- 1Department of Rheumatology, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands
- 2Rheumatology and Immunology Center (ARC), Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands
- 3Department of Internal Medicine, Section of Nephrology, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands
- 4Department of Rheumatology, Spaarne Gasthuis, Hoofddorp, Netherlands
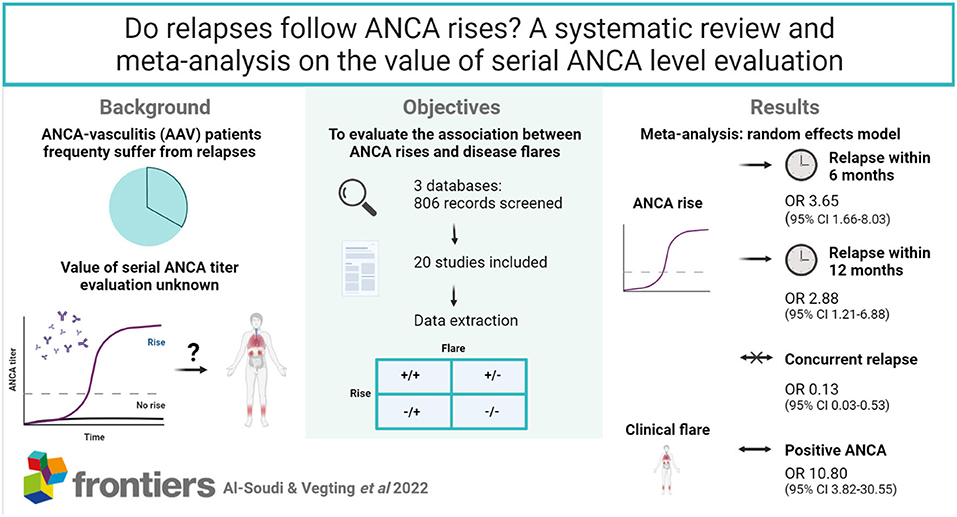
Objectives: ANCA-vasculitis (AAV) patients frequently suffer from relapses and risk subsequent organ damage. There is much debate on the value of serial ANCA level evaluation to monitor disease activity. We aimed to evaluate the association between ANCA rises and disease relapses at (I) moment of the rise, (II) within 6 months or (III) within a year from the rise.
Methods: 3 databases (MEDLINE, EMBASE, COCHRANE) were searched from 1993 through September 2021. We included studies that reported relapse incidence within 12 months after an ANCA rise measured by antigen-specific immunoassays in peripheral blood of AAV patients in remission. Quality assessment was performed using QUADAS-2. Finally, a meta-analysis was carried out to estimate average OR using a random effects model.
Results: Twenty unique studies were included. The methodological quality was limited due to risk of selection bias. An ANCA rise often preceded a disease relapse within 6 months (OR 3.65, 95% CI 1.66–8.03) and less often within 12 months (OR 2.88, 95% CI 1.21–6.88), while it was not indicative of a concurrent relapse (OR 0.13, 95% CI 0.03–0.53). Once a relapse is diagnosed, ANCA is significantly more often present than not (OR 10.80, 95% CI 3.82–30.55). As expected based on clinical, technical and methodological variability between studies, there was substantial heterogeneity across studies in all analyses (I2 = 70–87%).
Conclusion: In previously ANCA-positive patients, the ANCA test is often positive upon clinical suspicion of a disease relapse. Patients with a rise in ANCA are at risk of encountering disease relapses in the upcoming 6 or 12 months.
Key Messages
1. ANCA rises often precede disease relapses in the upcoming 6 or 12 months.
2. In previously ANCA-positive patients, the ANCA test is often positive upon clinical suspicion of a disease relapse.
Introduction
ANCA-associated vasculitis (AAV) refers to a group of vasculitides associated with the presence of antineutrophil cytoplasmic antibodies (ANCA). AAV comprises the clinical diagnoses of granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA). Although survival has improved over the past decades, mortality remains significantly increased, especially in patients with renal involvement (1, 2). Optimal treatment of AAV is challenging as the disease course is unpredictable. Some patients will experience relapses (after cessation of therapy) while others will not. Relapses induce and accelerate further organ damage, which is shown by the association between renal relapses and the incidence of end-stage kidney disease (ESRD) (3). Therefore, there is a pressing need for accurate biomarkers to monitor and predict disease activity in patients (4, 5). ANCA target predominantly proteinase-3 (PR3) and myeloperoxidase (MPO), both cytoplasmic components of neutrophils and monocytes. ANCA are being used extensively in diagnosing AAV, and are usually measured using immunoassays such as enzyme linked immunosorbent assays (ELISA) selectively measuring PR3- or MPO- antibodies. Although the place of ANCA testing in the diagnostic workup of AAV is undisputed, heterogeneous results have been obtained when investigating the predictive value of ANCA rises (6, 7). This heterogeneity is the result of clinical, technical and methodological factors that influence the correlation between ANCA rises and relapses. The disease severity of included patients, presence of renal involvement and persistence of ANCA positivity differs between cohorts (8). Technical and methodological factors comprise differences in sampling intervals, the use of both indirect immunofluorescence (IIF) and ELISA methods, and unclear chronological relations between ANCA changes and relapses. As a result, it remains unclear what ANCA rises mean when measured during remission, whether they precede subsequent relapses and/or if disease relapses are associated with positive ANCA levels.
