
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 24 May 2022
Sec. Family Medicine and Primary Care
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.858977
Background: Fertility awareness-based methods (FABMs) educate about reproductive health and enable tracking and interpretation of physical signs, such as cervical fluid secretions and basal body temperature, which reflect the hormonal changes women experience on a cyclical basis during the years of ovarian activity. Some methods measure relevant hormone levels directly. Most FABMs allow women to identify ovulation and track this “vital sign” of the menstrual or female reproductive cycle, through daily observations recorded on cycle charts (paper or electronic).
Applications: Physicians can use the information from FABM charts to guide the diagnosis and management of medical conditions and to support or restore healthy function of the reproductive and endocrine systems, using a restorative reproductive medical (RRM) approach. FABMs can also be used by couples to achieve or avoid pregnancy and may be most effective when taught by a trained instructor.
Challenges: Information about individual FABMs is rarely provided in medical education. Outdated information is widespread both in training programs and in the public sphere. Obtaining accurate information about FABMs is further complicated by the numerous period tracking or fertility apps available, because very few of these apps have evidence to support their effectiveness for identifying the fertile window, for achieving or preventing pregnancy.
Conclusions: This article provides an overview of different types of FABMs with a published evidence base, apps and resources for learning and using FABMs, the role FABMs can play in medical evaluation and management, and the effectiveness of FABMs for family planning, both to achieve or to avoid pregnancy.
Women's interest in learning to track their menstrual or reproductive cycle has increased dramatically over the last couple of decades, both for health monitoring and family planning purposes (1–3). This interest has been paralleled and fueled by the development of over 500 mobile health applications intended for cycle tracking, more than triple the number from only 5 years ago (4–6). By working with trained instructors or via other educational programs, women can learn how to track their cycles and observe specific external signs or biomarkers that reflect normal and abnormal hormonal patterns and reproductive function (7, 8). Women or couples can also use this information for family planning purposes (9, 10). The purpose of this review is to provide an overview of current evidence about fertility awareness-based methods (FABMs) in the context of women's health, and for achieving or avoiding pregnancy.
Historically, FABMs were most commonly referred to as natural family planning (NFP), which is defined by the World Health Organization as “methods for planning for avoiding pregnancies by observation of the natural signs and symptoms of the fertile and infertile phases of the menstrual cycle” (11). Some couples combine their knowledge of the fertile and infertile phase with the use of other methods during the fertile phase, such as barrier methods or withdrawal (12). As discussed later in this paper, we refer to this as FABMs combined with other methods. The term fertility awareness-based methods highlights that these methods may be used for more than family planning purposes and in recent decades, there has been additional focus on the value of using this information for medical evaluation and treatment (7, 8, 13, 14).
The menstrual cycle is increasingly recognized as a vital sign of health that women should have the opportunity to learn to monitor beginning in adolescence (15). Just as with other vital signs pointing to disease states, recognition of variations in menstrual patterns can improve early identification of potential health concerns that could become more severe if a timely diagnosis and appropriate treatment are not made (7, 15, 16). With most FABMs, women track vaginal bleeding and patterns of cervical fluid secretions and/or other biomarkers of health or fertility, such as basal body temperature (BBT) or urinary hormone measurements. Most FABMs employ a paper or electronic chart, which serves as a daily diary of the woman's own observations. Paradoxically, however, only 4% of physicians have received any formal training in FABMs (17). In addition, only 6% of physicians have correct knowledge about the perfect and typical use effectiveness of FABMs to avoid pregnancy (18). Without formal training in reading the female reproductive cycle chart, physicians and other clinicians may miss important information about this vital sign of health when providing care to their patients.
This article discusses FABMs that are frequently used in North America (Table 1), resources for clinicians to learn about FABMs, the role of FABMs in understanding women's health, and the effectiveness of FABMs for achieving or preventing pregnancy. Our intent is to provide information that physicians and other clinicians can use to guide patients who may benefit from learning FABMs. We also aim to provide information to clinicians about how FABMs can help with diagnosis and treatment of women's health conditions, including common conditions underlying female subfertility.
FABMs arise from an understanding of how the normally functioning reproductive age female produces observable external biomarkers, or ovulation indicators, that reflect internal hormonal changes. Figure 1 illustrates the relationship between a female's reproductive organs, hormones, and cyclic changes in ovulation indicators, including cervical mucus or fluid secretions, luteinizing hormone (LH), and basal body temperature (BBT). The uterine cervix plays a key role in producing the different types of cervical fluid that perform important functions related to sperm storage, transport and fertilization (19–21). Changes in cervical fluid, LH and BBT are each useful to identify the occurrence and timing of ovulation, which is usually the central event of the menstrual cycle (22).
Although the onset of menses is used to identify the beginning of the cycle, the menstrual bleed, or “menstrual period,” actually marks the end of the previous ovulatory cycle. Then, under the influence of gonadotropin-releasing hormone, the pituitary secretes follicle stimulating hormone (FSH) in the follicular phase (7, 13). Rising levels and changes in pulse frequency of FSH stimulate the growth of ovarian follicles that produce estradiol and related hormones (7, 13). In addition to building up the endometrium, estradiol also acts on crypt cells in the cervix, which results in the production of fertile type E cervical mucus, which is clear, stretchy, and/or slippery in sensation (13, 22–24). When estradiol rises and reaches a threshold, mid-cycle, it triggers a luteinizing hormone (LH) surge that results in ovulation (7, 13, 25).
Ovulation only occurs on 1 day in each cycle and the ovum or ova will survive <12–24 h if not fertilized (7, 25). Type E cervical fluid produced under the influence of estradiol in the peri-ovulatory period is critical for the effective transport, nurturing and survival of sperm (22, 23, 26). The last day of fertile type E cervical fluid, designated the mucus peak day, is a good external marker, as ovulation occurs within 2–3 days of the mucus peak day 87–98% of the time (23, 26, 27). After ovulation, the luteal phase begins. The ruptured follicle transforms into the corpus luteum and begins to secrete progesterone and estradiol (7, 28). The secretion of progesterone causes the cervical fluid to become thick and impermeable (Type G or gestagenic cervical fluid), and results in a change in sensation, typically causing dryness (21–23, 29). Progesterone also increases the metabolic rate and leads to a rise in the basal body temperature (BBT) (7, 12). Finally, progesterone also converts the endometrium from proliferative to secretory to prepare for possible implantation. In the case of implantation of an embryo, human chorionic gonadotropin (hCG) is produced, which stimulates the ovary to continue producing progesterone and estradiol (30). If implantation does not occur, in the absence of hCG, the corpus luteum atrophies and progesterone levels drop, which results in the shedding of the endometrial lining (menstruation) and the next cycle begins (7, 25).
With regard to cycle lengths, the follicular or preovulatory phase is inherently more variable than the luteal or postovulatory phase (31, 32). When considering past cycle lengths and prior estimated ovulation dates, it is possible to use evidence-based calendar formulas to estimate the start and end of the fertile window; however, calendar formulas are not precise enough to provide reasonable estimates of the day of ovulation. It must be emphasized that most calendar formulas in popular use, and even in most apps, are oversimplified, not individualized, and are not evidence-based (5, 33).
