
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 25 April 2022
Sec. Nephrology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.841876
 Giuseppe Tarantini1*
Giuseppe Tarantini1* Anand Prasad2
Anand Prasad2 Sudhir Rathore3
Sudhir Rathore3 Shweta Bansal4
Shweta Bansal4 Regine Gottfried5
Regine Gottfried5 Alexander R. Rosenkranz6
Alexander R. Rosenkranz6 Carlo Briguori7
Carlo Briguori7 Mohsen Yaghoubi8
Mohsen Yaghoubi8 Atefeh Mashayekhi9
Atefeh Mashayekhi9 Mehdi Javanbakht9,10
Mehdi Javanbakht9,10 Eoin Moloney9
Eoin Moloney9
Background: Contrast-associated acute kidney injury (CA-AKI) is an important adverse effect associated with injecting iodinated intra-arterial contrast media (CM) during coronary angiography. The DyeVert™ Contrast Reduction System is a medical device intended to reduce the intra-arterial CM volume (CMV) administered. The aim of this study was to assess DyeVert System clinical effectiveness and safety by implementing a systematic review and meta-analysis of existing evidence.
Methods: Systematic electronic literature searches were conducted in MEDLINE, Embase, the Cochrane Database of Systematic Reviews, ClinicalTrials.gov, and the International Clinical Trials Registry Platform database. Relevant data were extracted from included studies and meta-analyses were performed to synthesize evidence across studies.
Results: The review included 17 eligible studies involving 1,731 DyeVert System cases and 1,387 control cases (without the use of DyeVert). Meta-analyses demonstrated use of the DyeVert System reduced CMV delivered to the patient by 39.27% (95% CI, 36.10–42.48%, P < 0.001), reduced CMV/baseline renal function ratios (Hedges’s g, −0.56; 95% CI, −0.70 to −0.42, P < 0.001) and percentage of cases exceeding the maximum CMV threshold (risk difference −0.31, 95% CI, −0.48 to −0.13, P < 0.001) while maintaining adequate image quality in 98% of cases. DyeVert System cases demonstrated lower CA-AKI incidence vs. controls (absolute risk reduction 5.00% (95% CI, 0.40–9.80%; P = 0.03), relative risk 0.60 (95% CI, 0.40–0.90; P = 0.01) with a pooled estimate of the number needed to treat with the DyeVert System to avoid 1 CA-AKI event of 20.
Conclusion: DyeVert System use significantly reduces CMV delivered to the patient, CMV/baseline renal function ratios, and CA-AKI incidence while maintaining image quality. Accordingly, the device may serve as an adjunctive, procedure-based strategy to prevent CA-AKI. Future multi-center studies are needed to further assess effects of minimizing CMV on endpoints such as CA-AKI prevention, incidence of adverse cardiac and renal events, and health care costs.
Intra-arterial injection of contrast media (CM) is used to enhance image quality, allowing radiologists and clinicians to better distinguish between various body tissues when performing techniques such as coronary angiography and percutaneous coronary intervention (PCI). While beneficial for clinical interpretation and diagnosis, use of CM may result in adverse effects (1). One of the most important adverse effects associated with CM is contrast-associated acute kidney injury (CA-AKI) with incidence rates between 3 and 37% (2–6).
Currently, there is no treatment for CA-AKI. Therefore, clinical attention to CA-AKI focuses on prevention, especially for high-risk patients (7). Patients with comorbidities such as advanced age, co-existing chronic kidney disease (CKD), heart failure, anemia, and/or diabetes are at increased risk for CA-AKI, as are patients undergoing procedures with acute clinical presentation or high complexity as shown by previous CA-AKI risk prediction models (8). Higher contrast media volume (CMV) and, particularly, high CMV relative to individual patient baseline renal function are significantly associated with increased CA-AKI incidence (9–12). Clinical practice guidelines and recommendations for CA-AKI prevention have emphasized procedure-based strategies, including pre-procedural patient risk assessment, discontinuing potentially nephrotoxic medications prior to contrast administration, adequate volume expansion, and monitoring and minimizing CMV administered, particularly in patients with CKD (13–17). Yet despite a decade of clinical practice, these recommendations remain incompletely and inconsistently implemented (18, 19).
The FDA-cleared and CE-marked DyeVert™ Contrast Reduction System (Osprey Medical, Minnetonka, Minnesota, United States) is intended to reduce the CMV administered during diagnostic and interventional angiographic procedures while maintaining image quality. The DyeVert, DyeVert Plus, and DyeVert Plus EZ Contrast Reduction Systems are compatible with manual contrast injection, while the DyeVert Power XT Contrast Reduction System is compatible with automated contrast injection. By enabling accurate, real-time monitoring of CMV administered relative to a pre-determined maximum CMV threshold entered into the display, the DyeVert System provides clinicians in the catheterization laboratory with continuous data on CM use to facilitate intraprocedural decision-making. Additionally, the device diverts excess CM not required to accomplish procedural objectives, resulting in reduced contrast load to the patient.
We aimed to assess the effect of the DyeVert System in diagnostic coronary angiography and/or PCI procedures by pooling all relevant studies in a systematic review and performing meta-analyses to quantitatively synthesize evidence on outcomes.
We performed a systematic literature review to identify studies reporting the clinical effectiveness and/or safety of the DyeVert System. The literature review was conducted according to methodological guidance from the Center for Reviews and Dissemination on best practices for conducting systematic reviews in health care (20). We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) approach to report findings of the review and meta-analyses (21). The authors had full access to all data included in the study.
Systematic electronic searches were conducted July 2021 in MEDLINE, Embase, the Cochrane Database of Systematic Reviews, the ClinicalTrials.gov database, and the International Clinical Trials Registry Platform database. Additional Internet searches were performed to identify any further publications of interest. Supplementary Material provide search strategy details (Supplementary Tables 1–5). Records meeting the search criteria were downloaded from databases and imported into Microsoft® Excel® software, where duplicate records were removed.
Studies selected for inclusion in the review met these selection criteria: (1) patients were adults undergoing diagnostic coronary angiography, PCI, and/or a peripheral intervention procedure using intra-arterial injection of CM and (2) DyeVert, DyeVert Plus, DyeVert Plus EZ, or DyeVert Power XT systems were used as an intervention. Studies presented in a language other than English and studies or publications representing economic analyses, editorials, reviews, book chapters, or letters were excluded from this review and meta-analysis. Two reviewers systematically screened titles and abstracts. A third reviewer resolved any disagreements regarding potential exclusions. Remaining studies underwent full-text screening for eligibility. References of eligible studies were searched manually to identify additional relevant studies, although no further studies were found. The study selection process is depicted in a PRISMA diagram in Figure 1 (21).