Over the years the use of ELISA to primarily detect ANCA has increased and has resulted in an international consensus to use immunoassays as primary screening method, without the categorical need for IIF (9). A systematic review supported this notion and determined that especially the sensitivity of ANCA detection considerably increases with the use of immunoassays (10). The question whether an increase in ANCA levels leads to an increase in disease activity within a clinically relevant timeframe (e.g. within 6 months) remains open.
This systematic review provides a comprehensive literature search, followed by quality assessments and meta-analysis to explore whether ANCA level increases as measured by antigen-specific immunoassays associate with disease relapses at moment of the rise, within 6 months of the rise or within a year from the rise.
Methods
Literature Search Strategy
For this systematic review and meta-analysis, MEDLINE, EMBASE and COCHRANE were searched by two investigators (AA, MH) for 1) articles on ANCA measurements predicting relapse and 2) diagnosing disease activity in ANCA associated vasculitis (AAV). No restrictions were selected for publication date (inception to September 2021) and/or language. Articles were filtered on studies performed in humans. A combination of MeSH terms (Antibodies, Antineutrophil Cytoplasmic, Recurrence) and title + abstract (tiab) terms (ANCA, relapse, biomarker, disease activity) was used to identify relevant articles (Supplementary Table S1). Cross-references from reference lists of found studies and similar articles from PubMed were reviewed as well.
Study Selection
Found articles (last search on 01-09-2021) were screened on title and abstract by AA, MH and PLK independently. After title and abstract screening, selected studies were reviewed for final inclusion or exclusion (Figure 1). Inclusion criteria consisted of the use of antigen-specific immunoassays, a timeframe from measurement to relapse of a maximum of 12 months and a definition for a rise as either negative to positive conversion or a ANCA level increase. When patient cohort(s) were used in multiple studies, only one study was included. Since the majority of studies are performed using ELISA, we selected ELISA studies over alternative solid-phase immunoassays (such as FEIA) to increase the comparability between studies. No disagreements on study inclusion were found. In case of missing crucial data elements to calculate sensitivity, specificity and subsequent measures such as odds ratios, the study was excluded.
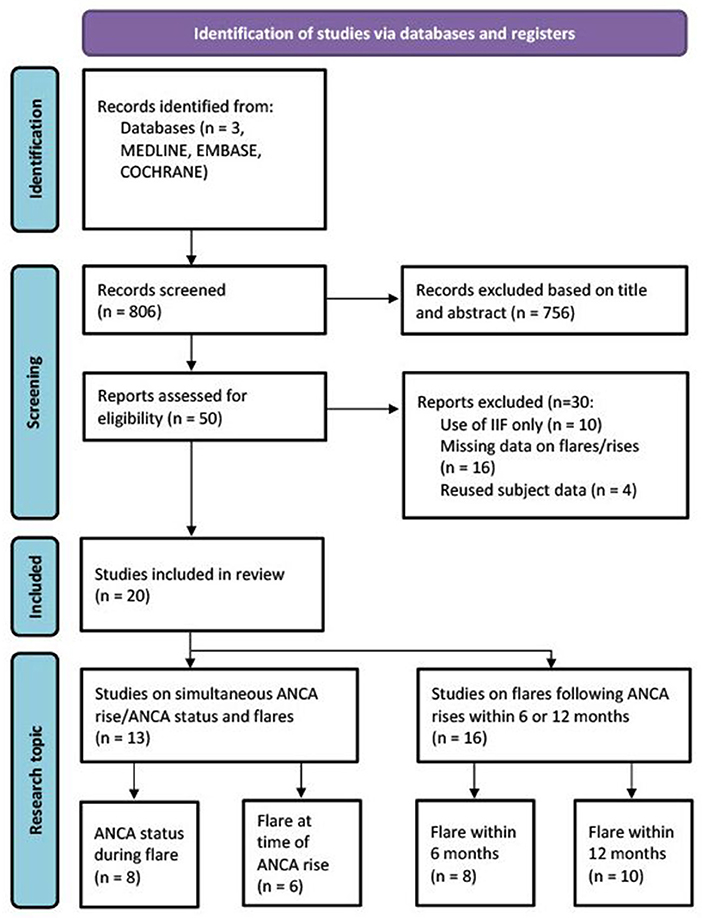
Figure 1. Schematic overview of study selection procedure (12).
Data Collection
Data extraction was performed by AA and YV independently. Name of first author, year of publication, study type, number of included patients, percentage of renal involvement, used immunoassay, definition of ANCA rise, and sampling interval were extracted. The slope of ANCA increase (%/month) was calculated by dividing the percentage of ANCA increase by the longest sampling interval. Studies were further separated on type of ANCA (PR3, MPO or pooled), time to relapse (rise during relapse, 1–6 months or 6–12 months), and the definition of a rise. From several studies we obtained data regarding ANCA rises during a relapse and prior to a relapse. From all selected studies the total number of relapses and total number of patients were extracted and four groups were created (rise+relapse+, rise-relapse+, rise+relapse-, rise-relapse-).
Quality Assessment
All included studies were assessed independently by AA and YV using the Quality Assessment Tool for Diagnostic Accuracy (QUADAS-2), in accordance with systematic review recommendations (11). According to QUADAS-2 guidelines, the content of the tool was first tailored to the two main research questions; to investigate the simultaneous presence of ANCA (rises) and relapses, and to investigate relapses that would follow ANCA rises within 6 or 12 months. This was followed by an independent pilot to reach agreement on the rating method by YV and MH. The QUADAS-2 supports a critical quality assessment of studies by providing four domains to assess: patient selection, index test, reference standard and timing; with subsequent questions in each domain. A study is regarded as having a high potential risk of bias when 2 or more negative points are found in each section. In case of discordance between quality assessments of AA and YV, the study was discussed with the other authors.
