- 1Department of Rheumatology, Hospital Dos de Maig, Barcelona, Spain
- 2Department of Rheumatology and Autoimmune Diseases, Hospital Universitari de la Santa Creu i Sant Pau, Barcelona, Spain
- 3Department of Medicine, Universitat Autònoma de Barcelona (UAB), Barcelona, Spain
- 4Unidad de Investigación, Spanish Society of Rheumatology, Madrid, Spain
- 5Department of Cardiology, Hospital Universitari de la Santa Creu i Sant Pau, Barcelona, Spain
- 6Department of Cardiology, Hospital Universitari Bellvitge, Hospitalet de Llobregat, Universitat de Barcelona (UB), Barcelona, Spain
Objective: To evaluate the evidence regarding the prevalence and risk of bundle branch block (BBB), atrioventricular block (AVB) and pacemaker implantation (PMI) in patients with spondyloarthritis compared to a control group without spondyloarthritis.
Methods: A systematic review of the literature was performed using Pubmed (Medline), EMBASE (Elsevier) and Cochrane Library (Wiley) databases until December 2021. The prevalence and risk for AVB, BBB and PMI were analyzed. Cohort, case control and cross-sectional studies in patients ≥18 years meeting the classification criteria for spondyloarthritis were included. The Odds ratio (OR), risk ratio (RR), or Hazard ratio (HR) and prevalence difference were considered as outcomes. Data was synthesized in a previously defined extraction form which included a risk of bias assessment using the Newcastle-Ottawa Scale.
Results: In total, eight out of 374 studies were included. None of the studies provided results regarding the risk of low grade AVB and BBB in SpA patients. Only indirect results comparing prevalences from low to medium quality studies were found. According to population based registries, the sex and age adjusted HR of AVB was 2.3 (95% CI 1.6–3.3) in ankylosing spondylitis, 2.9 (95% CI 1.8–4.7) in undifferentiated spondyloarthritis and 1.5 (95% CI 1.1 a 1.9) in psoriatic arthritis. The absolute risk for AVB was 0.4% (moderate to high; 95% CI 0.34%-0.69%) for AS, 0.33% (moderate to high; 95% CI 0.21%-0.53%) for uSpA and 0.34% (moderate to high; 95% CI 0.26%-0.45%) for PsA.The RR for PMI in AS patients was 1.3 (95% CI 1.16–1.46) for groups aged between 65 and 69 years, 1.33 (95% CI 1.22–1.44) for 70–75 years, 1.24 (95% CI 1.55–1.33) for 75–79 years and 1.11 (95% CI 1.06–1.17) for groups older than 80 years. The absolute risk for PMI in AS patients was 0.7% (moderate to high risk; 95% CI 0.6–0.8%) for groups aged between 65–69, 1.44% (high risk; 95% CI 1.33–1.6%) for 70–75 years, 2.09% (high risk; 95% CI 2.0–2.2%) for 75–79 years and 4.15% (high risk; 95% CI 4.0–4.3%) for groups older than 80 years
Conclusions: Very few cases of low grade AVB and BBB were observed in observational studies. No study evaluated association measures for low grade AVB and BBB but the differences of prevalence were similar in SpA and control groups even though studies lacked the power to detect statistical differences. According to population based registries there was an approximately two fold-increased risk of high grade AVB in SpA patients. RR for PMI was higher in younger age groups.
Introduction
Over the last 100 years, cardiac vasculature, valves, myocardium, pericardium, and conduction system disorders were observed in inflammatory rheumatic disease. The cardiac diseases most commonly described were cardiovascular ischaemic events, valve diseases, ventricular dysfunction and conduction disorders (1). The common hypothesis is that systemic inflammation accelerates tissue degeneration which in turn causes cardiovascular diseases. Nevertheless, it is unknown whether the different inflammatory rheumatic diseases have tropism for a specific cardiac structure or tissue (1).
The aortic valve attachment site and peripheral entheses are known to share histological similarities (2). Two experimental studies observed that enthesis tissue resident T cells were also present in the aortic root and valve while being absent from the myocardium. Overexpression of interleukin-23 in vivo resulted in a dense infiltrate of T cells, macrophages and neutrophils in the attachment site of the aortic valve leading to the aortic wall, as well as in the enthesis (3, 4). According to those findings, inflammation of the valve attachment site may produce tissue degeneration near the atrioventricular node, which may lead to electrical conduction disturbancesdistrubances, that is to say atrioventricular block (AVB) and bundle branch block (BBB).
