
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Med. , 17 June 2022
Sec. Dermatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.851208
This article is part of the Research Topic Learning from Cutaneous Manifestations in Systemic Infectious Diseases View all 8 articles
Background: Extranodal natural killer/T-cell lymphoma, nasal type is a syndrome of middle face destruction with an association to Epstein-Barr virus. Fungi have been recovered from the diseased tissue now and then but were often seen as a lymphoma-associated secondary infection. However, there are ENKTL-NT cases with the recoveries of fungi and complete recovery with antifungal therapy, which are quite similar to rhino-orbital-cerebral mycosis (ROCM) that often confuses the physicians.
Methods: We searched Medline for English-language manuscripts limited to “human” and “case reports,” “letters,” “reviews,” and “clinical conferences” from 1966 to 2022. We used MeSH terms “lymphoma, extranodal nk-t-cell” [MeSH Terms] or “lethal midline granuloma” [MeSH Terms], in combination with MeSH terms “microbiology” [subheading] or “microbiology” [all fields] or “fungi” [all fields] or “fungi” [MeSH Terms] for ENKTL-NT with infections. We used MeSH terms “Mycoses” in combination with “Nose” [Mesh] OR “Orbital Diseases” [Mesh] for rhino-orbital-cerebral fungal infections.
Results: We appraised 149 included articles and extracted references related to ENKTL-NT and/or ROCM. Themes and subcategories were subsequently derived. Our findings revealed that ROCM and ENKTL-NT are characterized by progressive and destructive ulcers in the midline face or rhino-orbital structures. ROCM is mainly caused by fungi in the order of Mucorales, and ENKTL-NT is usually associated with Epstein-Barr virus and sometimes fungi. Radiologically, both are characterized by non-specific features of sinusitis, soft tissue infection, and necrosis. Pathologically, ROCM and ENKTL-NT share the same characteristics of inflammation, necrosis, and granuloma. ROCM is characterized by the detection of fungi in tissue, while ENKTL-NT is typically positive for NK/T-cell markers and cytotoxic granule-associated proteins, proliferation, and vascular damage of angioinvasion, which could be incited by Mucor irregularis and Rhizopus arrhizus in patients and mice.
Conclusion: ENKTL-NT and ROCM share many similarities in clinical presentations, radiology, and histopathology, and might have the same etiology. This may explain why the two diseases are tangled together in the reported cases, and suggests the role that the fungi may play in the development of these ENKTL-NT/ROCM diseases. The reason why ENKTL-NT and ROCM are sometimes confused is that the main pathogens of ROCM, Mucor irregularis and Rhizopus arrhizus, are the fungal causative agents of ENKTL-NT.
Extranodal natural killer or/and T-cell lymphoma, nasal type (ENKTL-NT, syn. lethal midline granuloma, LMG) is a clinic-pathological entity characterized by progressive midline necrosis syndrome (MNS) that is predominantly reported in East Asia and parts of Central and South America with high mortality rate. Advanced ENKTL-NT is highly aggressive with survival periods spanning from days to months (1–3). The disease was first described as malignant granuloma associated with the rapid destruction of the face and nose by Mcbride in 1897 (4, 5). Since then, it had been renamed as non-healing granuloma, granuloma gangraenescens, malignant granuloma, idiopathic midline destructive disease, midline granuloma syndrome (MGS), and lethal midline granuloma, all of which are descriptive names with unknown etiological factors (6–9). In histology, the disease is described as inflammatory necrosis, associated with polymorphic cell infiltration and granulation that are mainly Wegener’s type, Stewart’s type, or Tsokos’s type (10, 11). In 1982, Ishii et al. first introduced the term “nasal T-cell lymphoma” when he observed T cells in the lesion, but later he named it as angiocentric T-cell lymphoma with vascular infiltration (12). Since 1999, the disease has been designated as an NK/T-cell lymphoma and is formally termed as “extranodal natural killer NK/T-cell lymphoma, nasal type (ENKTL-NT),” as the pleomorphic cells often express natural killer cells, and less frequently, T-cell markers with atypical cell type (3, 13). Therefore, it was formally incorporated into the classification of hematologic malignancies by World Health Organization (WHO) (3, 14). However, the diagnosis and treatment for ENKTL-NT have been challenging for physicians, as some cases resolved without any therapy (15) or without anticancer therapy and with antifungal therapy only, similar to primary rhino-orbital-cerebral mycosis (ROCM) (16, 17).
Similar to ENKTL-NT, ROCM is also characterized by progressive destruction of the sinus, nose, face, palate, orbit, and other midline structures; proptosis, ptosis, ophthalmoplegia, eyeball necrosis, and vision loss; and pathological features of inflammation, necrosis, and granulation (18, 19). ROCM is featured by the detection of fungi in the tissue, while ENKTL-NT is typically associated with NK/T-cell infiltration, hyperplasia, and angiocentric characteristics. Diagnosis is often confusing when both these features are present in the tissues of patients with facial destruction (16, 20, 21).
In published reports, there were ENKTL-NT patients with fungus recovery who continued to accept anticancer therapy and got worse or even died (2, 22). There were also ENKTL-NT patients who received chemotherapy, died, but turned out to have Mucorales fungal infection in the post-mortem of the nasal cavity (23, 24). In some ROCM patients, the detection of NK cells or/and T cells in tissue led to the change of diagnosis from ROCM to ENKTL-NT and then ended with fatal outcomes (1, 25). A physician reported two ROCM cases with NK/T cells and angiocentric characteristics. Diagnosis for one patient was from ENKTL-NT to ROCM, as fungal mycelium was seen and recovered from tissue, while the diagnosis for the other was from ROCM to NK/T-cell lymphoma because the NK/T cells were seen in the tissue. All these findings suggest an ambiguous understanding of the role of fungi in the development of ENKTL-NT (16, 21).
In the cases that we reported in 2012 and 2021, Mucor irregularis and Rhizopus arrhizus were identified as the cause of ENKTL-NT, and the patients got complete remission with amphotericin B (AMB) (16). Systemic literature reviewing study showed that M. irregularis and Rhizopus arrhizus infections in the central face share the same clinic-pathological characteristics with ENKTL-NT. Following our report, some dermatologists presented similar MNS cases, which were confirmed as M. irregularis-associated ROCM infections, and all achieved complete remission after receiving AMB (26, 27). With all the above-mentioned clinical evidence and relevant pathohistological and immunohistological information, ENTL-NT and ROCM seem to be tangled in terms of etiology, histopathology, and immunohistology, and the role of fungal infection has not yet been clarified and needs further investigation. We hereby reviewed comprehensive literature in order to explore the etiology, clinical presentation, histopathology, immunohistology, diagnosis, and treatment of the two diseases, the role of fungal infection, and challenges in the diagnosis of ENTL-NT and ROCM.
We searched Medline for English-language manuscripts limited to “human” and “case reports,” “letters,” “reviews,” and “clinical conferences” from 1966 to 2022. We used MeSH terms “lymphoma, extranodal nk-t-cell” [mesh terms] or “lethal midline granuloma”[MeSH Terms], in combination with MeSH term “microbiology” [subheading] or “microbiology” [all fields] or “fungi” [all fields] or “fungi” [mesh terms] for ENKTL-NT with infections. We also used MeSH terms “Mycoses” in combination with “Nose” [Mesh] OR “Orbital Diseases” [Mesh] for rhino-orbital-cerebral fungal infections. The results were combined with “humans” [mesh terms] for cases of human infections.
We appraised 149 included articles and extracted references related to ENKTL-NT and ROCM. Themes and subcategories were subsequently derived. Our findings revealed that the available literature describes that ENKTL-NT has many similarities to ROCM described in the existing literature, particularly that ROCM is caused by Mucor irregularis or Rhizopus arrhizus.
The etiological agent of ENKTL-NT has been known to be the Epstein-Barr virus (EBV) since Harabuchi et al. first showed the presence of EBV DNA and EBV nuclear antigen in the lymphoma cells obtained from five patients with ENKTL-NT and suggested that lethal midline granuloma is causally associated with EBV (28), which is regarded to be highly correlated with ENKTL-NT, and positive EBV is one of the diagnostic criteria according to the current understandings (3, 28–33). However, there were EBV-negative cases that had fatal outcomes (34–36). A recent meta-analysis of the association between EBV and ENKTL-NT showed that anti-EBV pre-treatment does not result in any benefit in the prognosis of patients with EBV-positive lymphoma but may bring an adverse effect on the survival outcome (31), while another research says that the results are controversial (37). Moreover, seroepidemiologic studies revealed that the worldwide seroprevalence of EBV infection is as high as 90% (38). These findings support doubts that EBV infection alone may be insufficient for tumor development.
The etiological agents of ROCM are a variety of, mostly fast-growing, fungi belonging to the order Mucorales (18, 19, 39, 40), which gave rise to its original name rhino-orbital-cerebral-mucormycosis (41, 42). As more patients were diagnosed, infections were found to be caused by fungi belonging to the class Zygomycetes (43), including Rhizopus arrhizus (Rhizopus oryzae) (40, 43, 44), R. homothallicus (45), R. rosporus var. oligosporus (46), R. microsporus var. rhizopodiformis (46), Mucor irregularis (16, 27, 26, 47), Apophysomyces elegans (41, 48–50), A. variabilis (48), Cunninghamella bertholletiae (40, 43, 51), Lichtheimia ramosa (Absidia corymbifera) (52), L. hongkongensis (53), Syncephalastrum racemosum (54), Saksenaea vasiformis (55–59), and Cokeromyces recurvatus (60). The disease was then renamed as rhino-orbital-cerebral zygomycosis, but then infections were identified with fungi outside Zygomycetes, even those in different phyla, such as Basidiobolus ranarum, Conidiobolus coronatus (61), C. incongruous (62, 63), Aspergillus flavus, A. fumigates, A. granulosus (64, 65), Alternaria infectoria (19), Arthrographis kalrae (66), or Schizophyllum commune (67). The disease was then renamed as rhino-orbital-cerebral mycosis. Research in 2014 (19) showed that the pathogenic fungi of ROCM have increased to 37 species in 12 orders, but the list expanded to 47 species in 15 orders with the addition of Tilletiopsis minor (68), Saksenaea erythrospora (69), Pleurostomophora richardsiae (70), Acremonium, Phoma sp. (71), Apophysomyces ossiformis (72), and Scedosporium apiospermum (73), Aspergillus nominae (74), Mucor menace (75), and Lichtheimia ornate (76) (Table 1).
Interestingly, Mucoralean fungi are frequently seen or isolated in ENKTL-NT cases (2, 16, 21, 22, 77, 78). In some patients, the detection of NK cells or/and T cells in the tissue led to the change of diagnosis from ROCM to ENKTL-NT (1, 25), or from ENKTL-NT to ROCM when fungal elements were observed (21), or ENKTL-NT patients died after chemotherapy due to the detection of Mucorales fungal infection on a post-mortem of the nasal cavity (23).
The first confirmed association of fungal infection in LMG has long been traced back to 1965 when Cowen et al. reported midline granuloma syndrome (MGS, previous name of LMG) in a 22-year-old white male Colorado farmer who turned out to be positive for infection with Cephalosporium fungus. The fungus eroded through his hard palate, resulting in destructive changes in the maxilla and mandible, with systemic manifestations of fever, weight loss, and hepatosplenomegaly. Failure of response to surgical drainage, teeth extraction, and treatment with iodides and sulfa necessitated intensive treatment with amphotericin B for 2 months, after which cultures for Cephalosporium became negative (79).
In a case that we reported in 2012 (16), Mucor irregularis was identified in ENKTL-NT (20). The fungus eroded through his sinuses and hard palate, resulting in destructive changes in mid-face, maxilla, mandible, and partial orbit with systemic manifestations of fever, weight loss, hepatosplenomegaly, and anemia. Failure of response to treatment with itraconazole, terbinafine, and steroids, the patient achieved complete recovery with AMB for 2 months, after which cultures for M. irregularis became negative. In the subsequent mural experiments, it was revealed, for the first time, that M. irregularis could induce NK/T-cell aggregation and atypical hyperplasia in experimental mice (80).
More recently, we reported an R. arrhizus infection in a 35-year-old Chinese male with ENKTL-NT (17). The R. arrhizus eroded through his hard palate, sinuses, nose, and face, resulting in destructive changes in the maxilla and mandible, with systemic manifestations of fever, weight loss, and hepatosplenomegaly. Failure of response to teeth extraction, surgical drainage, and treatment with itraconazole, it necessitated intensive treatment with AMB for 2 months. The destructed lesion was completely healed and was successfully transplanted with a thick skin graft from his thigh for the reconstruction of the nose and chin. In subsequent mural experiments, the Mucoralean fungus R. arrhizus could induce the expression of NK/T-cell markers (CD3+, CD8+, CD56+, TIA1+, GZMB+, and PRF+), proliferation (KI67+), and angioinvasion, as seen in ENKTL-NT that was suggested as the cause of ENKTL-NT (17, 80).
The predominant risk factor for ROCM is uncontrolled diabetes mellitus, particularly for those patients suffering from diabetic ketoacidosis (1, 16, 19, 39, 40, 44, 46, 68, 81–89), followed by leukemia and other hematologic malignancies (39, 40, 54, 71, 82). Other predisposing factors are malignancy, organ transplant, chronic organ failure, steroid use, burns, immune deficiency, AIDS, addiction to drugs (90, 91), and latent COVID-19 infection (92).
Trauma, wounds, surgeries, and other lesions of the midline face, particularly those of the nose or larynx, tooth extractions, local skin lesions, or cleaning the ear canal are all entries that have been reported to facilitate the entry of fungi into the body (86, 88, 93–95). Rhinitis and sinusitis are the common precursors of ENKTL-NT (1, 2, 12, 16, 25, 87, 96, 97) and ROCM (19, 39, 40, 82, 90, 98).
Both in ENKTL-NT and ROCM, signs of sinusitis, such as fluid density, air-fluid level, sinus expansion, and diffuse mucosal thickening, can be seen in the sinuses with radiographs, CT, and MRI. Extensive soft tissue enhancement, medial wall necrosis, palate perforation, and bone erosion can be revealed in some patients (16, 19, 39, 99, 100).
Orbit opacity with enhanced mass in the cavernous sinus (CS) can be seen if orbit involvement develops. Enhancements of the superior rectus, medial rectus, and lateral rectus can be seen in patients with painful ophthalmoplegia (16, 19, 101).
Thrombosis of the cavernous sinus, visible as an enhanced mass or filling defect, is the striking feature in ROCM that can be seen in ENKTL-NT (Supplementary Figure 3) (16, 19). The thromboses may extend into the optic foramen, often with narrowed or enlarged lumen, or aneurysm. Most commonly enhanced mass image and occlusion can be seen within the CS and internal carotid artery that may extend to other brain artery branches (16, 19, 101).
Clinically, ENKTL-NT is characterized by midline necrosis syndrome (MNS), that is, progressive necrosis of the face, nose, and upper airways with symptoms of swelling, ulceration, and perforation; destruction of the central face, the nose, and the orbit; and tissue defection (5, 16, 90, 91, 102–105). The disease usually begins as sinusitis, rhinitis, and rhinorrhea with symptoms of nasal congestion, discharge, epistaxis, nasal pain, and occasionally loss of sense (9, 25, 96, 106, 1, 2). As it progresses, it often spreads to adjacent areas with typical signs of MNS (16, 91, 104, 107).
