
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 17 February 2022
Sec. Nephrology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.842423
This article is part of the Research Topic Frailty and Sarcopenia in Various Cachectic Kidney Diseases, Volume II View all 9 articles
Background: Physical inactivity is an essential factor in the prognosis of patients with chronic kidney disease (CKD). Daily step count is a straightforward measure to assess physical activity levels. Understanding the step counts among different CKD stages is essential to change sedentary behavior.
Objectives: This systematic review and meta-analysis aimed to investigate the daily step counts in patients with CKD at a different stage.
Design: A systematic review and meta-analysis.
Data Sources: The literature search was performed in PubMed, Embase, and Web of Science from inception to November 3rd, 2021.
Review Methods: Observational studies (cross-sectional, case-control, or cohort studies) reported specific values of step counts in CKD patients by the wearable device were included. A random-effects model was used to pool the data. Subgroup analysis explored differences in outcomes by stage of CKD. Heterogeneity between studies was assessed using the χ2 test of Cochrane's Q statistic. A contour-enhanced funnel plot was conducted to investigate publication bias. Univariate and multivariate meta-regression was conducted to examine possible sources of heterogeneity.
Results: Twenty-eight articles were identified and used for quantitative analysis. The result showed that the daily step count in patients with CKD was 4642.47 (95% CI: 4274.18–5010.76), and significantly lower than the healthy population. Subgroup analysis revealed that the step counts decreased before dialysis, dropped to a freezing point at the hemodialysis phase, and increased after kidney transplantation. Meta-regression analysis showed that daily step counts were relatively higher in the Americas or younger than 60 or kidney transplant recipients.
Conclusion: The status of daily step counts in patients with CKD decreases with CKD severity and increases after kidney transplantation. Although studies have begun to focus on strategies to improve step counts in patients with CKD, future studies should focus more on step counts in pre-dialysis patients and changing their physically inactive lifestyle early to alleviate deteriorating renal function.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=291551, identifier: CRD42021291551.
Chronic kidney disease (CKD) has emerged as a major worldwide public health problem, with adverse physical, psychological, and economic outcomes (1). Being physically active is a healthy lifestyle considered a core part of the general and CKD populations (2). A recent Meta-analysis of a cohort study of patients with CKD showed that high physical activity was associated with a 14% reduction in all-cause mortality compared to low physical activity (3). Correspondingly, the UK Renal Association Clinical Practice Guideline recommends that patients with CKD engage in at least 30 min of moderate-intensity physical activity five times per week (4).
Walking is the most common physical activity for chronic diseases, including CKD (5). With the advent of pedometers, it has become feasible in the real world to accurately quantify the number of steps an individual walks each day. The number of daily steps reflects the amount of physical activity an individual is doing (6). Since the use of pedometers in medical research in the 1980s (7), numerous research studies have been conducted on the number of daily steps taken by individuals with CKD.
However, the mean or the median number of steps reported by different populations varies widely. Given the diversity of the results of these studies, this study evaluates the daily step counts in patients with CKD at a different stage by using a systematic review and meta-analysis to determine the differences between subgroups and provide a reference for promoting physical activity in patients with CKD.
This systematic review has been reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria statement (8). The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO), and the registration number was CRD42021291551.
We systematically searched PubMed, Embase, and Web of Science from inception to November 3rd, 2021. Free-text words and medical subject heading (MeSH) terms were used. The whole search strategy is presented in Supplementary Material 1. Moreover, we manually retrieved a relevant reference of systematic reviews to search for additional potential studies.
Published observation studies with the following criteria were considered eligible for inclusion in this meta-analysis: (1) participants: CKD patients. There is no limit to age and disease stage (pre-dialysis, peritoneal dialysis, hemodialysis, and kidney transplant recipients were eligible); (2) outcomes: the wearable device to measure the daily steps count; (3) studies: only observational study (cross-sectional, case-control, or cohort studies) designs were included; (4) studies reported specific values [mean (standard deviation, SD), median (quartile or range)] of patient steps were eligible. One literature was excluded if it met at least one of the following criteria: (1) patients affected by acute kidney failure; (2) clinical intervention trials, conference abstract, case reports, protocol, letters, commentaries, reviews, and editorials; (3) written in non-English.
Two authors (Yan Bai and Hui Wang) performed the literature selection independently, and a third author helped resolve any discrepancies if encountered. First, the titles/abstracts of all retrieved articles were reviewed, and studies that did not meet the inclusion criteria were excluded. Then, to determine if the articles meet final eligibility criteria, each author further reviewed the full text of the remaining studies.