Among a sea of similar information, it is necessary to critically review the available evidence up-to-date and acknowledge the downsides to guide further investigation of each of the cardiac manifestations and inflammatory rheumatic diseases. We have previously carried out a systematic review of the literature of the prevalence and risk of heart valve and aortic involvement in spondyloarthritis (SpA) (5). Higher risk for valvular heart disease was observed in SpA patients and a with an approximately HR 1.97 adjusted by confounders and RR of 1.2. A a smallsmall increase was observed for aortic valve procedures RR 1.1–1.3 but not for mitral valve. Most studies were not adjusted by potential confounders.
The aim of this systematic review of the literature is to evaluate the prevalence and risk of bundle branch block (BBB), atrioventricular block (AVB) and pacemaker implantation (PM) in patients with spondyloarthritis compared to a control group without spondyloarthritis.
Materials and Methods
A systematic review was conducted to identify all studies published up to December, 2021. This review was guided by the preferred reporting items for systematic review and meta-analysis (PRISMA) statements (Supplementary Table 1).
Research Question
The research question was defined using PICO (Population, Intervention, Comparison, Outcome and Study design) components.
Are patients ≥ 18 years who have spondyloarthritis at an increased risk or higher prevalence for AVB, BBB and PMI compared with a control group that do not have spondyloarthritis?
Inclusion Criteria
We included studies that met the following requirements:
• Study population: patients older than 18 years, diagnosed as Spondyloarthritis disease classified by ASAS, ESSG, Amor, modified New York, ARA, Moll & Wright or CASPAR criteria. Also patients diagnosed with spondyloarthritis following International Classification of Diseases (ICD) codes, or with a diagnosis confirmed by grade II bilateral sacroiliitis or grade III or superior if unilateral sacroiliitis was identified by X-Ray; and/or HLAB27 positivity; and fulfilling two or more clinical characteristics (peripheral arthritis, enthesitis, dactylitis, uveitis, inflammatory back pain, psoriasis, inflammatory bowel disease, history of urethritis or infective diarrhea) were included.
• Intervention: studies containing information about atrioventricular block, left and right bundle branch block measured by electrocardiogram (ECG) or holter.
• Outcome variables: studies containing information about association measures such as incidence ratio (IR), odds ratio (OR), risk ratio (RR) and Hazard Ratio (HR) and comparison of prevalences.
• Study design: Systematic review of the literature, meta-analysis, case control, cohort, cross sectional studies with more than 100 patients or population based registries.
• Language: English, French, Korean and Spanish.
Exclusion Criteria
Studies carried out on pregnant women, animals or ethnic minorities. Abstracts, posters, narrative reviews, letters, editorials and any type of unpublished study.
Search Strategy
A systematic search strategy was performed using the databases of Pubmed (Medline), EMBASE (Elsevier) and the Cochrane Library (Wiley) by a librarian (MG). The search strategy included MeSH terms and free text using different combinations (Supplementary Figure 1). Additional references were manually retrieved by reviewing the references of the included studies. Four studies were not available mainly because of antiquity (Supplementary Figure 2). They were previously consulted in two different national university library sources and the documentary collection of the Spanish Society of Rheumatology. An update of the systematic research was performed in December 2021 before the submission of this manuscript.
Article Selection
A total of 373 citations were peer reviewed by two rheumatologists (HSP & AL). The reviewers independently performed two-stage screening (title/abstract and full-text screening), data extraction, and a risk of bias assessment. EndNote X8 software was used to manage the literature references. Those articles that were not considered relevant for further checking were excluded and the reason for exclusion were listed (Supplementary Figure 3). Senior methodologists (PDC & MAM) and a cardiac electrophysiologist (CAM & JSV) were consulted to retrieve clinically relevant data and for the interpretation of the results.
Data Extraction and Data Analysis
For data extraction, a previously designed form in Word® format was used. Data collected from each study were: country of study, design, sample size, participant selection, period of recruitment, follow-up period, method applied for conduction disorder diagnosis, magnitude of association and confounder factors. Prevalence odds ratio (POR), OR or comparison of proportions were calculated with the information available if necessary. Data was extracted by one reviewer (HSP) and supervised by another reviewer (AL). Disagreements between the reviewers were solved by discussion. When there was no consensus a third reviewer was consulted (PDC or CAM).
After gathering information, a narrative synthesis was preferred over a meta-analysis due to heterogeneity in population, design and/or the outcome measure of the included studies.