Broadly, there are six different types or categories of FABMs, based on the biomarkers or fertility indicators that are used to identify ovulation and the fertile window (see Table 1 for overview of the types and the indicators used for each). These include cervical fluid (mucus) methods, BBT methods, urinary hormone methods, sympto-thermal methods, sympto-hormonal methods, and calendar-based (i.e., cycle length-based) methods. Finally, the lactational amenorrhea method is an effective natural method that a woman may use within the first 6 months post-partum as long as she has not had a return of menses and her baby is breastfeeding exclusively at the breast (34).
A woman's ovulatory function will vary normally throughout her reproductive years as part of healthy physiologic transitions, such as menarche, pregnancy, lactation, and menopause (35). Tracking of one or more indicators of ovulation can be used to detect and diagnose common underlying causes of ovulation disturbances. Menstrual cycle irregularities related to ovulation dysfunction are most commonly due to hormonal abnormalities, which may result from hypothalamic, pituitary, thyroid, adrenal, ovarian and metabolic disorders (7, 8, 15, 16). For example, hypothalamic disorders due to excessive exercise, disordered eating or stress may result in hypoestrogenic, anovulatory cycles and/or prolonged periods of amenorrhea (7, 13). Variations or changes in symptoms or parameters that women can observe through FABM charting are listed in Table 2, together with conditions that may underlie each of the patterns (26, 36–56). Two of these conditions, polycystic ovarian syndrome, and endometriosis, are each present in at least 10% of all women of reproductive age, and are among the most common underlying causes of subfertility (48, 57–59).
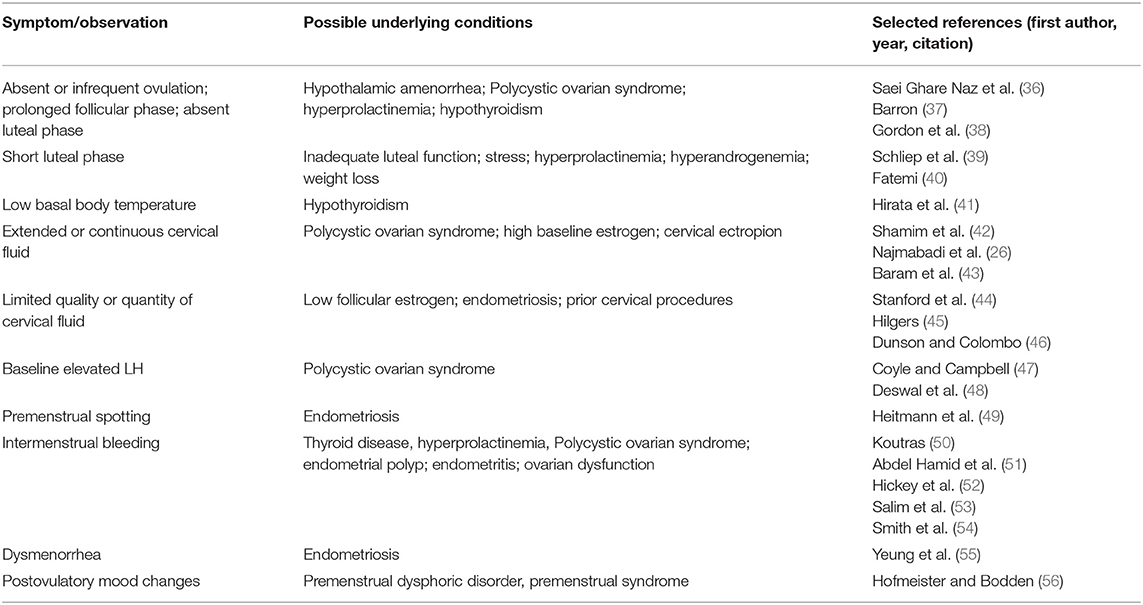
Table 2. Cycle parameters observable with fertility awareness-based methods and associated underlying health conditions.
There are several ways that FABMs can be used to enhance medical evaluation and treatment for women. (1) Women's observations on the FABM chart may suggest the presence of conditions that need further evaluation with diagnostic studies (Table 2). (2) Identifying the time of ovulation facilitates the scheduling and interpretation of time sensitive evaluations. For example, progesterone levels are very low prior to ovulation, and are normally at a maximum level 5–8 days following ovulation. Identifying ovulation allows the measurement of progesterone when it should be at its highest level (8). (3) Chart patterns may reflect intermediate outcomes from different types of fertility treatments. For example, a change from anovulatory to ovulatory cycles will be reflected in the woman's observation of her ovulation indicators (35).
Integrated medical evaluation and management protocols based on FABMs have been developed to address many women's health conditions that are related to the menstrual cycle, including subfertility or infertility. Natural Procreative Technology (also known as NaProTechnology) is a set of evaluation and treatment protocols developed based on women charting with the Creighton Model FertilityCare System (8, 45). It includes medical and surgical components. The Reproductive Health Research Institute (RHRI) has also published a set of medical evaluation and treatment protocols for women's health conditions, which are often related to FEMM (Fertility Education and Medical Management), but can also be used with any FABM that identifies ovulation accurately (7, 35, 60). A detailed or critical review of the components of each of these protocols is beyond the scope of this article; however, several resources for continuing medical education in FABMs are now available (see Table 3).
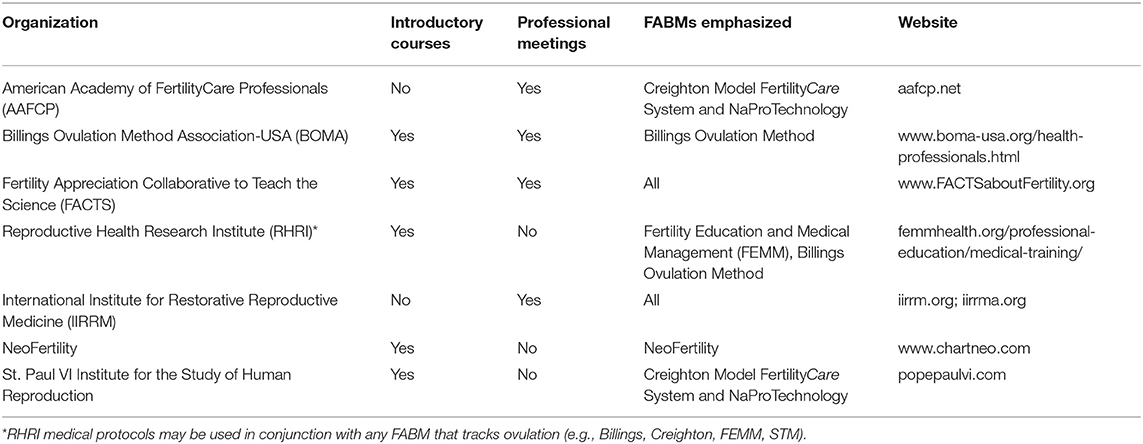
Table 3. Continuing medical education (CME) resources for medical applications of fertility awareness-based methods.
