
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 24 March 2022
Sec. Gastroenterology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.844915
Background: The increased antibiotic resistance of Helicobacter pylori (H. pylori) has led to the decreased efficacy of H. pylori regimens.
Aim: To evaluate the efficacy, safety, and compliance of susceptibility-guided therapy (SGT) vs. bismuth-containing quadruple therapy (BQT) as the first-line treatment for H. pylori infection.
Materials and Methods: This meta-analysis was performed in accordance with the PRISMA 2009 guidelines. A systematic search in PubMed, Embase, and Cochrane databases was conducted using the combination of “H. pylori or H. pylori or Hp,” “bismuth quadruple,” and “tailored eradication OR tailored therapy OR susceptibility-guided therapy OR personalized therapy OR antibiotic susceptibility testing.”
Results: Five studies with 2,110 H. pylori-infected patients were enrolled. The pooled eradication rates of SGT and BQT were 86 vs. 78% (p < 0.05) and 92 vs. 86% (p > 0.05) by intention-to-treat (ITT) and per-protocol (PP) analyses, respectively. SGT has a significantly superior efficacy than BQT [pooled risk ratio (RR) = 1.14, p < 0.05] in a subgroup of cultures with the susceptibility test. The pooled side effect rate was 20% in SGT and 22% in BQT, which showed no significant difference (p > 0.05). The compliances of SGT and BQT were 95 and 92%, respectively.
Conclusion: Compared with BQT, SGT showed a higher efficacy and similar safety as the first-line treatment of H. pylori infection in areas with high antibiotic resistance. The decision-making of first-line regimens for H. pylori infection should depend on the availability and cost-effectiveness of susceptibility tests and bismuth in local areas.
Helicobacter pylori (H. pylori) is acquired in childhood and can be transmitted person-to-person via oral–oral, fecal–oral, or gastro–oral transmission routes (1). H. pylori gastritis was defined as an infectious disease in the Kyoto global consensus report (2). As a type-1 carcinogen, H. pylori infection is closely associated with an incidence of gastric cancer (GC) (3). Due to the public health burden and threat caused by H. pylori infection and the thought that successfully clearing H. pylori could eliminate a carcinogen and reduce a source of infection, recent consensus reports have raised the importance of H. pylori screening and eradication for GC prevention (4, 5). They have also demonstrated that family-based H. pylori infection control and management are suitable approaches to prevent intrafamilial transmission and reduce the incidence of H. pylori-related diseases (6).
Widespread use of antibiotics leads to increased antimicrobial resistance, and the resistance rate of common antibiotics (clarithromycin, etc.) used in H. pylori regimens was reported to be more than 15% (7). Moreover, clarithromycin-resistant H. pylori was included in a high-priority tier for investment in new drugs (8). Compared with treatment-naive adults, previously treated adults showed a higher antibiotic resistance rate (9). Therefore, elevating the first-line efficacy of the H. pylori regimen is important to avoid secondary antibiotic resistance. Non-bismuth-containing quadruple therapies (including concomitant, sequential, and hybrid) are all influenced by dual antibiotic resistance (10). High-dose dual therapy has the advantages of less unnecessary antibiotic use and fewer side effects and shows a similar efficacy in comparison with the current mainstream guideline-recommended therapies (11), which is an alternative treatment for H. pylori infection. Bismuth-containing quadruple therapy (BQT) is currently the first-line treatment for H. pylori infection because it achieves a high efficacy in susceptible and resistant H. pylori strains (4, 12, 13).
Theoretically, any therapy for infectious diseases should be based on the results of susceptibility testing. Susceptibility-guided therapy (SGT) is an effective way to achieve high efficacy, have limited side effects, and to avoid unnecessary antibiotic use. Real-time PCR is a rapid method to diagnose H. pylori infection and detect the point mutations of clarithromycin, which guides the antibiotic choice for H. pylori eradication (14). However, the availability, accuracy, and cost-effectiveness of SGT should be considered in the clinical management of H. pylori infection (15). The H. pylori consensus reports have also demonstrated inconsistent statements regarding the application of SGT in the eradication of H. pylori (4, 5, 13, 16). Several clinical trials (17–19) were conducted to evaluate whether SGT (based on susceptibility testing or PCR results) shows a superior or similar efficacy in comparison with BQT.
Hence, this systematic review and meta-analysis were conducted to evaluate the efficacy, side effects, and compliance of SGT as the first-line treatment for H. pylori in comparison with BQT (the current mainstream guideline-recommended therapy), guiding the clinical choice of H. pylori regimens, especially in areas with high clarithromycin resistance.
