- 1Department of Gastroenterology, The Third Hospital of Hebei Medical University, Shijiazhuang, China
- 2Department of Pediatric Surgery, The Second Hospital of Hebei Medical University, Shijiazhuang, China
- 3Jitang College of North China University of Science and Technology, Tangshan, China
Objective: The purpose of this study is to establish an accurate prognostic model based on important clinical parameters to predict the overall survival (OS) of elderly patients with primary gastrointestinal diffuse large B-cell lymphoma (EGI DLBCL).
Methods: The Cox regression analysis is based on data from the Surveillance, Epidemiology, and End Results (SEER) database.
Results: A total of 1,783 EGI DLBCL cases were eligible for the study [median (interquartile range, IQR) age, 75 (68–82) years; 974 (54.63%) males], of which 1,248 were randomly assigned to the development cohort, while 535 were into the validation cohort. A more accurate and convenient dynamic prognostic nomogram based on age, stage, radiation, and chemotherapy was developed and validated, of which the predictive performance was superior to that of the Ann Arbor staging system [C-index:0.69 (95% CI:0.67–0.71) vs. 56 (95%CI:0.54–0.58); P < 0.001]. The 3- and 5-year AUC values of ROC curves for 3-year OS and 5-year OS in the development cohort and the validation cohort were were alll above 0.7.
Conclusion: We establish and validate a more accurate and convenient dynamic prognostic nomogram for patients with EGI DLBCL, which can provide evidence for individual treatment and follow-up.
Introduction
Primary gastrointestinal (GI) lymphoma is the most common type of extranodal lymphoma, accounting for approximately 25% of all primary extranodal lymphomas (1). However, primary gastrointestinal lymphoma accounts for only 1–4% of all gastrointestinal cancers (2). More than half of the cases occur in the stomach, followed by the small intestine and ileocecum (2). Histopathological findings showed the following types: marginal zone lymphoma (MALT), diffuse large B-cell lymphoma (DLBCL), enteropathy-associated lymphoma (EATL), mantle cell lymphoma (MCL), etc. According to histological type, DLBCL is the most common gastrointestinal lymphoma, with an estimated prevalence of 40–50% (2, 3).
Since 1993, the International Prognostic Index (IPI) has long been used for risk stratification, which can provide prognosis prediction and treatment guidance for patients with diffuse large B-cell lymphoma (DLBCL). The five factors of IPI score include Ann Arbor Stage III/IV, age > 60 years, elevated lactate dehydrogenase (LDH), Eastern Cooperative Oncology Group (ECOG) working status (PS) of ≥ 2, and at least one extranodal location are involved. The sum allows patients to be divided into four independent groups through each point of four factors, with a 5-year overall survival rate (OS) of 26–73% (4). GI DLBCL is usually diagnosed as low or medium IPI. Among the five factors of IPI score, age plays a non-negligible role in patients with GI DLBCL (5). Also, the National Comprehensive Cancer Network (NCCN) guidelines include the Ann Arbor staging of primary diffuse large B-cell lymphoma to guide clinical treatment and follow-up (6). As the conventional staging system for non-Hodgkin’s lymphoma (NHL), the Ann Arbor staging system considers the location of lymph node spreading as the basis for staging (7). It does not include other factors that may affect long-term survival, such as age and depth of tumor invasion. Also, the Ann Arbor staging system is not considered the best staging system for primary gastric diffuse large B-cell lymphoma (8). Elderly patients (≥60 years old), with primary gastrointestinal diffuse large B-cell lymphoma (EGI DLBCL), have a significantly higher risk of death compared with the adult population because they are much more likely to receive palliative rather than curative care. At the same time, elderly patients have poor physical condition and many complications and are less resistant to comprehensive treatment (9, 10). Therefore, it has a clinically significant role in predicting the survival of EGI DLBCL.
Therefore, based on the latest population-based data from Surveillance, Epidemiology, and End Results (SEER) database, this study investigated a large number of patients to develop a survival prediction nomogram and a web-based survival rate calculator that can dynamically predict the long-term survival of EGI DLBCL.
Materials and Methods
Data Collection
This study is a retrospective cohort study using the largest publicly available data set on the human cancers’ SEER database. The database contains information collected from different cancer registries in 18 geographic regions of the United States, which currently account for approximately 27.8% of the total population of the United States (11). The SEER database has a wide population coverage and high data accuracy. Since any information in the SEER database does not require the explicit consent of the patient, our research is not subject to the ethical permission requirements of the institutional review board, and informed consent was obtained from all participants.
Patient Selection
The inclusion criteria for this study are as follows: (1) Patients with pathological diagnosis of primary gastric diffuse large B-cell lymphoma from 2010 to 2015. (2) Pathological results support the diagnosis of the patient; (3) patients with ≥ 60 years old. Patients who meet any of the following criteria are excluded: (1) Ann Arbor staging was unknown; (2) Missing demographic information, including race, and marital status; (3) Missing clinical and treatment information; and (4) Missing survival information. In total, 1,783 elderly patients (≥ 60 years old), with primary gastric diffuse large B-cell lymphoma (EGI DLBCL), were included in the final analysis, randomly assigned to the development and validation cohorts with a ratio of 7:3.
Statistical Analysis
Overall survival (OS), measured from the date of diagnosis to the time of death from any cause or last follow-up, was employed as the outcome of interest. The Wilcoxon-Mann-Whitney test or Fisher’s exact test was performed to measure the distribution differences of variables between the development and validation cohorts, where appropriate. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated by the univariate Cox regression model to quantify the effect of potential prognostic predictors on OS. Then, predictors significantly associated with OS in the univariate analysis were entered into the multivariate analysis to validate their significance by applying a backward procedure based on the Akaike information criterion (AIC) (12).
A dynamic prognostic nomogram for OS was then generated based on the identified independent prognostic factors. Harrell’s concordance index (C-index) was used to assess the discrimination performance and compared between the dynamic prognostic nomogram and the Ann Arbor staging system. Calibration curves were adopted as indicators of internal calibration by plotting the nomogram-predicted probabilities against the observed probabilities via a bootstrap method with 1,000 resamples (13, 14). In the validation cohort, nomogram performance was assessed using the same methods as in the development cohort.
We further utilized the quartile to determine the optimal cut-off value for the total risk score (derived from the nomogram) and established a prognostic risk stratification to allocate patients of EGI DLBCL into groups at different risks. By comparing the prognostic score and the survival curve of the staging system grouping with the ROC curve at a specific time point, the discriminating power of the model was evaluated. In addition, the comparison of prediction effectiveness was measured using the receiver operating characteristics (ROC) curve at a specific time point between nomogram and a single meaningful variable (15). The above analyses were all performed using R software (version 3.6.3), and the statistically significant difference was determined to be two-sided P < 0.05.
