- 1Kirby Institute, The University of New South Wales (UNSW) Sydney, Sydney, NSW, Australia
- 2Department of Global Health and Development, London School of Hygiene and Tropical Medicine, London, United Kingdom
- 3Centre for Health Economics Research and Evaluation, University of Technology Sydney, Sydney, NSW, Australia
- 4Global Human immunodeficiency virus (HIV), Hepatitis and Sexually transmitted infections (STIs) Programmes, World Health Organization, Geneva, Switzerland
- 5Central Clinical School, Faculty of Medicine, Nursing and Health Sciences, Monash University, Melbourne, VIC, Australia
- 6Sydney Sexual Health Centre, Sydney, NSW, Australia
- 7School of Population Health, The University of New South Wales (UNSW) Sydney, Sydney, NSW, Australia
- 8Thorne Harbour Health, Melbourne, VIC, Australia
- 9ACON, Sydney, NSW, Australia
- 10Melbourne Sexual Health Centre, Alfred Health, Melbourne, VIC, Australia
- 11Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, The University of Melbourne, Melbourne, VIC, Australia
- 12Burnet Institute, Melbourne, VIC, Australia
- 13Centre for Social Research in Health, The University of New South Wales (UNSW) Sydney, Sydney, NSW, Australia
Background: In Australia, undiagnosed HIV rates are much higher among migrant gay, bisexual, or other men who have sex with men (GBMSM) than Australian-born GBMSM. HIV self-testing is a promising tool to overcome barriers to HIV testing and improve HIV testing uptake among migrant GBMSM. We compared the preferences for HIV testing services, including HIV self-testing, among migrant and Australian-born GBMSM.
Methods: Preferences were assessed via two discrete choice experiments (DCEs). Participants were recruited between December 2017 and January 2018 using online and offline advertising and randomly assigned to complete one of two online DCE surveys. Migrant GBMSM were classified as being born in a country with a reciprocal healthcare agreement (RHCA) with Australia (providing free or subsided health care) or not. Latent class analysis and mixed logit models were used to explore heterogeneity in preferences.
Findings: We recruited 1,606 GBMSM, including 583 migrant men of whom 419 (72%) were born in non-RHCA countries. Most participants preferred a free or cheap oral test with higher accuracy and a shorter window period to facilitate early detection of infections. Cost was more important for men born in non-RHCA countries than for men from RHCA countries or Australia. All groups preferred accessing kits through online distributers or off the shelf purchasing from pharmacies. Men born in RHCA countries least preferred accessing HIV self-testing kits from a medical clinic, while more than half of men from non-RHCA countries most preferred sourcing kits from a clinic. Sex-on-premises venues were the least preferred location to access test kits among all groups. In addition, two latent class analyses explored heterogeneity in preferences among men from non-RHCA countries and we found four latent classes for HIV testing services and two latent classes for HIVST distribution.
Interpretation: Our findings emphasise the need for high-performing and low-cost HIV self-testing kits that are accessible from a variety of distribution points as a component of Australia's HIV response, especially for those who do not have access to free or subsidised health care in Australia.
Introduction
Nearly one in 30 people in the world live in a country other than their place of birth (1). The focus on migrant health has been growing in recent years, with increasing recognition that the multi-faceted and heterogeneous nature of health risks, including those related to infectious disease prevention and control, can occur throughout the migration process (2). However, limited availability of quality data, and in some cases, access to publicly subsidised healthcare hamper efforts to address the health needs of migrants (3).
