- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, South Korea
- 2Division of Cardiology, Department of Internal Medicine, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, South Korea
Background: Non-alcoholic fatty liver disease (NAFLD) and Helicobacter pylori (Hp) infection have a close association with an increased risk of cardiovascular disease. Metabolic dysfunction-associated fatty liver disease (MAFLD) is characterized by metabolic dysfunction in NAFLD. We investigated the synergistic effects of NAFLD/MAFLD and Hp infection on the risk of arterial stiffness in an asymptomatic population.
Methods: We included individuals who underwent abdominal ultrasonography, anti-Hp IgG antibody evaluations and cardio-ankle vascular index (CAVI) during health screening tests between January 2013 and December 2017. Arterial stiffness was defined using CAVI. A logistic regression model was used to analyze the independent and synergistic effects of NAFLD/MAFLD and Hp infection on the risk of arterial stiffness.
Results: Among 3,195 subjects (mean age 54.7 years, 68.5% male), the prevalence of increased arterial stiffness was 36.4%. In the multivariate analysis, subjects with NAFLD but without Hp infection and those with both NAFLD and Hp infection had a significantly higher risk of increased arterial stiffness [odds ratio (OR) 1.61, 95% confidence interval (CI) 1.15–2.26, and OR 2.23, 95% CI 1.63–3.06, respectively], than subjects without Hp infection and NAFLD. Regarding MAFLD, Hp infection additively increased the risk of arterial stiffness in subjects with MAFLD (OR 2.13, 95% CI 1.64–2.78).
Conclusions: An interactive effect of Hp infection on the risk of arterial stiffness in individuals with NAFLD/MAFLD was observed. Hp infection additively increases the risk of arterial stiffness in subjects with NAFLD or MAFLD.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a substantial public health burden, with a prevalence of up to 25% of global population (1). NAFLD is closely associated with various metabolic conditions, including obesity, dyslipidemia, type 2 diabetes, and cardiovascular disease (2). In particular, NAFLD is known to be related to arterial stiffness, a surrogate marker of systemic atherosclerosis (3). Recently, in recognition of the close association between NAFLD and metabolic dysfunction, a new term “metabolic (dysfunction)-associated fatty liver disease (MAFLD)” has been introduced, and studies on its clinical significance have been conducted (4, 5).
Helicobacter pylori (Hp) is a gram-negative microorganism that infects more than half of the global population (6). While Hp is considered to cause many gastrointestinal diseases, such as chronic gastritis, peptic ulcers and gastric cancer (7, 8), its role in extragastric diseases, including metabolic syndrome and hematological and cardiovascular diseases, has also been studied (9). The association between Hp infection and cardiovascular risk factors or arterial stiffness has been reported (10–12). Some mechanisms, including chronic inflammation, free radical formation, and the immune response, may be the link between chronic Hp infection and atherogenesis (13). Also, Hp infection has been associated with insulin resistance (14), which is closely linked with increased arterial stiffness (15, 16).
Since both Hp infection and NAFLD are involved in the pathogenesis of insulin resistance and share proinflammatory conditions (17), they are known to be independently associated with arterial stiffness. Based on this background, we hypothesized that the combination of NAFLD and Hp infection increases the risk of arterial stiffness. Little has been reported about the association of MAFLD with arterial stiffness. Thus, we aimed to investigate the interactive effects of NAFLD/MAFLD and Hp infection on arterial stiffness in an asymptomatic population.
Methods
Study Population
This retrospective cohort study included individuals who underwent routine health check-ups, including abdominal ultrasonography, anti-Hp IgG antibody testing and cardio-ankle vascular index (CAVI) evaluations, on the same day at the Seoul National University Hospital Healthcare System Gangnam Center from January 2013 to December 2017. The subjects were mostly symptom-free and willfully underwent examinations either voluntarily or were supported by their employers for the check-ups. Among the total eligible subjects, those who met the following criteria were excluded from the study: a prior history of ischemic heart disease, peripheral artery disease or stroke (n = 132), significant arrhythmia or valvular heart disease (n = 31), indeterminate anti-Hp IgG antibody results (n = 43), and a history of gastrectomy (n = 20) or Hp eradication (n = 777) (12). Finally, 3,195 subjects were included in the analysis. For the NAFLD analysis, subjects who displayed any potential cause of chronic liver disease were additionally excluded: 67 were positive for the hepatitis B virus, 29 were positive for the hepatitis C virus, and 763 had significant alcohol intake (>20 g/day). As a result, 2,357 subjects were included in the NAFLD analysis (Figure 1).
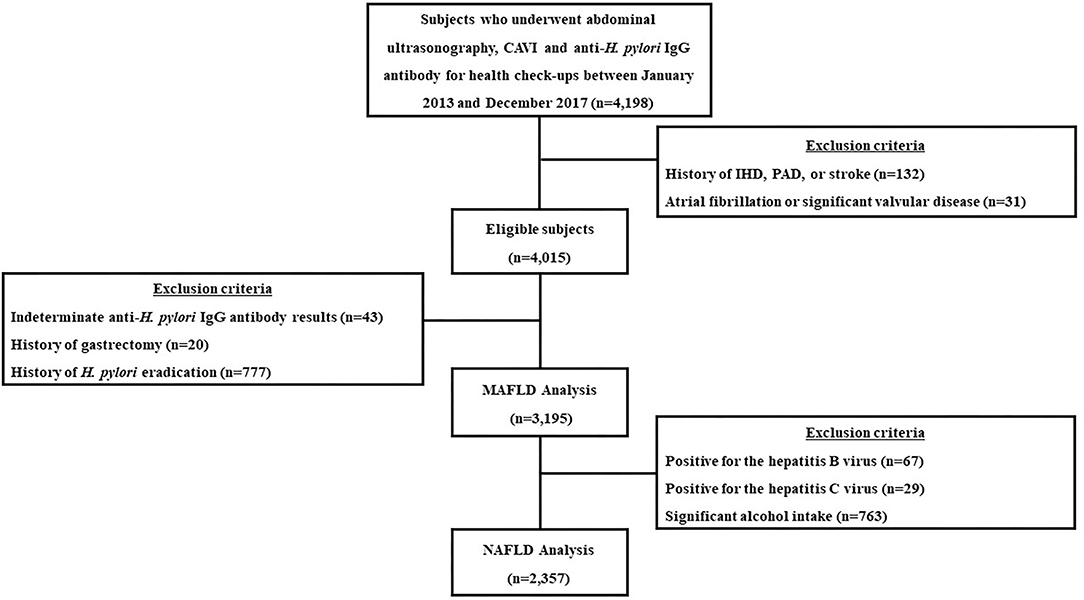
Figure 1. A flow diagram of the study population. CAVI, cardio-ankle vascular index; H. pylori, Helicobacter pylori; n, number; IHD, ischemic heart disease; PAD, peripheral artery disease; MAFLD, metabolic-dysfunction associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease.