Risk of Bias
The risk of bias was assessed by two reviewers (HSP & AL) using the Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies. The NOS contains eight items with an overall minimum score of one and a maximum of nine stars. There are three main quality dimensions: (1) selection of the study population; (2) comparability between the groups; and (3) outcome or exposure measures for cohort and case– control studies, respectively. The overall scores given were categorized into high (8–9 stars), medium (6–7 stars) and low quality (≤5 stars). We also categorized studies according to exposure assessment quality into high (three stars), medium (two stars) and low quality (one star). The complete checklist used is included in the Supplementary Material.
Results
Study Selection
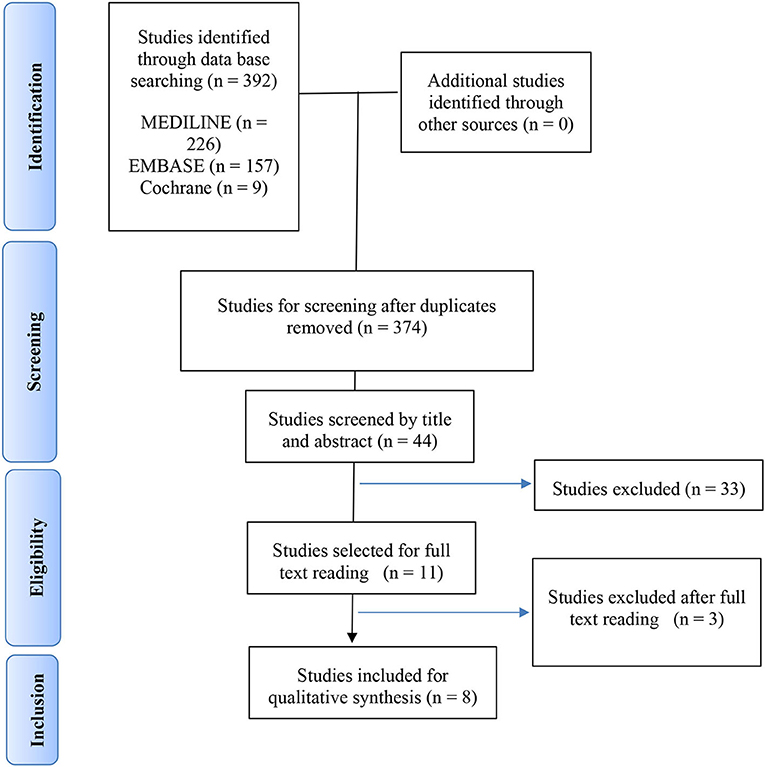
We selected 62 studies of the original 374 studies for further reading based on title and abstract screening. After excluding 53 studies following full text reading, we included eight studies in the analysis, as presented in the PRISMA flow-chart (Figure 1). The reasons for excluding the remaining studies after reading the full text are provided in Supplementary Figure 3. No additional study was retrieved after reviewing the bibliography of the articles included for a full text reading. The final selection included two population based cohort studies, two case control studies and four cross-sectional studies.
Characteristics of the Selected Studies
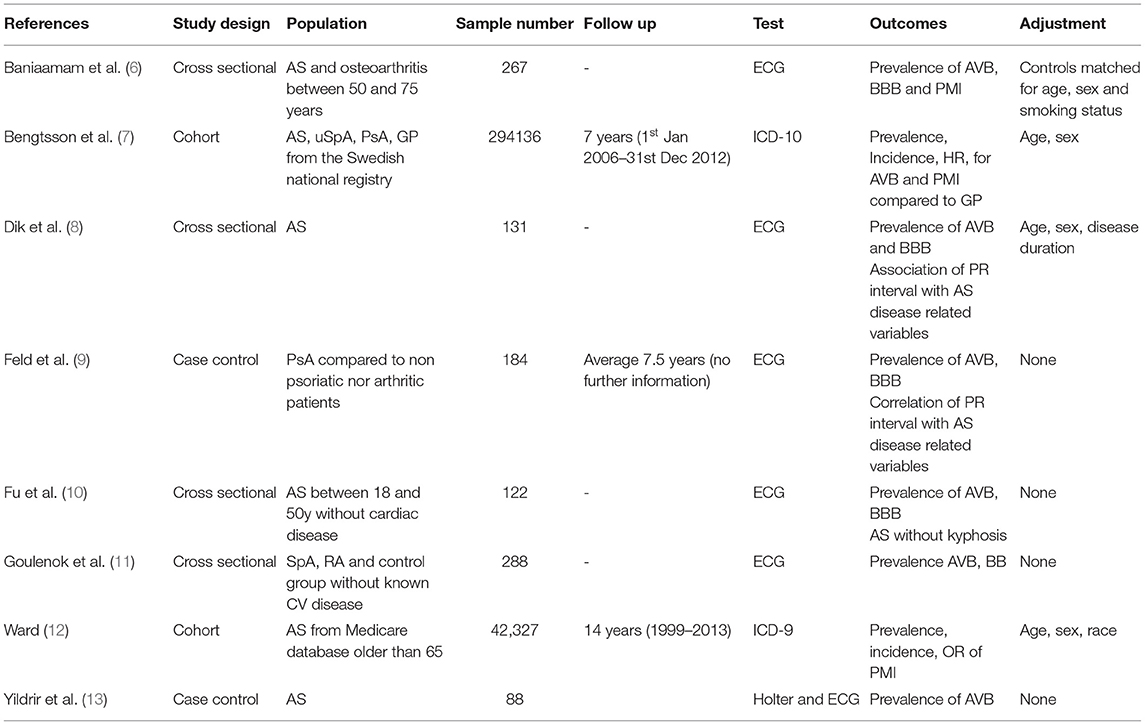
Detailed information regarding study design, location, sample size, follow up duration, exclusion and inclusion criteria, the methodology applied for the assessment of conduction disorder and outcome measures is summarized (Table 1).
