- 1Department of Pulmonary and Critical Care Medicine, China-Japan Friendship Hospital, Beijing, China
- 2National Center for Respiratory Medicine, Beijing, China
- 3Institute of Respiratory Medicine, Chinese Academy of Medical Sciences, Beijing, China
- 4National Clinical Research Center for Respiratory Diseases, Beijing, China
- 5Pulmonary and Critical Care Medicine, Capital Medical University, Beijing, China
- 6Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China
- 7Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 8Department of Respiratory Medicine, Capital Medical University, Beijing, China
Objective: To assess the efficacy and safety of tenecteplase in patients with pulmonary embolism (PE).
Methods: We completed the literature search on May 31, 2021 using PubMed, EMBASE and the Web of Science. Analyses were conducted according to PE risk stratification, study design and duration of follow-up. The pooled risk ratios (RRs) and its 95% confident intervals (CIs) for death and major bleeding were calculated using a random-effect model.
Results: A total of six studies, with four randomized controlled trials (RCTs) and two cohort studies, were included in this study out of the 160 studies retrieved. For patients with high-risk PE, tenecteplase increased 30-day survival rate (16% vs 6%; P = 0.005) and did not increase the incidence of bleeding (6% vs 5%; P = 0.73). For patients with intermediate-risk PE, four RCTs suggested that tenecteplase reduced right ventricular insufficiency at 24h early in the onset and the incidence of hemodynamic failure without affecting mortality in a short/long-term [<30 days RR = 0.83, 95% CI (0.47, 1.46);≥30 days RR = 1.04, 95% CI (0.88, 1.22)]. However, tenecteplase was associated with high bleeding risk [<30 days RR = 1.79, 95% CI (1.61, 2.00); ≥30 days RR = 1.28, 95% CI (0.62, 2.64)].
Conclusions: Tenecteplase may represent a promising candidate for patients with high risk PE. However, tenecteplase is not recommended for patients with intermediate-risk PE because of high bleeding risk. More large-scale studies focused on tenecteplase are still needed for PE patients.
Highlights
- Our study is the first, largest and most comprehensive meta-analysis of the efficacy and safety of tenecteplase on PE.
- For high-risk PE, tenecteplase may be beneficial in improving 30-day survival rate without increasing hemorrhage incidents.
- For intermediate-risk PE, tenecteplase could reduce the risk of hemodynamic decompensation, but was associated with high bleeding risk. Catheter-directed thrombolysis with low-dose teneteplase may be beneficial.
Background
Pulmonary embolism (PE) is a cardiovascular disease of major global burden after acute coronary syndrome and stroke (1). The estimated incidence of PE ranges from 39 to 115 per 100 000 population worldwide and PE is a major cause of death from cardiovascular disease (2–4). According to 2019 guideline of the European Society of Cardiology/the European Respiratory Society (2019 ESC/ERS), risk stratification of patients with acute PE is classified as high, intermediate and low risk (5). As the guideline recommends, real-world studies also emphasize the management of PE to be guided by risk stratification (3). Reduction of right ventricular dysfunction (RVD) and recurrent PE by reperfusion to reconstruct blood flow and stabilize hemodynamics are major goals in the treatment of acute PE, especially in intermediate-high/high risk PE (6). Conventional treatment of PE mainly refers to anticoagulation therapy including parenteral anticoagulation, such as low-molecular weight heparin (LMWH) or unfractionated heparin (UFH), and direct oral anticoagulants (DOACs). It has been reported that compared with anticoagulation, thrombolytic therapy may improve right-ventricular wall motion at 24 h from baseline (7). Evidence showed that for patients with high-risk PE, thrombolytic therapy reduced mortality and recurrence of PE significantly (8), while its application in intermediate-risk PE was still controversial (9).
Thrombolytic treatment has been shown to increase the risk of hemorrhage. In Pulmonary Embolism Thrombolysis (PEITHO) trial, they found fibrinolytic therapy was associated with a 2.0% rate of hemorrhagic stroke and a 6.3% rate of major extracranial hemorrhage for patients with intermediate-risk PE (10).
Tenecteplase, a genetically modified variant of alteplase, has been proved its potential in the treatment of stroke and cardiovascular disease (11–14). Compared with existing thrombolytic agents, such as alteplase (2-h infusions), tenecteplase can be administered in single intravenous bolus over 5 s due to its long half-life. In addition, unlike streptokinase, an antigenic thrombolytic agent, tenecteplase is less likely to cause allergic reactions (15–18).
Many studies have been conducted on PE patients with tenecteplase, but results were inconsistent (10, 19–23). Therefore, we aimed to summarize the efficacy and safety data of tenecteplase compared with anticoagulant therapy in patients with PE.
Methods
This meta-analysis was performed according to the guidelines of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.
Search Strategy
We searched the electronic bibliographic databases systematically, including EMBASE (Excerpta Medica database), PubMed (US National Library of Medicine National Institutes of Health) and Web of Science (including Science Citation Index and Social Sciences Citation Index). Boolean expressions were used when drafting search strategies specifically for each database search engine. The expression included the items (Supplementary Table 2 shows the full electronic search strategy for all database): (Tenecteplase OR TNK-tPA OR TNK-tissue plasminogen activator OR Metalyse OR TNKase) AND (Pulmonary Infarction OR pulmonary embolism OR PE OR venous thromboembolism OR venous thrombosis OR Pulmonary Veno-occlusive Disease) AND (Case Control OR clinical trial). We completed the literature search on May 31, 2021. The major articles were also checked for missing hits. The search retrieved 160 references for further filtration.
Selection Criteria
The retained articles for the meta-analysis should meet the following inclusion criteria simultaneously: (I) aimed to assess the efficacy and safety of tenecteplase on PE; (II) contained information including sample size, patients'outcomes (efficacy and safety outcomes), as well as the necessary statistical measures; (III) was written by English; (IV) Age>17 years; (V) randomized, controlled trials (RCTs) or cohort studies. The quality of RCTs was assessed by the Jadad scale and cohort studies were assessed by the Newcastle-Ottawa Scale (NOS) score. Studies with Jadad scale or NOS score <4 were excluded. Besides, review or meta-analysis, basic medical research, guideline/ case report, and articles with no available data were also excluded. The eligible articles were accessed independently by two members of the present study, according to the criteria above, and a third party was involved if there was any disagreement.