FABMs enable couples to achieve pregnancy by identifying the relatively few days during the female cycle when sexual intercourse will likely result in fertilization. This window of time, defined as the fertile window, usually begins about 5 days prior to ovulation, and ends within 12–24 h after ovulation (22, 61, 62). When the fertile window is estimated by cervical fluid and other biomarkers, it is usually varies from 1 day (in some subfertile populations) to more than 6 days long (26, 44, 61–65). In couples without subfertility, the highest probability of pregnancy per cycle is ~20–40%, depending on the characteristics of the population, including age and parity. It occurs when couples have intercourse 1–2 days before ovulation, particularly on days with the greatest estrogenic qualities of cervical fluid (clear, stretchy, slippery fluid), which optimizes sperm survival and transport (28, 64, 66). Data are sparse and mixed as to whether frequent intercourse decreases or actually increases overall sperm motility and concentration, and how this may impact the probability of pregnancy (67–69).
When couples regularly engage in acts of intercourse without attention to timing, approximately 85% of them will conceive by the end of 1 year (62). When couples can identify their fertile window and engage in fertility-focused intercourse, evidence suggests they can achieve similar pregnancy rates in less time. A number of studies have examined fertility focused intercourse with several different FABMs (Table 4) (70–79). Overall, these studies suggest that 85–90% of couples without subfertility can conceive within 6 months through fertility focused intercourse. Public awareness of the benefits of using FABMs to conceive is becoming more widespread in a recent large online study of couples in the USA and Canada trying to conceive, 75% of women were already using one or more FABM indicators to try to conceive (albeit not necessarily accurately), and 73% were using a menstrual and/or fertility tracker app (71).
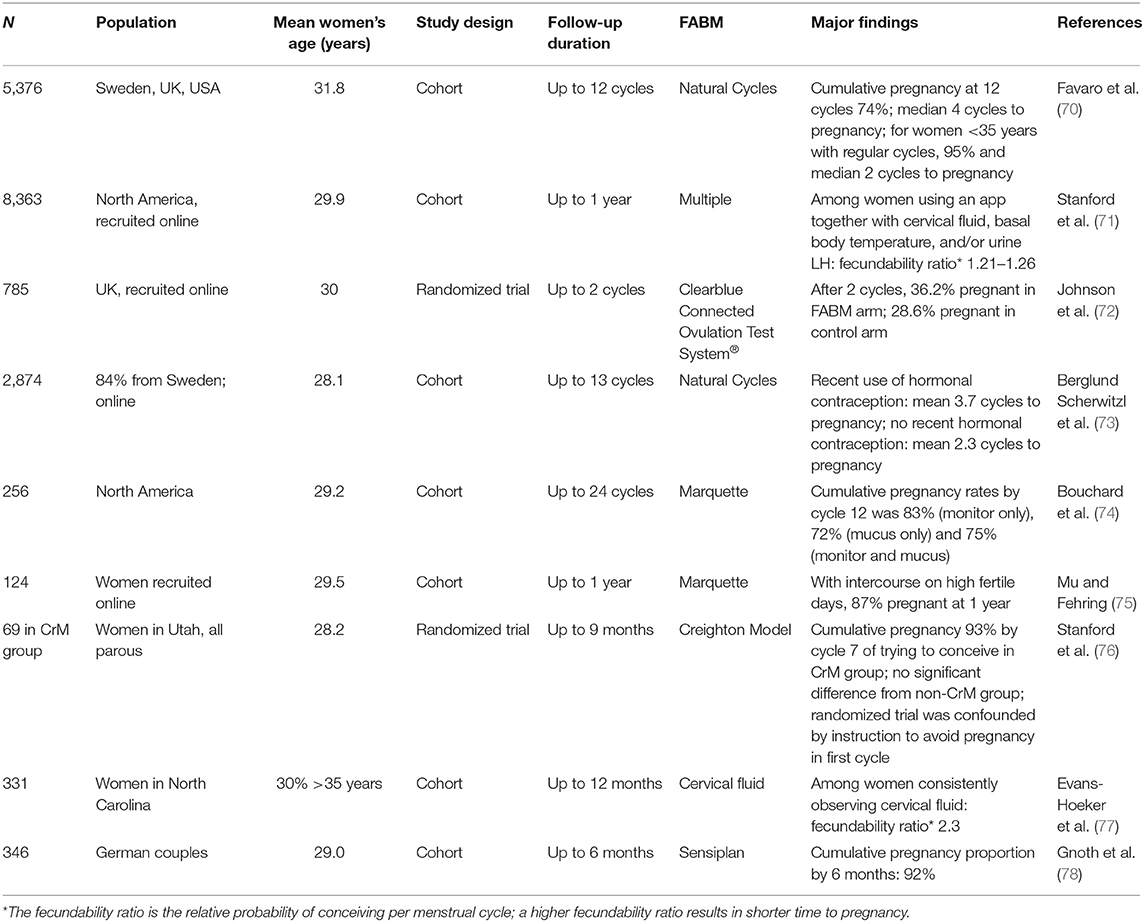
Table 4. Fertility awareness-based methods and time to pregnancy in women or couples with no known subfertility trying to conceive.
In women with cycle abnormalities or couples with subfertility or infertility, the female cycle chart may help a couple to identify a less frequent or narrower fertile window when trying to conceive (43, 79). It may also serve as a tool for clinicians trained in restorative reproductive medicine (RRM) to guide the work-up and management of multiple underlying causes of subfertility or recurrent pregnancy loss (7, 8, 50–53, 80).
There are several studies that document the outcomes of using FABMs to address subfertility, either with or without medical intervention (Table 5) (80–85). Four of these studies arise from practices of family physicians utilizing RRM techniques in addition to the FABM charting by the women and couples (80–83). Currently randomized comparisons of RRM vs. conventional fertility treatments are not available. It should also be noted that there are few randomized trials that compare different types of fertility treatments to each other. Most randomized trials involve adjustments within a particular treatment (e.g., different protocols for in vitro fertilization), rather than comparisons between different types or classes of treatment (86, 87). There are also practical and ethical considerations in studies of fertility therapies or family planning methods, in that women or couples who wish to choose which therapy or method of family planning they use may not be willing to be randomized to a different method.
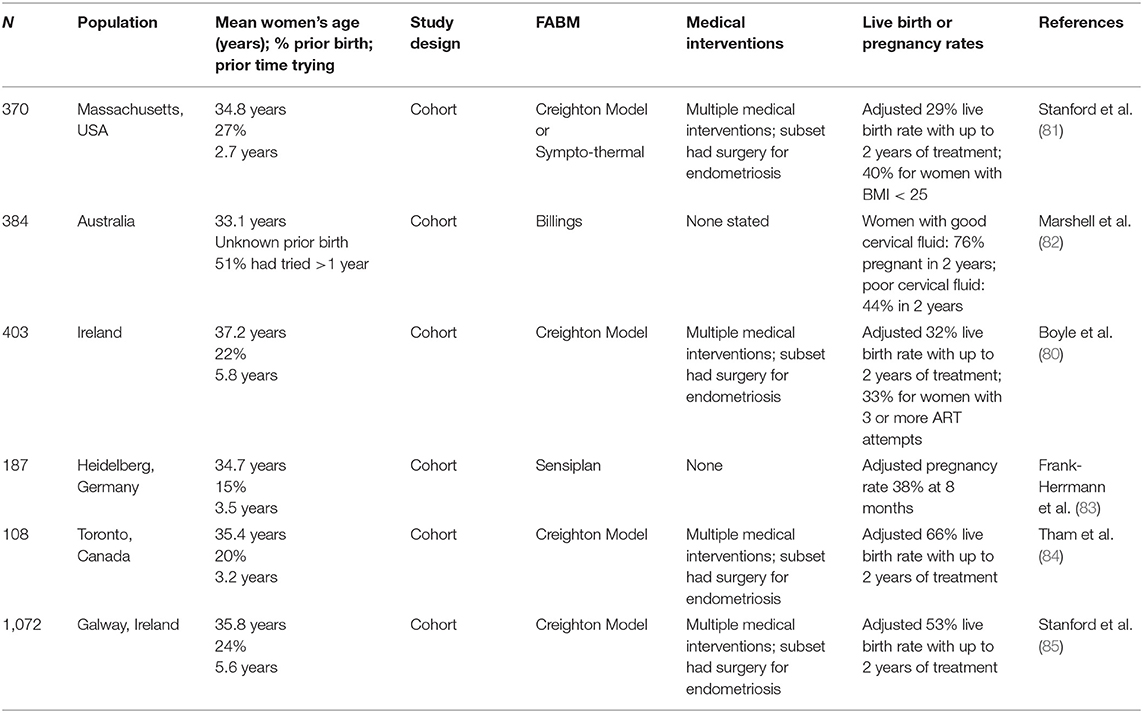
Table 5. Effectiveness of fertility awareness-based methods in women or couples with subfertility trying to conceive.