Results
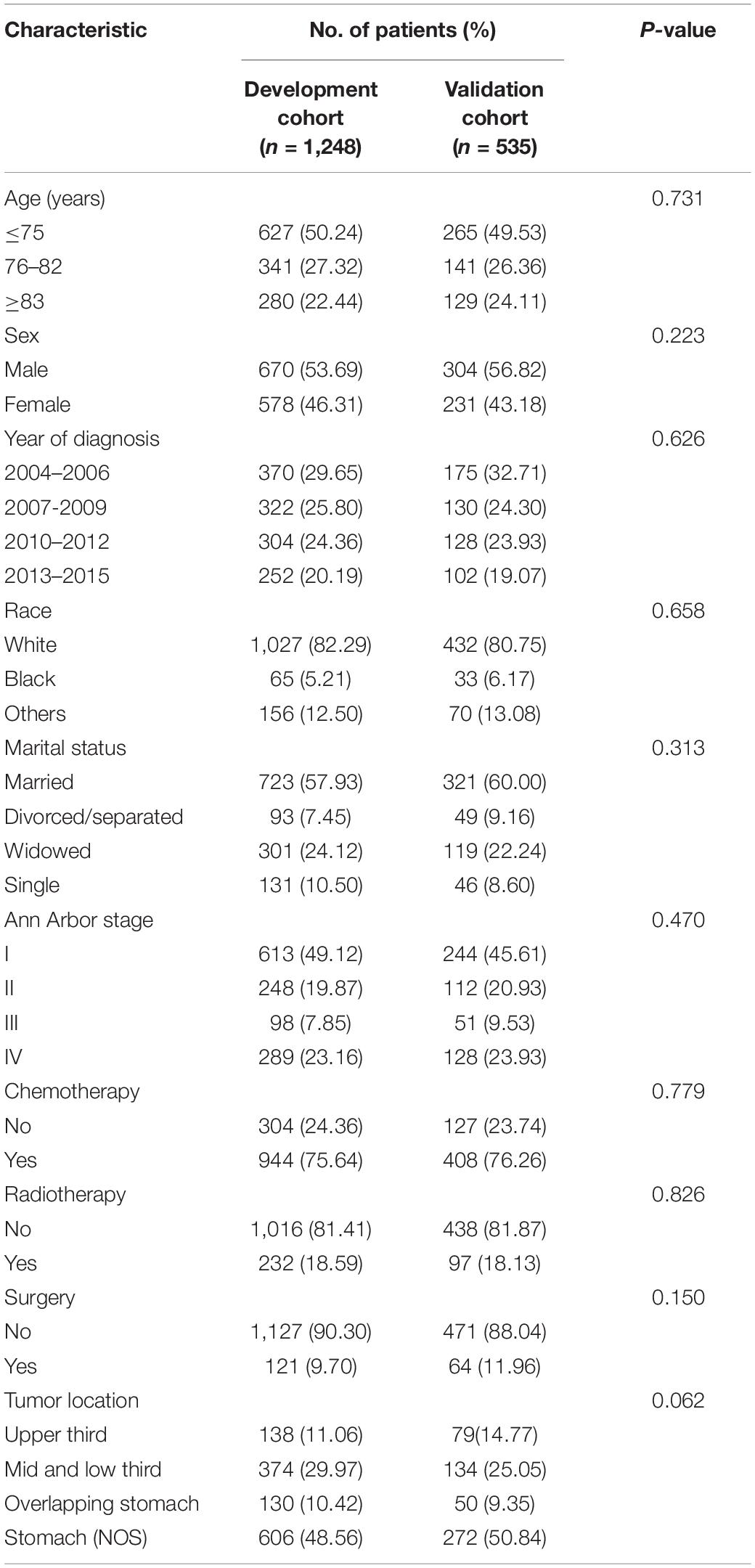
A total of 1,783 EGI DLBCL cases were eligible for the study [median (interquartile range, IQR) age, 75 (68–82) years; 974 (54.63%) males], of which 1,248 were randomly assigned to the development cohort, while 535 were into the validation cohort. The demographical and clinical characteristics of patients in the development and validation cohorts were all similar (Table 1). The median follow-up in the development cohort was 27 months (IQR, 6–77 months); the OS for 3- and 5-year were 50.68 and 45.44%, respectively. The median follow-up time in the validation cohort was 28 months (IQR, 5–78 months); 3- and 5-year OS rates were 50.56 and 42.05%, respectively.
Table 2 presents the results of univariate and multivariate analyses to include the nomogram variables for OS in the development cohort. In the univariate Cox model, except for sex, race, surgery, and tumor location, the remaining variables were significantly associated with OS. In the multivariate Cox model, all selected variables by applying the AIC-based backward procedure (age, stage, radiation, and chemotherapy) had significant effects on OS (all P < 0.05). At the same time, the survival curves were drawn separately for the meaningful variables of multivariate regression (Figure 1).
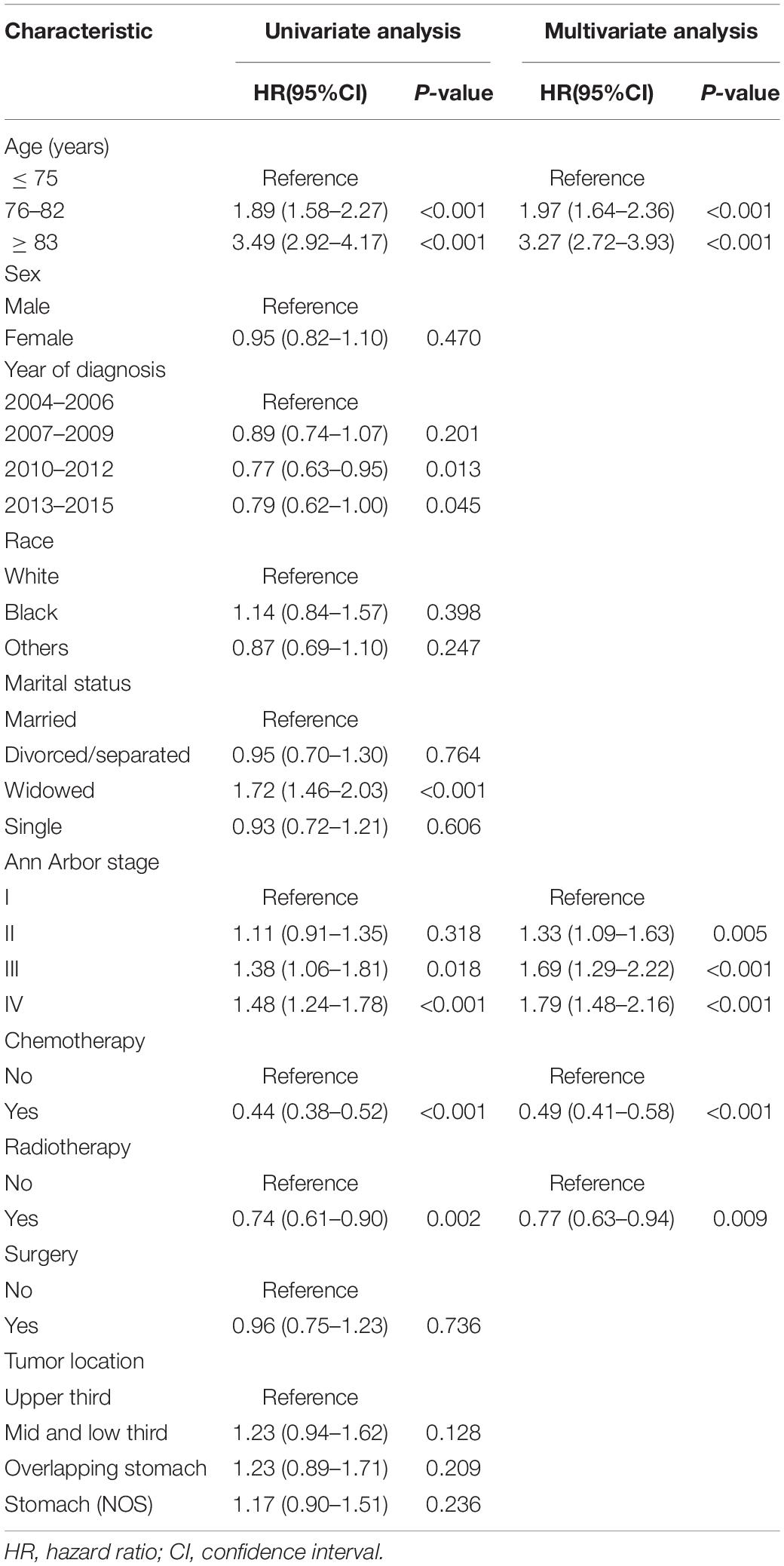
Table 2. Results of the univariate and multivariate cox models, including the nomogram variables for overall survival in the development cohort.