Over a quarter of people living in Australia (29.7%) were born overseas (4). In the early 2010s, migration-related HIV cases were predominantly diagnosed in people from sub-Saharan Africa (SSA), who acquired HIV before arriving in Australia (5). More recently, there has been an increase in the number of new HIV diagnoses in Australia among gay, bisexual or other men who have sex with men (GBMSM) from Asia (6). Migrants living in high-income countries are reported to have a lower self-perceived risk of HIV infection, language barriers, and concerns with confidentiality and privacy (7–10). Moreover, migrant GBMSM may face additional cultural and healthcare system barriers such as unfamiliarity with the local health system, distrust of health providers, and privacy concern, further hindering their access to HIV testing in conventional settings (11–13). Between 2014 and 2019, HIV diagnoses among Australian-born GBMSM declined by 44%, which likely occurred due to improved coverage of HIV testing and treatment, and implementation of PrEP across Australia (14). However, this success was offset by increased diagnoses among people born in other countries resulting in a relatively stable number of annual diagnoses overall (15). Surveillance data from 2018 suggest that migrant GBMSM in Australia are three times more likely to be undiagnosed for HIV and three times more likely to be diagnosed late than Australian-born GBMSM (14). Delays in testing lead to delayed treatment (16), and untreated HIV infections can disproportionately contribute to HIV transmissions (17–19). Together, this evidence underscores the urgent need to develop a better understanding of the needs of migrant GBMSM and improve access to earlier HIV testing, diagnosis, linkage to care, and bolster targeted prevention strategies.
Medicare, Australia's universal health care scheme, is available to all Australian citizens and permanent residents. Medicare also covers temporary migrants from ten countries in Europe and New Zealand through reciprocal healthcare agreements (RHCA) (20). With access to Medicare, migrants from RHCA countries have full access to free HIV testing. In comparison, migrants from non-RHCA countries can access free testing through public-funded programmes that are unevenly distributed across Australia or private insurance, which may require reimbursable upfront payments (21, 22). Previous studies found that migrants ineligible for subsidised healthcare through RHCA were more likely to be diagnosed with HIV later than those born in Australia or countries covered by the RHCA agreement (23, 24). In addition, studies on HIV and hepatitis B virus care in Australia have demonstrated that the issue of ineligibility for subsidised healthcare places additional psychological and financial pressure on migrants (25, 26).
HIV self-testing (HIVST) enables people to test for HIV conveniently and privately and is a promising tool to improve HIV testing uptake among migrant GBMSM (27, 28). Studies from several countries have confirmed that access to HIVST kits increases HIV testing uptake and frequency among GBMSM (29–32). More recently, HIVST kits have been successfully implemented in several countries to test underserved populations during the COVID-19 pandemic (33–35). To initiate or scale-up HIVST among GBMSM, various approaches to distribute HIVST kits have been evaluated globally, including through home delivery, pharmacies, vending machines, online purchasing, sexual or social networks, or social enterprise health campaigns—all of which have shown favourable outcomes (36–38). However, globally, little is known about the preferences of migrant GBMSM for accessing HIVST through these different channels.
Discrete choice experiments (DCEs) are a methodology to understand user preferences for goods and services that are not yet widely available in the market (39). Within a DCE, individuals are asked to state their preference between different goods or services on offer, with each of the goods or services described by their underlying characteristics or attributes (40). Data from DCEs can be used to identify the trade-offs that individuals are willing to make between the attributes describing a good or service (41). This method has been widely employed to quantitatively estimate user and provider preferences towards HIV testing services in various settings (42–46). In this paper, we compare the preferences for HIV testing services and HIVST kit distribution among GBMSM in Australia, assessing the impact on preferences of differences in country of birth and access to an RHCA for Medicare.
Methods
Study Population and Recruitment Procedures
This study used data from two DCEs (DCE-Test and DCE-Kits) administered through an online survey of GBMSM living in Australia (45). The recruitment procedures and details of the primary study are reported elsewhere (47). In brief, both surveys recruited GBMSM living in Australia, who were aged 18 years or over, and had not been diagnosed with HIV. Participants were recruited from December 2017 to January 2018 using online and offline advertising. The survey link was advertised in a dating application for GBMSM (Grindr) and social networking platforms, including the Facebook pages of two community-based organisations in Australia (ACON and Thorne Harbour Health, Australia's largest community-based HIV and LGBTI health organisations). For offline recruitment, GBMSM who attended the two largest public sexual health clinics in Sydney and Melbourne were invited by healthcare workers. In this study, migrants were defined as overseas-born people residing in Australia permanently or temporarily. Participants' sociodemographic characteristics and sexual histories were collected in the surveys, including their age, occupation, country of birth, duration of stay in Australia (if they were born outside Australia), condomless anal sex in the last 6 months, and HIV testing history. In this study, we focused our analyses primarily on migrant GBMSM and compared their preferences to men born in Australia. This study obtained ethical approval from the New South Wales South Eastern Sydney Local District Human Research Ethics Committee (17/147) and Alfred Health Human Research Ethics Committee (486/17).
