- 1Department of Corneal & External Eye Diseases, Singapore National Eye Centre, Singapore, Singapore
- 2Singapore Eye Research Institute, Singapore, Singapore
- 3Duke-NUS Medical School, Singapore, Singapore
- 4School of Materials Science and Engineering, Nanyang Technological University, Singapore, Singapore
Background: We evaluated the visual outcomes and complications of “endothelium-out” and “endothelium-in” Descemet membrane endothelial keratoplasty (DMEK) graft insertion techniques.
Materials and Methods: Electronic searches were conducted in CENTRAL, Cochrane databases, PubMed, EMBASE, ClinicalTrials.gov. Study designs included clinical trials, comparative observational studies, and large case series (≥25 eyes). PRISMA guidelines were used for abstracting data and synthesis. Random-effects models were employed for meta-analyses.
Results: 21,323 eyes (95 studies) were included. Eighty-six studies reported on “endothelium-out” techniques; eight studies reported on “endothelium-in” techniques. One study compared “endothelium-out” to “endothelium-in” techniques. Eighteen “endothelium-out” studies reported that 42.5–85% of eyes achieved best-corrected visual acuity (BCVA) ≥20/25 at 6 months; pooled proportion of eyes achieving BCVA ≥20/25 at 6 months was 58.7% (95% CI 49.4–67.7%,15 studies). Three “endothelium-in” studies reported that 44.7–87.5% of eyes achieved BCVA of ≥20/25 at 6 months; pooled proportion of eyes achieving BCVA ≥20/25 at 6 months was 62.4% (95% CI 33.9–86.9%). Pooled mean endothelial cell loss was lower in the “endothelium-in” studies (28.1 ± 1.3%, 7 studies) compared to “endothelium-out” studies (36.3 ± 6.9%,10 studies) at 6 months (p = 0.018). Graft re-bubbling rates were higher in the “endothelium-out” studies (26.2%, 95% CI 21.9–30.9%, 74 studies) compared to “endothelium-in” studies (16.5%, 95% CI 8.5–26.4%, 6 studies), although statistical significance was not reached (p = 0.440). Primary graft failure rates were comparable between the two groups (p = 0.552). Quality of evidence was considered low and significant heterogeneity existed amongst the studies.
Conclusion: Reported rates of endothelial cell loss were lower in “endothelium-in” DMEK studies at 6 months compared to “endothelium-out” studies. Outcomes of “endothelium-in” techniques were otherwise comparable to those reported in “endothelium-out” studies. Given the technical challenges encountered in “endothelium-out” procedures, surgeons may consider “endothelium-in” techniques designed for easier intra-operative DMEK graft unfolding. “Endothelium-in” studies evaluating outcomes at longer time points are required before conclusive comparisons between the two techniques can be drawn.
Introduction
Background
Loss of vision from diseases of the corneal endothelium is the predominant indication for corneal transplantations (1, 2). Over the past 20 years, selective replacement of damaged corneal endothelium using lamellar keratoplasty procedures has significantly changed the management of endothelial diseases (3–5). The first posterior lamellar keratoplasty procedure was described in the late 1990s (6). In this report, the surgeon only partially replaced the recipient’s diseased corneal endothelium, avoiding full-thickness or penetrating keratoplasty (PK). Ensuing developments to the procedure have resulted in more advanced techniques of endothelial keratoplasty (EK), which are associated with better visual outcomes, lower graft rejection risks, and improved graft survival rates (5, 7–9). Unlike PK, these EK techniques avoid full-thickness corneal trephination and intra-operative “open sky” situations associated the risks of severe blinding complications such as suprachoroidal hemorrhage. Endothelial keratoplasties also maintain corneal biomechanics and the overall strength of the globe, important in protecting the eye from external trauma. Data from national corneal graft registries have reported that EK procedures have now overtaken full-thickness PK as the leading procedure for treating corneal endothelial diseases in several countries (1, 2, 10).
Currently, there are two predominant techniques of EK performed worldwide: Descemet’s stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK) (3, 4, 11). In DSAEK, the transplanted corneal grafts are comprised of donor endothelium, Descemet’s membrane (DM), and some posterior stroma. Advancement of the DSAEK technique, such as the development of devices for graft insertion and techniques to cut thinner grafts, has greatly simplified DSAEK (12–15). With more predictable visual outcomes and faster visual recovery compared to PK (8, 16, 17), many corneal surgeons are now performing DSAEK as the primary technique to treat end-stage corneal endothelial diseases (18, 19).
Descemet membrane endothelial keratoplasty is the more recent advancement in EK surgery (20). In DMEK, only the DM and the corneal endothelium are harvested from donor corneal tissues and transplanted, rendering them anatomically more accurate. As corneal stroma is not transplanted, changes in corneal profiles are avoided. Faster visual recovery and improved visual outcomes compared to DSAEK can thus be achieved (21–25). Lower rates of graft rejection have also been reported in DMEK compared to DSAEK (26).
Rationale for This Review
Current methods of DMEK graft transfer into the anterior chamber involve inserting the graft through a small clear corneal wound. Different surgical instruments have been described for the insertion of DMEK grafts. Examples of such instruments include glass injectors (27, 28) and intraocular lens cartridges (29, 30). All these instruments are designed to shield the DMEK graft scroll from the surgical wound. Nevertheless, the majority of techniques reported in published literature involves the loading and insertion of the DMEK graft with the endothelium on the outer surface (“endothelium-out”). Thus, the grafts are potentially at risk of endothelial cell loss due to endothelial contact with the walls of the injection devices. Furthermore, “endothelium-out” DMEK techniques all involve the injection of the entire scrolled graft into the anterior chamber. The un-scrolling of the free floating graft, following its insertion, can be difficult and unpredictable (31, 32). Such challenges have hindered corneal surgeons from adopting DMEK as a primary treatment for corneal endothelial failure (2, 3). In a recent eye banking report, DSAEK still accounted for over 55% of EK procedures performed in the United States (2).
“Endothelium-in” DMEK graft insertion techniques have been described more recently (33–37) (Figure 1). In these techniques, the harvested DM is folded and prevented from adopting its natural scroll with its endothelium on the outside. By maintaining the orientation of the DMEK graft during graft insertion, these “endothelium-in” techniques aim to provide more control in graft unscrolling following insertion into the eye. Nevertheless, the differences in surgical outcomes of either technique for DMEK graft insertion, “endothelium-in” or “endothelium-out,” remains unclear.
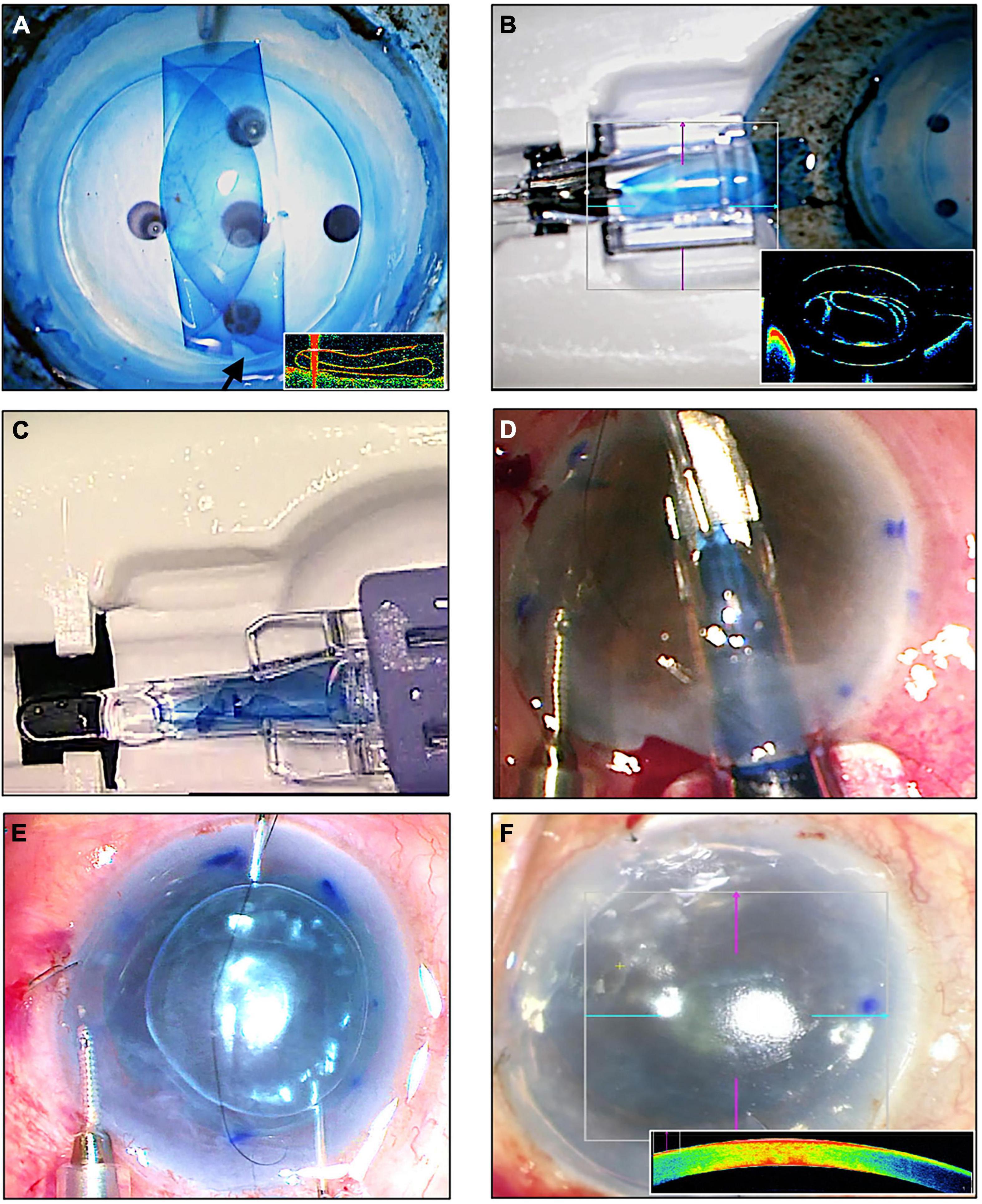
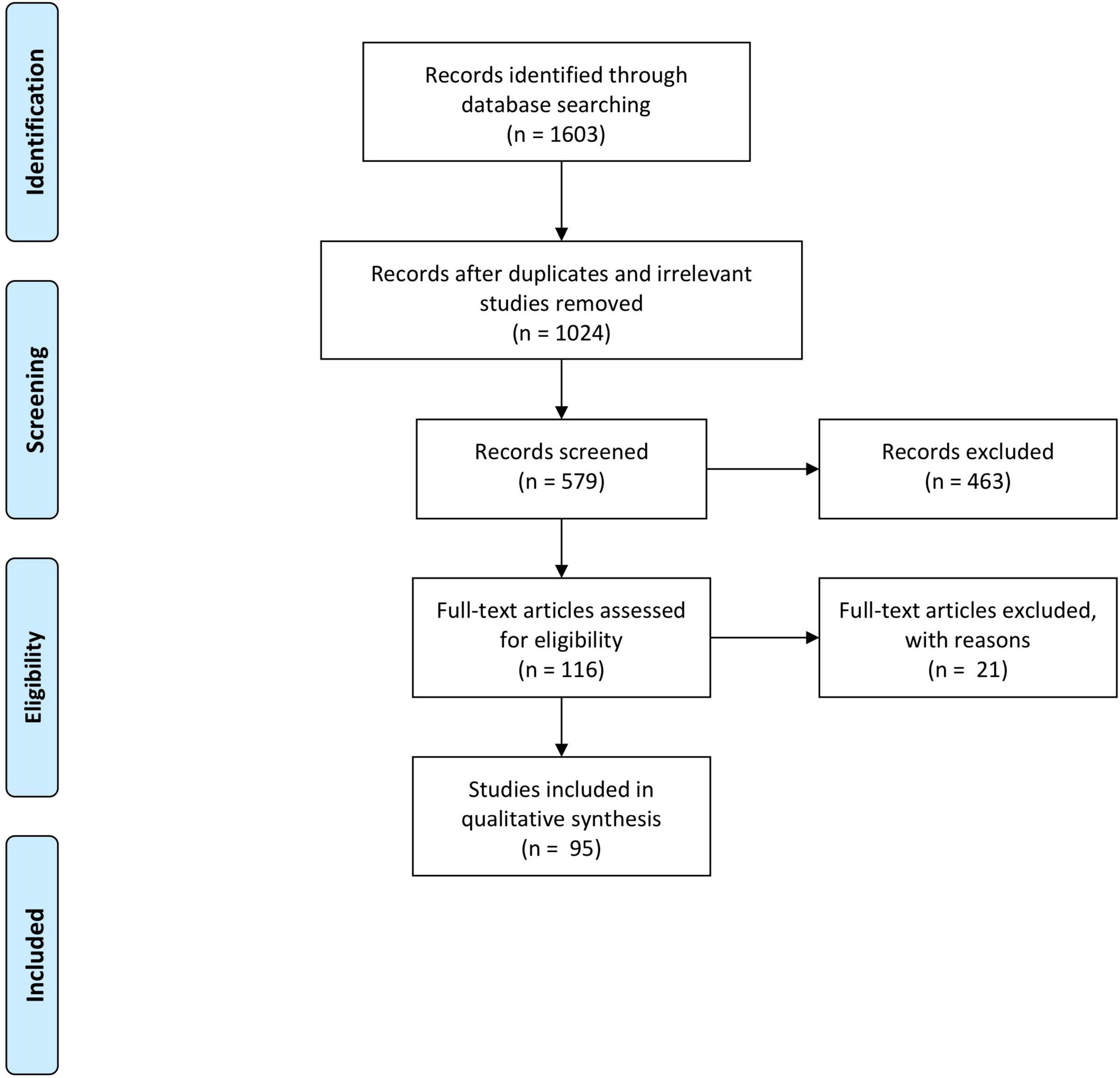
Figure 1. An “endothelium-in” surgical technique of Descemet membrane endothelial keratoplasty (DMEK) using the using the DMEK EndoGlide (Network Medical Products, United Kingdom). (A) DMEK graft is folded into a tri-fold with its endothelium in its inner surface; note the asymmetrical orientation marker (arrow); (inset) intraoperative optical coherence tomography (OCT) image of the tri-folded graft – note that the leaves of the tri-fold do not touch. (B) Graft is pulled and loaded into the EndoGlide; (inset) OCT image showing the tri-folded graft within the DMEK EndoGlide – note that the leaves of the tri-fold do not touch. (C) Customized clip fixed to the back of the EndoGlide; this creates a “closed system” after graft insertion maintaining anterior chamber stability. (D) Graft is drawn into the anterior chamber with micro-forceps with its endothelium facing down. (E) Unfolding of the graft with its orientation maintained whilst air is injected for tamponade. (F) Full air-gas tamponade of graft; (inset) intraoperative OCT showing a fully attached DMEK graft.
Objectives of This Review
This review aims to evaluate the published literature reporting the visual outcomes and complications of both “endothelium-out” and “endothelium-in” graft insertion techniques for DMEK.
Materials and Methods
This review was submitted to PROSPERO International prospective register of systematic reviews (reference ID: 160657)1. A study protocol for this systematic review is available in Supplementary Appendix 1.
Criteria for Considering Studies for This Review
Types of Intervention
