
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Med., 11 March 2022
Sec. Rheumatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.850858
This article is part of the Research TopicRheumatic Diseases and InfectionView all 7 articles
A correction has been applied to this article in:
Corrigendum: The Impact of Anti-rheumatic Drugs on the Seroprevalence of Anti-SARS-CoV-2 Antibodies in a Cohort of Patients With Inflammatory Arthritis: The MAINSTREAM Study
 Ennio Giulio Favalli1,2*†
Ennio Giulio Favalli1,2*† Andrea Gobbini3†
Andrea Gobbini3† Mauro Bombaci3†
Mauro Bombaci3† Gabriella Maioli1,2
Gabriella Maioli1,2 Martina Biggioggero1
Martina Biggioggero1 Elisa Pesce3
Elisa Pesce3 Andrea Favalli3
Andrea Favalli3 Martina Martinovic3
Martina Martinovic3 Tanya Fabbris3
Tanya Fabbris3 Edoardo Marchisio4
Edoardo Marchisio4 Alessandra Bandera5,6,7
Alessandra Bandera5,6,7 Andrea Gori5,6,7
Andrea Gori5,6,7 Sergio Abrignani3†
Sergio Abrignani3† Renata Grifantini3†
Renata Grifantini3† Roberto Caporali1,2†
Roberto Caporali1,2†Objectives: Given the high occurrence of asymptomatic subsets, the true prevalence of SARS-CoV-2 infection in rheumatic patients is still underestimated. This study aims to evaluate the seroprevalence of SARS-CoV-2 antibodies in rheumatic musculoskeletal diseases (RMD) patients receiving immunomodulatory drugs.
Methods: All consecutive patients with rheumatoid arthritis or spondyloarthritis receiving disease-modifying antirheumatic drugs (DMARDs) evaluated between 4th May and 16th June 2020 were included. All participants were tested for anti-SARS-CoV-2 antibodies (IgG, IgM, IgA) by ELISA and were questioned about previous COVID-19 symptoms and clinical course. Results were compared with healthy population from the same region and with a control group of healthy subjects diagnosed with confirmed COVID-19.
Results: The study population includes 358 patients. The overall prevalence of anti-SARS-CoV-2 antibodies (18.4%) was higher than prevalence rate based on swab-positivity (1.12%) or clinically suspected cases (10.6%), but consistent with seroprevalence observed in the healthy population. Among seropositive patients 58% were asymptomatic. Mean anti-SARS-CoV-2 titer was comparable with the control group. No differences in seroprevalence were observed according to age, sex, rheumatic disease and treatment with conventional, biologic or targeted synthetic DMARDs, whereas glucocorticoids and comorbidities resulted in higher seroprevalence rate.
Conclusions: The results of this study are reassuring about the low impact of RMDs and immunomodulatory therapies on the risk and clinical course of COVID-19 and on humoral immune response to SARS-CoV-2 infection.
SARS-CoV-2 infection, which exploded as a pandemic during 2020 causing over three million deaths worldwide, has an extremely variable spectrum of clinical presentation from pauci-symptomatic flu-like subsets to a critical disease with respiratory failure and multiorgan dysfunction, potentially resulting in death (1). In this scenario, it is imperative to clarify whether patients with rheumatic musculoskeletal diseases (RMDs) carry a higher risk of contracting Coronavirus Disease 2019 (COVID-19) or experience a more severe course of infection than the general population (2).
Several epidemiological studies based on the observation of infectious symptoms and nasopharyngeal swab (NPS) positivity have demonstrated a similar prevalence of COVID-19 in rheumatic patients and healthy population, suggesting that RMDs and their immunosuppressive treatment do not confer an increased susceptibility to infection (3–6). However, NPS is inaccurate in detecting the disease with a false-negativity rate of at least 20% (7), and a very large proportion of subjects have an asymptomatic infection that makes them escape common diagnostic procedures (8). Thus, studies based on the seroprevalence of anti-SARS-CoV-2 antibodies can decisively contribute to clarify the real spread of COVID-19 (9, 10). To date, such data in RMD patients are still very limited (11, 12), leaving many questions open that are essential for the proper management of these fragile patients. In addition, insights into the humoral response of RMD patients are of critical interest to optimize vaccination strategies.
To fill this gap, we conducted an observational seroprevalence study in a cohort of inflammatory arthritis patients receiving immunomodulatory drugs.