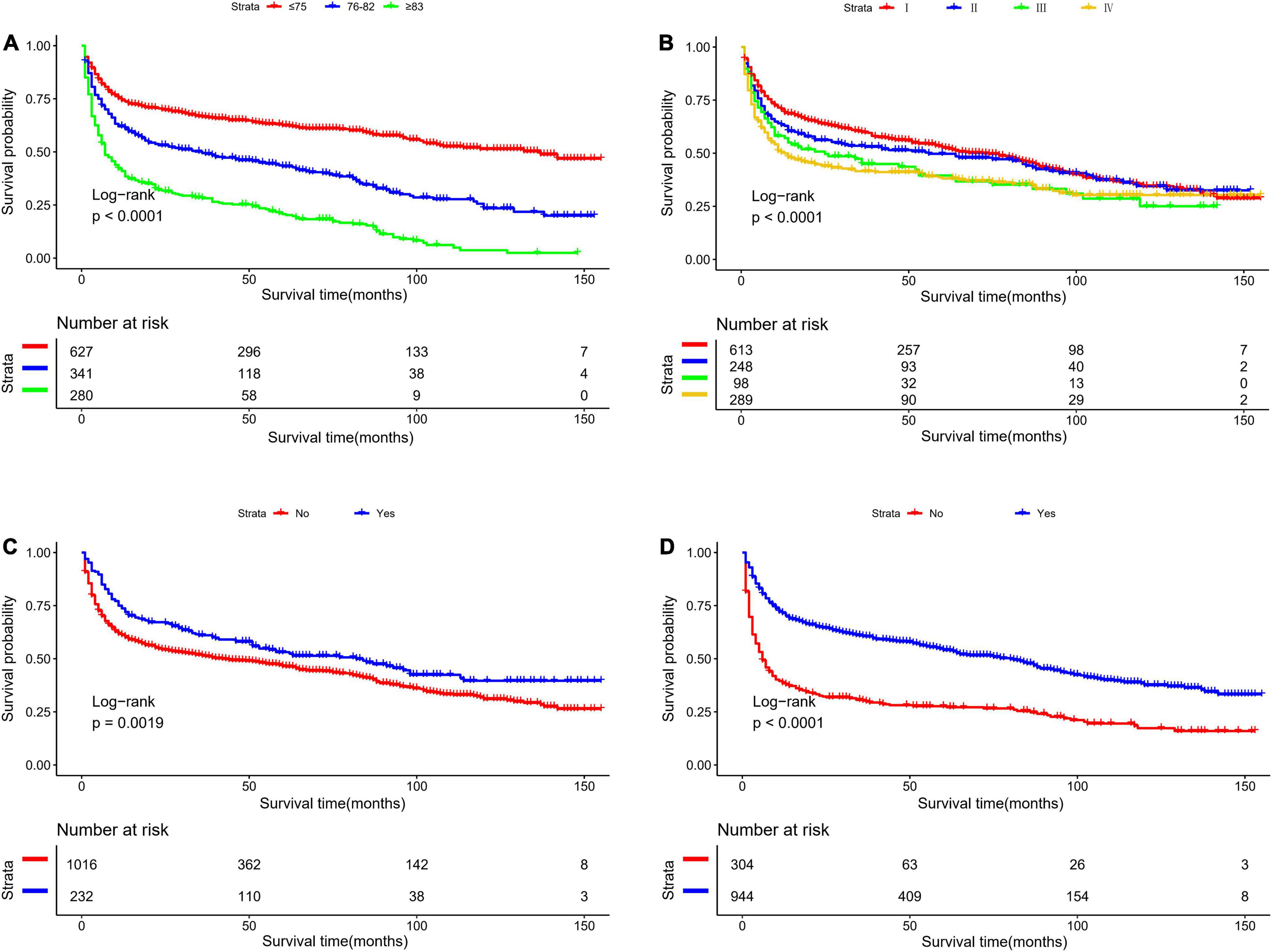
Figure 1. Kaplan-Meier survival curves for overall survival in the development cohort, as stratified by (A) Age, (B) stage, (C) radiation, (D) chemotherapy.