We included publications in which the visual outcomes and complications of DMEK performed for the treatment of endothelial dysfunction were reported.
Types of Studies
Study designs included controlled clinical trials, prospective or retrospective comparative observational studies, and large case series (≥25 eyes). Small case series (<25 eyes), letters, reviews, published abstracts, and laboratory-based studies were excluded.
Types of Participants (Study Population)
Studies reporting only surgical outcomes of DMEK performed for graft failure (including repeat DMEK surgery) or specific high-risk disease groups (e.g., glaucoma, cytomegalovirus endotheliitis, herpes simplex) were also excluded. To avoid duplicate reporting of similar study populations, where the same group of investigators published several studies, earlier smaller studies were excluded if more recent larger studies reporting the same outcome measures were available.
Information Sources
Information sources included all applicable electronic databases, all relevant articles in the reference list of any relevant articles, and all relevant articles which cite any relevant articles.
Search Methods for Identification of Studies
Electronic literature searches were conducted in the following databases: CENTRAL, Cochrane Library databases2, PubMed, EMBASE, ClinicalTrials.gov.3 No date or language restrictions were set in our electronic searches. Key search terms were the MeSH headings Descemet’s membrane endothelial keratoplasty, Descemet membrane endothelial keratoplasty, and DMEK. The last electronic database search was performed on 30 June 2021. The search strategies for the relevant databases can be found in Supplementary Appendix 2.
Data Collection and Analysis
Selection of Studies
Citations and abstracts obtained from electronic searches were examined. Replicated studies and evidently irrelevant studies were removed. Full text prints of relevant studies were retrieved; they were assessed against our inclusion criteria for this review.
Data Extraction and Management
Only data from eyes that had received DMEK surgeries were included. Where studies reported on the outcomes of eyes that had undergone surgeries other than DMEK, these eyes were excluded from the review. The following details of each study were extracted for this review: study participants’ characteristics, study design, DMEK graft insertion techniques, and surgical outcome measures.
Assessment of Risks of Bias in Included Studies
The study design of each article was assessed and rated according to its level of evidence. A rating scale adapted from the Oxford Centre for Evidence-Based Medicine was used (38) (Table 1).

Table 1. Level of evidence used to rate the design of each study (adapted from the Oxford Centre for Evidence-Based Medicine March) (38).
Studies meeting the inclusion criteria were also assessed for risk of bias using Chapter 8 of the Cochrane Handbook for Systematic Reviews of Intervention (39). The following domains for potential risk of bias were considered: (a) selection bias – random sequence generation, (b) selection bias – allocation concealment, (c) performance/detection bias – masking of outcome examiners and participants (to determine whether knowledge of the allocated intervention was adequately prevented during the study), (d) attrition bias incomplete outcome data, and (e) reporting bias – selective outcome reporting. Each study was graded as “low risk” of bias, “high risk” of bias, or “unclear risk.” Any differences between the authors were resolved by discussion.
Outcome Measures
Data on the following surgical outcome measures were obtained: visual outcomes, endothelial cell loss, and complications including graft detachment/re-bubbling, graft rejection, and graft failure. For direct comparison of visual outcomes, measures of visual acuities in Snellen were converted to the respective logarithm of the minimum angle of resolution (LogMAR) equivalents. The proportion of eyes that achieved a best-corrected visual acuity (BCVA) of 20/25 or better at a specific time points were also evaluated.
Measures of Treatment Effect
All outcome measures (proportion of eyes achieving ≥20/25 BCVA, re-bubbling rates for graft detachments, graft rejection rates, and graft failure rates) were discrete data, except mean endothelial cell loss where outcome measures were continuous data. Outcomes for eyes rather than individuals were used as the unit of analysis. Studies where both eyes received the same treatment were included.
Managing Missing Data
All relevant data were extracted from the published studies. These included the details of studies and their quantitative results, without having to request these data from the original investigators.
Data Synthesis
Data analyses were performed according to Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (39). As published studies were performed in different institutions at various times, it is likely that variations exist amongst the patient populations included in this review. We therefore employed a random-effects model for our meta-analyses as the true effect size might differ between studies. Where we could not perform a meta-analysis, narrative syntheses describing the directions, magnitude, and consistencies of effects across the studies has been presented. MedCalc software was used for providing the meta-analyses results (MedCalc® Statistical Software version 20.014; MedCalc Software Ltd., Ostend, Belgium; 2021).4
Assessment of Heterogeneity
We identified dissimilarities between published studies which are expected to introduce heterogeneities. As some degree of heterogeneity would always exist due to the diversities in methodologies of studies, where appropriate, we employed the Chi2 test and I2 statistic to quantify heterogeneities across reports. Significant heterogeneity was defined as an I2 statistic of ≥50% and a Chi2 test p-value of <0.1. If all the effects of an outcome measure were in a similar direction, then we considered data-pooling to be acceptable even in the existence of heterogeneities.
Results
Results of Search
Electronic searches generated a total of 1,603 citations. Publications not relevant to the review were removed. After removal of duplicated publications, abstracts of 579 records were screened and a further 463 records were removed. Full text copies of 116 articles were obtained and reviewed. We included 95 studies in this review; 21 studies that failed to meet the inclusion criteria were excluded. The PRISMA flow diagram is illustrated in Figure 2.
Characteristics of Included Studies
Studies included in this review are summarized in Supplementary Table 1. A total of 21,323 eyes in 95 studies that had undergone DMEK were included. Eighty-six studies (19,945 eyes) reported on “endothelium-out” insertion techniques; eight studies (624 eyes) reported on “endothelium-in” insertion techniques, respectively. Only one study (36) compared “endothelium-out” to “endothelium-in” DMEK graft insertion techniques; this study was a large comparative series of 754 eyes (36).
Levels of Evidence and the Risks of Bias in Included Studies
Using the Oxford Centre for Evidence-Based Medicine rating (38) of the “endothelium-out” studies included, 4/86 (4.7%) were rated level I, 17/86 (19.8%) were rated level II, 22/86 (25.6%) were rated level III, and 43/86 (50.0%) were rated level IV evidence. Of the eight “endothelium-in” studies included, 5/8 (62.5%) were rated level III evidence and 3/8 (37.5%) were rated level IV evidence. The study that included both “endothelium-out” and “endothelium-in” techniques was rated level III evidence.
Figure 3 summarizes the judgments of each risk of bias domain of all studies included. Five of 95 included studies (5.3%) were assessed as “low risk” and 90/95 (94.7%) as “high risk” of random sequence generation (selection bias). Four of 95 studies (4.2%) were assessed as “low risk” and 91/95 (95.8%) as “high risk” of allocation concealment (selection bias). Two of 95 studies (2.1%) and two studies (2.1%) were assessed as “low risk” of performance bias and detection bias, respectively. Fifty-six of 95 studies (58.9%), 28/95 (29.5%), and 11/76 (11.6%) were assessed as “low risk,” “high risk,” and “unclear risk” of attrition bias, respectively. When assessing selective reporting (reporting bias), it was noted that all included studies reported results on some of the pre-specified outcome measures for this review. No study reported results for every outcome measure. All included studies did not state whether the published methods used in the analysis of outcomes were pre-specified in a protocol. Thus, 55/95 (57.9%) and 40/95 (42.1%) of studies were assessed as “high risk” or “unclear risk” for selective reporting, respectively. The authors’ judgments of each risk of bias item for each included study is found in Supplementary Appendix 3.