Design of the DCE and Selection of Attributes
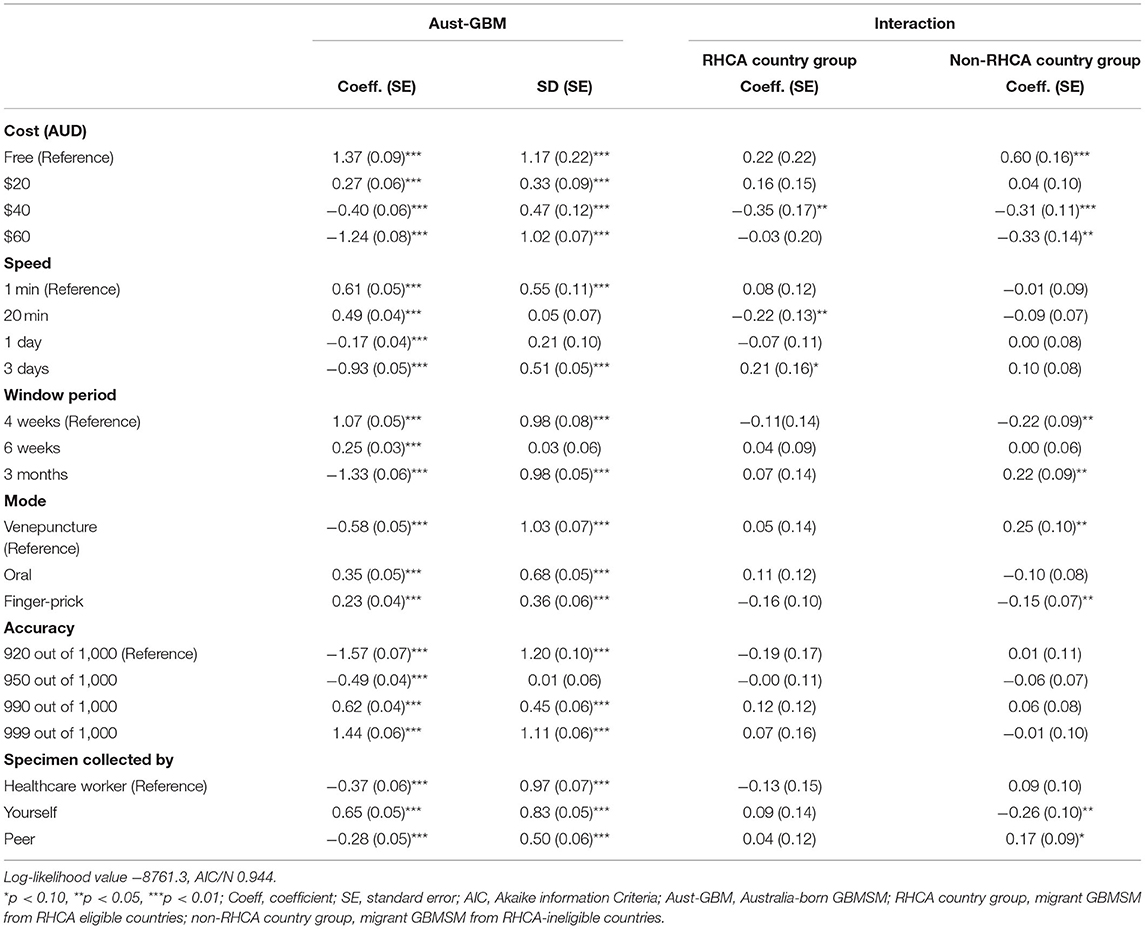
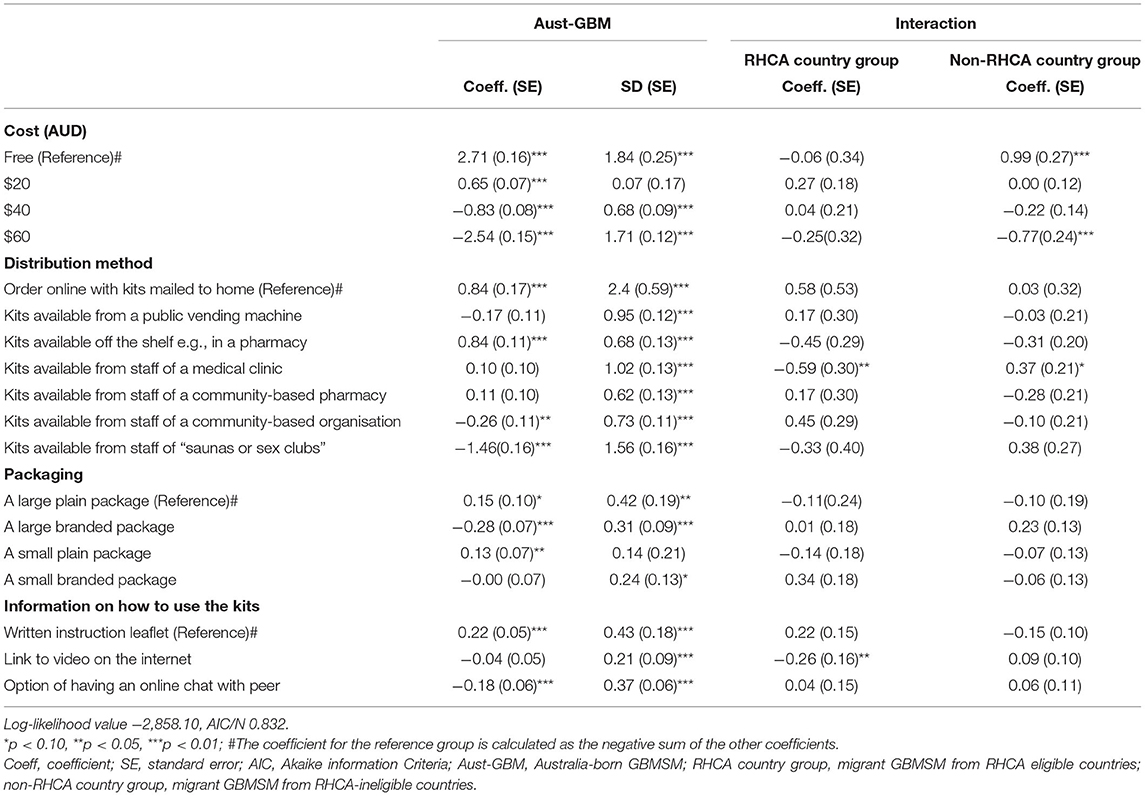
After obtaining informed consent, participants were randomly assigned to complete one of two online DCE surveys (in a 2:1 ratio for DCE-Test and DCE-Kits). All surveys were in English. Attributes and levels for the DCEs were based on a review of the literature, qualitative interviews, and policy review (47). In the first DCE, participants were asked to choose between different aspects of HIV testing services to identify preferences for HIVST relative to other methods of HIV testing (DCE-Test). These attributes included the cost of the test, the length of the window period, the length of time to receive results, the accuracy of the HIV test, the type of sample used for testing, and who was responsible for collecting the sample. The second DCE examined preferences related to HIVST kit distribution (DCE-Kits). These attributes included the cost of the HIVST kit, where the kits were distributed, type of packaging, and type of instructions for use.
Statistical Analysis
Participants' sociodemographic characteristics, sexual behaviours and HIV testing behaviours were characterised using descriptive statistics and compared between Australian-born and migrant GBMSM using Mann-Whitney U-tests for continuous variables and chi-squared tests for categorical variables. These analyses were conducted using Stata version 14 (StataCorp, College Station, Texas, USA).
Effects coding was used for all preference data. Mixed logit and latent class analysis models were used to estimate the relative utility of each attribute level (48). The mixed logit model assumed a continuous, normal distribution for all attributes, while the latent class analysis model assumed a discrete distribution based on latent constructs (49). We conducted two mixed logit models (one for HIV testing, one for HIVST distribution) that includes an interaction term for each attribute level to examine the differences in preferences for HIV testing services among Australian born men (Australian-born group), migrants from RHCA countries (RHCA country group) and migrants not from RHCA countries (non-RHCA country group). All attributes were set as random in the mixed logit model and treated as ordinal. In addition, the coefficient range of the levels for each attribute was used to calculate the relative weight of the attributes: the attribute with the largest range is likely to be the most important attribute in influencing testing behaviours (48). Latent class analysis models classify groups of responses that indicate homogenous preference patterns according to unobserved (latent) constructs; these groups can then be further characterised by using observable respondent characteristics to test the likelihood of respondents contributing to each class of response (49). We conducted two latent class analysis models to examine the heterogeneity of preferences for HIV testing services and HIVST distribution among the non-RHCA country group.