Data Extraction
From each eligible article, the following information was extracted: surname of the first author, year of publication, country, study design, demographic characteristics of the study population including age, sex and weight, treatment protocols and tenecteplase doses, duration of follow-up, sample size, vital signs such as heart rate and systolic blood pressure, improvement of clinical symptom or RVD index, incidence of recurrent PE, patients who needed to upgrade treatment and all-cause mortality (<30 days and ≥30 days), incidence of hemorrhage (<30 days and ≥30 days) and chronic thromboembolic pulmonary hypertension (CTEPH). Of note, patients requiring upgraded treatment were defined as those with circulatory or respiratory failure but excluding those who died. Discrepancies were solved by discussion among the authors of this study.
Statistical Analysis
The pooled RRs and 95% CI for death and major bleeding were calculated using a random-effect model. Analyses were conducted according to duration of follow-up (<30 days or ≥30 days). The heterogeneity between studies was assessed by the inconsistency index I2 statistic (ranging from 0 to 100%) on the basis of the Cochrane Q test. Heterogeneity is considered to be low between the studies if I2 ranged from 0 to 25%, moderate from 25 to 75% and high from 75 to 100%. All the statistical analyses above were performed using STATA software (StataCorp, Texas, USA, version 14.0 for Windows).
Results
Qualified Studies
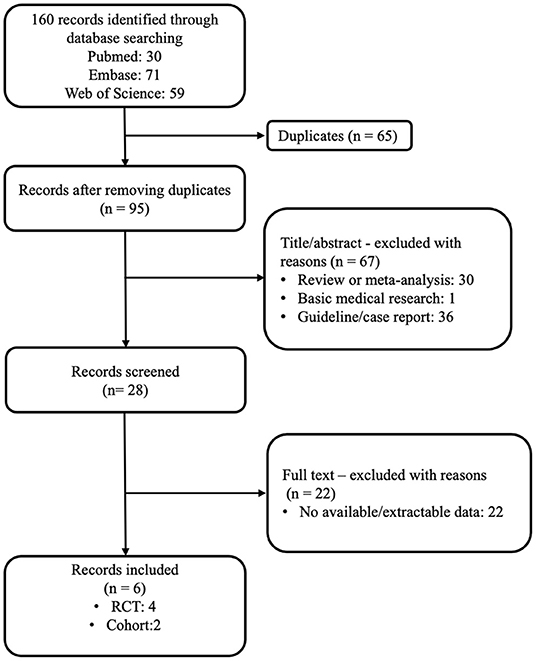
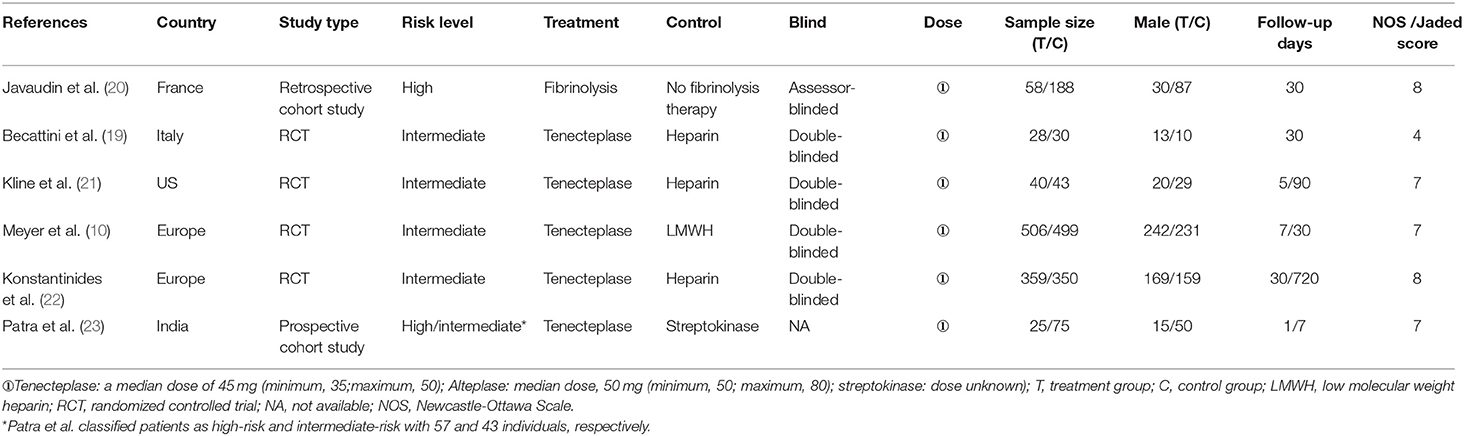
According to our search strategy, 160 articles were obtained by the primary literature retrieval from databases. After screening title, abstract and full-text, six articles were identified according to the inclusion criteria. The selection process was visually shown in detail in a flow diagram (Figure 1). The baseline characteristics of the six qualified studies are shown in Table 1. All studies were PE-related. The years of publication ranged from 2010 to 2019, with a total of 2201 patients. Four studies were RCTs (10, 19, 21, 22), and two studies were retrospective/prospective cohort studies. (20, 23) Among the six studies, only one study focused on high-risk PE, four RCT studies included patients with intermediate risk, and one study included both intermediate-risk and high-risk PE patients. (10, 19, 21–23) Doses of tenecteplase ranged from 30 to 50 mg (0.5 mg/kg), with a 5 mg step-up for every 10 kg increase from 60 to 90 kg. All studies scored ≥4 by the Jadad scale or NOS score, where appropriate.
Tenecteplase May Be Beneficial for Survival Rate Without Increasing Hemorrhage Events in High Risk PE
A study focused on patients with PE and out-of-hospital cardiac arrest from France included 246 patients with PE (Table 1) (20). They followed patients up to 30 days and found that patients receiving thrombolysis therapy during cardiopulmonary resuscitation had a higher 30-day survival rate (16 vs 6%, p = 0.005) without association to thrombolytic agents. Tenecteplase was the most used agent in the study (74%). Among 9 survivors in thrombolysis group, tenecteplase was administered to five patients and alteplase was administered to four patients. Moreover, thrombolysis therapy did not increase the mortality rate due to hemorrhage (6 vs 5%; P = 0.73).
