- Qingdao Women and Children's Hospital Affiliated to Qingdao University, Qingdao, China
Objective: To evaluate whether the intrauterine perfusion of platelet-rich plasma (PRP) before frozen-thawed embryo transfer (FET) improves the pregnancy outcomes of patients with repeated implantation failure (RIF).
Methods: This retrospective study included 288 infertile women with RIF after undergoing in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) treatment from October 1, 2019, to January 1, 2021, at Qingdao Women and Children's Hospital. Patients were divided into two groups according to whether they received PRP intrauterine perfusion before embryo transfer in FET cycles. 138 women were in the PRP group, 150 women were in the control group. The primary outcome measure was live birth rates and the secondary outcome were clinical pregnancy, positive β hCG, miscarriage and implantation rates.
Results: No significant differences in baseline demographic and clinical characteristics were observed between the two groups. Overall, significantly more women in the PRP group than in the control group achieved a live birth rate (41 women; 29.71% vs. 27 women; 18%) and a clinical pregnancy (50 women; 36.23% vs. 37 women; 24.67%). The PRP group had a higher implantation rate and lower spontaneous miscarriage rate than the control group, but these differences were not statistically significant. No pregnancy outcome difference between two groups in PCOS patients with RIF.
Conclusion: Our results showed that intrauterine perfusion of PRP before embryo transfer in FET cycles can significantly increase the live birth and clinical pregnancy rates in patients with RIF.
Introduction
In the context of in vitro fertilization-embryo transfer (IVF-ET), RIF refers to patients for whom implantation fails after repeated transfers of morphologically good-quality embryos into a normal uterus (1). Despite great progress in the application of IVF-ET to infertile couples, recurrent implantation failure (RIF) following embryo transfer (ET) continues to be a major problem affecting about 10% of women causing great psychological and economic burden to individuals and their families (2, 3), New strategies to improve the pregnancy likelihoods are urgently needed for these patients.
The successful implantation depends on embryo quality and endometrial receptivity. Endometrial receptivity is a complex process that involves the participation of and regulation by many molecules, including cytokines, growth factors, adhesion molecules, and cytoskeletal proteins, among other factors (4). Patients with RIF are in most due to endometrial receptivity disorders. numerous studies have investigated ways to improve endometrial receptivity, and the methods examined have included low-dose aspirin therapy, intrauterine infusion of human chorionic gonadotrophin (HCG) or colony cell-stimulating factor (CSF), endometrial injury and so like (5–8), however, the optimal technique remains unclear.
PRP is an autologous blood-derived concentrate of platelets from peripheral blood with a considerable concentration of growth factors and cytokines. Since its introduction in 1970, PRP has been widely used as a form of regenerative treatment in multiple medical fields, such as sports medicine, maxillofacial surgery and cosmetology. Studies in recent years have shown great promise in reproduction fields. Many studies have demonstrated that autologous PRP can regenerate and thicken the thin endometrium and decrease the likelihood of implantation failure leading to good pregnancy outcomes (9–11), however the patient's heterogeneity and small sample size of the subjects led to different research results, therefore this research analyzed the use of PRP before embryo transfer in RIF patients of frozen thawed cycles and its efficacy in this population.
Materials and Methods
Study Design
This retrospective study was carried at reproductive center of Qingdao Women and Children's Hospital. We retrospectively analyzed the records of 410 patients with history of RIF underwent FET in Qingdao Women and Children's Hospital from October 1, 2019, to January 1, 2021. 138 patients (screened from 168 patients) who received PRP intrauterine perfusion before embryo transfer in frozen thawed cycles were collected, 150 patients (screened from 242 patients) with history of RIF did not receive PRP intrauterine perfusion as the control group. We excluded 122 patients who did not meet the criteria and had incomplete data. No repeat treatment cycle for all patients. Clinicians and this PRP intrauterine treatment study were authorized by the ethics committee of Qingdao Women and Children's Hospital. Informed consent was obtained from all patients with PRP intrauterine perfusion on endometrial transformation day, follow-up was complete in all patients.