With identification of the fertile window, couples can modify their sexual behavior to avoid pregnancy, e.g., by abstaining from sexual contact during the fertile window. Two recent systematic reviews have summarized the evidence for pregnancy rates when FABMs are used to avoid pregnancy, based on cohort studies from around the world (9, 10). From these reviews, we present a summary overview of the studies and their pregnancy rates that were judged of reasonable methodologic quality in at least one of the reviews, were published since 1990, had at least 100 women, and which involve FABMs that are currently readily available in North America (see Table 6). With regard to the Marquette Model, we only included those study populations using the Clearblue® monitor. We also included three more recent studies that we believe may meet the same level of methodologic quality, but which have not yet been vetted in a systematic review. Correct use pregnancy rates (also called “perfect use” pregnancy rates) are observed during consistent and correct application of the FABM to avoid pregnancy (101) (These rates have often been calculated incorrectly based on all participant time in the study; we present in the table those which have been calculated correctly based on the participant time of correct use). Typical use pregnancy rates are observed during actual use of the FABM to avoid pregnancy, including both correct and incorrect use. Because the use of FABM to avoid pregnancy requires adaptations in sexual behavior, and because strength of motivation to avoid pregnancy may vary between populations or change over time, there is a difference between pregnancy rates with correct use and typical use, which may vary substantially based on underlying characteristics and motivations of the population being studied (102). Furthermore, some studies assessed pregnancy intentions only once at the beginning of the study, whereas others did so at the beginning of each cycle.
With correct use to avoid pregnancy, the pregnancy rate is <5 per 100 women years for all methods included, and for some methods, it is <1% (Table 6). Typical use pregnancy rates vary depending on the characteristics of the population studied, and at least to some extent the individual method, ranging from about 2 to 23 per 100 woman-years, with the majority of studies showing typical use pregnancy rates of <15 per 100 woman-years.
It's important to recognize that pregnancy rates reported in studies to avoid pregnancy may not necessarily correlate with real world pregnancy rates, as effectiveness depends on adequate training or instruction, user motivation for correct use to avoid pregnancy, and sufficient cooperation or support from the male partner (10, 12, 103). To date, most FABM effectiveness studies for pregnancy prevention have been done with couples who learned the method from a trained instructor, with the exception of the Natural Cycles and DOT apps, which have electronic instruction resources (98, 99). It should also be noted that while the actual fertile window is generally considered to be about 6 days in healthy couples, there is some normal variability in the biomarkers, which means that the observed fertile window for FABMs to avoid pregnancy is usually about 12 days that are considered potentially fertile (26, 31, 64, 104).
It is more difficult to identify the beginning of the fertile window than the end of it, in part because of the inherent variability of the length of the follicular phase. Therefore, for couples who wish to have the least possible chance of pregnancy while using an FABM, it is prudent to recommend that they restrict intercourse to the postovulatory infertile phase, and that they consider using two indicators to confirm the end of the fertile window (10, 91, 105, 106). For example, the sympto-thermal method uses the mucus peak and the BBT shift combined to confirm the end of the fertile window (12, 88). As another example, the mucus peak and postovulatory measurement of progesterone may also confirm the end of the fertile window (10, 106, 107). This may result in a longer period of time when couples consider themselves to be potentially fertile.
The concomitant use of barrier methods (e.g., condoms) or withdrawal during fertile days may influence pregnancy rates, in comparison to abstinence from genital contact during the fertile window. There are a few studies that have examined this question systematically. In a study of the Standard Days Method (n = 373), the correct use pregnancy rate with abstinence in the fertile time was 4.8% at 1 year, while the correct use pregnancy rate including barriers or withdrawal during the fertile time was 5.7% (96). Similarly, in a study of the TwoDay Method (n = 450), the correct use pregnancy rate with abstinence in the fertile time was 3.5%, while the correct use pregnancy rate including barriers or withdrawal during the fertile time was 6.3% (95). In a study of 900 women using Sensiplan, the 13-cycle cumulative typical use pregnancy rates were 1.6% for Sympto-Thermal only, and 2.0% for occasional use of barriers in the fertile time (88). Overall, these data do support the logic that use of a barrier method or withdrawal during a fertile day should be expected to have at least a slightly higher chance of pregnancy than no sexual contact during that same fertile day.
In the last decade there has been an explosion in the number of fertility apps for smart phones and other mobile devices, available for women to track their cycle, with more than 500 apps available via Google and the Apple app store when using the keyword fertility to search for apps (6). Although many apps claim to be useful for avoiding or achieving pregnancy, a 2016 systematic review of apps marketed for avoiding pregnancy demonstrated that the large majority are not concordant with evidenced-based methods of fertility awareness. The few apps that were rated highly were associated with established FABM methods (see Table 1) (5). Similar results were reported in a 2020 scoping review, namely that few apps accurately predict the fertile window (108). To date, two apps, Natural Cycles and Clue, the former based primarily on basal body temperature, and the latter using the dynamic optimal timing (DOT) algorithm, have received FDA clearance for use as a contraceptive device (99, 100). Both apps support an approach of FABM combined with barrier methods, as they stipulate that correct use includes the possible use of barrier methods on fertile days. One app interprets urine estrogen metabolites and LH to define the fertile window, and has been shown effective for trying to conceive (see Table 4) (72). Additional apps are available or being developed that may integrate artificial intelligence to interpret hormones or metabolites in urine, including estradiol, LH, and progesterone, but as yet there is no published research on their effectiveness to avoid pregnancy or to conceive (109).
FABMs or natural methods are unique among family planning options, in the level of encouraging understanding, involvement or assent from both partners, and communication between them (9, 12, 103, 110). As behavioral methods of family planning, FABMs rely on people learning to track the observable female biomarkers on a daily basis to determine whether they may be fertile and when they are not (9, 12). They can then share this information with their partner and depending on their family planning goals follow the rules of their chosen method for preventing or achieving pregnancy (111). These methods may positively influence relationships and body literacy. One study of over 2500 sympto-thermal users found large majorities of women and men felt NFP improved their relationship and sex-life, and three-fourths of them were satisfied with how often they had sexual intercourse. Fully 95% of women reported using a natural method improved their body literacy (103).