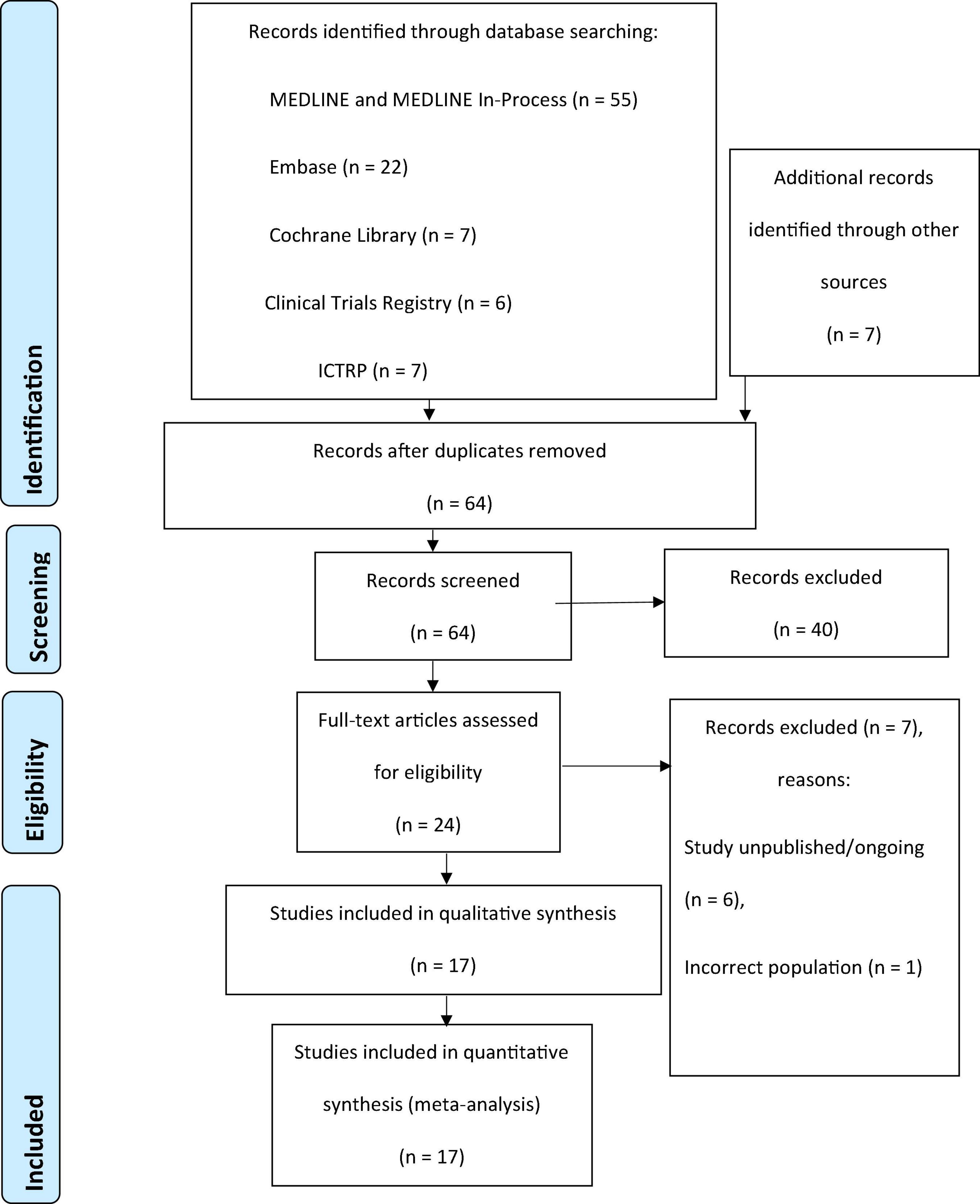
Figure 1. PRISMA flow diagram for the systematic review. ICTRP, International Clinical Trials Registry Platform; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
All eligible studies were reviewed, and relevant data were extracted by 1 reviewer; another independent reviewer performed quality control. Disagreements between the reviewers regarding extracted data were resolved by discussion, and a third reviewer participated as needed to reach consensus. Extracted data included study design, location, and setting; type of intervention and comparators; characteristics of the patient population (including details related to patient follow-up and withdrawal); main outcomes; and results reported (including clinical effectiveness and safety of the intervention).
Outcomes of interest included CMV delivered to the patient, CMV diverted with the DyeVert System, CMV threshold management, CMV/baseline renal function ratios, image quality, CA-AKI incidence, and DyeVert System-related adverse events.
We performed meta-analyses to quantitatively synthesize the findings related to CMV use, CMV threshold management, CMV/baseline renal function ratios, image quality and CA-AKI incidence. The number of studies included in each meta-analysis varied depending on outcomes reported in each study. Fixed- and random-effects analyses were performed. The Mantel-Haenszel method was used to calculate the fixed-effects estimate, with inverse-variance weighting using the DerSimonian and Laird method used to account for heterogeneity in the random-effects model (22, 23). Cochran’s Q statistic and the I2 statistic were calculated to test for heterogeneity across studies, with an I2 statistic > 50% indicating a moderate to strong (> 75%) presence of heterogeneity (24, 25). Egger’s test and Begg’s test statistics were used to evaluate potential publication bias (26). Forest plots were produced to show pooled estimates. All p-values were 2-tailed with statistical significance set at < 0.05. Computations were performed using StataCorp and MedCalc.
All included studies were quality-assessed using an appropriate critical appraisal tool.
The initial systematic, electronic search identified 97 studies, and Supplementary Internet searches identified 7 additional potentially relevant articles. From these 104 studies, 40 duplicates were removed. The remaining 64 studies were screened for eligibility, of which 40 studies were excluded based on screening of titles and abstracts. Of 24 studies that underwent full-text screening, 7 were deemed ineligible based on exclusion criteria. The remaining 17 studies were included in the qualitative review and quantitative meta-analysis (27, 28). Results of the study selection process are depicted in Figure 1.