Rhino-orbital-cerebral mycosis (ROCM) is more likely characterized by rhino (facial)-orbital-cerebral mycosis syndrome (ROCMS), which is progressive rhinofacial destruction, palate perforation (sometimes associated with proptosis, ptosis, and ophthalmoplegia), and symptoms of the brain (19, 61, 85, 87, 89, 108). Initial signs include sinusitis, rhinitis with nasal stuffiness, epistaxis, and facial swelling with pain (83). As the infection progresses, it becomes progressively necrotic in the nose, face, and orbit, which are the signs of MNS. All of these are signs of ENKTL-NT and were seen in M. irregularis- and R. arrhizus-associated ENKTL-NT/ROCM cases (17, 19, 44, 85).
Necrosis and pleomorphic inflammatory granulation are the main features of ENKTL-NT and ROCM (10, 16, 18, 19, 109). Besides, ROCM is featured by the detection of fungi in the tissue (16, 19, 69), while ENKTL-NT is typically associated with NK/T cells, hyperplasia, and angiodestruction (3, 110, 13, 14, 33, 111). In some cases, both features were present in patients with MNS, which made the diagnosis rather confused (16, 20–22, 27).
Necrosis, acute or progressive, focal or diffuse, commonly ischemic with inclusive vasculitis and giant cell infiltration, is the predominant feature of ENKTL-NT that may follow thrombosis or embolism (12, 16, 13, 19, 20, 112, 113). Sometimes, necrosis occurs suddenly like an acute vascular event (3, 9, 16, 114, 115).
Ischemic necrosis with inclusive vasculitis, thrombosis, or embolism is usually seen in ROCM (39, 16, 19, 48, 51, 52, 62, 64, 116–118). Purulent masses can be detected in the cavernous sinus, ophthalmic artery, and other vessels with inflamed or infracted orbital or cerebral tissue or nerves (19). Under a microscope, angioinvasive fungus can be seen with endothelial injury, vessel wall necrosis, or thrombosis (19).
Pleomorphic inflammatory granulation is featured in ENKTL-NT involving predominantly giant cells, lymphocytes, plasma cells, and multinucleated giant cells (16). Sometimes, bacteria, actinomycetes, mycobacteria, and even Treponema pallidum may be detected when fungi are the most commonly isolated pathogens (16, 115, 119). Following an infection, inflammatory cell infiltration and granulation are seen in all the patients with ROCM (19). The inflammatory response comprises abundant giant cells, lymphocytes, plasma cells, and multinucleated giant cells, which may be associated with phagocytosed fungal hyphae or spores (19). Occasionally, microabscesses mixed with hyphae could be observed (19, 101, 120).
Angioinvasive, angiocentric, and angiodestructive infiltration is a well-known pathological feature of ENKTL-NT (13, 3, 12, 16, 18, 20, 106, 112, 113) and ROCM (Figures 1–3) (12, 13, 16, 19, 20, 39, 69, 88, 101, 112, 113). Sometimes, hyphae and spores were seen growing in the artery lumens, on the artery walls, and in the tissues along the destructed arteries (Figures 1–3). Fungal thromboses and artery inclusion were seen in the newly involved tissues, some of which were ischemic necrosis (Figures 5, 6) (16, 19). Meanwhile, fungal elements could be endocytosed and destructed by multinucleate macrophages and lymphoid cells. Angioinvasion, angiocentricity, angiodestruction, and onion-skin lesions could be observed involving destroyed artery, and sometimes hyphae could be seen on the artery wall (Figures 1–4) (9, 16, 121).
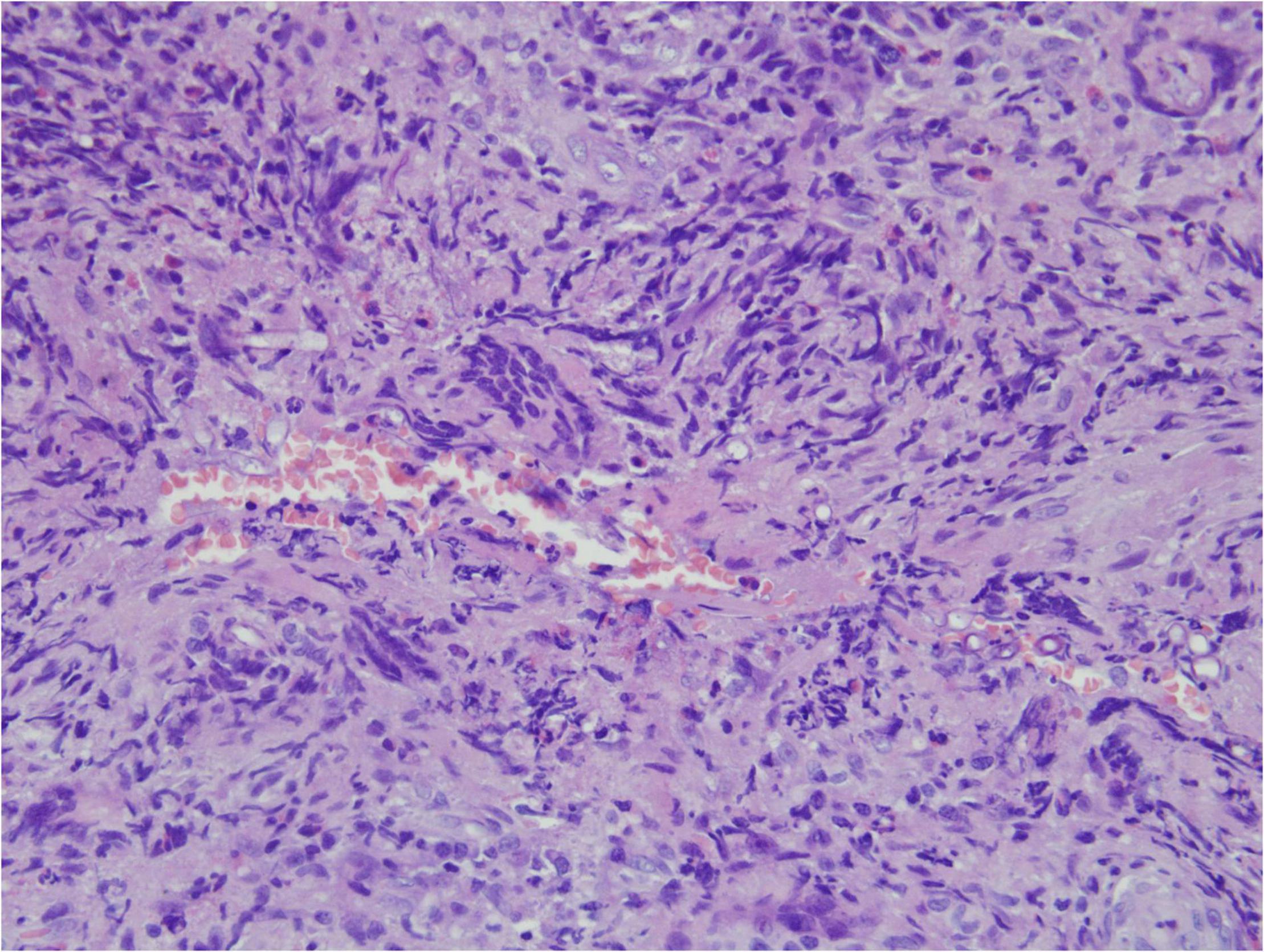
Figure 1. “Extranodal natural killer/T-cell lymphoma, nasal type/rhino-orbital-cerebral mycosis.” Mucor irregularis angioinvasion and angiodestruction (H&E, original magnification ×100).
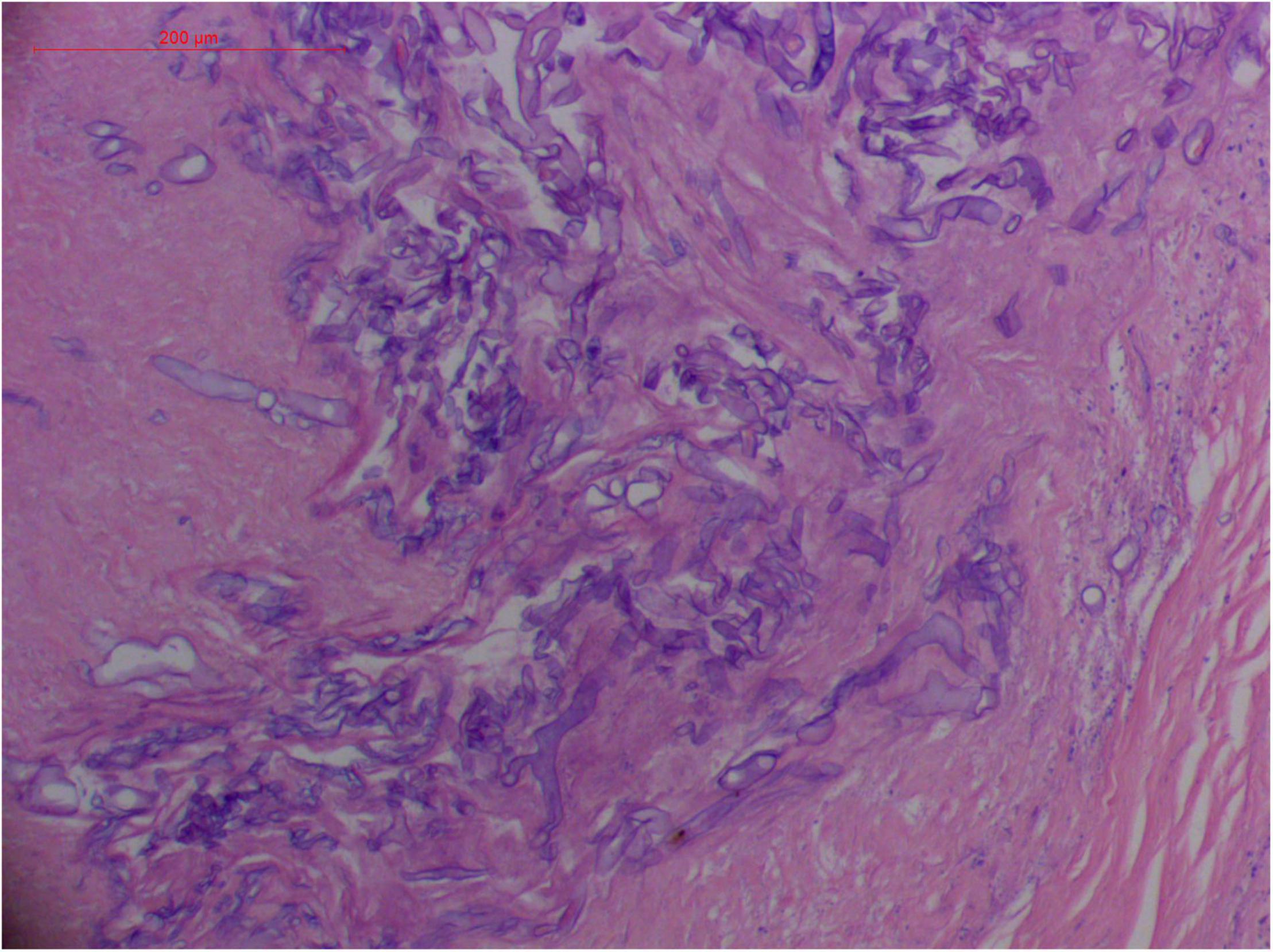
Figure 2. “Extranodal natural killer/T-cell lymphoma, nasal type/rhino-orbital-cerebral mycosis.” Broad thin-walled hyphae of Rhizopus arrhizus (H&E, original magnification ×400).
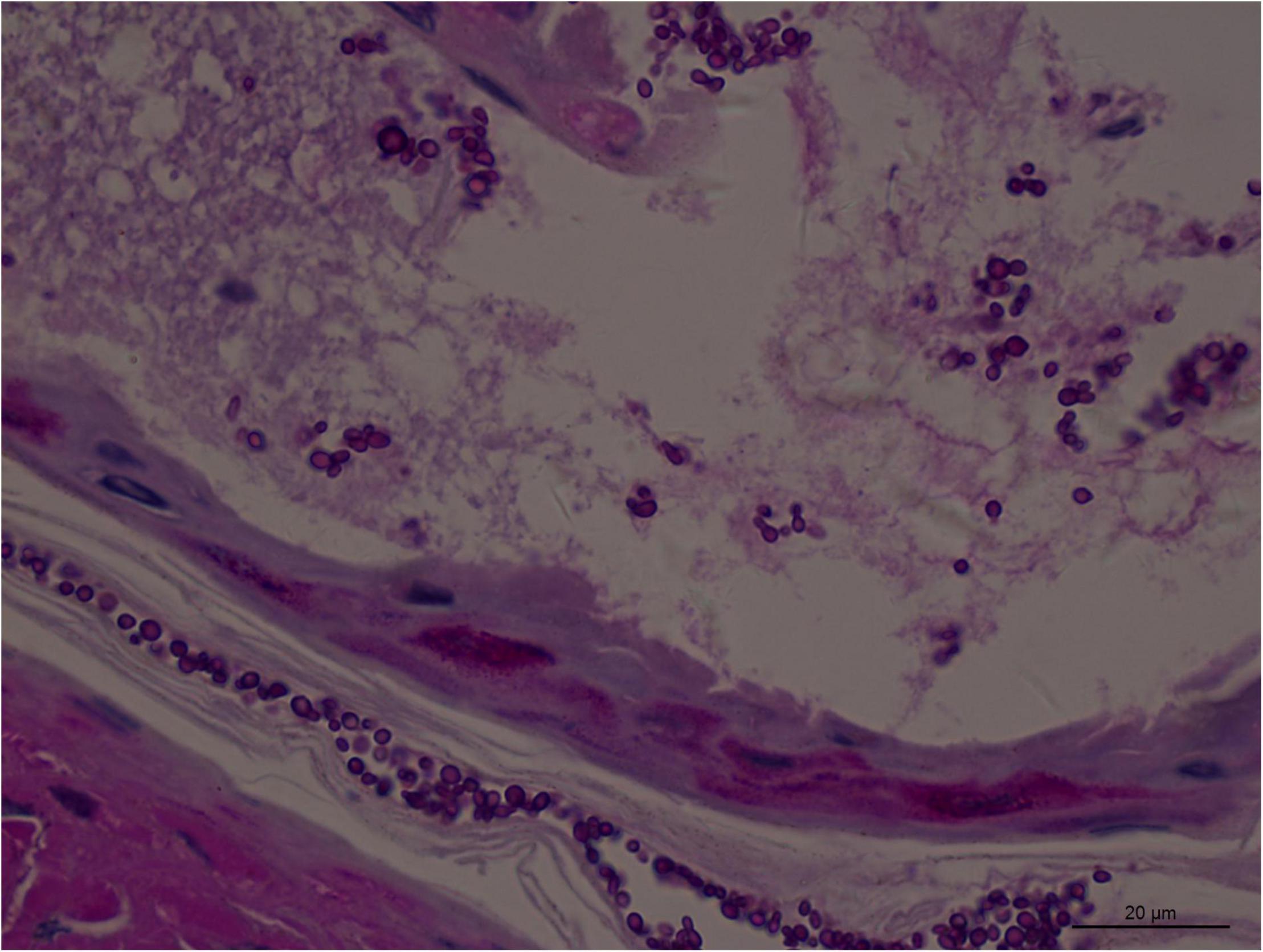
Figure 3. Numerous spores of Aspergillus sydowii in the artery lumen and artery wall in rhino-orbital-cerebral mycosis (periodic acid-Schiff staining, original magnification ×1,000).
