
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Med. , 24 February 2022
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.862953
This article is part of the Research Topic Current Insights into Complex Post-Infection Fatigue Syndromes with Unknown Aetiology: the Case of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Beyond View all 11 articles
Editorial on the Research Topic
Current Insights Into Complex Post-infection Fatigue Syndromes With Unknown Aetiology: The Case of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Beyond
Black plague epidemics in Medieval Europe, the Spanish Flu pandemic during the first world war, and the pandemic of COVID-19 disease are just three devastating examples of the fragile co-existence between human beings and the microbial world. Remarkably, the human immune system with its innate and adaptive arms recognizes and clears the invading pathogens in most cases. However, like a scar after an injury, some people who had suffered from acute infections remain ill long after the clearance of the pathogen itself. These individuals develop complex fatigue-related syndromes whose pathological mechanisms remain poorly understood. A prime example of such syndromes is the Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) characterized by persistent fatigue and post-exertional malaise among other symptoms (1). Unfortunately, its diagnosis remains challenging due to the inexistence of objective biomarkers that could identify cases. However, researchers are gathering around multidisciplinary networks, such as the US ME/CFS Clinician Coalition and the European Network on ME/CFS, with the aim of fostering collaboration, standardizing research and clinical practices, while accelerating biomarker discovery (2–5). Less-known fatigue-related syndromes have been recently reported after the outbreaks of Ebola virus, Dengue virus, and Chikungunya virus in the Tropics (6–8). However, it is still unclear whether these syndromes constitute clinical entities beyond ME/CFS itself.
In this scenario, we invited the research community to contribute with studies on these complex fatigue-related syndromes. Our primary objective was to take the pulse of current data and hypotheses about how these syndromes are initiated and maintained over time. Our second objective was to understand how current insights can lead to successful treatments for patients. With the WHO notification of the COVID-19 as a pandemic on March 11, 2020, our third and final objective was to debate for the first time about ME/CFS as a sequela of post-SARS-CoV-2 infections. The graphical summary of all the contributions received is shown in Figure 1.
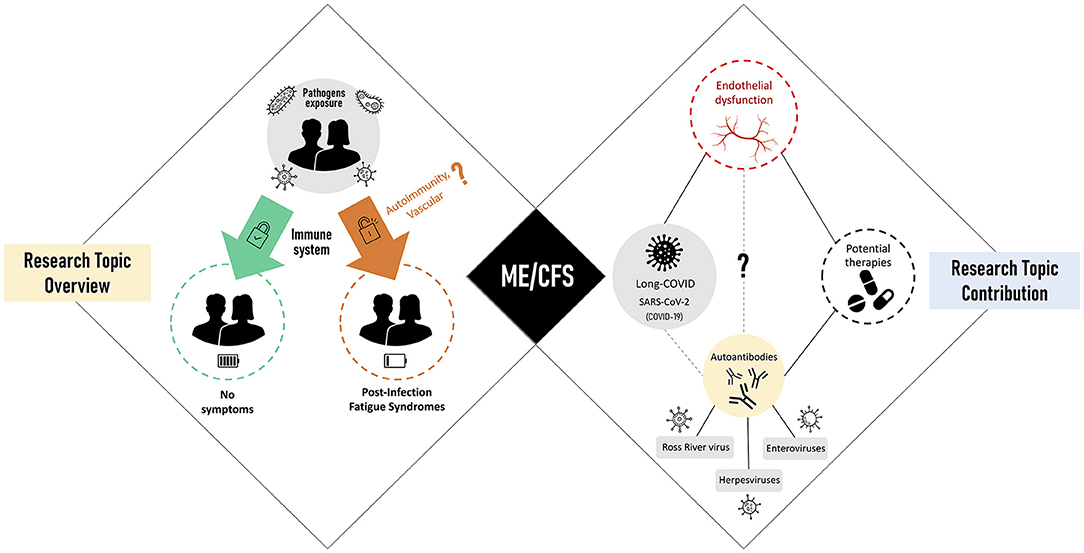
Figure 1. Research Topic overview (left side) linked to post-infectious fatigue syndromes with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) as the main representative (center). Research Topic contribution (right) focused on viral triggers of ME/CFS, disease-related pathomechanisms, and potential therapies.