Table 1 summarizes the 17 included studies from 8 full text articles and 9 abstracts; all studies were published or presented (in the case of poster abstracts) from 2017 through 2020. Collectively, the studies involved 1,731 DyeVert System cases and 1,387 control cases. All 17 studies referred to use of the DyeVert System as an intervention implemented in combination with CM injection systems used routinely at each site (DyeVert group). Fifteen studies involved the use of manual CM injection systems (Table 1). Two studies reported use of the DyeVert System with an automated CM injection system, of which Bruno et al. was a sub-set of Amoroso et al. data and reported the author’s single-center experience (28, 43). Nine studies included a control group composed of cases using routine CM injection systems and imaging practices without the DyeVert System (control group). Eight studies did not involve a control group. Most publications include real-world data reflecting the modern health care system, current practices for care delivery, and complexity of patient demographics. Kutschman et al. published two reports of a 6-month hospital quality improvement project—one involving the overall population (33) and one focused on a subgroup with CKD (32).
Table 2 summarizes patient baseline characteristics. Patients were predominantly male with advanced age and many studies included patients with co-morbidities such as CKD, hypertension, diabetes, heart failure, peripheral arterial disease, prior myocardial infarction, and prior PCI. Table 3 summarizes procedure characteristics. Cases involving radial as well as femoral access are represented. Most procedures involved PCI (either exclusively or combined with CA). Four studies involved chronic total occlusions in which one or two contrast injection lines may be used; and therefore, one or two DyeVert Systems may be used depending on the technique employed. Two studies involved peripheral vascular interventions. Iso-osmolar and low osmolar contrast agents were used. Supplementary Material contain additional reported baseline and procedure characteristics (Supplementary Table 6) and CA-AKI prevention strategies (Supplementary Table 7).
Table 4 summarizes CMV use and threshold management. Mean CMV “attempted,” defined as the CMV that would have been delivered if the DyeVert System had not been used, calculated by summing the actual CMV delivered to the patient and the CMV diverted (or “saved”) by the DyeVert System, ranged from 112 ml in a study involving 65% diagnostic coronary angiography cases to 342 ml in a PCI study. In cases involving DyeVert System use, the mean CMV diverted ranged from 42 to 126 ml, which equated to a mean 34–47% of the attempted CMV being diverted with the DyeVert System. Overall mean CMV delivered to the patient ranged from 37 to 216 ml in DyeVert group cases and 63–250 ml in control group cases.
Because of the CMV diverted by the DyeVert System, a larger proportion of cases delivered CMV to the patient at or below the maximum CMV dose threshold prespecified by the physician and resulted in lower mean CMV/baseline renal function ratios as well as a larger proportion of cases achieving lower CMV/baseline renal function ratios (Table 4 and Supplementary Table 8, respectively).
Image quality was assessed during DyeVert System use in 9 studies by the physician at the time of the procedure (Supplementary Table 9) and was reported to be adequate in 96–100% of cases. Two of these studies additionally involved the use of independent reviewers to assess image quality of DyeVert group cases compared to control group cases and reported non-inferior image quality (27, 36).
CA-AKI incidence was reported in 11 publications (Supplementary Table 10). CA-AKI definitions were not reported in 2 studies (35, 38); however, 9 studies did report the definition used, which ranged from worsening renal function to serum creatinine (SCr) increase ≥ 0.3 mg/dL or 50% within 48 h of the procedure. In studies involving a control group, observed CA-AKI incidence was lower in DyeVert group cases. Briguori et al. involved the use of propensity matching of DyeVert and control group in acute coronary syndrome patients. Tucker et al. and Cameron et al. reported overall CA-AKI incidence from hospital quality improvement efforts demonstrating a meaningful decrease in CA-AKI between the initial and final follow-up intervals. Kutschman et al. reported an observed 33% relative reduction in CA-AKI in the DyeVert group compared to the control group in the overall population included in a hospital quality improvement program and a 57% relative reduction in the CKD subgroup. Tajti et al. studied DyeVert System use in chronic total occlusion cases and additionally reported a post-procedure CA-AKI incidence though the CA-AKI definition was not reported.
Nine studies reported on adverse events and no DyeVert System-related adverse events were reported (27, 28, 30, 35–39, 42). Corcione et al. described a case of contrast-induced nephropathy in a patient who underwent a combined carotid angiography and angioplasty and experienced elevated serum creatinine levels that returned to baseline 3 days after the procedure; the authors noted the event was not device-related (38). Briguori et al. and Tajti et al. further reported frequencies of reported adverse events were similar in the DyeVert and control Groups, with no adverse events identified as being related to use of the DyeVert System (30, 35).
The pooled estimate of the standardized mean difference in absolute CMV (mL) delivered to the patient between the DyeVert group and control group in 2 randomized controlled trials was -2.27 (95% CI, −2.62 to 1.92; P < 0.001) (Figure 2A and Supplementary Table 11). In this meta-analysis, there was no evidence of heterogeneity (I2 = 0%) and Egger’s test was significant for publication bias (P < 0.0001). In 3 retrospective, observational, 2-arm studies, the pooled estimate of the standardized mean difference in absolute CMV (mL) between the DyeVert group and control group was −0.53 (95% CI, −0.81 to −0.25; P < 0.0.001) (Figure 2B and Supplementary Table 12). Two studies that reported mean differences without standard deviations were excluded from this meta-analysis (31, 34). This analysis showed moderate evidence of heterogeneity (I2 = 67%), and Egger’ test was significant for publication bias (P = 0.049). These results indicate DyeVert System use resulted in a significant decrease in CMV relative to the control group.