Patient Enrolment Criteria
Patients aged 23 to 40 years who had three or more consecutive failed embryo implantations with good-quality embryos (at least 6 cleavage-stage embryos or three blastocysts) were included in the study (12). The exclusion criteria were an abnormal karyotype from either partner, evidence of uterine defects, ultrasonographic evidence of hydrosalpinx, infections, endocrine problems, coagulation defects or autoimmune defects.
Endometrial preparation for frozen-thawed cycle included hormone replacement treatment (HRT) protocol (estrogen-progesterone cycle, EP) and natural cycle (NC). PRP intrauterine perfusion was performed 48–72 h before embryo transfer. One or two good quality embryos were transferred. The rates of live birth, clinical pregnancy, positive-hCG, miscarriages and implantation will be assessed. Serum β-hCG was measured 14 days after embryo transfer and was considered positive for β- hCG level ≥ 5IU. Clinical pregnancy was confirmed by transvaginal ultrasonography 30 days after embryo transfer.
Plate-Rich Plasma Preparation and Intrauterine Perfusion
Twenty milliliters of peripheral blood were collected from each patient and centrifuged at 500 × g for 10 min at 18°C. After centrifugation, three layers were visible, namely, the serum, platelets and red blood cells. The serum and platelets were transferred to another tube and centrifuged at 500 × g for another 10 min at 18°C. The supernatant was discarded, the remaining 1 mL PRP was infused into the uterine cavity using artificial insemination catheter 2 days before embryo transfer (13). The mean number of platelets in PRP was 1513.45 ± 322.18 × 109/L (Automated Hematology Analyzer sysmex xs-500i, Sysmex Corporation, Kobe, Japan). All perfusions were prepared and performed by a single operator.
Statistical Analysis
All statistical analyses were performed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Student's t-test was used for comparisons of continuous variables between the groups. The chi-squared test or Fisher's exact test, where appropriate, was used for comparisons of categorical variables. The results are presented as the mean ± standard deviation (SD). Statistical significance was set at a probability (P) value < 0.05.
Results
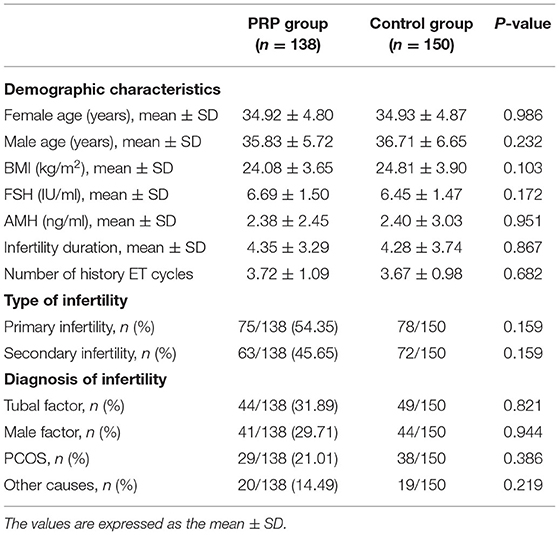
A total of 288 patients with an RIF history were included in this study; 138 patients undergoing intrauterine PRP perfusion before embryo transfer in frozen-thawed cycles were included in the PRP group, and the remaining 150 women who were not undergoing PRP perfusion were included in the control group. The mean age was 35.7 ± 4.6 years, the mean duration of infertility was 7.8 ± 3.7 years and the mean number of history embryo transfer cycles was 3.72 ± 1.09 and the control was 3.67 ± 0.98. No local or systemic side effects relating to the use of PRP perfusion were reported (Table 1).
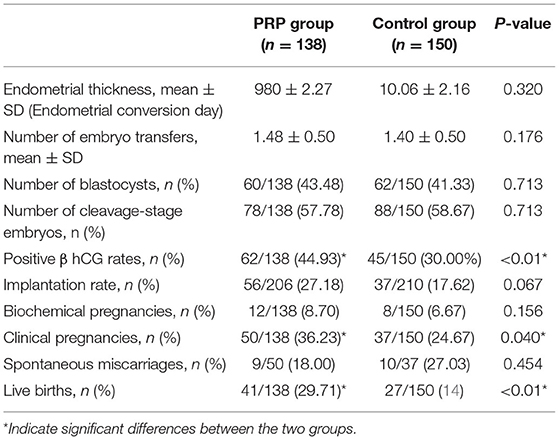
Regarding the patients' baseline demographic characteristics, we found no differences between the two groups in age, body mass index (BMI), duration of infertility, number of history cycles transferred or infertility diagnosis between the two groups. There were also no differences in cycle characteristics, such as endometrial thickness, number of days embryo transfer, or number of blastocysts or cleavage-stage embryos transferred between the two groups (Table 2).