Statistical Analysis
To study ANCA positivity or rise during a relapse, odds ratios were calculated by dividing the odds of having a clinical flare accompanied by a positive or rising ANCA test, divided by the odds of having a clinical flare accompanied by a negative or stable ANCA test. To study relapses following ANCA rises, odds ratios were calculated by dividing the odds of having an ANCA rise in 6 or 12 months before a clinical flare is diagnosed, divided by the odds of having an ANCA rise without the presence of a clinical flare in the following months. Finally, a meta-analysis based on the odds ratio was performed to estimate the odds of having a relapse when ANCA rises as opposed to a non-ANCA-rise. A random effects model was used and 95% confidence intervals (CI) were calculated. Four meta-analyses were performed: ANCA positivity during a relapse, having a relapse when ANCA rises, having a relapse within 6 months of an ANCA rise and having a relapse within 12 months of an ANCA rise. Statistical heterogeneity was assessed by Chi-squared test (Chi2) and between-study inconsistency was quantified by the I2 statistic. In subsequent analyses, studies were pooled based on whether they were PR3-ANCA-only, MPO-ANCA-only or pooled ANCA. Forest plots were generated using Review Manager 5.4 software.
Results
Final Study Selection and Characteristics
The literature search provided 806 results. These included all articles found in the last known meta-analysis on this subject from 2012(6). After title and abstract screening, 50 studies were selected for full-text review (Figure 1) (12). Inclusion and exclusion criteria were reviewed and 30 studies were excluded due to the use of IIF only (N = 10), missing information to complete calculations or to subdivide studies in 6 or 12 month prediction analyses (N = 16) or due to reuse of patient cohorts (N = 4) (Supplementary Table S2). 20 studies were included (Table 1). In total, 13 studies were used for simultaneous ANCA rise and disease relapse analysis, with eight studies investigating whether ANCA is positive during disease relapse and six studies investigating whether a relapse is simultaneously present with an ANCA rise. A total of 16 studies were used for the meta-analyses of ANCA rises preceding subsequent relapses within 6 or 12 months. Some studies provided information on both simultaneous ANCA rise and disease relapse, as well as prediction of disease relapses. Selected studies were published between 1993 and 2019. Nine of 20 studies were prospectively conducted. Detailed characteristics of included studies are provided (Table 1).
Quality Assessment of Selected Studies
A risk of bias inventory was made and schematically overviewed for each of the conditions (Supplementary Tables S3–5). Reasons for scoring risk of bias are annotated (Supplementary Tables S6, S7).
What Is the ANCA Status When a Relapse Is Diagnosed?
Eight studies investigated the moment of relapse and tested for the positivity of ANCA after patients had been in remission prior (13, 16, 17, 21, 25, 28, 31, 32). None of these studies had included individuals with newly diagnosed AAV. Only one of the seven studies, Miloslavsky et al. (25), demonstrated an insignificant OR of 2.07 (CI 0.92–4.69) (25). All other studies favor the presence of a positive ANCA when a relapse is present, with a grand total OR of 10.80 (3.82–30.55) (Figure 2). Dolman et al. (17) and Segelmark et al. (31) included only PR3-ANCA positive individuals, whereas Ara et al. (13) and Terrier et al. (28) only included MPO-ANCA positive individuals. Ara et al. (13) and Dolman et al. (17) demonstrated the highest OR with 71.67 (2.26–2276.12) and 44.20 (1.80–1088.14), respectively, but had the lowest number of patients. The results significantly favor the detectability of ANCA at the moment of disease relapse (Figure 2).
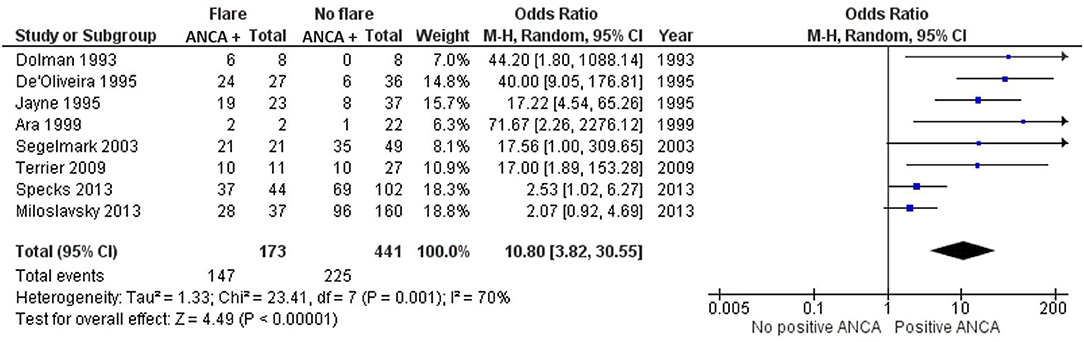
Figure 2. Meta-analysis summarizing the data regarding ANCA positivity once a relapse is diagnosed. Odds ratio with 95% confidence interval is displayed in the forest plot.
What Does a Rise in ANCA Indicate?
ANCA is often used to monitor AAV patients, even though fluctuations are common during follow-up. We reviewed the literature to understand whether a concurrent ANCA rise indicates a disease relapse or might associate with disease relapses in the future. Six studies investigated whether an ANCA rise is simultaneously associated with a disease relapse (8, 18–21, 26). In these studies, an ANCA rise was defined as 1.3–4-fold elevation in ANCA level. Jayne et al. (21), Han et al. (20), Finkielman et al. (18), Kemna et al. (8), and Fussner et al. (19) strongly favor an absence of a disease relapse, as opposed to the presence of a disease relapse, simultaneously with an ANCA increase. Nowack et al. (26) slightly trends toward favoring a relapse instead, with an OR of 1.62 (0.75–3.48). Overall there is an OR of 0.13 (0.03–0.53) that significantly favors not having a relapse, as opposed to the presence of a relapse, simultaneously with an ANCA increase (Figure 3).