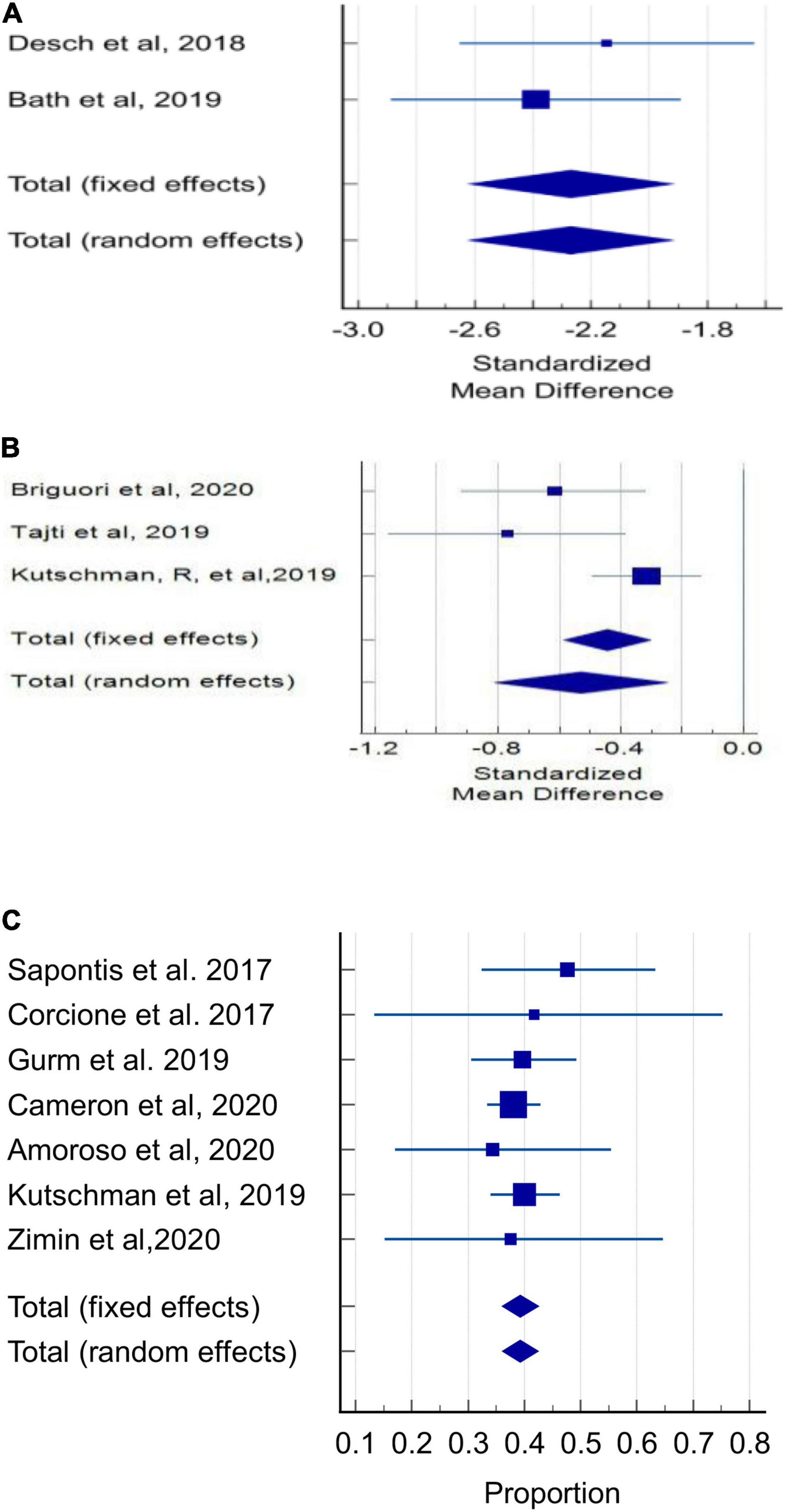
Figure 2. Contrast media volume use. (A) Forest plot of mean difference in absolute contrast volume (mL) in randomized controlled trials. (B) Forest plot of mean difference in absolute contrast volume (mL) in observational studies. (C) Forest plot of proportion of attempted CMV that was diverted (%) in observational studies.
Among 7 observational studies, in the DyeVert group, the pooled estimate of the percentage of the total attempted CMV diverted by the DyeVert System was 39.27% (95% CI, 36.10–42.48%; P < 0.01, Figure 2C and Supplementary Table 13A) for all studies and 39.47% (95% CI, 36.24–42.73%; P < 0.01) for studies using manual CM injection systems (Supplementary Figure 1 and Supplementary Table 13B). In both analyses, there was no evidence of heterogeneity (I2 = 0%) and Egger’s test was not significant for publication bias (P = 0.48 and P = 0.24, respectively).
In DyeVert group cases, at the beginning of each case, the physician enters the predefined maximum CMV threshold into the display. This analysis compared the predetermined CMV threshold with the CMV attempted and CMV delivered to the patient across 5 studies (Figure 3). Results indicated use of the DyeVert System resulted in significantly reduced risk of a patient receiving a CMV that exceeded their maximum CMV threshold (risk difference, −0.31; 95% CI, −0.48 to −0.13, P < 0.001).
In DyeVert group cases, 6 studies explored the actual CMV/estimated glomerular filtration rate (eGFR) ratio vs. attempted CMV/eGFR ratio (Figures 4A–D). Results indicated use of the DyeVert System resulted in significantly reduced actual CMV/eGFR compared with the attempted CMV/eGFR (Hedges’s g, −0.56; 95% CI, −0.70 to −0.42, P < 0.001) (Figure 4A). Five studies reported additional outcomes by 3 different CMV/eGFR ratios. Results indicate DyeVert System use significantly reduced the risk of receiving a CMV exceeding each of the 3 CMV/eGFR ratio subgroups (Figures 4B–D). All 3 analyses showed strong evidence of heterogeneity (I2 ≥ 88%).