Early on, it was immediately recognized the impact of herpesviruses on the pathology of ME/CFS (9–11). Follow-up studies made clear that other viruses could also elicit the disease (12). However, the respective pathological mechanisms remain to be uncovered. In this regard, O'Neal and Hanson offered a critical review about past research on enteroviruses as causative agents of ME/CFS. Another interesting review was conducted by Lidbury who discussed the immune evasion strategies of the Ross River virus, which is an arbovirus endemic to Australia, Papua New Guinea, and other islands in the South Pacific. We foresee this review to be useful for understanding post-infection fatigue syndromes due to other arboviruses, such as the Chikungunya, Dengue, and Zika. In this regard, it is a priority to study the burden of post-infection fatigue among Brazilian or Cape Verdean survivors who suffered from recent outbreaks of these arboviruses (13, 14). Finally, Lee et al. and Domingues et al. provided new research on herpesviruses in patients from the United Kingdom ME/CFS biobank. The first study is a rare longitudinal analysis of multiple herpesviruses in patients with ME/CFS; such studies should become standard given the natural fluctuations in disease dynamics. The second study concerns a re-analysis of published serological data using a stratification based on infection and non-infection triggers. The findings of this study clearly show the necessity of stratifying patients adequately, as suggested by Jason et al. (15).
With the onset of the COVID-19 pandemic, a new viral trigger of ME/CFS is currently spreading across the world: SARS-CoV-2. Past experience with the “original” SARS-CoV pandemic suggested this coronavirus as another trigger of ME/CFS (16). Before any mainstream discussion about “long-COVID” or “post-acute sequelae SARS-CoV-2 infection”, Komaroff and Bateman on behalf of the US ME/CFS clinician coalition drafted a sort of memorandum alerting for the devastating long-term consequences in survivors of SARS-CoV-2 infections. In turn, Petracek et al. reported probably the first three ME/CFS cases after 6 months of SARS-CoV-2 infections. Other studies published elsewhere provide further evidence that some long-COVID patients suffer from ME/CFS (17, 18) and, as such, there is a window of opportunity to improve the understanding of both conditions.
A key challenge of investigating ME/CFS is that the disease is likely to be multifactorial and heterogeneous and, therefore, patients might show different pathological pathways that could explain their symptoms. To resolve this, many theoretical papers about possible disease mechanisms emerged in the literature over the years (19–23). In this Research Topic, Stanculescu et al. followed the footsteps of these early theoretical papers by paralleling the pathological mechanisms suggested for patients in an intensive care unit (ICU) and patients with ME/CFS. Their research premise is that the same “vicious circle” between inflammation, oxidative and nitrosative stress, and low thyroid hormone function is operating in both clinical populations. In a follow-up paper, Stanculescu et al. made a comprehensive review of available treatments for ICU patients with the idea of being repurposed to stop that “vicious circle” in patients with ME/CFS. Given the heterogeneous nature of ME/CFS, it is likely that the suggested parallelism might only hold true for some but not all the patients. In another theoretical paper, O'Boyle et al. provided a general discussion about treatment and case management using a previously proposed framework for the natural progression of the disease (24). These authors suggested that pre-disease and early disease call for rehabilitation strategies that could avoid long-term co-morbidity while the management of the established disease should be more holistic and tailored to the specific needs of each patient. The basic question is whether clinicians are able to estimate accurately at which disease stage a patient is.
As a follow-up from early clinical trials in Norwegian patients with ME/CFS (25, 26), Sørland et al. evaluated endothelial function in patients with ME/CFS at baseline and after a therapeutic intervention with cyclophosphamide, an immunosuppressive drug used in cancer. This evaluation was motivated by the growing evidence of vascular abnormalities in ME/CFS (27, 28). The authors also found endothelial dysfunction at baseline, which persisted after treatment irrespective of the clinical response of the patients. Interestingly, the authors also reported a significant correlation between high symmetric dimethylarginine (SDMA) levels and low flow-mediated dilation values. Thus, given that SDMA has been described to reduce the production of nitric oxide (NO) in endothelial cells (29), this study raises a new perspective to address endothelial dysfunction in ME/CFS by combining clinical and metabolic parameters. Endothelial dysfunction and inadequate regulation of blood flow resulting in hypoperfusion of the brain and muscles are considered as key pathological mechanisms in ME/CFS as further outlined in two recent papers (21, 30). There is increasing evidence that autoantibodies directed against vasoregulatory receptors contribute to the vascular dysregulation in ME/CFS (21, 30). These findings open perspectives for therapy. For example, one can target autoreactive B cells or autoantibodies, and preliminary studies provide evidence for clinical efficacy [reviewed in ref. (30)]. The use of drugs that help regulating vascular function is another possibility to treat patients with ME/CFS.
In conclusion, this Research Topic collects further pieces of evidence about how various viruses including SARS-CoV-2 can trigger ME/CFS. The neglect of research in ME/CFS during the last decades has left patients, carers, and clinicians alike adrift without a licensed drug to use in the disease. On the one hand, the COVID-19 pandemic will result in an unprecedented explosion of ME/CFS cases. At the same time, this pandemic is the perfect storm that can motivate different stakeholders, including funders and clinicians, to take the necessary steps to accelerate research on ME/CFS and other post-infectious syndromes. If taken, these steps will bring hope to all those outstanding patients who have been homebound or even bedridden for many years but neglected by national health authorities.