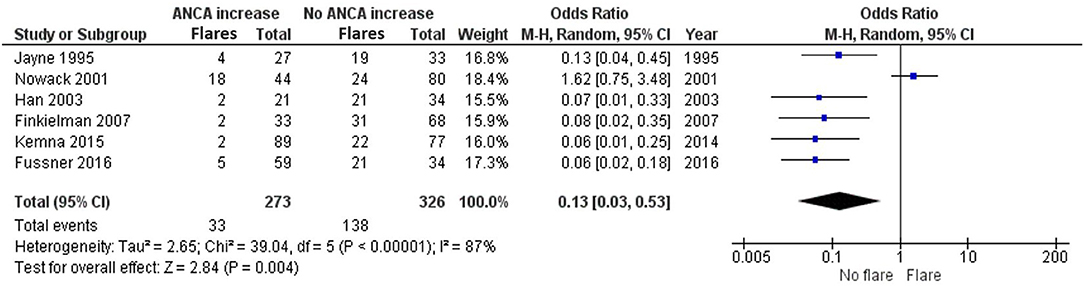
Figure 3. Meta-analysis summarizing the data regarding having a relapse when ANCA increases. Odds ratio with 95% confidence interval is displayed in the forest plot.
Do ANCA Increases Precede Future Disease Relapses?
ANCA increases could be indicative of an ongoing or starting inflammatory process that could indicate future relapses. We set out to investigate, within clinically relevant time frames, if this was the case. Eight studies investigating ANCA rises with subsequent relapse within 6 months of the rise are included in the meta-analysis (8, 14, 16, 17, 23, 26, 29, 30) (Figure 4).
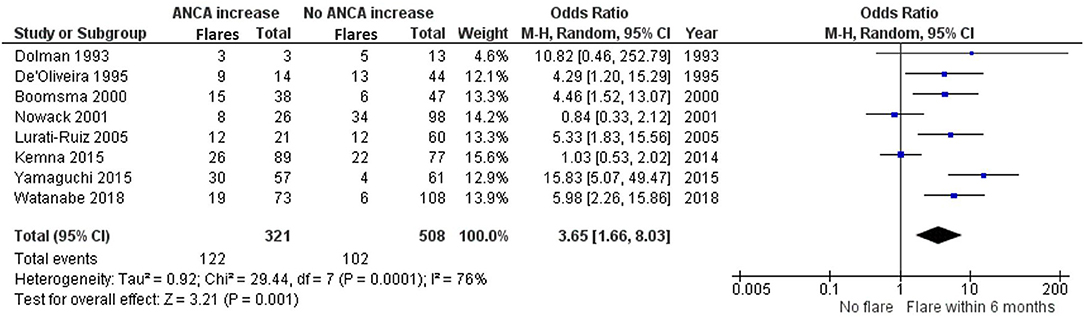
Figure 4. Meta-analysis summarizing the data regarding having a relapse within 6 months of an ANCA increase. Odds ratio with 95% confidence interval is displayed in the forest plot.
De'Oliveira et al. (16), Boomsma et al. (14), Lurati-Ruiz et al. (23), Yamaguchi et al. (30) and Watanabe et al. (29) all significantly favor a relapse within 6 months, as opposed to no relapse, after a measured ANCA increase. Additionally, the study by Dolman et al. (17) trends toward a relapse within 6 months with an OR of 10.82 (0.46–252.79). The studies by Nowack et al. (26) and Kemna et al. (8) remain inconclusive. The grand total OR of having a relapse within 6 months of an ANCA increase compared with not having a relapse is 3.65 (1.66–8.03).
Ten studies investigated ANCA rises with subsequent relapses within 12 months of the rise (8, 14, 15, 18–22, 24, 28) (Figure 5). Part of these studies overlap with the 6 months analysis, but have looked separately at relapses within 12 months. Boomsma et al. (14), Terrier et al. (28), Damoiseaux et al. (15), and McClure et al. (24) significantly favor the presence of a relapse as opposed to no relapse, within 12 months of an ANCA increase. Additionally, Jayne et al. (21), Han et al. (20), Kemna et al. (8), and Jones et al. (22) trend toward favoring a relapse within 12 months of an ANCA increase. However, Finkielman et al. (18) and Fussner et al. (19) favor no relapse instead, making the 12-month data slightly more variable compared with the 6-month data. This also resulted in a slightly lower, yet significant grand total OR of 2.88 (1.21–6.88), favoring a relapse as opposed to no relapse within 12 months of an ANCA increase.