The study included all consecutive patients aged ≥18 years and living in Lombardy, diagnosed with rheumatoid arthritis (RA) or spondyloarthritis (SpA), with a follow-up visit scheduled between 4th May and 16th June 2020 at the outpatient rheumatology clinic of ASST Gaetano Pini-CTO Institute in Milan, a tertiary referral medical institution. All patients were receiving disease-modifying antirheumatic drugs (DMARDs) including conventional synthetic DMARDs (csDMARDs) (methotrexate, leflunomide, sulfasalazine, hydroxychloroquine, cyclosporin), biologic DMARDs (bDMARDs) (anti-TNFα mAbs; anti-IL-6R mAbs: CTLA-4-Ig; anti-IL 23 mAb; anti-IL 17A mAbs: anti-CD 20 mAb; IL1-RA/anti-IL 1β) or targeted synthetic (tsDMARDs) (Janus kinase inhibitors or PDE4 inhibitor), alone or in combination. The analysis was approved by the Ethics Committee Milano Area 2. All included patients signed an informed consent to participate in the study. A cohort of healthy residents in Lombardy (n = 64.660) included in a nationwide seroprevalence study conducted by the National Institute of Statistics (ISTAT) during the same timeframe was used as a control group (external control group) for seroprevalence comparison (13). In addition, a group of non-RMD subjects (n = 13) recovered from mild-to-moderate COVID-19 confirmed by NPS was used as a comparator for anti-SARS-CoV-2 antibody titer analysis.
Demographic and clinical data were collected during the scheduled visit. In addition, with regard to the period between the onset of the pandemic in Lombardy (25th February 2020) and the visit, we recorded the occurrence of signs/symptoms suggestive of COVID-19; a potential diagnosis of COVID-19 based on NPS; any admission to ordinary hospital or intensive care unit (ICU) because of COVID-19; any close contact with established COVID-19 cases; the maintenance of usual rheumatological therapy throughout the selected period. We defined patients with positive RT-PCR NPS as confirmed COVID-19, whereas patients who developed respiratory symptoms compatible with a mild viral infection but had no access to NPS were defined as highly suspicious COVID-19, according to the relevance of registered signs/symptoms as reported in Supplementary Table 1 (two major signs/symptoms or one major sign/symptom plus at least two minor signs/symptoms).
Sera obtained from both the participants and the internal control group were processed by using ELISA assays-based tests from Diapro (Milan, Italy). We tested IgG, IgM and IgA against SARS-CoV-2 receptor binding domain (RBD) and nucleocapsid (N), whose detection is more sensitive than spike subunits (S1/S2) (14). According to manufacturer's instructions, the cut-off for the definition of a positive sample was set at S/Co > 1.5. In the ISTAT control group seroprevalence was determined as positivity for anti-N IgG (by Abbott Architect anti-SARS-CoV-2 ELISA kit).
The comparative analysis of anti-SARS-CoV-2 titer vs. the internal control group and the search for factors associated with SARS-CoV-2 IgG positivity was conducted only on anti-RBD which has been shown to provide the best specificity for SARS-CoV-2 with limited cross-reactivity with other coronaviruses. Differences between subgroups were analyzed by a chi-squared test for categorical variables. The comparison between different subgroups was analyzed with Kruskal-Wallis test or Mann-Whitney test. Comparison of anti-N IgG seroprevalence with the ISTAT cohort was performed by a chi-square test. Statistical analyses were performed using SPSS statistical software, version 20.0 (SPSS, Chicago, IL, USA). P-values equal to or < 0.05 were considered statistically significant.
The study population included 358 patients diagnosed with RA (n = 200, 56%) or SpA (n = 158, 44%). Mean age was 54.2 (± 13.9) years, and 64% were female. Age and gender distribution were as expected according to the specific type of arthritis.
All patients were on stable treatment with DMARDs for at least 6 months, comprising b/tsDMARDs (N = 300), alone or in combination with conventional treatment, and csDMARDs alone (N = 58) (mainly methotrexate) (Table 1). Among targeted therapies, anti-tumor necrosis factor (TNFα), anti-interleukin-6 receptor (IL6R) and Cytotoxic T-lymphocyte associated antigen-4 immunoglobulin fusion proteins (CTLA4-Ig) were the most commonly used (48.3, 11.7, and 9.8%, respectively). Approximately one-third of the patients were on concurrent chronic treatment with glucocorticoids (GC, mean dose 4 mg daily, prednisone equivalent). The majority of enrolled patients had a long-term established disease (median disease duration 15 years).