To evaluate whether PRP improves the reproductive outcomes of patients with RIF, we first calculated the live birth rates and the clinical pregnancy rates in two groups. In our study, there were 41 live birth in the PRP intrauterine perfusion group (41/138) and 27 in the control group (27/150), the live birth rate was significantly higher in the PRP group (29.71%) than in the control group 18% (P < 0.01). Additionally, the clinical pregnancy rate was also higher in the PRP group 36.23% than in the control group 24.67% (P = 0.040). Further analysis of the secondary outcomes showed that the Positive β hCG rates is significantly higher in PRP group 44.93% (62/138) than the control group 28.66% (P < 0.01). None of other secondary outcome indices were significantly different between the two groups, that the implantation rates were 27.18% and 17.62% (P = 0.067), respectively; that the biochemical pregnancy rates were 7.97% and 6.675 (P = 0.156), respectively; and that the miscarriage rates were 18.0% and 27.03% (P = 0.454), respectively (Table 2). There is also no in clinical pregnancy and live birth rate difference between PRP group and control group in RIF PCOS patients (Table 2).
Discussion
This retrospective cohort study was performed to evaluate the pregnancy outcomes of RIF patients who underwent PRP intrauterine perfusion before embryo transfer and compare these results to a cohort of RIF patients who were not treated with PRP. We found there is significant different in live birth and clinical pregnancy rates, we have also found there is significantly different in positive β-hCG rates, no significantly difference were found in the implantation rates and miscarriages.
Yajie Chang, Sunita R, Tandulwadkar, and Eftekhar have demonstrated that autologous PRP intrauterine perfusion could expanded the thin endometrium and increase the clinical pregnancy and implantation rates in FET cycles (15–17), they think that PRP could regenerate and improve the endometrium receptivity leading to good pregnancy outcomes. Hakan Coksuer also found that PRP intrauterine perfusion could thicken the endometrium leading to good endometrial lining and optimal pregnancy outcomes in RIF patients (18). Xiaohan Wang and colleagues found that PRP significantly stimulated the growth, migration and adhesion of endometrial mesenchymal stem cells, these effects are probably because it contains or stimulates several growth factors such as transforming growth factor, Platelet-derived growth factor, vascular endothelial growth factor, insulin like growth factor, epithelial growth factor and bioactive-cytokines (14). However, these majority of studies had small sample cases, most less than 50 patients and almost all fewer than 100 patients. the live birth rate was not reported in most studies. Our study is relatively larger sample size of 238 patients, we found no difference in endometrium thickness between groups, the live birth rate, clinical pregnancy, positive β-HCG rates are higher than the control group, the results are consistent with the literature. We think those biological factors may acts in most by improving and triggering endometrial receptivity through the improvement of cell proliferation, vascularization, anti-inflammatory properties, rather than expanded endometrial growth.
However, contradictory views on the usefulness of PRP perfusion treatment in IVF reproductive medicine persists. Tehraninejad and Allahveisi found that the pregnancy outcomes, namely, the clinical and ongoing pregnancy rates, live birth rates were similar between the PRP intrauterine infusion group and the control group in RIF patients of FET cycles (10, 11). The reason for the inconsistency between our results and those of others might be attributable to the different subject cohorts, different definitions of RIF and wide variety of PRP preparation protocols. The PRP in our study contains leukocytes in addition to the platelets and plasma, it is categorized as leucocyte-poor PRP, not pure PRP. leukocytes might be also a source of several growth factors and cytokines (19). The concentrations of platelets and leukocytes obtained in PRP preparation depending on how the blood sample was treated, which may in turn impact the amount of various types of growth factors playing a key part in the treatment.