The study protocol followed the guidelines of the Declaration of Helsinki of 1975 and its revision in 1983. The protocol was approved by the Institutional Review Board of Seoul National University Hospital (No. 2005-051-1121). The requirement for informed consent was waived by the board, as researchers only accessed and analyzed deidentified data.
Measurement of Anthropometric and Laboratory Parameters
The methods employed in this study have been described previously in detail (18). Anthropometric and laboratory parameters were measured on the same day as the health check-ups. Body weight and height were measured using a digital scale, and body mass index (BMI) was calculated by dividing weight (kg) by the squared value of height (m2). Waist circumference (WC) was measured at the midpoint between the lower costal margin and the anterior superior iliac crest by a well-trained person using a tape measure. Data regarding past medical history, comorbidities, and medication history were obtained using subject-recorded questionnaires. Based on smoking status, subjects were categorized as never or ever-smokers. The amount of alcohol each patient consumed was calculated. Blood pressure was measured at least twice, and mean values of the measurements were recorded. Hypertension was defined as blood pressure ≥140/90 mmHg or receiving antihypertensive medications. Diabetes was defined as a fasting blood glucose level ≥126 mg/dL or glycated hemoglobin A1c (HbA1c) level ≥6.5% or treatment with glucose-lowering agents. Dyslipidemia was defined as a total cholesterol level ≥240 mg/dL and/or triglyceride level ≥200 mg/dL and/or high-density lipoprotein (HDL) cholesterol level <40 mg/d or the use of anti-dyslipidemic medications (19).
All blood samples were collected after a 12-h overnight fast. Laboratory tests included serum fasting glucose, total cholesterol, triglyceride, HDL cholesterol, HbA1c, and high-sensitivity C-reactive protein (hs-CRP) levels. All of these tests were performed using standard laboratory methods. The diagnosis of Hp infection was based on the results of a serum anti-Hp IgG antibody test using a commercially available chemiluminescent microparticle immunoassay kit (Immulite® 2000 CMIA, Siemens, Germany) as described previously (12). Values >1.10 IU/mL were considered positive (20). The Hp IgG kit has a sensitivity of 91% and a specificity of 100% (6).
Measurement of NAFLD/MAFLD and Advanced Fibrosis
Abdominal ultrasonography (Acuson Sequoia 512; Siemens, Mountain View, CA) was performed to diagnose fatty liver by experienced radiologists who were unaware of the clinical information of the individuals. Fatty liver was diagnosed based on characteristic ultrasonographic findings consistent with a “bright liver” and evident contrast between hepatic and renal parenchyma, focal sparing, vessel blurring, and narrowing of the lumen of the hepatic veins (21). MAFLD was diagnosed as the presence of hepatic steatosis with 1 or more of the following: (1) overweight or obese (BMI ≥ 23 kg/m2) (2) diabetes mellitus (3) at least 2 metabolic risk abnormalities. Metabolic risk abnormalities consisted of (1) WC ≥ 90 cm for men and 80 ≥ cm for women, (2) blood pressure ≥ 130/85 mmHg or specific drug treatment, (3) fasting plasma triglycerides ≥ 150 mg/dl or specific drug treatment, (4) plasma HDL-cholesterol <40 mg/dl for men and <50 mg/dl for women or specific drug treatment, (5) prediabetes (fasting glucose 100–125 mg/dl or hemoglobin A1c 5.7–6.4%, (6) homeostasis model assessment of insulin resistance score ≥ 2.5, (7) plasma hs-CRP level > 2 mg/L (5, 22).
For subjects with NAFLD or MAFLD, the Fibrosis-4 (FIB-4) index was used as a surrogate marker for advanced liver fibrosis. FIB-4 was calculated as age (years) × AST (U/L)/platelet (109/L) × √ALT (U/L) and three risk categories (low, intermediate, high) for FIB-4 were based on the 2 cut points (1.30 and 2.67).We used the lower cutoff of FIB-4 index <1.30 to exclude advanced liver fibrosis (23).