Tenecteplase Could Improve the Right Heart Function but Increase the Major Bleeding Risk for Intermediate-Risk PE Patients
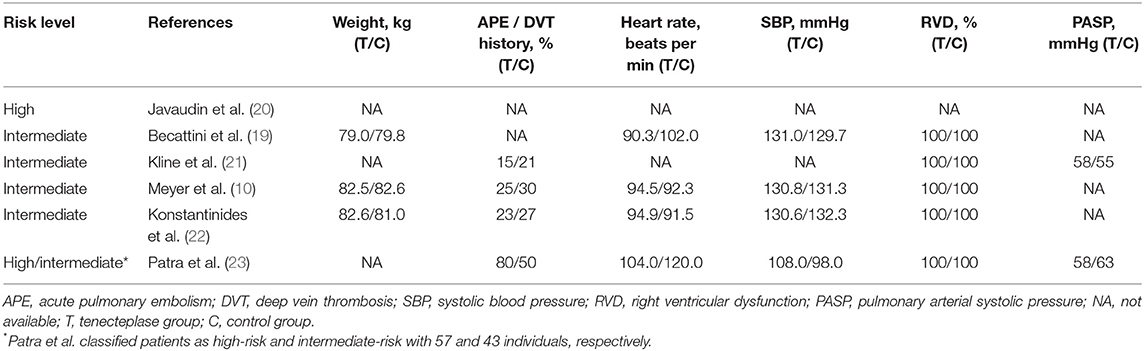
Among the five qualified studies for intermediate-risk PE, four studies were RCTs and one was a cohort study (10, 19, 21–23). Tenecteplase was used in all studies, combined with heparin in four studies and with LMWH in one study. Table 1 shows the main information of the five studies. Patients were followed up in all studies, with duration of follow-up ranging from 5 days to 720 days. Meyer et al. (10) collected data on the largest number of patients of 1,005, of whom 506 received tenecteplase. The study of Patra et al. (23) was the only cohort study including both high- and intermediate-risk PE patients. Table 2 shows the baseline information of the patients included. Kline et al. (21) and Patra et al. (23) reported the pulmonary arterial systolic pressure (PASP).
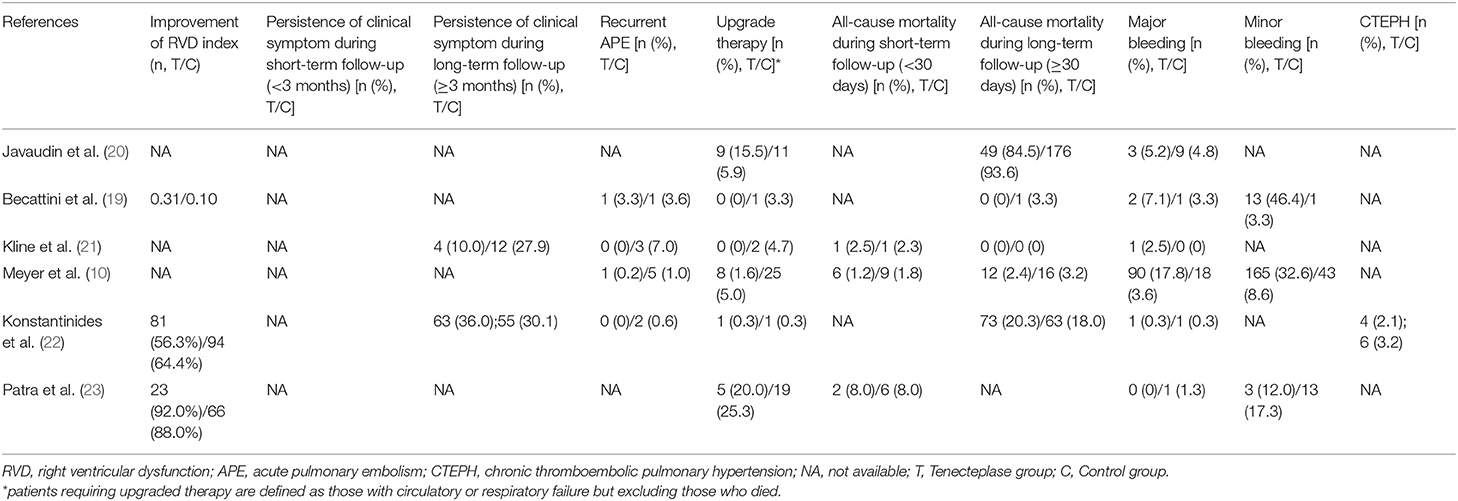
Table 3 contains information on the clinical events and prognosis of studies involved. In terms of the major efficacy outcome, Meyer et al. (10) concluded that tenecteplase reduced hemodynamic decompensation based on the largest dataset from intermediate-risk PE patients [upgrade therapy: 8(1.6%) in tenecteplase group vs 25(5.0%) in placebo group, p = 0.002)]. Similarly, different studies verified that tenecteplase could improve right ventricle function for patients with intermediate-risk PE. Some studies found that RVD index and PASP at 24 h/7-day reduced to a greater extent in the tenecteplase group than the control group. (19, 23) Moreover, they found lower mean duration of intensive care unit (ICU) stay in the tenecteplase group (p = 0.04) (23). In addition to objective indicators such as RVD index, we also collected relatively subjective indicators such as persistence of clinical symptoms. Kline et al. (22) found that 12 (27.9%) patients remained clinically symptomatic in the control group at 90-day follow-up compared to only 4(10.0%) patients in the tenecteplase group (p = 0.039). With regard to long-term prognosis, Konstantinides et al. (22), the study with the longest follow-up duration (720 days), found that tenecteplase use was not correlated with persistent clinical symptoms[63(36.0%) vs 55 (30.1%), p = 0.23)], RVD index improvement[81(56.3%) vs 94(64.4%), p = 0.20)] or CTEPH morbidity[4(2.1%) vs 6(3.2%), p = 0.79)]. Furthermore, currently available data did not demonstrate a clear association between tenecteplase and recurrent PE events.