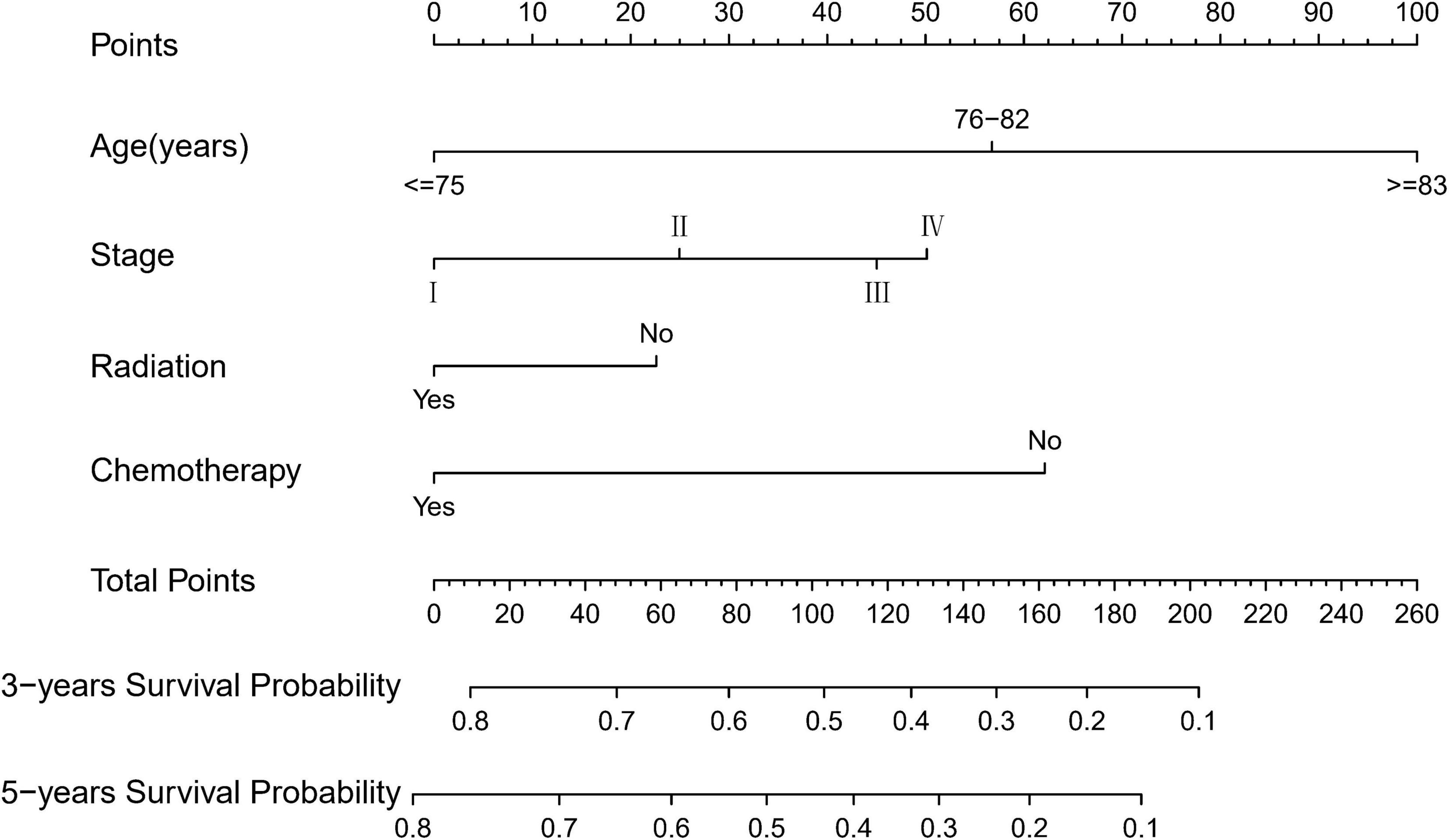
Based on the proven independent prognostic factors, a user-friendly and accurate dynamic prognostic nomogram1 was established (Figure 2A); a point assignment for each factor and total risk score calculation for each patient is described in detail in Supplementary Table 1 and Figure 3. The dynamic prognostic nomogram would conveniently provide the accurately predicted probability of OS based on the total risk score, which was calculated automatically according to the input characteristics of subjects (Figure 2B). The C-indices of the nomogram in the development cohort and the validation cohort were 0.69 (95% CI, 0.67–0.71) and 0.69 (95% CI, 0.66–0.72), respectively. Figure 4 shows the nomogram calibration curves for 3- and 5- years in the development cohort and validation cohort. Calibration plots revealed that the nomogram was well-calibrated, with the superb agreement between the nomogram-predicted probabilities and the observed probabilities of 3- and 5-year OS.
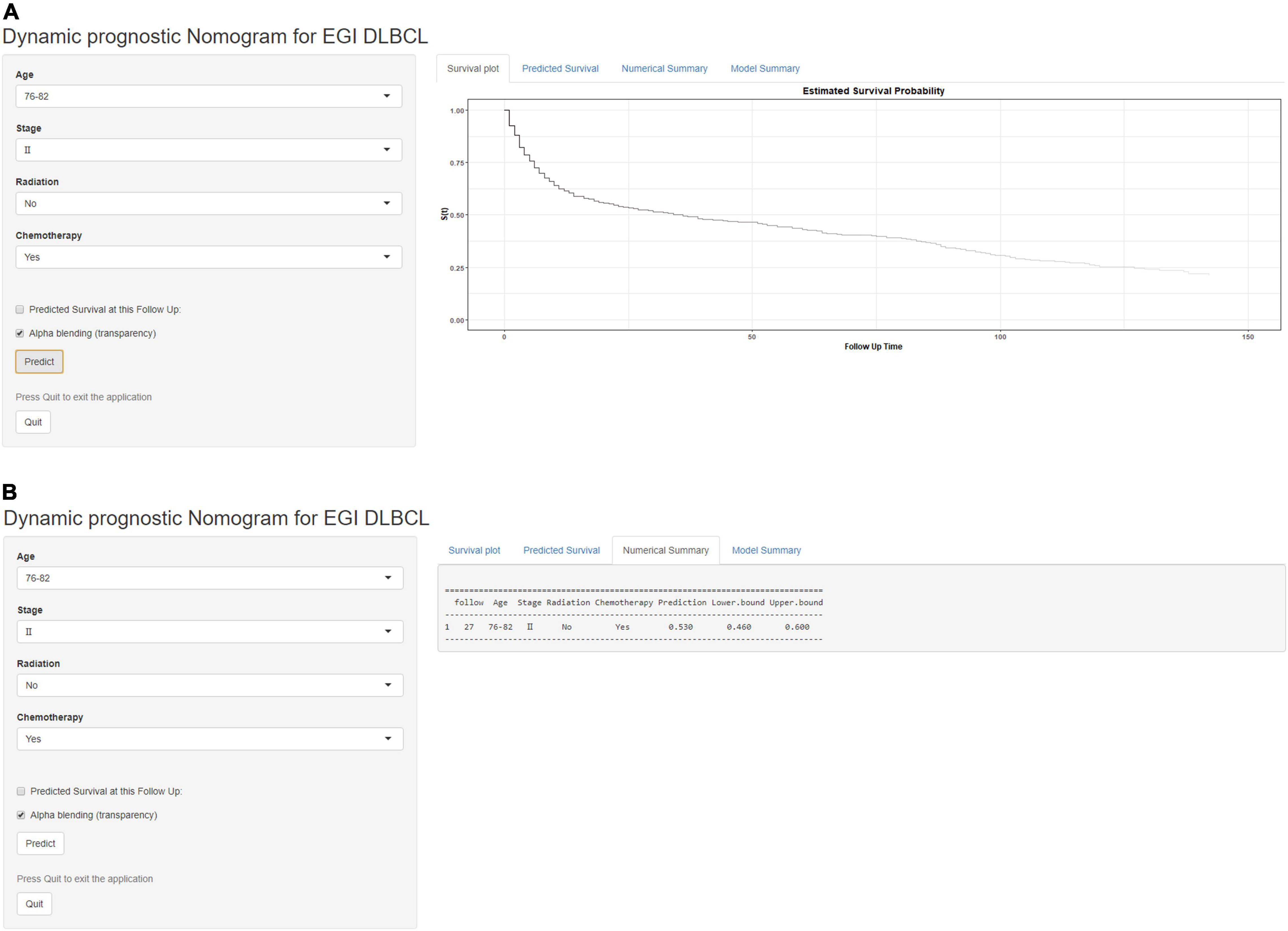
Figure 2. A web-based dynamic prognostic nomogram for overall survival (OS) estimation in elderly patients with primary gastrointestinal diffuse large B-cell lymphoma (EGI DLBCL). (A) Patient with an Ann Arbor stage of III, age at 76–82 years, no radiation, who received chemotherapy according to the web survival rate calculator (95% CI 46–60%). (B) 95% confidence interval (CI) according to the web survival rate calculator.
