
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 22 March 2022
Sec. Translational Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.841202
 Xi Chen1†
Xi Chen1† Xingyu Liu2,3,4,5†
Xingyu Liu2,3,4,5† Yiou Wang1
Yiou Wang1 Ruichen Ma6
Ruichen Ma6 Shibai Zhu7
Shibai Zhu7 Shanni Li1
Shanni Li1 Songlin Li1
Songlin Li1 Xiying Dong8
Xiying Dong8 Hairui Li9
Hairui Li9 Guangzhi Wang4
Guangzhi Wang4 Yaojiong Wu3
Yaojiong Wu3 Yiling Zhang5*
Yiling Zhang5* Guixing Qiu1*
Guixing Qiu1* Wenwei Qian1*
Wenwei Qian1*
Background: Accurate preoperative planning is essential for successful total hip arthroplasty (THA). However, the requirements of time, manpower, and complex workflow for accurate planning have limited its application. This study aims to develop a comprehensive artificial intelligent preoperative planning system for THA (AIHIP) and validate its accuracy in clinical performance.
Methods: Over 1.2 million CT images from 3,000 patients were included to develop an artificial intelligence preoperative planning system (AIHIP). Deep learning algorithms were developed to facilitate automatic image segmentation, image correction, recognition of preoperative deformities and postoperative simulations. A prospective study including 120 patients was conducted to validate the accuracy, clinical outcome and radiographic outcome.
Results: The comprehensive workflow was integrated into the AIHIP software. Deep learning algorithms achieved an optimal Dice similarity coefficient (DSC) of 0.973 and loss of 0.012 at an average time of 1.86 ± 0.12 min for each case, compared with 185.40 ± 21.76 min for the manual workflow. In clinical validation, AIHIP was significantly more accurate than X-ray-based planning in predicting the component size with more high offset stems used.
Conclusion: The use of AIHIP significantly reduced the time and manpower required to conduct detailed preoperative plans while being more accurate than traditional planning method. It has potential in assisting surgeons, especially beginners facing the fast-growing need for total hip arthroplasty with easy accessibility.
– Develop an artificial intelligent preoperative planning system for THA (AIHIP) with increased inefficiency.
– Conduct a prospective clinical study to validated the efficacy of AIHIP.
– Convolutional neural networks automated the processing of CT images and achieved satisfactory accuracy.
– AIHIP significantly reduced the time and manpower required to conduct detailed preoperative planning.
Total hip arthroplasty (THA) is the primary surgical procedure performed for the treatment of pain and impaired function associated with osteoarthritis, osteonecrosis, fracture and other diseases. It is among the top 5 most commonly performed procedures and the top 5 fastest growing procedures (1). By 2030, the annual counts of THA are estimated to be 572–1,385 thousand (2).
The goals of THA are to minimize discomfort, improve hip function and prolong implant survival. However, leg length discrepancy (LLD), dislocation and implant failure remain primary challenges to success. Accurate preoperative planning may help surgeons achieve successful THA because it provides a detailed assessment of preoperative deformities, predicts implant sizes, provides intraoperative references and simulates postoperative outcomes such as leg length (3). Preoperative planning based on X-ray remains one of the most common methods. However, the accuracy of X-ray-based planning is controversial, ranging from 40.7 to 99.2% (3–7). Many factors may limit the accuracy of X-ray-based planning: surgeon experience, magnification error, patient position and the nature that X-ray images can only provide 2-dimensional information (8).
CT-based preoperative planning offers more detailed information on a three-dimensional scale (9). However, CT-based planning requires a complex workflow that includes image segmentation, pelvis correction, deformity recognition and postoperative simulation. Therefore, the application of CT-based planning systems is limited in that they are especially time-consuming for each case and require a group of experienced engineers, programmers and doctors to work closely together (10).
Artificial intelligence (AI) techniques, including convolutional neural networks (CNNs), have shown promising results in processing medical images with high accuracy and significantly reduced time requirements (11, 12). However, the clinical application of artificial intelligence has mainly focused on diagnosing diseases (13–15). Research to date has not yet validated the use of AI in preoperative planning systems for THA.
With the help of artificial intelligence, it is possible to develop efficient and accurate preoperative THA planning system. The purpose of this study is described as follows. 1. A comprehensive artificial intelligent preoperative planning system for THA (AIHIP) was developed, which included automatic image segmentation, preoperative deformity recognition and real-time postoperative outcome simulation. 2. A prospective clinical study was conducted to compare the efficacy between AIHIP planning and X-ray-based planning.
The AIHIP planning system included preoperative assessment and postoperative outcome simulations. Image segmentation was required to differentiate the femur from the pelvis. Featured anatomic landmarks were identified to serve as references. Then, the pelvis was corrected to a neutral position in the sagittal and coronal planes according to the identified landmarks. Assessment of preoperative deformity was also conducted referring to the identified landmarks. Postoperative outcome simulations showed the planned implant size, implant coverage and to what degree the preoperative deformity could be corrected. The comprehensive workflow was integrated into the AIHIP software. The accuracy of the AIHIP was validated through a clinical study. The research workflow is shown in Figure 1.