Baseline Characteristics of the Participants
Mean age at enrollment was 33–54 years in five of the eight studies. Three studies were based on a specific age population: one study was based on patients between 50 and 75 years old (6), one study was based on patients older than 65 of age (12) and another study was based on patients between 18 and 50 of age (10). The proportion of women ranged from 8 to 72% depending on the type of spondyloarthritis. Mean disease duration was 3.9–22 years (6, 8, 9, 13). Hypertension was observed in 24.1–66% of patients (6–8, 11). Ischaemic heart disease was observed in 7.3–9% (6, 7, 9). Mean BASDAI was 3.1–5.9 (6, 8, 11). Uveitis was observed in 1.6–20.8% (7, 11, 13) depending on the type of spondyloarthritis.
Risk of Bias Assessment
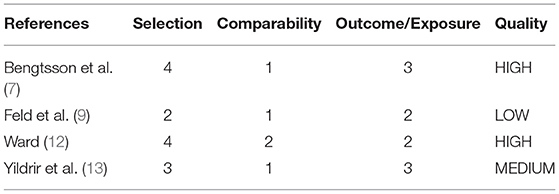
Four longitudinal observational studies were evaluated using the NOS scale (Table 2). One was of medium quality (13), another was of low quality (9) as the other two studies were of high quality. One study showed a possible selection bias as eligibility was not clearly described (9). All studies were matched or adjusted by sex and age but none evaluated possible confounding factors. Only one study also adjusted for race (12). Two studies did not provide information about loss to follow-up (12) or nonresponse rate (9).
None of the four cross sectional studies evaluated possible confounding factors. The statistical method was not clearly described in two studies (8, 11).
Description and Analysis of the Results
Five studies reported data regarding the risk of spondyloarthritis for bundle branch block, atrioventricular block or pacemaker implantation when compared to the population without spondyloarthritis.
Bundle Branch Block
No studies provided information regarding the risk of BBB in patients with SpA. Three studies compared the prevalence of BBB between SpA patients and control groups (6, 11, 13).
Only one or two cases of RBBB and LBBB were observed in the SpA group of each of the studies. The proportion of SpA patients with RBBB was between 0.9% and 1.14%, and the proportion of LBBB was between 1 and 2.7%. The proportion of control groups with RBBB was 0 and 4.1% (p = 1.0), and 0 and 2.7% for groups with LBBB (p = 0.1). In all studies the comparison of prevalence or the OR were neither clinically nor statistically significant.
Atrioventricular Block
Only one study provided information regarding the risk of high grade AVB in SpA (7). Three studies compared the prevalence of low and high grade AVB in SpA and control groups (6, 9, 11, 13).
When using (6, 9, 11) ECG the prevalence of SpA patients with 1st degree AVB was 1–2.8% and no case of 2nd/3rd degree AVB was observed in any of the studies. No case of AVB was observed in the control groups. In all studies the comparison of the prevalence or the OR were neither clinically nor statistically significant (p = 1.0).
When using a Holter monitor, transient cases of AVB were observed (13). The prevalence of SpA patients with 1st degree AVB was 3.41%, that of Mobitz II 2nd degree AVB was 1.13% and that of asymptomatic 3rd degree AVB was 1.13%. Only one person from control group had transient Mobitz I 2nd degree AVB but none other type of AVB was observed. Once again, the differences in prevalence and OR were neither clinically nor statistically significant (p = 0.56).