This systematic review and meta-analysis were registered in PROSPERO (registration no.: CRD42022284258) and performed in accordance with the PRISMA 2009 guidelines (20). Relative records were searched through PubMed, Embase, and Cochrane (Cochrane Central Register of Controlled Trials) databases. The following search terms were used: “Helicobacter pylori or H. pylori or Hp,” “bismuth quadruple,” and “tailored eradication OR tailored therapy OR susceptibility-guided therapy OR personalized therapy OR antibiotic susceptibility testing.” Only English articles published from 1983 to December 13, 2021 were evaluated. The search strategy for each database is shown in detail in Supplementary Table S1.
Two researchers (OY and ZW) independently evaluated all relevant records in two stages, and any disagreement was discussed until a consensus was reached; otherwise, the decision was made by a third researcher (HC). In the first stage, irrelevant records were excluded by evaluating their titles and abstracts. In the second stage, the full text of the remaining studies was assessed with the following criteria. Inclusion criteria: P (Patients), H. pylori-infected adult patients without a history of H. pylori eradication; I (Intervention), SGT, defined as a consensus recommended triple and/or quadruple therapy based on the antimicrobial susceptibility test to clarithromycin and/or metronidazole and/or levofloxacin and other antibiotics (4); C (Comparator), empiric BQT recommended by the current guidelines (4, 12); O (Outcome), the eradication rate, side effects and compliance of SGT and BQT; S (Study design), only randomized controlled trials (RCTs) were included. The exclusion criteria were as follows: studies in children or the elderly, basic studies, case reports, conference abstracts, reviews, meta-analyses, and studies with insufficient published data.
Two investigators (OY and ZW) independently extracted the data from the included studies, and discrepancies were fully discussed until an approval was reached; otherwise, the decision was made by a third investigator (HC). The following data were extracted from the included studies: study year of publication; first author; country; patient characteristics; tests to confirm H. pylori infection and eradication (including the time interval between an end of the treatment and the confirmation of successful H. pylori eradication test); eradication regimen, H. pylori eradication rate (intention-to-treat analysis, ITT; per-protocol analysis, PP), side effects, and the compliance of SGT and BQT.
The methodological quality of the enrolled studies was assessed. RCTs were assessed by the Cochrane Risk of Bias Assessment Tool for the following categories: selection bias, performance bias (4), detection bias, attrition bias, reporting bias, and other bias (21).
The primary endpoint of this study was to compare the eradication rate of SGT and BQT by the ITT and PP analysis, and the secondary endpoint was to compare the side effects and the compliance of both groups.
This meta-analysis was performed by Stata software (version 12; Stata Corp LP), and p < 0.05 was regarded as a significant difference. Risk ratios (RRs) and corresponding 95% CIs were used to determine the effect of SGT and BQT. A random effect model was used to reduce the potential bias caused by heterogeneity. Heterogeneity was detected by the chi-squared test and I2 test, with the value of p < 0.10 of the chi-squared test or I2 > 50% defined as a significant heterogeneity. Publication bias was estimated by a funnel plot, Begg's test, and Egger's test. The value of p < 0.05 of Begg's test and Egger's test was deemed to indicate a significant publication bias. When heterogeneity was large, studies were removed one by one, and changes in heterogeneity were observed to find the source of heterogeneity.
As shown in Figure 1, 656 records were identified, and 64 were removed due to duplication. In the first stage, 579 studies were removed after the title and abstract screening. In the second stage, 13 studies were reviewed by full text, and 8 studies were removed for the lack of empirical BQT control group (n = 4), lack of initial treatment (n = 1), a non-RCT study design (n = 2), and a treatment duration not equal to 10 or 14 days (n = 1). Finally, 5 studies (17–19, 22, 23) and 2,110 H. pylori-infected patients were enrolled; 1,100 patients were assigned to SGT, and 1,010 were assigned to BQT.
The study characteristics are shown in Table 1. All of the studies were published between 2015 and 2021; 4 studies were conducted in China, and 1 was conducted in Korea. In these 5 studies, antimicrobial susceptibility was determined by the E-test in 2 studies, the agar-dilution test in 2 studies, and the dual-priming oligonucleotide-based multiplex PCR (DPO-PCR) test in 1 study. Susceptibility-based triple and/or quadruple therapy was used in the SGT group in all studies.
As demonstrated in Supplementary Figure S1, the 5 RCTs showed a low risk of detection bias, attrition bias, reporting bias, and random sequence generation. Two studies showed an unclear risk in allocation concealment, and three studies showed a low risk. Regarding performance bias, one study showed a high risk, another study showed a low risk, and the remaining three studies showed an unclear risk. As presented in Supplementary Figures S2–S4, there was no significant publication bias in this meta-analysis, as determined by the funnel plot, Begg's test (p = 0.24), and Egger's test (p = 0.23).