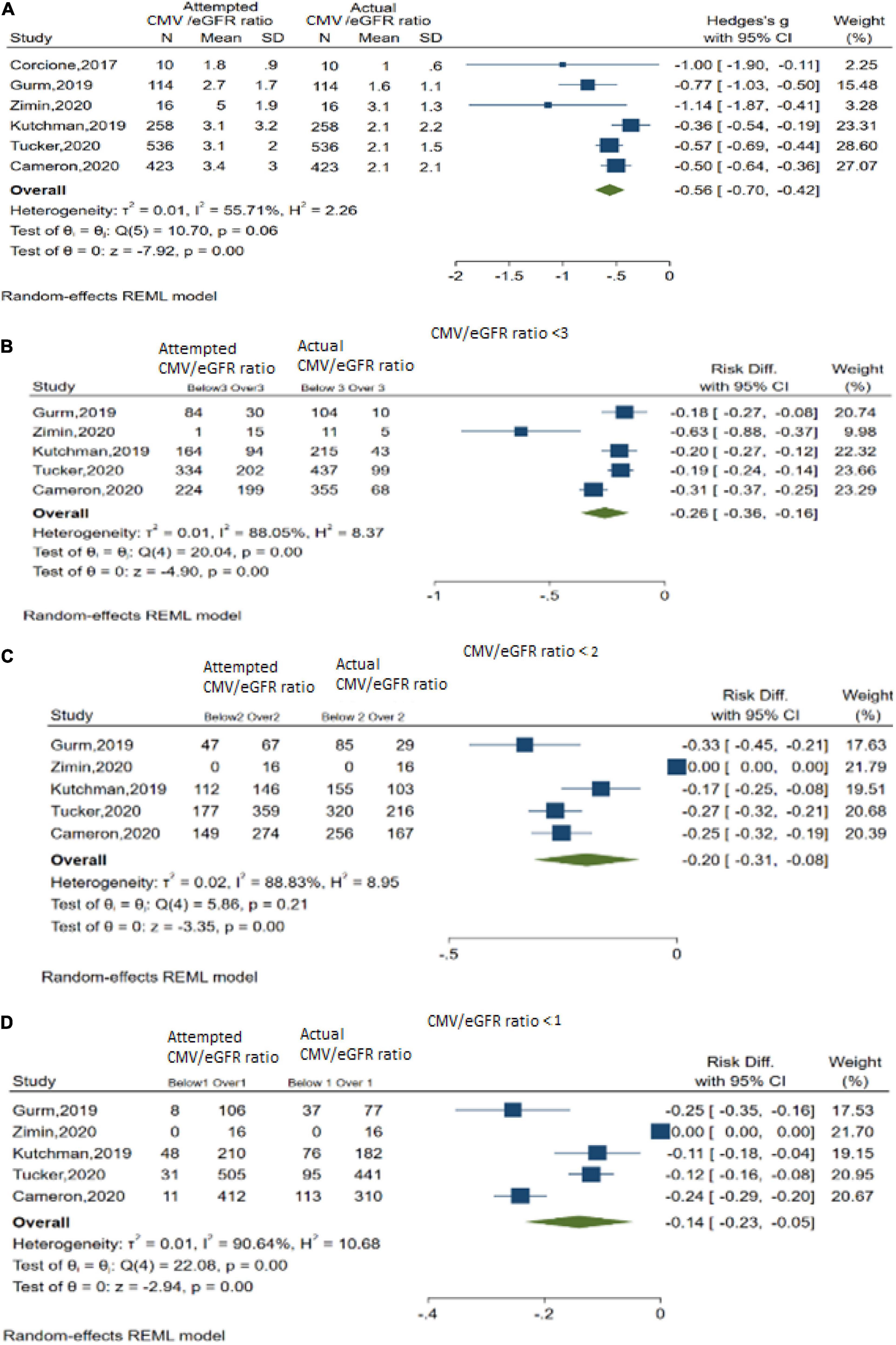
Figure 4. Contrast media volume/baseline renal function ratios. (A) Overall meta-analysis across 6 studies. (B) Meta-analysis for CMV/eGFR ratio < 3. (C) Meta-analysis for CMV/eGFR ratio < 2. (D) Meta-analysis for CMV/eGFR ratio < 1.
In DyeVert group cases, 7 studies reported image quality based on clinician feedback during the case (Figure 5 and Supplementary Table 14). The pooled estimate of percent cases in which clinicians reported adequate fluoroscopic image quality during DyeVert System use was 98.21% (95% CI, 96.54–99.34%). This analysis demonstrated low evidence of heterogeneity (I2 = 1.74%), and Egger’s test was not significant for publication bias (P = 0.27). Results indicate the overall image quality was adequate in a majority of cases.
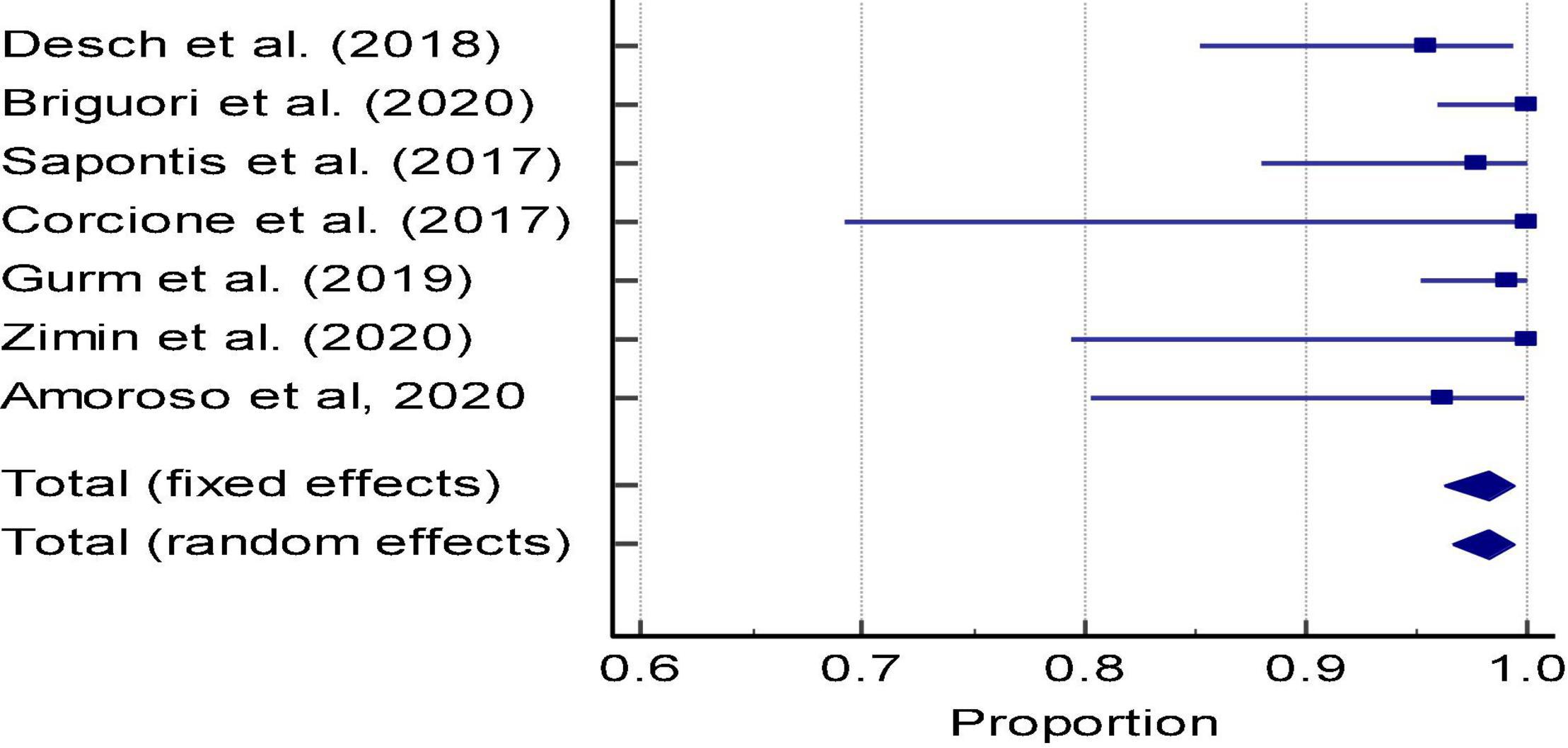
Figure 5. Meta-analysis: Forest plot of image quality in the DyeVert group (Proportion of cases with adequate image quality per physician assessment).