The risk of high grade AVB (2nd and 3rd degree) was evaluated using ICD-9 codes by a population based registry (7). The sex and age adjusted IR of AVB was 0.9 (95% CI 0.6–1.3) in AS, 1.2 (95% CI 0.5–1.9) in uSpA and 0.7 (95% CI 0.5–0.9) in PsA. The sex and age adjusted HR of AVB was 2.3 (95% CI 1.6–3.3) in AS, 2.9 (95% CI 1.8–4.7) in uSpA and 1.5 (95% CI 1.1 a 1.9) in PsA.
Pacemaker Implantation
Two population based registries evaluated the risk of pacemaker implantation in spondyloarthritis compared to a control group.
The incidence, absolute risk, IR (1,000 person-years) and HR adjusted for age and sex in the Swedish population were reported[|2]. The incidence (1,000 person-years) was 0.86 in AS, 0.61 in uSpA and 0.6 in PsA. The IR was 2.0 (95% CI 1.3–2.7) in AS, 1.5 (95% CI 0.7–2.2) in uSpA and 1.5 (95% CI 1.2–1.8) for PsA. The absolute risk for AVB was 0.4% (moderate to high; 95% CI 0.34–0.69%) for AS, 0.33% (moderate to high; 95% CI 0.21–0.53%) for uSpA and 0.34% (moderate to high; 95% CI 0.26–0.45%) for PsA. The sex and age adjusted HR was 2.0 (95% CI 1.6–2.8) in AS, 1.9 (95% CI 1.2–2.8) in uSpA and 1.6 (95% CI 1.3–1.9) for PsA.
Incidence, absolute risk, OR and RR adjusted for sex and race were reported in an American AS population older than 65 years of age stratified by age intervals (12). The incidence (1,000 person-years) was 3.59 (95% CI 3.26–4.10) for the group aged between 65 and 69 years, 6.68 (95% CI 6.28–7.36) for the 70–75 group, 10.04 (CI 95% 9.66–11.04) for the 75–79 group, 14.6 (95% CI 14.21–15.61) for groups older than 80 years of age. The absolute risk for PMI in AS patients was 0.7% (moderate to high risk; 95% CI 0.6–0.8%) for groups aged between 65 and 69, 1.44% (high risk; 95% CI 1.33–1.6%) for 70–75 years, 2.09% (high risk; 95% CI 2.0–2.2%) for 75–79 years and 4.15% (high risk; 95% CI 4.0–4.3%) for groups older than 80 years. The OR was 1.38 (95% CI 0.97–1.96) for the group aged 65–69 years, 1.28 (95% CI 0.82–1.67) for the 70–75 group, 1.18 (95% CI 0.94–1.48) for the 75–79 group, and 1.23 (95% CI 1.1–1.39) for the group older than 80 years of age. The RR was 1.3 (95% CI 1.16–1.46) for the group aged between 65 and 69 years, 1.33 (95% CI 1.22–1.44) for the 70–75 group, 1.24 (95% CI 1.55–1.33) for the 75–79 group and 1.11 (95% CI 1.06–1.17) for the group older than 80 years of age. The causes for PMI were sinoatrial dysfunction in 41.8%, AVB in 16.6% and atrial fibrillation in 7.6%.
Spondyloarthritis Related Prognostic Factors Associated to Conduction Disorders
Even though it was not the main objective of this systematic review, we synthesized the data retrieved on SpA disease related prognostic factors for AVB or BBB. Three studies evaluated indirect association (8–10).
Two studies evaluated AVB by studying the possible association between the clinical factors of SpA and PR interval duration (8, 9). PR interval duration was associated with age (B = 0.6, p = 0.001), disease duration (B = 0.75, p ≤ 0.001) and BMI (B = 1.23, p = 0.016) by univariate analysis (8). Age and disease duration was statistically significant by multivariate analysis but no further data was provided. On the contrary another study did not find any correlation with disease duration, subtype of PsA or uveitis but the authors concluded that the sample size was too small to detect possible associations (9).
The prevalence of RBBB was similar in AS patients between 18 and 50 years of age with and without kyphosis due to more evolved disease (10). In patients with kyphosis 3 (5.3%) cases of RBBB were observed while in patients without kyphosis four cases (6.2%) were observed. The differences in prevalences were neither clinically nor statistically significant (p = 0.86).