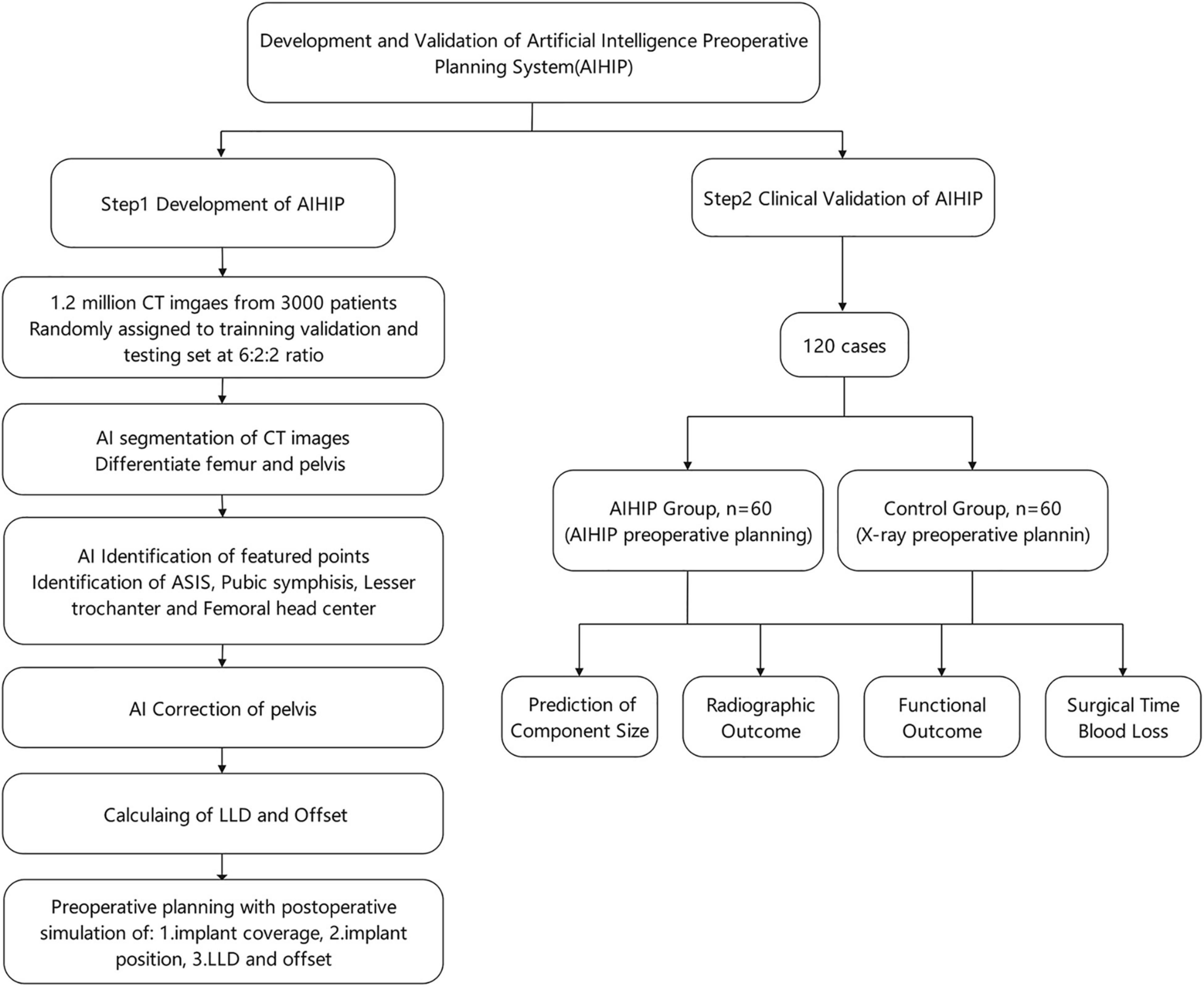
Figure 1. Flow chart of the development and clinical validation of artificial intelligence preoperative planning system for THA (AIHIP).
Over 1.2 million qualified CT images from 3,000 anonymized patients were included in this study. All patients were scheduled to receive total hip arthroplasty. The primary diagnosis included osteonecrosis, osteoarthritis, rheumatoid arthritis and developmental dysplasia of the hip. Standardized pelvic CTs were conducted prior to the operation. The range of each CT scan began from the highest point of the pelvis to 15 cm below the lesser trochanter at 1 mm intervals. All CTs were stored according to the DICOM protocol.
CT images were manually segmented by a group of engineers and three orthopedic surgeons using Mimics Software (Materialise NV, Leuven, Belgium). All engineers and surgeons had performed manual segmentation for at least 50 cases prior to this study. The contours of the femur and pelvis were manually annotated. Featured anatomic landmarks were manually marked, which included the anterior superior iliac spine (ASIS), pubic symphysis, center of the femoral head, medial edge of the lesser trochanter, and anatomic axis of the femur.
The complete dataset was randomly assigned to a training set, validation set and testing set at a ratio of 6:2:2. All images were resized to 512 × 512 pixels. The neural network structure was developed based on the attention U-Net with a point rend module. The U-Net convolutional neural network can automatically segment CT images and has achieved high accuracy in recognizing abdominal organs and tissues (16). The point rend module was used to provide point-based predictions to further enhance segmentation performance (17). An attention U-Net was developed based on a U-Net with added skip connections and attention gates (18). The use of an attention gate enables the network to automatically focus on target structures without requiring large computational power and model parameters. A skip connection was conducted between the corresponding encoder and decoder layers. Implementing skip connections provided segmentation results with a higher level of accuracy because more graphic features from the basic level were preserved and integrated into the output feature. The increased number of decoders also provides a more detailed segmentation (Figure 2A). The Dice similarity coefficient (DSC) and loss was used to assess the model performance of the AIHIP in the segmentation of CT images. DSC and loss were calculated for every 100 iterations.