Several sociodemographic and sexual behaviour characteristics of participants recognised as key determinants for HIV testing in the published literature (23, 50, 51) were included in the latent class analysis models: young migrant (age ≤ 25-year-old); born in Southeast Asia; recent migrant (arriving Australia <5 years ago); ever engaged in condomless anal intercourse with casual partners in the last 6 months, and naive HIV tester (never tested for HIV). We used the participant's country of birth to determine if they were born in a country that has an RHCA with Australia, referred to as an RHCA country vs. a non-RHCA country (23, 24). The log-likelihood and Akaike Information Criteria (AIC) were used to evaluate the goodness-of-fit of the models. All mixed logit and latent class analysis models were estimated using NLOGIT statistical software (version 6, Econometric Software Inc, Plainview, NY, USA).
Role of the Funding Source
The funder had no role in study design, data collection and analysis, or manuscript preparation.
Results
Of the 1,606 men recruited between December 2017 and January 2018, 583 were migrants born overseas, with 420 and 163 participated in the DCE-Test survey and the DCE-kits survey, respectively. Table 1 presents the participants' sociodemographic characteristics, sexual behaviours, and HIV testing behaviours. Among the 419 (72%) migrant men born in non-RHCA countries, 127 (30%) were from Southeast Asia, 101 from other Asian countries (24%), 25 from Sub-Saharan Africa (6%) and 166 from other non-RHCA countries (40%). For migrants, the median time since arrival in Australia was seven years. Overall, migrant participants were younger and had higher education levels than those born in Australia. Compared to Australian-born men, a lower proportion of migrants had tested for HIV at a general practise and a higher proportion had tested at community services. In addition, 141 (24%) migrant men and 197 (19%) Australian-born men reported having delayed their HIV testing because no HIVST was available.

Table 1. Sociodemographic characteristics of all respondents completing DCETest (HIV testing method) and DCEKits (HIVST access), 2018 (N = 1,606) compared with Australia born GBMSM and migrant GBMSM.
Comparison Between Groups: Preferences for HIV Testing Services (DCE-Test)
Figure 1 shows the relative importance of each attribute among the Australian-born group, RHCA country group and non-RHCA country group according to the findings of the mixed logit models. The mixed logit model for the DCE-Test survey indicated that participants across the three groups generally preferred a free or low-cost oral test with higher accuracy and a shorter window period (Table 2, Figure 3). Notably, men from the non-RHCA country group expressed a significantly stronger preference for a free test kit compared to men in Australian-born and RHCA country groups (β = 0.60, p < 0.01). The finding was also confirmed in the importance of attributes across the three groups. In the Australian-born and RHCA country groups, the attribute with the highest relative importance was the accuracy of the test, followed by the cost of the test, window period, speed of receiving HIV results, person conducting the test, and the way a test kit was obtained. However, the cost of the test was the most important attribute for the non-RHCA country group.
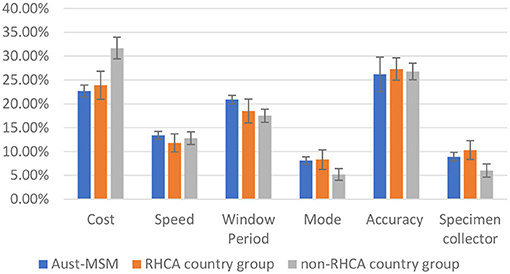
Figure 1. Relative importance of HIV testing attributes among Aust-MSM, RHCA country group, and non-RHCA country group.
Furthermore, although respondents across the three groups all preferred HIV self-testing over testing by health workers or peers, those from the non-RHCA country group were significantly less likely to choose self-testing than men in the other two groups (β = −0.26, p < 0.05).