As presented in Supplementary Figures S5, S6, the pooled H. pylori eradication rate was 86% (95% CI, 80–92) in SGT and 78% (95% CI, 70–85) in BQT by an ITT analysis. As shown in Figure 2, SGT had a superior eradication efficacy compared to BQT, and the difference was statistically significant (pooled RR = 1.10, 95% CI, 1.01–1.21, p = 0.03). In addition, a significant heterogeneity (chi-squared test, p =0.009, I2 = 70.5%) was identified between the enrolled studies.
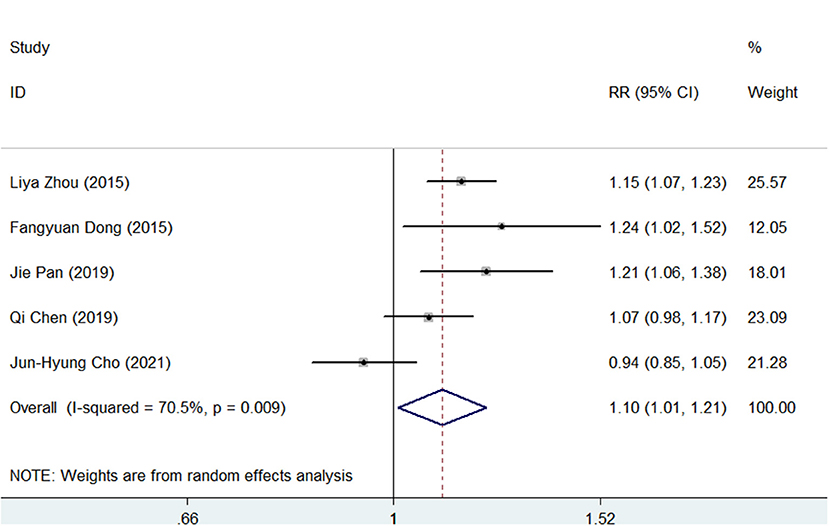
Figure 2. Forest plots for the comparison of susceptibility-guided therapy (SGT) vs. bismuth-containing quadruple therapy (BQT) in Helicobacter pylori eradication by an intention-to-treat (ITT) analysis.
For the PP analysis, the pooled H. pylori eradication rate was 92% (95% CI, 87–97) in the SGT group and 86% (95% CI, 77–94) in the BQT group (Supplementary Figures S7, S8). As shown in Figure 3, SGT had a superior eradication efficacy compared to BQT although there was no statistical significance (pooled RR = 1.07, 95% CI, 0.98–1.16, p = 0.147) and a significant heterogeneity (chi-squared test, p = 0, I2 = 85.7%) was also identified.
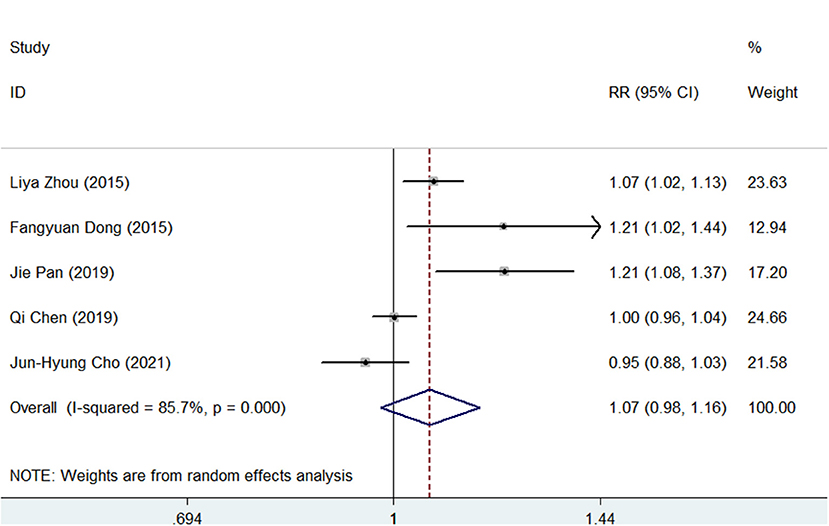
Figure 3. Forest plots for the comparison of SGT vs. BQT in H. pylori eradication by a per-protocol (PP) analysis.
Next, we analyzed the eradication rate of SQT and BQT in patients with dual clarithromycin-metronidazole resistance, which was recorded in two studies (17, 18). The eradicate rate was 93% (112/121) in SGT and 80% (93/116) in BQT (SGT vs. BQT; RR = 1.15, 95%CI: 1.02–1.29).