The four RCT studies had low heterogeneity (I2 = 0.0%), all of which suggested that tenecteplase did not affect short- and long-term mortality in PE patients (10, 19, 21, 22). Compared with coagulation treatment in patients with intermediate-risk PE, the pooled RRs of tenecteplase in all-cause mortality were 0.83 [95% CI (0.47, 1.46)] with a follow-up of <30 days (Figure 2) and 1.04 [95% CI (0.88, 1.22)] with a follow-up of ≥30 days, respectively (Figure 2). Additionally, the pooled RRs of tenecteplase in major bleeding were 1.79[95% CI (1.61, 2.00)] with a follow-up of <30 days (Figure 3) and 1.28 [95% CI (0.62, 2.64)] with a follow-up of ≥30 days, respectively (Figure 3). However, Meyer et al. (10) is the largest trial and may have some influence on overall analysis. We also performed overall mortality and bleeding rates excluding the Meyer study. The all-cause mortality rate RRs were 1.04 [95% CI (0.26, 4.23)] with a follow-up of <30 days (Figure 4) and 1.07 [95% CI (0.90, 1.28)] with a follow-up of ≥30 days, respectively (Figure 4). The major bleeding rates RRs were 1.40 [95% CI (0.81, 2.42)] with a follow-up of <30 days (Figure 5) and 1.28 [95% CI (0.62, 2.64)] with a follow-up of ≥30 days, respectively (Figure 5).
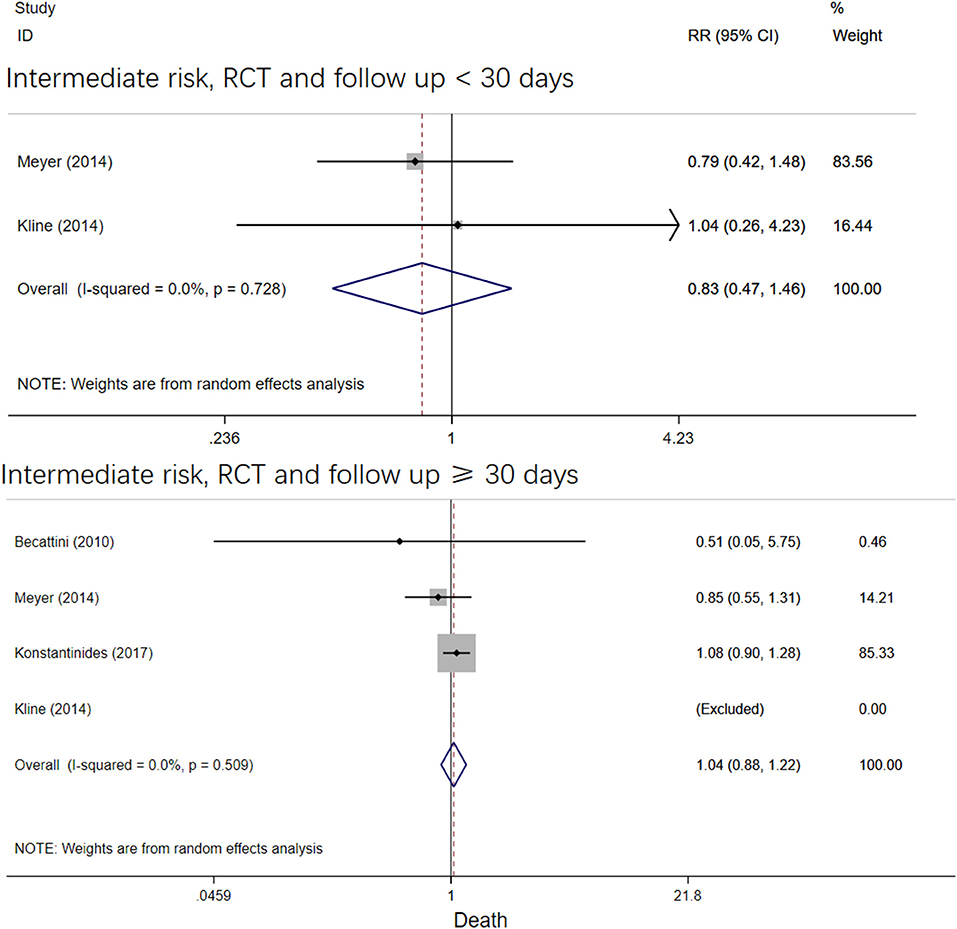
Figure 2. Forest plots of tenecteplase vs. anticoagulation treatment grouped by all-cause mortality and duration of follow-up (<30 days or≥30 days) for patients with intermediate-risk PE.
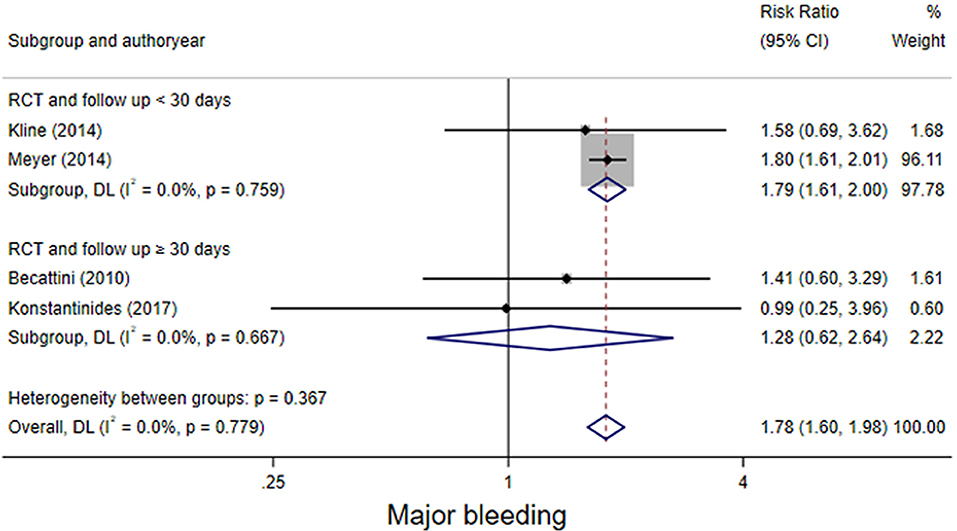
Figure 3. Forest plots of tenecteplase vs. anticoagulation treatment grouped by hemorrhage rates (<30 days or≥30 days) for patients with intermediate-risk PE.