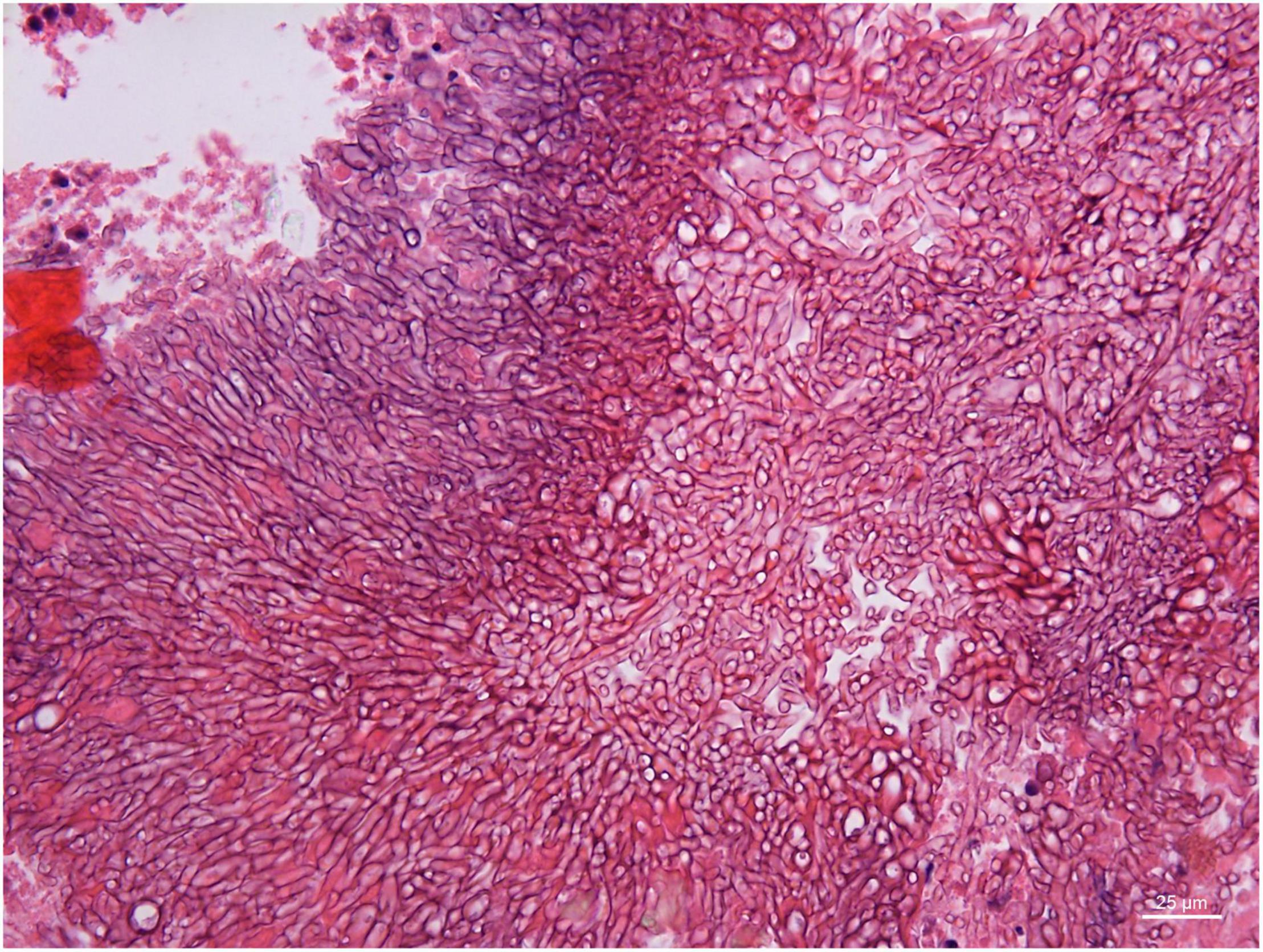
Figure 4. Branched and septate hyphae of Penicillium sp. in the cavernous sinus of rhino-orbital-cerebral mycosis (periodic acid-Schiff staining, original magnification ×400).
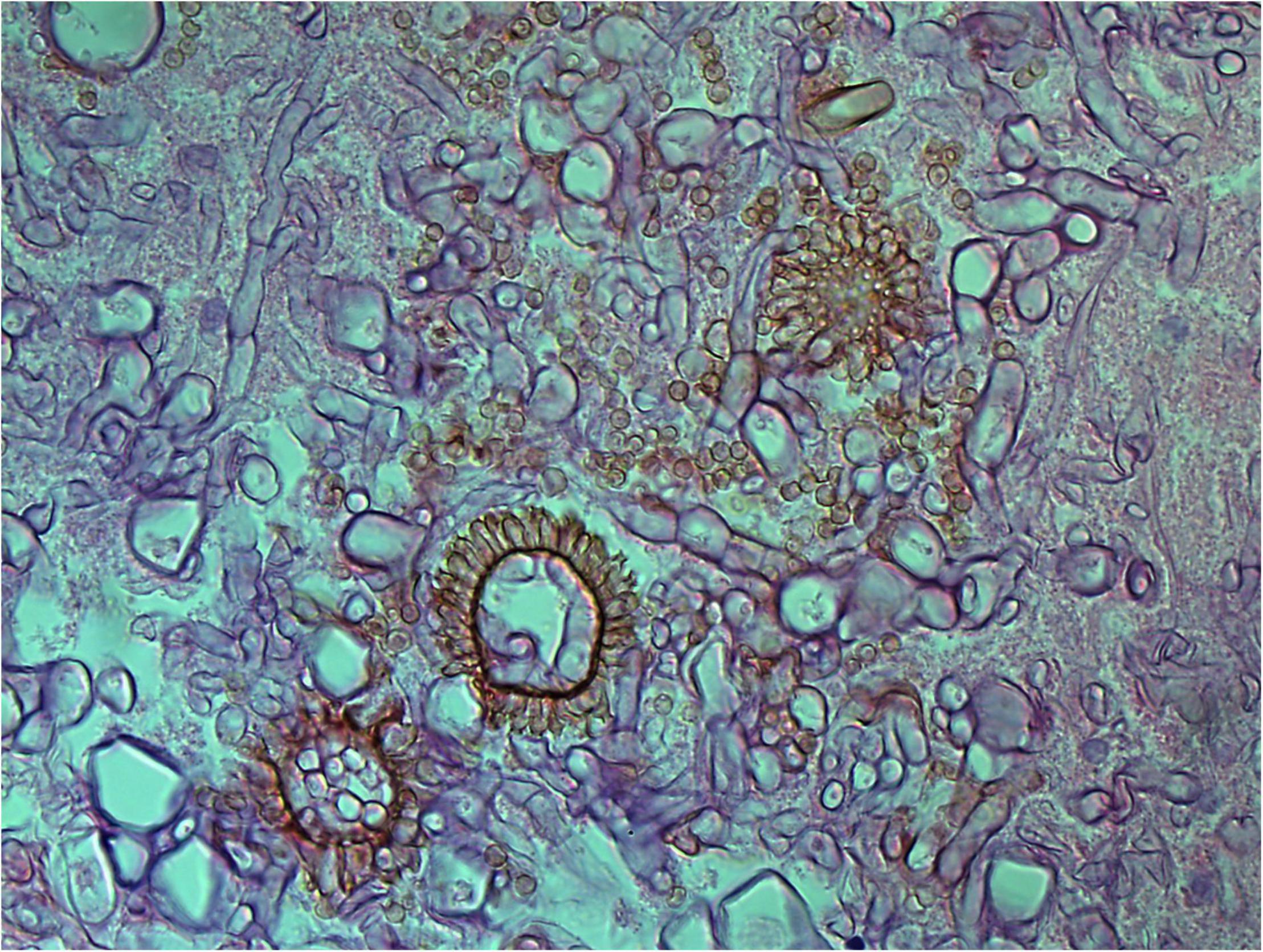
Figure 5. Aspergillus heads in the cavernous sinus of rhino-orbital-cerebral mycosis (periodic acid-Schiff staining, original magnification ×400).
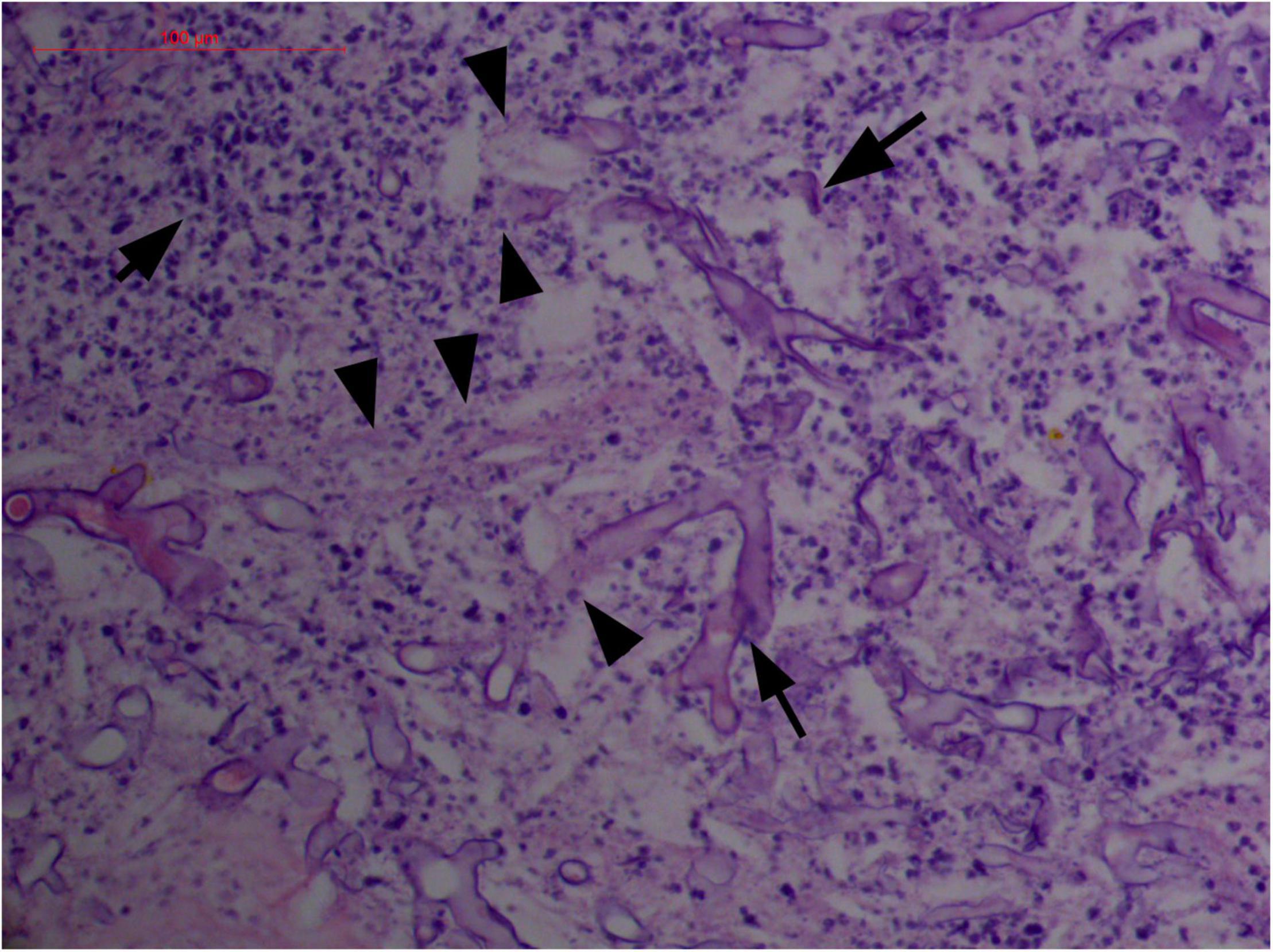
Figure 6. “Extranodal natural killer/T-cell lymphoma, nasal type-orbital-cerebral mycosis.” Distorted, broken, and disintegrated hyphae of Mucor irregularis (H&E, original magnification ×400).
On a hematoxylin-eosin stain, Mucorales would be seen as broad hyphae without septa that could be stained intensely with Periodic acid-Schiff, Grocott’s Methenamine Silver, or Congo red stain, or revealed by fluorescence microscopy after Blankophor staining (26, 39, 69, 85, 46, 16, 40). If branched and septate hyphae are present, the fungus is non-Mucorales, likely an Aspergillus sp., Alternaria spp., and other non-Mucoralean fungi (Figures 1–6) (19, 120).
Granulation with atypical hyperplasia with high Ki-67 expression is usually seen in ENKTL-NT that could either represent neoplastic proliferation (Figures 7–9) (9, 122, 123) or as a marker of activated NK and/or T cells in host defense against pathogens (124). Recently, we reported the inducement of proliferation in ENKTL-NT patients with M. irregularis or R. arrhizus infection (16) (Figures 7–9). We retrieved, from the peers, a series of reports about the proliferation of cytotoxic T lymphocytes induced by Staphyloccus aureus (125), Nocardia farcinica (126), Mycobacterium fortuitum, M. marinum (127), and Leishmania infantum (128), all of which were reported to induce ENKTL-NT-like syndrome (129–132). Still, another research reported the recovery of ENKTL-NT following treatment with sulfamethoxazole and levofloxacin when the infections were confirmed in the blood cultures (133).
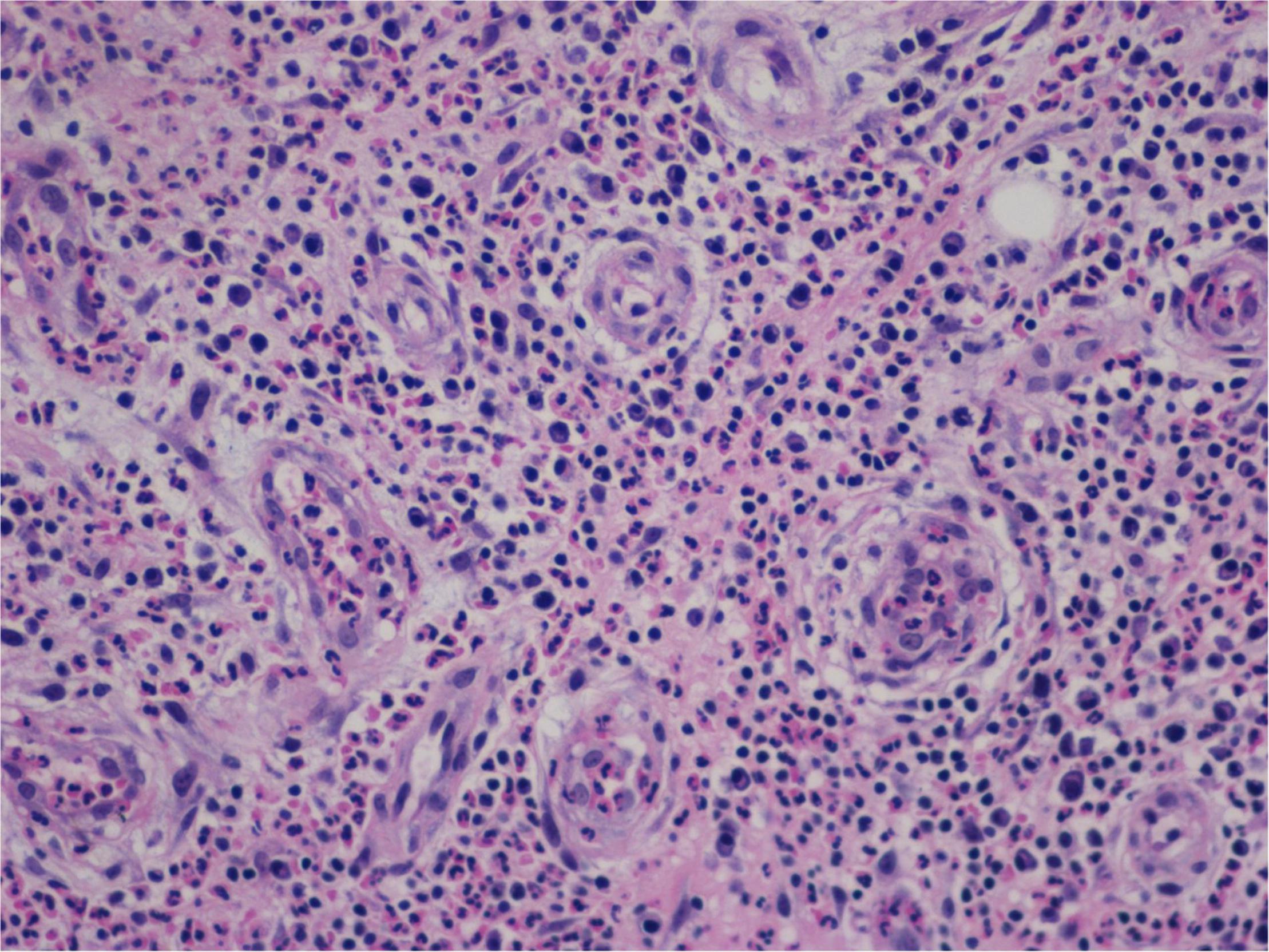
Figure 7. “Extranodal natural killer/T-cell lymphoma, nasal type-orbital-cerebral mycosis.” Mucor irregularis-induced atypical hyperplasia (H&E, original magnification ×200).