Two independent authors (Yan Bai and Hui Wang) extracted the relevant information as follows: (1) first authors; (2) publication year; (3) sample size; (4) participant's age; (5) daily step counts; (6) assessment tool. We tried to contact the corresponding author for unclear data to determine whether to include this literature. Any disagreement about data extraction was resolved through consulting a third author.
For quantitative analysis, the mean, standard deviation (or standard error), and sample size were extracted from the included article. When results were presented in figures, data were extracted using the GetData software (getdata-graph-digitizer.com) (9–11). When results were described in terms of median, quartiles (or range) (12–20), data were calculated using the formula proposed by Luo et al. and Wan et al. (https://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html) (21, 22). If data were reported for subgroups (e.g., male vs. female) (23–25), we calculated a pooled mean (standard deviation) by pooling the data of the two different subgroups (26). Furthermore, if a study reported results for different stages of CKD separately, both data were included in the meta-analysis.
The quality of cross-sectional and longitudinal studies was assessed using the JBI Meta-Analysis of Statistics Assessment and Review Instrument (JBI-MAStARI) (27). The JBI-MAStARI includes eight questions. Two authors (Yan Bai and Hui Wang) performed the quality assessment procedure independently, with disagreements discussed by both authors or with the help of a third author.
Quantitative data synthesis was used to present the data extracted from each study. Given the potential heterogeneity across the included studies, a random-effects model was used with the DerSimonian-Laird method. Subgroup analysis explored differences in outcomes by stage of CKD.
Heterogeneity between studies was assessed using the χ2 test of Cochrane's Q statistic, and I-square estimates >75% were considered highly heterogeneous (28). Contour-enhanced funnel plots, the Egger's test, and the trim-and-fill method were conducted to investigate publication bias across studies.
Univariate and multivariate meta-regression were conducted to examine possible sources of heterogeneity using the following variables: region (Asia, Europe, and America), stage of CKD (pre-dialysis, peritoneal dialysis, hemodialysis, and kidney transplant recipients), samples (>100 and ≤100), bias (score < 7 and ≥7), age (<60 and ≥60), and measurement tools (Accelerometer, Armband, Pedometer, and Fitbit).
P < 0.05 was considered statistically significant. Stata software (version 12.0) was used to analyze the data.
The initial search yielded 464 articles and abstracts, and 198 pieces were excluded due to duplication. After screening titles and abstracts, 196 irrelevant articles were excluded. The remaining 36 articles were searched in full text for review based on inclusion and exclusion criteria. Eight articles were excluded, and the reasons for exclusion were listed in Supplementary Material 2. Finally, 28 articles were identified and used for quantitative analysis (9–20, 23–25, 29–41). The flow chart is based on PRISMA 2020 Flow Diagram (8) (Figure 1).
Hemodialysis patients were the most reported population. Study sample sizes ranged from 20 to 1,163 (Table 1), with a total sample size of 3,769. The age range across the studies was 15.1–70.9 years. Two studies used a longitudinal design, and 22 were cross-section studies, and four were cohort studies. The details of the quality assessment are shown in Supplementary Material 3.
Figure 2 shows a forest plot of studies included in the meta-analysis. The results based on the random-effects model showed an overall step count of 4642.47 (95% CI 4274.18–5010.76) for patients with CKD. The considerable heterogeneity (I2 = 93.5%) may be a specific result of differences in patient age, stage of disease, and sample size.
Subgroup analysis was performed to determine the overall step counts in different disease stages. Two studies reported step counts for pre-dialysis (5638.37, 95% CI 4052.48–7224.25) patients. Three and 22 studies reported step counts for peritoneal dialysis (4264.08, 95% CI 3546.29–4981.87) and hemodialysis patients (4111.82, 95% CI 3760.87–4462.77), respectively. Furthermore, four studies reported step counts for kidney transplant recipients (8689.83, 95% CI 6702.46–10677.20).
Ten studies reported steps on dialysis days and non-dialysis days in hemodialysis patients (Figure 3). A pooled analysis showed that the overall step counts on a dialysis day was 3413.24 (95% CI 2825.61–4000.88), with high heterogeneity (I2 = 93.1%); while the overall step counts on a non-dialysis day was 4197.83 (95% CI 3631.92–4763.75), also with high heterogeneity (I-squared = 89.0%).