To maximize effectiveness of any FABM, it is important that people receive adequate instruction, which clearly identifies biomarkers of interest and how they may be tracked to understand fertility. We believe this is particularly true for using FABMs in medical applications. To date, most studies of FABMs to avoid pregnancy or to conceive have delivered this instruction via trained teachers, usually in person (9). More recently, online models of instruction have proven effective for some methods (102). Some simpler methods have delivered their instruction through online resources, such as videos (11, 99, 100).
FABMs serve as a useful tool for people to track daily external observations that reflect ovulation and the internal hormonal changes women experience throughout their cycle. Physicians and other clinicians may learn to interpret the female cycle chart to identify potential abnormalities of the menstrual cycle and inform a differential diagnosis and management plan to address a range of reproductive health issues, such as abnormal uterine bleeding, subfertility, and other conditions associated with abnormalities in ovulation or reproductive hormone levels. When clinicians are knowledgeable about the range of FABMs and their effectiveness, they can also offer patients a wider array of options for seeking pregnancy or avoiding pregnancy (both aspects of family planning), which will meet the needs of more people. Adding FABMs to the mix of available contraceptive methods has been demonstrated to expand the proportion of women using family planning, without any increase of unplanned pregnancy rates (112). Unfortunately, most physicians are currently not well-versed in modern FABMs, the science underlying their use, or the medical applications of these methods (18). This article offers an introduction to FABMs and their medical applications for physicians and other clinicians. For more information, we encourage our colleagues to pursue continuing medical education options in restorative reproductive medicine, such as those outlined in Table 3.
MD and JS designed the review, conducted the literature search, and drafted the initial manuscript. CP provided a critical review of the manuscript and assisted with the creation of the tables. PV assisted with writing the manuscript and provided critical review of the content. All authors reviewed and approved the final manuscript.
This project is supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) as part of an award totaling $ $1,572,177 over 5 years with zero percentage financed with non-governmental sources. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by HRSA, HHS or the U.S. Government.
The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by HRSA, HHS or the U.S. Government.
MD serves as a paid part-time Director of the Fertility Appreciation Collaborative to Teach the Science (FACTS), a collaborative project of the Family Medicine Education Consortium. JS serves without compensation on the Boards of the International Institute of Restorative and Reproductive Medicine and the Fertility Care Centers of America. CP serves on the board of the International Board of Lactation Consultant Examiners. PV serves as the medical director of the Reproductive Health Research Institute.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors wish to acknowledge the following individuals for their contributions to this manuscript: Virginia Martinez and Grace Stotzer were instrumental in preparation of the manuscript and verifying references. Padmaja Sundaram also assisted with verifying references. We would also like to acknowledge Nick Mayeaux, illustrator of Figure 1.
1. Leonard CJ, Chavira W, Coonrod DV, Hart KW, Bay RC. Survey of attitudes regarding natural family planning in an urban Hispanic population. Contraception. (2006) 74:313–7. doi: 10.1016/j.contraception.2006.05.075
2. Stanford JB, Lemaire JC, Thurman PB. Women's interest in natural family planning. J Fam Pract. (1998) 46:65–71.
3. Smith A. Fertility awareness based methods (FABMs): evaluating and promoting female interest for purposes of health monitoring and family planning [Master's thesis]. University of Alabama, Fayetteville, AL, United States (2019). Available online at: https://scholarworks.uark.edu/etd/3279 (accessed August 9, 2021).
4. Ali R, Gürtin ZB, Harper JC. Do fertility tracking applications offer women useful information about their fertile window? Reprod Biomed Online. (2020) 42:273–81. doi: 10.1016/j.rbmo.2020.09.005
5. Duane M, Contreras A, Jensen ET, White A. The performance of fertility awareness-based method apps marketed to avoid pregnancy. J Am Board Fam Med. (2016) 29:508–11. doi: 10.3122/jabfm.2016.04.160022
6. Costa Figueiredo M, Huynh T, Takei A, Epstein DA, Chen Y. Goals, life events, and transitions: examining fertility apps for holistic health tracking. JAMIA Open. (2021) 4:1–12. doi: 10.1093/jamiaopen/ooab013
7. Vigil P, Lyon C, Flores B, Rioseco H, Serrano F. Ovulation, a sign of health. Linacre Q. (2017) 84:343–55. doi: 10.1080/00243639.2017.1394053
9. Manhart MD, Duane M, Lind A, Sinai I, Golden-Tevald J. Fertility awareness-based methods of family planning: a review of effectiveness for avoiding pregnancy using SORT. Osteopath Fam Phys. (2013) 5:2–8. doi: 10.1016/j.osfp.2012.09.002
10. Peragallo Urrutia R, Polis CB, Jensen ET, Greene ME, Kennedy E, Stanford JB. Effectiveness of fertility awareness-based methods for pregnancy prevention: a systematic review. Obstet Gynecol. (2018) 132:591–604. doi: 10.1097/AOG.0000000000002784
12. Simmons RG, Jennings V. Fertility awareness-based methods of family planning. Best Pract Res Clin Obstet Gynaecol. (2020) 66:68–82. doi: 10.1016/j.bpobgyn.2019.12.003
13. Vigil P, Blackwell LF, Cortés ME. The importance of fertility awareness in the assessment of a woman's health: a review. Linacre Q. (2012) 79:426–50. doi: 10.1179/002436312804827109
14. Vigil P, Ceric F, Cortés ME, Klaus H. Usefulness of monitoring fertility from menarche. J Pediatr Adolesc Gynecol. (2006) 19:173–9. doi: 10.1016/j.jpag.2006.02.003
15. ACOG Committee Opinion No. 651: menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. (2015) 126:143–6. doi: 10.1097/AOG.0000000000001215
16. Popat VB, Prodanov T, Calis KA, Nelson LM. The menstrual cycle: a biological marker of general health in adolescents. Ann N Y Acad Sci. (2008) 1135:43–51. doi: 10.1196/annals.1429.040
17. Fehring RJ. The future of professional education in natural family planning. J Obstet Gynecol Neonatal Nurs. (2004) 33:34–43. doi: 10.1177/0884217503258549
18. Choi J, Chan S, Wiebe E. Natural family planning: physicians' knowledge, attitudes, and practice. J Obstet Gynaecol Can. (2010) 32:673–8. doi: 10.1016/S1701-2163(16)34571-6
19. Martyn F, McAuliffe FM, Wingfield M. The role of the cervix in fertility: is it time for a reappraisal? Hum Reprod. (2014) 29:2092–8 doi: 10.1093/humrep/deu195
20. Billings EL, Brown JB, Billings JJ, Burger HG. Symptoms and hormonal changes accompanying ovulation. Lancet. (1972) 1:282–4. doi: 10.1016/S0140-6736(72)90291-7
21. Hilgers TW, Prebil AM. The ovulation method–vulvar observations as an index of fertility/infertility. Obstet Gynecol. (1979) 53:12–22.
22. Ecochard R, Boehringer H, Rabilloud M, Marret H. Chronological aspects of ultrasonic, hormonal, and other indirect indices of ovulation. Int J Gynaecol Obstet. (2001) 108:822–9. doi: 10.1016/S0306-5456(00)00194-7
24. Ceric F, Silva D, Vigil P. Ultrastructure of the human periovulatory cervical mucus. J Electron Microsc. (2005) 54:479–84. doi: 10.1093/jmicro/dfh106
25. Fritz MA, Speroff L. “The Regulation of the Menstrual Cycle.” Clinical Gynecologic Endocrinology and Infertility. 8th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins (2011). p. 199–242.