Throughout the study period, 38 patients were defined as highly suspicious COVID-19, four as confirmed COVID-19, 33 reported close contacts with established COVID-19 cases. The most frequently reported symptoms were cough (12.8%), asthenia (12.5%), fever (10.9%), and ageusia/anosmia (4.5%). Five patients (four confirmed COVID-19 and one with negative NPS) required hospitalization with low-flow oxygen supplementation. No patient has been admitted to ICU and no deaths have been reported (Supplementary Figure 1).
Antibody specificity data are reported in Table 2. Briefly, 66 (18.4%) out of 358 patients tested positive for at least one anti-SARS-CoV-2 antibody (IgG/IgM/IgA, anti-RBD/N), compared with four out of 358 patients (1.12%) with swab-confirmed COVID-19 (p < 0.0001). Most seropositive patients tested positive for a single antibody class (37/66, 56%), while one-third tested triple positivity (IgG+IgM+IgA). Among patients who tested positive to serological test, the majority (57.5%) reported no symptoms in the 2 months prior to the blood draw. Ten patients (15.4%) referred one or two symptoms, 8 (12.3%) three symptoms, 9 (13.8%) four or more symptoms. The most frequently reported symptoms were cough (23%), fever (18.5%), asthenia (18.5%), and smell or taste loss (15.4%). Four patients (6%) were hospitalized (Supplementary Figure 1). The difference in prevalence rate (seroprevalence vs. swab-confirmed cases) confirms that the spread of COVID-19 among the population is much higher than that clinically observed. At the same time, the detection of high fraction of asymptomatic cases among RMD patients under immunosuppressant therapies is quietly reassuring about the severity and clinical outcomes of COVID-19 in this population.
To compare the seroprevalence rate of SARS-CoV-2 between RMD patients and general population, we extrapolated data from the ISTAT survey (13), where the seroprevalence was defined by the detection of IgG against N protein. In our cohort, selecting the subjects positive for IgG anti-N, the seroprevalence rate was 7.8% (95% CI 5.2–11.3), thus comparable to that estimated in the general population resident in Lombardy region in the same period, that was 7.5% (95% CI 6.8–8.3%) (p = 0.50), with a similar rate of asymptomatic infections (28.5 vs. 27.3%, respectively; p = 0.81).
The magnitude of the antibody response to SARS-CoV-2 proteins in the 66 RMD seropositive patients, in terms of antibody levels against RBD and N proteins, was comparable between patients treated with b/ts-DMARDs and cs-DMARDs (Figure 1A), and it was not significantly impaired by concomitant treatment with GCs (data not shown, p = 0.56) (Figure 1A). Furthermore, the level of anti-RBD IgG titer in the RMD seropositive patients was similar to non-RMD control group (6.8 vs. 7.2 S/Co, respectively; p = 0.78) (Figure 1B). These data confirm the hypothesis that DMARDs treatment do not significantly impair the elicitation of antibody response.
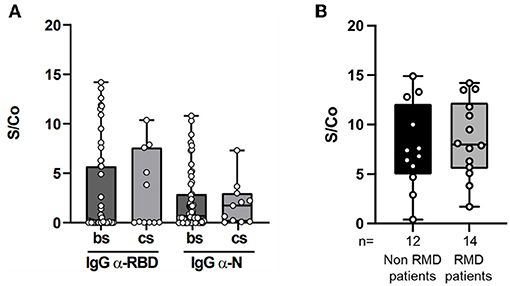
Figure 1. Magnitude of the anti-RBD and anti-N antibody response in RMD patients. (A) IgG levels against SARS-CoV-2 RBD (left panel) and N (right panel), expressed as Signal (S) vs. Control (Co) measured by ELISA in sera of RMD patients treated with b/ts-DMARD and cs-DMARD. (B) IgG levels against SARS-CoV-2 RBD in sera of RMD patients treated with b/ts-DMARD and cs-DMARD and in non-RMD patients not under 290 immunosuppressant treatment and RMD patients undergoing b/ts- or cs-DMARD treatment. Each dot into the box represent individual values, and bar min and max values. Statistical analyses were performed using Mann-Whitney t-test to compare two classes.
Factors associated with anti-RBD IgG positivity are shown in Table 3.