All authors contributed to this editorial and approved the final version.
FW received funding from ME Research UK (SCIO charity number SC036942). EL received funding from the National Institutes of Health (ref. NIH 2R01AI103629), and the ME Association (UKMEB–ME Association, Grant PF8947_ME Association). CS received funding from the Weidenhammer Zoebele Foundation, Germany. NS received funding from Fundação para a Ciência e Tecnologia, Portugal (ref. UIDB/00006/2020), and the Polish National Agency for Academic Exchange, Poland (ref. PPN/ULM/2020/1/00069/U/00001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Lígia Pasqualin (MA, MBA) for helping in the design of the graphical summary. Some elements and templates of the graphical summary were downloaded from the vecteezy site (vecteezy.com) as “Free Content” under a “Free License” (digital use for unlimited views with no sales involved).
1. Rivera MC, Mastronardi C, Silva-Aldana CT, Arcos-Burgos M, Lidbury BA. Myalgic encephalomyelitis/chronic fatigue syndrome: a comprehensive review. Diagnostics. (2019) 9:91. doi: 10.3390/diagnostics9030091
2. Scheibenbogen C, Freitag H, Blanco J, Capelli E, Lacerda E, Authier J, et al. The European ME/CFS biomarker landscape project: an initiative of the European network EUROMENE. J Transl Med. (2017) 15:162. doi: 10.1186/s12967-017-1263-z
3. Pheby DFH, Araja D, Berkis U, Brenna E, Cullinan J, de Korwin J-D, et al. The development of a consistent Europe-wide approach to investigating the economic impact of Myalgic Encephalomyelitis (ME/CFS): a report from the European Network on ME/CFS (EUROMENE). Healthcare. (2020) 8:88. doi: 10.3390/healthcare8020088
4. Nacul L, Authier FJ, Scheibenbogen C, Lorusso L, Helland IB, Martin JA, et al. European Network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): expert consensus on the diagnosis, service provision, and care of people with ME/CFS in Europe. Medicina (Kaunas). (2021) 57:510. doi: 10.3390/MEDICINA57050510
5. U.S. ME/CFS Clinician Coalition. Available online at: https://mecfscliniciancoalition.org/ (accessed January 13, 2022).
6. Seet RCS, Quek AML, Lim ECH. Post-infectious fatigue syndrome in dengue infection. J Clin Virol. (2007) 38:1–6. doi: 10.1016/J.JCV.2006.10.011
7. Duvignaud A, Fianu A, Bertolotti A, Jaubert J, Michault A, Poubeau P, et al. Rheumatism and chronic fatigue, the two facets of post-chikungunya disease: the TELECHIK cohort study on Reunion island. Epidemiol Infect. (2018) 146:633–41. doi: 10.1017/S0950268818000031
8. Sneller M, Reilly C, Badio M, Bishop R, Eghrari A, Moses S, et al. A longitudinal study of Ebola Sequelae in Liberia. N Engl J Med. (2019) 380:924–34. doi: 10.1056/NEJMOA1805435
9. Koo D. Chronic fatigue syndrome. A critical appraisal of the role of Epstein-Barr virus. West J Med. (1989) 150:590–6.