A subgroup analysis was conducted according to the different types of antimicrobial susceptibility tests used in the 5 studies. As shown in Figure 4, SGT had a significantly superior efficacy than BQT (pooled RR = 1.14, 95% CI, 1.08–1.20, p < 0.05) in a subgroup of cultures with susceptibility tests. However, in a subgroup of DPO-PCR tests, SGT tended to have a similar efficacy as BQT (RR = 0.94, 95% CI, 0.85–1.05).
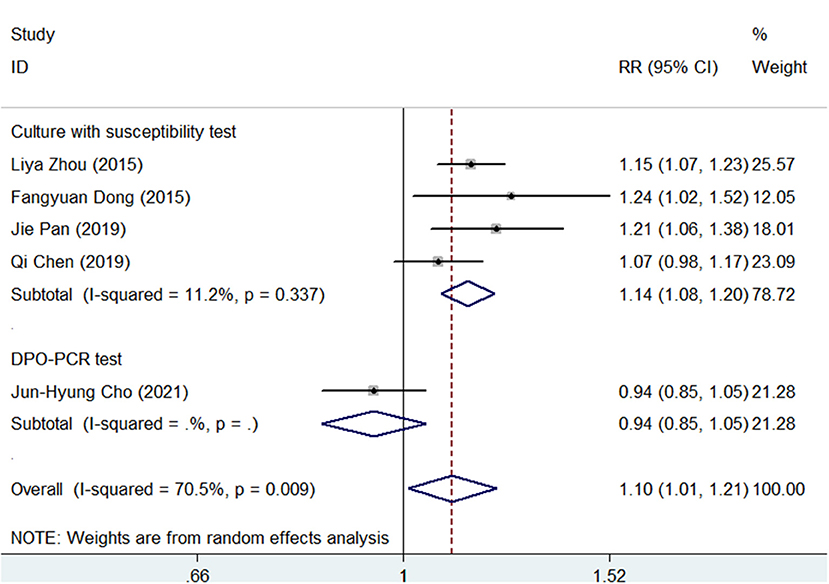
Figure 4. Forest plots for the comparison of SGT vs. BQT using the different types of antimicrobial susceptibility tests in H. pylori eradication.
Next, we performed a subgroup analysis according to different treatment durations (10 or 14 days) and antibiotic combinations (amoxicillin + clarithromycin or amoxicillin + metronidazole) of BQT in the 5 studies. As shown in Supplementary Figures S9, S10, SGT had a significantly superior efficacy than BQT (pooled RR = 1.16, 95% CI, 1.10–1.23, p < 0.05) in the amoxicillin + clarithromycin subgroup, while in the amoxicillin + clarithromycin subgroup, a significant difference was not found (pooled RR = 1.01, 95% CI, 0.89–1.15, p > 0.05). For the treatment duration subgroup, SGT showed a superior efficacy than BQT in both 10- (pooled RR = 1.15, 95% CI, 1.07–1.23) and 14-day (pooled RR = 1.09, 95% CI, 0.97–1.23) subgroups.
The side effects were recorded in all studies. As shown in Supplementary Figures S11, S12, the pooled side effect rate was 20% (95% CI, 10–30) in SGT and 22% (95% CI, 11–33) in BQT. There were no significant differences between the two groups regarding the side effects (RR = 0.90, 95% CI, 0.72–1.14, p = 0.383), as presented in Figure 5.
Compliance was recorded in 4 of the 5 studies and defined as patients who took more than 80% of the treatment drugs. As shown in Supplementary Figures S13, S14, the pooled compliance rate was 95.0% (95% CI, 93–98) in SGT and 92% (95% CI, 89–95) in BQT. As shown in Figure 6, although SGT tended to have a higher rate of compliance vs. BQT, there was no significant difference (RR = 1.03, 95% CI, 1.00–1.07, p = 0.07).
The susceptibility-guided therapy of H. pylori can be based on the results of culture with susceptibility tests or a molecular determination of genotype resistance, which is recommended after the failure of the second-line treatment in the Maastricht V/Florence Consensus Report (4). H. pylori resistance to antibiotics has become a serious global problem in recent years, mainly because of increased antibiotic use in the community (7, 24). Rational first-line regimens with a high efficacy should be applied to eradicate H. pylori to avoid secondary antibiotic resistance and failures of second- or third-line treatment, especially in areas with high antibiotic resistance. Theoretically, a susceptibility test is the best choice for guiding a tailored therapy for H. pylori infection. If not available, empiric BQT is recommended, and sensitive antibiotic combinations should be used in the regimens based on the region or population-specific antibiotic susceptibility data (13, 25). A recent Taipei global consensus also emphasized the importance of susceptibility testing for guiding decision-making for H. pylori regimens (5).