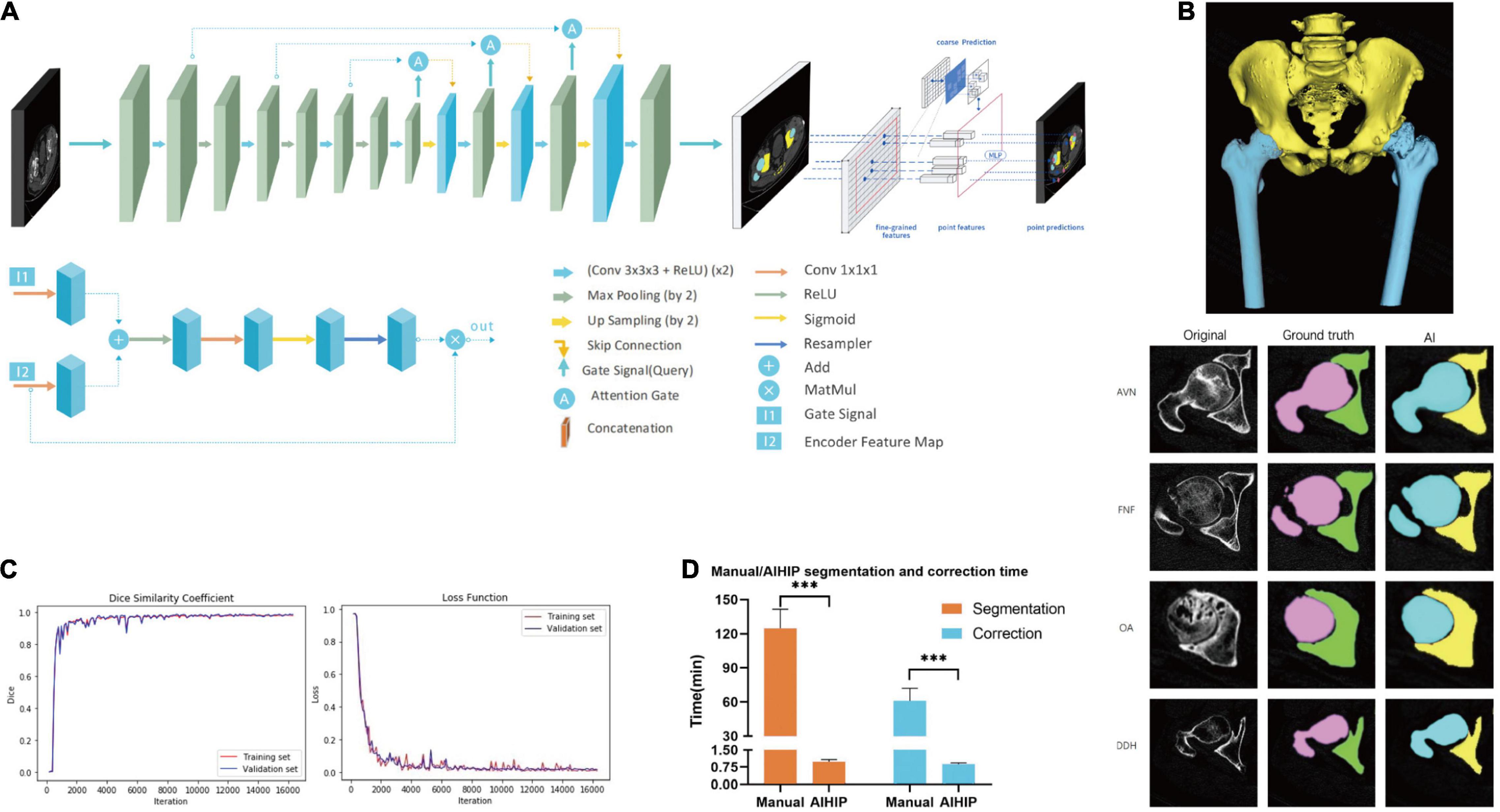
Figure 2. Development of artificial intelligence preoperative planning system for THA (AIHIP): image segmentation. (A) Net-work structure; (B) segmentation of pelvis and femur. Images of original CT, manual segmentation, and automatic segmentation with AIHIP in four primary diseases: avascular necrosis (AVN), femoral neck fracture (FNF), osteoarthritis (OA), and developmental dysplasia of hip (DDH). 3D reconstruction of the CT was completed after segmentation; (C) performance of AIHIP in automatic segmentation. Dice similarity coefficient (DSC) of training set and validation set. Loss of training set and validation set; (D) time comparison between manual segmentation and artificial intelligence (AI) segmentation. Time comparison between manual correction and AI correction. ***p < 0.001.
An example of manual identification of feature anatomic landmarks and correction of pelvis is shown in Figure 3A. Based on the segmented pelvis, the automatic recognition of featured anatomic landmarks was conducted with a stacked hourglass network, which has been used in human pose estimation (19). Repeated bottom-up and top-down inference is beneficial in predicting the coordinates of the featured anatomic structure. The stacked hourglass network automatically recognized featured anatomic structures, including the bilateral anterior superior iliac spine (ASIS), the pubic symphysis and the center of the femoral head (Figure 3B). The least square method was used to determine the anatomic axis of the femur. The pelvis could be adjusted, and the preoperative deformity could be measured based on these identified points (Figure 3C).
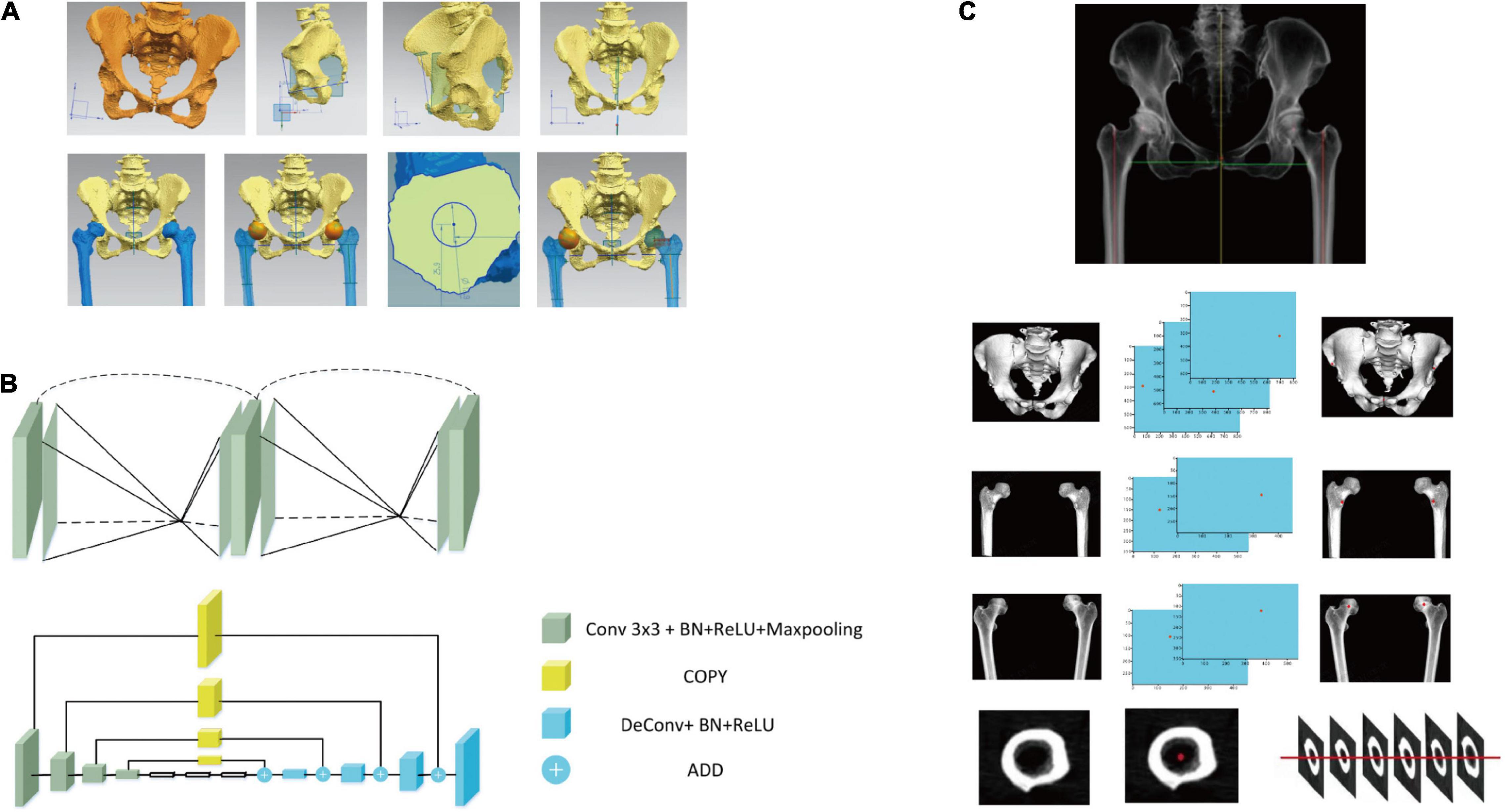
Figure 3. Development of artificial intelligence preoperative planning system for THA (AIHIP): correction of pelvis, identification of anatomical landmarks and recognition of preoperative deformities. (A) Manual correction and measurement of pelvis and femur; (B) network structure used to identify featured anatomic landmarks; (C) examples of automatic identification of anterior superior iliac spine (ASIS), medial point of lesser trochanter and center of femoral head. The anatomic axis of femur was identified using least square method.