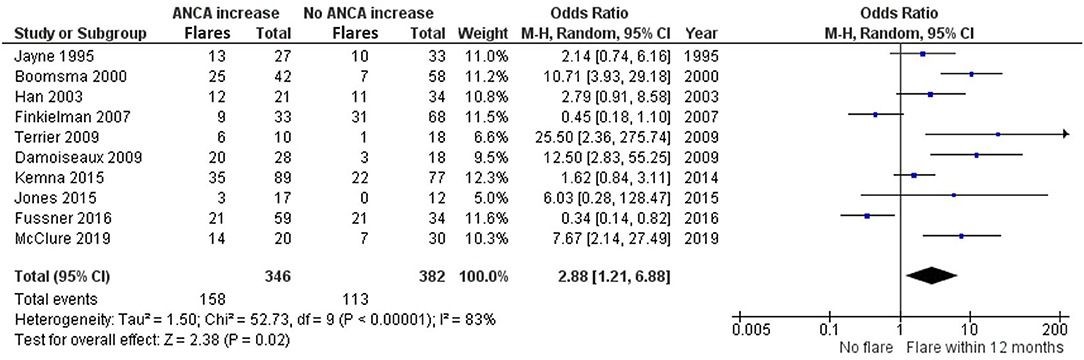
Figure 5. Meta-analysis summarizing the data regarding having a relapse within 12 months of an ANCA increase. Odds ratio with 95% confidence interval is displayed in the forest plot.
Differences Between PR3-ANCA and MPO-ANCA Positive Patients
In previous analyses PR3-ANCA and MPO-ANCA patients were pooled. To extrapolate our findings to patient groups, it would be of interest to understand whether disease relapses are predicted more accurately by changes in one of the two ANCA types. Within 6 months of a ANCA rise, Yamaguchi et al. (30) and Watanabe et al. (29) investigated MPO-ANCA only. A grand total OR of 9.29 (3.59–24.07) was reached in favor of having a relapse, as opposed to no relapse, within 6 months after MPO-ANCA increase (Supplementary Figure S1). In contrast, Dolman et al. (17), Boomsma et al. (14) and Nowack et al. (26), all investigating PR3-ANCA positive patients, only showed a trend toward favoring having a relapse within 6 months of a PR3-ANCA increase at an OR of 2.46 (0.57–10.62) (Supplementary Figure S2).
At 12 months, Boomsma et al. (14) and Terrier et al. (28) both favor a relapse within 12 months of an MPO-ANCA increase with an OR of 27.11 (4.14–177.41) (14, 28) (Supplementary Figure S3). However, both studies have studied a low number of patients. For PR3-ANCA positive patients only, the results are heterogeneous. Finkielman et al. (18) and Fussner et al. (19) favor no relapse within 12 months of a PR3-ANCA increase with OR of 0.45 (0.18–1.10) and 0.34 (0.14–0.82) respectively. However, Boomsma et al. (14), Damoiseaux et al. (15) and McClure et al. (24) significantly favor a relapse within 12 months of a PR3-ANCA increase, shifting the grand total of the five studies toward favoring a relapse within 12 months at an OR of 2.53 (0.52–12.44) (Supplementary Figure S4). Although the results are widespread, clinical characteristics of the five studies are similar for ANCA increase definition, but the two studies that trend toward no association are prospective cohort studies (18, 19) (Table 1).
Discussion
The use of serial serum ANCA level evaluation during remission is questionable and varies in daily clinical practice. In this systematic review and meta-analysis we demonstrate that during follow-up of AAV patients who are in remission, an ANCA rise often preceded a disease relapse within 6 months and to a lower extent within 12 months, while an increase in ANCA level is not indicative of an ongoing relapse. Once a relapse is diagnosed, ANCA is significantly more often present than not. In further follow-up of patients, MPO-ANCA increases were more significantly associated with future disease relapses than PR3-ANCA increases.
The complexity of ANCA level evaluation has frequently been discussed and is influenced by many factors, such as disease severity, follow-up, treatment and variation in ANCA test methods (8). Whether the serological subtype affects the association between ANCA rises and relapses remains unclear since ANCA response is heavily influenced by induction regimens (19). While data from our meta-analysis suggests that there was a strong association between ANCA rises and relapses in MPO-ANCA positive patients, a recent cohort study of Van Dam et al., (33) did not find an association with reappearance of MPO-ANCA, but only of PR3-ANCA. All studies on MPO-ANCA included in our meta-analysis used cyclophosphamide for remission-induction therapy, while Rituximab was used in the latter study. These results indicate differences in the role of MPO- and PR3-ANCA during disease reactivation (34) and treatment-specific interactions, but the exact mechanisms remain to be elucidated.
The variable association between ANCA and relapses could also be explained by modulation of immune responses on a tissue level. While it is known that ANCA activate the innate immune system leading to necroinflammation and endothelial cell damage (35, 36), disease manifestations are not always present in patients with elevated ANCA levels. Therefore, we speculate that the autoimmune cascade induced by ANCA is counterbalanced by mechanisms that attempt to maintain homeostasis. For example, serum anti-inflammatory cytokine IL-10 levels are increased in ANCA patients (27, 37, 38) and inhibit inflammation caused by neutrophils (39). In many cases, ANCA level fluctuations do not lead to substantial organ damage and no relapse is following. However, when these feedback mechanisms are overwhelmed and local damage is increasing, complaints develop and a relapse is diagnosed. Therefore, ANCA rises and relapses are not exhibited at the same time, but are clearly linked.
Results of this meta-analysis extend our knowledge of the utility of ANCA level evaluation and are consistent with a previous systematic review (6), demonstrating that ANCA rises have a significant association with future disease relapses. Key strengths are first, that studies are selected for the use of a similar antigen-specific immunoassay, which minimizes bias introduced by comparing multiple testing techniques. It has to be noted that no recommendations exist on what assay is preferred for monitoring or follow-up of AAV patients (9). Second, relapses are categorized according to clinically relevant timeframes from ANCA rise to relapse. And third, the PR3-ANCA and MPO-ANCA subtypes are separately studied in sub-analyses. Therefore, the combination of clinically relevant inclusion criteria combined with an extensive quality assessment provides clinicians with information on the value of ANCA testing during a relapse and the association with future disease relapses.