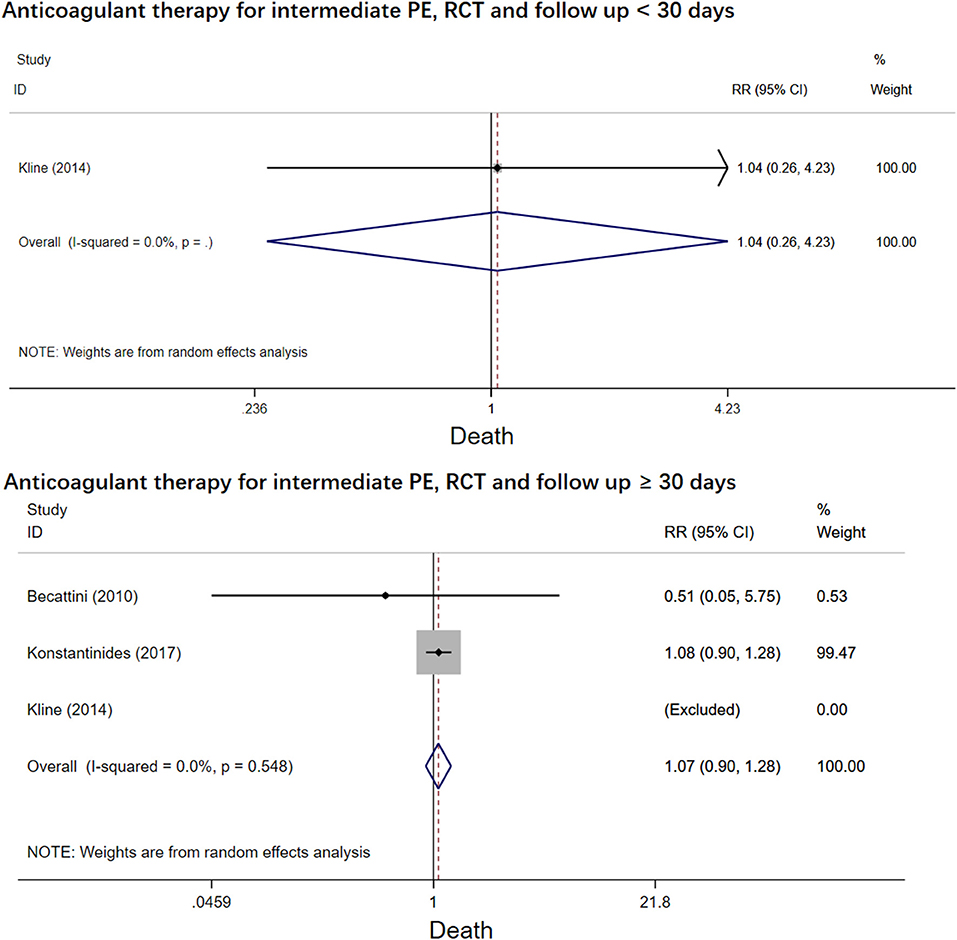
Figure 4. Forest plots of tenecteplase vs. anticoagulation treatment grouped by all-cause mortality and duration of follow-up (<30 days or≥30 days) for patients with intermediate-risk PE (excluding Meyer et al. study).
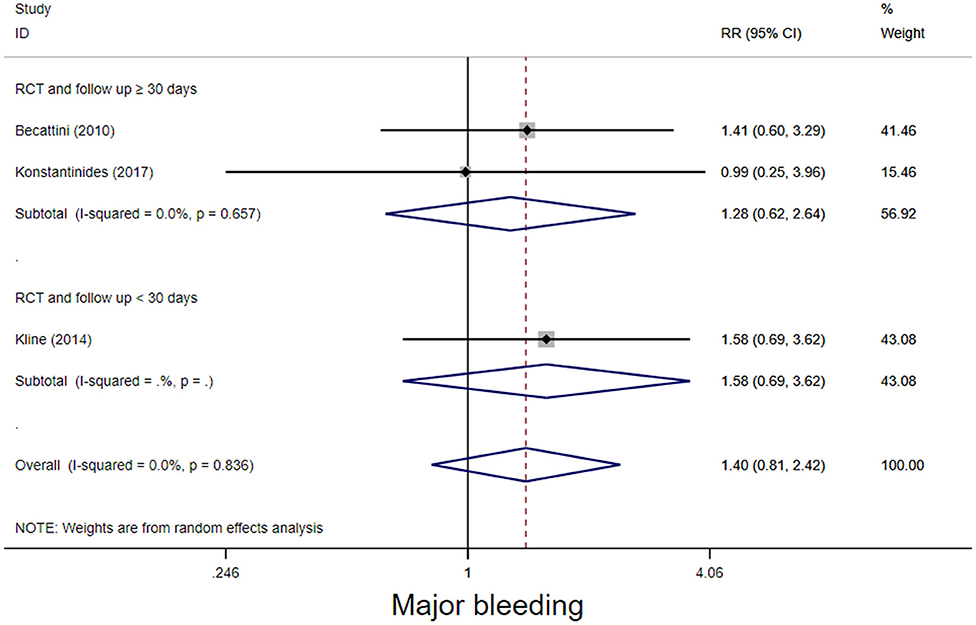
Figure 5. Forest plots of tenecteplase vs. anticoagulation treatment grouped by hemorrhage rates (<30 days or≥30 days) for patients with intermediate-risk PE. (excluding Meyer et al. study).
Discussion
To our knowledge, the present study is the first, largest and most comprehensive meta-analysis of the efficacy and safety of tenecteplase in PE patients, summarizing multiple RCT and cohort studies. There are several key points from this meta-analysis and systematic review. First, for patients with high-risk PE, tenecteplase could improve patient survival over 30 days without increasing major bleeding rates. Second, for patients with intermediate-risk PE, tenecteplase could prevent the disease progression and improve the clinical symptoms rapidly, decreasing the length of ICU stay and cost. Furthermore, tenecteplase has some unique advantages such as high fibrin specificity and convenient usage. However, tenecteplase could increase the major bleeding risk in the short term as could other thrombolytic agents. In summary, we believe tenecteplase is a promising candidate for patients with high risk PE. Further studies related to tenecteplase are quite necessary, especially for patients with high risk PE.