A previous meta-analysis (26–28) was conducted to explore a tailored therapy vs. an empirically chosen treatment for H. pylori eradication, which showed higher efficacy of the tailored therapy. The empiric treatments included standard or quadruple therapies, and standard therapy was not recommended for eradicating H. pylori because of its high resistance rate. Moreover, heterogeneity might exist because first-line or rescue treatments were included. DPO-PCR is a novel method applied in clinical practice. As such, this updated systematic review and meta-analysis included 5 RCTs to evaluate the efficacy of SGT vs. BQT for 10 or 14 days (recommended by the current consensus reports) as the first-line treatment for H. pylori. In total, SGT showed higher efficacy than BQT according to the ITT and PP analysis because the resistance rate of H. pylori was high in the included studies. We further conducted a subgroup analysis according to the different types of antimicrobial susceptibility tests of SGT, treatment durations, and BQT antibiotic combinations. Interestingly, SGT had a significantly superior efficacy than BQT in a subgroup of cultures with susceptibility tests. Cultures with susceptibility tests included the E-test, agar dilution test, and disk diffusion method. The agar dilution method is considered the gold standard for detecting the antibiotic resistance of H. pylori according to the Clinical and Laboratory Standards Institute (29). The E-test (different concentrations of antibiotics in a single strip were used) is simpler, has lower cost, and is less time-consuming than the agar dilution method (30), which is an acceptable alternative for antimicrobial susceptibility testing for H. pylori. Several studies (31–33) evaluated the accuracy of the E-test for H. pylori antibiotic resistance using the agar dilution method as the gold standard, and the results showed an acceptable agreement between the two methods. The E-test or agar dilution test can provide accurate antibiotic resistance information for H. pylori, guiding a tailored therapy for eradicating H. pylori. As such, a higher eradication rate of SGT was observed in comparison with BQT in a subgroup analysis of cultures with susceptibility tests.
Considering the time-consuming, cost-effectiveness, and false-negative results of culture and clarithromycin being the most common antibiotics in the first-line regimens for H. pylori, DPO-PCR is applied in clinical practice because of its straightforward and rapid test for H. pylori infection and clarithromycin resistance (34). A2142G and A2143G mutations were most frequent and account for ~80–90% of clarithromycin resistance (35). If mutations exist, the other antibiotics will be used in the H. pylori regimens. Otherwise, clarithromycin will be used in the regimens for eradicating H. pylori. Our results showed no differences in the eradication rate between SGT and BQT in a subgroup analysis of DPO-PCR. Several factors might explain this result: (1) Other mutations (36–39) of clarithromycin resistance also exist, including A2142C, A2115G, and G2141A. (2) The A2142C point mutation was reported to show a higher efficacy than A2142G and A2143G for inducing clarithromycin resistance (40). (3) DPO-PCR only provides information on clarithromycin resistance, which could not guide the choice of other antibiotics used in the H. pylori regimens.
Currently, the prevalence of H. pylori primary resistance to clarithromycin is high (7, 24), especially in China. Clarithromycin-resistant H. pylori is classified by the WHO as a high-priority bacterium (8). Moreover, clarithromycin resistance cannot be overcome by increasing the dose, frequency, or duration of the antibiotic. As such, SGT has significantly superior efficacy than BQT in the amoxicillin + clarithromycin combination subgroup.
Side effects and compliance were also analyzed in our study, and the results showed that the side effects of both groups were below 25%, and no serious side effects occurred. These data indicate the safety of SGT and BQT in the clinical practice of H. pylori eradication. Compliance is an important factor influencing the eradication rate of H. pylori. Several studies (41–43) were conducted in China to evaluate useful methods to improve compliance in the management of H. pylori infection. Interestingly, short-message-based reeducation or WeChat platform (a social media platform) reminders two times daily were the efficient ways to improve the eradication rate of H. pylori infection and elevate the compliance of H. pylori regimens. Our results also showed good compliance of SGT and BQT in the management of H. pylori infection.
There are limitations regarding this review. First, the included studies were conducted in China and Korea and also in areas with a high resistance rate to antibiotics, which may have limited the applicability of these findings outside Asian countries. The comparison between SGT and BQT in areas with moderate or low resistance rates to antibiotics remains unclear. Second, the results were not applied for patients who were allergic to penicillin. Third, the sample size of the included studies was limited, especially in a subgroup analysis. Fourth, heterogeneity existed when data from different studies were combined, which were probably attributed to the regimens, the method to detect antibiotic resistance, and the populations included in the enrolled studies. Fifth, our study aimed to compare the efficacy of SGT and BQT as the first-line treatment of H. pylori, which could not be applied in the second- or third-line treatment of H. pylori. Sixth, an unclear risk in allocation concealment and performance bias existed in a subset of the included studies, and a selection bias might have existed in this study. Seventh, the studies that did not mention the keywords in titles/abstracts or were published in other languages might have been missed in this review.