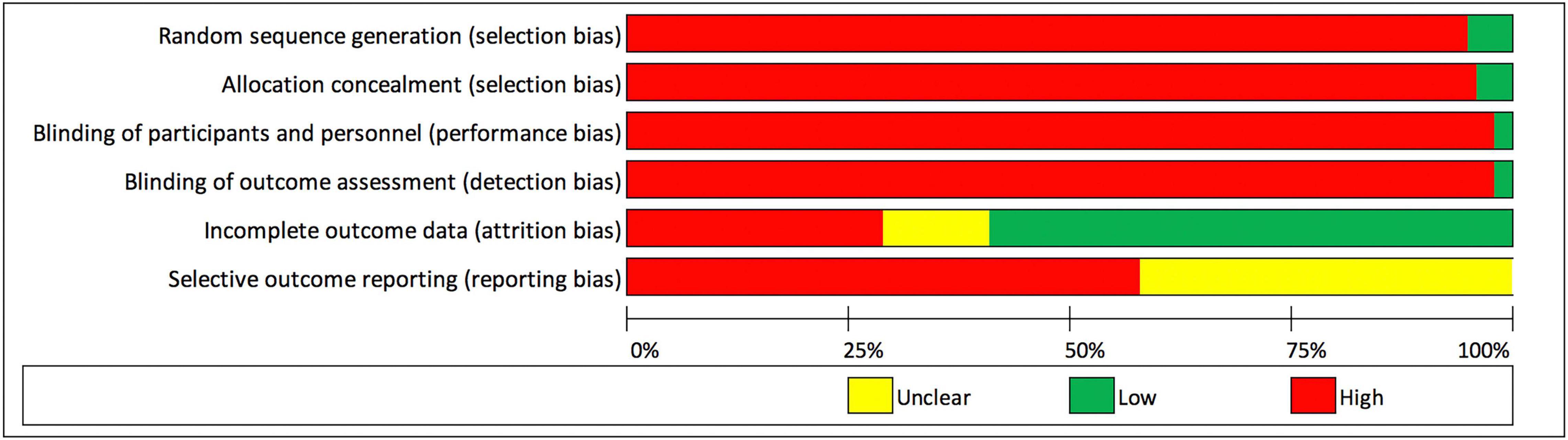
Figure 3. Risk of bias graph: review authors’ judgments about each risk of bias domain presented as percentages across all included studies.
Visual Outcomes and Complications Reported in Studies
The visual outcomes and complications reported in studies included are summarized in Supplementary Table 1.
Follow-Up
The reported mean length of follow-up of all studies ranged from 0 to 60 months (mean 12.8 ± 12.2 months).
Visual Outcomes
“Endothelium-out” studies: Thirty-four of the 87 studies (39.1%) reported the mean BCVA at 6 months after DMEK surgery; BCVA ranged from 0.0 to 0.49 LogMAR.
Fifteen studies (17.2%) reported that 42.5–85% of eyes achieved a BCVA of 20/25 or better at 6 months. The random pooled proportion of eyes achieving BCVA of 20/25 or better at 6 months was 58.7% (95% CI 49.4–67.7%) (15 studies).
“Endothelium-in” studies: Two of the nine studies (22.2%) reported the mean BCVA at 6 months after DMEK surgery; BCVA ranged from 0.09 to 0.10 LogMAR. Three studies (33.3%) reported that 44.7–87.5% of eyes achieved a BCVA of 20/25 or better at 6 months. The random pooled proportion of eyes achieving BCVA of 20/25 or better at 6 months was 62.4% (95% CI 33.9–86.9%) (3 studies).
Endothelial Cell Loss
“Endothelium-out” studies: 67/87 (77.0%) studies reported data on percentage endothelial cell loss following DMEK surgery at various time points. The mean endothelial cell loss ranged from 19 to 53%. One study (40), reported a rate of 5.6–6.4% endothelial cell loss per year. The random pooled mean endothelial cell loss was 36.3 ± 6.4% at 6 months (27 studies) and 38.7 ± 7.2% at 12 months (12 studies).
“Endothelium-in” studies: Percentage endothelial cells loss data following DMEK surgery were reported in eight out of the nine studies (88.9%) at various time points. The reported mean endothelial cell loss range from 26.6 to 56.0%. The random pooled mean endothelial cell loss was 28.1 ± 1.3% at 6 months (7 studies) and 29.6 ± 1.2% at 12 months (1 studies).
Comparing outcomes of “endothelium-out” to “endothelium-in” techniques, pooled mean endothelial cell loss was lower in the “endothelium-in” group, compared to “endothelium-out” group at 6 months (p = 0.018). However, this was not statistically computable at 12 months as there was only 1 study for the “endothelium in” group.
Rates of Complications
“Endothelium-out” studies: Re-bubbling rates to treat DMEK graft detachments were reported in 77/87 (88.5%) studies and ranged from 0 to 82%. Fifty-eight (66.7%) studies reported primary graft failure rates which ranged from 0 to 21.0%. Thirty-five (40.2%) studies reported secondary graft failure rates which ranged from 0 to 7.0% at 15.3 ± 13.9 months. Fifty (57.5%) studies reported graft rejection rates; rates ranged from 0 to 7.0%.
“Endothelium-in” studies: Re-bubbling rates to treat DMEK graft detachments were reported in all nine studies and ranged from 4.7 to 45.7%. Six of the nine studies (66.7%) reported primary graft failure rates which ranged from 0 to 3.0%. Three of the nine studies (33.3%) reported on secondary graft failures rates which ranged from 0 to 6.5%.
The random pooled graft re-bubbling rates for “endothelium-out” and “endothelium-in” techniques were 26.2% (95% CI 21.9–30.9%) (74 studies) and 16.5% (95% CI 8.5–26.4%) (6 studies), respectively. Comparing outcomes of “endothelium-out” to “endothelium-in” techniques, graft re-bubbling rates were not statistically significant in the “endothelium-out” group (p = 0.440). The random pooled primary graft failure rates for “endothelium-out” and “endothelium-in” techniques were 2.9% (95% CI 2.03–4.02%) (58 studies) and 1.5% (95% CI 0.6–2.7%) (5 studies), respectively. Comparing outcomes of “endothelium-out” to “endothelium-in” techniques, there was no significant difference in primary graft failure rates between the two groups (p = 0.552).
Discussion
Although DMEK offers the advantages of faster visual rehabilitation, better visual and refractive outcomes (21–25), and lower risks of graft rejection compared to DSAEK (26), many transplant surgeons have been slow to adopt DMEK as procedure of choice for the management of endothelial diseases (2, 3). Indeed, DSAEK still accounts for approximately 57% of EK surgeries performed in the United States (2). This has been ascribed to: the technical difficulties in DMEK donor preparation and surgical technique, with the reported higher risks of early complications, namely graft detachment and iatrogenic graft failure due to inadvertent up-side-down graft (25, 26, 31, 41–45) (Figure 4). The insertion and un-scrolling of the DMEK graft, once inside the anterior chamber, are indeed the most demanding steps in DMEK. The challenges occur as the DM, once detached from the cornea stromal surface, has an intrinsic propensity to adopt a scrolled configuration with the endothelial surface on its outside (46, 47). This is particularly the case for DMEK grafts harvested from young donors (46). Unlike conventional DSAEK, an alternative surgical skill set is needed by the corneal surgeon (42). The surgeon should understand the different described techniques to unscroll the DMEK graft once in the eye (48–50). Such techniques include methodological approaches to unfolding a double scrolled graft by tapping the cornea in a shallow anterior chamber, and the use of air bubbles to assist in tight or single scrolls (49, 50). In situations, for example tight scrolls or deep anterior chambers, the unscrolling of the graft can be technically demanding (46). Consequently, many corneal surgeons still reserve DMEK for more straightforward cases of endothelial diseases and DSAEK for more challenging cases (e.g., advanced bullous keratopathy, aphakia, large iris defects, vitrectomized eyes, previous glaucoma filtration surgery) (51–54).
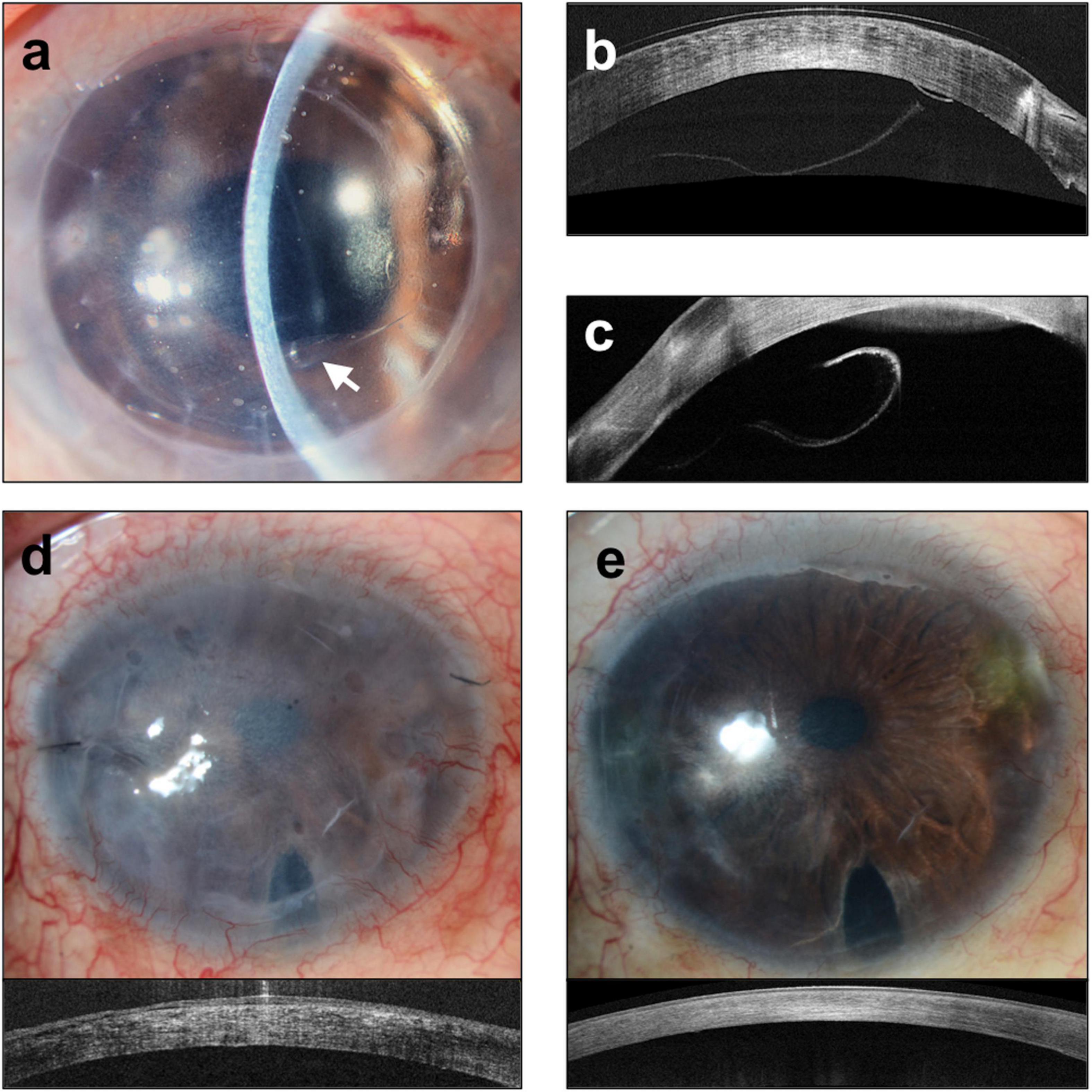
Figure 4. Complications of Descemet membrane endothelial keratoplasty (DMEK). (a) Slit lamp image of graft detachment (arrow) at post-operative day 7 and corresponding anterior segment optical coherence tomography (ASOCT) (Optovue, Oculus, CA, United States). Images (b,c) showing detached graft. (d) Iatrogenic graft failure likely a result of inadvertent graft eversion showing a hazy and thick cornea. (e) Repeated DMEK surgery with correct graft orientation showing rapid clearance of cornea and reduction in corneal thickness.
