-00664-9
6. Hernando N, Wagner CA. Mechanisms and regulation of intestinal phosphate absorption. Compr Physiol. (2018) 8:1065–90. doi: 10.1002/cphy.c170024
7. Subramanian R, Khardori R. Severe hypophosphatemia. Pathophysiologic implications, clinical presentations, and treatment. Medicine. (2000) 79:1–8. doi: 10.1097/00005792-200001000-00001
8. Block GA, Port FK. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: recommendations for a change in management. Am J Kidney Dis. (2000) 35:1226–37. doi: 10.1016/s0272-6386(00)70064-3
9. Jung SY, Kwon J, Park S, Jhee JH, Yun HR, Kim H, et al. Phosphate is a potential biomarker of disease severity and predicts adverse outcomes in acute kidney injury patients undergoing continuous renal replacement therapy. PLoS One. (2018) 13:e0191290. doi: 10.1371/journal.pone.0191290
10. Moon H, Chin HJ, Na KY, Joo KW, Kim YS, Kim S, et al. Hyperphosphatemia and risks of acute kidney injury, end-stage renal disease, and mortality in hospitalized patients. BMC Nephrol. (2019) 20:362. doi: 10.1186/s12882-019-1556-y
11. Broman M, Wilsson AMJ, Hansson F, Klarin B. Analysis of hypo- and hyperphosphatemia in an intensive care unit cohort. Anesth Analg. (2017) 124:1897–905. doi: 10.1213/ANE.0000000000002077
12. Wang H, Zhang L, Liao W, Huang J, Xu J, Yang J, et al. Hyperphosphatemia rather than hypophosphatemia indicates a poor prognosis in patients with sepsis. Clin Biochem. (2021) 91:9–15. doi: 10.1016/j.clinbiochem.2021.01.016
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg (London, England). (2010) 8:336–41.
14. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
15. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
16. Chen Y, Luo M, Xu H, Zhao W, He Q. Association between serum phosphate and mortality in critically ill patients: a large retrospective cohort study. BMJ Open. (2021) 11:e044473. doi: 10.1136/bmjopen-2020-044473
17. Al Harbi SA, Al-Dorzi HM, Al Meshari AM, Tamim H, Abdukahil SAI, Sadat M, et al. Association between phosphate disturbances and mortality among critically ill patients with sepsis or septic shock. BMC Pharmacol Toxicol. (2021) 22:30. doi: 10.1186/s40360-021-00487-w
18. Kuo G, Lee CC, Yang SY, Hsiao YC, Chuang SS, Chang SW, et al. Hyperphosphatemia is associated with high mortality in severe burns. PLoS One. (2018) 13:e0190978. doi: 10.1371/journal.pone.0190978
19. Miller CJ, Doepker BA, Springer AN, Exline MC, Phillips G, Murphy CV. Impact of serum phosphate in mechanically ventilated patients with severe sepsis and septic shock. J Intensive Care Med. (2020) 35:485–93. doi: 10.1177/0885066618762753
20. Rugg C, Bachler M, Kammerlander R, Niederbrunner D, Bösch J, Schmid S, et al. ICU-Admission Hyperphosphataemia is related to shock and tissue damage, indicating injury severity and mortality in polytrauma patients. Diagnostics (Basel). (2021) 11:1548. doi: 10.3390/diagnostics11091548
21. Suzuki S, Egi M, Schneider AG, Bellomo R, Hart GK, Hegarty C. Hypophosphatemia in critically ill patients. J Crit Care. (2013) 28:536.e539-519.
22. Palafox-Serdán F, Luna-Montiel OA, Pablo-Franco SE, Guillen-Tejada DL, Carreño-Vázquez SD, Silva Pereira TS, et al. Nutritional guideline for the management of Mexican patients with CKD and hyperphosphatemia. Nutrients. (2020) 12:3289. doi: 10.3390/nu12113289
23. Comité Nacional de Hematología. [Guideline for management of tumor lysis syndrome]. Arch Argent Pediatr. (2011) 109:77–82.
24. Dundar ZD, Cander B, Gul M, Karabulut KU, Kocak S, Girisgin S, et al. Serum intestinal fatty acid binding protein and phosphate levels in the diagnosis of acute intestinal ischemia: an experimental study in rabbits. J Emerg Med. (2012) 42:741–7. doi: 10.1016/j.jemermed.2011.05.051
25. Jung YH, Lee BK, Jeung KW, Youn CS, Lee DH, Lee SM, et al. Prognostic value of serum phosphate level in adult patients resuscitated from cardiac arrest. Resuscitation. (2018) 128:56–62. doi: 10.1016/j.resuscitation.2018.04.026
26. Zettervall SL, Soden PA, Ultee KH, Seldon C, Oh J, McGann K, et al. Elevated serum phosphate levels are associated with decreased amputation-free survival after interventions for critical limb ischemia. J Vasc Surg. (2017) 65:431–7. doi: 10.1016/j.jvs.2016.06.097
27. Kwiatkowska M, Chomicka I, Malyszko J. Rhabdomyolysis - induced acute kidney injury - an underestimated problem. Wiadomosci Lekarskie. (2020) 73:2543–8. doi: 10.36740/wlek202011137
28. Huijing F. Hyperphosphatemia and lactic acidosis. N Engl J Med. (1978) 298:112–3. doi: 10.1056/nejm197801122980217
29. Kebler R, McDonald FD, Cadnapaphornchai P. Dynamic changes in serum phosphorus levels in diabetic ketoacidosis. Am J Med. (1985) 79:571–6. doi: 10.1016/0002-9343(85)90053-1
Keywords: hyperphosphatemia, critically ill, mortality, meta-analysis, prognosis
Citation: Zheng W-H, Yao Y, Zhou H, Xu Y and Huang H-B (2022) Hyperphosphatemia and Outcomes in Critically Ill Patients: A Systematic Review and Meta-Analysis. Front. Med. 9:870637. doi: 10.3389/fmed.2022.870637
Received: 07 February 2022; Accepted: 05 April 2022;
Published: 17 May 2022.
Edited by:
Stefan J. Schaller, Charité Universitätsmedizin Berlin, GermanyReviewed by:
Annika Reintam Blaser, University of Tartu, EstoniaCopyright © 2022 Zheng, Yao, Zhou, Xu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Bin Huang, aGhiYTAyOTIyQGJ0Y2guZWR1LmNu; Yuan Xu, eHl1YW43NkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.