In conclusion, compared with BQT, SGT showed a higher efficacy and similar safety as the first-line treatment of H. pylori infection in areas with high antibiotic resistance. The decision-making of first-line regimens for H. pylori infection should depend on the availability and cost-effectiveness of susceptibility tests and bismuth in local areas, which is consistent with the recommendations of the Taipei global consensus (5).
YH was guarantor of the article. YO, WZ, and CH performed the literature search with a systematic review and meta-analysis and extracted the data. YO and YH wrote the manuscript. YZ, NL, and YH designed a systematic review and edited this manuscript. All authors approved the final version of this manuscript.
This study was supported by the National Natural Science Foundation of China (No. 82000531), the Project for Academic and Technical Leaders of Major Disciplines in Jiangxi Province (No. 20194BCJ22016), the Key Research and Development Program of Jiangxi Province (No. 20212BBG73018), the Youth Project of the Jiangxi Natural Science Foundation (No. 20202BABL216006), the Key Fund of the Jiangxi Education Department (No. GJJ190007), the Scientific Research of Health Commission of Jiangxi Province (No. 20213019), Scientific Research of Traditional Chinese Medicine of Jiangxi Province (No. 2020A0047), and the Young Teachers' Scientific Research and Cultivation Fund of the Medical Department of Nanchang University (PY201919).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.844915/full#supplementary-material
Supplementary Figure S1. Quality assessment of randomized controlled trials (RCT) studies.
Supplementary Figure S2. Publication bias evaluated by a funnel plot.
Supplementary Figure S3. Publication bias evaluated by Begg's test.
Supplementary Figure S4. Publication bias evaluated by Egger's test.
Supplementary Figure S5. Forest plots for the pooled eradication rate of susceptibility-guided therapy (SGT) by an intention-to-treat (ITT) analysis.
Supplementary Figure S6. Forest plots for the pooled eradication rate of bismuth-containing quadruple therapy (BQT) by an ITT analysis.
Supplementary Figure S7. Forest plots for the pooled eradication rate of SGT by per-protocol (PP) analysis.
Supplementary Figure S8. Forest plots for the pooled eradication rate of BQT by PP analysis.
Supplementary Figure S9. Forest plots for the comparison of SGT vs. BQT by different treatment duration in Helicobacter pylori eradication.
Supplementary Figure S10. Forest plots for the comparison of SGT vs. BQT by different antibiotic combinations in H. pylori eradication.
Supplementary Figure S11. Forest plots for the pooled side effect rate of SGT.
Supplementary Figure S12. Forest plots for the pooled BQT side effect rate.
Supplementary Figure S13. Forest plots for the pooled compliance of SGT.
Supplementary Figure S14. Forest plots for the pooled compliance of BQT.
Supplementary Table S1. Full search strategy in PubMed, Embase, and Cochrane Central.
Supplementary Table S2. The PRISMA 2009 checklist.