Only four studies (three hemodialysis patients and one kidney transplant recipient) evaluated step counts in patients with CKD compared with healthy controls (Figure 4). A random-effects model showed that step counts were lower in CKD patients than healthy controls (Mean difference: −4034.10; 95% CI −5662.73 to −2405.46), and the difference being statistically significant.
Table 2 shows the results of univariate and multivariate analyses. Univariate analyses showed that the daily step counts were higher across studies with those carried out in the Americas and among the kidney transplant recipients. Patients were aged <60 years had significantly higher step counts than patients aged ≥60 years. Therefore, these three variables (region, stage of CKD, and age) were eligible for inclusion in the multivariable regression analysis.
The multivariable model was statistically significant [R2 = 51.12%; F(6,25); P < 0.001] and reduced the I2 from 93.5 to 89.5%. However, there were no variables that remained statistically significant.
As shown in Supplementary Material 4, the contour-enhanced funnel plot was asymmetric. The Egger's test indicates a publication bias (z = 3.67, P = 0.004, Supplementary Material 5 provides the Egger's publication bias plot). Although it is usually impossible to know the precise mechanism for funnel plot asymmetry, publication bias could explain the presence of an asymmetrical funnel plot (26). No significant change in effect size was found when reanalyzing publication bias using the trim-and-fill computation (Supplementary Material 6).
To our knowledge, this is the first systematic review of the literature to examine daily step counts in patients with the full spectrum of CKD. The key findings including (1) daily step counts in patients with CKD was 4642.47 (95% CI: 4274.18–5010.76); (2) patients with CKD have significantly lower daily step counts than healthy population, and the step counts began to decrease before dialysis, drops to a freezing point at the hemodialysis phase, and increases after kidney transplantation (Figure 5); (3) relatively high daily step counts in cases in the Americas or younger than 60 or kidney transplant recipients.
Physical activity is a cornerstone of renal rehabilitation in patients with CKD (42). Evidence suggests that regular physical activity is beneficial at all stages of CKD, including improvements in physical fitness, muscle functions, and health-related quality of life (43). More importantly, higher levels of physical activity are associated with a reduced risk of cardiovascular disease, all-cause mortality in this population (44). In comparing subjective methods (e.g., diaries, questionnaires) for physical activity assessment, activity monitors are regarded as the gold standard in detecting daily step counts and quantifying the volume of physical activity (45). A survey that included 5,656 participants reported that most CKD patients tend to lead sedentary and inactive lifestyles (46). A significantly higher risk of premature death has been reported in patients with <4,000 steps per day compared to hemodialysis patients with more than 4,000 steps per day (HR: 2.37; 95% CI 1.22–4.60) (40). A recent dose-response meta-analysis showed a 12% reduction in the risk of all-cause mortality for every 1,000 increase in step count (HR: 0.88; 95% CI 0.83–0.93) (47). However, most of the currently published studies were limited to assessing daily step counts taken by patients with CKD at a certain stage, and it would be helpful to pool different studies to understand trends to develop countermeasures to increase their physical activity.
In the current study, the average daily step counts for patients with CKD was 4642.47 (95% CI 4274.18–5010.76). The global physical activity recommendations issued by the American College of Sports Medicine (48) and the World Health Organization (49) recommend ~30 min of moderate physical activity per day as the minimum physical activity target value for maintaining and promoting health. Walking is recommended as the most basic physical activity for people with all types of chronic diseases. As a recommendation, Tudor-Locke et al. translated the above-recommended values (30 min or more of moderate physical activity per day) into a daily step count and recommended that patients with chronic diseases walk at least 7,000–10,000 steps per day (50). It can be seen that the physical activity level of CKD patients is much lower than recommended. Furthermore, a study by Chen et al. examined the relationship between walking and survival and renal replacement therapy in patients with CKD, reporting a 33% lower overall risk of death (HR: 0.67; 95% CI, 0.53–0.84) and a 21% lower risk of RRT (HR: 0.79; 95% CI, 0.73–0.85) (5). Additional studies have shown that walking is associated with an improved renal function (OR: 2.23; 95% CI, 1.91–2.60) in patients with CKD (51) and an inflammatory environment (52).
From the subgroup analysis of this study, the pooled analysis results further clarified the trend in the number of daily steps in patients with CKD at different stages, i.e., post-transplantation > pre-dialysis > peritoneal dialysis > hemodialysis. This result is similar to the findings of Wilkinson et al. on physical activity in patients with the whole spectrum of CKD (46). Predictably, physical activity levels increase with progressive physical function after kidney transplantation (53); for hemodialysis patients, the combination of dialysis-related fatigue, muscle atrophy, and other comorbidities (e.g., shortness of breath) further decreases physical activity over time in such patients (54). Only renal transplant recipients obtained daily step counts roughly equivalent to the recommended value, indirectly reflecting that most CKD patients do not reach the WHO recommended physical activity level. Therefore, further population-based research is needed to assess the impact of different measures on increasing walking to improve the prognosis of patients with CKD, which should be a priority in renal rehabilitation.