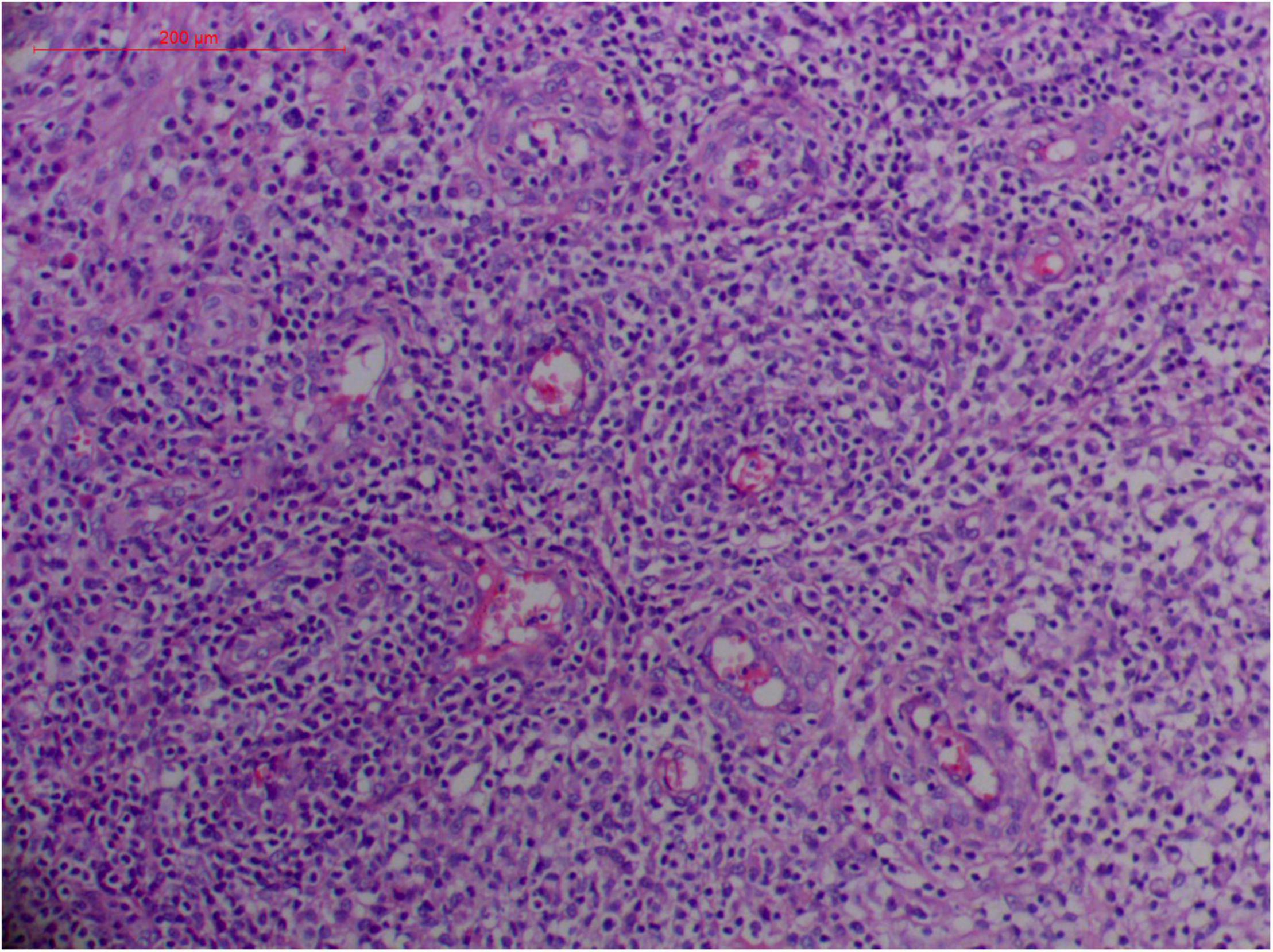
Figure 8. “Extranodal natural killer/T-cell lymphoma, nasal type/rhino-orbital-cerebral mycosis.” Mucor irregularis-induced angioinvasion (H&E, original magnification ×200).
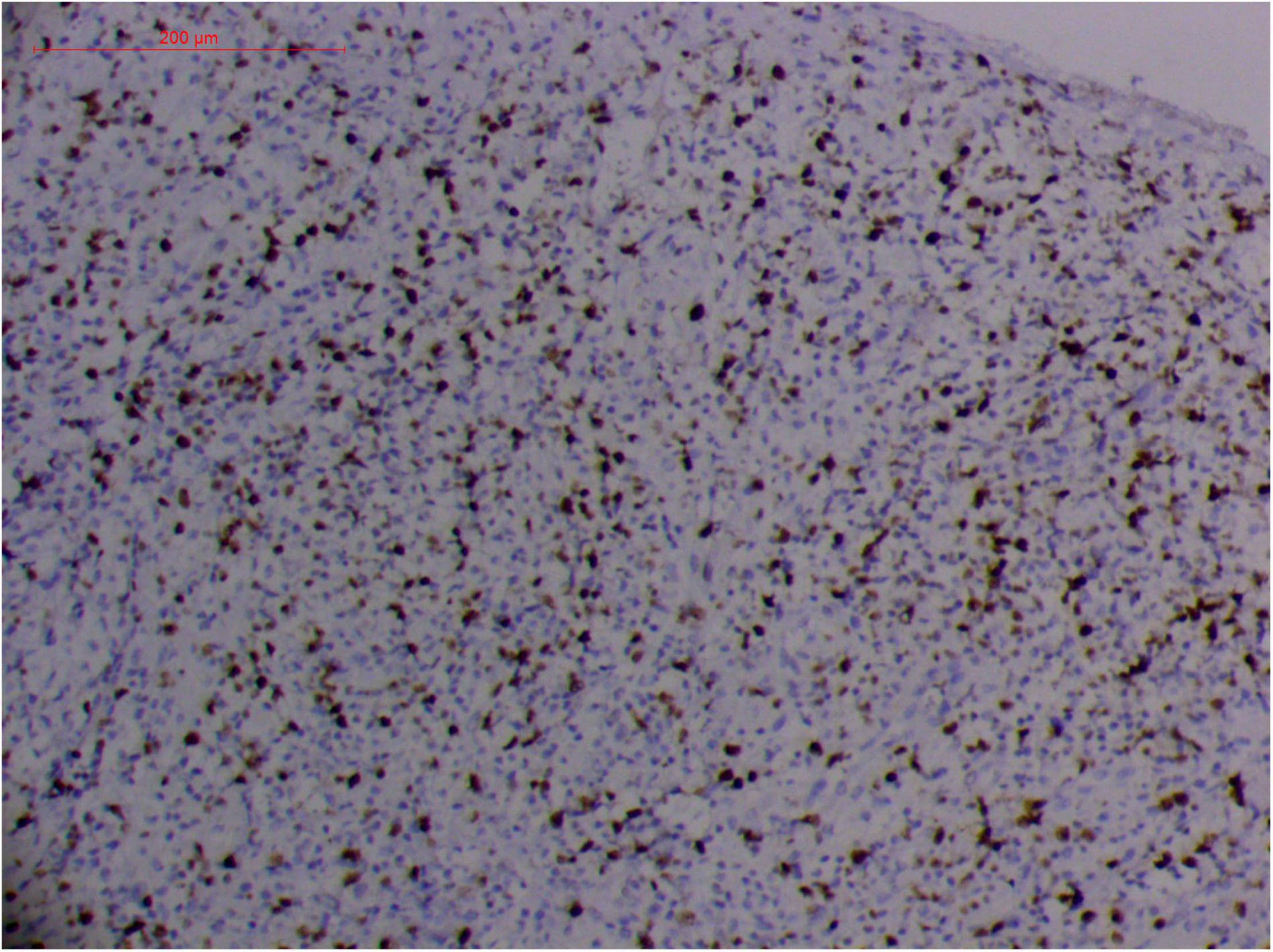
Figure 9. “Extranodal natural killer/T cell lymphoma, nasal type/rhino-orbital-cerebral mycosis.” Rhizopus arrhizus-induced positive expression of KI67 from granulomatous tissues (envision method, original magnification ×200).
Fungal-associated atypical hyperplasia with high Ki-67 expression has been reported previously. Terayama et al. reported the inducement of proliferation in the representative forestomach sections by Candida albicans in rats (134). In M. irregularis or R. arrhizus cases, the proliferation was measured using the Ki-67 labeling index which was observed to be as high as 50%, accompanied by high loads of fungal elements (Figure 14). In the mouse model, when the isolated M. irregularis or R. arrhizus was introduced, high Ki-67 expression was duplicated. A more significant observation is that Ki-67 expression was observed in the cells around the hyphae, indicating that it was probably the fungal infection that led to hyperplasia.
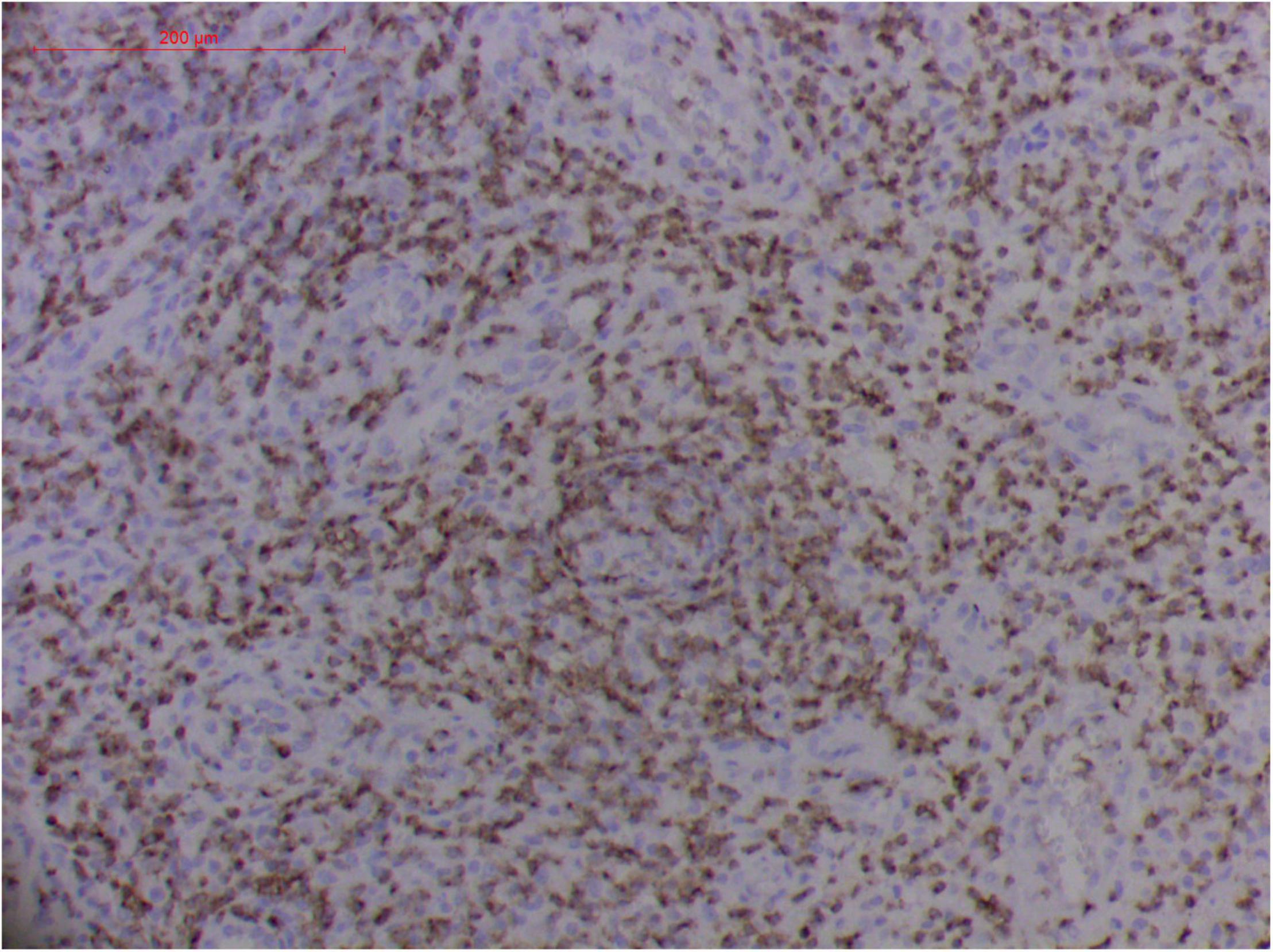
Figure 10. Extranodal natural killer/T cell lymphoma, nasal type/rhino-orbital-cerebral mycosis.” Rhizopus arrhizus-induced positive expression of CD2 from granulomatous tissues (envision method, original magnification ×200).