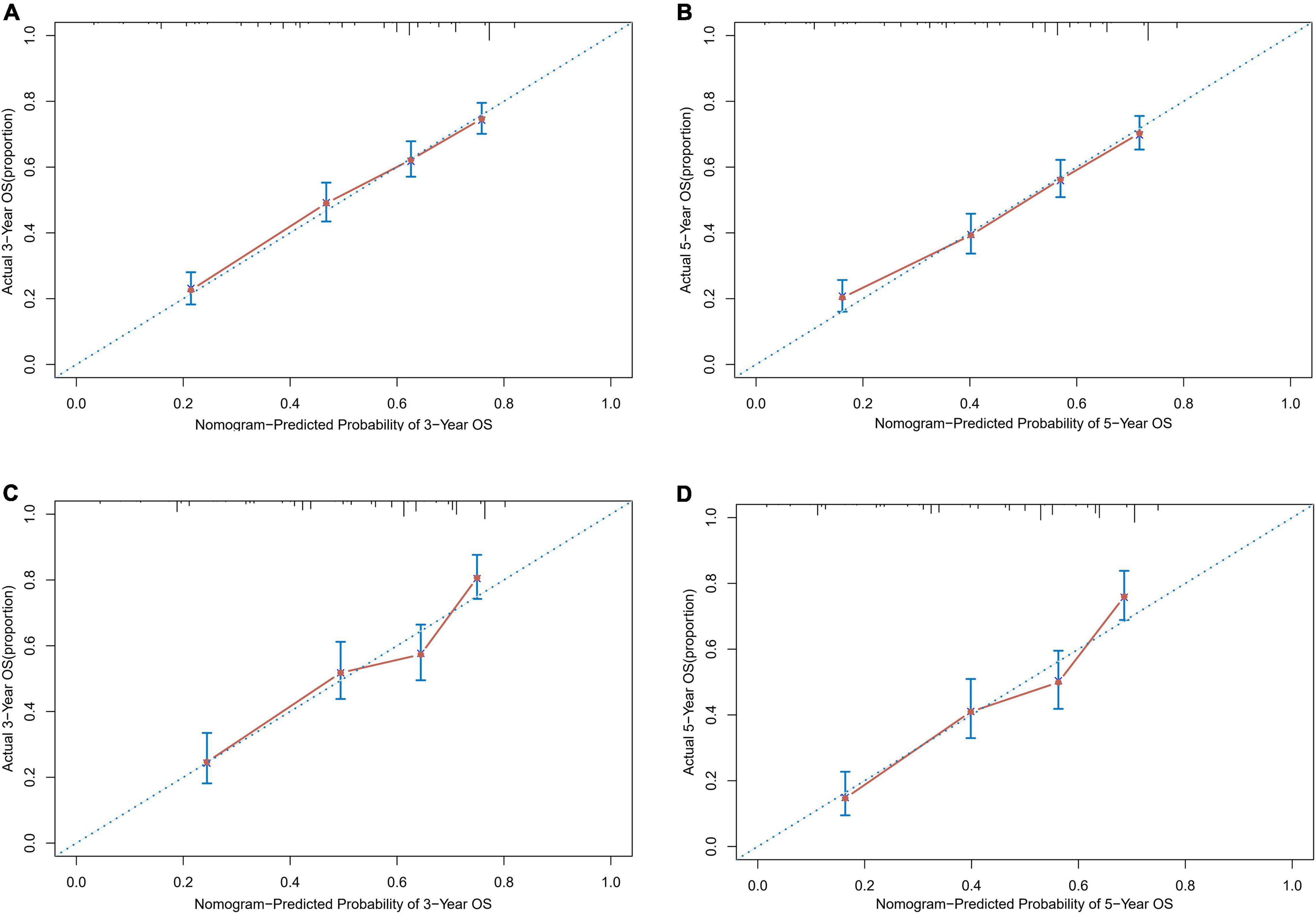
Figure 4. Calibration plots of survival probabilities in patients with EGI DLBCL. (A) At 3-year and (B) 5-year in the development cohort. (C) At 3-year and (D) 5-year in the validation cohort.
Table 3 shows the comparison of the dynamic prognostic nomogram and the Ann Arbor staging system concerning prognostic accuracy for OS in patients of EGI DLBCL. The C-indices of the nomogram were significantly higher than those of the Ann Arbor staging system ([-index (95% CI) for the development cohort, 0.56 (0.54–0.58); C-index (95% CI) for the validation cohort, 0.54 (0.51–0.58)], with the P-values both less than 0.001 for the development cohort and the validation cohort.
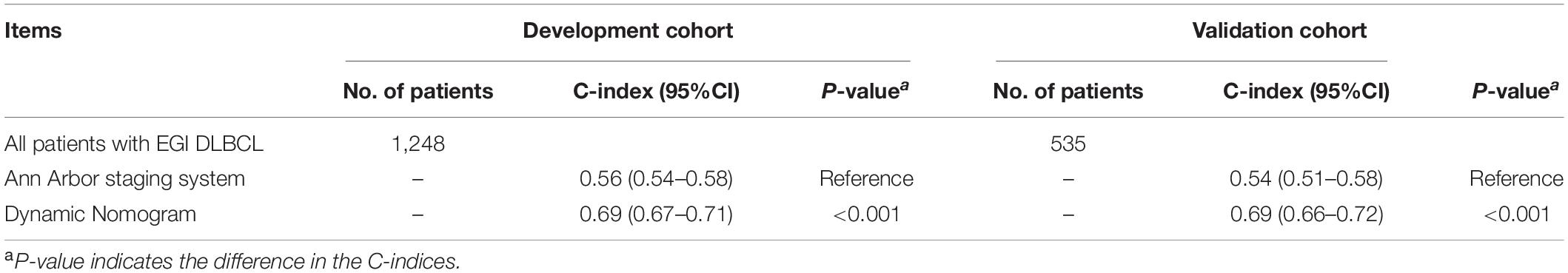
Table 3. C-indices of the nomograms and Ann Arbor staging system in elderly patients with primary gastrointestinal diffuse large B-cell lymphoma (EGI DLBCL).
The optimal cut-off value for the total risk score was calculated based on the quartile of the patient’s prognostic score. By comparing the survival curves between the quartiles of risk scores and the Ann Arbor staging, we can see that the difference between the survival curves of the quartiles in the development cohort and the validation cohort is significantly better than that of the Ann Arbor staging (Figure 5). In addition, we compared the 3- and 5-year ROC curves of the nomogram in the development cohort and the validation cohort with a single multivariate meaningful variable at specific time points. The results show that the predictive power of the nomogram is higher than that of a single multivariate meaningful variable (Figure 6).