Moreover, elderly patients with CKD have a lower step count and should be prioritized. Frailty is a common comorbidity in CKD patients, and its prevalence increases with age (55). Data support the concept of a spiral of frailty, whereby elder patients with CKD are predisposed to inactivity and frailty (56), and frailty can cause further inactivity and disability (57). However, while disease and aging contribute to frailty, there are also potentially reversible factors, such as inflammation (58). Evidence that increased walking improves indicators of inflammation is well-established (59). Walking reflects physical activity in daily life in the elderly population (60); therefore, low step counts in elderly CKD patients suggest the urgency and need for further strengthening of walking management in this population.
We also noted that the assessment of step counts in CKD patients was mostly limited to the dialysis population, largely ignoring the pre-dialysis group, even though there were indications of physical inactivity already in the early stages of CKD. In this regard, of the 28 publications included, only four studies assessed step counts in pre-dialysis patients. Moreover, compared to healthy controls, step counts were at a disadvantage in dialysis patients and kidney transplant recipients. This result is expected, with a reduced step count associated with disease progression.
Based on a search of the literature, we obtained randomized control trials on increasing daily step counts in patients with CKD (Table 3). Overall, feasible strategies included: (1) pedometer-based intervention based on goal-setting theory; (2) physical activity increase based on exercise interventions to improve physical function; (3) changing the dialysis model. Different interventions can achieve their effect through different mechanisms of action.
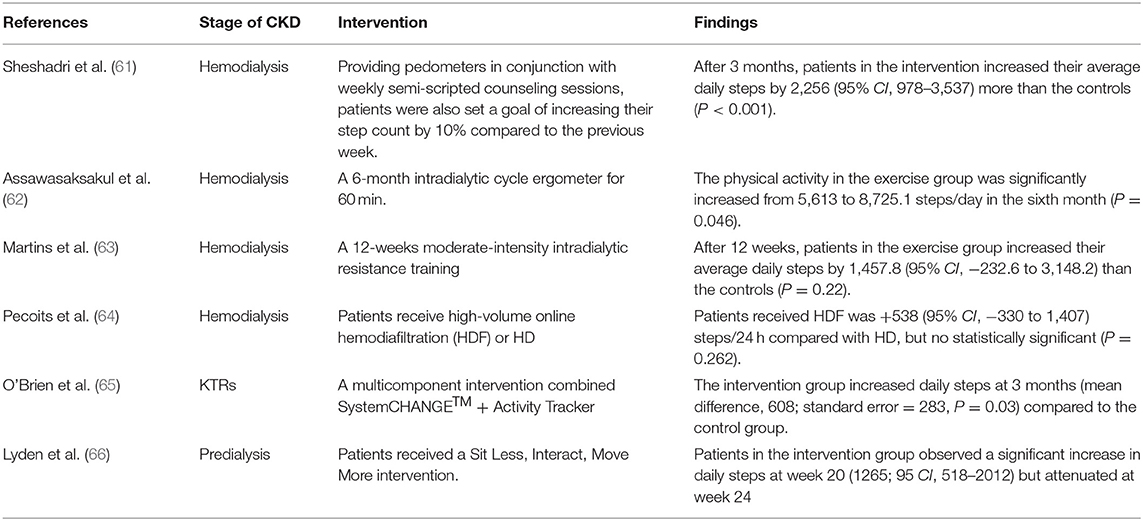
Table 3. A summary of randomized control trials on increasing daily step counts in patients with CKD.
There are some shortcomings of this study that should be mentioned. First, like other meta-analysis (67, 68), the statistical heterogeneity of the present meta-analysis was high and remained in the subgroup analysis, which is difficult to avoid in a meta-analysis of observational studies. For this result, we performed a meta-regression, heterogeneity may be associated with region, stage of disease, and age. However, a large portion of the high heterogeneity remains unexplained. Several other factors, such as duration of CKD disease, gender, etc., remain largely unknown and may be responsible for this unexplained heterogeneity. Secondly, although we conducted an exhaustive search of the three primary databases, the lack of non-English publications and unpublished studies may have contributed to the publication bias. We tried to account for this phenomenon by trim-and-fill in our analysis, but it remained unexplained. Thirdly, the current meta-analysis focused on literatures retrieved in standard databases, so gray literature and unpublished articles were not included. Although there may be additional studies of CKD patient step counts in the gray literature that may affect the estimation of pooled effects, we focused on the literature published with peer-reviewed outcomes.