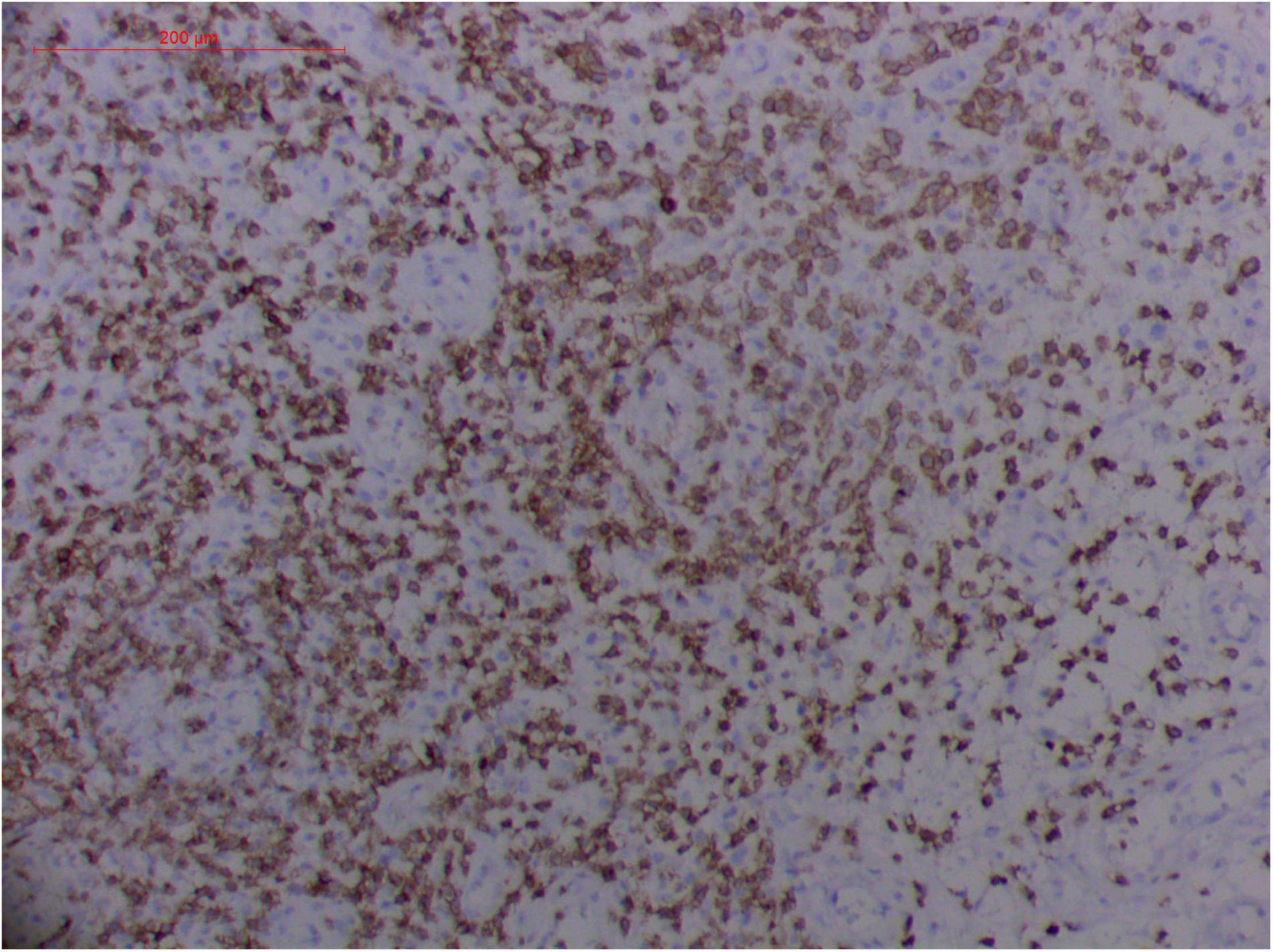
Figure 11. Extranodal natural killer/T cell lymphoma, nasal type/rhino-orbital-cerebral mycosis.” Rhizopus arrhizus-induced positive expression of CD3 from granulomatous tissues (envision method, original magnification ×200).
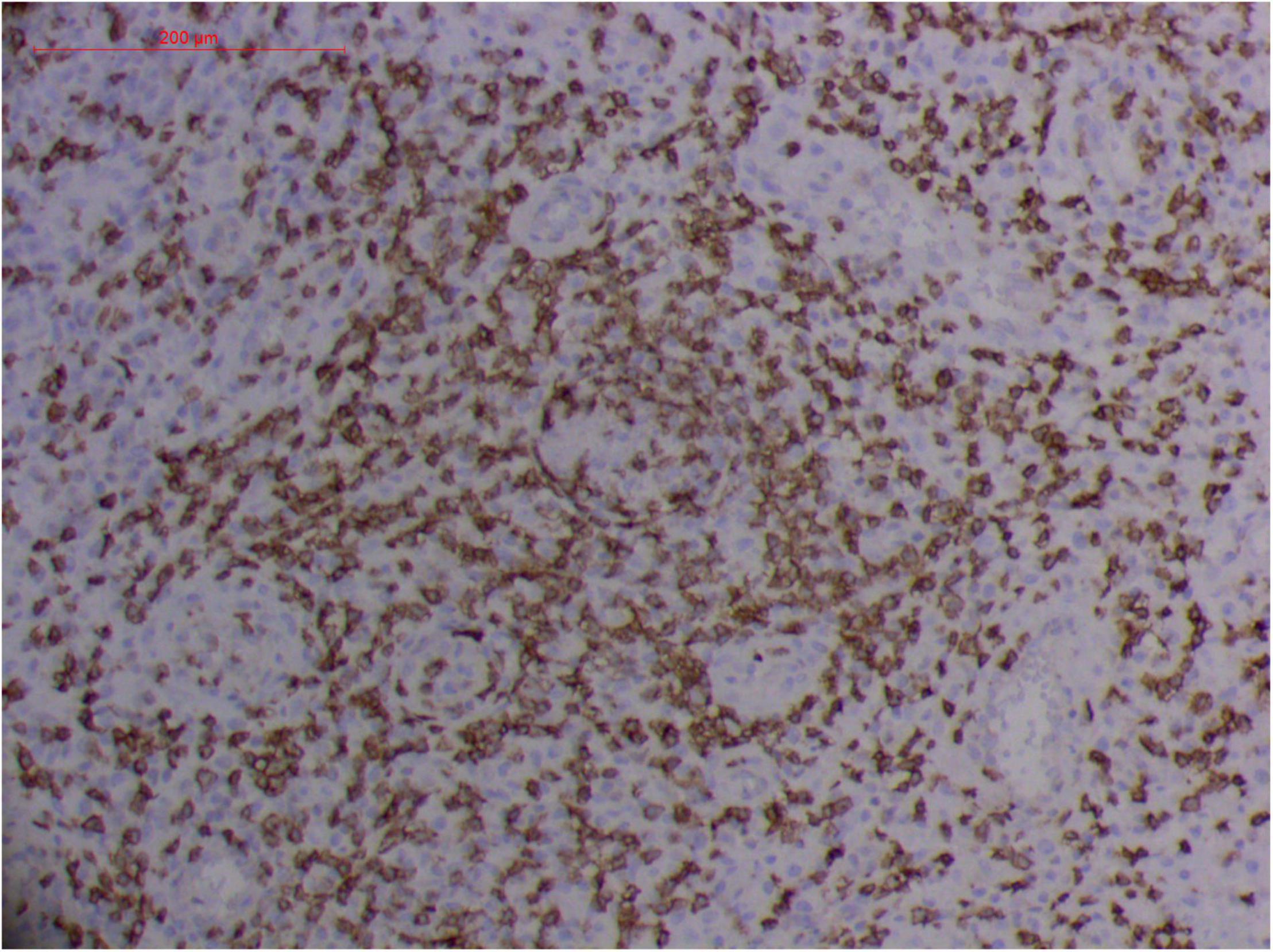
Figure 12. Extranodal natural killer/T cell lymphoma, nasal type-orbital-cerebral mycosis.” Rhizopus arrhizus-induced positive expression of CD8 from granulomatous tissues (envision method, original magnification ×200).
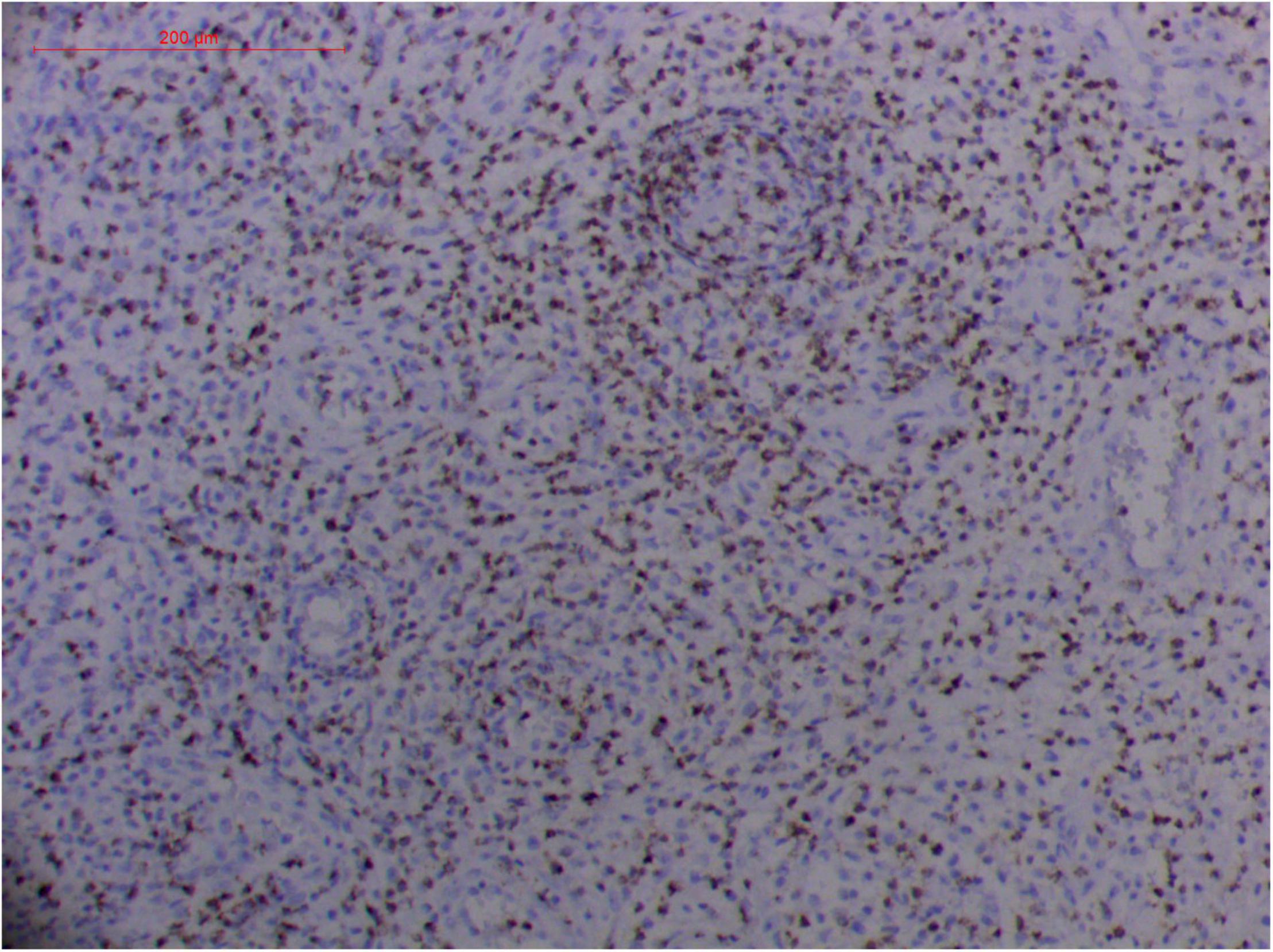
Figure 13. Extranodal natural killer/T cell lymphoma, nasal type-orbital-cerebral mycosis.” Rhizopus arrhizus-induced positive expression of GZMB from granulomatous tissues (envision method, original magnification ×200).
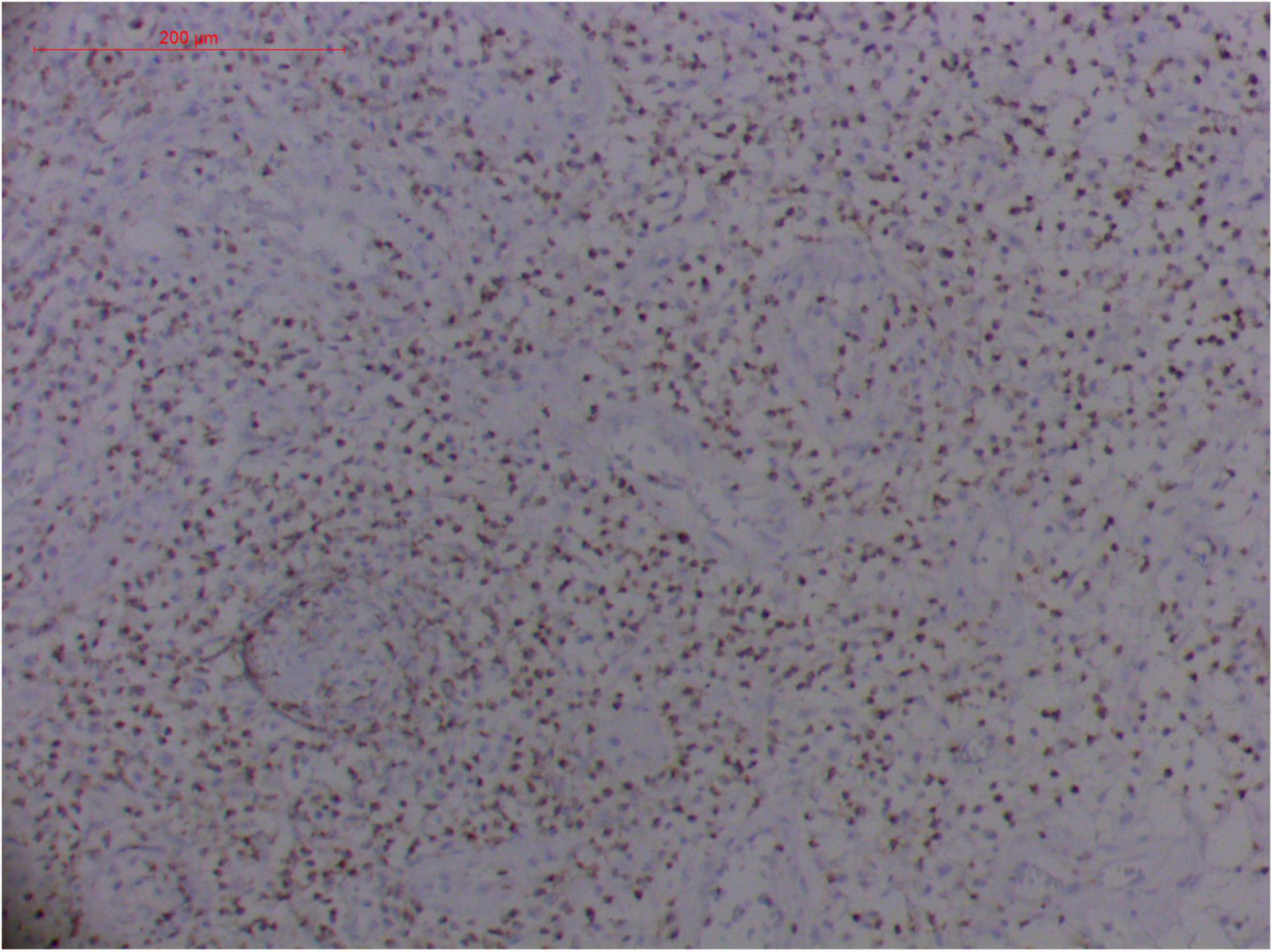
Figure 14. Extranodal natural killer/T cell lymphoma, nasal type/rhino-orbital-cerebral mycosis.” Rhizopus arrhizus-induced positive expression of TIA1 from granulomatous tissues (envision method, original magnification ×200).