Preoperative planning was conducted by two orthopedic surgeons using AIHIP software (Version 3.0, Longwood Valley Technology, China). Planning was carried out by first determining the position and size of the acetabular component. Inclination, anteversion, and coverage of the acetabular component were planned as well. Then, the position and size of the femoral component were determined, and the level of femoral resection was determined. Different types of acetabular components, femoral components, and femoral heads could be chosen. A simulation of the postoperative effect was generated, which showed the postoperative leg length, offset and coverage of the acetabular component. Leg length and offset of the contralateral side were also shown so that changes in the surgical plan could be made accordingly. The distance between the tip of the lesser trochanter and the tip of the femoral stem (neck length) and the distance between the tip of the lesser trochanter and the resection line of the femoral neck (calcar length) were measured in preoperative planning as references. Then, the operating surgeon measured the neck length and calcar length intraoperatively for verification (Figures 4A–C).
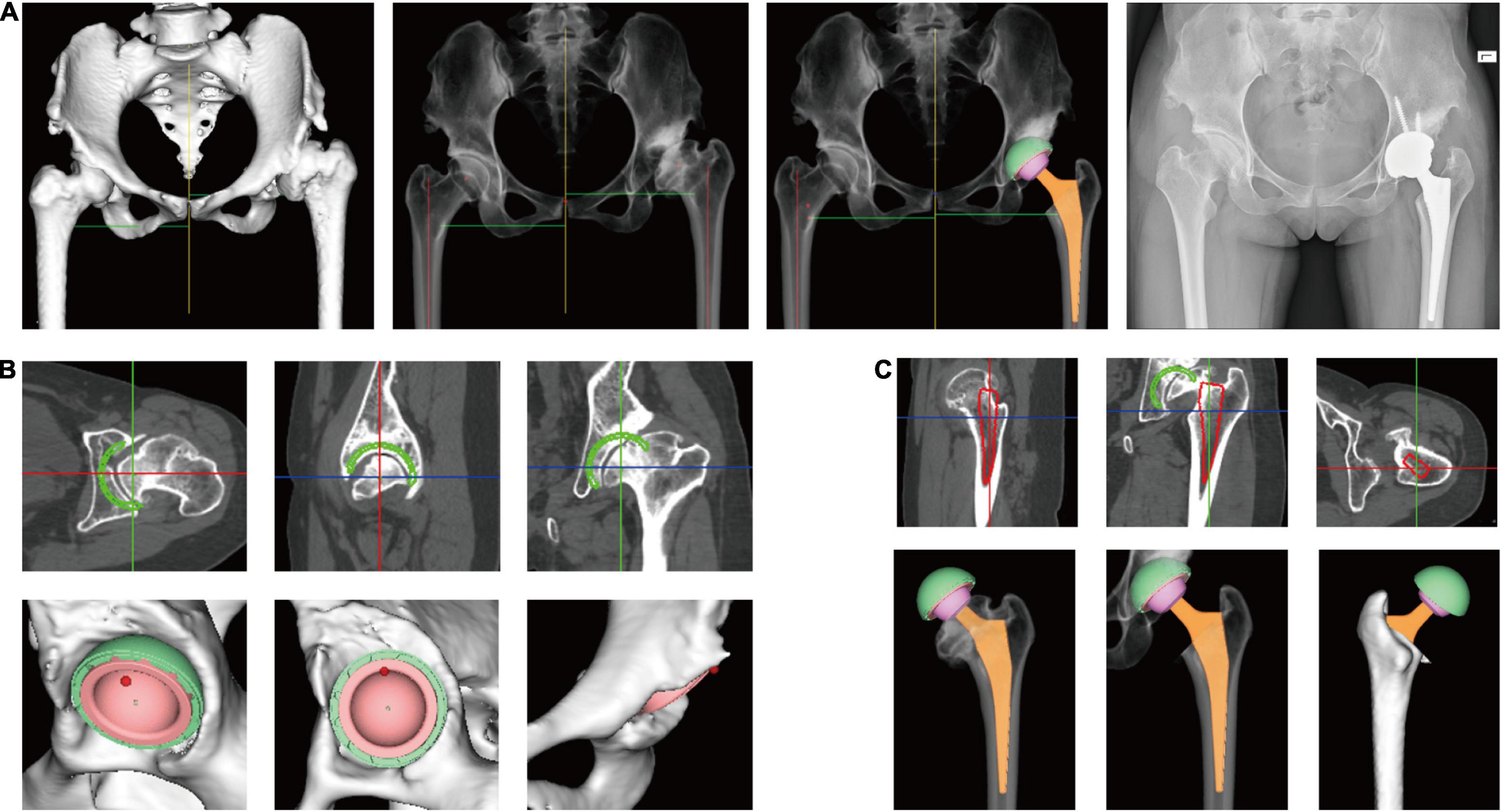
Figure 4. Preoperative planning using artificial intelligent preoperative planning system for THA (AIHIP). (A) From left to right: 3D reconstructed pelvis and femur; simulated hip X-ray; simulated postoperative outcome; postoperative X-ray; (B) preoperative planning of acetabular component. The green circle shows the planned component position in real-time. Bone coverage was calculated once the size, position, inclination, and anteversion of acetabular component is determined; (C) preoperative planning of femoral component. The red circle shows the planned position of femoral component in real-time.