Yet, weaknesses in our systematic review may have also arisen from the choice to include all types of study designs. The patients, methods and techniques used in the included studies were diverse, leading to statistical heterogeneity. The study of Kemna et al. (8) identified multiple factors that influence the association between ANCA rises and relapses, such as renal involvement, persistent ANCA positivity, slope, and sampling interval. As can be seen from Table 1, these factors differed between the included patients cohorts, likely affecting outcomes. Also, over half of the studies showed a high risk of bias by quality assessment (Supplementary Tables S6, S7), which can lead to under- or overestimation of the observed effect. For example, case-control studies may lead to overestimation of the diagnostic accuracy (40), the majority of studies did not specify which patients remained ANCA-positive in remission, therefore, inclusion of these patients could affect the results on ANCA status during a relapse. Furthermore, ANCA rises and time intervals were frequently not pre-defined, nor blinding for the ANCA test was performed. Since individual patient data was rarely available, we could not adjust for potential confounders or look into patient-specific effects, therefore, we provided estimates of the odds ratios for the total group of ANCA patients.
What do our results mean for clinical practice? In case of a disease relapse the ANCA is likely to be positive, similar to the situation at time of AAV diagnosis. Therefore, in case of a negative ANCA test, alternative diagnoses should logically be considered. Second, an ANCA rise is not necessarily related to a concurrent relapse, but patients are more likely to experience a disease relapse in the 6 months following an ANCA increase. These results provide further support to monitor patients with an ANCA rise more closely and raise the question if patients should be treated pre-emptively to prevent a potential disease relapse with subsequent organ damage. On the other hand, over half of the patients with an ANCA rise do not experience a relapse within a year, and these patients risk complications of overtreatment such as infections. Starting or escalating immunosuppressive treatment could be beneficial for high-risk patients. This is confirmed in a small randomized trial which showed that there were significantly fewer major relapses in the group that was pre-emptively treated with high dose cyclophosphamide and prednisolone after an ANCA rise (41). In addition, two retrospective cohorts found a significant reduction in the incidence of relapses in patients in whom maintenance therapy was intensified (20, 30). These studies are limited by small sample sizes and differences in treatment options. Based on our findings and these promising results, we believe pre-emptive treatment will form the future clinical practice guided by prediction models including specific patient characteristics (severity of disease, renal involvement, persistence of ANCA, type of ANCA, glycosylation profiles), environmental characteristics (seasonal influences) and testing characteristics (immunoassay used, sampling interval) to minimize potential side effects of overtreatment and maximize efficacy in selecting individuals at high risk of developing a vasculitis flare (42–44). Further research will investigate potential applicability, benefits and cost-effectiveness of changing this clinical practice and tailoring therapy for specific patient groups.
Conclusion
Although an AAV relapse is associated with positive ANCA, rises in ANCA level are not indicative of a concurrent disease relapse. However, ANCA increases do associate significantly with higher odds of having a disease relapse within the first 6 and 12 months after measurement. This association is strongest in MPO-ANCA positive patients and loses significance in PR3-ANCA positive patients. Our meta-analysis confirms that in previously ANCA-positive patients, the ANCA test is often positive upon clinical suspicion of a disease relapse and an increasing ANCA may be helpful to identify patients that are more at risk of encountering disease relapses in the upcoming 6 or 12 months, and for whom pre-emptive treatment could be a realistic possibility.
Author Contributions
AA and MH set up the literature search strategy and performed the search. Study selection was performed by AA, MH, and PK. The quality of studies was assessed, data extraction, and data analysis was performed by AA and YV. All authors contributed in writing the manuscript and approved the final manuscript.
Funding
This work was supported by the Dutch Kidney Foundation [Grant number 19OK007 to MH and YV] and ZonMW [VENI grant number 91617058 to PK].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Graphical abstract was created with BioRender.com.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.844112/full#supplementary-material