Assessment of Arterial Stiffness Using CAVI
CAVI was measured using a VaSera VS-1000 (Fukuda Denshi Co Ltd, Tokyo, Japan) as described in previous studies to evaluate arterial stiffness (3, 12, 24). Briefly, the brachial pulse pressure was measured using an automated cuff oscillometer in seated individuals after 5 min of rest. The average value of two measurements was calculated to determine the systolic and diastolic pressures and pulse pressure. While the individuals were resting in a supine position, the cuffs were applied to ankles and both upper arms. After a 10 min rest period, the measurement was recorded. A phonocardiogram used for the detection of heart sounds was placed over the right sternum between the second intercostal spaces, and electrocardiogram electrodes were applied on both wrists. The pulse wave velocity was calculated as the vascular length (L) divided by the time (T) required for the pulse wave to propagate from the aortic valve to the ankle. Because the initiation of blood release from the aortic valve is difficult to identify based on the opening sound of the valve, T is difficult to determine; thus, the T value was calculated by summing the interval between the initiation of the brachial pulse waveform and the initiation of the ankle pulse waveform and the interval between the closing sound of the aortic valve and the notch of the brachial pulse waveform. Measurements were performed by a well-trained staff member. The CAVI was determined using the following equation:
where Ps and Pd are the systolic and diastolic blood pressures, respectively, ΔP is Ps–Pd, ρ is the blood density, and a and b are constants. The mean values of the left and right CAVI were used. We used a cutoff value of 8 to define increased arterial stiffness based on previous studies (12, 25, 26).
Statistical Analysis
Continuous variables with a normal distribution are reported as the means ± SD or medians (with interquartile ranges) and categorical variables are reported as numbers and percentages. To test for normality, the Kolmogorov-Smirnov test and the normal Q-Q plots were used. Student's t-test was used when the data were normally distributed, and Mann–Whitney U-test was used otherwise. The differences between nominal variables were compared with the chi-square test or Fisher exact test. We divided participants into four groups according to the presence of NAFLD/MAFLD and/or Hp infection. A logistic regression analysis was utilized to analyze the association between NAFLD or MAFLD with Hp infection and increased arterial stiffness after adjusting for potential confounders. Among variables with a P <0.05 in the univariate analysis, those with clinical importance were subjected to multivariate analyses. All statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA), and P <0.05 were considered statistically significant.
Results
Study Population
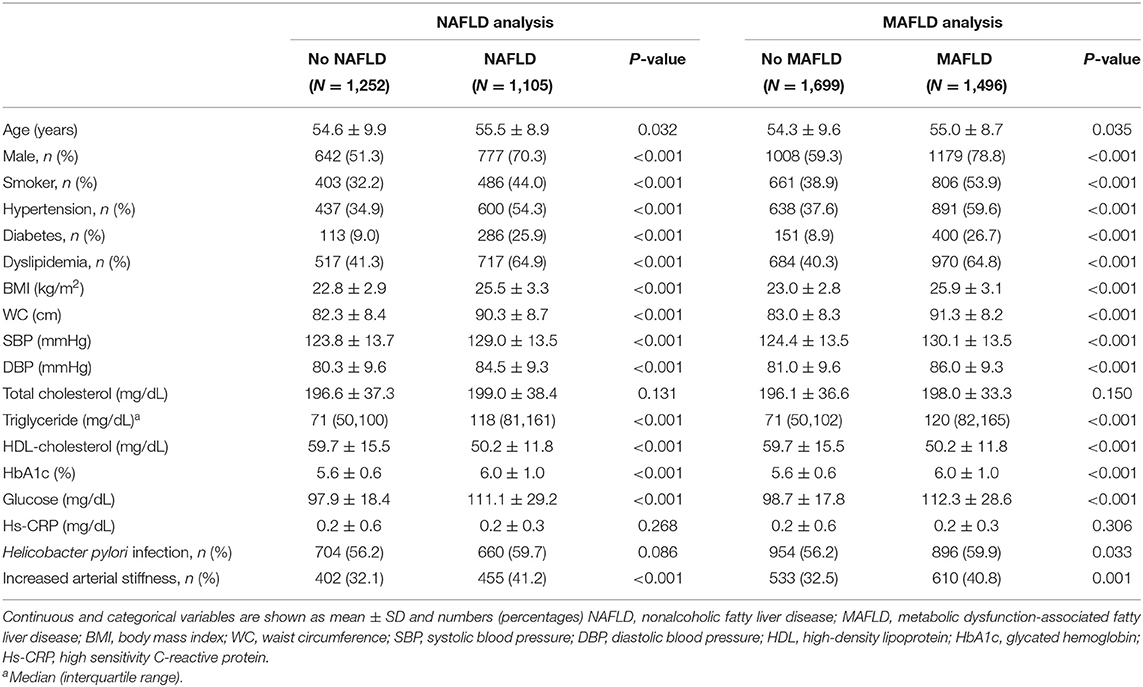
The mean age of 3,195 subjects was 54.7 years, and the proportion of males was 68.5%. The prevalence rate of increased arterial stiffness (CAVI ≥ 8) was 36.4%. Table 1 shows the baseline characteristics of the study population according to the presence of NAFLD or MAFLD. Individuals with NAFLD or MAFLD have been observed more frequently in male (70.3 vs. 51.3% in NAFLD vs. no-NAFLD and 78.8 vs. 59.3% in MAFLD vs. no-MAFLD, respectively, P <0.001), and ever smokers (44.0 vs. 32.2% in NAFLD vs. no-NAFLD and 53.9 vs. 38.9% in MAFLD vs. no-MAFLD, respectively, P <0.001). In individuals with NAFLD or MAFLD, traditional risk factors of atherosclerosis were significantly more common compared to those without NAFLD or MAFLD: hypertension (54.3 vs. 34.9% in NAFLD vs. no-NAFLD and 59.6 vs. 37.6% in MAFLD vs. no-MAFLD, respectively, P <0.001), diabetes (25.9 vs. 9.0% in NAFLD vs. no-NAFLD and 26.7 vs. 8.9% in MAFLD vs. no-MAFLD, respectively, P <0.001), and dyslipidemia (64.9 vs. 41.3% in NAFLD vs. no-NAFLD and 64.8 vs. 40.3% in MAFLD vs. no-MAFLD, respectively, P <0.001). In addition, most of the body measurements and laboratory results (including WC, BMI, systolic or diastolic blood pressure, triglyceride, HDL cholesterol, fasting glucose, and HbA1c levels) were less favorable in terms of metabolism in individuals with NAFLD or MAFLD (P <0.001). The prevalence of increased arterial stiffness was significantly higher in both patients with NAFLD and MAFLD than those without NAFLD/MAFLD (41.2 vs. 32.1% in NAFLD vs. no-NAFLD and 40.8 vs. 32.5% in MAFLD vs. no-MAFLD, respectively).