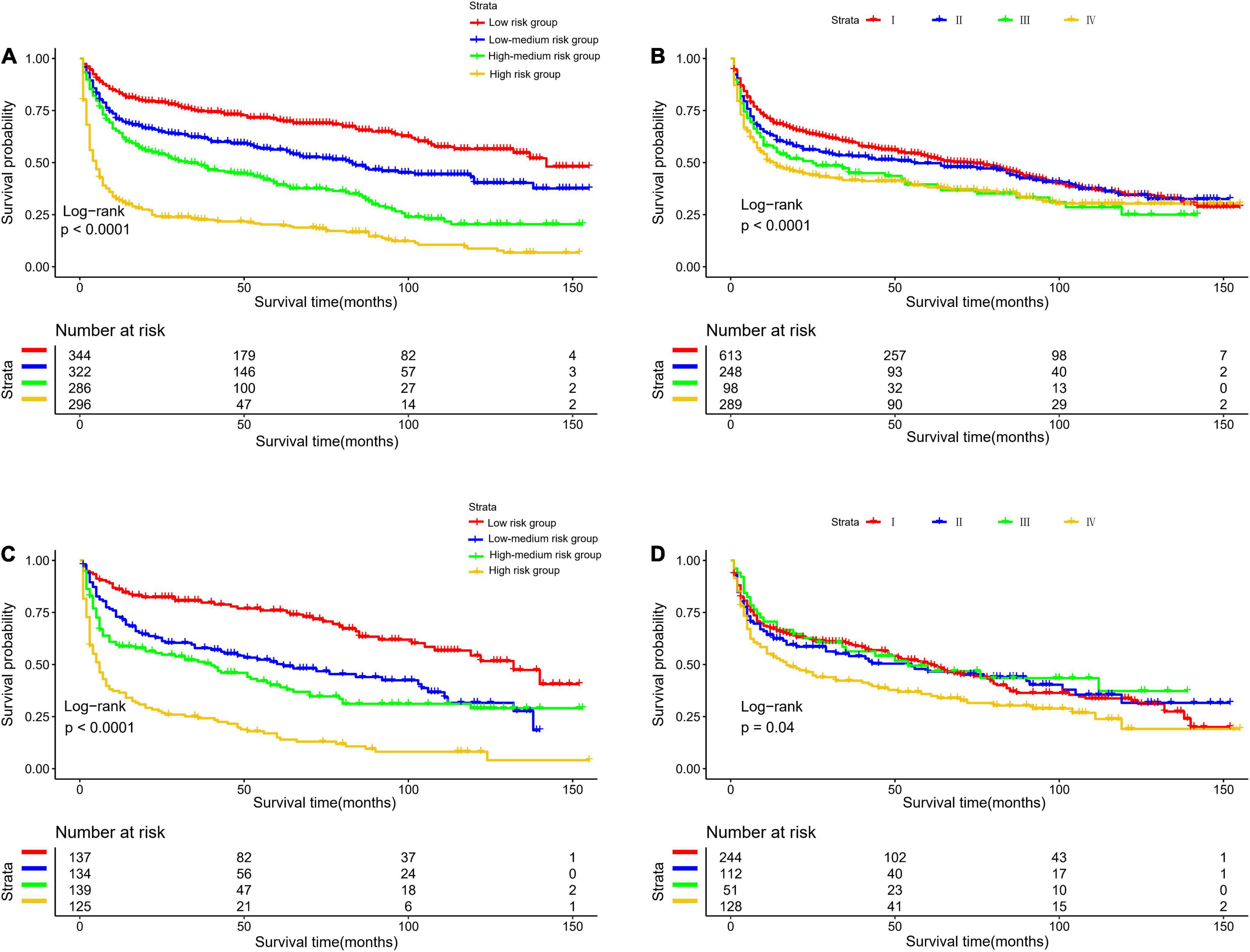
Figure 5. Kaplan-Meier survival curves for risk stratification in the development cohort (A) nomogram (B) stage and validation cohort (C) nomogram (D) stage.
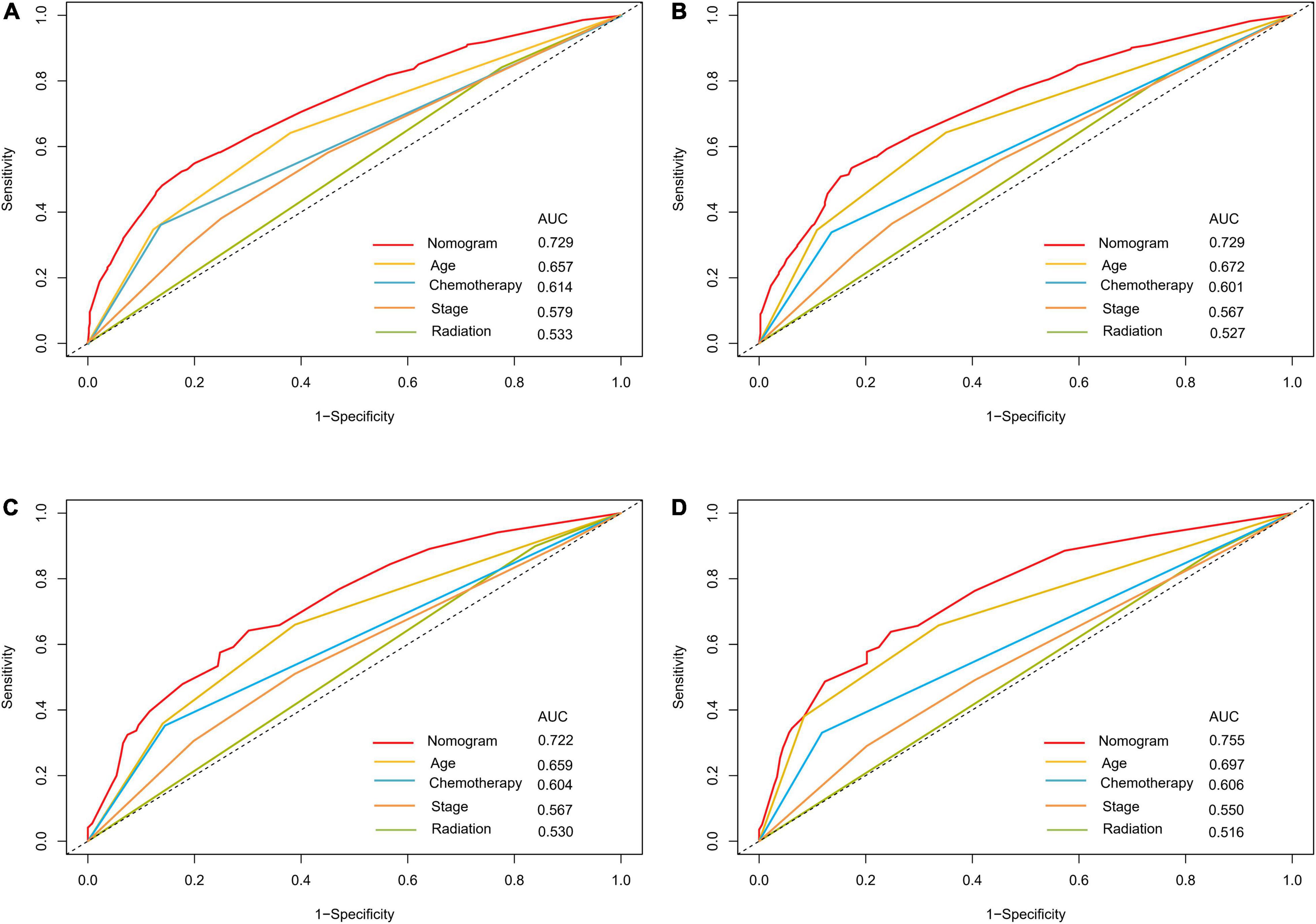
Figure 6. ROC curves for predictions of overall survival in the development cohort (A,C) and validation cohort (B,D) at 3, 5-year.