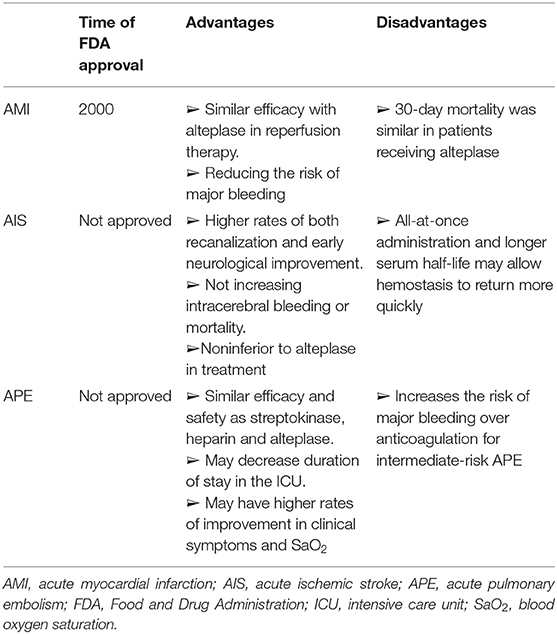
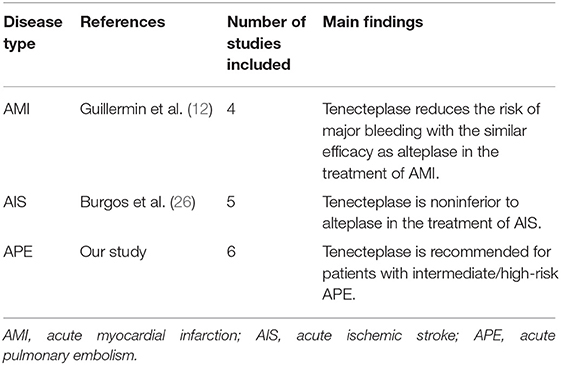
As a third-generation thrombolytic agent, tenecteplase has been widely studied in thrombotic diseases due to its unique advantages. We summarized the advantages and disadvantages of the different thrombolytic agents in Supplementary Table 1. (24) In comparison, tenecteplase has more advantages. First of all, tenecteplase demonstrates the greatest fibrin specificity, decreasing the risk of major bleeding. Secondly, the clearance of tenecteplase is approximately eight-fold slower than alteplase. In contrast, alteplase requires a continuous intravenous infusion for 2 h while tenecteplase is administered in 5–10 min by a single bolus (11, 25). Moreover, tenecteplase has been under research in other thrombotic studies such as acute myocardial infarction (AMI) and acute ischemic stroke (AIS) (Tables 4, 5). In 2000, tenecteplase has been approved to treat AMI by the Food and Drug Administration (FDA), as it reduces the risk of major bleeding with the similar efficacy compared to alteplase (12). Although tenecteplase has not yet received FDA approval for AIS, a meta-analysis found tenecteplase was noninferior to alteplase and improved the neurologic function in the early stage (26). Also, tenecteplase may reduce the delay in endovascular thrombectomy and may be more suitable for large vessel occlusions because of convenient usage (26). These studies provide a basis and demonstrate the potential for tenecteplase in PE studies.
High-risk PE, defined by sustained hypotension or cardiogenic shock, is associated with a 24-h short-term mortality rate>20%. Despite limited study, high-risk PE is a clear indication for thrombolytic therapy according to guidelines including the 2019 ESC/ERS and American College of Chest Physicians (5, 27). Pooled data from several systematic reviews and meta-analyses support an increased survival benefit with thrombolytic therapy when used in patients with high-risk PE (28). A large prospective cohort study concluded that thrombolysis during cardiopulmonary resuscitation was associated with higher 30-day survival rate without increasing the rate of hemorrhage in high-risk PE patients, whether the thrombolytic agent was tenecteplase or alteplase (20). Therefore, the tenecteplase may benefit patients with high-risk PE in efficacy and safety aspects and need further studies to verify the point in the future.
The current evidence included studies that mainly focused on intermediate-risk PE group. The PEITHO study, the largest randomized, placebo-controlled trial of fibrinolysis for intermediate-risk PE to date, found tenecteplase was associated with reduced hemodynamic decompensation at 7 days (1.6 vs. 5.0%, p = 0.002). From Table 3, we concluded that tenecteplase could reduce the risk of hemodynamic failure for these normotensive PE patients (10), which indicated that tenecteplase may prevent the further disease progression. Additionally, tenecteplase was better than UFH at reducing RVD in the early stage (19) but did not affect short/long-term mortality. Compared with streptokinase, studies have found that tenecteplase could improve the clinical symptoms rapidly and enable patients to obtain better self-assessment of overall health function, especially for those with comorbid conditions such as recurrent venous thromboembolism or heart failure, which was also verified by Stewart et al. and Agrawal et al. (21, 23, 29–31). Similarly, an observational study found that tenecteplase could reduce heart rate, increase the systolic blood pressure and oxygen saturation (29). Furthermore,tenecteplase could decrease the dependency for ICU and the length of stay, therefore, the application of tenecteplase may reduce the cost of therapy (21, 23).
However, current results on the risk of bleeding with tenecteplase are controversial. Clinicians are cautious about thrombolytic therapy mainly because of the concerns of bleeding. It is noted that the risk of bleeding generally remains elevated for a period of 12–24 h after thrombolytic infusion (28). Becattini et al. (19) found that tenecteplase did not increase excessive major bleeding rates. While data from PEITHO trial (10) believed that teneteplase increased the risk of major bleeding, including intracranial hemorrhage, within 7 days [90(17.8%) vs. 18(3.6%), p < 0.001)]. Pooled data also showed that the frequency of major bleeding in patients treated with systemic fibrinolytic therapy is 0–33% and the incidence of intracranial hemorrhage is 0–7.4% (32). According to our meta-analysis, tenecteplase was associated with higher bleeding risk in 7 days for intermediate-risk PE patients and did not affect long-term bleeding events. As the guideline indicated, we believed tenecteplase, similar to other thrombolytic agents, increased the risk of bleeding for aged patients who have more comorbidities. However, some retrospective studies showed that tenecteplase did not increase, but reduced the hemorrhagic rates (23, 31). We speculated that the differences in some studies was associated with drug doses and its administration. The current doses of tenecteplase were 0.5 mg/kg in most studies involved, with a 5 mg step-up for every 10 kg increase from 60 to 90 kg; however, the 0.25 mg/kg dose of tenecteplase was found to be associated with early neurological improvement and reduced tendency of intracranial hemorrhage compared to other thrombolytic agents in the treatment of stroke (33). Our previous study on thrombolysis also showed that half-dose thrombolysis reduced the risk of bleeding with similar efficacy (34) Moreover, applying catheter-directed thrombolysis with tenecteplase to treat PE patients with RVD appeared to improve right ventricle function without increasing bleeding risk (35). Recently, the HI-PEITHO study launched and started enrollment, which aims to assess whether ultrasound-facilitated, catheter-directed thrombolysis and standard anticoagulation are associated with adverse outcomes for patients with intermediate-high risk PE (36). Therefore, catheter-guided administration of low-dose teneteplase may benefit patients with intermediate-risk PE. In conclude, we do not recommend tenecteplase for intermediate-risk PE patients based on current evidence, further studies would be necessary to validate the efficacy and safety of tenecteplase at a lower dose or the different methods of administration.