Risk of Arterial Stiffness According to NAFLD/MAFLD and Hp Infection
We investigated the risk of increased arterial stiffness according to NAFLD/MAFLD and Hp infection. In the univariate analysis, subjects with Hp infection but without NAFLD had a significantly higher risk of increased arterial stiffness [odds ratio (OR) 1.33, 95% confidence interval (CI) 1.04–1.69] than subjects without Hp infection and NAFLD (used as a control group). Subjects with NAFLD but without Hp infection and those with both NAFLD and Hp infection also had a significantly higher OR for increased arterial stiffness (OR 1.47, 95% CI 1.12–1.92 and OR 1.95, 95% CI 1.53–2.48, respectively). When adjusting for multiple metabolic factors, including age, BMI, hypertension, diabetes, dyslipidemia, and smoking, the higher risk of increased arterial stiffness in the NAFLD (+) Hp (−) and NAFLD (+) Hp (+) groups remained (OR 1.61, 95% CI 1.15–2.26 and OR 2.23, 95% CI 1.63–3.06, respectively, Table 2). Regarding MAFLD, subjects with MAFLD but without Hp infection and subjects with both MAFLD and Hp infection exhibited significantly higher risks of increased arterial stiffness in a dose-dependent manner (OR 1.80, 95% CI 1.36–2.39 and OR 2.13, 95% CI 1.64–2.78, respectively). Meanwhile, in subjects without NAFLD or MAFLD, the risk of arterial stiffness tended to increase in the Hp (±) group compared to Hp (−), but statistical significance was not observed (OR 1.23, 95% CI 0.92–1.65, P = 0.170 and OR 1.16, 95% CI 0.91–1.48, P = 0.237, respectively).
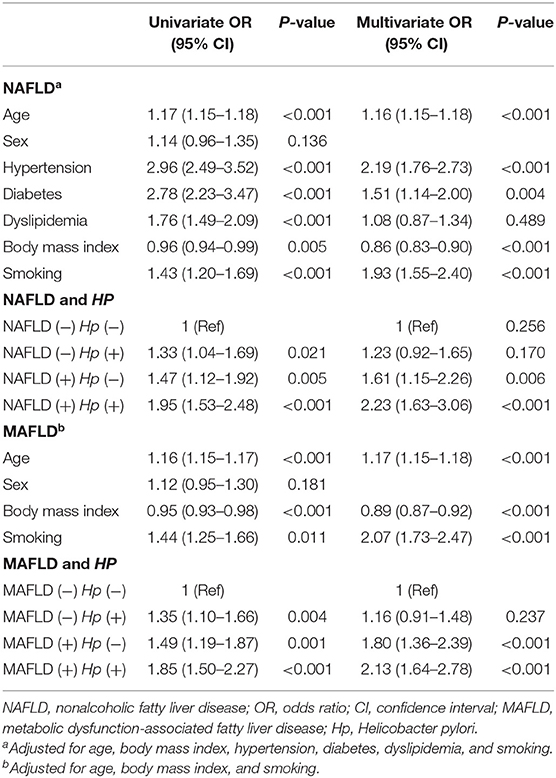
Table 2. Univariate and multivariate analyses of the risk for arterial stiffness in the total population.
When we performed an analysis stratified according to sex, the higher risk of increased arterial stiffness in subjects with both NAFLD and Hp infection persisted in both men and women (OR 2.28, 95% CI 1.52–3.42 and OR 2.18, 95% CI 1.29–3.68, respectively, Supplementary Table 1), and similar trends were observed for men and women subjects with MAFLD (OR 1.95, 95% CI 1.43–2.66 and OR 2.66, 95% CI 1.60–4.42, respectively).
Advanced Fibrosis, Hp Infection, and Increased Arterial Stiffness
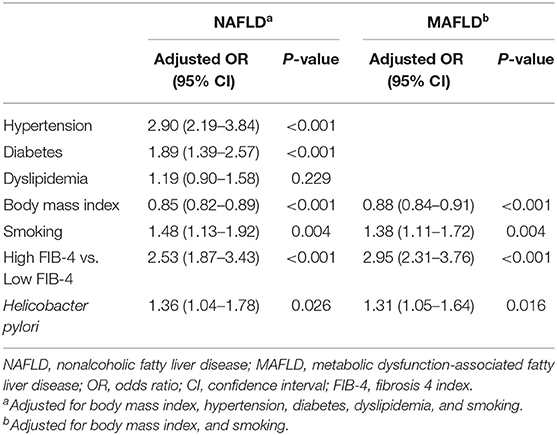
Next, we performed subgroup analysis in patients with NAFLD/MAFLD for the association between advanced fibrosis, Hp infection and increased arterial stiffness. When participants with NAFLD/MAFLD were categorized according to the presence of advanced fibrosis using the FIB-4 index, high FIB-4 index was significantly associated with increased risk of arterial stiffness compared to low FIB-4 index in both patients with NAFLD and MAFLD (NAFLD: OR 2.53, 95% CI, 1.87–3.43, P <0.001 and MAFLD: OR 2.95, 95% CI, 2.31–3.76, P <0.001). Hp infection was independently associated with arterial stiffness in both patients with NAFLD and MAFLD (NAFLD: OR 1.36, 95% CI, 1.04–1.78, P = 0.026 and MAFLD: OR 1.31, 95% CI, 1.05–1.64, P = 0.016, Table 3).