Discussion
Primary gastric lymphoma (PGL) is a rare tumor that accounts for 2–8% of all gastric tumors (16). DLBCL accounts for 45–50% of all primary gastric lymphomas (PGL). To the best of our knowledge, the present population-based study is the first time to establish and validate a dynamic prognostic nomogram for survival prediction in patients of EGI DLBCL, which can incorporate sociodemographic and clinicopathologic characteristics. Importantly, the dynamic prognostic nomogram was further utilized to generate individualized risk stratifications to further individualize the choice of the treatment strategy.
Precision medicine has developed rapidly in recent years. Clinicians must formulate personalized treatment and follow-up strategies for patients, which requires more accurate and convenient survival models. The line graph integrates tumor staging and multiple prognostic factors into a simple and practical tool, which has been widely used to predict the long-term survival of patients with malignant tumors. The accuracy is usually better than traditional tumor staging systems (17–21). In addition, to increase the convenience of the prediction model, some scholars have used a web-based calculator to predict the long-term survival of cancer patients (21–23). Primary gastric diffuse large B-cell lymphoma is a rare malignant tumor, and its incidence is less than 5% of gastric malignant tumors (24). There is no previous study on a web-based calculator that can be used to predict the survival rate of elderly patients with primary gastric diffuse large B-cell lymphoma. Therefore, this study included 1,783 patients in the SEER database to analyze factors affecting long-term survival and used a survival rate calculator based on the nomogram to dynamically predict the prognosis of elder patients with primary gastric diffuse large B cell lymphoma, which can determine individual treatment and follow-up strategies.
To the best of our knowledge, this is the largest retrospective case series of major EGI DLBCL, which aims to obtain a prognostic model for predicting OS (25–29). In this study, we developed a nomogram to predict the prognosis of patients with EGI DLBCL based on the following four important factors: age at diagnosis, Ann Arbor staging, chemotherapy, and radiotherapy. In the present study, based on an AIC-backward procedure, we identified four independent predictors for OS, which were in keeping with the previous findings and could be easily obtained from routine clinical measurements. By incorporating these four independent predictors, we successfully established and validated a dynamic prognostic nomogram for OS, with calibration curves showing good accordance between nomogram-based and actual overall survival probability. In this study, the 5-year survival rate of patients 76–82 years old and in stage II, who received chemotherapy without radiotherapy, was 53% (95% CI, 46.0–60.0%). Compared with other web-based survival rate calculators, web-based calculators can provide better visualization. It can dynamically predict the cancer-specific survival rate of patients with EGI DLBCL at different time points and help identify patients at high risk of cancer-specific death. Because of its easy-to-use clinical applicability, we do believe this tool would be widespread acceptance and serves as a practical calculator in routine clinical practice. The clinicians could calculate the variable scores and sum them according to the values of 4 meaningful variables for each patient, and then predict the possible incidence of different survival periods based on the total score.
In the past few decades, several staging systems have been developed to improve the prognostic stratification of NHL. The Ann Arbor staging system is widely used in NHL staging. The Lugano staging system is an improved version of the original Ann Arbor staging system designed for the staging of GI lymphoma. The purpose of this staging system is to combine the depth of invasion and the measurement of distal lymph node invasion. The most widely used classification is the Lugano staging system, which has been adopted by the eighth edition of the Cancer Staging Manual of the American Joint Committee on Cancer (30). The SEER database only provides data on the Ann Arbor staging. Our survival analysis shows that Ann Arbor staging is an independent prognostic indicator of GI DLBCL. When compared with the Ann Arbor staging system, the dynamic prognostic nomogram showed superior prognostic accuracy, with both higher C-indices for the nomogram in EGI DLBCL.
Despite these strengths, this study still has several limitations that need to be taken into consideration. First and most importantly, it is regrettable that the SEER database could not provide information about the extranodal extension (ENE) in patients with EGI DLBCL, thus, we fail to reassess them according to the eighth edition of the Ann Arbor staging system. Further study is encouraged to overcome this shortcoming. Second, the SEER database lacks information on overall comorbidity and lifestyle habits, which may impact the prognosis of patients and lead to a change in subsequent therapeutic decisions. Accordingly, the dynamic prognostic nomogram, to some extent, may be limited by the failure to include these prognostic predictors. Finally, although the precise and easy-to-use dynamic prognostic nomogram was established with the latest data from a large population-based US database, we did not perform external validation, leaving its clinical applicability unknown in non-US population, and our prediction model needs an independent validation in different population before any clinical application.