In current clinical practice, the vast majority of DMEK surgeries performed are “endothelium-out” techniques. This was reflected in this systematic review. Of the 21,323 included eyes that underwent DMEK, 19,945 (93.5%) received their grafts through various “endothelium-out” insertion techniques. In these techniques, the DMEK graft is loaded into an injector and inserted into the anterior chamber as a scroll, with the endothelium on its outer surface. Injectors used included modified intraocular lens cartridges, implantable contact lens cartridges, intravenous tubing, or glass injectors (Supplementary Table 1). Direct contact of the endothelium of the DMEK graft to the walls of the injectors can potentially cause endothelial cell damage and loss. Studies have indicated that plastic graft injectors are associated with higher rates of post-operative graft detachments, compared to glass devices (55, 56). Such observations have been explained by more damage to the corneal endothelium with plastic materials, and intra-operative alterations in the morphologies of the grafts during insertion and un-scrolling, which may be caused by electro-static forces produced by plastic (55). Nonetheless, not all reports have found similar effects (57).
Moreover, in “endothelium-out” techniques, there is often no control of the scrolled graft during insertion. Despite the use of intraoperative imaging (58), orientation markers such as S-stamps (59) or other asymmetrical indicators (60), determining the orientation of the graft in the anterior chamber can sometimes be difficult. Especially in cases of prolonged surgery, DMEK grafts in the eye can lose their pre-stained trypan blue stains, making visualization of graft orientation even more difficult. This is especially so in patients with dark irides (Figure 5).
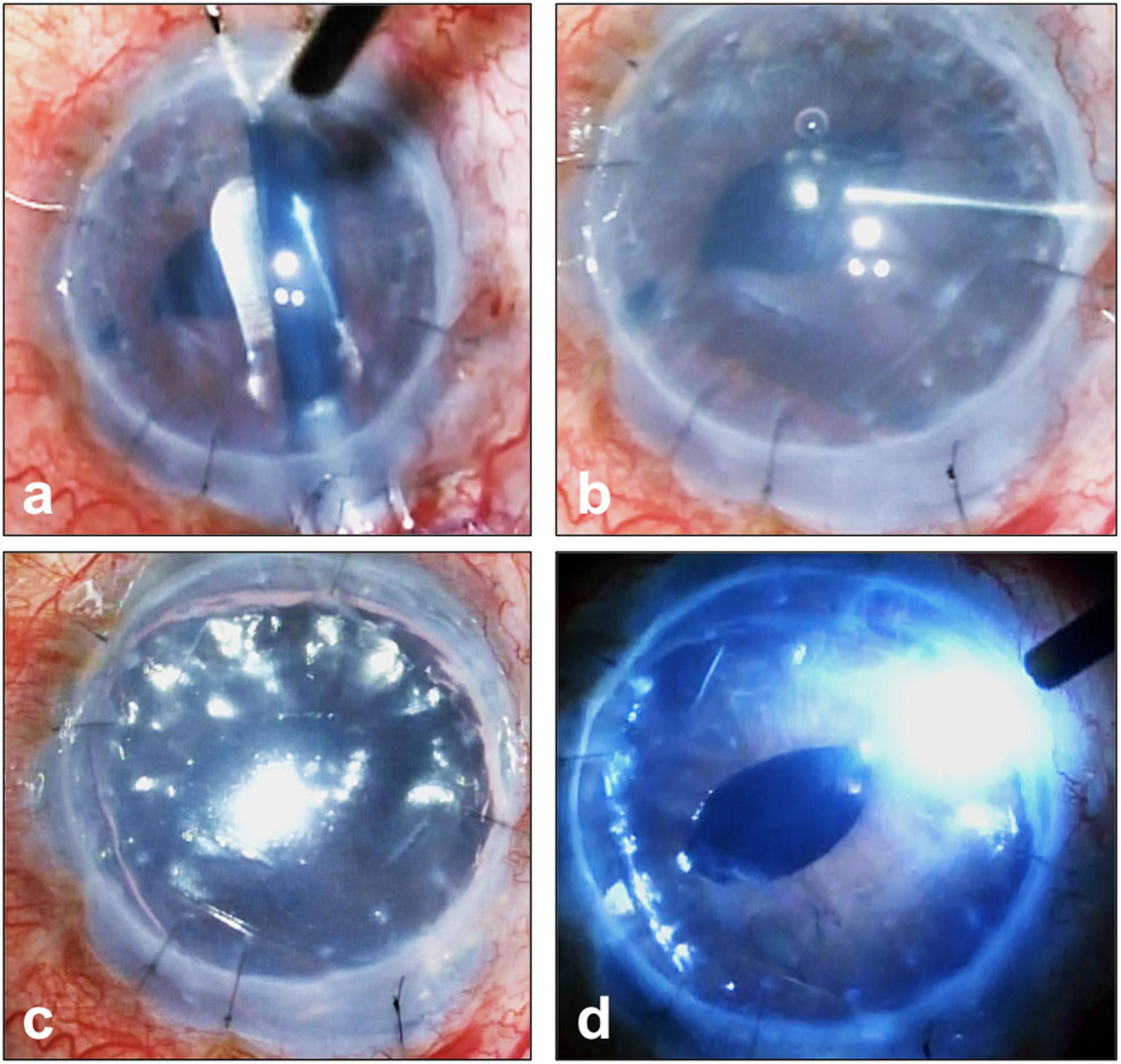
Figure 5. Complex Descemet membrane endothelial keratoplasty (DMEK) surgery performed in an eye with previously failed penetrating keratoplasty graft and iridocorneal endothelial (ICE) syndrome. (a) DMEK graft pre-stained with Membrane Blue Dual (D.O.R.C., Netherlands) and inserted into the eye. (b) Prolonged surgery has resulted in the loss of the blue stain making visualization of graft orientation and attachment difficult; (c) this is made more difficult given the patient’s dark iris (d) full air-gas tamponade of graft and the use of an external light pipe to assist the surgeon in graft orientation and attachment.
The unfolding of a scrolled DMEK graft and its central positioning on the recipient’s posterior stromal surface can also be problematic and time-consuming. To unfold the DMEK scroll after insertion into the anterior chamber, numerous approaches such as using air bubbles or jets of balanced salt solution in the presence of a shallow anterior chamber and the stroking of the corneal surface have been described (48). To overcome these difficulties of intracameral DMEK graft unfolding, different groups have investigated various alternative techniques. An example of such alternative techniques is the transplantation of DMEK tissue of various shapes (61). Authors have showed that certain DMEK graft shapes, such as the Maltese cross graft design, may be less prone to tight scrolling.
The concept of “endothelium-in” DMEK insertion techniques have been recently introduced (33–37, 44, 45). The grafts are folded, usually in a trifold, with the endothelium on the inside. These “endothelium-in” techniques prevent the DMEK grafts from adopting their natural scrolls with the endothelium on the outside. These “endothelium-in” techniques are believed to have the benefits of minimizing endothelial cell damage from the mechanical stress of the endothelial cells on the walls of the injectors. Moreover, in “endothelium-in” techniques, the grafts are pulled into the eyes with the endothelium facing downward. Once in the eye, the graft begins to unfold to acquire its physiological “endothelium-out” configuration, effectively “aiding” the surgeon in graft unfolding. Pre-clinical laboratory studies have also reported significantly shorter graft unfolding times for “endothelium-in” compared to “endothelium-out” techniques (62). These factors in “endothelium-in” techniques reduce the technical difficulties of intracameral graft orientation and unscrolling, making DMEK procedures more controlled and predictable. Some of these “endothelium-in” techniques also use devices created to mimic DSAEK techniques, which many corneal surgeons are accustomed to (33, 44, 45). Various laboratory studies have reported no significant differences in endothelial cell loss when DMEK grafts were loaded “endothelium-in” and pulled-through or loaded “endothelium-out” and injected-through different graft insertion devices (62–64). In this review, the surgical outcomes of both “endothelium-out” and “endothelium-in” techniques were evaluated.
Summary of Evidence
This review included a total of 95 studies (Supplementary Table 1). Eighty-six studies using “endothelium-out” insertion techniques, eight studies using “endothelium-in” insertion techniques, and one study comparing “endothelium-out” to “endothelium-in” techniques. The majority of studies, 73/95 (76.8%), were rated as level III or level IV evidence. Only 4/95 (4.2%) studies were rated as level I evidence.
Evaluating the outcomes of “endothelium-out” techniques, the mean BCVA at 6 months after DMEK surgery ranged from 0.0 to 0.49 LogMAR (34 studies); 42.5–85% of eyes (15 studies) achieved a best-corrected visual acuity (BCVA) of 20/25 or better at 6 months. The mean endothelial cell loss ranged from 19 to 53%. The random pooled mean endothelial cell loss was 36.3 ± 6.4% at 6 months (27 studies) and 38.7 ± 7.2% at 12 months (12 studies). Rates of re-bubbling for graft detachments, primary graft failure rates, secondary graft failure rates, and graft rejection rates ranged from 0 to 82%, 0 to 21.0%, 0 to 7.0%, and 0 to 7.0%, respectively. The random pooled graft re-bubbling rates for “endothelium-out” techniques were 26.2% (95% CI 21.9–30.9%) (74 studies). The random pooled primary graft failure rates for “endothelium-out” techniques was 2.9% (95% CI 2.03–4.02%) (58 studies).