Five two-arm studies assessed CA-AKI incidence (Table 5). Pooled incidence CA-AKI in the DyeVert group was 7.30% (95% CI, 5.11–9.85%). There was no evidence of heterogeneity in this analysis (I2 = 0%), and Egger’s test was not significant for publication bias (P = 0.93; Supplementary Table 15A). Pooled incidence of CA-AKI in the control group was 10.65% (95% CI, 6.60–15.52%). The control group analysis showed strong evidence of heterogeneity based on the Q statistic (P < 0.001) and I2 = 78.54%; Egger’s test was non-significant for publication bias (P = 0.71; Supplementary Table 16A).
Pooled estimate of the absolute risk reduction for CA-AKI associated DyeVert System use was 5.00% (95% CI, 0.40–9.80%; P = 0.03) and the pooled relative risk of CA-AKI was 0.60 (95% CI, 0.40–0.90; P = 0.01) (Supplementary Table 17A). There was no evidence of heterogeneity in the analysis of absolute risk reduction (I2 = 0%; Supplementary Table 17A). The pooled estimate of the number of patients needed to be treated to avoid 1 CA-AKI event was 20 (Supplementary Table 17A).
Tajti et al. was different from the other four studies in that it was performed exclusively in chronic total occlusion cases and did not report the CA-AKI definition used. The meta-analysis also was repeated with this study excluded and overall results were similar (Supplementary Tables 15B, 16B, 17B).
See Supplementary Material for additional tables and figures.
Results of the quality assessment of included studies are presented in Supplementary Tables 18–34.
This is the first systematic review and meta-analysis of the DyeVert System. This review included data from 17 recent studies encompassing 1,731 DyeVert System cases and 1,387 control cases. Meta-analyses demonstrated that DyeVert System use: (a) reduced CMV delivered to the patient and CMV/baseline renal function ratios; (b) reduced the percentage of cases exceeding the maximum CMV threshold; and (c) maintained adequate image quality.
Additionally, DyeVert System use reduced CA-AKI incidence, resulting in a number needed to treat to avoid 1 CA-AKI event of 20. CA-AKI is associated with increased morbidity, mortality, and length of stay (6). Briguori et al. was the first report of a procedure-based CA-AKI prevention strategy resulting in significant CA-AKI reduction as well as a significantly shorter length of stay of about 2 days (30). Given the high cost of CA-AKI events (6), the overall economic value of the DyeVert System may be derived from CA-AKI avoidance based on recent hospital budget impact evaluations (32, 40, 41) and a modeling study (44).
Various intra-procedural contrast-sparing strategies have been suggested including limiting CMV per injection to 2 mL, use of optical coherence tomography, roadmap dynamic software, and biplane angiography (7). Additionally, use of automated CM injection systems may result in slightly less CMV over manual CM injection systems though the authors concluded it was unlikely to impact contrast-induced renal complications (45). Publications reviewed in this study demonstrate DyeVert System use as an additive strategy for reducing CMV and diversion of excess CMV is still significant even when other modalities are deployed.
Primary limitations of our review and meta-analysis include heterogenous definitions of CA-AKI, lack of reporting outcomes on potential subgroups of interest preclude further assessment of a potential treatment effect, small sample sizes of some studies, lack of long-term follow-up, clinical event committees not used, and lack of randomization in some studies. High I2 values (>75%), indicative of strong heterogeneity, were also seen in the meta-analyses of CMV threshold management in the intervention group, CMV/eGFR ratio, and in the analysis of CA-AKI incidence in the control group. However, given the small number of studies identified in the review and included in these primary analyses, further sub-group analyses of studies were not considered appropriate. Also, the scope of CA-AKI prevention strategies used in each case was not well reported in all studies and are often not specifically cited in the medical record, which precluded the ability to adjust for these potential variables. Additionally, we did not have access to patient-level data; and therefore, cannot confirm whether patients received optimal medical therapy for CA-AKI prevention. Despite these shortcomings, this exhaustive systematic review encompassing all relevant scientific databases identified numerous studies that demonstrated the clinical effectiveness of interventional use of the DyeVert System. Additionally, we used appropriate meta-analysis methods to synthesize outcomes of the included studies to thoroughly assess the efficacy and safety of the intervention.
In conclusion, DyeVert System use significantly reduces CMV delivered to the patient, CMV/baseline renal function ratios, and CA-AKI incidence while maintaining image quality. Accordingly, the device may serve as an adjunctive, procedure-based strategy to prevent CA-AKI. Future multi-center studies are needed to further assess effects of minimizing CMV on endpoints such as CA-AKI prevention, incidence of adverse cardiac and renal events, and health care costs.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
AM, MJ, MY, and EM were responsible for performing the systematic review and meta-analysis. All other authors provided clinical input to the manuscript. All authors were responsible for developing the final, submitted version of the manuscript.
This report describes independent research funded by the Osprey Medical Corporation.
EM, AM, and MJ were employees of Optimax Access United Kingdom Ltd., and MY was employee of Optimax Access United Kingdom Ltd. and is now an Assistant Professor at Mercer University. AP, CB, and SB were paid consultants for Osprey Medical Corporation. AR and AP received honoraria from GE Healthcare as a consultant. RG has previously received speaker fees from Shockwave Medical Inc. Osprey Medical provided funding for the professional services of Optimax/Device Access, Wendy Mills Writing LLC, and Vita Medical LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Wendy Mills of Wendy Mills Medical Writing LLC and Kimberly Knish of VitaMedical LLC provided medical writing and editing support.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.841876/full#supplementary-material
CA, coronary angiography; CA-AKI, contrast-associated acute kidney injury; CM, contrast media; CMV, contrast media volume; eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary intervention; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; RD, risk difference.