Conclusion
We establish and validate a more accurate and convenient dynamic prognostic nomogram for patients with EGI DLBCL, which can provide evidence for individual treatment and follow-up. Further independent studies are warranted to externally evaluate and validate our dynamic prognostic nomogram.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
WR and JW conceived and designed the study. All authors wrote the article, downloaded, screened the data from the SEER database, participated in analyzing the data, read, and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.860993/full#supplementary-material
Footnotes
References
1. Foukas PG, de Leval L. Recent advances in intestinal lymphomas. Histopathology. (2015) 66:112–36. doi: 10.1111/his.12596
2. Peng JC, Zhong L, Ran ZH. Primary lymphomas in the gastrointestinal tract. J Dig Dis. (2015) 16:169–76. doi: 10.1111/1751-2980.12234
3. Howell JM, Auer-Grzesiak I, Zhang J, Andrews CN, Stewart D, Urbanski SJ. Increasing incidence rates, distribution and histological characteristics of primary gastrointestinal non-Hodgkin lymphoma in a North American population. Can J Gastroenterol. (2012) 26:452–6. doi: 10.1155/2012/480160
4. International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. (1993) 329:987–94. doi: 10.1056/NEJM199309303291402
5. López-Guillermo A, Colomo L, Jiménez M, Bosch F, Villamor N, Arenillas L, et al. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol. (2005) 23:2797–804. doi: 10.1200/JCO.2005.07.155
6. Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, et al. Diffuse large B-cell lymphoma version 1.2016. J Natl Compr Cancer Netw. (2016) 14:196–231. doi: 10.6004/jnccn.2016.0023
7. Lu P. Staging and classification of lymphoma. Semin Nucl Med. (2005) 35:160–4. doi: 10.1053/j.semnuclmed.2005.02.002
8. Ruskoné-Fourmestraux A, Dragosics B, Morgner A, Wotherspoon A, De Jong D. Paris staging system for primary gastrointestinal lymphomas. Gut. (2003) 52:912–3. doi: 10.1136/gut.52.6.912
9. Morschhauser F, Feugier P, Flinn IW, Gasiorowski R, Greil R, Illés Á, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. (2021) 137:600–9. doi: 10.1182/blood.2020006578
10. Shen QD, Zhu HY, Wang L, Fan L, Liang JH, Cao L, et al. Gemcitabine-oxaliplatin plus rituximab (R-GemOx) as first-line treatment in elderly patients with diffuse large B-cell lymphoma: a single-arm, open-label, phase 2 trial. Lancet Haematol. (2018) 5:e261–9. doi: 10.1016/S2352-3026(18)30054-1
11. Feng TC, Zai HY, Jiang W, Zhu Q, Jiang B, Yao L, et al. Survival and analysis of prognostic factors for hepatoblastoma: based on SEER database. Ann Transl Med. (2019) 7:555. doi: 10.21037/atm.2019.09.76
12. Li Z, Lin Y, Cheng B, Zhang Q, Cai Y. Prognostic model for predicting overall and cancer-specific survival among patients with cervical squamous cell carcinoma: a SEER based study. Front Oncol. (2021) 11:651975. doi: 10.3389/fonc.2021.651975
13. Wang Y, Song J, Wen S, Zhang X. A visual model for prognostic estimation in patients with primary diffuse large B-cell lymphoma of small intestine and colon: analysis of 1,613 cases from the SEER database. Transl Cancer Res. (2021) 10:1842–55. doi: 10.21037/tcr-20-3086
14. Wang W, Liu J, Liu L. Development and validation of a prognostic model for predicting overall survival in patients with bladder cancer: a SEER-based study. Front Oncol. (2021) 11:692728. doi: 10.3389/fonc.2021.692728
15. Wang C, Yang C, Wang W, Xia B, Li K, Sun F, et al. A prognostic nomogram for cervical cancer after surgery from SEER database. J Cancer. (2018) 9:3923–8. doi: 10.7150/jca.26220
16. Kuo SH, Cheng AL. Helicobacter pylori and mucosa-associated lymphoid tissue: what’s new. Hematology. (2013) 2013:109–17. doi: 10.1182/asheducation-2013.1.109
17. Han DS, Suh YS, Kong SH, Lee HJ, Choi Y, Aikou S, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol. (2012) 30:3834–40. doi: 10.1200/JCO.2012.41.8343
18. Buettner S, van Vugt JLA, Gaspersz MP, Coelen RJS, Roos E, Labeur TA, et al. Survival after resection of perihilar cholangiocarcinoma in patients with lymph node metastases. HPB. (2017) 19:735–40. doi: 10.1016/j.hpb.2017.04.014
19. Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D, et al. Development and validation of a nomogram for predicting survival in patients with resected non–small-cell lung cancer. J Clin Oncol. (2015) 33:861–9. doi: 10.1200/JCO.2014.56.6661
20. Kattan MW, Karpeh MS, Mazumdar M, Brennan MF. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol. (2003) 21:3647–50. doi: 10.1200/JCO.2003.01.240
21. Battersby NJ, Bouliotis G, Emmertsen KJ, Juul T, Glynne-Jones R, Branagan G, et al. Development and external validation of a nomogram and online tool to predict bowel dysfunction following restorative rectal cancer resection: the POLARS score. Gut. (2018) 67:688–96. doi: 10.1136/gutjnl-2016-312695
22. Renfro LA, Grothey A, Xue Y, Saltz LB, André T, Twelves C, et al. ACCENT-based web calculators to predict recurrence and overall survival in stage III colon cancer. J Natl Cancer Inst. (2014) 106:dju333. doi: 10.1093/jnci/dju333
23. Hippisley-Cox J, Coupland C. Development and validation of risk prediction equations to estimate survival in patients with colorectal cancer: cohort study. BMJ. (2017) 357:j2497. doi: 10.1136/bmj.j2497
24. Boot H, de Jong D. Diagnosis, treatment decisions, and follow up in primary gastric lymphoma. Gut. (2002) 51:621–2. doi: 10.1136/gut.51.5.621
25. Han Y, Yang J, Liu P, He X, Zhang C, Zhou S, et al. Prognostic nomogram for overall survival in patients with diffuse large B−cell lymphoma. Oncologist. (2019) 24:e1251. doi: 10.1634/theoncologist.2018-0361
26. Yin X, Xu A, Fan F, Huang Z, Cheng Q, Zhang L, et al. Incidence and mortality trends and risk prediction nomogram for extranodal diffuse large B-cell lymphoma: an analysis of the surveillance, epidemiology and end results database. Front Oncol. (2019) 9:1198. doi: 10.3389/fonc.2019.01198
27. Jiang S, Qin Y, Liu P, Yang J, Yang S, He X, et al. A prognostic nomogram constructed for relapsed or refractory diffuse large B-cell lymphoma patients. Asia Pac J Clin Oncol. (2019) 18:e11–6. doi: 10.1111/ajco.13222
28. Jiang S, Qin Y, Liu P, Yang J, Yang S, He X, et al. Prognostic nomogram and predictive factors in refractory or relapsed diffuse large B-cell lymphoma patients failing front-line R-CHOP regimens. J Cancer. (2020) 11:1516. doi: 10.7150/jca.36997
29. Go SI, Park S, Kim JH, Kim HR, Kim M, Moon K, et al. A new prognostic model using the NCCN-IPI and neutrophil-to-lymphocyte ratio in diffuse large B-cell lymphoma. Tumori J. (2018) 104:292–9. doi: 10.5301/tj.5000694
Keywords: primary gastric diffuse large B-cell lymphoma, prognostic nomogram, overall survival, dynamically predict, elderly patients
Citation: Wang J, Ren W, Zhang C and Wang X (2022) A New Staging System Based on the Dynamic Prognostic Nomogram for Elderly Patients With Primary Gastrointestinal Diffuse Large B-Cell Lymphoma. Front. Med. 9:860993. doi: 10.3389/fmed.2022.860993
Received: 24 January 2022; Accepted: 31 March 2022;
Published: 02 May 2022.
Edited by:
Alberto Mussetti, Catalan Institute of Oncology, SpainReviewed by:
Wenqiang Zhan, Shanghai Jiao Tong University, ChinaDong-Ta Zhong, Fujian Medical University Union Hospital, China
Copyright © 2022 Wang, Ren, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junmin Wang, c29tYXRvc3RhdGluQDEyNi5jb20=
 Junmin Wang
Junmin Wang Weirui Ren1
Weirui Ren1