References
1. Berti A, Cornec-Le Gall E, Cornec D, Casal Moura M, Matteson EL, Crowson CS, et al. Incidence, prevalence, mortality and chronic renal damage of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis in a 20-year population-based cohort. Nephrol Dial Transplant. (2019) 34:1508–17. doi: 10.1093/ndt/gfy250
2. Wallace ZS, Lu N, Unizony S, Stone JH, Choi HK. Improved survival in granulomatosis with polyangiitis: a general population-based study. Semin Arthritis Rheum. (2016) 45:483–9. doi: 10.1016/j.semarthrit.2015.07.009
3. Wester Trejo MAC, Flossmann O, Westman KW, Hoglund P, Hagen EC, Walsh M, et al. Renal relapse in antineutrophil cytoplasmic autoantibody-associated vasculitis: unpredictable, but predictive of renal outcome. Rheumatology (Oxford). (2019) 58:103–9. doi: 10.1093/rheumatology/key260
4. Draibe JB, Fulladosa X, Cruzado JM, Torras J, Salama AD. Current and novel biomarkers in anti-neutrophil cytoplasm-associated vasculitis. Clin Kidney J. (2016) 9:547–51. doi: 10.1093/ckj/sfw056
5. Monach PA. Biomarkers in vasculitis. Curr Opin Rheumatol. (2014) 26:24–30. doi: 10.1097/BOR.0000000000000009
6. Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis–a meta-analysis. Rheumatology. (2012) 51:100–9. doi: 10.1093/rheumatology/ker280
7. Birck R, Schmitt WH, Kaelsch IA, van der Woude FJ. Serial ANCA determinations for monitoring disease activity in patients with ANCA-associated vasculitis: systematic review. Am J Kidney Dis. (2006) 47:15–23. doi: 10.1053/j.ajkd.2005.09.022
8. Kemna MJ, Damoiseaux J, Austen J, Winkens B, Peters J, van Paassen P, et al. ANCA as a predictor of relapse: useful in patients with renal involvement but not in patients with nonrenal disease. J Am Soc Nephrol. (2015) 26:537–42. doi: 10.1681/ASN.2013111233
9. Bossuyt X, Cohen Tervaert JW, Arimura Y, Blockmans D, Flores-Suarez LF, Guillevin L, et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol. (2017) 13:683–92. doi: 10.1038/nrrheum.2017.140
10. Guchelaar NAD, Waling MM, Adhin AA, van Daele PLA, Schreurs MWJ, Rombach SM. The value of anti-neutrophil cytoplasmic antibodies (ANCA) testing for the diagnosis of ANCA-associated vasculitis, a systematic review and meta-analysis. Autoimmun Rev. (2021) 20:102716. doi: 10.1016/j.autrev.2020.102716
11. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
13. Ara J, Mirapeix E, Rodriguez R, Saurina A, Darnell A. Relationship between ANCA and disease activity in small vessel vasculitis patients with anti-MPO ANCA. Nephrol Dial Transplant. (1999) 14:1667–72. doi: 10.1093/ndt/14.7.1667
14. Boomsma MM, Stegeman CA, van der Leij MJ, Oost W, Hermans J, Kallenberg CG, et al. Prediction of relapses in Wegener's granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: a prospective study. Arthritis Rheum. (2000) 43:2025–33. doi: 10.1002/1529-0131(200009)43:9<2025::AID-ANR13>3.0.CO;2-O
15. Damoiseaux J, Dahnrich C, Rosemann A, Probst C, Komorowski L, Stegeman CA, et al. A novel enzyme-linked immunosorbent assay using a mixture of human native and recombinant proteinase-3 significantly improves the diagnostic potential for antineutrophil cytoplasmic antibody-associated vasculitis. Ann Rheum Dis. (2009) 68:228–33. doi: 10.1136/ard.2007.086579
16. De'Oliviera J, Gaskin G, Dash A, Rees AJ, Pusey CD. Relationship between disease activity and anti-neutrophil cytoplasmic antibody concentration in long-term management of systemic vasculitis. Am J Kidney Dis. (1995) 25:380–9. doi: 10.1016/0272-6386(95)90098-5
17. Dolman KM, Stegeman CA, van de Wiel BA, Hack CE., von dem Borne AE, Kallenberg CG, et al. Relevance of classic anti-neutrophil cytoplasmic autoantibody (C-ANCA)-mediated inhibition of proteinase 3-alpha 1-antitrypsin complexation to disease activity in Wegener's granulomatosis. Clin Exp Immunol. (1993) 93:405–10. doi: 10.1111/j.1365-2249.1993.tb08192.x
18. Finkielman JD, Merkel PA, Schroeder D, Hoffman GS, Spiera R, St Clair EW, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med. (2007) 147:611–9. doi: 10.7326/0003-4819-147-9-200711060-00005
19. Fussner LA, Hummel AM, Schroeder DR, Silva F, Cartin-Ceba R, Snyder MR, et al. Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthritis Rheumatol. (2016) 68:1700–10. doi: 10.1002/art.39637
20. Han WK, Choi HK, Roth RM, McCluskey RT, Niles JL. Serial ANCA titers: useful tool for prevention of relapses in ANCA-associated vasculitis. Kidney Int. (2003) 63:1079–85. doi: 10.1046/j.1523-1755.2003.00821.x
21. Jayne DR, Gaskin G, Pusey CD, Lockwood CM. ANCA and predicting relapse in systemic vasculitis. QJM. (1995) 88:127–33.
22. Jones RB, Furuta S, Tervaert JW, Hauser T, Luqmani R, Morgan MD, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis. (2015) 74:1178–82. doi: 10.1136/annrheumdis-2014-206404
23. Lurati-Ruiz F, Spertini F. Predictive value of antineutrophil cytoplasmic antibodies in small-vessel vasculitis. J Rheumatol. (2005) 32:2167–72.