Approval from the Institutional Review Board and written informed consent was acquired to conduct a prospective clinical study from October 2019 to February 2021. Patients were included if they 1. were diagnosed with osteonecrosis, osteoarthritis and developmental dysplasia of the hip (Crowe I) and received THA; 2. provided written informed consent to participate in the study. Patients were excluded if 1. the preoperative or postoperative radiographs were not standardized or 2. different types of prostheses were used during surgery. A total of 120 cases were included. X-ray-based planning was conducted in 60 cases (control group) according to the method described by Della Valle et al. (3), where planning was completed directly over the printed X-ray using templates. AIHIP planning was conducted in 60 cases (AIHIP group).
Leg length discrepancy, offset, neck length, and calcar length were measured on postoperative radiographs with the patients’ names concealed. Each measurement was made and recorded by the two observers at least 4 weeks after the operation to avoid any recollection bias. The mean value of the two measurements was used for statistical analysis. Inter-observer reliability of radiographic measurement was assessed with intraclass correlation coefficient (ICC).
Functional outcome was assessed by Hip Disability and Osteoarthritis Score Joint Replacement (HOOS JR) (20) and EuroQol 5 Dimensions Questionnaire (EQ-5D) (21, 22). Patients were followed up until 12 weeks postoperatively. Surgical time and blood loss were also recorded.
All patients underwent total hip arthroplasty through a posterior approach by one experienced orthopedic surgeon in one facility. The prostheses used were Pinnacle Cup (DePuyOrthopaedics, Warsaw, IN, United States), Corail Stem (DePuyOrthopaedics, Warsaw, IN, United States) and Trilock stem (DePuyOrthopaedics, Warsaw, IN, United States). Standard perioperative care and patient education were administered to all patients.
Statistical analysis was performed with SPSS version 25 (IBM, New York, NY, United States) and GraphPad Prism version 8 (GraphPad Software, San Diego, CA, United States). According to previous literatures (23–25), accurate prediction was defined as the predicted size to be within ± 1 size from the implanted size. Absolute error was defined as the absolute difference between planned and implanted component size and the difference between plan and postoperative radiographic measurement. Mean error was defined as the average value of the planned component size minus the implanted component size. A p-value less than 0.05 was considered statistically significant. Discontinuous variables were recorded as incidence and rate. The chi-square test was used to compare the discontinuous variables between groups. Continuous variables were recorded as the means and standard deviation. A general linear model was used to test whether there was a statistically significant difference between the two groups considering confounding factors, including age, sex, BMI, and primary diagnosis.
The effect of manual segmentation and AI segmentation from four common primary diagnoses are shown in Figure 2B. The DSC curves and loss from the training set and validation set are shown in Figure 2C. Both curves reached convergence by 15,800 iterations, which indicated optimal DSC and loss. At 15,800 iterations, the DSC of the training set was 0.983, and the DSC of the validation set was 0.987. The losses were 0.008 and 0.013 for the training set and validation set, respectively (Figure 2C). The testing set was used to validate algorithm performance. The testing set achieved a DSC of 0.973 and loss of 0.012, which was comparable to that of the training set and validation set.
The average time consumption was 0.99 ± 0.94 min for AIHIP segmentation and 0.87 ± 0.07 min for AIHIP correction and deformity assessment. The average time was 124.55 ± 16.87 min for manual segmentation and 60.85 ± 11.11 min for manual correction and deformity assessment (Figure 2D). The total time required for the AIHIP algorithm to process the CT for one case was 1.86 ± 0.12 min on average, compared with 185.40 ± 21.76 min for the manual workflow (P < 0.001).
A total of 120 cases were included in the study. The demographic characteristics are listed in Table 1, which include age, sex, height, weight, BMI, and primary diagnosis. The difference in age between the control group (mean: 53.75, range: 24–78) and the AIHIP group (mean: 47.62, range: 23–78) was statistically significant (P = 0.033). There were no statistically significant differences in terms of sex, weight, height, or BMI. Demographic characteristics were considered as confounding variables. Their influences on the prediction accuracy were assessed and adjusted with generalized linear models.
The predicted cup size and implanted cup size were exactly the same in 66.67% of the AIHIP cases and 20% of the control cases (P < 0.001). For femoral stem, the exact same size was achieved in 55% of the AIHIP cases and 31.67% of the control cases. The cup size was accurately predicted to within ± 1 size in 55.00 and 96.67% of patients in the control group and AIHIP group, respectively (P < 0.001). Stem size was accurately predicted to within ± 1 size in 65.00 and 96.67% of the control group and AIHIP group, respectively (P < 0.001). Further analysis showed more detailed comparison in Figures 5A, B. The tendency toward overestimation or underestimation of component size was assessed by mean error. Comparing with AIHIP planning, acetate templating tended to underestimate cup size by 2.13 ± 2.11 (P < 0.001) and underestimate the stem size by 0.53 ± 1.28 (P = 0.013). The comparison of the mean absolute error between the two groups is shown in Table 2. Compared with the control group, high offset/varus stems were more commonly used in the AIHIP group (P = 0.004) (Figure 5C). The average time it took to conduct X-ray planning was 7.37 ± 1.32 min and the average time it took to conduct AIHIP planning was 8.11 ± 0.98 min (P = 0.001).