Discussion
To the best of our knowledge, our study is the first to show an interactive effect of NAFLD/MAFLD and Hp infection on arterial stiffness. In the present study, a significantly increased risk of arterial stiffness was observed in subjects with NAFLD/MAFLD and Hp infection compared with subjects without these conditions. Hp infection additively increased the risk of arterial stiffness in subjects with NAFLD or MAFLD.
Arterial stiffness is one of the major indicators of systemic atherosclerosis and is closely related to cardiovascular risk (27). Since increased arterial stiffness is associated with adverse cardiovascular outcomes, even in the general population (28), measurements of arterial stiffness may be helpful to identify high-risk groups for cardiovascular diseases. As a novel indicator of arterial stiffness, CAVI represents the stiffness of the entire arterial segment and is independent of blood pressure, making it highly reproducible and easy to measure (29). Thus, CAVI has been used as a screening tool to evaluate the subclinical atherosclerotic risk in asymptomatic individuals (30). In the present study, we used CAVI as a tool to measure arterial stiffness and revealed that both the presence of NAFLD/MAFLD and Hp infection were independently associated with increased arterial stiffness.
Previous studies have investigated the association between NAFLD and increased arterial stiffness. Arterial stiffness indicated by CAVI was associated with the ultrasonography-diagnosed presence and severity of NAFLD (3), and arterial stiffness measured using the augmentation index was associated with more severe NAFLD histology in the biopsy-proven NAFLD cohort (31, 32). NAFLD defined using controlled attenuation parameters also showed a significant association with increased arterial stiffness (24, 33). Consistent with previous results, the presence of NAFLD/MAFLD and advanced fibrosis were independently associated with arterial stiffness in our study.
Several possible mechanisms supporting the association between NAFLD and arterial stiffness are plausible. NAFLD has been recognized as a hepatic manifestation of metabolic syndrome and is closed associated with hyperglycemia, dyslipidemia, and insulin resistance (34), all of which are associated with subclinical inflammation, vascular endothelial cells damage, prothrombotic status, and hemodynamic changes that may increase the risk of atherosclerosis (35). Increased oxidative stress (36), chronic subclinical inflammation (37), reduced levels of adiponectin (38) and altered production of coagulant factors can be involved in the pathogenesis of atherosclerosis in patients with NAFLD.
On the other hand, several studies have suggested that Hp infection increases cardiovascular disease, including coronary artery disease and peripheral arterial stiffness (39–41). Choi et al. found that Hp seropositivity was significantly associated with increased arterial stiffness (12). Yoshikawa et al. reported that Hp infection accelerated the effects of impaired glucose metabolism and increased arterial stiffness (42). Chronic Hp infection has been reported to trigger an inflammatory reaction and release inflammatory cytokines, which lead to endothelial dysfunction (43). According to Yu et al. the combination of Hp infection and NAFLD increases carotid artery plaque formation, a surrogate marker of atherosclerosis (OR = 1.93). The risk of atherosclerosis was significantly increased in the fatty liver (±) Hp (±) group, but not in the fatty liver (±) Hp (−) group in the previous study (44). Consistently, Hp infection additively increased the risk of arterial stiffness in subjects with NAFLD/MAFLD, and the risk was higher than that of previous study with OR = 2.23 and 2.13 in our study. Moreover, the risk of atherosclerosis showed a dose-dependent relationship in Hp (−) NAFLD/MAFLD and Hp (±) NAFLD/MAFLD [OR (95% CI), 1.61 (1.15–2.26) and 1.80 (1.36–2.39) in Hp (−) NAFLD/MAFLD vs. 2.23 (1.63–3.06) and 2.13 (1.64–2.78) in Hp (±) NAFLD/MAFLD, respectively]. Collectively, arterial stiffness, measured using CAVI, is a novel approach in the present study, and this association was probably attributed to the synergistic effect of Hp infection and NAFLD/MAFLD on atherosclerosis.
Because NAFLD is a sexually dimorphic disease with respect to epidemiological and clinical features (45), we performed an analysis stratified according to sex. The increased risk of arterial stiffness in subjects with both NAFLD/MAFLD and Hp infection persisted in both men and women, suggesting the additive effect of Hp infection and NAFLD/MAFLD on arterial stiffness in both sexes.
Liver fibrosis is a crucial prognostic factor for cardiovascular outcomes in NAFLD (46). When we evaluated the association between FIB-4 index and increased arterial stiffness in subjects with NAFLD or MAFLD, high FIB-4 index was associated with increased arterial stiffness compared to low FIB-4 index in both NAFLD and MAFLD, suggesting the role of advanced fibrosis in the subclinical atherosclerosis. In line with our results, advanced fibrosis was associated with carotid atherosclerosis in patient with NAFLD (47, 48).
Interestingly, BMI showed an inverse correlation with arterial stiffness in this study. This phenomenon has also been reported in previous studies, and part of this complex association can be explained by the obesity paradox (49, 50). That is, it is explained that some of the patients with elevated BMI benefit from the preservation of arterial stiffness by increased metabolic reserves, attenuated response to renin–angiotensin–aldosterone system, greater muscular strength, potentially protective cytokines and neuroendocrine factors (51, 52). However, there are studies showing that obesity is associated with high CAVI levels and insulin resistance, an independent predictor of vascular stiffness, so additional studies are needed (53, 54).