Pathologic findings of ENKTL-NT show a diffuse infiltration of pleomorphic large and small lymphoma cells mixed with various inflammatory cells on the necrotic background. The lymphoma cells express T-cell markers, such as cytoplasmic CD3 (CD3ε), CD2, and CD8, as well as the NK-cell marker CD56. Cytotoxic granule-associated proteins (GZMB, PRF, and TIA1), Fas ligand, intercellular adhesion molecule-1 (ICAM-1), and perforin are also expressed in the ENKTL-NT cells, which constitute the diagnostic criteria (3, 10, 12, 13, 14, 33, 91, 103, 111, 115).
However, the expression of these T-cell or/and NK-cell markers can be seen in ROCM or ROCM-associated ENKTL-NT, although the roles of these immune cells remain ambiguous (16, 22, 91). In both M. irregularis and R. arrhizus related ROCM/ENKTL-NT cases, T-cell and NK-cell infiltration were observed (Figures 8–12) (16, 19). Other researchers have reported that in some ENKTL-NT patients, these immunopathological markers were induced by microbes like Staphyloccus aureus (131), Nocardia sp., Mycobacterium fortuitum, M marinum, Pseudomonas aeruginosa, and Leishmania spp. (77, 129–133). All these cases achieved complete resolution after anti-infection therapy. This might be explained by the findings of some previous studies. Potenza et al. observed that Mucorales-specific T cells emerged during the course of infection in patients with invasive mucormycosis and that they exhibited direct antifungal activity comparable to that of either polymorphonuclear leukocytes or antigen-presenting cells (135). Other experiments showed that human NK cells or T cells could damage R. arrhizus and other fungi by perforin- and granzyme B-mediated apoptosis and by inducing other immune response cells to bind and then kill fungi (136, 137). Our report showed that the expression of NK/T cells and proliferation were intensified around fungal hyphae, which also suggested that immune response induced cell aggregation and proliferation (16, 17, 80).
The clinical-pathological manifestations of ENKTL-NT and ROCM share similarities, including necrotizing lesions; destruction in the upper aerodigestive tract, sinuses, orbits, hard palate, and middle face; inflammation; granulation; and vascular damage (3, 13, 14, 16, 19, 33, 85, 104, 110, 111, 138). The main distinguishing feature between the two is that ROCM is characterized by the detection of fungi in the tissue, while ENKTL-NT is typically associated with NK/T-cell infiltration, with cytotoxic granule-associated proteins of GZMB, PRF, and TIA1 and atypical dysplasia. If the pathology is positive for fungi, the diagnosis is often ROCM; if positive for NK/T cells plus cytotoxic granule-associated proteins, vascular damage, and atypical dysplasia, the diagnosis is confirmed as ENKTL-NT (3, 13, 14, 16, 20, 21, 33, 110, 111).
However, what is challenging is not the established diagnostic criteria but the interesting cases presented by the author and other researchers, where for one reason or another, the decisive evidence, that is, positivity for NK/T cells and positivity for fungi, was found to be coexisting. Whatever diagnosis was made in these cases, anti-infection treatments alone or in combination with anticancer therapy were associated with positive health and life, whereas anticancer treatments alone were associated with fatal outcomes (1, 16, 20, 21, 23, 25, 139). These cases made us ponder the fungal etiology of ENKTL-NT, and further its treatment and prognosis, specifically the relationship between ENKTL-NT and fungal infection.
One major challenge in the dilemma related to the diagnosis of ROCM/ENKTL-NT is the detection and recovery of fungi from the patient specimen (78). As in the cases of M. irregularis and R. arrhizus infections, it was rather difficult to recognize the broad thin-walled hyphae in the granulomatous tissue or in the necrotic areas. By comparing multiple specimens of biopsied samples obtained from the edge of newly infected tissues with those obtained from necrotic tissues that were infected for a long period, we found that typical hyphae could be seen clearly only in the newly infected tissues but were hardly recognized in the necrotic tissues (Figures 4–7, 13) (16, 17, 140). However, they could sometimes be recovered in the culture (16). This observation was in accordance with Wang’s experiment, who observed that M. irregularis could not be recognized but could be transferred from the infected tissue to the mouse (140). When M. irregularis and R. arrhizus were inoculated into the mice, some hyphae were broken down and some were surrounded by giant cells. But more importantly, the inoculated fungi induced the expression of NK/T-cell markers and hyperplasia (CD3+, CD8+, CD56+, TIA1+, GZMB+, and PRF+) in mice, which is considered a diagnostic feature of ENKTL-NT.
As Koch’s four postulates defined, the causal relationship between a microbe and a disease is established when a microorganism can be isolated from the diseased tissue, grown on a pure culture medium, and be introduced into healthy organisms, and subsequently can be re-isolated from the inoculated diseased experimental host (141, 142). In the ENKTL-NT cases, microbes M. irregularis and R. arrhizus were isolated from the diseased tissue of the ENKTL-NT patients, grown on a pure culture medium, and could be introduced into a healthy mouse, and could be further re-isolated from the inoculated diseased experimental host, indicating the possible causal relationship between the Mucoralean fungi and ENKTL-NT. The causal relationship was further confirmed by the fact that the lesions got completely resolved after treatment with AMB alone.
In the field of oncology, the direct causal relationship between cancer and a microbe was traced back to 1908 when Ellermann and Bang suggested a viral etiology by employing cell-free tumor extracts (143). In 1911, Peyton Rous established an association between cancer and an infectious agent, the Rous sarcoma virus (144). In 1964, Anthony Epstein, Bert Achong, and Yvonne Barr discovered the first human tumor virus, which was later named EBV, from equatorial African pediatric patients with Burkitt’s lymphoma by observing viral particles in the cell cultures (145). Further experiments confirmed EBV as the causative agent of endemic Burkitt’s lymphoma and many neoplasms, including ENKTL (146). In 1990, Harabuchi et al. first showed the presence of EBV DNA and EBV nuclear antigen in the lymphoma cells extracted from five patients with ENKTL-NT and suggested that lethal midline granuloma is causally associated with EBV (28). To date, EBV, Kaposi’s sarcoma-associated herpesvirus, human high-risk papillomaviruses, Merkel cell polyomavirus, hepatitis B virus, hepatitis C virus, human T-cell lymphotropic virus type 1 (HTLV-1), and the Gram-negative bacterium Helicobacter pylori have been classified as type 1 carcinogenic agents (147). In recent years, Marseillevirus, Campylobacter jejuni, Chlamydia psittaci, Borrelia burgdorferi, and Coxiella burnetii have also been added to the cohort of causal inference between microbes and lymphoma (148). In this study, M. irregularis and R. arrhizus were demonstrated in the lymphoma cells extracted from ENKTL-NT and experimental mice, and hence should be added to the list of causal microbes of ENKTL-NT, in addition to EBV (16, 17, 80).
Treatment options for ENKTL-NT and ROCM are quite different. While ENKTL-NT is considered a malignancy, its treatment is often anticancer therapy, which includes chemotherapy, radiotherapy, radiochemotherapy, and/or stem cell transplantation. In the last few years, chemotherapy (dexamethasone, etoposide, ifosfamide, and carboplatin), concomitant with local radiotherapy (RT-2/3DeVIC), was conducted as a phase I/II trial (JCOG0211) and showed a good clinical outcome. More recent experimental data show good results by employing pembrolizumab, nivolumab, or tofacitinib (149).
Treatment regimens for ROCM include antifungal therapy, reversal of the underlying predisposing risk factors, and surgical debridement. A combination of surgery and potent antifungal therapy was significantly better than antifungal therapy alone (19, 84). Since most of the ROCM cases are caused by Mucoralean fungi, AMB is the common choice of potent antifungal drug (52, 88). In occasional refractory cases, posaconazole or isavuconazole may serve as salvage therapy (44). An antifungal susceptibility test is often performed to decide the choice of drug.
In conclusion, ENKTL-NT and ROCM share many similarities in clinical presentations, radiology, histopathology, and even immunopathology, and may have the same etiology. This may explain why the two diseases are tangled together in the reported cases, and suggests a role that the fungi may play in the development of these ENKTL-NT/ROCM diseases. The reason why ENKTL-NT and ROCM are sometimes confused is that the main pathogens of ROCM, Mucor irregularis and Rhizopus arrhizus, are the fungal causative agents of ENKTL-NT. Further research on the role of fungi in ENKTL-NT is needed. Based on the above-mentioned evidence, we strongly suggest screening for fungal infection in patients diagnosed with ENKTL-NT and propose antifungal therapy for ENKTL-NT patients with fungal infections.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
DL and LL: conceptualization, methodology, data curation, original draft preparation, and illustration. DL: conceptualization, editing, and reviewing. Both authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (C32070019 and C31770013).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We express our thanks particularly to Jian Zhang for the revision of the manuscript and Xiao Kang Lun for scientific revision.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.851208/full#supplementary-material
1. Taali L, Abou-Elfadl M, Fassih M, Mahtar M. Nasal NK/T-cell lymphoma: a tragic case. Eur Ann Otorhinolaryngol Head Neck Dis. (2017) 134:121–2. doi: 10.1016/j.anorl.2016.08.006
2. Thakur JS, Mahajan A, Saluja M, Mohindroo NK. Deceptive nasal NK/T-cell lymphoma. Trop Doct. (2017) 47:268–71. doi: 10.1177/0049475516684088
3. Haverkos BM, Pan Z, Gru AA, Freud AG, Rabinovitch R, Xu-Welliver M, et al. Extranodal NK/T cell lymphoma, nasal type (ENKTL-NT): an update on epidemiology, clinical presentation, and natural history in north american and european cases. Curr Hematol Malig Rep. (2016) 11:514–27. doi: 10.1007/s11899-016-0355-9
4. Woods R. Observations on malignant granuloma of the nose. Br Med J. (1921) 2:65–6. doi: 10.1136/bmj.2.3159.65
5. Mcbride P. Photographs of a case of rapid destruction of the nose and face. 1897. J Laryngol Otol. (1991) 105:1120. doi: 10.1017/s0022215100118407
8. Harrison DF. Non-healing granulomata of the upper respiratory tract. Br Med J. (1974) 4:205–9. doi: 10.1136/bmj.4.5938.205
9. Friedmann I, Sando I, Balkany T. Idiopathic pleomorphic midfacial granuloma (Stewart’s type). J Laryngol Otol. (1978) 92:601–11. doi: 10.1017/s0022215100085819
10. Tsokos M, Fauci AS, Costa J. Idiopathic midline destructive disease (Imdd): a subgroup of patients with the “midline granuloma” syndrome. Am J Clin Pathol. (1982) 77:162–8. doi: 10.1093/ajcp/77.2.162
11. Califano L, Zupi A, Maremonti P, De Rosa G. Sinonasal lymphoma presenting as a lethal midline granuloma: case report. J Oral Maxillofac Surg. (1998) 56:667–71. doi: 10.1016/s0278-2391(98)90471-2
12. Ishii Y, Yamanaka N, Ogawa K, Yoshida Y, Takami T, Matsuura A, et al. Nasal T-cell lymphoma as a type of so-called “lethal midline granuloma”. Cancer. (1982) 50:2336–44.
13. Lim MS, De Leval L, Quintanilla-Martinez L. Commentary on the 2008 Who classification of mature T- and NK-cell neoplasms. J Hematop. (2009) 2:65–73. doi: 10.1007/s12308-009-0034-z
14. William BM, Armitage JO. International analysis of the frequency and outcomes of NK/T-cell lymphomas. Best Pract Res Clin Haematol. (2013) 26:23–32. doi: 10.1016/j.beha.2013.04.003
15. Isobe Y, Aritaka N, Sasaki M, Oshimi K, Sugimoto K. Spontaneous regression of natural killer cell lymphoma. J Clin Pathol. (2009) 62:647–50. doi: 10.1136/jcp.2008.062976
16. Li DM, Lun LD. Mucor irregularis infection and lethal midline granuloma: a case report and review of published literature. Mycopathologia. (2012) 174:429–39. doi: 10.1007/s11046-012-9559-2
17. Li DM, Lun L, Ge J, Zhang GJ, Li XL, De Hoog GS. Case report: rhizopus arrhizus rhino-orbital-cerebral mycosis and lethal midline granuloma: another fungal etiological agent. Front Med (Lausanne). (2021) 8:578684. doi: 10.3389/fmed.2021.578684
18. Li DM, De Hoog GS. Cerebral phaeohyphomycosis–a cure at what lengths? Lancet Infect Dis. (2009) 9:376–83. doi: 10.1016/S1473-3099(09)70131-8
19. Li DM, Shang PP, Zhu L, De Hoog GS. Rhino-orbital-cerebral mycosis and cavernous thromboses. Eur J Inflamm. (2014) 12:1–10.
20. Li Z, Liu C, Huang X, Gao Z. Nasal Nk/T cell lymphoma with severe facial disfigurement in a 37-year-old male. Int J Oral Maxillofac Surg. (2013) 42:102–5. doi: 10.1016/j.ijom.2012.06.021
21. Zhang Y, Wang T, Liu GL, Li J, Gao SQ, Wan L. Mucormycosis or extranodal natural killer/T cell lymphoma, similar symptoms but different diagnosis. J Mycol Med. (2016) 26:277–82. doi: 10.1016/j.mycmed.2016.04.005
22. Zhang Xf YQ, Yang Xc, Deng J, Hao F, Song Q. One case of nasal tye NK/T - cell lymphoma. J Clin Dermatol (Chinese). (2015) 34:752–3.
23. Aydogdu I, Sari R, Mizrak B. Case report. Rhinocerebral zygomycosis. Mycoses. (2001) 44:59–60. doi: 10.1046/j.1439-0507.2001.00615.x
24. Kameh DS, Gonzalez OR, Pearl GS, Walsh AF, Gambon T, Kropp TM. Fatal rhino-orbital-cerebral zygomycosis. South Med J. (1997) 90:1133–5. doi: 10.1097/00007611-199711000-00015
25. Luemsamran P, Pornpanich K, Uiprasertkul M, Sakolsatayadorn N, Vangveeravong S. NK/T-cell lymphoma of the nasal cavity causing contralateral dacryoadenitis. Orbit. (2013) 32:250–2. doi: 10.3109/01676830.2013.788665
26. Kang D, Jiang X, Wan H, Ran Y, Hao D, Zhang C. Mucor irregularis infection around the inner canthus cured by amphotericin B: a case report and review of published literatures. Mycopathologia. (2014) 178:129–33. doi: 10.1007/s11046-014-9770-4
27. Xia ZK, Wang WL, Yang RY. Slowly progressive cutaneous, rhinofacial, and pulmonary mucormycosis caused by Mucor irregularis in an immunocompetent woman. Clin Infect Dis. (2013) 56:993–5. doi: 10.1093/cid/cis1045
28. Harabuchi Y, Yamanaka N, Kataura A, Imai S, Kinoshita T, Mizuno F, et al. Epstein-Barr virus in nasal T-cell lymphomas in patients with lethal midline granuloma. Lancet. (1990) 335:128–30. doi: 10.1016/0140-6736(90)90002-m
29. Chan JK, Yip TT, Tsang WY, Ng CS, Lau WH, Poon YF, et al. Detection of Epstein-Barr viral Rna in malignant lymphomas of the upper aerodigestive tract. Am J Surg Pathol. (1994) 18:938–46. doi: 10.1097/00000478-199409000-00009
30. Kawa-Ha K, Ishihara S, Ninomiya T, Yumura-Yagi K, Hara J, Murayama F, et al. CD3-negative lymphoproliferative disease of granular lymphocytes containing Epstein-Barr viral Dna. J Clin Invest. (1989) 84:51–5. doi: 10.1172/JCI114168
31. Fei Q, Tian XK, Wu J, Zhu HM, Wang Y, Peng FY, et al. Prognostic significance of Epstein-Barr virus Dna in Nk/T-cell lymphoma: a meta-analysis. Onco Targets Ther. (2018) 11:997–1004. doi: 10.2147/OTT.S153942
32. Tomoka T, Powers E, Van Der Gronde T, Amuquandoh A, Dhungel BM, Kampani C, et al. Extranodal natural killer/T-cell lymphoma in Malawi: a report of three cases. Bmc Cancer. (2017) 17:633. doi: 10.1186/s12885-017-3612-y
33. Haverkos BM, Coleman C, Gru AA, Pan Z, Brammer J, Rochford R, et al. Emerging insights on the pathogenesis and treatment of extranodal Nk/T cell lymphomas (Enktl). Discov Med. (2017) 23:189–99.