The current systematic review reveals the status of daily step counts in patients with CKD, which decreases with increasing CKD severity and increases after kidney transplantation. Although studies have begun to focus on strategies to improve step counts in patients with CKD, the inclusion of participants and the short follow-up period require further elucidation of the effects of long-term changes in physical activity in this population. In addition, future studies should focus more on step counts in pre-dialysis patients, and changing their physically inactive lifestyle early in the disease is central to slowing the progression of CKD.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
FZ and YR: conceptualization and data analysis. YB and HW: retrieved literature and data extraction. FZ and LH: writing—original draft. LH: writing—review and editing. All authors have read and approved the final draft.
This study was supported by the Third Batch of Specialist Nurse Education Program of Longhua Hospital (RC-2021-03-04).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.842423/full#supplementary-material
1. Coresh J. Update on the Burden of CKD. J Am Soc Nephrol. (2017) 28:1020–2. doi: 10.1681/ASN.2016121374
2. Saint-Maurice PF, Troiano RP, Bassett DR Jr, Graubard BI, Carlson SA, Shiroma EJ, et al. Association of daily step count and step intensity with mortality among US adults. JAMA. (2020) 323:1151–60. doi: 10.1001/jama.2020.1382
3. Martins P, Marques EA, Leal DV, Ferreira A, Wilund KR, Viana JL. Association between physical activity and mortality in end-stage kidney disease: a systematic review of observational studies. BMC Nephrol. (2021) 22:227. doi: 10.1186/s12882-021-02407-w
4. Clinical Practice Guideline: Exercise and Lifestyle in Chronic Kidney Disease. Available online at: https://renal.org/sites/renal.org/files/Exercise%20and%20Lifestyle%20in%20CKD%20clinical%20practice%20guideline33_v4_FINAL_0.pdf (accessed January 26, 2021).
5. Chen IR, Wang S-M, Liang C-C, Kuo H-L, Chang C-T, Liu J-H, et al. Association of walking with survival and RRT among patients with CKD stages 3-5. Clin J Am Soc Nephrol. (2014) 9:1183–9. doi: 10.2215/CJN.09810913
6. Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. (2007) 298:2296–304. doi: 10.1001/jama.298.19.2296
7. Bohannon RW. Number of pedometer-assessed steps taken per day by adults: a descriptive meta-analysis. Phys Ther. (2007) 87:1642–50. doi: 10.2522/ptj.20060037
8. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
9. Williams S, Han M, Ye X, Zhang H, Meyring-Wösten A, Bonner M, et al. Physical activity and sleep patterns in hemodialysis patients in a suburban environment. Blood Purif. (2017) 43:235–43. doi: 10.1159/000452751
10. Han M, Ye X, Preciado P, Williams S, Campos I, Bonner M, et al. Relationships between neighborhood walkability and objectively measured physical activity levels in hemodialysis patients. Blood Purif. (2018) 45:236–44. doi: 10.1159/000485161
11. Malhotra R, Kumar U, Virgen P, Magallon B, Garimella PS, Chopra T, et al. Physical activity in hemodialysis patients on nondialysis and dialysis days: prospective observational study. Hemodial Int. (2021) 25:240–8. doi: 10.1111/hdi.12913
12. Zhang Q, Zhang J, Zhang W, Wang M, Huang B, Zhang M, et al. Risk factors for decreased upper-limb muscle strength and its impact on survival in maintenance hemodialysis patients. Int Urol Nephrol. (2020) 52:1143–53. doi: 10.1007/s11255-020-02468-0
13. Sheshadri A, Kittiskulnam P, Johansen KL. Higher physical activity is associated with less fatigue and insomnia among patients on hemodialysis. Kidney Int Rep. (2019) 4:285–92. doi: 10.1016/j.ekir.2018.10.014
14. Panaye M, Kolko-Labadens A, Lasseur C, Paillasseur JL, Guillodo MP, Levannier M, et al. Phenotypes influencing low physical activity in maintenance dialysis. J Ren Nutr. (2015) 25:31–9. doi: 10.1053/j.jrn.2014.07.010
15. Matsuzawa R, Matsunaga A, Kutsuna T, Ishii A, Abe Y, Yoneki K, et al. Association of habitual physical activity measured by an accelerometer with high-density lipoprotein cholesterol levels in maintenance hemodialysis patients. Sci World J. (2013) 2013:780783. doi: 10.1155/2013/780783