Our study has several limitations. First, the cross-sectional nature of the study design limits the ability to assess cause and effect. Thus, we were unable to infer causal relationships from this study. Second, the NAFLD diagnosis was exclusively based on ultrasonography, but was not confirmed by liver biopsy, which is the standard diagnostic modality for confirming NAFLD. Ultrasonography has a high specificity but underestimates hepatic steatosis when the fat content is <20% and is unable to quantify fibrosis (55). However, liver biopsy is not typically performed in asymptomatic individuals, and radiographic techniques such as ultrasonography or magnetic resonance imaging are used to diagnose NAFLD in clinical practice. Third, we could not exclude patients with chronic liver disease due to causes other than viral and alcoholic hepatitis. In addition, subjects who take steatogenic drugs could not excluded from this study. However, since this study is based on health check-up examination data targeting asymptomatic adults, the prevalence of this area is thought to be low. Fourth, although the serological test does not discriminate current and past Hp infections (56), the Hp infection status was assessed only with serology and not other assessment methods, such as the urease breath test or a rapid urease test, in the present study. Due to its cost-effectiveness and invasiveness, serology tests are a common method used in health screening centers that conduct routine blood sampling. We thoroughly investigated the history of Hp eradication therapy to supplement the shortcomings of serological tests and overcome this limitation. Fifth, although significant alcohol consumption is considered to be >30 g for men and 20 g for women per day according to the Korean Association for the Study of the Liver Clinical Practice Guideline for NAFLD (57), sex-specific criteria could not be applied to the amount of alcohol consumed in this study. Last, our study population of those who underwent health evaluations upon their own initiative may not represent the majority of the general Korean population, which may contribute to selection bias.
Conclusions
We demonstrated the synergistic effect of Hp infection and NAFLD/MAFLD on the risk of arterial stiffness among asymptomatic Koreans. Hp infection additively increases the risk of arterial stiffness in subjects with NAFLD or MAFLD. Therefore, evaluating Hp infection status in patients with NAFLD/MAFLD may be helpful in cardiovascular risk assessment. Further studies are needed to determine whether eradication of Hp and adequate management of NAFLD/MAFLD helps to improve arterial stiffness and prevent cardiovascular disease.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Seoul National University Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
GC: conceptualization. GC, JC, HP, YH, JL, HL, SC, and SL: data curation. JC: formal analysis. SC, JY, and SL: supervision. JC and GC: writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.844954/full#supplementary-material
References
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
2. Marchesini G, Marzocchi R. Metabolic syndrome and NASH. Clin Liver Dis. (2007) 11:105–117. doi: 10.1016/j.cld.2007.02.013
3. Chung GE, Choi SY, Kim D, Kwak MS, Park HE, Kim MK, et al. Nonalcoholic fatty liver disease as a risk factor of arterial stiffness measured by the cardioankle vascular index. Medicine. (2015) 94:e654. doi: 10.1097/MD.0000000000000654
4. Kang SH, Cho Y, Jeong SW, Kim SU, Lee JW, Korean NAFLD Study Group. From nonalcoholic fatty liver disease to metabolic-associated fatty liver disease: big wave or ripple? Clin Mol Hepatol. (2021) 27:257–69. doi: 10.3350/cmh.2021.0067
5. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. (2020) 73:202–9. doi: 10.1016/j.jhep.2020.07.045
6. Lim SH, Kim N, Kwon JW, Kim SE, Baik GH, Lee JY, et al. Trends in the seroprevalence of Helicobacter pylori infection and its putative eradication rate over 18 years in Korea: a cross-sectional nationwide multicenter study. PLoS ONE. (2018) 13:e0204762. doi: 10.1371/journal.pone.0204762
7. Kuipers EJ. Review article: relationship between Helicobacter pylori, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. (1998) 12 (Suppl. 1):25–36. doi: 10.1111/j.1365-2036.1998.00009.x
8. Zhang C, Yamada N, Wu YL, Wen M, Matsuhisa T, Matsukura N. Helicobacter pylori infection, glandular atrophy and intestinal metaplasia in superficial gastritis, gastric erosion, erosive gastritis, gastric ulcer and early gastric cancer. World J Gastroenterol. (2005) 11:791–6. doi: 10.3748/wjg.v11.i6.791
9. Gravina AG, Priadko K, Ciamarra P, Granata L, Facchiano A, Miranda A, et al. Extra-Gastric manifestations of Helicobacter pylori infection. J Clin Med. (2020) 9:3887. doi: 10.3390/jcm9123887
10. Sung KC, Rhee EJ, Ryu SH, Beck SH. Prevalence of Helicobacter pylori infection and its association with cardiovascular risk factors in Korean adults. Int J Cardiol. (2005) 102:411–7. doi: 10.1016/j.ijcard.2004.05.040
11. Ohnishi M, Fukui M, Ishikawa T, Ohnishi N, Ishigami N, Yoshioka K, et al. Helicobacter pylori infection and arterial stiffness in patients with type 2 diabetes mellitus. Metabolism. (2008) 57:1760–4. doi: 10.1016/j.metabol.2008.08.001