Of the eight “endothelium-in” studies reporting visual acuity data, the mean BCVA at 6 months after DMEK surgery ranged from 0.09 to 0.10 LogMAR (2 studies); 44.7–87.5% of eyes (3 studies) achieved a best-corrected visual acuity (BCVA) of 20/25 or better at 6 months. The mean endothelial cell loss ranged from 26.6 to 56.0% (7 studies). The random pooled mean endothelial cell loss was 28.1 ± 1.3% at 6 months (7 studies) and 29.6 ± 1.2% at 12 months (1 study). Graft detachment re-bubbling rates and primary graft failure rates ranged from 4.7 to 45.7% (nine studies) and 0 to 3.0% (six studies), respectively. Only one study reported on secondary graft failure rates in which there were none. None of the studies reporting on “endothelium-in” techniques reported the graft rejection rates. The random pooled graft re-bubbling rates for “endothelium-in” techniques was 16.5% (95% CI 8.5–26.4%) (6 studies). The random pooled primary graft failure rates for “endothelium-in” techniques was 1.5% (95% CI 0.6–2.7%) (six studies).
Comparing outcomes of “endothelium-out” to “endothelium-in” techniques, pooled mean endothelial cell loss was lower in the “endothelium-in” studies compared to “endothelium-out” studies at 6 months (p = 0.018); this was not statistically computable at 12 months as there was only 1 study for “endothelium in” group. Although re-bubbling rates for graft detachments were higher in the “endothelium-out” studies compared to “endothelium-in” studies, statistical significance was not achieved (p = 0.440). There was no significant difference in primary graft failure rates between the two groups (p = 0.552).
Limitations of This Review
This review has several limitations. The quality of available evidence was considered low (grade III and IV) with a significant number of studies judged as having high risks of bias (Figure 3 and Supplementary Appendix 3). Significant heterogeneity existed in the studies, such as study designs, study population, surgical techniques, surgeon experience, outcome measures, and duration of follow-up. Studies published after the date of the pre-defined search strategy have also not been included. Furthermore, there was a smaller number of studies that reported on outcomes using “endothelium-in” DMEK surgeries that met the inclusion criteria for this review. This makes it difficult to provide any definitive conclusions through a comparative meta-analysis, especially in longer post-operative time points. Thus, the evidence to compare “endothelium-out” to “endothelium-in” techniques cannot be considered complete with this review.
Conclusion
The rates of endothelial cell loss were reported to be significantly lower in “endothelium-in” DMEK surgeries at 6 months following surgery compared to “endothelium-out” surgeries. Despite the above-mentioned limitations, visual outcomes and rates of complications of “endothelium-in” techniques from the small number of studies were noted to be comparable to those reported in “endothelium-out” studies. Given the intra-operative challenges following graft insertion encountered using “endothelium-out” techniques, surgeons may consider “endothelium-in” techniques designed for easier intra-operative DMEK graft unfolding after graft insertion. However, further well-conducted, adequately powered, randomized controlled trials and studies with longer duration of follow-up are needed before conclusive comparisons between the two techniques can be made.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
HO and JM: conceptualization and supervision. HO and HH: data curation. HO, HH, MA, and JM: formal analysis, investigation, methodology, writing draft, and review and editing. All authors approved the manuscript.
Funding
The costs of publication of this article was funded by a grant from SingHealth Fund-SNEC (SHF-SNEC).
Conflict of Interest
JM holds a patent on the EndoGlide and receive royalties.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.868533/full#supplementary-material
Footnotes
- ^ https://www.crd.york.ac.uk/PROSPERO
- ^ www.thecochranelibrary.com
- ^ www.clinicaltrials.gov
- ^ https://www.medcalc.org
References
1. Australian Corneal Graft Registry. The Australian Graft Registry 2018 Report. Adelaide, SA: Flinders University South Australian Health and Medical Research Institute (2018).
2. EBAA. Eye Banking Statistical Report 2019. Washington, DC: Eye Bank Association of America (2020).
3. Park CY, Lee JK, Gore PK, Lim CY, Chuck RS. Keratoplasty in the United States: a 10-year review from 2005 through 2014. Ophthalmology. (2015) 122:2432–42. doi: 10.1016/j.ophtha.2015.08.017
5. Ong HS, Ang M, Mehta JS. Evolution of therapies for the corneal endothelium: past, present and future approaches. Br J Ophthalmol. (2020) 105:454–67. doi: 10.1136/bjophthalmol-2020-316149
6. Melles GR, Eggink FA, Lander F, Pels E, Rietveld FJ, Beekhuis WH, et al. A surgical technique for posterior lamellar keratoplasty. Cornea. (1998) 17:618–26.
7. Koenig SB, Covert DJ, Dupps WJ Jr, Meisler DM. Visual acuity, refractive error, and endothelial cell density six months after Descemet stripping and automated endothelial keratoplasty (DSAEK). Cornea. (2007) 26:670–4. doi: 10.1097/ICO.0b013e3180544902
8. Aung TT, Yam JK, Lin S, Salleh SM, Givskov M, Liu S, et al. Biofilms of pathogenic nontuberculous mycobacteria targeted by new therapeutic approaches. Antimicrob Agents Chemother. (2016) 60:24–35. doi: 10.1128/AAC.01509-15
9. Woo JH, Ang M, Htoon HM, Tan DT. Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol. (2019) 207:288–303. doi: 10.1016/j.ajo.2019.06.012
10. Guell JL, El Husseiny MA, Manero F, Gris O, Elies D. Historical review and update of surgical treatment for corneal endothelial diseases. Ophthalmol Ther. (2014) 3:1–15. doi: 10.1007/s40123-014-0022-y
11. Price FW Jr, Feng MT, Price MO. Evolution of endothelial keratoplasty: where are we headed? Cornea. (2015) 34:S41–7. doi: 10.1097/ICO.0000000000000505
12. Ang M, Saroj L, Htoon HM, Kiew S, Mehta JS, Tan D. Comparison of a donor insertion device to sheets glide in Descemet stripping endothelial keratoplasty: 3-year outcomes. Am J Ophthalmol. (2014) 157:1163–1169.e3. doi: 10.1016/j.ajo.2014.02.049
13. Ang M, Htoon HM, Cajucom-Uy HY, Tan D, Mehta JS. Donor and surgical risk factors for primary graft failure following Descemet’s stripping automated endothelial keratoplasty in Asian eyes. Clin Ophthalmol. (2011) 5:1503–8. doi: 10.2147/OPTH.S25973
14. Ang M, Mehta JS, Anshu A, Wong HK, Htoon HM, Tan D. Endothelial cell counts after Descemet’s stripping automated endothelial keratoplasty versus penetrating keratoplasty in Asian eyes. Clin Ophthalmol. (2012) 6:537–44. doi: 10.2147/OPTH.S26343
15. Ang M, Mehta JS, Lim F, Bose S, Htoon HM, Tan D. Endothelial cell loss and graft survival after Descemet’s stripping automated endothelial keratoplasty and penetrating keratoplasty. Ophthalmology. (2012) 119:2239–44. doi: 10.1016/j.ophtha.2012.06.012
16. Ang M, Lim F, Htoon HM, Tan D, Mehta JS. Visual acuity and contrast sensitivity following Descemet stripping automated endothelial keratoplasty. Br J Ophthalmol. (2016) 100:307–11. doi: 10.1136/bjophthalmol-2015-306975
17. Fuest M, Ang M, Htoon HM, Tan D, Mehta JS. Long-term visual outcomes comparing Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol. (2017) 182:62–71. doi: 10.1016/j.ajo.2017.07.014
18. Bose S, Ang M, Mehta JS, Tan DT, Finkelstein E. Cost-effectiveness of Descemet’s stripping endothelial keratoplasty versus penetrating keratoplasty. Ophthalmology. (2013) 120:464–70. doi: 10.1016/j.ophtha.2012.08.024
19. Tan D, Htoon HM, Ang M. Descemet’s stripping automated endothelial keratoplasty with anterior chamber intraocular lenses. Br J Ophthalmol. (2014) 98:1462.
20. Melles GR, Ong TS, Ververs B, van der Wees J. Descemet membrane endothelial keratoplasty (DMEK). Cornea. (2006) 25:987–90.
21. Singh A, Zarei-Ghanavati M, Avadhanam V, Liu C. Systematic review and meta-analysis of clinical outcomes of Descemet membrane endothelial keratoplasty versus Descemet stripping endothelial keratoplasty/Descemet stripping automated endothelial keratoplasty. Cornea. (2017) 36:1437–43. doi: 10.1097/ICO.0000000000001320
22. Droutsas K, Lazaridis A, Papaconstantinou D, Brouzas D, Moschos MM, Schulze S, et al. Visual outcomes after Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial keratoplasty-comparison of specific matched Pairs. Cornea. (2016) 35:765–71. doi: 10.1097/ICO.0000000000000822
23. Tourtas T, Laaser K, Bachmann BO, Cursiefen C, Kruse FE. Descemet membrane endothelial keratoplasty versus descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. (2012) 153:1082–90.e2.
24. Guerra FP, Anshu A, Price MO, Price FW. Endothelial keratoplasty: fellow eyes comparison of Descemet stripping automated endothelial keratoplasty and Descemet membrane endothelial keratoplasty. Cornea. (2011) 30:1382–6. doi: 10.1097/ICO.0b013e31821ddd25
25. Stuart AJ, Romano V, Virgili G, Shortt AJ. Descemet’s membrane endothelial keratoplasty (DMEK) versus Descemet’s stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst Rev. (2018) 6:CD012097. doi: 10.1002/14651858.CD012097.pub2
26. Marques RE, Guerra PS, Sousa DC, Goncalves AI, Quintas AM, Rodrigues W. DMEK versus DSAEK for Fuchs’ endothelial dystrophy: a meta-analysis. Eur J Ophthalmol. (2018) 29:15–22. doi: 10.1177/1120672118757431
27. Dapena I, Moutsouris K, Droutsas K, Ham L, van Dijk K, Melles GR. Standardized “no-touch” technique for Descemet membrane endothelial keratoplasty. Arch Ophthalmol. (2011) 129:88–94.
28. Arnalich-Montiel F, Munoz-Negrete FJ, De Miguel MP. Double port injector device to reduce endothelial damage in DMEK. Eye (Lond). (2014) 28:748–51. doi: 10.1038/eye.2014.67
29. Kruse FE, Laaser K, Cursiefen C, Heindl LM, Schlotzer-Schrehardt U, Riss S, et al. A stepwise approach to donor preparation and insertion increases safety and outcome of Descemet membrane endothelial keratoplasty. Cornea. (2011) 30:580–7. doi: 10.1097/ico.0b013e3182000e2e
30. Kim EC, Bonfadini G, Todd L, Zhu A, Jun AS. Simple, inexpensive, and effective injector for descemet membrane endothelial keratoplasty. Cornea. (2014) 33:649–52. doi: 10.1097/ICO.0000000000000121
31. Maier AK, Gundlach E, Schroeter J, Klamann MK, Gonnermann J, Riechardt AI, et al. Influence of the difficulty of graft unfolding and attachment on the outcome in Descemet membrane endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol. (2015) 253:895–900. doi: 10.1007/s00417-015-2939-9
32. Ang M, Ting DSJ, Kumar A, May KO, Htoon HM, Mehta JS. Descemet membrane endothelial keratoplasty in Asian eyes: intraoperative and postoperative complications. Cornea. (2020) 39:940–5. doi: 10.1097/ICO.0000000000002302
33. Ang M, Mehta JS, Newman SD, Han SB, Chai J, Tan D. Descemet membrane endothelial keratoplasty: preliminary results of a donor insertion pull-through technique using a donor mat device. Am J Ophthalmol. (2016) 171:27–34. doi: 10.1016/j.ajo.2016.08.023
34. Busin M, Leon P, D’Angelo S, Ruzza A, Ferrari S, Ponzin D, et al. Clinical outcomes of preloaded Descemet membrane endothelial keratoplasty grafts with endothelium tri-folded inwards. Am J Ophthalmol. (2018) 193:106–13. doi: 10.1016/j.ajo.2018.06.013
35. Leon P, Parekh M, Nahum Y, Mimouni M, Giannaccare G, Sapigni L, et al. Factors associated with early graft detachment in primary Descemet membrane endothelial keratoplasty. Am J Ophthalmol. (2018) 187:117–24.