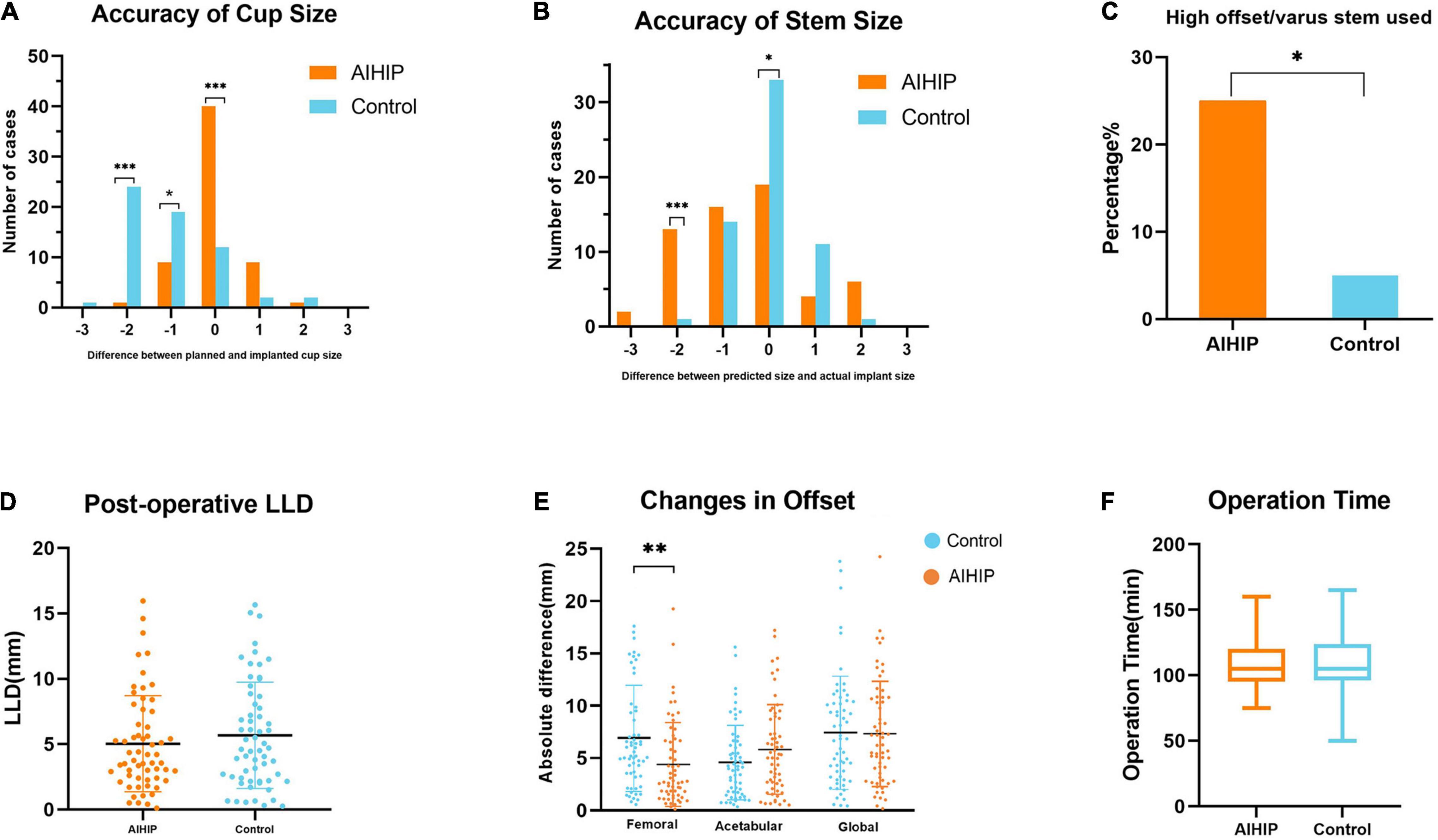
Figure 5. Clinical validation of artificial intelligent preoperative planning system for THA (AIHIP). (A) Plan accuracy of cup size; (B) plan accuracy of stem size; (C) proportion of high offset/varus stem used; (D) postoperative leg length discrepancy (LLD); (E) difference between preoperative and postoperative offset; (F) operation time. *, **, *** P < 0.05, 0.01, 0.001.
Neck length, calcar length, LLD, and offset were measured on radiographs. The difference between preoperative planning and postoperative radiographic measurements was recorded as the mean absolute error and is shown in Table 2. The ICCs for all radiographic measurement were above 0.9, which indicated substantial inter-observer agreement (Table 3). The mean absolute error for neck length was 6.13 ± 3.16 mm in the control group and 5.49 ± 4.40 mm in the AIHIP group (P = 0.813). The mean absolute error of calcar length was 4.51 ± 2.96 mm in the control group and 3.92 ± 2.79 mm in the AIHIP group (P = 0.249). The average postoperative LLD for the control group and AIHIP group was 5.68 ± 4.06 mm and 5.03 ± 3.67 mm, respectively (P = 0.360) (Figure 5D). Although a trend was observed in the above radiographic outcomes, the differences were not statistically significant. Changes in acetabular offset and global offset were not significantly different between the two groups. Femoral offset was more accurately restored in the AIHIP group than in the control group (P = 0.001) (Figure 5E).
The average operation time was 106.83 ± 18.20 min in the AIHIP group and 109.58 ± 21.98 min in the control group (P = 0.457) (Figure 5F). The average blood loos was 285.00 ± 127.33 ml in the AIHIP group and 315.67 ± 164.68 ml in the control group (P = 0.256). There were no statistically significant differences in HOOS score preoperatively (P = 0.605) and 12 weeks postoperatively (P = 0.22) between the two groups. There were no statistically significant differences in EQ5D index preoperatively (P = 0.846) and 12 weeks postoperatively (P = 0.203) between the two groups.
We developed an artificial intelligence-based system (AIHIP) to enhance the efficiency and accuracy of preoperative planning for THA. Deep learning algorithms were used for the comprehensive workflow. In the segmentation module, the attention U-Net with point rend features achieved satisfactory DSC and loss in the training set, validation set and testing set, which indicated satisfactory segmentation performance. Based on segmentation, a stacked hourglass network was applied to recognize featured anatomic landmarks. The identified landmarks served as a reference for pelvis correction and recognition of preoperative deformities. The comprehensive workflow including automatic segmentation, pelvis correction, deformity assessment and real-time simulation of postoperative outcomes was integrated into the AIHIP software.
Segmentation is the foundation for building an accurate preoperative planning system. Previous studies have reported deep learning as a valuable tool for automatic segmentation in abdominal CT (12), head CT (26), and CT angiography (27). Although the role of deep learning in joint segmentation remains largely unexamined, the results of this study were comparable to the abovementioned studies. A stacked hourglass network was first used to identify featured bony landmarks in cephalograms in 2020 (28). Cephalograms are two-dimensional X-ray images, while CT offers three-dimensional information, which complicates the coordinate prediction of featured points. This study further investigated its application in hip CT. The use of artificial intelligence has also greatly reduced the time and manpower required to conduct detailed preoperative plans for patients.