12. Choi JM, Lim SH, Han YM, Lee H, Seo JY, Park HE, et al. Association between Helicobacter pylori infection and arterial stiffness: results from a large cross-sectional study. PLoS ONE. (2019) 14:e0221643. doi: 10.1371/journal.pone.0221643
13. Patel P, Carrington D, Strachan DP, Leatham E, Goggin P, Northfield TC, et al. Fibrinogen: a link between chronic infection and coronary heart disease. Lancet. (1994) 343:1634–5. doi: 10.1016/S0140-6736(94)93084-8
14. Polyzos SA, Kountouras J, Zavos C, Deretzi G. The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter. (2011) 16:79–88. doi: 10.1111/j.1523-5378.2011.00822.x
15. Urbina EM, Gao Z, Khoury PR, Martin LJ, Dolan LM. Insulin resistance and arterial stiffness in healthy adolescents and young adults. Diabetologia. (2012) 55:625–31. doi: 10.1007/s00125-011-2412-1
16. Nakagomi A, Sunami Y, Kawasaki Y, Fujisawa T, Kobayashi Y. Sex difference in the association between surrogate markers of insulin resistance and arterial stiffness. Diabetes Complic. (2020) 34:107442. doi: 10.1016/j.jdiacomp.2019.107442
17. Li M, Shen Z, Li YM. Potential role of Helicobacter pylori infection in nonalcoholic fatty liver disease. World J Gastroenterol. (2013) 19:7024–31. doi: 10.3748/wjg.v19.i41.7024
18. Park HE, Lee H, Choi SY, Kwak MS, Yang JI, Yim JY, et al. Clinical significance of hepatic steatosis according to coronary plaque morphology: assessment using controlled attenuation parameter. J Gastroenterol. (2019) 54:271–80. doi: 10.1007/s00535-018-1516-5
19. Committee for the Korean Guidelines for the Management of Dyslipidemia. 2015 Korean Guidelines for the management of dyslipidemia: executive summary (English translation) Korean Circ J. (2016) 46:275–306. doi: 10.4070/kcj.2016.46.3.275
20. Han YM, Chung SJ, Choi JM, Lee C, Kim JS. Long-term outcome of group D patients with negative serum anti-Helicobacter pylori antibody and positive serum pepsinogen test in healthy Koreans. J Dig Dis. (2018) 19:529–39. doi: 10.1111/1751-2980.12660
21. Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. (2002) 123:745–50. doi: 10.1053/gast.2002.35354
22. Seo JY, Bae JH, Kwak MS, Yang JI, Chung SJ, Yim JY, et al. The risk of colorectal adenoma in nonalcoholic or metabolic-associated fatty liver disease. Biomedicines. (2021) 9:1401. doi: 10.3390/biomedicines9101401
23. Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. (2013) 145:782–9. doi: 10.1053/j.gastro.2013.06.057
24. Park HE, Lee H, Choi SY, Kwak MS, Yang JI, Yim JY, et al. Usefulness of controlled attenuation parameter for detecting increased arterial stiffness in general population. Dig Liver Dis. (2018) 50:1062–7. doi: 10.1016/j.dld.2018.04.027
25. Nakamura K, Tomaru T, Yamamura S, Miyashita Y, Shirai K, Noike H. Cardio-ankle vascular index is a candidate predictor of coronary atherosclerosis. Circ J. (2008) 72:598–604. doi: 10.1253/circj.72.598
26. Park JB, Park HE, Choi SY, Kim MK, Oh BH. Relation between cardio-ankle vascular index and coronary artery calcification or stenosis in asymptomatic subjects. J Atheroscler Thromb. (2013) 20:557–67. doi: 10.5551/jat.15149
27. Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. (2011) 57:1511–22. doi: 10.1016/j.jacc.2010.12.017
28. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the framingham heart study. Circulation. (2010) 121:505–11. doi: 10.1161/CIRCULATIONAHA.109.886655
29. Shirai K, Hiruta N, Song M, Kurosu T, Suzuki J, Tomaru T, et al. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb. (2011) 18:924–38. doi: 10.5551/jat.7716
30. Choi SY. Clinical application of the cardio-ankle vascular index in asymptomatic healthy Koreans. Pulse. (2017) 4 (Suppl. 1):17–20. doi: 10.1159/000448462
31. Kim HL, Koo BK, Joo SK, Kim W. Association of arterial stiffness with the histological severity of nonalcoholic fatty liver disease. Hepatol Int. (2020) 14:1048–56. doi: 10.1007/s12072-020-10108-z
32. Bilgin BO, Sunbul M, Kani HT, Demirtas CO, Keklikkiran C, Yilmaz Y. Arterial stiffness is associated independently with liver stiffness in biopsy-proven nonalcoholic fatty liver disease: a transient elastography study. Eur J Gastroenterol Hepatol. (2020) 32:54–7. doi: 10.1097/MEG.0000000000001471
33. Yu XY, Song XX, Tong YL, Wu LY, Song ZY. Usefulness of controlled attenuation parameter and liver stiffness measurement for detecting increased arterial stiffness in asymptomatic populations in China. Medicine. (2020) 99:e23360. doi: 10.1097/MD.0000000000023360
34. Xu X, Lu L, Dong Q, Li X, Zhang N, Xin Y, et al. Research advances in the relationship between nonalcoholic fatty liver disease and atherosclerosis. Lipids Health Dis. (2015) 14:158. doi: 10.1186/s12944-015-0141-z
35. Loria P, Lonardo A, Targher G. Is liver fat detrimental to vessels?: intersections in the pathogenesis of NAFLD and atherosclerosis. Clin Sci. (2008) 115:1–12. doi: 10.1042/CS20070311
36. Yesilova Z, Yaman H, Oktenli C, Ozcan A, Uygun A, Cakir E, et al. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. (2005) 100:850–05. doi: 10.1111/j.1572-0241.2005.41500.x
37. Targher G, Bertolini L, Scala L, Zoppini G, Zenari L, Falezza G. Non-alcoholic hepatic steatosis and its relation to increased plasma biomarkers of inflammation and endothelial dysfunction in non-diabetic men. Role of visceral adipose tissue. Diabet Med. (2005) 22:1354–8. doi: 10.1111/j.1464-5491.2005.01646.x