26. Najmabadi S, Schliep KC, Simonsen SE, Porucznik CA, Egger MJ, Stanford JB. Cervical mucus patterns and the fertile window in women without known subfertility: a pooled analysis of three cohorts. Hum Reprod. (2021) 36:1784–95. doi: 10.1093/humrep/deab049
27. Stanford JB, Schliep KC, Chang CP, O'Sullivan JP, Porucznik CA. Comparison of woman-picked, expert-picked, and computer-picked Peak Day of cervical mucus with blinded urine luteinising hormone surge for concurrent identification of ovulation. Paediatr Perinat Epidemiol. (2020) 34:105–13. doi: 10.1111/ppe.12642
28. Blackwell LF, Brown JB, Vigil P, Gross B, Sufi S, D'Arcangues C. Hormonal monitoring of ovarian activity using the Ovarian Monitor, part I. Validation of home and laboratory results obtained during ovulatory cycles by comparison with radioimmunoassay. Steroids. (2003) 68:465–76. doi: 10.1016/S0039-128X(03)00049-7
29. Ecochard R, Duterque O, Leiva R, Bouchard T, Vigil P. Self-identification of the clinical fertile window and the ovulation period. Fertil Steril. (2015) 103:1319–25. doi: 10.1016/j.fertnstert.2015.01.031
30. Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med. (2010) 28:5–16. doi: 10.1055/s-0029-1242988
31. Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs. (2006) 35:376–84. doi: 10.1111/j.1552-6909.2006.00051.x
32. Symul L, Wac K, Hillard P, Salathé M. Assessment of menstrual health status and evolution through mobile apps for fertility awareness. NPJ Digit Med. (2019) 2:64. doi: 10.1038/s41746-019-0139-4
33. Johnson S, Marriott L, Zinaman M. Can apps and calendar methods predict ovulation with accuracy?. Curr Med Res Opin. (2018) 34:158794. doi: 10.1080/03007995.2018.1475348
34. Peterson AE, Perez-Escamilla R, Labbok MH, Hight V, von Hertzen H, Van Look P. Multicenter study of the lactational amenorrhea method (LAM) III: effectiveness, duration, and satisfaction with reduced client-provider contact. Contraception. (2000) 62:221–30. doi: 10.1016/S0010-7824(00)00171-2
35. Brown JB. Types of ovarian activity in women and their significance: the continuum (a reinterpretation of early findings). Hum Reprod Update. (2011) 17:141–58. doi: 10.1093/humupd/dmq040
36. Saei Ghare Naz M, Rostami Dovom M, Ramezani Tehrani F. The menstrual disturbances in endocrine disorders: a narrative review. Int J Endocrinol Metab. (2020) 18:e106694. doi: 10.5812/ijem.106694
37. Barron ML. Proactive management of menstrual cycle abnormalities in young women. J Perinat Neonatal Nurs. (2004) 18:81–92. doi: 10.1097/00005237-200404000-00003
38. Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, et al. Functional hypothalamic amenorrhea: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. (2017) 102:1413–39. doi: 10.1210/jc.2017-00131
39. Schliep KC, Mumford SL, Hammoud AO, Stanford JB, Kissell KA, Sjaarda LA, et al. Luteal phase deficiency in regularly menstruating women: prevalence and overlap in identification based on clinical and biochemical diagnostic criteria. J Clin Endocrinol Metab. (2014) 99:E1007–14. doi: 10.1210/jc.2013-3534
40. Fatemi HM. Assessment of the luteal phase in stimulated and substituted cycles. Facts Views Vis Obgyn. (2009) 1:30–46.
41. Hirata Y, Fukuoka H, Iguchi G, Iwahashi Y, Fujita Y, Hari Y, et al. Median-lower normal levels of serum thyroxine are associated with low triiodothyronine levels and body temperature in patients with central hypothyroidism. Eur J Endocrinol. (2015) 173:247–56. doi: 10.1530/EJE-15-0130
42. Shamim N, Usala SJ, Biggs WC, McKenna GB. The elasticity of cervical-vaginal secretions is abnormal in polycystic ovary syndrome: case report of five PCOS women. Indian J Endocrinol Metab. (2012) 16:1019–21. doi: 10.4103/2230-8210.103030
43. Baram A, Paz GF, Peyser MR, Schachter A, Homonnai ZT. Treatment of cervical ectropion by cryosurgery: effect on cervical mucus characteristics. Fertil Steril. (1985) 43:86–9. doi: 10.1016/S0015-0282(16)48323-8
44. Stanford JB, Smith KR, Dunson DB. Vulvar mucus observations and the probability of pregnancy. Obstet Gynecol. (2003) 101:1285–93. doi: 10.1097/00006250-200306000-00025
45. Hilgers TW. The Medical and Surgical Practice of NaPro Technology. Omaha: Pope Paul VI Institute Press (2004).
46. Dunson DB, Colombo B. Bayesian modeling of markers of day-specific fertility. J Am Stat Assoc. (2003) 98:28–37. doi: 10.1198/016214503388619067
47. Coyle C, Campbell RE. Pathological pulses in PCOS. Mol Cell Endocrinol. (2019) 498:110561. doi: 10.1016/j.mce.2019.110561
48. Deswal R, Narwal V, Dang A, Pundir CS. The prevalence of polycystic ovary syndrome: a brief systematic review. J Hum Reprod Sci. (2020) 13:261–71. doi: 10.4103/jhrs.JHRS_95_18
49. Heitmann RJ, Langan KL, Huang RR, Chow GE, Burney RO. Premenstrual spotting of ≥2 days is strongly associated with histologically confirmed endometriosis in women with infertility. Am J Obstet Gynecol. (2014) 211:358.e1–6. doi: 10.1016/j.ajog.2014.04.041
50. Koutras DA. Disturbances of menstruation in thyroid disease. Ann N Y Acad Sci. (1997) 816:280–4. doi: 10.1111/j.1749-6632.1997.tb52152.x
51. Abdel Hamid AM, Borg TF, Madkour WA. Prevalence of hyperprolactinemia and thyroid disorders among patients with abnormal uterine bleeding. Int J Gynaecol Obstet. (2015) 131:273–6. doi: 10.1016/j.ijgo.2015.05.035
52. Hickey M, Karthigasu K, Agarwal S. Abnormal uterine bleeding: a focus on polycystic ovary syndrome. Womens Health. (2009) 5:313–24. doi: 10.2217/WHE.09.20
53. Salim S, Won H, Nesbitt-Hawes E, Campbell N, Abbott J. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. (2011) 18:569–81. doi: 10.1016/j.jmig.2011.05.018
54. Smith M, Hagerty KA, Skipper B, Bocklage T. Chronic endometritis: a combined histopathologic and clinical review of cases from 2002 to 2007. Int J Gynecol Pathol. (2010) 29:44–50. doi: 10.1097/PGP.0b013e3181ae81bb
55. Yeung P, Gupta S, Grieg S. Endometriosis in adolescents: a systematic review. J Endometriosis Pelvic Pain Disord. (2017) 9:17–29. doi: 10.5301/je.5000264
56. Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Phys. (2016) 94:236–40.