10. Buchwald D, Cheney PR, Peterson DL, Henry B, Wormsley SB, Geiger A, et al. A chronic illness characterized by fatigue, neurologic and immunologic disorders, and active human herpesvirus type 6 infection. Ann Intern Med. (1992) 116:103–13. doi: 10.7326/0003-4819-116-2-103
11. Ariza ME. Myalgic encephalomyelitis/chronic fatigue syndrome: the human herpesviruses are back! Biomolecules. (2021) 11:185. doi: 10.3390/biom11020185
12. Rasa S, Nora-Krukle Z, Henning N, Eliassen E, Shikova E, Harrer T, et al. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med. (2018) 16:268. doi: 10.1186/s12967-018-1644-y
13. Zanotto PM de A, Leite LC de C. The challenges imposed by Dengue, Zika, and Chikungunya to Brazil. Front Immunol. (2018) 9:1964. doi: 10.3389/FIMMU.2018.01964
14. Lourenço J, Monteiro M, Tomás T, Monteiro Rodrigues J, Pybus O, Rodrigues Faria N. Epidemiology of the Zika Virus Outbreak in the Cabo Verde Islands, West Africa. PLoS Curr. (2018). doi: 10.1371/CURRENTS.OUTBREAKS.19433B1E4D007451C691F138E1E67E8C
15. Jason LA, Corradi K, Torres-Harding S, Taylor RR, King C. Chronic fatigue syndrome: the need for subtypes. Neuropsychol Rev. (2005) 15:29–58. doi: 10.1007/s11065-005-3588-2
16. Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. (2011) 11:37. doi: 10.1186/1471-2377-11-37
17. Mantovani E, Mariotto S, Gabbiani D, Dorelli G, Bozzetti S, Federico A, et al. Chronic fatigue syndrome: an emerging sequela in COVID-19 survivors? J Neurovirol. (2021) 27:631–7. doi: 10.1007/S13365-021-01002-X
18. Kedor C, Freitag H, Meyer-Arndt L, Wittke K, Zoller T, Steinbeis F, et al. Chronic COVID-19 Syndrome and Chronic Fatigue Syndrome (ME/CFS) following the first pandemic wave in Germany – a first analysis of a prospective observational study. medRxiv. (2021) 2021.02.06.21249256. doi: 10.1101/2021.02.06.21249256
19. Blomberg J, Gottfries CG, Elfaitouri A, Rizwan M, Rosén A. Infection elicited autoimmunity and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: an explanatory model. Front Immunol. (2018) 9:229. doi: 10.3389/fimmu.2018.00229
20. Sepúlveda N, Carneiro J, Lacerda E, Nacul L. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome as a hyper-regulated immune system driven by an interplay between regulatory T cells and chronic human herpesvirus infections. Front Immunol. (2019) 10:2684. doi: 10.3389/fimmu.2019.02684
21. Wirth K, Scheibenbogen C A. Unifying hypothesis of the pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): recognitions from the finding of autoantibodies against ß2-adrenergic receptors. Autoimmun Rev. (2020) 19:102527. doi: 10.1016/j.autrev.2020.102527
22. Wyller VB, Eriksen HR, Malterud K. Can sustained arousal explain the chronic fatigue syndrome? Behav Brain Funct. (2009) 5:10. doi: 10.1186/1744-9081-5-10
23. Hatziagelaki E, Adamaki M, Tsilioni I, Dimitriadis G, Theoharides TC. Myalgic encephalomyelitis/chronic fatigue syndrome—metabolic disease or disturbed homeostasis due to focal inflammation in the hypothalamus? J Pharmacol Exp Ther. (2018) 367:155–67. doi: 10.1124/jpet.118.250845
24. Nacul L, O'Boyle S, Palla L, Nacul FE, Mudie K, Kingdon CC, et al. How Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Progresses: the natural history of ME/CFS. Front Neurol. (2020) 11:826. doi: 10.3389/fneur.2020.00826
25. Fluge Ø, Rekeland IG, Lien K, Thürmer H, Borchgrevink PC, Schäfer C, et al. B-lymphocyte depletion in patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. (2019) 170:585–93. doi: 10.7326/M18-1451
26. Rekeland IG, Fosså A, Lande A, Ktoridou-Valen I, Sørland K, Holsen M, et al. Intravenous cyclophosphamide in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. An open-label phase II study. Front Med. (2020) 7:162. doi: 10.3389/FMED.2020.00162
27. Newton DJ, Kennedy G, Chan KKF, Lang CC, Belch JJF, Khan F. Large and small artery endothelial dysfunction in chronic fatigue syndrome. Int J Cardiol. (2012) 154:335–6. doi: 10.1016/J.IJCARD.2011.10.030
28. Scherbakov N, Szklarski M, Hartwig J, Sotzny F, Lorenz S, Meyer A, et al. Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Fail. (2020) 7:1064–71. doi: 10.1002/ehf2.12633
29. Feliers D, Lee DY, Gorin Y, Kasinath BS. Symmetric dimethylarginine alters endothelial nitric oxide activity in glomerular endothelial cells. Cell Signal. (2015) 27:1–5. doi: 10.1016/J.CELLSIG.2014.09.024
Keywords: ME/CFS, Ross River virus (RRV), SARS-CoV-2, long-COVID, post COVID syndrome herpesviruses, enteroviruses, endothelial (dys)function, ICU—rehabilitation
Citation: Westermeier F, Lacerda EM, Scheibenbogen C and Sepúlveda N (2022) Editorial: Current Insights Into Complex Post-infection Fatigue Syndromes With Unknown Aetiology: The Case of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Beyond. Front. Med. 9:862953. doi: 10.3389/fmed.2022.862953
Received: 26 January 2022; Accepted: 01 February 2022;
Published: 24 February 2022.
Edited and reviewed by: Marc Jean Struelens, Université Libre de Bruxelles, Belgium
Copyright © 2022 Westermeier, Lacerda, Scheibenbogen and Sepúlveda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nuno Sepúlveda, N.Sepulveda@mini.pw.edu.pl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.