38. Pagano C, Soardo G, Esposito W, Fallo F, Basan L, Donnini D, et al. Plasma adiponectin is decreased in nonalcoholic fatty liver disease. Eur J Endocrinol. (2005) 152:113–8. doi: 10.1530/eje.1.01821
39. Sun J, Rangan P, Bhat SS, Liu L. A meta-analysis of the association between Helicobacter pylori infection and risk of coronary heart disease from published prospective studies. Helicobacter. (2016) 21:11–23. doi: 10.1111/hel.12234
40. Yang YF, Li Y, Liu JH, Wang XM, Wu BH, He CS, et al. Relation of Helicobacter pylori infection to peripheral arterial stiffness and 10-year cardiovascular risk in subjects with diabetes mellitus. Diab Vasc Dis Res. (2020) 17:1479164120953626. doi: 10.1177/1479164120953626
41. Chung J, Min KW, Son BK, Kim D-H, Kim H-L. Association between histological severity of Helicobacter pylori infection and cardiovascular risk scores in the Korean population. Atherosclerosi. (2021) 333:124–30. doi: 10.1016/j.atherosclerosis.2021.08.019
42. Yoshikawa H, Aida K, Mori A, Muto S, Fukuda T. Involvement of Helicobacter pylori infection and impaired glucose metabolism in the increase of brachial-ankle pulse wave velocity. Helicobacter. (2007) 12:559–66. doi: 10.1111/j.1523-5378.2007.00523.x
43. Blum A, Tamir S, Mualem K, Ben-Shushan RS, Keinan-Boker L, Paritsky M. Endothelial dysfunction is reversible in Helicobacter pylori-positive subjects. Am J Med. (2011) 124:1171–4. doi: 10.1016/j.amjmed.2011.08.015
44. Yu LY, Hu KC, Liu CJ, Hung CL, Bair MJ, Chen MJ, et al. Helicobacter pylori infection combined with non-alcoholic fatty liver disease increase the risk of atherosclerosis: focus in carotid artery plaque. Medicine. (2019) 98:e14672. doi: 10.1097/MD.0000000000014672
45. Lonardo A, Suzuki A. Sexual dimorphism of NAFLD in adults. Focus on clinical aspects and implications for practice and translational research. J Clin Med. (2020) 9:1278. doi: 10.3390/jcm9051278
46. Polyzos SA, Kechagias S, Tsochatzis EA. Review article: non-alcoholic fatty liver disease and cardiovascular diseases: associations and treatment considerations. Aliment Pharmacol Ther. (2021) 54:1013–25. doi: 10.1111/apt.16575
47. Arai T, Atsukawa M, Tsubota A, Kato K, Kato K, Abe H, et al. Liver fibrosis is associated with carotid atherosclerosis in patients with liver biopsy-proven nonalcoholic fatty liver disease. Sci Rep. (2021) 11:15938. doi: 10.1038/s41598-021-95581-8
48. Xin Z, Zhu Y, Wang S, Liu S, Xu M, Wang T, et al. Associations of subclinical atherosclerosis with nonalcoholic fatty liver disease and fibrosis assessed by non-invasive score. Liver Int. (2020) 40:806–14. doi: 10.1111/liv.14322
49. Choi SY, Oh BH, Bae Park J, Choi DJ, Rhee MY, Park S. Age-associated increase in arterial stiffness measured according to the cardio-ankle vascular index without blood pressure changes in healthy adults. J Atheroscler Thromb. (2013) 20:911–23. doi: 10.5551/jat.18267
50. Nagayama D, Imamura H, Sato Y, Yamaguchi T, Ban N, Kawana H, et al. Inverse relationship of cardioankle vascular index with BMI in healthy Japanese subjects: a cross-sectional study. Vasc Health Risk Manag. (2017) 13:1–9. doi: 10.2147/VHRM.S119646
51. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. (2009) 53:1925–32. doi: 10.1016/j.jacc.2008.12.068
52. Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. (2004) 43:1439–44. doi: 10.1016/j.jacc.2003.11.039
53. Nagayama D, Endo K, Ohira M, Yamaguchi T, Ban N, Kawana H, et al. Effects of body weight reduction on cardio-ankle vascular index (CAVI). Obes Res Clin Pract. (2013) 7:139–145. doi: 10.1016/j.orcp.2011.08.154
54. Jia G, Sowers JR. Endothelial dysfunction potentially interacts with impaired glucose metabolism to increase cardiovascular risk. Hypertension. (2014) 64:1192–3. doi: 10.1161/HYPERTENSIONAHA.114.04348
55. Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. (2009) 51:1061–7. doi: 10.1016/j.jhep.2009.09.001
56. Lee JH, Kim N, Chung JI, Kang KP, Lee SH, Park YS, et al. Long-term follow up of Helicobacter pylori IgG serology after eradication and reinfection rate of H. pylori in South Korea. Helicobacter. (2008) 13:288–94. doi: 10.1111/j.1523-5378.2008.00616.x
Keywords: Helicobacter, hepatic steatosis, arterial stiffness, risk, atherosclerosis
Citation: Choi JM, Park HE, Han YM, Lee J, Lee H, Chung SJ, Lim SH, Yim JY and Chung GE (2022) Non-alcoholic/Metabolic-Associated Fatty Liver Disease and Helicobacter pylori Additively Increase the Risk of Arterial Stiffness. Front. Med. 9:844954. doi: 10.3389/fmed.2022.844954
Received: 07 January 2022; Accepted: 01 February 2022;
Published: 25 February 2022.
Edited by:
Giuseppe Losurdo, University of Bari Medical School, ItalyReviewed by:
Alberto Ferrarese, Integrated University Hospital Verona, ItalyAngelo Armandi, University of Turin, Italy
Copyright © 2022 Choi, Park, Han, Lee, Lee, Chung, Lim, Yim and Chung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Goh Eun Chung, Z29od29tQHNudS5hYy5rcg==
 Ji Min Choi
Ji Min Choi Hyo Eun Park
Hyo Eun Park Yoo Min Han
Yoo Min Han Jooyoung Lee
Jooyoung Lee Heesun Lee
Heesun Lee Su Jin Chung
Su Jin Chung Seon Hee Lim
Seon Hee Lim Jeong Yoon Yim
Jeong Yoon Yim Goh Eun Chung
Goh Eun Chung