57. Costello MF, Misso ML, Balen A, Boyle J, Devoto L, Garad RM, et al. A brief update on the evidence supporting the treatment of infertility in polycystic ovary syndrome. Aust N Z J Obstet Gynaecol. (2019) 59:867–73. doi: 10.1111/ajo.13051
58. Fadhlaoui A, Bouquet de la Jolinière J, Feki A. Endometriosis and infertility: how and when to treat?. Front Surg. (2014) 1:24. doi: 10.3389/fsurg.2014.00024
59. Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, et al. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. (2011) 96:360–5. doi: 10.1016/j.fertnstert.2011.05.087
60. Portales MDPV, González FAS, Díaz HAR, López JLA, Errázuriz MCL, Castro PHC, et al. Reproductive Health Research Institute: Guidelines. 9th ed. Santiago: Asociación Instituto de Investigación en Medicina Reproductiva (2019).
61. Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on probability of conception, survival of the pregnancy, and sex of baby. N Engl J Med. (1995) 333:1517–21. doi: 10.1056/NEJM199512073332301
62. Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril. (2017) 107:52–8. doi: 10.1016/j.fertnstert.2016.09.029
63. Keulers MJ, Hamilton CJCM, Franx A, Evers JLH, Bots RSGM. The length of the fertile window is associated with the chance of spontaneously conceiving an ongoing pregnancy in subfertile couples. Hum Reprod. (2007) 22:1652–6. doi: 10.1093/humrep/dem051
64. Colombo B, Masarotto G. Daily fecundability: first results from a new data base. Demogr Res. (2000) 3. doi: 10.4054/DemRes.2000.3.5
65. Lynch CD, Jackson LW, Buck Louis GM. Estimation of the day-specific probabilities of conception: current state of the knowledge and the relevance for epidemiological research. Paediatr Perinat Epidemiol. (2006) 20:3–12. doi: 10.1111/j.1365-3016.2006.00765.x
66. Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod. (2004) 19:889–92. doi: 10.1093/humrep/deh173
67. Wang N, Gu H, Gao Y, Li X, Yu G, Lv F, et al. Study on influencing factors of semen quality in fertile men. Front Physiol. (2022) 13:813591 doi: 10.3389/fphys.2022.813591
68. Valsa J, Skandhan KP, Gusani P, Khan PS, Amith S, Gondalia M. Effect of daily ejaculation on semen quality and calcium and magnesium in semen. Rev Int Androl. (2013) 11:94–9. doi: 10.1016/j.androl.2013.03.001
69. Tur-Kapsa I, Maor Y, Levran D, Yonish M, Mashiach S, Dor J. How often should infertile men have intercourse to achieve conception? Fertil Steril. (1994) 62:370–5. doi: 10.1016/S0015-0282(16)56893-9
70. Favaro C, Pearson JT, Rowland SP, Jukic AM, Chelstowska M, Scherwitzl EB, et al. Time to pregnancy for women using a fertility awareness based mobile application to plan a pregnancy. J Womens Health. (2021) 30:1538–45. doi: 10.1089/jwh.2021.0026
71. Stanford JB, Willis SK, Hatch EE, Rothman KJ, Wise LA. Fecundability in relation to use of mobile computing apps to track the menstrual cycle. Hum Reprod. (2020) 35:2245–52. doi: 10.1093/humrep/deaa176
72. Johnson S, Stanford JB, Warren G, Bond S, Bench-Capon S, Zinaman MJ. Increased likelihood of pregnancy using an app-connected ovulation test system: a randomized controlled trial. J Womens Health. (2020) 29:84–90. doi: 10.1089/jwh.2019.7850
73. Berglund Scherwitzl E, Lundberg O, Kopp Kallner H, Rowland SP, Holte J, Trussell J, et al. Short- and long-term effect of contraceptive methods on fecundity. Eur J Contracept Reprod Health Care. (2019) 24:260–5. doi: 10.1080/13625187.2019.1621999
74. Bouchard TP, Fehring RJ, Schneider MM. Achieving pregnancy using primary care interventions to identify the fertile window. Front Med. (2018) 4:250. doi: 10.3389/fmed.2017.00250
75. Mu Q, Fehring RJ. Efficacy of achieving pregnancy with fertility-focused intercourse. MCN Am J Matern Child Nurs. (2014) 39:35–40. doi: 10.1097/NMC.0b013e3182a76b88
76. Stanford JB, Smith KR, Varner MW. Impact of instruction in the Creighton model FertilityCare system on time to pregnancy in couples of proven fecundity: results of a randomised trial. Paediatr Perinat Epidemiol. (2014) 28:391–9. doi: 10.1111/ppe.12141
77. Evans-Hoeker E, Pritchard DA, Long DL, Herring AH, Stanford JB, Steiner AZ. Cervical mucus monitoring prevalence and associated fecundability in women trying to conceive. Fertil Steril. (2013) 100:1033–8.e1. doi: 10.1016/j.fertnstert.2013.06.002
78. Gnoth C, Godehardt D, Godehardt E, Frank-Herrmann P, Freundl G. Time to pregnancy: results of the German prospective study and impact on the management of infertility. Hum Reprod. (2003) 18:1959–66. doi: 10.1093/humrep/deg366
79. Thijssen A, Meier A, Panis K, Ombelet W. ‘Fertility Awareness-Based Methods' and subfertility: a systematic review. Facts Views Vis Obgyn. (2014) 6:113–23.
80. Boyle PC, de Groot T, Andralojc KM, Parnell TA. Healthy singleton pregnancies from Restorative Reproductive Medicine (RRM) after failed IVF. Front Med. (2018) 5:210. doi: 10.3389/fmed.2018.00210
81. Stanford JB, Carpentier PA, Meier BL, Rollo M, Tingey B. Restorative reproductive medicine for infertility in two family medicine clinics in New England, an observational study. BMC Pregn Childbirth. (2021) 21:495. doi: 10.1186/s12884-021-03946-8
82. Marshell M, Corkill M, Whitty M, Thomas A, Turner J. Stratification of fertility potential according to cervical mucus symptoms: achieving pregnancy in fertile and infertile couples. Hum Fertil. (2021) 24:353–9. doi: 10.1080/14647273.2019.1671613
83. Frank-Herrmann P, Jacobs C, Jenetzky E, Gnoth C, Pyper C, Baur S, et al. Natural conception rates in subfertile couples following fertility awareness training. Arch Gynecol Obstet. (2017) 295:1015–24. doi: 10.1007/s00404-017-4294-z
84. Tham E, Schliep K, Stanford J. Natural procreative technology for infertility and recurrent miscarriage: outcomes in a Canadian family practice. Can Fam Phys. (2012) 58:e267–74.