36. Price MO, Lisek M, Kelley M, Feng MT, Price FW Jr. Endothelium-in versus endothelium-out insertion with Descemet membrane endothelial keratoplasty. Cornea. (2018) 37:1098–101. doi: 10.1097/ICO.0000000000001650
37. Ong HS, Mehta JS. Descemet’s membrane endothelial keratoplasty (DMEK)—why Surgeons should consider adopting endothelium-in techniques. US Ophthalmic Rev. (2019) 12:65–8.
39. Higgins JPT, Altman DG, Sterne JAC. Chapter 8: assessing risk of bias in included studies. In: Higgins, JPT Green S editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. (London: The Cochrane Collaboration) (2011).
40. Price MO, Scanameo A, Feng MT, Price FW Jr. Descemet’s membrane endothelial keratoplasty: risk of immunologic rejection episodes after discontinuing topical corticosteroids. Ophthalmology. (2016) 12:1232–6. doi: 10.1016/j.ophtha.2016.02.001
41. Hamzaoglu EC, Straiko MD, Mayko ZM, Sales CS, Terry MA. The first 100 eyes of standardized Descemet stripping automated endothelial keratoplasty versus standardized Descemet membrane endothelial keratoplasty. Ophthalmology. (2015) 122:2193–9. doi: 10.1016/j.ophtha.2015.07.003
42. Phillips PM, Phillips LJ, Muthappan V, Maloney CM, Carver CN. Experienced DSAEK Surgeon’s transition to DMEK: outcomes comparing the last 100 DSAEK surgeries with the first 100 DMEK surgeries exclusively using previously published techniques. Cornea. (2017) 36:275–9. doi: 10.1097/ICO.0000000000001069
43. Rose-Nussbaumer J, Alloju S, Chamberlain W. Clinical outcomes of Descemet membrane endothelial keratoplasty during the Surgeon learning curve versus Descemet stripping endothelial keratoplasty performed at the same time. J Clin Exp Ophthalmol. (2016) 7:599. doi: 10.4172/2155-9570.1000599
44. Romano V, Kazaili A, Pagano L, Gadhvi KA, Titley M, Steger B, et al. Eye bank versus Surgeon prepared DMEK tissues: influence on adhesion and re-bubbling rate. Br J Ophthalmol. (2022) 106:177–83. doi: 10.1136/bjophthalmol-2020-317608
45. Parekh M, Pedrotti E, Viola P, Leon P, Neri E, Bosio L, et al. Factors affecting the success rate of pre-loaded DMEK with endothelium-inwards technique: a multi-centre clinical study. Am J Ophthalmol. (2022). doi: 10.1016/j.ajo.2022.03.009 [Epub ahead of print].
46. Heinzelmann S, Bohringer D, Haverkamp C, Lapp T, Eberwein P, Reinhard T, et al. Influence of postoperative intraocular pressure on graft detachment after Descemet membrane endothelial keratoplasty. Cornea. (2018) 37:1347–50.
47. Tan TE, Devarajan K, Seah XY, Lin SJ, Peh GSL, Cajucom-Uy HY, et al. Lamellar dissection technique for Descemet membrane endothelial keratoplasty graft preparation. Cornea. (2019) 39:23–9. doi: 10.1097/ICO.0000000000002090
48. Ang M, Wilkins MR, Mehta JS, Tan D. Descemet membrane endothelial keratoplasty. Br J Ophthalmol. (2016) 100:15–21.
49. Liarakos VS, Dapena I, Ham L, van Dijk K, Melles GR. Intraocular graft unfolding techniques in Descemet membrane endothelial keratoplasty. JAMA Ophthalmol. (2013) 131:29–35. doi: 10.1001/2013.jamaophthalmol.4
50. Yoeruek E, Bayyoud T, Hofmann J, Bartz-Schmidt KU. Novel maneuver facilitating Descemet membrane unfolding in the anterior chamber. Cornea. (2013) 32:370–3. doi: 10.1097/ICO.0b013e318254fa06
51. Ang M, Ho H, Wong C, Htoon HM, Mehta JS, Tan D. Endothelial keratoplasty after failed penetrating keratoplasty: an alternative to repeat penetrating keratoplasty. Am J Ophthalmol. (2014) 158:1221–1227.e1. doi: 10.1016/j.ajo.2014.08.024
52. Ang M, Li L, Chua D, Wong C, Htoon HM, Mehta JS, et al. Descemet’s stripping automated endothelial keratoplasty with anterior chamber intraocular lenses: complications and 3-year outcomes. Br J Ophthalmol. (2014) 98:1028–32.
53. Ang M, Sng CCA. Descemet membrane endothelial keratoplasty and glaucoma. Curr Opin Ophthalmol. (2018) 29:178–84. doi: 10.1097/ICU.0000000000000454
54. Ang M, Sng CC. Descemet membrane endothelial keratoplasty developing spontaneous ‘malignant glaucoma’ secondary to gas misdirection. Clin Exp Ophthalmol. (2018) 46:811–3. doi: 10.1111/ceo.13150
55. Monnereau C, Quilendrino R, Dapena I, Liarakos VS, Alfonso JF, Arnalich-Montiel F, et al. Multicenter study of descemet membrane endothelial keratoplasty: first case series of 18 Surgeons. JAMA Ophthalmol. (2014) 132:1192–8. doi: 10.1001/jamaophthalmol.2014.1710
56. Dirisamer M, van Dijk K, Dapena I, Ham L, Oganes O, Frank LE, et al. Prevention and management of graft detachment in descemet membrane endothelial keratoplasty. Arch Ophthalmol. (2012) 130:280–91. doi: 10.1001/archophthalmol.2011.343
57. Oellerich S, Baydoun L, Peraza-Nieves J, Ilyas A, Frank L, Binder PS, et al. Multicenter study of 6-month clinical outcomes after Descemet membrane endothelial keratoplasty. Cornea. (2017) 36:1467–76. doi: 10.1097/ICO.0000000000001374
58. Ang M, Dubis AM, Wilkins MR. Descemet membrane endothelial keratoplasty: intraoperative and postoperative imaging spectral-domain optical coherence tomography. Case Rep Ophthalmol Med. (2015) 2015:506251. doi: 10.1155/2015/506251
59. Veldman PB, Dye PK, Holiman JD, Mayko ZM, Sales CS, Straiko MD, et al. The S-stamp in Descemet membrane endothelial keratoplasty safely eliminates upside-down graft implantation. Ophthalmology. (2016) 123:161–4. doi: 10.1016/j.ophtha.2015.08.044
60. Bhogal M, Maurino V, Allan BD. Use of a single peripheral triangular mark to ensure correct graft orientation in Descemet membrane endothelial keratoplasty. J Cataract Refract Surg. (2015) 41:2022–4. doi: 10.1016/j.jcrs.2015.08.005
61. Modabber M, Talajic JC, Mabon M, Mercier M, Jabbour S, Choremis J. The role of novel DMEK graft shapes in facilitating intraoperative unscrolling. Graefes Arch Clin Exp Ophthalmol. (2018) 256:2385–90. doi: 10.1007/s00417-018-4145-z
62. Parekh M, Ruzza A, Ferrari S, Ahmad S, Kaye S, Ponzin D, et al. Endothelium-in versus endothelium-out for Descemet membrane endothelial keratoplasty graft preparation and implantation. Acta Ophthalmol. (2017) 95:194–8. doi: 10.1111/aos.13162
63. Barnes K, Chiang E, Chen C, Lohmeier J, Christy J, Chaurasia A, et al. Comparison of tri-folded and scroll-based graft viability in preloaded Descemet membrane endothelial keratoplasty. Cornea. (2019) 38:392–6. doi: 10.1097/ICO.0000000000001831
64. Romano V, Ruzza A, Kaye S, Parekh M. Pull-through technique for delivery of a larger diameter DMEK graft using endothelium-in method. Can J Ophthalmol. (2017) 52:e155–6. doi: 10.1016/j.jcjo.2017.03.006
65. Price MO, Price FW Jr, Kruse FE, Bachmann BO, Tourtas T. Randomized comparison of topical prednisolone acetate 1% versus fluorometholone 0.1% in the first year after Descemet membrane endothelial keratoplasty. Cornea. (2014) 33:880–6. doi: 10.1097/ICO.0000000000000206
66. Price MO, Feng MT, Scanameo A, Price FW Jr. Loteprednol etabonate 0.5% Gel Vs. prednisolone acetate 1% solution after Descemet membrane endothelial keratoplasty: prospective randomized trial. Cornea. (2015) 34:853–8. doi: 10.1097/ICO.0000000000000475
67. Chamberlain W, Lin CC, Austin A, Schubach N, Clover J, McLeod SD, et al. Descemet endothelial thickness comparison trial: a randomized trial comparing ultrathin Descemet stripping automated endothelial keratoplasty with Descemet membrane endothelial keratoplasty. Ophthalmology. (2019) 126:19–26.