Discussion
Summary of the Main Findings
The main findings of this systematic review showed that there are no significant differences in prevalences of BBB and low grade AVB in SpA patients when compared to a control group. The risk of high grade AVB and PMI was approximately double-fold increased in all types of SpA adjusted for sex and age according to a population based registry. The risk of PMI in AS population older than 65 years was smaller lower according to another population based registry possibly because PMI risk is increased in older populations. The RR was about 1.3 but showed a decreasing tendency with older age intervals adjusted for sex and race.
BBB
Isolated BBB is a conduction delay that does not require treatment in itself (14). The prevalence of BBB is difficult to estimate because it is frequently underdiagnosed in asymptomatic patients. In the Framingham study (15) the prevalence of BBB was between 0.5 and 1% in subjects under 50 years of age but prevalence was almost 11% in subjects between 80 and 90 years of age.
In this systematic review, no study that evaluated risk of BBB in SpA was found. Two cross sectional and case control studies provided indirect evidence that compared prevalence in SpA group and control group. Very few cases were observed in each group. The sample sizes were not adequate for evaluating the low prevalence of BBB in the young population under 65. Also important information that may have shed some light on, such as cardiovascular risk factors, cardiac disease, drugs and SpA disease related characteristics were missing.
AVB and PM
First degree, Mobitz I and transient AVB are asymptomatic and benign so they may not be detected if not sought. The prevalence of low degree AVB increases at older age (16, 17) and may progress over time to a higher grade AVB. The overall prevalence for low grade and transient AVB was around 1–3%, The prevalence of low grade AVB was similar in control groups according to two case control studies. Once again, due to the scarce number of cases, small sample size and lack of confounder analysis, the results do not allow us to draw conclusions about the risk of SpA for low grade AVB. It would be of interest to evaluate if SpA patients present AVB at a younger age and detect progression to a higher grade AVB through a longitudinal cohort study with a long follow-up.
In contrast, high grade AVB (Mobitz II or 3rd degree) (16) is a potentially life-threatening conduction disease that may lead to a cardiac arrest. High grade AVB usually requires hospitalization for PM implantation (18). Thus a population based registry based on discharge information would adequately reflect the real incidence and risk of high grade AVB if adjusted for appropriate confounders. According to the results of this systematic review, there is a two-fold increase in the risk of high grade AVB in the SpA population compared to the control population according to a population based registry (7). Accordingly, the same study also observed a two-fold increase in the risk of PM in the SpA population compared to the control population (7).
Another registry based on population older than 65 years of age (12) did not observe a relevant risk of PMI (RR between 1.1 and 1.3) compared to the control population. Interestingly, this study also showed a decreasing risk in higher age intervals practically matching the control population in populations older than 80 years of age. High grade AVB and PM implantation are conditions that occur in older age. According to these studies, it may be speculated that in SpA patients high grade AVB and PM implantation occur at younger ages. However when compared to a longitudinal cohort study conducted in a hospital setting, population registry sources lack clinical follow-up information which is essential to study confounders. Results must be adjusted for drugs, cardiovascular risk factors or previous cardiac diseases.
SpA Related Prognostic Factors for Conduction Disorders
As for possible SpA related prognostic factors for conduction disorders, only one study provided some relevant information. However, this study evaluated indirect outcome measures using the PR interval. The PR interval was associated with age and disease duration when adjusted for in a multiple regression (8). Other studies were not adjusted and had a very small number of cases.
Strength and Limitations
This systematic review aimed to verify and challenge rooted concepts using an exhaustive bibliographic search of the existing literature without time limit. This is the first systematic review of the literature to evaluate the prevalences and risk of AVB, BBB, and PMI in SpA. Meta-analysis was not carried out due to the heterogeneity of the population, design, diagnostic test and outcome measures of the few studies retrieved. Indirect outcomes were reported such as comparisons of prevalence with the aim of being as comprehensive as possible given the information provided even though most studies lacked sufficient statistical power.
Conclusions
None of the studies provided results about the risk of low grade AVB and BBB in SpA patients. Indirect results comparing prevalence from low to medium quality studies were found. These studies conclude that there was no difference in prevalence of low grade AVB and BBB in SpA patients when compared to control groups. According to population based registries, a higher risk of high degree AVB and PM implantation in younger aged SpA patients compared to control groups when adjusted by age and sex. However these results were not adjusted by clinically relevant confounders.