In this series, AIHIP planning was significantly more accurate in predicting implant sizes than X-ray-based planning. X-ray-based planning underestimated cup size by an average of 2.13. The accuracy of X-ray-based planning varies in different studies. The reported accuracy of X-ray-based planning ranged from 40.68 to 90% (24, 29, 30). The wide variety of reported accuracies for X-ray-based planning is subjected to many factors, including the quality of the radiograph, magnification error and surgeon experience (31). In this series, the accuracy to within ± 1 size of X-ray-based planning was 55% for cup size and 65.00% for stem size, while the accuracy to within ± 1 size of AIHIP planning was above 95% for cup size and stem size. Acetate templating tended to underestimate stem size and cup size. Planners might be more conservative in X-ray-based planning because less information is provided in 2-dimensional X-rays than in 3-dimensional CT. During AIHIP planning, the simulation of prothesis position and its relation to surrounding bone can be visualized in coronal, sagittal and axial plane, allowing surgeons to adjust the plan from more angles.
Both X-ray-based planning and AIHIP planning were carried out by two orthopedic residents. This suggested the potential benefit of AIHIP in assisting beginners because it provides more information than traditional methods. In AIHIP planning, real-time simulation of the position, coverage and to what degree the deformity could be corrected were visualized, allowing for improved understanding and simulation of the case prior to surgery. AIHIP planning also facilitated accurate measurement of offset and LLD. Femoral offset was more accurately restored in AIHIP planning comparing with X-ray planning, which may be related to the fact that more high offset/varus stems were selected in AIHIP planning. It has been reported that reduced femoral offset negatively affected range of motion due to impingement and reduced abductor lever (32). Gait analysis has shown that changes in femoral offset also influence the function of external rotator, extensor and short flexor muscle during different phase of gait (33). On the other hand, one study found that significantly increased femoral offset was an important source of elevated ions in metal on polyethylene THA, suggesting possible increased contact force and accelerated wear (34). Therefore, accurate restoration of femoral offset might provide potential benefit in avoiding impingement and implant biomechanics. Although the changes of femoral offset might be compensated by changes in acetabular offset in certain aspects, a significant decrease in femoral offset might result in impingement and possible dislocation irrespective of acetabular offset. The limitations of this study are as follows. 1. Postoperative radiographic assessment was conducted on X-ray images rather than CT, which is less accurate. 2. There was no statistical significant differences in terms of clinical outcome. 3. Different planning methods were applied in the two groups, which could lead to potential bias in comparing the accuracy of both methods. However, general linear model was applied to minimize the influence of confounding factors.
The use of AIHIP greatly reduced the time and manpower required to conduct detailed preoperative plans while being more accurate than traditional planning methods. It has potential in assisting surgeons, especially beginners facing the fast-growing need for total hip arthroplasty with easy accessibility.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
WQ, GQ, YZ, and XC conceived and designed the study. YZ and XL developed, trained, validated, and tested the neural networks. RM, YiW, SZ, XD, and XC conducted the radiographic measurement. XC, ShL, and SoL analyzed the data. HL, GW, and YaW checked the data and methodology. XC and XL wrote the manuscript and conducted critical analysis. All authors read and approved the final manuscript.
XL and YZ are employed by Longwood Valley Medical Technology Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
THA, Total hip arthroplasty; AIHIP, Artificial intelligent preoperative planning system for THA; LLD, Leg length discrepancy; DSC, Dice similarity coefficient.
1. Schwartz AM, Farley KX, Guild GN, Bradbury TL Jr. Projections and epidemiology of revision hip and knee arthroplasty in the United States to 2030. J Arthroplasty. (2020) 35:S79–85. doi: 10.1016/j.arth.2020.02.030
2. Singh JA, Yu S, Chen L, Cleveland JD. Rates of total joint replacement in the United States: future projections to 2020-2040 using the national inpatient sample. J Rheumatol. (2019) 46:1134–40. doi: 10.3899/jrheum.170990
3. Della Valle AG, Padgett DE, Salvati EA. Preoperative planning for primary total hip arthroplasty. J Am Acad Orthop Surg. (2005) 13:455–62. doi: 10.5435/00124635-200511000-00005
4. Petretta R, Strelzow J, Ohly NE, Misur P, Masri BA. Acetate templating on digital images is more accurate than computer-based templating for total hip arthroplasty. Clin Orthop Relat Res. (2015) 473:3752–9. doi: 10.1007/s11999-015-4321-y
5. Shaarani SR, McHugh G, Collins DA. Accuracy of digital preoperative templating in 100 consecutive uncemented total hip arthroplasties: a single surgeon series. J Arthroplasty. (2013) 28:331–7. doi: 10.1016/j.arth.2012.06.009
6. Gamble P, de Beer J, Petruccelli D, Friesenbichler J, Maurer-Ertl W, Leithner A. The accuracy of digital templating in uncemented total hip arthroplasty. J Arthroplasty. (2010) 25:529–32. doi: 10.1016/j.arth.2009.04.011
7. Efe T, El Zayat BF, Heyse TJ, Timmesfeld N, Fuchs-Winkelmann S, Schmitt J. Precision of preoperative digital templating in total hip arthroplasty. Acta Orthop Belg. (2011) 77:616–21.