68. Dunker SL, Dickman MM, Wisse RPL, Nobacht S, Wijdh RHJ, Bartels MC, et al. Descemet membrane endothelial keratoplasty versus ultrathin Descemet stripping automated endothelial keratoplasty: a multicenter randomized controlled clinical trial. Ophthalmology. (2020) 127:1152–9. doi: 10.1016/j.ophtha.2020.02.029
69. Santander-Garcia D, Peraza-Nieves J, Muller TM, Gerber-Hollbach N, Baydoun L, Liarakos VS, et al. Influence of intraoperative air tamponade time on graft adherence in Descemet membrane endothelial keratoplasty. Cornea. (2019) 38:166–72. doi: 10.1097/ICO.0000000000001795
70. Price MO, Giebel AW, Fairchild KM, Price FW Jr. Descemet’s membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology. (2009) 116:2361–8. doi: 10.1016/j.ophtha.2009.07.010
71. Rudolph M, Laaser K, Bachmann BO, Cursiefen C, Epstein D, Kruse FE. Corneal higher-order aberrations after Descemet’s membrane endothelial keratoplasty. Ophthalmology. (2012) 119:528–35. doi: 10.1016/j.ophtha.2011.08.034
72. Feng MT, Burkhart ZN, Price FW Jr, Price MO. Effect of donor preparation-to-use times on Descemet membrane endothelial keratoplasty outcomes. Cornea. (2013) 32:1080–2. doi: 10.1097/ICO.0b013e318292a7e5
73. Chaurasia S, Price FW Jr, Gunderson L, Price MO. Descemet’s membrane endothelial keratoplasty: clinical results of single versus triple procedures (combined with cataract surgery). Ophthalmology. (2014) 121:454–8. doi: 10.1016/j.ophtha.2013.09.032
74. Cabrerizo J, Livny E, Musa FU, Leeuwenburgh P, van Dijk K, Melles GR. Changes in color vision and contrast sensitivity after descemet membrane endothelial keratoplasty for fuchs endothelial dystrophy. Cornea. (2014) 33:1010–5. doi: 10.1097/ICO.0000000000000216
75. Guell JL, Morral M, Gris O, Elies D, Manero F. Comparison of sulfur hexafluoride 20% versus air tamponade in Descemet membrane endothelial keratoplasty. Ophthalmology. (2015) 122:1757–64. doi: 10.1016/j.ophtha.2015.05.013
76. Heinzelmann S, Maier P, Bohringer D, Huther S, Eberwein P, Reinhard T. Cystoid macular oedema following Descemet membrane endothelial keratoplasty. Br J Ophthalmol. (2015) 99:98–102. doi: 10.1136/bjophthalmol-2014-305124
77. Heinzelmann S, Bohringer D, Eberwein P, Reinhard T, Maier P. Outcomes of Descemet membrane endothelial keratoplasty, Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty from a single centre study. Graefes Arch Clin Exp Ophthalmol. (2016) 254:515–22. doi: 10.1007/s00417-015-3248-z
78. Schaub F, Enders P, Snijders K, Schrittenlocher S, Siebelmann S, Heindl LM, et al. One-year outcome after Descemet membrane endothelial keratoplasty (DMEK) comparing sulfur hexafluoride (SF6) 20% versus 100% air for anterior chamber tamponade. Br J Ophthalmol. (2017) 101:902–8. doi: 10.1136/bjophthalmol-2016-309653
79. Aravena C, Yu F, Deng SX. Outcomes of Descemet membrane endothelial keratoplasty in patients with previous glaucoma surgery. Cornea. (2017) 36:284–9. doi: 10.1097/ICO.0000000000001095
80. Tourtas T, Schlomberg J, Wessel JM, Bachmann BO, Schlotzer-Schrehardt U, Kruse FE. Graft adhesion in descemet membrane endothelial keratoplasty dependent on size of removal of host’s descemet membrane. JAMA Ophthalmol. (2014) 132:155–61. doi: 10.1001/jamaophthalmol.2013.6222
81. Gundlach E, Maier AK, Tsangaridou MA, Riechardt AI, Brockmann T, Bertelmann E, et al. DMEK in phakic eyes: targeted therapy or highway to cataract surgery? Graefes Arch Clin Exp Ophthalmol. (2015) 253:909–14. doi: 10.1007/s00417-015-2956-8
82. Rock T, Bramkamp M, Bartz-Schmidt KU, Rock D, Yoruk E. Causes that influence the detachment rate after Descemet membrane endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol. (2015) 253:2217–22.
83. Hoerster R, Stanzel TP, Bachmann BO, Siebelmann S, Felsch M, Cursiefen C. Intensified topical steroids as prophylaxis for macular Edema after posterior lamellar keratoplasty combined with cataract surgery. Am J Ophthalmol. (2016) 163:174–179.e2. doi: 10.1016/j.ajo.2015.12.008
84. Schaub F, Pohl L, Enders P, Adler W, Bachmann BO, Cursiefen C, et al. Impact of corneal donor lens status on two-year course and outcome of Descemet membrane endothelial keratoplasty (DMEK). Graefes Arch Clin Exp Ophthalmol. (2017) 255:2407–14. doi: 10.1007/s00417-017-3827-2
85. Regnier M, Auxenfans C, Maucort-Boulch D, Marty AS, Damour O, Burillon C, et al. Eye bank prepared versus Surgeon cut endothelial graft tissue for Descemet membrane endothelial keratoplasty: an observational study. Medicine (Baltimore). (2017) 96:e6885. doi: 10.1097/MD.0000000000006885
86. Botsford B, Vedana G, Cope L, Yiu SC, Jun AS. Comparison of 20% sulfur hexafluoride with air for intraocular tamponade in Descemet membrane endothelial keratoplasty (DMEK). Arq Bras Oftalmol. (2016) 79:299–302. doi: 10.5935/0004-2749.20160086
87. Rickmann A, Opitz N, Szurman P, Boden KT, Jung S, Wahl S, et al. Clinical comparison of two methods of graft preparation in Descemet membrane endothelial keratoplasty. Curr Eye Res. (2018) 43:12–7. doi: 10.1080/02713683.2017.1368086
88. Schrittenlocher S, Bachmann B, Cursiefen C. Impact of donor tissue diameter on postoperative central endothelial cell density in Descemet membrane endothelial keratoplasty. Acta Ophthalmol. (2018) 97:e618–22. doi: 10.1111/aos.13943
89. Brockmann T, Brockmann C, Maier AB, Schroeter J, Bertelmann E, Torun N. Primary Descemet’s membrane endothelial keratoplasty for fuchs endothelial dystrophy versus bullous keratopathy: histopathology and clinical results. Curr Eye Res. (2018) 43:1221–7. doi: 10.1080/02713683.2018.1490773
90. Kocluk Y, Kasim B, Sukgen EA, Burcu A. Descemet membrane endothelial keratoplasty (DMEK): intraoperative and postoperative complications and clinical results. Arq Bras Oftalmol. (2018) 81:212–8. doi: 10.5935/0004-2749.20180043
91. Rickmann A, Szurman P, Jung S, Boden KT, Wahl S, Haus A, et al. Impact of 10% SF6 gas compared to 100% air tamponade in descemet’s membrane endothelial keratoplasty. Curr Eye Res. (2018) 43:482–6. doi: 10.1080/02713683.2018.1431286
92. von Marchtaler PV, Weller JM, Kruse FE, Tourtas T. Air versus sulfur hexafluoride gas tamponade in Descemet membrane endothelial keratoplasty: a fellow eye comparison. Cornea. (2018) 37:15–9. doi: 10.1097/ICO.0000000000001413
93. Rickmann A, Wahl S, Hofmann N, Haus A, Michaelis R, Petrich T, et al. Precut DMEK using dextran-containing storage medium is equivalent to conventional DMEK: a prospective pilot study. Cornea. (2019) 38:24–9. doi: 10.1097/ICO.0000000000001778
94. Shahnazaryan D, Hajjar Sese A, Hollick EJ. Endothelial cell loss after Descemet’s membrane endothelial keratoplasty for Fuchs’ endothelial dystrophy: DMEK compared to triple DMEK. Am J Ophthalmol. (2020) 218:1–6. doi: 10.1016/j.ajo.2020.05.003
95. Koechel D, Hofmann N, Unterlauft JD, Wiedemann P, Girbardt C. Descemet membrane endothelial keratoplasty (DMEK): clinical results of precut versus Surgeon-cut grafts. Graefes Arch Clin Exp Ophthalmol. (2021) 259:113–9. doi: 10.1007/s00417-020-04901-7
96. Potts LB, Bauer AJ, Xu DN, Chen SY, Alqudah AA, Sanchez PJ, et al. The last 200 Surgeon-loaded descemet membrane endothelial keratoplasty tissue versus the first 200 preloaded descemet membrane endothelial keratoplasty tissue. Cornea. (2020) 39:1261–6. doi: 10.1097/ICO.0000000000002400
97. Bohm MS, Wylegala A, Leon P, Ong Tone S, Ciolino JB, Jurkunas UV. One-year clinical outcomes of preloaded descemet membrane endothelial keratoplasty versus non-preloaded descemet membrane endothelial keratoplasty. Cornea. (2021) 40:311–9. doi: 10.1097/ICO.0000000000002430
98. Zwingelberg SB, Buscher F, Schrittenlocher S, Rokohl AC, Loreck N, Wawer-Matos P, et al. Long-term outcome of descemet membrane endothelial keratoplasty in eyes with fuchs endothelial corneal dystrophy versus pseudophakic bullous keratopathy. Cornea. (2021) 41:304–9. doi: 10.1097/ICO.0000000000002737
99. Jansen C, Zetterberg M. Descemet membrane endothelial keratoplasty versus Descemet stripping automated keratoplasty – outcome of one single Surgeon’s more than 200 initial consecutive cases. Clin Ophthalmol. (2021) 15:909–21. doi: 10.2147/OPTH.S289730
100. Fajardo-Sanchez J, de Benito-Llopis L. Clinical outcomes of descemet membrane endothelial keratoplasty in pseudophakic eyes compared with triple-DMEK at 1-year follow-up. Cornea. (2021) 40:420–4. doi: 10.1097/ICO.0000000000002636
101. Guerra FP, Anshu A, Price MO, Giebel AW, Price FW. Descemet’s membrane endothelial keratoplasty: prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology. (2011) 118:2368–73. doi: 10.1016/j.ophtha.2011.06.002
102. Laaser K, Bachmann BO, Horn FK, Cursiefen C, Kruse FE. Descemet membrane endothelial keratoplasty combined with phacoemulsification and intraocular lens implantation: advanced triple procedure. Am J Ophthalmol. (2012) 154:47–55e2. doi: 10.1016/j.ajo.2012.01.020
103. Parker J, Dirisamer M, Naveiras M, Tse WH, van Dijk K, Frank LE, et al. Outcomes of Descemet membrane endothelial keratoplasty in phakic eyes. J Cataract Refract Surg. (2012) 38:871–7.