The highest rate of seropositivity was observed in patients classified as highly suspicious COVID-19 (18.4%; OR = 4.92, 95% CI = 1.84–13.1; p = 0.001) and those who had close contacts with confirmed COVID-19 cases (24.2%; OR = 7.66, 95% CI = 2.9–20.2; p < 0.0001). Seroprevalence was similar between woman and men (6.1 vs. 5.5%, p = ns) and was independent of age. No significant difference was identified between RA and SpA patients and anti-citrullinated protein antibodies (ACPA)/rheumatoid factor (RF) positivity did not influence the seroprevalence rate. An increased risk of seropositivity was observed in patients with at least one comorbidity (OR = 4.0, 95% CI = 1.57–10.19; p = 0.003), especially including hypertension (OR = 3.64, 95% CI = 1.48–8.88; p = 0.004) and obesity (OR = 4.77, 95% CI = 1.7–13.33; p = 0.003). The lack of comorbidities was associated with a significantly lower probability of anti-RBD positivity (OR = 0.25, 95% CI = 0.09–0.63; p = 0.004). Among ongoing anti-rheumatic drugs, no correlation emerged between anti-RBD positivity and therapy with conventional synthetic (including MTX), biological or targeted synthetic DMARDs. Conversely, GC therapy was associated with a dose-dependent progressive increase in the positivity rate resulting in a high OR for doses above 10 mg/day (OR = 6.97, 95% CI = 1.2–38.2, p = 0.02).
Finally, no factor has been found to correlate with the development of symptoms related to COVID-19 (Supplementary Table 2).
This seroprevalence study confirmed that the spread of SARS-CoV-2 infection among RMD patients was significantly wider than the prevalence estimated from the detection of symptomatic cases. In addition to the epidemiological results, the observation of a large proportion of asymptomatic cases and mild infections has great relevance with practical clinical implications, especially in the management of immunosuppressive therapy in this pandemic contest. In particular, no deaths or admissions to ICU were observed out of 66 subjects who tested positive for anti-SARS-CoV-2 antibodies in our cohort. These findings had not emerged from most of the studies conducted to date, which analyzed registry data of patients with confirmed COVID-19 only and selected mainly symptomatic infections with a more severe clinical course (15, 16). Furthermore, we have shown that both the seroprevalence of SARS-CoV-2 and the proportion of asymptomatic COVID-19 in RMD patients were consistent with that observed in the general population. Data from the seroprevalence survey on SARS-CoV-2 in Italy, conducted by ISTAT from 25th May to 15th July 2020, showed that the seroprevalence rate in the Italian general population (estimated on a sample of 64.660 persons) was 2.5% (95% CI 2.3–2.6) but reached a rate of 7.5% (95% CI 6.8–8.3%) in Lombardy, with maximum values recorded in the province of Bergamo (24%) and Cremona (19%). Our finding confirmed by a serological analysis what had already emerged in previous epidemiological studies conducted in the same and other geographical areas but limited by the lack of serological data on asymptomatic subjects (3–6). Other seroprevalence studies have been conducted worldwide during the first wave of the pandemic, but none of these was focused on the comparison between RMD and non-RMD patients (17–19). In addition, we demonstrated that the magnitude of the immune response in RMD patients was not significantly altered by different classes of DMARDs and it seemed to be comparable to a non-immunosuppressed control group.
Despite the theoretical fragility of RMD patients related to immunosuppressive therapies, our results showed that treatment with any kind of DMARD is not associated with an increased risk of SARS-CoV-2 infection. However, our serological analysis confirms the increased dose-dependent infectious risk associated with the chronic use of GCs (20), which is already known from several studies conducted on other types of infection and also emerged in a recent epidemiological analysis conducted by our group (21). Beyond the anti-rheumatic treatment, in our cohort the risk factors for SARS-CoV-2 infection were confirmed as those already known in the general population, namely the presence of comorbidities such as hypertension and obesity (22). The comparative analysis and the search for factors associated with SARS-CoV-2 seropositivity was conducted only on anti-RBD IgG which has been shown to provide the best specificity for SARS-CoV-2 and appear to decrease more slowly over time than levels of other classes of antibody (23, 24). IgM and IgA antibodies have been included to define the seropositivity rate but no comparative analysis has been performed on these antibody classes. However, we found that patients who tested positive to IgM or IgA and negative to IgG had higher rate of asymptomatic infections than IgG positive patients. Since both IgM and IgA have been shown to appear in the first 3 to 4 days of SARS-CoV-2 infection (25), our hypothesis is that we identified patients in the very early phase of COVID-19, before they developed symptoms or even seroconverted to IgG.