1. Kayali S, Manfredi M, Gaiani F, Bianchi L, Bizzarri B, Leandro G, et al. Helicobacter pylori, transmission routes and recurrence of infection: state of the art. Acta Biomed. (2018) 89:72–6. doi: 10.23750/abm.v89i8-S.7947
2. Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. (2015) 64:1353–67. doi: 10.1136/gutjnl-2015-309252
3. Yang L, Kartsonaki C, Yao P, de Martel C, Plummer M, Chapman D, et al. The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: a case-cohort study. Lancet Public Health. (2021) 6:e888–96. doi: 10.1016/S2468-2667(21)00164-X
4. Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the maastricht V/florence consensus report. Gut. (2017) 66:6–30. doi: 10.1136/gutjnl-2016-312288
5. Liou JM, Malfertheiner P, Lee YC, Sheu BS, Sugano K, Cheng HC, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. (2020) 69:2093–112. doi: 10.1136/gutjnl-2020-322368
6. Ding SZ, Du YQ, Lu H, Wang WH, Cheng H, Chen SY, et al. Chinese consensus report on family-based Helicobacter pylori infection control and management (2021 edition). Gut. (2021) 71:238–53. doi: 10.1136/gutjnl-2021-325630
7. Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. (2018) 155:1372–82.e17. doi: 10.1053/j.gastro.2018.07.007
8. Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. (2018) 18:318–27. doi: 10.1016/S1473-3099(17)30753-3
9. Liu DS, Wang YH, Zhu ZH, Zhang SH, Zhu X, Wan JH, et al. Characteristics of Helicobacter pylori antibiotic resistance: data from four different populations. Antimicrob Resist Infect Control. (2019) 8:192. doi: 10.1186/s13756-019-0632-1
10. Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. (2014) 12:177–86.e3; discussion: e12–3. doi: 10.1016/j.cgh.2013.05.028
11. Gao CP, Zhang D, Zhang T, Wang JX, Han SX, Graham DY, et al. PPI-amoxicillin dual therapy for Helicobacter pylori infection: an update based on a systematic review and meta-analysis. Helicobacter. (2020) 25:e12692. doi: 10.1111/hel.12692
12. Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, et al. The Toronto consensus for the treatment of Helicobacter pylori Infection in adults. Gastroenterology. (2016) 151:51–69.e14. doi: 10.1053/j.gastro.2016.04.006
13. Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, et al. Fifth Chinese National Consensus report on the management of Helicobacter pylori infection. Helicobacter. (2018) 23:e12475. doi: 10.1111/hel.12475
14. Pohl D, Keller PM, Bordier V, Wagner K. Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing. World J Gastroenterol. (2019) 25:4629–60. doi: 10.3748/wjg.v25.i32.4629
15. Matsumoto H, Shiotani A, Graham DY. Current and future treatment of Helicobacter pylori infections. Adv Exp Med Biol. (2019) 1149:211–25. doi: 10.1007/5584_2019_367
16. Shah SC, Iyer PG, Moss SF. AGA clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology. (2021) 160:1831–41. doi: 10.1053/j.gastro.2020.11.059
17. Zhou L, Zhang J, Song Z, He L, Li Y, Qian J, et al. Tailored versus triple plus bismuth or concomitant therapy as Initial Helicobacter pylori treatment: a randomized trial. Helicobacter. (2016) 21:91–9. doi: 10.1111/hel.12242
18. Chen Q, Long X, Ji Y, Liang X, Li D, Gao H, et al. Randomised controlled trial: susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment Pharmacol Ther. (2019) 49:1385–94. doi: 10.1111/apt.15273
19. Dong F, Ji D, Huang R, Zhang F, Huang Y, Xiang P, et al. Multiple genetic analysis system-based antibiotic susceptibility testing in Helicobacter pylori and high eradication rate with phenotypic resistance-guided quadruple therapy. Medicine. (2015) 94:e2056. doi: 10.1097/MD.0000000000002056
20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
21. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
22. Pan J, Shi Z, Lin D, Yang N, Meng F, Lin L, et al. Is tailored therapy based on antibiotic susceptibility effective? A multicenter, open-label, randomized trial. Front Med. (2020) 14:43–50. doi: 10.1007/s11684-019-0706-8
23. Cho JH, Jin SY, Park S. Comparison of tailored Helicobacter pylori eradication versus modified bismuth quadruple therapy in Korea: a randomized controlled trial. Expert Rev Anti Infect Ther. (2021) 20:1–7. doi: 10.1080/14787210.2022.2017280
24. Hu Y, Zhu Y, Lu NH. Primary antibiotic resistance of Helicobacter pylori in China. Dig Dis Sci. (2017) 62:1146–54. doi: 10.1007/s10620-017-4536-8
25. El-Serag HB, Kao JY, Kanwal F, Gilger M, LoVecchio F, Moss SF, et al. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol. (2018) 16:992–1002.e6. doi: 10.1016/j.cgh.2018.03.013
26. Lopez-Gongora S, Puig I, Calvet X, Villoria A, Baylina M, Munoz N, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. (2015) 70:2447–55. doi: 10.1093/jac/dkv155
27. Chen H, Dang Y, Zhou X, Liu B, Liu S, Zhang G. Tailored therapy versus empiric chosen treatment for Helicobacter pylori eradication: a meta-analysis. Medicine. (2016) 95:e2750. doi: 10.1097/MD.0000000000002750
28. Gingold-Belfer R, Niv Y, Schmilovitz-Weiss H, Levi Z, Boltin D. Susceptibility-guided versus empirical treatment for Helicobacter pylori infection: a systematic review and meta-analysis. J Gastroenterol Hepatol. (2021) 36:2649–58. doi: 10.1111/jgh.15575
29. CLSI. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically. 10th ed. Wayne, MI: Clinical and Laboratory Standards Institute (2015). p. M07.