To date, only a few RCT studies on intrauterine infusion of PRP have been performed in the reproductive field. Eftekhar and Nazari suggested that PRP could expand the thin endometrium and improve clinical pregnancy outcomes (17, 20), while Allahveisi's study concluded that intrauterine infusion of PRP did not improve pregnancy outcomes in patients with recurrent implantation failure (11). Design of placebo in the control group was inconsistent across studies. The intrauterine perfusion of PRP is associated with the potential effect of the endometrial injury, is it possible the beneficial effect is due to the endometrial injury of the catheter? So we should design studies carefully, especially RCTs, larger scales, the standard PRP preparation procedure and the strict indication of the PRP treatment should be all considered.
Another notable point is that PRP intrauterine perfusion did not improve pregnancy outcomes in RIF PCOS patients. There is no significant difference between the two groups. This might be caused by the small sample size in this study. Besides, PCOS is a special group where obesity, insulin resistance, abnormal glucose metabolism and metabolic syndrome may alter the competence of oocytes, embryos and endometrium (21). Palomba's comprehensive review shows that among several interventions to improve endometrial receptivity in women with PCOS, only lifestyle modification, metformin and bariatric surgery have the scientific evidence for clinical benefit (22).
The strength of this study is that we included a large sample of RIF patients with the PRP intervention before embryo transfer in our reproductive center, which allows us to draw a conclusion on the efficacy of this strategy. The main drawback of this study was retrospective and its non-randomized design. The findings provide evidence for future randomized, controlled trials with large sample size in this field. One further prospective and high-quality randomized controlled trial is ongoing in our center to identify subpopulations that would benefit most from PRP therapy.
In summary, we analyzed the effect of intrauterine PRP treatment before embryo transfer in patients with RIF and found that the PRP intervention improved the live birth rate and clinical pregnancy rate.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Qingdao Women and Children's Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YX took part in the first draft and preparation of the manuscript. JF, XL, PX, and RM collected material and data. CH contributed to the concept, design and preparation of the manuscript. All authors were involved in the study conception and design and read and approved the final manuscript.
Funding
This study was supported by Qingdao Key Health Discipline Development Fund.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reprod Biomed Online. (2014) 28:14–38. doi: 10.1016/j.rbmo.2013.08.01
2. Busnelli A, Reschini M, Cardellicchio L, Vegetti W, Somigliana E, Vercellini P. How common is real repeated implantation failure? An indirect estimate of the prevalence. Reprod Biomed Online. (2020) 40:91–7. doi: 10.1016/j.rbmo.2019.10.014
3. Simon A, Laufer N. Assessment and treatment of repeated implantation failure (RIF). J Assist Reprod Genet. (2012) 29:1227–39. doi: 10.1007/s10815-012-9861-4
4. Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, et al. Molecular cues to implantation. Endocr Rev. (2004) 25:341–73. doi: 10.1210/er.2003-0020
5. Blois SM, Klapp BF, Barrientos G. Decidualization and angiogenesis in early pregnancy: unravelling the functions of DC and NK cells. J Reprod Immunol. (2011) 88:86–92. doi: 10.1016/j.jri.2010.11.002
6. Volovsky MHM, Maclachlan VB. Intrauterine human chorionic gonadotropin (HCG) infusion prior to embryo transfer (ET) may be detrimental to pregnancy rate. Fertility Sterility. (2016) 106:e52. doi: 10.1016/j.jaad.2019.07.024
7. Vlachadis N, Tsamadias V, Economou E. Aspirin to improve IVF unexplained implantation rates: time for an individualized approach. Reprod Biomed Online. (2014) 28:133. doi: 10.1016/j.rbmo.2013.09.007