16. Lunney M, Wiebe N, Kusi-Appiah E, Tonelli A, Lewis R, Ferber R, et al. Wearable fitness trackers to predict clinical deterioration in maintenance hemodialysis: a prospective cohort feasibility study. Kidney Med. (2021) 3:768–75.e761. doi: 10.1016/j.xkme.2021.04.013
17. Kittiskulnam P, Sheshadri A, Johansen KL. Validation of a new physical activity instrument against pedometers among dialysis patients. J Ren Nutr. (2019) 29:498–503. doi: 10.1053/j.jrn.2019.03.081
18. Brys ADH, Bossola M, Lenaert B, Biamonte F, Gambaro G, Di Stasio E. Daily physical activity in patients on chronic haemodialysis and its relation with fatigue and depressive symptoms. Int Urol Nephrol. (2020) 52:1959–67. doi: 10.1007/s11255-020-02578-9
19. Avesani CM, Trolonge S, Deléaval P, Baria F, Mafra D, Faxén-Irving G, et al. Physical activity and energy expenditure in haemodialysis patients: an international survey. Nephrol Dial Transplant. (2012) 27:2430–4. doi: 10.1093/ndt/gfr692
20. Akber A, Portale AA, Johansen KL. Pedometer-assessed physical activity in children and young adults with CKD. Clin J Am Soc Nephrol. (2012) 7:720–6. doi: 10.2215/CJN.06330611
21. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
22. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
23. Mafra D, Deleaval P, Teta D, Cleaud C, Arkouche W, Jolivot A, et al. Influence of inflammation on total energy expenditure in hemodialysis patients. J Ren Nutr. (2011) 21:387–93. doi: 10.1053/j.jrn.2010.09.006
24. D'Alessandro C, Barsotti M, Cianchi C, Mannucci C, Morganti R, Tassi S, et al. Nutritional aspects in diabetic CKD patients on tertiary care. Medicina. (2019) 55:427. doi: 10.3390/medicina55080427
25. Cobo G, Gallar P, Di Gioia C, García Lacalle C, Camacho R, Rodriguez I, et al. Hypogonadism associated with muscle atrophy, physical inactivity and ESA hyporesponsiveness in men undergoing haemodialysis. Nefrologia. (2017) 37:54–60. doi: 10.1016/j.nefro.2016.04.009
26. Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane (2021). Available online at: www.training.cochrane.org/handbook
27. Ma L-L, Wang Y-Y, Yang Z-H, Huang D, Weng H, Zeng X-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. (2020) 7:7. doi: 10.1186/s40779-020-00238-8
28. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
29. Cupisti A, Capitanini A, Betti G, D'Alessandro C, Barsotti G. Assessment of habitual physical activity and energy expenditure in dialysis patients and relationships to nutritional parameters. Clin Nephrol. (2011) 75:218–25. doi: 10.5414/CNP75218
30. Cobo G, Gallar P, Gama-Axelsson T, Di Gioia C, Qureshi AR, Camacho R, et al. Clinical determinants of reduced physical activity in hemodialysis and peritoneal dialysis patients. J Nephrol. (2015) 28:503–10. doi: 10.1007/s40620-014-0164-y
31. Yamamoto S, Matsuzawa R, Hoshi K, Harada M, Watanabe T, Suzuki Y, et al. Impact of physical activity on dialysis and nondialysis days and clinical outcomes among patients on hemodialysis. J Ren Nutr. (2021) 31:380–8. doi: 10.1053/j.jrn.2020.07.007
32. Dontje ML, de Greef MH, Krijnen WP, Corpeleijn E, Kok T, Bakker SJ, et al. Longitudinal measurement of physical activity following kidney transplantation. Clin Transplant. (2014) 28:394–402. doi: 10.1111/ctr.12325
33. Hamiwka LA, Cantell M, Crawford S, Clark CG. Physical activity and health related quality of life in children following kidney transplantation. Pediatr Transplant. (2009) 13:861–7. doi: 10.1111/j.1399-3046.2009.01195.x
34. Lou X, Li Y, Shen H, Juan J, He Q. Physical activity and somatic symptoms among hemodialysis patients: a multi-center study in Zhejiang, China. BMC Nephrol. (2019) 20:477. doi: 10.1186/s12882-019-1652-z
35. Carvalho EV, Reboredo MM, Gomes EP, Teixeira DR, Roberti NC, Mendes JO, et al. Physical activity in daily life assessed by an accelerometer in kidney transplant recipients and hemodialysis patients. Transplant Proc. (2014) 46:1713–7. doi: 10.1016/j.transproceed.2014.05.019
36. Oishi D, Koitabashi K, Hiraki K, Imai N, Sakurada T, Konno Y, et al. Physical activity is associated with serum albumin in peritoneal dialysis patients. Adv Perit Dial. (2012) 28:148–52.