30. Valdivieso-Garcia A, Imgrund R, Deckert A, Varughese BM, Harris K, Bunimov N, et al. Cost analysis and antimicrobial susceptibility testing comparing the E test and the agar dilution method in Campylobacter jejuni and Campylobacter coli. Diagn Microbiol Infect Dis. (2009) 65:168–74. doi: 10.1016/j.diagmicrobio.2009.07.008
31. Piccolomini R, Di Bonaventura G, Catamo G, Carbone F, Neri M. Comparative evaluation of the E test, agar dilution, and broth microdilution for testing susceptibilities of Helicobacter pylori strains to 20 antimicrobial agents. J Clin Microbiol. (1997) 35:1842–6. doi: 10.1128/jcm.35.7.1842-1846.1997
32. Ogata SK, Gales AC, Kawakami E. Antimicrobial susceptibility testing for Helicobacter pylori isolates from Brazilian children and adolescents: comparing agar dilution, E-test, and disk diffusion. Braz J Microbiol. (2014) 45:1439–48. doi: 10.1590/S1517-83822014000400039
33. Miftahussurur M, Fauzia KA, Nusi IA, Setiawan PB, Syam AF, Waskito LA, et al. E-test versus agar dilution for antibiotic susceptibility testing of Helicobacter pylori: a comparison study. BMC Res Notes. (2020) 13:22. doi: 10.1186/s13104-019-4877-9
34. Lehours P, Siffre E, Megraud F. DPO multiplex PCR as an alternative to culture and susceptibility testing to detect Helicobacter pylori and its resistance to clarithromycin. BMC Gastroenterol. (2011) 11:112. doi: 10.1186/1471-230X-11-112
35. Megraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. (2004) 53:1374–84. doi: 10.1136/gut.2003.022111
36. Hao Q, Li Y, Zhang ZJ, Liu Y, Gao H. New mutation points in 23S rRNA gene associated with Helicobacter pylori resistance to clarithromycin in northeast China. World J Gastroenterol. (2004) 10:1075–7. doi: 10.3748/wjg.v10.i7.1075
37. Kim JM, Kim JS, Kim N, Kim YJ, Kim IY, Chee YJ, et al. Gene mutations of 23S rRNA associated with clarithromycin resistance in Helicobacter pylori strains isolated from Korean patients. J Microbiol Biotechnol. (2008) 18:1584–9.
38. Rimbara E, Noguchi N, Kawai T, Sasatsu M. Novel mutation in 23S rRNA that confers low-level resistance to clarithromycin in Helicobacter pylori. Antimicrob Agents Chemother. (2008) 52:3465–6. doi: 10.1128/AAC.00445-08
39. Zhen-Hua Z, De-Qiang H, Yong X, Lin-Lin L, Nong-Hua L. Characterization of 23S rRNA gene mutation in primary and secondary clarithromycin-resistant Helicobacter pylori strains from East China. Turk J Gastroenterol. (2013) 24:5–9. doi: 10.4318/tjg.2013.0525
40. Wang YH, Wang FF, Gong XL, Yan LL, Zhao QY, Song YP, et al. Genotype profiles of Helicobacter pylori from gastric biopsies and strains with antimicrobial-induced resistance. Therap Adv Gastroenterol. (2020) 13:1756284820952596. doi: 10.1177/1756284820952596
41. Wang T, Yang X, Li Y, Li L, Liu J, Ji C, et al. Twice daily short-message-based re-education could improve Helicobacter pylori eradication rate in young population: a prospective randomized controlled study. Helicobacter. (2019) 24:e12569. doi: 10.1111/hel.12569
42. Luo M, Hao Y, Tang M, Shi M, He F, Xie Y, et al. Application of a social media platform as a patient reminder in the treatment of Helicobacter pylori. Helicobacter. (2020) 25:e12682. doi: 10.1111/hel.12682
Keywords: Helicobacter pylori, susceptibility-guided therapy, bismuth containing quadruple therapy, efficacy, meta-analysis
Citation: Ouyang Y, Zhang W, He C, Zhu Y, Lu N and Hu Y (2022) Susceptibility-Guided Therapy vs. Bismuth-Containing Quadruple Therapy as the First-Line Treatment for Helicobacter pylori Infection: A Systematic Review and Meta-Analysis. Front. Med. 9:844915. doi: 10.3389/fmed.2022.844915
Received: 29 December 2021; Accepted: 01 February 2022;
Published: 24 March 2022.
Edited by:
Zongxin Ling, Zhejiang University, ChinaReviewed by:
Rocco Maurizio Zagari, University of Bologna, ItalyCopyright © 2022 Ouyang, Zhang, He, Zhu, Lu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Hu, bmR5ZnkwNjIwMkBuY3UuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.