8. Kunicki M, Lukaszuk K, Woclawek-Potocka I, Liss J, Kulwikowska P, Szczyptańska J. Evaluation of granulocyte colony-stimulating factor effects on treatment-resistant thin endometrium in women undergoing in vitro fertilization. Biomed Res Int. (2014) 2014:913235. doi: 10.1155/2014/913235
9. Coksuer H, Akdemir Y, Ulas Barut M. Improved in vitro fertilization success and pregnancy outcome with autologous platelet-rich plasma treatment in unexplained infertility patients that had repeated implantation failure history. Gynecol Endocrinol. (2019) 35:815–8. doi: 10.1080/09513590.2019.1597344
10. Tehraninejad ES, Kashani NG, Hosseini A, Tarafdari A. Autologous platelet-rich plasma infusion does not improve pregnancy outcomes in frozen embryo transfer cycles in women with history of repeated implantation failure without thin endometrium. J Obstet Gynaecol Res. (2021) 47:147–51. doi: 10.1111/jog.14445
11. Allahveisi A, Seyedoshohadaei F, Rezaei M, Bazrafshan N, Rahimi K. The effect of platelet-rich plasma on the achievement of pregnancy during frozen embryo transfer in women with a history of failed implantation. Heliyon. (2020) 6:e03577. doi: 10.1016/j.heliyon2020.e03577
12. Simon A, Laufer N. Repeated implantation failure: clinical approach. Fertil Steril. (2012) 97:1039–43. doi: 10.1016/j.fertnstert.2012.03.010
13. Cengiz IF, Oliveira JM, Reis RL. PRP Therapy. Adv Exp Med Biol. (2018) 1059:241–53. doi: 10.1007/978-3-319-76735-2_11
14. Wang X, Liu L, Mou S, Zhao H, Fang J, Xiang Y, et al. Investigation of platelet-rich plasma in increasing proliferation and migration of endometrial mesenchymal stem cells and improving pregnancy outcome of patients with thin endometrium. J Cell Biochem. (2018). doi: 10.1002/jcb.28014
15. Chang Y, Li J, Wei LN, Pang J, Chen J, Liang X. Autologous platelet-rich plasma infusion improves clinical pregnancy rate in frozen embryo transfer cycles for women with thin endometrium. Medicine. (2019) 98:e14062. doi: 10.1097/MD.0000000000014062
16. Tandulwadkar SR, Naralkar MV, Surana AD, Selvakarthick M, Kharat AH. Autologous intrauterine platelet-rich plasma instillation for suboptimal endometrium in frozen embryo transfer cycles: a pilot study. J Hum Reprod Sci. (2017) 10:208–2. doi: 10.4103/jhrs.JHRS_28_17
17. Eftekhar M, Neghab N, Naghshineh E, Khani P. Can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial Taiwan. J Obstet Gynecol. (2018) 57:810–3. doi: 10.1016/j.tjog.2018.10.007
18. Coksuer H, Akdemir Y, Ulas Barut M. Improved in vitro fertilization success and pregnancy outcome with autologous platelet-rich plasma treatment in unexplained infertility patients that had repeated implantation failure history. Gynecol Endocrinol. (2019) 35:815–8. doi: 10.1080/095135902019.1597344
19. Cerza F, Carnì S, Carcangiu A, Di Vavo I, Schiavilla V, Pecora A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. (2012) 40:2822–7. doi: 10.1177/0363546512461902
20. Nazari L, Salehpour S, Hoseini S, Zadehmodarres S, Azargashb E. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: a double-blind RCT. Int J Reprod Biomed. (2019) 17:443–8. doi: 10.18502/ijrm.v17i6.4816
21. Palomba S. Is fertility reduced in ovulatory women with polycystic ovary syndrome? An opinion paper. Hum Reprod. (2021) 36:2421–8. doi: 10.1093/humrep/deab181
Keywords: platelet-rich plasma (PRP), frozen-thawed embryo transfer (FET), intrauterine perfusion, live birth rate, clinical pregnancy rate (CPR)
Citation: Xu Y, Hao C, Fang J, Liu X, Xue P and Miao R (2022) Intrauterine Perfusion of Autologous Platelet-Rich Plasma Before Frozen-Thawed Embryo Transfer Improves the Clinical Pregnancy Rate of Women With Recurrent Implantation Failure. Front. Med. 9:850002. doi: 10.3389/fmed.2022.850002
Received: 07 January 2022; Accepted: 07 March 2022;
Published: 29 March 2022.
Edited by:
Isabella Fabietti, Bambino Gesù Children's Hospital (IRCCS), ItalyReviewed by:
Svend Lindenberg, Copenhagen Fertility Center, DenmarkStefano Palomba, University of Catanzaro, Italy
Copyright © 2022 Xu, Hao, Fang, Liu, Xue and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cuifang Hao, aGFvY3VpZmFuZyYjeDAwMDQwO3FkZmUuY29t
 Yangying Xu
Yangying Xu Cuifang Hao*
Cuifang Hao*