34. Jin X, Xu Y, Zhang J, Li G, Huang D, Yang Y, et al. Aggressive natural killer cell leukemia or extranodal Nk/T cell lymphoma? a case with nasal involvement. Diagn Pathol. (2017) 12:46. doi: 10.1186/s13000-017-0636-1
35. Tian C, Wang Y, Zhu L, Yu Y, Zhang Y. Primary bone natural killer/T cell lymphoma, nasal type without EBV infection: a case report. Int J Clin Exp Pathol. (2015) 8:14836–9.
36. Matsuda M, Iwanaga T, Hashimoto S, Uesugi T, Itagaki N. Primary Epstein-Barr virus-negative nasal-type natural killer/T cell lymphoma of the testis. Leuk Res. (2009) 33:e119–20. doi: 10.1016/j.leukres.2009.02.019
37. Feng Y, Rao H, Lei Y, Huang Y, Wang F, Zhang Y, et al. Cd30 expression in extranodal natural killer/T-cell lymphoma, nasal type among 622 cases of mature T-cell and natural killer-cell lymphoma at a single institution in South China. Chin J Cancer. (2017) 36:43. doi: 10.1186/s40880-017-0212-9
39. Hilal AA, Taj-Aldeen SJ, Mirghani AH. Rhinoorbital mucormycosis secondary to Rhizopus oryzae: a case report and literature review. Ear Nose Throat J. (2004) 556:558–60.
40. Gumral R, Yildizoglu U, Saracli MA, Kaptan K, Tosun F, Yildiran ST. A case of rhinoorbital mucormycosis in a leukemic patient with a literature review from Turkey. Mycopathologia. (2011) 172:397–405. doi: 10.1007/s11046-011-9449-z
41. Fairley C, Sullivan TJ, Bartley P, Allworth T, Lewandowski R. Survival after rhino-orbital-cerebral mucormycosis in an immunocompetent patient. Ophthalmology. (2000) 107:555–8. doi: 10.1016/s0161-6420(99)00142-6
42. Haliloglu NU, Yesilirmak Z, Erden A, Erden I. Rhino-orbito-cerebral mucormycosis: report of two cases and review of the literature. Dentomaxillofac Radiol. (2008) 37:161–6. doi: 10.1259/dmfr/14698002
43. Diwakar A, Dewan RK, Chowdhary A, Randhawa HS, Khanna G, Gaur SN. Zygomycosis–a case report and overview of the disease in India. Mycoses. (2007) 50:247–54. doi: 10.1111/j.1439-0507.2007.01382.x
44. Ervens J, Ghannoum M, Graf B, Schwartz S. Successful isavuconazole salvage therapy in a patient with invasive mucormycosis. Infection. (2014) 42:429–32. doi: 10.1007/s15010-013-0552-6
45. Chakrabarti A, Singh R. Mucormycosis in India: unique features. Mycoses. (2014) 57(Suppl. 3):85–90. doi: 10.1111/myc.12243
46. Ribeiro LC, Wanke B, Da Silva M, Dias LB, Mello R, Canavarros FA, et al. Mucormycosis in Mato Grosso, Brazil: a case reports, caused by Rhizopus microsporus var. oligosporus and Rhizopus microsporus var. rhizopodiformis. Mycopathologia. (2012) 173:187–92. doi: 10.1007/s11046-011-9472-0
47. Hemashettar BM, Patil RN, O’donnell K, Chaturvedi V, Ren P, Padhye AA. Chronic rhinofacial mucormycosis caused by Mucor irregularis (Rhizomucor variabilis) in India. J Clin Microbiol. (2011) 49:2372–5. doi: 10.1128/JCM.02326-10
48. Wolkow N, Jakobiec FA, Stagner AM, Cunnane ME, Piantadosi AL, Basgoz N, et al. Chronic orbital and calvarial fungal infection with Apophysomyces variabilis in an immunocompetent patient. Surv Ophthalmol. (2017) 62:70–82. doi: 10.1016/j.survophthal.2016.05.006
49. Garcia-Covarrubias L, Bartlett R, Barratt DM, Wassermann RJ. Rhino-orbitocerebral mucormycosis attributable to Apophysomyces elegans in an immunocompetent individual: case report and review of the literature. J Trauma. (2001) 50:353–7. doi: 10.1097/00005373-200102000-00027
50. Liang KP, Tleyjeh IM, Wilson WR, Roberts GD, Temesgen Z. Rhino-orbitocerebral mucormycosis caused by Apophysomyces elegans. J Clin Microbiol. (2006) 44:892–8.
51. Hirayama Y, Yajima N, Kaimori M, Akagi T, Kubo K, Saito D, et al. Disseminated infection and pulmonary embolization of Cunninghamella bertholletiae complicated with hemophagocytic lymphohistiocytosis. Intern Med. (2013) 52:2275–9. doi: 10.2169/internalmedicine.52.0171
52. Eucker J, Sezer O, Lehmann R, Weber JR, Graf B, Denkert C, et al. Disseminated mucormycosis caused by Absidia corymbifera leading to cerebral vasculitis. Infection. (2000) 28:246–50. doi: 10.1007/s150100070047
53. Woo PC, Lau SK, Ngan AH, Tung ET, Leung SY, To KK, et al. Lichtheimia hongkongensis sp. nov., a novel Lichtheimia spp. associated with rhinocerebral, gastrointestinal, and cutaneous mucormycosis. Diagn Microbiol Infect Dis. (2010) 66:274–84. doi: 10.1016/j.diagmicrobio.2009.10.009
54. Rodriguez-Gutierrez G, Carrillo-Casas EM, Arenas R, Garcia-Mendez JO, Toussaint S, Moreno-Morales ME, et al. Mucormycosis in a Non-hodgkin lymphoma patient caused by Syncephalastrum racemosum: case report and review of literature. Mycopathologia. (2015) 180:89–93. doi: 10.1007/s11046-015-9878-1
55. Taj-Aldeen SJ, Falamarzi A, Almuzrkchi A, Guarro J. Rare pediatric rhino-orbital infection caused by Saksenaea vasiformis. Infection. (2012) 40:703–7. doi: 10.1007/s15010-012-0338-2
56. Dean DF, Ajello L, Irwin RS, Woelk WK, Skarulis GJ. Cranial zygomycosis caused by Saksenaea vasiformis, Case report. J Neurosurg. (1977) 46:97–103. doi: 10.3171/jns.1977.46.1.0097
57. Baradkar VP, Mathur M, Taklikar S, Rathi M, Kumar S. Fatal rhino-orbito-cerebral infection caused by Saksenaea vasiformis in an immunocompetent individual: first case report from India. Indian J Med Microbiol. (2008) 26:385–7. doi: 10.4103/0255-0857.43572
58. Kaufman L, Padhye AA, Parker S. Rhinocerebral zygomycosis caused by Saksenaea vasiformis. J Med Vet Mycol. (1988) 26:237–41.
59. Shatriah I, Mohd-Amin N, Tuan-Jaafar TN, Khanna RK, Yunus R, Madhavan M. Rhino-orbito-cerebral mucormycosis in an immunocompetent patient: case report and review of literature. Middle East Afr J Ophthalmol. (2012) 19:258–61. doi: 10.4103/0974-9233.95269
60. Gomes MZ, Lewis RE, Kontoyiannis DP. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin Microbiol Rev. (2011) 24:411–45.
62. Wuppenhorst N, Lee MK, Rappold E, Kayser G, Beckervordersandforth J, De With K, et al. Rhino-orbitocerebral zygomycosis caused by Conidiobolus incongruus in an immunocompromised patient in Germany. J Clin Microbiol. (2010) 48:4322–5. doi: 10.1128/JCM.01188-10
63. Pornpanich K, Uiprasertkul M, Luemsamran P, Jantharaworamet B, Vangveeravong S. Entomophthoramycosis: a rare fungal orbital infection presenting with dacryocystitis. Orbit. (2011) 30:21–3. doi: 10.3109/01676830.2010.535640
64. Sutton DA, Wickes BL, Romanelli AM, Rinaldi MG, Thompson EH, Fothergill AW, et al. Cerebral aspergillosis caused by Aspergillus granulosus. J Clin Microbiol. (2009) 47:3386–90.
65. Neil JA, Orlandi RR, Couldwell WT. Malignant fungal infection of the cavernous sinus: case report. J Neurosurg. (2016) 124:861–5. doi: 10.3171/2015.2.JNS142668
66. Xi L, Fukushima K, Lu C, Takizawa K, Liao R, Nishimura K. First case of Arthrographis kalrae ethmoid sinusitis and ophthalmitis in the People’s Republic of China. J Clin Microbiol. (2004) 42:4828–31. doi: 10.1128/JCM.42.10.4828-4831.2004
67. Roh ML, Tuazon CU, Mandler R, Kwon-Chung KJ, Geist CE. Sphenocavernous syndrome associated with Schizophyllum commune infection of the sphenoid sinus. Ophthal Plast Reconstr Surg. (2005) 21:71–4. doi: 10.1097/01.iop.0000148407.34784.6e
68. Godfrey KJ, Mcconville TH, Miko BA, Kazim M. Sino-orbital fungal infection by Tilletiopsis minor, a rare human pathogen, diagnosed by internal transcribed spacer sequencing. Ophthalmic Plast Reconstr Surg. (2018) 34:e162–4.
69. Mukherjee B, Kundu D. Necrotizing fungal infection due to Saksenaea erythrospora: a case report and review of literature. Indian J Ophthalmol. (2018) 66:1513–6.
70. Alam MS, Vaidehi D, Therese KL, Ali MJ. Rare Pleurostomophora richardsiae mass causing transient nasolacrimal duct obstruction. Ophthalmic Plast Reconstr Surg. (2017) 33:e154–6. doi: 10.1097/IOP.0000000000000919
71. Roehm CE, Salazar JC, Hagstrom N, Valdez TA. Phoma and Acremonium invasive fungal rhinosinusitis in congenital acute lymphocytic leukemia and literature review. Int J Pediatr Otorhinolaryngol. (2012) 76:1387–91. doi: 10.1016/j.ijporl.2012.06.026
72. Martínez-Herrera E, Frías-De-LeóN MG, JuliáN-CastrejóN A, Cruz-Benítez L, Xicohtencatl-Cortes J, Hernández-Castro R. Rhino-orbital mucormycosis due to Apophysomyces ossiformis in a patient with diabetes mellitus: a case report. Bmc Infect Dis. (2020) 20:614. doi: 10.1186/s12879-020-05337-4
73. Kishimoto I, Shinohara S. Orbital apex syndrome secondary to a fungal nasal septal abscess caused by Scedosporium apiospermum in a patient with uncontrolled diabetes: a case report. BMC Infect Dis. (2017) 17:649. doi: 10.1186/s12879-017-2753-6
74. Zhou YB, Li DM, Houbraken J, Sun TT, De Hoog GS. Fatal rhinofacial mycosis due to Aspergillus nomiae: case report and review of published literature. Front Microbiol. (2020) 11:595375. doi: 10.3389/fmicb.2020.595375
75. Bhatt M, Soneja M, Fazal F, Vyas S, Kumar P, Jorwal P, et al. Two cases of osteoarticular mucor menace: a diagnostic and management conundrum. Drug Discov Ther. (2018) 12:374–8.
76. Pan J, Tsui C, Li M, Xiao K, de Hoog GS, Verweij PE, et al. First case of rhinocerebral mucormycosis caused by Lichtheimia ornata, with a review of Lichtheimia infections. Mycopathologia. (2020) 185:555–67.
77. Crovetto-Martinez R, Aguirre-Urizar JM, Orte-Aldea C, Araluce-Iturbe I, Whyte-Orozco J, Crovetto-De La Torre MA. Mucocutaneous leishmaniasis must be included in the differential diagnosis of midline destructive disease: two case reports. Oral Surg Oral Med Oral Pathol Oral Radiol. (2015) 119:e20–6.
78. Li LL, Liu HG, Piao YS, He CY, Zhou Q, Zhang Y. [Clinicopathologic study of malignant tumors in head and neck region complicated by fungal infection]. Zhonghua Bing Li Xue Za Zhi. (2010) 39:508–12.
79. Cowen DE. Cephalosporium midline granuloma. Trans Indiana Acad Ophthalmol Otolaryngol. (1965) 48:78–82.
80. Ge J, Zhou YB, Li DM. Studies of atypical hyperplasia in mice caused by Mucor irregularis and Rhizopus oryzae isolated from lethal midline granuloma. Mycosystema. (2019) 38:1314–22.
81. Torre V, Bucolo S, Galletti B, Cavallari V. Midfacial granuloma syndrome or an inflammatory non-specific disease? A case report. J Oral Pathol Med. (2001) 30:190–2. doi: 10.1034/j.1600-0714.2001.300310.x
82. Bakhshaee M, Bojdi A, Allahyari A, Majidi MR, Tavakol S, Najafzadeh MJ, et al. Acute invasive fungal rhinosinusitis: our experience with 18 cases. Eur Arch Otorhinolaryngol. (2016) 273:4281–7. doi: 10.1007/s00405-016-4109-z
83. Bhansali A, Bhadada S, Sharma A, Suresh V, Gupta A, Singh P, et al. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J. (2004) 80:670–4. doi: 10.1136/pgmj.2003.016030
84. Bala K, Chander J, Handa U, Punia RS, Attri AK. A prospective study of mucormycosis in north India: experience from a tertiary care hospital. Med Mycol. (2015) 53:248–57. doi: 10.1093/mmy/myu086
85. Erami M, Shams-Ghahfarokhi M, Jahanshiri Z, Sharif A, Razzaghi-Abyaneh M. Rhinocerebral mucormycosis due to Rhizopus oryzae in a diabetic patient: a case report. J Mycol Med. (2013) 23:123–9.
86. Motaleb HY, Mohamed MS, Mobarak FA. A fatal outcome of rhino-orbito-cerebral mucormycosis following tooth extraction: a case report. J Int Oral Health. (2015) 7:68–71.