High-risk PE patients may be suitable for tenecteplase, however, for patients with intermediate-risk PE, it was not appropriate to apply tenecteplase with the same dose or regimen as with high-risk PE patients. Studies have reported that normotensive PE patients with elevated troponin and BNP, or lactate≥ 2 mmol/L were at a higher risk of the adverse outcomes, and indicated a potential need for more aggressive systemic thrombolytic treatment instead of anticoagulants alone in these patients. In this way, these patients should be closely monitored, and teneteplase could be beneficial when hemodynamic instability occurs.
We acknowledge some limitations of our analysis. First, a publication bias is possible, however, as the number of studies included was limited, no filled funnel plot for publication bias or Egger's test was generated or performed. Second, the sample sizes of some subgroups were too small to assess heterogeneity between studies and draw an accurate conclusion. Third, the PE risk level was not available for all involved studies, which may lead to misclassification. Also, only one study focused on the treatment of tenecteplase in high-risk PE patients may cause bias.
Conclusion
In conclusion, our study indicated that tenecteplase would be suitable for high-risk PE patients because it could be beneficial for 30-day survival rate without increasing hemorrhagic incidents. However, tenecteplase is not recommended for patients with intermediate-risk PE because of high bleeding risk. More large-scale studies focused on catheter-directed thrombolysis involving intermediate-high/high risk PE are needed to validate the efficacy and safety of tenecteplase on short/long-term outcomes.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
ZZhai and CW have full access to all the data in the study and take responsibility for the content of the manuscript. ZZhan conceived and designed the study. ZZhan and LX integrated data, analyzed the data, and wrote the manuscript. GF provided methodological support. PY participated in editing of the manuscript. SZ, YZ, XT, QG, and WX contributed to the interpretation of the data and clinical inputs. All authors were involved in the revision of the manuscript for important intellectual content and approved the final version.
Funding
This study is funded by the by CAMS Innovation Fund for Medical Sciences(CIFMS) (2021-I2M-1-061) and The National Key Research and Development Program of China (No. 2016YFC0905600).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.860565/full#supplementary-material
Abbreviations
PE, pulmonary embolism; RRs, risk ratios; PEITHO, Pulmonary Embolism Thrombolysis; CI, confident intervals; RCTs, randomized controlled trials; RVD, right ventricular dysfunction; LMWH, low molecular weight heparin; UFH, unfractionated heparin; DOACs, direct oral anticoagulants; tPA, tissue plasminogen activator; 2019 ESC/ERS, 2019 Guideline of the European Society of Cardiology/the European Respiratory Society; PRISMA, preferred reporting items for systematic reviews and meta-analyses; NOS, Newcastle-ottawa scale; CTEPH, chronic thromboembolic pulmonary hypertension; PASP, pulmonary artery systolic pressure; ICU, intensive care unit; AMI, acute myocardial infarction; AIS, acute ischemic stroke; FDA, Food and Drug Administration.
References
1. Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. (2016) 388:3060–73. doi: 10.1016/S0140-6736(16)30514-1
2. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. (2016) 118:1340–7. doi: 10.1161/CIRCRESAHA.115.306841
3. Zhai Z, Wang D, Lei J, Yang Y, Xu X, Ji Y, et al. Trends in risk stratification, in-hospital management and mortality of patients with acute pulmonary embolism: an analysis from China pUlmonary thromboembolism REgistry Study (CURES). Eur Respir J. (2021). doi: 10.1183/13993003.02963-2020
4. Zhang Z, Lei J, Shao X, Dong F, Wang J, Wang D, et al. Trends in hospitalization and in-hospital mortality from VTE, 2007 to 2016, in China. Chest. (2019) 155:342–53. doi: 10.1016/j.chest.2018.10.040
5. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. (2019) 54. doi: 10.1183/13993003.01647-2019
6. Ullmann M, Hemmer W, Hannekum A. The urgent pulmonary embolectomy: mechanical resuscitation in the operating theatre determines the outcome. Thorac Cardiovasc Surg. (1999) 47:5–8. doi: 10.1055/s-2007-1013099
7. Goldhaber SZ, Haire WD, Feldstein ML, Miller M, Toltzis R, Smith JL, et al. Alteplase vs. heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet. (1993) 341:507–11. doi: 10.1016/0140-6736(93)90274-K
8. Marti C, John G, Konstantinides S, Combescure C, Sanchez O, Lankeit M, et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. (2015) 36:605–14. doi: 10.1093/eurheartj/ehu218
9. Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. (2014) 311:2414–21. doi: 10.1001/jama.2014.5990
10. Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. (2014) 370:1402–11. doi: 10.1056/NEJMoa1302097
11. Keyt BA, Paoni NF, Refino CJ, Berleau L, Nguyen H, Chow A, et al. A faster-acting and more potent form of tissue plasminogen activator. Proc Natl Acad Sci U S A. (1994) 91:3670–4. doi: 10.1073/pnas.91.9.3670
12. Guillermin A, Yan DJ, Perrier A, Marti C. Safety and efficacy of tenecteplase vs. alteplase in acute coronary syndrome: a systematic review and meta-Analysis of randomized trials. Archives of Medical Science. (2016) 12:1181–7. doi: 10.5114/aoms.2016.58929
13. Van De Werf F, Adgey J, Ardissino D, Armstrong PW, Aylward P, Barbash G, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. (1999) 354:716–22. doi: 10.1016/S0140-6736(99)07403-6