In conclusion, given the still pressing need to optimize the management of RMD patients during the pandemic and to organize their prioritization of access to the vaccine programme, the results of this study are reassuring about the low impact of RMDs and immunomodulatory therapies on the risk and clinical course of COVID-19. Our results provide a further contribution to the management strategy of RMD patients, in the direction of reaffirming the importance of maintaining ongoing anti-rheumatic therapy even during the most critical phases of the pandemic.
Moreover, the antibody titer measured in RMD patients, which was comparable to that of the control population, suggests an adequate humoral response to SARS-CoV-2 infection and, presumably, to vaccination.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee Milano Area 2. The patients/participants provided their written informed consent to participate in this study.
EF, MBi, and GM collected clinical data and blood samples. EF and GM drafted the manuscript. MBo, EP, AF, TF, and MM contributed to experimental data and related analysis. AG made data elaboration and statistical analysis. EM provided the ELISA kits for analysis. RG, SA, and RC drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
The project was co-financed by Lombardy 2014–2020 Operational Program under the European Regional Development Fund (MAINSTREAM project).
EM is employed by Dia.Pro, Diagnostic Bioprobes Srl.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Dr. Elena Agape, Dr. Tommaso Schioppo, Dr. Isabella Scotti, and Dr. Tania Ubiali of ASST Gaetano Pini-CTO for the contribution on sampling collection and to the survey and patients' interviews and Elena Zagato of Istituto Nazionale Genetica Molecolare for the contribution to the final analysis. We are grateful to patients that have generously consented to the study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.850858/full#supplementary-material
1. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. (2020) 395:1763–70. doi: 10.1016/S0140-6736(20)31189-2
2. Favalli EG, Ingegnoli F, De Lucia O, Cincinelli G, Cimaz R, Caporali R. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun Rev. (2020) 19:102523. doi: 10.1016/j.autrev.2020.102523
3. Favalli EG, Ingegnoli F, Cimaz R, Caporali R. What is the true incidence of COVID-19 in patients with rheumatic diseases? Ann Rheum Dis. (2021) 80:e18–e18. doi: 10.1136/annrheumdis-2020-217615
4. Michelena X, Borrell H, López-Corbeto M, López-Lasanta M, Moreno E, Pascual-Pastor M, et al. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheu. (2020) 50:564–70. doi: 10.1016/j.semarthrit.2020.05.001
5. Monti S, Balduzzi S, Delvino P, Bellis E, Quadrelli VS, Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. (2020) 79:667. doi: 10.1136/annrheumdis-2020-217424
6. Fredi M, Cavazzana I, Moschetti L, Andreoli L, Franceschini F. COVID-19 in patients with rheumatic diseases in northern Italy: a single-centre observational and case–control study. Lancet Rheu. (2020) 2:e549–56. doi: 10.1016/S2665-9913(20)30169-7
7. Gopaul R, Davis J, Gangai L, Goetz L. Practical diagnostic accuracy of nasopharyngeal swab testing for novel coronavirus disease 2019 (COVID-19). West J Emerg Med. (2020) 21:1–4. doi: 10.5811/westjem.2020.8.48420
8. Kronbichler A, Kresse D, Yoon S, Lee KH, Effenberger M, Shin JI. Asymptomatic patients as a source of COVID-19 infections: a systematic review and meta-analysis. Int J Infect Dis. (2020) 98:180–6. doi: 10.1016/j.ijid.2020.06.052
9. Markewitz R, Torge A, Wandinger K-P, Pauli D, Franke A, Bujanda L. Clinical correlates of anti-SARS-CoV-2 antibody profiles in Spanish COVID-19 patients from a high incidence region. Sci Rep-uk. (2021) 11:4363. doi: 10.1038/s41598-021-83969-5
10. Sood N, Simon P, Ebner P, Eichner D, Reynolds J, Bendavid E, et al. Seroprevalence of SARS-CoV-2-Specific antibodies among adults in Los Angeles County, California, on april 10-11, 2020. JAMA. (2020) 323:2425–7. doi: 10.1001/jama.2020.8279
11. Simon D, Tascilar K, Krönke G, Kleyer A, Zaiss MM, Heppt F, et al. Patients with immune-mediated inflammatory diseases receiving cytokine inhibitors have low prevalence of SARS-CoV-2 seroconversion. Nat Commun. (2020) 11:3774. doi: 10.1038/s41467-020-17703-6
12. Teng J, Dai J, Su Y, Zhou Z, Chi H, Wan L, et al. Detection of IgM and IgG antibodies against SARS-CoV-2 in patients with autoimmune diseases. Lancet Rheu. (2020) 2:e384–5. doi: 10.1016/S2665-9913(20)30128-4
13. Primi risultati dell'indagine di sieroprevalenza sul SARS-CoV-2. Available online at: https://www.istat.it/it/archivio/246156 (accessed January 13, 2021).