87. Teixeira CA, Medeiros PB, Leushner P, Almeida F. Rhinocerebral mucormycosis: literature review apropos of a rare entity. Bmj Case Rep. (2013) 2013. doi: 10.1136/bcr-2013-008552
88. Thajeb P, Thajeb T, Dai D. Fatal strokes in patients with rhino-orbito-cerebral mucormycosis and associated vasculopathy. Scand J Infect Dis. (2004) 36:643–8. doi: 10.1080/00365540410020794
89. Barrak HA. Hard palate perforation due to mucormycosis: report of four cases. J Laryngol Otol. (2007) 121:1099–102. doi: 10.1017/S0022215107006354
90. Goldberg RA, Weisman JS, Mcfarland JE, Krauss HR, Hepler RS, Shorr N. Orbital inflammation and optic neuropathies associated with chronic sinusitis of intranasal cocaine abuse. Possible role of contiguous inflammation. Arch Ophthalmol. (1989) 107:831–5. doi: 10.1001/archopht.1989.01070010853028
91. Rodrigo JP, Suarez C, Rinaldo A, Devaney KO, Carbone A, Barnes L, et al. Idiopathic midline destructive disease: fact or fiction. Oral Oncol. (2005) 41:340–8. doi: 10.1016/j.oraloncology.2004.10.007
92. Rao R, Shetty AP, Nagesh CP. Orbital infarction syndrome secondary to rhino-orbital mucormycosis in a case of Covid-19: clinico-radiological features. Indian J Ophthalmol. (2021) 69:1627–30. doi: 10.4103/ijo.IJO_1053_21
93. Zwang NA, Van Wagner LB, Rose S. A case of levamisole-induced systemic vasculitis and cocaine-induced midline destructive lesion: a case report. J Clin Rheumatol. (2011) 17:197–200. doi: 10.1097/RHU.0b013e31821cb9d5
94. Kim J, Fortson JK, Cook HE. A fatal outcome from rhinocerebral mucormycosis after dental extractions: a case report. J Oral Maxillofac Surg. (2001) 59:693–7. doi: 10.1053/joms.2001.23407
95. Takata K, Hong ME, Sitthinamsuwan P, Loong F, Tan SY, Liau JY, et al. Primary cutaneous NK/T-cell lymphoma, nasal type and CD56-positive peripheral T-cell lymphoma: a cellular lineage and clinicopathologic study of 60 patients from Asia. Am J Surg Pathol. (2015) 39:1–12. doi: 10.1097/PAS.0000000000000312
96. Ketharanathan N, Van Kipshagen PJ, Vasmel W, Barbe E, De Vries N. T/Nk cell lymphoma presenting as a “blocked nose”. Eur Arch Otorhinolaryngol. (2008) 265:1131–4.
97. Fletcher JW, Ramolia P, Wells MJ. Jaad Grand Rounds quiz. History of sinus infection and necrotic lesions. J Am Acad Dermatol. (2012) 67:328–30. doi: 10.1016/j.jaad.2011.12.013
98. Santos Gorjon P, Blanco Perez P, Batuecas Caletrio A, Munoz Herrera AM, Sanchez Gonzalez F, De La Fuente Canibano R. Rhino-orbito-cerebral mucormycosis, a retrospective study of 7 cases. Acta Otorrinolaringol Esp. (2010) 61:48–53. doi: 10.1016/j.otorri.2009.07.001
99. Koc Z, Koc F, Yerdelen D, Ozdogu H. Rhino-orbital-cerebral mucormycosis with different cerebral involvements: infarct, hemorrhage, and ophthalmoplegia. Int J Neurosci. (2007) 117:1677–90. doi: 10.1080/00207450601050238
100. Drake-Lee AB, Milford CA. A review of the role of radiology in non-healing granulomas of the nose and nasal sinuses. Rhinology. (1989) 27:231–6.
101. Melsom SM, Khangure MS. Craniofacial mucormycosis following assault: an unusual presentation of an unusual disease. Australas Radiol. (2000) 44:104–6. doi: 10.1046/j.1440-1673.2000.00751.x
102. Bhatt VR, Koirala B, Terjanian T. Extranodal natural killer/T cell lymphoma, nasal type presenting as a palatal perforation and naso-oral fistula. BMJ Case Rep. (2011). doi: 10.1136/bcr.11.2010.3511
103. Metgud RS, Doshi JJ, Gaurkhede S, Dongre R, Karle R. Extranodal NK/T-cell lymphoma, nasal type (angiocentric T-cell lymphoma): A review about the terminology. J Oral Maxillofac Pathol. (2011) 15:96–100. doi: 10.4103/0973-029X.80016
104. Al-Hakeem DA, Fedele S, Carlos R, Porter S. Extranodal NK/T-cell lymphoma, nasal type. Oral Oncol. (2007) 43:4–14.
105. Rachapalli SM, Kiely PD. Cocaine-induced midline destructive lesions mimicking Ent-limited Wegener’s granulomatosis. Scand J Rheumatol. (2008) 37:477–80. doi: 10.1080/03009740802192043
106. Ostri C, Heegaard S, Prause JU. Sclerosing Wegener’s granulomatosis in the orbit. Acta Ophthalmol. (2008) 86:917–20. doi: 10.1111/j.1755-3768.2008.01179.x
107. Mendenhall WM, Olivier KR, Lynch JW Jr., Mendenhall NP. Lethal midline granuloma-nasal natural killer/T-cell lymphoma. Am J Clin Oncol. (2006) 29:202–6. doi: 10.1097/01.coc.0000198738.61238.eb
108. Bonifaz A, Macias B, Paredes-Farrera F, Arias P, Ponce RM, Araiza J. Palatal zygomycosis: experience of 21 cases. Oral Dis. (2008) 14:569–74. doi: 10.1111/j.1601-0825.2007.01433.x
109. Au WY, Ma SY, Chim CS, Choy C, Loong F, Lie AK, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. (2005) 16:206–14. doi: 10.1093/annonc/mdi037
110. Vose J, Armitage J, Weisenburger D, International TCLP. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. (2008) 26:4124–30. doi: 10.1200/JCO.2008.16.4558
111. Kohrt H, Advani R. Extranodal natural killer/T-cell lymphoma: current concepts in biology and treatment. Leuk Lymphoma. (2009) 50:1773–84. doi: 10.3109/10428190903186502
112. Royer M, Cervera P, Kahan A, Menkes CJ, Puechal X. Mucormycosis cerebral arteritis mimicking a flare in ANCA-associated vasculitis. Lancet Infect Dis. (2013) 13:182. doi: 10.1016/S1473-3099(12)70143-3
113. Ding W, Wang J, Zhao S, Yang Q, Sun H, Yan J, et al. Clinicopathological study of pulmonary extranodal nature killer/T-cell lymphoma, nasal type and literature review. Pathol Res Pract. (2015) 211:544–9. doi: 10.1016/j.prp.2015.04.002
114. Martin-Moro JG, Calleja JM, Garcia MB, Carretero JL, Rodriguez JG. Rhinoorbitocerebral mucormycosis: a case report and literature review. Med Oral Patol Oral Cir Bucal. (2008) 13:E792–5.
115. Yanagi H, Nakamura Y, Takagi D, Kubota K. Extranodal natural killer/T-cell lymphoma: a diagnostic dilemma. Rhinology. (2012) 50:325–31. doi: 10.4193/Rhino11.245
116. Segundo JB, Da Silva MA, Filho WE, Nascimento AC, Vidal FC, Bezerra GF, et al. Cerebral aspergillosis in a patient with leprosy and diabetes: a case report. BMC Res Notes. (2014) 7:689. doi: 10.1186/1756-0500-7-689
117. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. (2012) 54(Suppl. 1):S16–22.
118. Muhm M, Zuckermann A, Prokesch R, Pammer J, Hiesmayr M, Haider W. Early onset of pulmonary mucormycosis with pulmonary vein thrombosis in a heart transplant recipient. Transplantation. (1996) 62:1185–7. doi: 10.1097/00007890-199610270-00029
119. Reategui Schwarz E, Oikonomou KG, Reynolds M, Kim J, Balmiki RL, Sterling SA. Extranodal NK/T-cell lymphoma, nasal type, presenting as refractory Pseudomonas aeruginosa facial cellulitis. J Investig Med High Impact Case Rep. (2017) 5:2324709617716471. doi: 10.1177/2324709617716471
120. Seton M, Pless M, Fishman JA, Caruso PA, Hedley-Whyte ET. Case records of the massachusetts general hospital. Case 18-2008. A 68-year-old man with headache and visual changes after liver transplantation. N Engl J Med. (2008) 358:2619–28. doi: 10.1056/NEJMcpc0802849
121. Chen CS, Miller NR, Lane A, Eberhart C. Third cranial nerve palsy caused by intracranial extension of a sino-orbital natural killer T-cell lymphoma. J Neuroophthalmol. (2008) 28:31–5. doi: 10.1097/WNO.0b013e3181674228
122. Gonzalez-Sistal A, Baltasar-Sanchez A, Menendez P, Arias JI, Ruibal A. Breastfeeding and Immunohistochemical Expression of ki-67, p53 and Bcl2 in Infiltrating Lobular Breast Carcinoma. PLoS One. (2016) 11:e0151093. doi: 10.1371/journal.pone.0151093
123. Trihia H, Murray S, Price K, Gelber RD, Golouh R, Goldhirsch A, et al. Ki-67 expression in breast carcinoma: its association with grading systems, clinical parameters, and other prognostic factors–a surrogate marker? Cancer. (2003) 97:1321–31. doi: 10.1002/cncr.11188
124. Ruibal P, Oestereich L, Ludtke A, Becker-Ziaja B, Wozniak DM, Kerber R, et al. Unique human immune signature of Ebola virus disease in Guinea. Nature. (2016) 533:100–4. doi: 10.1038/nature17949
125. Borg AJ, Kumagai-Braesch M, Moller E. 15-Deoxyspergualin inhibits interleukin 6 production in in vitro stimulated human lymphocytes. Transpl Immunol. (1996) 4:133–43. doi: 10.1016/s0966-3274(96)80007-4
126. Eisenblatter M, Buchal A, Gayum H, Jasny E, Renner Viveros P, Ulrichs T, et al. Nocardia farcinica activates human dendritic cells and induces secretion of interleukin-23 (IL-23) rather than IL-12p70. Infect Immun. (2012) 80:4195–202. doi: 10.1128/IAI.00741-12
127. Mehrotra J, Bisht D, Tiwari VD, Sinha S. Serological distinction of integral plasma membrane proteins as a class of mycobacterial antigens and their relevance for human T cell activation. Clin Exp Immunol. (1995) 102:626–34. doi: 10.1111/j.1365-2249.1995.tb03863.x
128. Junior AM, De Amorim Carvalho FA, De Oliveira Dantas W, Gomes LC, Da Silva AB, De Sousa Cavalcante MM, et al. Does Leishmaniasis disease alter the parenchyma and protein expression in salivary glands? Exp Biol Med (Maywood). (2016) 241:359–66. doi: 10.1177/1535370215614658
129. Nicodemo AC, Duailibi DF, Feriani D, Duarte MIS, Amato VS. Mucosal leishmaniasis mimicking T-cell lymphoma in a patient receiving monoclonal antibody against Tnfalpha. PLoS Negl Trop Dis. (2017) 11:e0005807. doi: 10.1371/journal.pntd.0005807
130. Shibahara T, Mitarai Y, Ishikawa Y, Sato M, Kadota K. Bovine nasal eosinophilic, granuloma with blood eosinophilia caused by Nocardia species. Aust Vet J. (2001) 79:363–5. doi: 10.1111/j.1751-0813.2001.tb12016.x
131. Van Putten JW, Van Haren EH, Lammers JW. Association between Wegener’s granulomatosis and Staphylococcus aureus infection? Eur Respir J. (1996) 9:1955–7.
132. Asakura T, Ishii M, Kikuchi T, Kameyama K, Namkoong H, Nakata N, et al. Disseminated Mycobacterium marinum infection with a destructive nasal lesion mimicking extranodal Nk/T cell lymphoma: a case report. Medicine (Baltimore). (2016) 95:e3131. doi: 10.1097/MD.0000000000003131
133. Cong J, Wang C, Ma L, Zhang S, Wang J. Septicemia and pneumonia due to Mycobacterium fortuitum infection in a patient with extronodal NK/T-cell lymphoma, nasal type: a case report. Medicine (Baltimore). (2017) 96:e6800. doi: 10.1097/MD.0000000000006800
134. Terayama Y, Matsuura T, Uchida M, Narama I, Ozaki K. Probiotic (yogurt) containing Lactobacillus gasseri Oll2716 is effective for preventing Candida albicans-induced mucosal inflammation and proliferation in the forestomach of diabetic rats. Histol Histopathol. (2016) 31:689–97. doi: 10.14670/HH-11-710
135. Potenza L, Vallerini D, Barozzi P, Riva G, Forghieri F, Zanetti E, et al. Mucorales-specific T cells emerge in the course of invasive mucormycosis and may be used as a surrogate diagnostic marker in high-risk patients. Blood. (2011) 118:5416–9. doi: 10.1182/blood-2011-07-366526
136. Ibrahim AS, Spellberg B, Avanessian V, Fu Y, Edwards JEJr Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro. Infect Immun. (2005) 73:778–83. doi: 10.1128/IAI.73.2.778-783.2005
137. Schmidt S, Tramsen L, Perkhofer S, Lass-Florl C, Hanisch M, Roger F, et al. Rhizopus oryzae hyphae are damaged by human natural killer (Nk) cells, but suppress Nk cell mediated immunity. Immunobiology. (2013) 218:939–44. doi: 10.1016/j.imbio.2012.10.013
138. Teixeira MJ, Fonoff ET, Machado Ldos R, Nobrega JP, Sterman-Neto H, Amorim RL. Paracoccidioidomycosis: intralesional therapy. Arq Neuropsiquiatr. (2010) 68:458–9. doi: 10.1590/s0004-282x2010000300024
139. Egelund EF, Egelund TA, Ng JS, Wassil SK, Peloquin CA. Posaconazole pharmacokinetics in a 2-year-old boy with rhino-cerebral-orbital zygomycosis. Pharmacotherapy. (2013) 33:e1–8. doi: 10.1002/phar.1172
140. Wang A, Li RY, Wang DL. Pathogenesis of Rhizomucor variabilis in mouse. J Clin Dermatol [in Chinese]. (1996) 96:264–72.
141. Fredricks DN, Relman DA. Sequence-based identification of microbial pathogens: a reconsideration of Koch’s postulates. Clin Microbiol Rev. (1996) 9:18–33. doi: 10.1128/CMR.9.1.18
142. Evans AS. Causation and disease: a chronological journey. The thomas parran lecture. Am J Epidemiol. (1978) 108:249–58.
143. Ellermann V, Bang O. Experimentelle leukamie bei huhnern. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. (1908) 46:595–7.
144. Rous P. A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J Exp Med. (1911) 13:397–411. doi: 10.1084/jem.13.4.397
145. Epstein MA, Achong BG, Barr YM. Virus Particles in cultured lymphoblasts from burkitt’s lymphoma. Lancet. (1964) 1:702–3. doi: 10.1016/s0140-6736(64)91524-7
147. Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. (2010) 10:878–89. doi: 10.1038/nrc2961
148. Aherfi S, Colson P, Audoly G, Nappez C, Xerri L, Valensi A, et al. Marseillevirus in lymphoma: a giant in the lymph node. Lancet Infect Dis. (2016) 16:e225–34. doi: 10.1016/S1473-3099(16)30051-2
Keywords: lethal midline granuloma, mucor irregularis, Rhizopus arrhizus, rhino-orbital-cerebral mycosis, facial destruction, extranodal nK/T-cell lymphoma, nasal type
Citation: Li DM and Lun LD (2022) Rhino-Orbital-Cerebral Mycosis and Extranodal Natural Killer or/and T-Cell Lymphoma, Nasal Type. Front. Med. 9:851208. doi: 10.3389/fmed.2022.851208
Received: 09 January 2022; Accepted: 06 May 2022;
Published: 17 June 2022.
Edited by:
Giusto Trevisan, University of Trieste, ItalyReviewed by:
Nicola Di Meo, University of Trieste, ItalyCopyright © 2022 Li and Lun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Ming Li, ZG9yaXNsaUAxMjYuY29t, RG9uZ21pbmdsaUBiam11LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.