1. Pomara C, Pascale N, Maglietta F, Neri M, Riezzo I, Turillazzi E. Use of contrast media in diagnostic imaging: medico-legal considerations. Radiol Med. (2015) 120:802–9. doi: 10.1007/s11547-015-0549-6
2. Faucon AL, Bobrie G, Clément O. Nephrotoxicity of iodinated contrast media: from pathophysiology to prevention strategies. Eur J Radiol. (2019) 116:231–41. doi: 10.1016/j.ejrad.2019.03.008
3. Lun Z, Liu L, Chen G, Ying M, Liu J, Wang B, et al. The global incidence and mortality of contrast-associated acute kidney injury following coronary angiography: a meta-analysis of 1.2 million patients. J Nephrol. (2021) 34:1479–89. doi: 10.1007/s40620-021-01021-1
4. Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR cath-PCI registry. JACC Cardiovasc Interv. (2014) 7:1–9. doi: 10.1016/j.jcin.2013.06.016
5. Brown JR, Rezaee ME, Nichols EL, Marshall EJ, Siew ED, Matheny ME. Incidence and in-hospital mortality of acute kidney injury (AKI) and dialysis-requiring AKI (AKI-D) after cardiac catheterization in the national inpatient sample. J Am Heart Assoc. (2016) 5:e002739. doi: 10.1161/JAHA.115.002739
6. Prasad A, Rosenthal NA, Kartashov A, Knish K, Dreyfus J. Contemporary trend of acute kidney injury incidence and incremental costs among US patients undergoing percutaneous coronary procedures. Catheter Cardiovasc Interv. (2020) 96:1184–97. doi: 10.1002/ccd.28824
7. Almendarez M, Gurm HS, Mariani J Jr, Montorfano M, Brilakis ES, Mehran R, et al. Procedural strategies to reduce the incidence of contrast-induced acute kidney injury during percutaneous coronary intervention. JACC Cardiovasc Interv. (2019) 12:1877–88. doi: 10.1016/j.jcin.2019.04.055
8. Allen DW, Ma B, Leung KC, Graham MM, Pannu N, Traboulsi M, et al. Risk prediction models for contrast-induced acute kidney injury accompanying cardiac catheterization: systematic review and meta-analysis. Can J Cardiol. (2017) 33:724–36. doi: 10.1016/j.cjca.2017.01.018
9. Amin AP, Bach RG, Caruso ML, Kennedy KF, Spertus JA. Association of variation in contrast volume with acute kidney injury in patients undergoing percutaneous coronary intervention. JAMA Cardiol. (2017) 2:1007–12. doi: 10.1001/jamacardio.2017.2156
10. Gurm HS, Dixon SR, Smith DE, Share D, Lalonde T, Greenbaum A, et al. Renal function-based contrast dosing to define safe limits of radiographic contrast media in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. (2011) 58:907–14. doi: 10.1016/j.jacc.2011.05.023
11. Gurm HS, Seth M, Mehran R, Cannon L, Grines CL, LaLonde T, et al. Impact of contrast dose reduction on incidence of acute kidney injury (AKI) among patients undergoing PCI: a modeling study. J Invasive Cardiol. (2016) 28:142–6.
12. Gurm HS, Seth M, Dixon SR, Michael Grossman P, Sukul D, Lalonde T, et al. Contemporary use of and outcomes associated with ultra-low contrast volume in patients undergoing percutaneous coronary interventions. Catheter Cardiovasc Interv. (2019) 93:222–30. doi: 10.1002/ccd.27819
13. Klein LW, Sheldon MW, Brinker J, Mixon TA, Skelding K, Strunk AO, et al. The use of radiographic contrast media during PCI: a focused review: a position statement of the society of cardiovascular angiography and interventions. Catheter Cardiovasc Interv. (2009) 74:728–46. doi: 10.1002/ccd.22113
14. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American college of cardiology foundation/American heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2011;124:e574-e651. Corrected Circ. (2012) 125:e412. doi: 10.1161/CIR.0b013e31823ba622
15. Naidu SS, Abbott JD, Bagai J, Blankenship J, Garcia S, Iqbal SN, et al. SCAI expert consensus update on best practices in the cardiac catheterization laboratory: this statement was endorsed by the American college of cardiology (ACC), the American heart association (AHA), and the heart rhythm society (HRS) in april 2021. Catheter Cardiovasc Interv. (2021) 98:255–76. doi: 10.1002/ccd.29744
16. Bashore TM, Balter S, Barac A, Byrne JG, Cavendish JJ, Chambers CE, et al. 2012 American college of cardiology foundation/society for cardiovascular angiography and interventions expert consensus document on cardiac catheterization laboratory standards update: a report of the American college of cardiology foundation task force on expert consensus documents developed in collaboration with the society of thoracic surgeons and society for vascular medicine. J Am Coll Cardiol. (2012) 59:2221–305. doi: 10.1016/j.jacc.2012.02.010
17. Nallamothu BK, Tommaso CL, Anderson HV, Anderson JL, Cleveland JC Jr, Dudley RA, et al. ACC/AHA/SCAI/AMA-convened PCPI/NCQA 2013 performance measures for adults undergoing percutaneous coronary intervention: a report of the American college of cardiology/American heart association task force on performance measures, the society for cardiovascular angiography and interventions, the American medical association-convened physician consortium for performance improvement, and the national committee for quality assurance. Circulation. (2014) 129:926–49. doi: 10.1161/01.cir.0000441966.31451.3f
18. Prasad A, Sohn A, Morales J, Williams K, Bailey SR, Levin D, et al. Contemporary practice patterns related to the risk of acute kidney injury in the catheterization laboratory: results from a survey of society of cardiovascular angiography and intervention (SCAI) cardiologists. Catheter Cardiovasc Interv. (2017) 89:383–92. doi: 10.1002/ccd.26628
19. Valdenor C, McCullough PA, Paculdo D, Acelajado MC, Dahlen JR, Noiri E, et al. Measuring the variation in the prevention and treatment of CI-AKI among interventional cardiologists. Curr Probl Cardiol. (2021) 46:100851. doi: 10.1016/j.cpcardiol.2021.100851
20. Centre for Reviews and Dissemination.Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York (2009).
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10:89. doi: 10.1186/s13643-021-01626-4
22. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. (1959) 22: 719–48.