104. Anshu A, Price MO, Price FW Jr. Risk of corneal transplant rejection significantly reduced with Descemet’s membrane endothelial keratoplasty. Ophthalmology. (2012) 119:536–40. doi: 10.1016/j.ophtha.2011.09.019
106. Burkhart ZN, Feng MT, Price FW Jr, Price MO. One-year outcomes in eyes remaining phakic after Descemet membrane endothelial keratoplasty. J Cataract Refract Surg. (2014) 40:430–4. doi: 10.1016/j.jcrs.2013.08.047
107. Maier AK, Wolf T, Gundlach E, Klamann MK, Gonnermann J, Bertelmann E, et al. Intraocular pressure elevation and post-DMEK glaucoma following Descemet membrane endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol. (2014) 252:1947–54. doi: 10.1007/s00417-014-2757-5
108. Feng MT, Price MO, Miller JM, Price FW Jr. Air reinjection and endothelial cell density in Descemet membrane endothelial keratoplasty: five-year follow-up. J Cataract Refract Surg. (2014) 40:1116–21. doi: 10.1016/j.jcrs.2014.04.023
109. Deng SX, Sanchez PJ, Chen L. Clinical outcomes of descemet membrane endothelial keratoplasty using eye bank-prepared tissues. Am J Ophthalmol. (2015) 159:590–6. doi: 10.1016/j.ajo.2014.12.007
110. Rodriguez-Calvo-de-Mora M, Quilendrino R, Ham L, Liarakos VS, van Dijk K, Baydoun L, et al. Clinical outcome of 500 consecutive cases undergoing Descemet’s membrane endothelial keratoplasty. Ophthalmology. (2015) 122:464–70. doi: 10.1016/j.ophtha.2014.09.004
111. Bhandari V, Reddy JK, Relekar K, Prabhu V. Descemet’s stripping automated endothelial keratoplasty versus descemet’s membrane endothelial keratoplasty in the fellow eye for fuchs endothelial dystrophy: a retrospective study. Biomed Res Int. (2015) 2015:750567. doi: 10.1155/2015/750567
112. Schoenberg ED, Price FW Jr, Miller J, McKee Y, Price MO. Refractive outcomes of descemet membrane endothelial keratoplasty triple procedures (combined with cataract surgery). J Cataract Refract Surg. (2015) 41:1182–9. doi: 10.1016/j.jcrs.2014.09.042
113. Gorovoy IR, Gorovoy MS. Descemet membrane endothelial keratoplasty postoperative year 1 endothelial cell counts. Am J Ophthalmol. (2015) 159:597–600e2. doi: 10.1016/j.ajo.2014.12.008
114. Ham L, Dapena I, Liarakos VS, Baydoun L, van Dijk K, Ilyas A, et al. Midterm results of descemet membrane endothelial keratoplasty: 4 to 7 years clinical outcome. Am J Ophthalmol. (2016) 171:113–21. doi: 10.1016/j.ajo.2016.08.038
115. Siggel R, Adler W, Stanzel TP, Cursiefen C, Heindl LM. Bilateral descemet membrane endothelial keratoplasty: analysis of clinical outcome in first and fellow eye. Cornea. (2016) 35:772–7. doi: 10.1097/ICO.0000000000000811
116. van Dijk K, Rodriguez-Calvo-de-Mora M, van Esch H, Frank L, Dapena I, Baydoun L, et al. Two-year refractive outcomes after descemet membrane endothelial keratoplasty. Cornea. (2016) 35:1548–55. doi: 10.1097/ICO.0000000000001022
117. Schlogl A, Tourtas T, Kruse FE, Weller JM. Long-term clinical outcome after descemet membrane endothelial keratoplasty. Am J Ophthalmol. (2016) 169:218–26.
118. Bhandari V, Reddy JK, Chougale P. Descemet’s membrane endothelial keratoplasty in south Asian population. J Ophthalmic Vis Res. (2016) 11:368–71. doi: 10.4103/2008-322X.194072
119. Debellemaniere G, Guilbert E, Courtin R, Panthier C, Sabatier P, Gatinel D, et al. Impact of surgical learning curve in descemet membrane endothelial keratoplasty on visual acuity gain. Cornea. (2017) 36:1–6. doi: 10.1097/ICO.0000000000001066
120. Peraza-Nieves J, Baydoun L, Dapena I, Ilyas A, Frank LE, Luceri S, et al. Two-year clinical outcome of 500 consecutive cases undergoing descemet membrane endothelial keratoplasty. Cornea. (2017) 36:655–60. doi: 10.1097/ICO.0000000000001176
121. Showail M, Obthani MA, Sorkin N, Einan-Lifshitz A, Boutin T, Borovik A, et al. Outcomes of the first 250 eyes of descemet membrane endothelial keratoplasty: canadian centre experience. Can J Ophthalmol. (2018) 53:510–7. doi: 10.1016/j.jcjo.2017.11.017
122. Basak SK, Basak S, Pradhan VR. Outcomes of descemet membrane endothelial keratoplasty (DMEK) using Surgeon’s prepared donor DM-roll in consecutive 100 Indian eyes. Open Ophthalmol J. (2018) 12:134–42. doi: 10.2174/1874364101812010134
123. Kurji KH, Cheung AY, Eslani M, Rolfes EJ, Chachare DY, Auteri NJ, et al. Comparison of visual acuity outcomes between nanothin descemet stripping automated endothelial keratoplasty and descemet membrane endothelial keratoplasty. Cornea. (2018) 37:1226–31. doi: 10.1097/ICO.0000000000001697
124. Fajgenbaum MAP, Kopsachilis N, Hollick EJ. Descemet’s membrane endothelial keratoplasty: surgical outcomes and endothelial cell count modelling from a UK centre. Eye (Lond). (2018) 32:1629–35. doi: 10.1038/s41433-018-0152-x
125. Newman LR, DeMill DL, Zeidenweber DA, Mayko ZM, Bauer AJ, Tran KD, et al. Preloaded descemet membrane endothelial keratoplasty donor tissue: surgical technique and early clinical results. Cornea. (2018) 37:981–6.
126. Price DA, Kelley M, Price FW Jr, Price MO. Five-year graft survival of descemet membrane endothelial keratoplasty (EK) versus descemet stripping EK and the effect of donor sex matching. Ophthalmology. (2018) 125:1508–14. doi: 10.1016/j.ophtha.2018.03.050
127. Schrittenlocher S, Schaub F, Hos D, Siebelmann S, Cursiefen C, Bachmann B. Evolution of consecutive descemet membrane endothelial keratoplasty outcomes throughout a 5-year period performed by two experienced Surgeons. Am J Ophthalmol. (2018) 190:171–8. doi: 10.1016/j.ajo.2018.03.036
128. Droutsas K, Lazaridis A, Giallouros E, Kymionis G, Chatzistefanou K, Sekundo W. Scheimpflug densitometry after dmek versus dsaek-two-year outcomes. Cornea. (2018) 37:455–61. doi: 10.1097/ICO.0000000000001483
129. Godin MR, Boehlke CS, Kim T, Gupta PK. Influence of lens status on outcomes of descemet membrane endothelial keratoplasty. Cornea. (2019) 38:409–12.
130. Rickmann A, Wahl S, Katsen-Globa A, Szurman P. Safety analysis and results of a borosilicate glass cartridge for no-touch graft loading and injection in descemet membrane endothelial keratoplasty. Int Ophthalmol. (2019) 39:2295–301. doi: 10.1007/s10792-018-01067-4
131. Sarnicola C, Sabatino F, Sarnicola E, Perri P, Cheung AY, Sarnicola V. Cannula-assisted technique to unfold grafts in descemet membrane endothelial keratoplasty. Cornea. (2019) 38:275–9. doi: 10.1097/ICO.0000000000001827
132. Brockmann T, Pilger D, Brockmann C, Maier AB, Bertelmann E, Torun N. Predictive factors for clinical outcomes after primary descemet’s membrane endothelial keratoplasty for fuchs’ endothelial dystrophy. Curr Eye Res. (2019) 44:147–53. doi: 10.1080/02713683.2018.1538459
133. Schaub F, Gerber F, Adler W, Enders P, Schrittenlocher S, Heindl LM, et al. Corneal densitometry as a predictive diagnostic tool for visual acuity results after descemet membrane endothelial keratoplasty. Am J Ophthalmol. (2019) 198:124–9. doi: 10.1016/j.ajo.2018.10.002
134. Livny E, Bahar I, Levy I, Mimouni M, Nahum Y. “PI-less DMEK”: results of DESCEMET’S membrane endothelial keratoplasty (DMEK) without a peripheral iridotomy. Eye (Lond). (2019) 33:653–8.
135. Basak SK, Basak S, Gajendragadkar N, Ghatak M. Overall clinical outcomes of descemet membrane endothelial keratoplasty in 600 consecutive eyes: a large retrospective case series. Indian J Ophthalmol. (2020) 68:1044–53. doi: 10.4103/ijo.IJO_1563_19
136. Siddharthan KS, Shet V, Agrawal A, Reddy JK. Two-year clinical outcome after descemet membrane endothelial keratoplasty using a standardized protocol. Indian J Ophthalmol. (2020) 68:2408–14.
137. Lekhanont K, Pisitpayat P, Cheewaruangroj N, Jongkhajornpong P, Nonpassopon M, Anothaisintawee T. Outcomes of descemet membrane endothelial keratoplasty in Bangkok, Thailand. Clin Ophthalmol. (2021) 15:2239–51. doi: 10.2147/OPTH.S310873
138. Marchand M, El-Khoury J, Harissi-Dagher M, Robert MC. Outcomes of first cases of DMEK at a canadian university hospital centre. Can J Ophthalmol. (2021) 57:214–5. doi: 10.1016/j.jcjo.2021.05.010
139. Studeny P, Hlozankova K, Krizova D, Netukova M, Veith M, Mojzis P, et al. Long-term results of a combined procedure of cataract surgery and descemet membrane endothelial keratoplasty with stromal rim. Cornea. (2021) 40:628–34. doi: 10.1097/ICO.0000000000002574
140. Tan TE, Devarajan K, Seah XY, Lin SJ, Peh GSL, Cajucom-Uy HY, et al. Descemet membrane endothelial keratoplasty with a pull-through insertion device: surgical technique, endothelial cell loss, and early clinical results. Cornea. (2020) 39:558–65. doi: 10.1097/ICO.0000000000002268
141. Yu AC, Myerscough J, Spena R, Fusco F, Socea S, Furiosi L, et al. Three-year outcomes of tri-folded endothelium-in descemet membrane endothelial keratoplasty with pull-through technique. Am J Ophthalmol. (2020) 219:121–31. doi: 10.1016/j.ajo.2020.07.004
142. Woo JH, Htoon HM, Tan D. Hybrid descemet membrane endothelial keratoplasty (H-DMEK): results of a donor insertion pull-through technique using donor stroma as carrier. Br J Ophthalmol. (2020) 104:1358–62. doi: 10.1136/bjophthalmol-2019-314932
143. Ighani M, Dzhaber D, Jain S, De Rojas JO, Eghrari AO. Techniques, outcomes, and complications of preloaded, trifolded descemet membrane endothelial keratoplasty using the DMEK endoglide. Cornea. (2021) 40:669–74. doi: 10.1097/ICO.0000000000002648
Keywords: endothelial keratoplasty, Descemet’s membrane endothelial keratoplasty, DMEK, bullous keratopathy, cornea, corneal transplants, outcomes, surgical techniques
Citation: Ong HS, Htoon HM, Ang M and Mehta JS (2022) “Endothelium-Out” and “Endothelium-In” Descemet Membrane Endothelial Keratoplasty (DMEK) Graft Insertion Techniques: A Systematic Review With Meta-Analysis. Front. Med. 9:868533. doi: 10.3389/fmed.2022.868533
Received: 02 February 2022; Accepted: 10 May 2022;
Published: 14 June 2022.
Edited by:
Mohit Parekh, University College London, United KingdomReviewed by:
Vito Romano, University of Brescia, ItalyHannah Levis, University of Liverpool, United Kingdom
Copyright © 2022 Ong, Htoon, Ang and Mehta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jodhbir S. Mehta, am9kbWVodGFAZ21haWwuY29t; Hon Shing Ong, aG9uc2hpbmdAZ21haWwuY29t
 Hon Shing Ong
Hon Shing Ong Hla M. Htoon2,3
Hla M. Htoon2,3 Marcus Ang
Marcus Ang Jodhbir S. Mehta
Jodhbir S. Mehta