The majority of the studies that evaluate the association between cardiac diseases and inflammatory rheumatic diseases are small scaled observational studies or population based registries. Small scaled studies may not have sufficient power to detect differences of an infrequent manifestation but may find small subclinical changes in an asymptomatic stage. Population based registries may be useful for studying infrequent manifestations but lack clinically relevant information to adjust for confounders. Prospective cohort studies with long follow-up are the most accurate design, as for example, the CARMA project (19) for cardiovascular mortality. However this kind of study is rare due to the considerable research effort and budget needed. Among a sea of similar information, it is necessary to critically review the available evidence to date and acknowledge the downsides to guide further investigation of each of the cardiac manifestations and inflammatory rheumatic diseases.
The results obtained in our review give rise to many questions that remain to be answered. We suggest that future research into on conduction disorder in SpA should look deeper into the influence of age and identify which are the prognostic factors. Further studies must analyze the severity of conduction disorders and evaluate the influence of obesity, cardiovascular risk factors and medication in collaboration with cardiologists. From then on studies may evaluate the usefulness of screening (ECG, Holter monitor or even wearable devices) for early detection of conduction disorders in patients that are at higher risk. the, the progression of low grade to higher grade AVB and screening with and identify possible prognostic factors.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
MG-R performed the systematic literature search. HP and AL were involved in data screening, extraction, analysis and drafted the manuscript. PD, HC, and MM contributed substantially to the study conceptions, design and critical revision of the article. JS-V and CA-M were consulted for interpretation of the extracted data as referent cardiologists. All authors read and approved the final manuscript.
Conflict of Interest
AL has received speaker fees/honoraria from Abbvie, Lilly, Novartis, Pfizer and UCB. HC has received speaker fees/honoraria from BMS, Gebro, MSD, Lilly, Novartis, Pfizer, Roche, Sanofi, and UCB, participated in consulting for Abbvie, Amgen, Biogen, Celgene, Gilead, Kern, Pfizer, and Sanofi.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank David Bridgewater and David Moore for the English editing of the article.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.851483/full#supplementary-material
Abbreviations
AS, Ankylosing Spondylitis; AVB, Atrioventricular Block BBB, Bundle Branch Block; BMI, Body Mass Index; ECG, Electrocardiogram ICD, International Classifica IR, Incidence Rate HR, Hazard Ratio LBBB, Left Bundle Branch Block PM, Pacemaker OR, Odds Ratio; ReA, Reactive arthritis; POR, Prevalence Odds Ratio PsA, Psoriatic Arthritis; RBBB, Right Bundle Branch Block RR, Risk Ratio SpA, spondyloarthritis; uSpA, undifferentiated spondyloarthritis.
References
1. Prasad M, Hermann J, Gabriel SE, Weyand CM, Mulvagh S, Mankad R, et al. Cardiorheumatology: cardiac involvement in systemic rheumatic disease. Nat Rev Cardiol. (2015) 12:168–76. doi: 10.1038/nrcardio.2014.206
2. Gensler LS. Axial spondyloarthritis: the heart of the matter. Clin Rheumatol. (2015) 34:995–8. doi: 10.1007/s10067-015-2959-1
3. Reinhardt A, Yevsa T, Worbs T, Lienenklaus S, Sandrock I, Oberdöfer L, et al. Interleukin-23-Dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol. (2016) 68:2476–86. doi: 10.1002/art.39732
4. Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. (2012) 18:1069–76. doi: 10.1038/nm.2817
5. Park HS, Laiz A, Sanchez-Vega J, Díaz Del Campo P, Martín-Martínez MA, et al. Valve abnormalities, risk factors for heart valve disease and valve replacement surgery in spondyloarthritis. a systematic review of the literature. Front Cardiovasc Med. (2021) 8:719523. doi: 10.3389/fcvm.2021.719523
6. Baniaamam M, Heslinga SC, Boekel L, Konings TC, Louis Handoko M, Kamp O, et al. The prevalence of cardiac diseases in a contemporary large cohort of dutch elderly ankylosing spondylitis patients— the cardas study. J Clin Med. (2021) 10:5069. doi: 10.3390/jcm10215069