85. Stanford JB, Parnell TA, Boyle PC. Outcomes from treatment of infertility with natural procreative technology in an Irish general practice [published correction appears in J Am Board Fam Med. (2008) 21:583. J Am Board Fam Med. (2008) 21:375–84. doi: 10.3122/jabfm.2008.05.070239
86. Zhang Y, Zhang C, Shu J, Guo J, Chang HM, Leung PCK, et al. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: a systematic review and network meta-analysis. Hum Reprod Update. (2020) 26:247–63. doi: 10.1093/humupd/dmz046
87. Pandian Z, Gibreel A, Bhattacharya S. In vitro fertilisation for unexplained subfertility. Cochrane Database Syst Rev. (2012) 4:CD003357. doi: 10.1002/14651858.CD003357.pub3
88. Frank-Herrmann P, Heil J, Gnoth C, Toledo E, Baur S, Pyper C, et al. The effectiveness of a fertility awareness based method to avoid pregnancy in relation to a couple's sexual behaviour during the fertile time: a prospective longitudinal study. Hum Reprod. (2007) 22:1310–9. doi: 10.1093/humrep/dem003
89. Fehring RJ, Schneider M, Raviele K, Rodriguez D, Pruszynski J. Randomized comparison of two Internet-supported fertility-awareness-based methods of family planning. Contraception. (2013) 88:24–30. doi: 10.1016/j.contraception.2012.10.010
90. Fehring RJ, Schneider M. Effectiveness of a natural family planning service program. MCN Am J Matern Child Nurs. (2017) 42:43–9. doi: 10.1097/NMC.0000000000000296
91. Fehring RJ, Schneider M, Raviele K, Barron ML. Efficacy of cervical mucus observations plus electronic hormonal fertility monitoring as a method of natural family planning. J Obstet Gynecol Neonatal Nurs. (2007) 36:152–60. doi: 10.1111/j.1552-6909.2007.000129.x
92. Bhargava H, Bhatia JC, Ramachandran L, Rohatgi P, Sinha A. Field trial of Billings ovulation method of natural family planning. Contraception. (1996) 53:69–74. doi: 10.1016/0010-7824(95)00269-3
93. Trussell J, Grummer-Strawn L. Contraceptive failure of the ovulation method of periodic abstinence. Fam Plann Perspect. (1990) 22:65–75. doi: 10.2307/2135511
94. Howard MP, Stanford JB. Pregnancy probabilities during use of the Creighton Model Fertility Care System. Arch Fam Med. (1999) 8:391–402. doi: 10.1001/archfami.8.5.391
95. Arévalo M, Jennings V, Nikula M, Sinai I. Efficacy of the new TwoDay Method of family planning. Fertil Steril. (2004) 82:885–92. doi: 10.1016/j.fertnstert.2004.03.040
96. Arévalo M, Jennings V, Sinai I. Efficacy of a new method of family planning: the Standard Days Method. Contraception. (2002) 65:333–8. doi: 10.1016/S0010-7824(02)00288-3
97. Burkhart MC, de Mazariegos L, Salazar S, Lamprecht VM. Effectiveness of a standard-rule method of calendar rhythm among Mayan couples in Guatemala. Int Perspect Sex Reprod Health. (2000) 26:131–6. doi: 10.2307/2648302
98. Pearson JT, Chelstowska M, Rowland SP, Mcilwaine E, Benhar E, Berglund Scherwitzl E, et al. Natural Cycles app: contraceptive outcomes and demographic analysis of UK users. Eur J Contracept Reprod Health Care. (2021) 26:105–110. doi: 10.1080/13625187.2020.1867844
99. Pearson JT, Chelstowska M, Rowland SP, Benhar E, Kopp-Kallner H, Berglund Scherwitzl E, et al. Contraceptive effectiveness of an FDA-Cleared birth control app: results from the Natural Cycles U.S. cohort. J Womens Health. (2021) 30:782–8. doi: 10.1089/jwh.2020.8547
100. Jennings V, Haile LT, Simmons RG, Spieler J, Shattuck D. Perfect- and typical-use effectiveness of the Dot fertility app over 13 cycles: results from a prospective contraceptive effectiveness trial. Eur J Contracept Reprod Health Care. (2019) 24:148–53. doi: 10.1080/13625187.2019.1581164
101. Lamprecht V, Trussell J. Natural family planning effectiveness: evaluating published reports. Adv Contracept. (1997) 13:155–65. doi: 10.1023/A:1006595703472
102. Fehring RJ, Schneider M, Barron ML, Pruszynski J. Influence of motivation on the efficacy of natural family planning. MCN Am J Matern Child Nurs. (2013) 38:352–8. doi: 10.1097/NMC.0b013e3182a1ecc0
103. Unseld M, Rötzer E, Weigl R, Masel EK, Manhart MD. Use of Natural Family Planning (NFP) and its effect on couple relationships and sexual satisfaction: a multi-country survey of NFP users from US and Europe. Front Public Health. (2017) 5:42. doi: 10.3389/fpubh.2017.00042
104. Colombo B, Mion A, Passarin K, Scarpa B. Cervical mucus symptom and daily fecundability: first results from a new database. Stat Methods Med Res. (2006) 15:161–80. doi: 10.1191/0962280206sm437oa
105. Frank-Herrmann P, Gnoth C, Baur S, Strowitzki T, Freundl G. Determination of the fertile window: reproductive competence of women–European cycle databases. Gynecol Endocrinol. (2005) 20:305–12. doi: 10.1080/09513590500097507
106. Hilgers TW. The identification of postovulation infertility with the measurement of early luteal phase (Peak Day +3) progesterone production. Linacre Q. (2020) 87:78–84. doi: 10.1177/0024363919885551
107. Leiva R, McNamara-Kilian M, Niezgoda H, Ecochard R, Bouchard T. Pilot observational prospective cohort study on the use of a novel home-based urinary pregnanediol 3-glucuronide (PDG) test to confirm ovulation when used as adjunct to fertility awareness methods (FAMs) stage 1. BMJ Open. (2019) 9:e028496. doi: 10.1136/bmjopen-2018-028496
108. Earle S, Marston HR, Hadley R, Banks D. Use of menstruation and fertility app trackers: a scoping review of the evidence. BMJ Sex Reprod Health. (2021) 47:90–101. doi: 10.1136/bmjsrh-2019-200488
109. Bouchard TP, Fehring RJ, Mu Q. Quantitative versus qualitative estrogen and luteinizing hormone testing for personal fertility monitoring. Expert Rev Mol Diagn. (2021) 21:1349–60. doi: 10.1080/14737159.2021.2000393
110. Lundgren R, Cachan J, Jennings V. Engaging men in family planning services delivery: experiences introducing the Standard Days Method® in four countries. World Health Popul. (2012) 14:44–51. doi: 10.12927/whp.2013.23097
111. Sinai I, Arévalo M. It's all in the timing: coital frequency and fertility awareness-based methods of family planning. J Biosoc Sci. (2006) 38:763–77. doi: 10.1017/S0021932005027227
Keywords: fertility awareness, women's health, family planning, infertility, menstrual cycle, fertility apps, natural family planning, reproductive health
Citation: Duane M, Stanford JB, Porucznik CA and Vigil P (2022) Fertility Awareness-Based Methods for Women's Health and Family Planning. Front. Med. 9:858977. doi: 10.3389/fmed.2022.858977
Received: 20 January 2022; Accepted: 20 April 2022;
Published: 24 May 2022.
Edited by:
Mary M. Schneider, Marquette University, United StatesReviewed by:
Rene Antonio Leiva, Bruyère Continuing Care, CanadaCopyright © 2022 Duane, Stanford, Porucznik and Vigil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marguerite Duane, RHJEdWFuZUBGQUNUU2Fib3V0RmVydGlsaXR5Lm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.