37. Shibata S, Tsutou A, Shiotani H. Relation between sleep quality and daily physical activity in hemodialysis outpatients. Kobe J Med Sci. (2014) 59:E161–166.
38. Katayama A, Miyatake N, Nishi H, Ujike K, Hashimoto H, Kurato R, et al. Relationship between changes in physical activity and changes in health-related quality of life in patients on chronic hemodialysis with 1-year follow-up. Acta Med Okayama. (2016) 70:353–61. doi: 10.18926/AMO/54593
39. Han M, Williams S, Mendoza M, Ye X, Zhang H, Calice-Silva V, et al. Quantifying physical activity levels and sleep in hemodialysis patients using a commercially available activity tracker. Blood Purif. (2016) 41:194–204. doi: 10.1159/000441314
40. Matsuzawa R, Roshanravan B, Shimoda T, Mamorita N, Yoneki K, Harada M, et al. Physical activity dose for hemodialysis patients: where to begin? Results from a Prospective Cohort Study. J Ren Nutr. (2018) 28:45–53. doi: 10.1053/j.jrn.2017.07.004
41. Raymond J, Johnson ST, Diehl-Jones W, Vallance JK. Walking, Sedentary Time and health-related quality life among kidney transplant recipients: an exploratory study. Transplant Proc. (2016) 48:59–64. doi: 10.1016/j.transproceed.2015.12.022
42. Hoshino J. Renal rehabilitation: exercise intervention and nutritional support in dialysis patients. Nutrients. (2021) 13:14444. doi: 10.3390/nu13051444
43. Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. (2014) 64:383–93. doi: 10.1053/j.ajkd.2014.03.020
44. MacKinnon HJ, Wilkinson TJ, Clarke AL, Gould DW, O'Sullivan TF, Xenophontos S, et al. The association of physical function and physical activity with all-cause mortality and adverse clinical outcomes in nondialysis chronic kidney disease: a systematic review. Ther Adv Chronic Dis. (2018) 9:209–26. doi: 10.1177/2040622318785575
45. Yang C-C, Hsu Y-L. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors. (2010) 10:7772–88. doi: 10.3390/s100807772
46. Wilkinson TJ, Clarke AL, Nixon DGD, Hull KL, Song Y, Burton JO, et al. Prevalence and correlates of physical activity across kidney disease stages: an observational multicentre study. Nephrol Dial Transplant. (2021) 36:641–9. doi: 10.1093/ndt/gfz235
47. Jayedi A, Gohari A, Shab-Bidar S. Daily step count and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. Sports Med. (2021) 52:89–99. doi: 10.1007/s40279-021-01536-4
48. Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. (2007) 116:1081–93. doi: 10.1161/CIRCULATIONAHA.107.185649
49. Global Recommendations on Physical Activity for Health. (2010). Available online at: https://www.who.int/publications/i/item/9789241599979 (accessed January 26, 2021).
50. Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. (2011) 8:80. doi: 10.1186/1479-5868-8-80
51. Yu D, Chen Y, Chen T, Cai Y, Qin R, Jiang Z, et al. Walking, but not other physical activity at a higher intensity, is associated with improved kidney function: a cross-sectional health survey of general adult population. J Phys Act Health. (2018) 15:600–4. doi: 10.1123/jpah.2017-0412
52. Viana JL, Kosmadakis GC, Watson EL, Bevington A, Feehally J, Bishop NC, et al. Evidence for anti-inflammatory effects of exercise in CKD. J Am Soc Nephrol. (2014) 25:2121–30. doi: 10.1681/ASN.2013070702
53. Takahashi A, Hu SL, Bostom A. Physical activity in kidney transplant recipients: a review. Am J Kidney Dis. (2018) 72:433–43. doi: 10.1053/j.ajkd.2017.12.005
54. Delgado C, Johansen KL. Barriers to exercise participation among dialysis patients. Nephrol Dial Transplant. (2012) 27:1152–7. doi: 10.1093/ndt/gfr404
55. McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. (2013) 61:896–901. doi: 10.1111/jgs.12266
56. Zelle DM, Klaassen G, van Adrichem E, Bakker SJL, Corpeleijn E, Navis G. Physical inactivity: a risk factor and target for intervention in renal care. Nat Rev Nephrol. (2017) 13:152–68. doi: 10.1038/nrneph.2016.187
57. Portilla Franco ME, Tornero Molina F, Gil Gregorio P. Frailty in elderly people with chronic kidney disease. Nefrologia. (2016) 36:609–15. doi: 10.1016/j.nefroe.2016.12.005
58. Lorenz EC, Kennedy CC, Rule AD, LeBrasseur NK, Kirkland JL, Hickson LJ. Frailty in CKD and transplantation. Kidney Int Rep. (2021) 6:2270–80. doi: 10.1016/j.ekir.2021.05.025
59. Carney EF. Chronic kidney disease. Walking reduces inflammation in predialysis CKD. Nat Rev Nephrol. (2014) 10:300. doi: 10.1038/nrneph.2014.75
60. Aggio D, Papachristou E, Papacosta O, et al. Twenty-year trajectories of physical activity types from midlife to old age. Med Sci Sports Exerc. (2019) 51:481–9. doi: 10.1249/MSS.0000000000001802
61. Sheshadri A, Kittiskulnam P, Lazar AA, Johansen KL. A walking intervention to increase weekly steps in dialysis patients: a pilot randomized controlled trial. Am J Kidney Dis. (2020) 75:488–96. doi: 10.1053/j.ajkd.2019.07.026
62. Assawasaksakul N, Sirichana W, Joosri W, Kulaputana O, Eksakulkla S, Ketanun C, et al. Effects of intradialytic cycling exercise on daily physical activity, physical fitness, body composition, and clinical parameters in high-volume online hemodiafiltration patients: a pilot randomized-controlled trial. Int Urol Nephrol. (2021) 53:359–71. doi: 10.1007/s11255-020-02677-7
63. Martins do Valle F, Valle Pinheiro B, Almeida Barros AA, Ferreira Mendonça W, de Oliveira AC, de Oliveira Werneck G, et al. Effects of intradialytic resistance training on physical activity in daily life, muscle strength, physical capacity and quality of life in hemodialysis patients: a randomized clinical trial. Disabil Rehabil. (2020) 42:3638–44. doi: 10.1080/09638288.2019.1606857
64. Pecoits-Filho R, Larkin J, Poli-de-Figueiredo CE, Cuvello-Neto AL, Barra ABL, Gonçalves PB, et al. Effect of hemodiafiltration on measured physical activity: primary results of the HDFIT randomized controlled trial. Nephrol Dial Transplant. (2021) 36:1057–70. doi: 10.1093/ndt/gfaa173
65. O'Brien T, Russell CL, Tan A, Mion L, Rose K, Focht B, et al. A pilot randomized controlled trial using system CHANGE™ approach to increase physical activity in older kidney transplant recipients. Prog Transplant. (2020) 30:306–14. doi: 10.1177/1526924820958148
66. Lyden K, Boucher R, Wei G, Zhou N, Christensen J, Chertow GM, et al. Targeting sedentary behavior in CKD: a pilot and feasibility randomized controlled trial. Clin J Am Soc Nephrol. (2021) 16:717–26. doi: 10.2215/CJN.12300720
67. Fellmeth G, Rose-Clarke K, Zhao C, Busert LK, Zheng Y, Massazza A, et al. Health impacts of parental migration on left-behind children and adolescents: a systematic review and meta-analysis. Lancet. (2018) 392:2567–82. doi: 10.1016/S0140-6736(18)32558-3
Keywords: chronic kidney disease, daily step counts, physical activity, systematic review, meta-analysis
Citation: Zhang F, Ren Y, Wang H, Bai Y and Huang L (2022) Daily Step Counts in Patients With Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Observational Studies. Front. Med. 9:842423. doi: 10.3389/fmed.2022.842423
Received: 23 December 2021; Accepted: 26 January 2022;
Published: 17 February 2022.
Edited by:
Yoshiyuki Morishita, Jichi Medical University Saitama Medical Center, JapanReviewed by:
Wisit Kaewput, Phramongkutklao Hospital, ThailandCopyright © 2022 Zhang, Ren, Wang, Bai and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liuyan Huang, aGx5MTk4MEAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.