14. Brochot E, Demey B, Touzé A, Belouzard S, Dubuisson J, Schmit JL, et al. Anti-spike, anti-nucleocapsid and neutralizing antibodies in SARS-CoV-2 inpatients and asymptomatic individuals. Front Microbiol. (2020) 11:584251. doi: 10.3389/fmicb.2020.584251
15. Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. (2020) 79:859–66. doi: 10.1136/annrheumdis-2020-217871
16. Scirè CA, Carrara G, Zanetti A, Landolfi G, Chighizola C, Alunno A, et al. COVID-19 in rheumatic diseases in Italy: first results from the Italian registry of the Italian Society for Rheumatology (CONTROL-19). Clin Exp Rheumatol. (2020) 38:748–53.
17. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernàn MA, Pérez-Olmeda B, et al. Prevalence of SARS-CoV-2 in 71 Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. (2020) 396:535–44. doi: 10.1016/S0140-6736(20)31483-5
18. Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. (2020) 396:313–9. doi: 10.1016/S0140-6736(20)31304-0
19. Eviatar T, Furer V, Polachek A, Levartovsky D, Elalouf O, Zisapel M, et al. Seroprevalence of SARS-CoV-2 antibodies in patients with autoimmune inflammatory rheumatic diseases. Clin Exp Rheumatol. (2021). doi: 10.1136/annrheumdis-2021-eular.2107. [Epub ahead of print].
20. Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheumatic Dis Clin North Am. (2015) 42:157–76, ix–x. doi: 10.1016/j.rdc.2015.08.004
21. Favalli EG, Bugatti S, Klersy C, Biggioggero M, Rossi S, De Lucia O, et al. Impact of corticosteroids and immunosuppressive therapies on symptomatic SARS-CoV-2 infection in a large cohort of patients with chronic inflammatory arthritis. Arthritis Res Ther. (2020) 22:290. doi: 10.1186/s13075-020-02395-6
22. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
23. Qu J, Wu C, Li X, Zhang G, Jiang Z, Li X, et al. Profile of immunoglobulin G and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. (2020) 71:2255–8. doi: 10.1093/cid/ciaa489
24. Iyer AS, Jones FK, Nodoushani A, Kelly M, Becker M, Slater D, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. (2020) 5:eabe0367. doi: 10.1126/sciimmunol.abe0367
Keywords: seroprevalence, SARS-CoV-2, humoral response, rheumatic musculoskeletal diseases, disease-modifying anti-rheumatic drugs, risk of infection, COVID-19
Citation: Favalli EG, Gobbini A, Bombaci M, Maioli G, Biggioggero M, Pesce E, Favalli A, Martinovic M, Fabbris T, Marchisio E, Bandera A, Gori A, Abrignani S, Grifantini R and Caporali R (2022) The Impact of Anti-rheumatic Drugs on the Seroprevalence of Anti-SARS-CoV-2 Antibodies in a Cohort of Patients With Inflammatory Arthritis: The MAINSTREAM Study. Front. Med. 9:850858. doi: 10.3389/fmed.2022.850858
Received: 08 January 2022; Accepted: 10 February 2022;
Published: 11 March 2022.
Edited by:
Lingli Dong, Huazhong University of Science and Technology, ChinaReviewed by:
Waleed Mahallawi, Taibah University, Saudi ArabiaCopyright © 2022 Favalli, Gobbini, Bombaci, Maioli, Biggioggero, Pesce, Favalli, Martinovic, Fabbris, Marchisio, Bandera, Gori, Abrignani, Grifantini and Caporali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ennio Giulio Favalli, ZW5uaW9mYXZhbGxpQG1lLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.