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
24. Fleiss JL. Analysis of data from multiclinic trials. Control Clin Trials. (1986) 7:267–75. doi: 10.1016/0197-2456(86)90034-6
25. Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the effective health care program. J Clin Epidemiol. (2011) 64:1187–97. doi: 10.1016/j.jclinepi.2010.08.010
26. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. (2018) 74:785–94. doi: 10.1111/biom.12817
27. Desch S, Fuernau G, Pöss J, Meyer-Saraei R, Saad M, Eitel I, et al. Impact of a novel contrast reduction system on contrast savings in coronary angiography – the DyeVert randomised controlled trial. Int J Cardiol. (2018) 257:50–3. doi: 10.1016/j.ijcard.2017.12.107
28. Bruno RR, Nia AM, Wolff G, Erkens R, Kelm M, Westenfeld R, et al. Early clinical experiences with a novel contrast volume reduction system during invasive coronary angiography. Int J Cardiol Heart Vasc. (2019) 23:100377. doi: 10.1016/j.ijcha.2019.100377
29. Bath A, Bobba K, Gautam S, Gupta V. Use of DyeVert plus to reduce contrast exposure in high-risk patients undergoing coronary angiography. J Am Coll Cardiol. (2019) 73(Suppl. 1):1193. doi: 10.1016/S0735-1097(19)31800-5
30. Briguori C, Golino M, Porchetta N, Scarpelli M, De Micco F, Rubino C, et al. Impact of a contrast media volume control device on acute kidney injury rate in patients with acute coronary syndrome. Catheter Cardiovasc Interv. (2021) 98:76–84. doi: 10.1002/ccd.29136
31. Sattar A, Schnatz R, Darby M, El-Hamdani M. Impact of using DyeVert plus on incidence of acute kidney injury after cardiac catheterization with coronary interventions in high-risk patients. In: Poster Presented at the American College of Cardiology Annual Meeting, Poster 88–89. Charleston, WV (2018).
32. Kutschman R. Clinical and economic outcomes of a comprehensive clinical quality initiative for reducing acute kidney injury in chronic kidney disease patients undergoing coronary angiography. CA J Am Coll Cardiol. (2019) 74(Suppl.):B605.
33. Kutschman R, Davison L, Beyer J. Comprehensive clinical quality initiative for reducing acute kidney injury in at-risk patients undergoing diagnostic coronary angiogram and/or percutaneous coronary interventions. In: Poster Presented at the Society for Cardiac Angiography & Interventions 2019 Scientific Sessions. Las Vegas, NV (2019).
34. Bunney R, Saenger E, Shah C, Harris S, Hill-Herrera K, Leopold D, et al. Contemporary use of contrast dye reduction technology in a tertiary academic hospital: patient characteristics and acute kidney injury outcomes following percutaneous coronary interventions. In: Poster Presented at the American College of Cardiology (ACC) Quality Summit, Poster 2018-063. New Orleans, LA (2019).
35. Tajti P, Xenogiannis I, Hall A, Burke MN, Chavez I, Garcia S, et al. Use of the DyeVert system in chronic total occlusion percutaneous coronary intervention. J Invasive Cardiol. (2019) 31:253–9.
36. Zimin VN, Jones MR, Richmond IT, Durieux JC, Alaiti AM, Pereira G, et al. A feasibility study of the DyeVert™ plus contrast reduction system to reduce contrast media volumes in percutaneous coronary procedures using optical coherence tomography. Cardiovasc Revasc Med. (2021) 30:40–6. doi: 10.1016/j.carrev.2020.09.040
37. Sapontis J, Barron G, Seneviratne S, Fuernau G, Eitel I, Ledwoch J, et al. A first in human evaluation of a novel contrast media saving device. Catheter Cardiovasc Interv. (2017) 90:928–34. doi: 10.1002/ccd.27033
38. Corcione N, Biondi-Zoccai G, Ferraro P, Messina S, Maresca G, Avellino R, et al. Contrast minimization with the new-generation DyeVert plus system for contrast reduction and real-time monitoring during coronary and peripheral procedures: first experience. J Invasive Cardiol. (2017) 29:259–62.
39. Gurm HS, Mavromatis K, Bertolet B, Kereiakes DJ, Amin AP, Shah AP, et al. Minimizing radiographic contrast administration during coronary angiography using a novel contrast reduction system: a multicenter observational study of the DyeVert™ plus contrast reduction system. Catheter Cardiovasc Interv. (2019) 93:1228–35. doi: 10.1002/ccd.27935
40. Turner C, Tucker PA. Real-world impact of a quality improvement program for AKI prevention in the cardiac cath lab. Catheter Cardiovasc Interv. (2020) 95(Suppl. 2):S112–3.
41. Cameron A, Espinosa TJ. Reducing contrast-induced acute kidney injury in a cardiac catherization laboratory: a quality improvement initiative. Catheter Cardiovasc Interv. (2020) 95(Suppl. 2):1–34. doi: 10.1002/ccd.28864
42. Rao S. DyeVert plus contrast reduction system use in patients undergoing highly complex peripheral vascular interventions. J Vasc Interv Radiol. (2019) 30:e16. doi: 10.1016/j.jvir.2018.11.033
43. Amoroso G, Christian J, Christopher A. First European experience using a novel contrast reduction system during coronary angiography with automated contrast injection. Eurointervention. (2020) 16(Suppl. AC):Euro20A-POS426.
44. Javanbakht M, Hemami MR, Mashayekhi A, Branagan-Harris M, Zaman A, Al-Najjar Y, et al. DyeVert™ plus EZ system for preventing contrast-induced acute kidney injury in patients undergoing diagnostic coronary angiography and/or percutaneous coronary intervention: a UK-based cost-utility analysis. Pharmacoecon Open. (2020) 4:459–72. doi: 10.1007/s41669-020-00195-x
45. Gurm HS, Smith D, Share D, Wohns D, Collins J, Madala M, et al. Impact of automated contrast injector systems on contrast use and contrast-associated complications in patients undergoing percutaneous coronary interventions. JACC Cardiovasc Interv. (2013) 6:399–405. doi: 10.1016/j.jcin.2012.11.008
Keywords: DyeVert System, acute kidney injury, contrast media, contrast induced nephropathy, coronary angiography, percutaneous coronary intervention, systematic review, meta-analysis
Citation: Tarantini G, Prasad A, Rathore S, Bansal S, Gottfried R, Rosenkranz AR, Briguori C, Yaghoubi M, Mashayekhi A, Javanbakht M and Moloney E (2022) DyeVert Contrast Reduction System Use in Patients Undergoing Coronary and/or Peripheral Angiography: A Systematic Literature Review and Meta-Analysis. Front. Med. 9:841876. doi: 10.3389/fmed.2022.841876
Received: 29 December 2021; Accepted: 14 March 2022;
Published: 25 April 2022.
Edited by:
Piyameth Dilokthornsakul, Naresuan University, ThailandReviewed by:
Surasak Saokaew, University of Phayao, ThailandCopyright © 2022 Tarantini, Prasad, Rathore, Bansal, Gottfried, Rosenkranz, Briguori, Yaghoubi, Mashayekhi, Javanbakht and Moloney. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Tarantini, Z2l1c2VwcGUudGFyYW50aW5pLjFAdW5pcGQuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.