14. Kvistad CE, Novotny V, Kurz MW, Rønning OM, Thommessen B, Carlsson M, et al. Safety and outcomes of tenecteplase in moderate and severe ischemic stroke. Stroke. (2019) 50:1279–81. doi: 10.1161/STROKEAHA.119.025041
15. McGrath KG, Zeffren B, Alexander J, Kaplan K, Patterson R. Allergic reactions to streptokinase consistent with anaphylactic or antigen-antibody complex-mediated damage. J Allergy Clin Immunol. (1985) 76:453–7. doi: 10.1016/0091-6749(85)90726-2
16. McGrath KG, Patterson R. Anaphylactic reactivity to streptokinase. JAMA. (1984) 252:1314–7. doi: 10.1001/jama.1984.03350100044028
17. Lee HS. How safe is the readministration of streptokinase? Drug safety. (1995) 13:76–80. doi: 10.2165/00002018-199513020-00002
18. Tebbi C, Costanzi J, Shulman R, Dreisbach L, Jacobs BR, Blaney M, et al. A phase III, open-label, single-arm study of tenecteplase for restoration of function in dysfunctional central venous catheters. JVIR. (2011) 22:1117–23. doi: 10.1016/j.jvir.2011.02.034
19. Becattini C, Agnelli G, Salvi A, Grifoni S, Pancaldi LG, Enea I, et al. Bolus tenecteplase for right ventricle dysfunction in hemodynamically stable patients with pulmonary embolism. Thromb Res. (2010) 125:e82–6. doi: 10.1016/j.thromres.2009.09.017
20. Javaudin F, Lascarrou JB, Le Bastard Q, Bourry Q, Latour C, De Carvalho H, et al. Thrombolysis during resuscitation for out-of-hospital cardiac arrest caused by pulmonary embolism increases 30-day survival: findings from the french national cardiac arrest registry. Chest. (2019) 156:1167–75. doi: 10.1016/j.chest.2019.07.015
21. Kline JA, Nordenholz KE, Courtney DM, Kabrhel C, Jones AE, Rondina MT, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost. (2014) 12:459–68. doi: 10.1111/jth.12521
22. Konstantinides SV, Vicaut E, Danays T, Becattini C, Bertoletti L, Beyer-Westendorf J, et al. Impact of thrombolytic therapy on the long-term outcome of intermediate-risk pulmonary embolism. J Am Coll Cardiol. (2017) 69:1536–44. doi: 10.1016/j.jacc.2016.12.039
23. Patra S, Nagesh CM, Reddy B, Srinivas BC, Agrawal N, Manjunath CN, et al. Thrombolysis with single bolus tenecteplase compared with streptokinase infusion in the treatment of acute pulmonary embolism: a pilot study. Clin Appl Thromb-Hemost. (2015) 21:550–7. doi: 10.1177/1076029613511524
24. Cross DB, White HD. Allergic reactions to streptokinase. Clin Immunother. (1994) 2:415–20. doi: 10.1007/BF03259042
25. Davydov L, Cheng JWM. Tenecteplase: a review. Clin Ther. (2001) 23:982–97. doi: 10.1016/S0149-2918(01)80086-2
26. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. (2019) 50:2156–62. doi: 10.1161/STROKEAHA.119.025080
27. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ, American American College of Chest Physicians Antithrombotic T. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (2012) 141:7S−47S. doi: 10.1378/chest.141.4.1129a
28. Stewart LK, Kline JA. Fibrinolytics for the treatment of pulmonary embolism. Transl Res. (2020) 225:82–94. doi: 10.1016/j.trsl.2020.05.003
29. Bhuvaneswaran JS, Premchand RK, Iyengar SS, Khare R, Chabra CB, Padmanabhan TNC, et al. Tenecteplase in the treatment of acute pulmonary thrombo-embolism. J Thromb Thrombolysis. (2011) 31:445–8. doi: 10.1007/s11239-010-0524-y
30. Stewart LK, Peitz GW, Nordenholz KE, Courtney DM, Kabrhel C, Jones AE, et al. Contribution of fibrinolysis to the physical component summary of the SF-36 after acute submassive pulmonary embolism. J Thromb Thrombolysis. (2015) 40:161–6. doi: 10.1007/s11239-014-1155-5
31. Agrawal A, Kamila S, Donepudi A, Premchand R. Tenecteplase compared with streptokinase and heparin in the treatment of pulmonary embolism: an observational study. J Drug Assess. (2017) 6:33–7. doi: 10.1080/21556660.2017.1419957
32. Daley MJ, Murthy MS, Peterson EJ. Bleeding risk with systemic thrombolytic therapy for pulmonary embolism: scope of the problem. Ther Adv Drug Saf. (2015) 6:57–66. doi: 10.1177/2042098615572333
33. Xu N, Chen Z, Zhao C, Xue T, Wu X, Sun X, et al. Different doses of tenecteplase vs alteplase in thrombolysis therapy of acute ischemic stroke: evidence from randomized controlled trials. Drug Des Devel Ther. (2018) 12:2071–84. doi: 10.2147/DDDT.S170803
34. Wang C, Zhai Z, Yang Y, Wu Q, Cheng Z, Liang L, et al. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest. (2010) 137:254–62. doi: 10.1378/chest.09-0765
35. Bagla S, Smirniotopoulos JB, van Breda A, Sheridan MJ, Sterling KM. Ultrasound-accelerated catheter-directed thrombolysis for acute submassive pulmonary embolism. J Vasc Interv Radiol. (2015) 26:1001–6. doi: 10.1016/j.jvir.2014.12.017
Keywords: tenecteplase, thrombolysis, meta-analysis, pulmonary embolism, efficacy and safety
Citation: Zhang Z, Xi L, Zhang S, Zhang Y, Fan G, Tao X, Gao Q, Xie W, Yang P, Zhai Z and Wang C (2022) Tenecteplase in Pulmonary Embolism Patients: A Meta-Analysis and Systematic Review. Front. Med. 9:860565. doi: 10.3389/fmed.2022.860565
Received: 23 January 2022; Accepted: 09 March 2022;
Published: 31 March 2022.
Edited by:
Alejandro Lazo-Langner, Western University, CanadaReviewed by:
Carlos Jerjes-Sanchez, Escuela de Medicina y Ciencias de la Salud Tec Salud, Tecnológico de Monterrey, MexicoFatimah Al-Ani, Dalhousie University, Canada
Copyright © 2022 Zhang, Xi, Zhang, Zhang, Fan, Tao, Gao, Xie, Yang, Zhai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenguo Zhai, emhhaXpoZW5ndW8yMDExQDEyNi5jb20=; Chen Wang, d2FuZ2NoZW5AcHVtYy5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Zhu Zhang
Zhu Zhang Linfeng Xi
Linfeng Xi Shuai Zhang
Shuai Zhang Yunxia Zhang
Yunxia Zhang Guohui Fan
Guohui Fan Xincao Tao
Xincao Tao Qian Gao
Qian Gao Wanmu Xie
Wanmu Xie Peiran Yang
Peiran Yang Zhenguo Zhai
Zhenguo Zhai Chen Wang
Chen Wang