8. Asnis SE, Heller YY. Total hip arthroplasty templating: a simple method to correct for radiograph magnification. Orthopedics. (2019) 42:e322–5. doi: 10.3928/01477447-20190307-01
9. Osmani FA, Thakkar S, Ramme A, Elbuluk A, Wojack P, Vigdorchik JM. Variance in predicted cup size by 2-dimensional vs 3-dimensional computerized tomography-based templating in primary total hip arthroplasty. Arthroplasty Today. (2017) 3:289–93. doi: 10.1016/j.artd.2016.09.003
10. Chu C, Chen C, Liu L, Zheng G. FACTS: fully automatic CT segmentation of a hip joint. Ann Biomed Eng. (2015) 43:1247–59. doi: 10.1007/s10439-014-1176-4
11. Zhou XY, Guo Y, Shen M, Yang GZ. Application of artificial intelligence in surgery. Front Med. (2020) 14:417–30. doi: 10.1007/s11684-020-0770-0
12. Wang Z, Meng Y, Weng F, Chen Y, Lu F, Liu X, et al. An effective CNN method for fully automated segmenting subcutaneous and visceral adipose tissue on CT scans. Ann Biomed Eng. (2020) 48:312–28. doi: 10.1007/s10439-019-02349-3
13. Wang P, Liu X, Xu J, Li T, Sun W, Li Z, et al. Deep learning for diagnosing osteonecrosis of the femoral head based on magnetic resonance imaging. Comput Methods Programs Biomed. (2021) 208:106229. doi: 10.1016/j.cmpb.2021.106229
14. Rouzrokh P, Wyles CC, Philbrick KA, Ramazanian T, Weston AD, Cai JC, et al. A deep learning tool for automated radiographic measurement of acetabular component inclination and version after total hip arthroplasty. J Arthroplasty. (2021) 36:2510–7.e6. doi: 10.1016/j.arth.2021.02.026
15. Xue P, Tang C, Li Q, Li Y, Shen Y, Zhao Y, et al. Development and validation of an artificial intelligence system for grading colposcopic impressions and guiding biopsies. BMC Med. (2020) 18:406. doi: 10.1186/s12916-020-01860-y
16. Weston AD, Korfiatis P, Philbrick KA, Conte GM, Kostandy P, Sakinis T, et al. Complete abdomen and pelvis segmentation using U-net variant architecture. Med Phys. (2020) 47:5609–18. doi: 10.1002/mp.14422
17. Kirillov A, Wu Y, He K, Girshick R. PointRend: image segmentation as rendering. arXiv [Preprint]. (2020). arXiv:191208193. doi: 10.1109/CVPR42600.2020.00982
18. Oktay O, Schlemper J, Folgoc LL, Lee M, Heinrich M, Misawa K, et al. Attention U-net: learning where to look for the pancreas. arXiv [Preprint]. (2018). arXiv:180403999
19. Newell A, Yang K, Jia D. Stacked hourglass networks for human pose estimation. In: B Leibe, J Matas, N Sebe, M Welling editors. Proceedings of the European Conference on Computer Vision. Cham: Springer (2016). p. 483–99. doi: 10.1007/978-3-319-46484-8_29
20. Lyman S, Lee YY, Franklin PD, Li W, Mayman DJ, Padgett DE. Validation of the HOOS, JR: a short-form hip replacement survey. Clin Orthop Relat Res. (2016) 474:1472–82. doi: 10.1007/s11999-016-4718-2
21. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. (2001) 33:337–43. doi: 10.3109/07853890109002087
22. Luo N, Liu G, Li M, Guan H, Jin X, Rand-Hendriksen K. Estimating an EQ-5D-5L value set for China. Value Health. (2017) 20:662–9. doi: 10.1016/j.jval.2016.11.016
23. Mainard D, Barbier O, Knafo Y, Belleville R, Mainard-Simard L, Gross JB. Accuracy and reproducibility of preoperative three-dimensional planning for total hip arthroplasty using biplanar low-dose radiographs : a pilot study. Orthop Traumatol Surg Res. (2017) 103:531–6. doi: 10.1016/j.otsr.2017.03.001
24. Huo J, Huang G, Han D, Wang X, Bu Y, Chen Y, et al. Value of 3D preoperative planning for primary total hip arthroplasty based on artificial intelligence technology. J Orthop Surg Res. (2021) 16:156. doi: 10.1186/s13018-021-02294-9
25. Wu P, Liu Q, Fu M, Zhang Z, He S, Liao W, et al. Value of computed tomography-based three-dimensional pre-operative planning in cup placement in total hip arthroplasty with dysplastic acetabulum. J Investig Surg. (2019) 32:607–13. doi: 10.1080/08941939.2018.1444828
26. Chilamkurthy S, Ghosh R, Tanamala S, Biviji M, Campeau NG, Venugopal VK, et al. Deep learning algorithms for detection of critical findings in head CT scans: a retrospective study. Lancet. (2018) 392:2388–96. doi: 10.1016/S0140-6736(18)31645-3
27. Fu F, Wei J, Zhang M, Yu F, Xiao Y, Rong D, et al. Rapid vessel segmentation and reconstruction of head and neck angiograms using 3D convolutional neural network. Nat Commun. (2020) 11:4829. doi: 10.1038/s41467-020-18606-2
28. Kim H, Shim E, Park J, Kim Y-J, Lee U, Kim Y. Web-based fully automated cephalometric analysis by deep learning. Comput Methods Programs Biomed. (2020) 194:105513. doi: 10.1016/j.cmpb.2020.105513
29. Knight JL, Atwater RD. Preoperative planning for total hip arthroplasty. quantitating its utility and precision. J Arthroplasty. (1992) 7 Suppl:403–9. doi: 10.1016/S0883-5403(07)80031-3
30. Carter LW, Stovall DO, Young TR. Determination of accuracy of preoperative templating of noncemented femoral prostheses. J Arthroplasty. (1995) 10:507–13. doi: 10.1016/S0883-5403(05)80153-6
31. Wako Y, Nakamura J, Miura M, Kawarai Y, Sugano M, Nawata K. Interobserver and intraobserver reliability of three-dimensional preoperative planning software in total hip arthroplasty. J Arthroplasty. (2018) 33: 601–7. doi: 10.1016/j.arth.2017.08.031
32. Patel AB, Wagle RR, Usrey MM, Thompson MT, Incavo SJ, Noble PC. Guidelines for implant placement to minimize impingement during activities of daily living after total hip arthroplasty. J Arthroplasty. (2010) 25: 1275–81.e1. doi: 10.1016/j.arth.2009.10.007
33. Hu X, Zheng N, Chen Y, Dai K, Dimitriou D, Li H, et al. Optimizing the femoral offset for restoring physiological hip muscle function in patients with total hip arthroplasty. Front Bioeng Biotechnol. (2021) 9:645019. doi: 10.3389/fbioe.2021.645019
Keywords: arthroplasty, artificial intelligence, hip, convolutional neural network, preoperative planning
Citation: Chen X, Liu X, Wang Y, Ma R, Zhu S, Li S, Li S, Dong X, Li H, Wang G, Wu Y, Zhang Y, Qiu G and Qian W (2022) Development and Validation of an Artificial Intelligence Preoperative Planning System for Total Hip Arthroplasty. Front. Med. 9:841202. doi: 10.3389/fmed.2022.841202
Received: 22 December 2021; Accepted: 11 February 2022;
Published: 22 March 2022.
Edited by:
Bing Yue, Shanghai Jiao Tong University, ChinaReviewed by:
Guoan Li, Newton Wellesley Hospital, United StatesCopyright © 2022 Chen, Liu, Wang, Ma, Zhu, Li, Li, Dong, Li, Wang, Wu, Zhang, Qiu and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiling Zhang, eWx6aGFuZ0BjaGFuZ211Z3UuY29t; Guixing Qiu, cWl1Z3hfcHVtY2hAMTI2LmNvbQ==; Wenwei Qian, cWlhbnd3MDA3QDE2My5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.