24. McClure ME, Wason J, Gopaluni S, Tieu J, Smith RM, Jayne DR, et al. Evaluation of PR3-ANCA status after rituximab for ANCA-associated vasculitis. J Clin Rheumatol. (2019) 25:217–23. doi: 10.1097/RHU.0000000000001030
25. Miloslavsky EM, Specks U, Merkel PA, Seo P, Spiera R, Langford CA, et al. Clinical outcomes of remission induction therapy for severe antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. (2013) 65:2441–9. doi: 10.1002/art.38044
26. Nowack R, Grab I, Flores-Suarez LF, Schnulle P, Yard B, van der Woude FJ, et al. titres, even of IgG subclasses, and soluble CD14 fail to predict relapses in patients with ANCA-associated vasculitis. Nephrol Dial Transplant. (2001) 16:1631–7. doi: 10.1093/ndt/16.8.1631
27. Ohlsson S, Wieslander J, Segelmark M. Circulating cytokine profile in anti-neutrophilic cytoplasmatic autoantibody-associated vasculitis: prediction of outcome? Mediators Inflamm. (2004) 13:275–83. doi: 10.1080/09629350400003100
28. Terrier B, Saadoun D, Sene D, Ghillani P, Amoura Z, Deray G, et al. Antimyeloperoxidase antibodies are a useful marker of disease activity in antineutrophil cytoplasmic antibody-associated vasculitides. Ann Rheum Dis. (2009) 68:1564–71. doi: 10.1136/ard.2008.094714
29. Watanabe H, Sada KE, Matsumoto Y, Harigai M, Amano K, Dobashi H, et al. Association between reappearance of myeloperoxidase-antineutrophil cytoplasmic antibody and relapse in antineutrophil cytoplasmic antibody-associated vasculitis: subgroup analysis of nationwide prospective cohort studies. Arthritis Rheumatol. (2018) 70:1626–33. doi: 10.1002/art.40538
30. Yamaguchi M, Ando M, Kato S, Katsuno T, Kato N, Kosugi T, et al. Increase of antimyeloperoxidase antineutrophil cytoplasmic antibody (ANCA) in patients with renal ANCA-associated vasculitis: association with risk to relapse. J Rheumatol. (2015) 42:1853–60. doi: 10.3899/jrheum.141622
31. Segelmark M, Phillips BD, Hogan SL, Falk RJ, Jennette JC. Monitoring proteinase 3 antineutrophil cytoplasmic antibodies for detection of relapses in small vessel vasculitis. Clin Diagn Lab Immunol. (2003) 10:769–74. doi: 10.1128/CDLI.10.5.769-774.2003
32. Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. (2013) 369:417–27. doi: 10.1056/NEJMoa1213277
33. van Dam LS, Dirikgil E, Bredewold EW, Ray A, Bakker JA, van Kooten C, et al. PR3-ANCAs predict relapses in ANCA-associated vasculitis patients after rituximab. Nephrol Dial Transplant. (2021) 36:1408–17. doi: 10.1093/ndt/gfaa066
34. Hilhorst M, Van Paassen P, Cohen Tervaert JW, Registry LR. Proteinase-3 ANCA vasculitis versus myeloperoxidase-ANCA vasculitis. J Am Soc Nephrol. (2015) 26:2314–27. doi: 10.1681/ASN.2014090903
35. Kitching AR, Anders HJ, Basu N, Brouwer E, Gordon J, Jayne DR, et al. ANCA-associated vasculitis. Nat Rev Dis Primers. (2020) 6:71. doi: 10.1038/s41572-020-0204-y
36. Vegting Y, Vogt L, Anders HJ, de Winther MPJ, Bemelman FJ, Hilhorst ML. Monocytes and macrophages in ANCA-associated vasculitis. Autoimmun Rev. (2021) 20:102911. doi: 10.1016/j.autrev.2021.102911
37. Wikman A, Fagergren A, Gunnar OJS, Lundahl J, Jacobson SH. Monocyte activation and relationship to anti-proteinase 3 in acute vasculitis. Nephrol Dial Transplant. (2003) 18:1792–9. doi: 10.1093/ndt/gfg216
38. Hoffmann JC, Patschan D, Dihazi H, Muller C, Schwarze K, Henze E, et al. Cytokine profiling in anti neutrophil cytoplasmic antibody-associated vasculitis: a cross-sectional cohort study. Rheumatol Int. (2019) 39:1907–17. doi: 10.1007/s00296-019-04364-y
39. Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. (2012) 12:109–16. doi: 10.1016/j.chom.2012.05.015
40. Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JH, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA. (1999) 282:1061–6. doi: 10.1001/jama.282.11.1061
41. Tervaert JW, Huitema MG, Hene RJ, Sluiter WJ, The TH, van der Hem GK. Prevention of relapses in Wegener's granulomatosis by treatment based on antineutrophil cytoplasmic antibody titre. Lancet. (1990) 336:709–11. doi: 10.1016/0140-6736(90)92205-V
42. Kemna MJ, Cohen Tervaert JW, Broen K, Timmermans S, van Paassen P, Damoiseaux J. Seasonal influence on the risk of relapse at a rise of antineutrophil cytoplasmic antibodies in vasculitis patients with renal involvement. J Rheumatol. (2017) 44:473–81. doi: 10.3899/jrheum.160066
43. Kemna MJ, Plomp R, van Paassen P, Koeleman CAM, Jansen BC, Damoiseaux J, et al. Galactosylation and sialylation levels of IgG predict relapse in patients with PR3-ANCA associated vasculitis. EBioMedicine. (2017) 17:108–18. doi: 10.1016/j.ebiom.2017.01.033
Keywords: ANCA-associated vasculitis (AAV), anti-neutrophil cytoplasmic antibodies (ANCA), biomarker (BM), relapse, flare
Citation: Al-Soudi A, Vegting Y, Klarenbeek PL and Hilhorst ML (2022) Do Relapses Follow ANCA Rises? A Systematic Review and Meta-Analysis on the Value of Serial ANCA Level Evaluation. Front. Med. 9:844112. doi: 10.3389/fmed.2022.844112
Received: 27 December 2021; Accepted: 10 June 2022;
Published: 04 July 2022.
Edited by:
Gian Marco Ghiggeri, Giannina Gaslini Institute (IRCCS), ItalyReviewed by:
Augusto Vaglio, University of Parma, ItalyJan Willem Cohen Tervaert, University of Alberta, Canada
Nobuyuki Ono, Kyushu University, Japan
Copyright © 2022 Al-Soudi, Vegting, Klarenbeek and Hilhorst. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc L. Hilhorst, bS5sLmhpbGhvcnN0QGFtc3RlcmRhbXVtYy5ubA==
†These authors have contributed equally to this work and share first authorship
 Aram Al-Soudi
Aram Al-Soudi Yosta Vegting
Yosta Vegting Paul L. Klarenbeek
Paul L. Klarenbeek Marc L. Hilhorst
Marc L. Hilhorst