7. Bengtsson K, Forsblad-d'Elia H, Lie H, Klingberg E., Dehlin M, Exarchou S, et al. Risk of cardiac rhythm disturbances and aortic regurgitation in different spondyloarthritis subtypes in comparison with general population: a register-based study from Sweden. Ann Rheum Dis. (2017) 77:541–8. doi: 10.1136/annrheumdis-2017-212189
8. Dik VK, Peters MJ, Dijkmans PA, Van der Weijden MA, De Vries MK, Dijkmans BA, et al. The relationship between disease-related characteristics and conduction disturbances in ankylosing spondylitis. Scand J Rheumatol. (2010) 39:38–41. doi: 10.3109/03009740903096101
9. Feld J, Weiss G, Rosner I, Rozenbaum M, Laor A, Rimar D, et al. Electrocardiographic findings in psoriatic arthritis: a case-controlled study. J Rheumatol. (2008) 35:2379–82. doi: 10.3899/jrheum.080314
10. Fu J, Wu M, Liang Y, Song K, Ni M, Zhang Y, et al. Differences in cardiovascular manifestations between ankylosing spondylitis patients with and without kyphosis. Clin Rheumatol. (2016) 35:2003−8. doi: 10.1007/s10067-016-3324-8
11. Goulenok TM, Meune C, Gossec L, Dougados M, Kahan A, Allanore Y, et al. Usefulness of routine electrocardiography for heart disease screening in patients with spondyloarthropathy or rheumatoid arthritis. Joint Bone Spine. (2010) 77:146–50. doi: 10.1016/j.jbspin.2010.01.001
12. Ward MM. Lifetime risks of valvular heart disease and pacemaker use in patients with ankylosing spondylitis. J Am Heart Assoc. (2018) 7:e010016. doi: 10.1161/JAHA.118.010016
13. Yildirir A, Aksoyek S, CAlguneri M, Oto A, Kes S, et al. Echocardiographic evidence of cardiac involvement in ankylosing spondylitis. Clin Rheumatol. (2002) 21:129–34. doi: 10.1007/s10067-002-8271-x
14. Glikson M, Niehlsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. (2021). ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA). Eur Heart J. (2021) 42:3427–520. doi: 10.1093/eurheartj/ehab699
15. Eriksson P, Hansson PO, Eriksson H, Dellborg M. Bundle-branch block in a general male population: the study of men born 1913. Circulation. (1998) 98:2494–500. doi: 10.1161/01.CIR.98.22.2494
16. Shan R, Ning Y, Ma Y, Liu S, Wu J, Fan X, et al. Prevalence and risk factors of atrioventricular block among 15 million Chinese health examination participants in 2018: a nation-wide cross-sectional study. BMC Cardiovasc Disord. (2021) 21:289. doi: 10.1186/s12872-021-02105-3
17. Upshaw Jr CB. Comparison of the prevalence of first-degree atrioventricular block in African-American and in Caucasian patients: an electrocardiographic study III. J Natl Med Assoc. (2004) 96:756–60.
18. Epstein AE, DiMarco JP, Ellenbogen KA, Estes III NA, Freedman RA, Gettes LS, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. (2013) 61:e6–75. doi: 10.1016/j.jacc.2012.11.007
19. Martín-Martínez MA, Castañeda S, Sánchez-Alonso F, García-Gómez C, González-Juanatey C, Sánchez-Costa JT, et al. Cardiovascular mortality and cardiovascular event rates in patients with inflammatory rheumatic diseases in the CARdiovascular in rheuMAtology (CARMA) prospective study-results at 5 years of follow-up. Rheumatology (Oxford). (2021) 60:2906–15. doi: 10.1093/rheumatology/keaa737
Keywords: spondylarthritis, spondyloarthritis, cardiac conduction system disease, pacemaker, epidemiology, systematic review
Citation: Park HS, Laiz A, Díaz del Campo P, Martín Martínez MA, Guerra-Rodriguez M, Alonso-Martin C, Sanchez-Vega J and Corominas H (2022) Prevalence and Risk for Bundle Branch Block, Atrioventricular Block and Pacemaker Implantation in Spondyloarthritis. A Systematic Review of the Literature. Front. Med. 9:851483. doi: 10.3389/fmed.2022.851483
Received: 09 January 2022; Accepted: 16 February 2022;
Published: 25 March 2022.
Edited by:
Clementina López-Medina, Reina Sofía Hospital, SpainReviewed by:
Michael T. Nurmohamed, VU Medical Center, NetherlandsJulianna Desmarais, Oregon Health and Science University, United States
Copyright © 2022 Park, Laiz, Díaz del Campo, Martín Martínez, Guerra-Rodriguez, Alonso-Martin, Sanchez-Vega and Corominas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hector Corominas, aGNvcm9taW5hc0BzYW50cGF1LmNhdA==
 Hye Sang Park
Hye Sang Park Ana Laiz
Ana Laiz Petra Díaz del Campo4
Petra Díaz del Campo4 Hector Corominas
Hector Corominas