Comparison Between Groups: Preferences for HIVST Distribution (DCE-Kits)
Table 3 show the mixed logit model results for the DCE-Kits survey, and Figure 2 shows the relative importance of each attribute. The most important attribute for all groups was the cost of the test, with a preference for low-cost self-testing kits. As in the results for the DCE-Test survey, men from the non-RHCA country group expressed a significantly stronger preference for a free self-test kit than those in Australian-born and RHCA country groups (β = 0.99, p < 0.01). The location where HIVST kits could be accessed was the second most important attribute. All groups preferred accessing kits through online distributors or off the shelf purchasing from pharmacies, less preferred getting kits from the staff of a community-based organisation, and getting kits from sex-on-premises-venues was least preferred. Men from the RHCA country group least preferred kits from medical clinics compared with men from non-RHCA country and Australian-born groups (β = −0.59, p < 0.05). On the contrary, the non-RHCA country group were more likely to access kits at a medical clinic, although the difference between this group and the Australian-born group was not significant. We also found differences between the groups in their preferences for usage instructions. For participants from the RHCA country group, accessing usage instruction through watching a video online was less preferred (β = −0.26, p < 0.05; Figure 3).

Figure 2. Relative importance of HIV-self testing distribution attributes among Aust-MSM, RHCA-eligible group, and RHCA-ineligible group.

Figure 3. Scales estimated of HIV testing and HIV self-testing attributes among Aust-MSM, RHCA country group and non-RHCA country group. (A) Scaled estimates of HIV testing preferences, by country of birth. (B) Scaled estimates of HIV self-testing distribution preferences, by country of birth. Note: Triangle denotes estimate is statistically significantly different from the mean preference of Aust-MSM.
Non-RHCA Group: Latent Class Analyses
Given the small number of respondents born in RHCA countries, latent class analysis models were only created for respondents from the non-RHCA country group to further understand the heterogeneous preferences for HIV testing services and HIVST distribution in this group. The goodness-of-fit for the latent class analysis models are presented and compared in Supplementary Figures 1, 2. In the DCE-Test survey, 23% of participants belonged to Class 1 (“accuracy-oriented”). This group preferred free, faster, and more accurate tests with a shorter window period. Men in the second class (“cost first,” 24%) were most influenced by the cost of the test and tended to be aged above 25 years or were born in Southeast Asia. The second class preferred a more accurate oral test with a shorter window period. Men in Class 3 (“self-tester,” 25%) were strongly influenced by who collected the specimen. They preferred testing themselves and were more likely to be aged above 25 years. Men in Class 4 (“timing is crucial,” 28%) were most sensitive towards the test window period. They preferred a free, faster, and more accurate test with a shorter window period (Table 4, Figure 4).

Table 4. Latent class analysis of HIV testing preferences of Australian migrant men who have sex with men born in non-RHCA countries (N = 298).

Figure 4. (A) Scaled estimates of HIV testing preferences among non-RHCA country group, by classes. (B) Scaled estimates of HIV self-testing distribution preferences among non-RHCA country group, by classes. Note: Triangle denotes mean p < 0.05
In the DCE-Kits survey, nearly half of the respondents belonged to Class 1 (“the cheaper the better,” 48%) and were most influenced by the cost of the test. They had no significant preference for where to access the test but least preferred a test kit with a small plain package. Men who had never been tested were more likely to belong to this class. The second class (“location is key,” 52%) preferred kits purchased off the shelf in a pharmacy, followed by online distribution and asking for a kit from a clinic's staff. They least preferred accessing kits from sex-on-premises venues (Table 5, Figure 4).

Table 5. Latent class analysis of HIVST distribution preferences of Australian migrant men who have sex with men born in non-RHCA countries (N = 121).
Discussion
In light of the increasing number of HIV diagnoses among migrant GBMSM in Australia, expanding HIV testing coverage among this group is critical to prevent onward transmission in undiagnosed men. Previous research emphasises the need to identify testing strategies that effectively target migrants who may face various obstacles to accessing HIV testing (52). Our study extends the limited literature on HIV testing for migrant populations by identifying heterogeneous preferences towards HIV testing services. To our knowledge, this is the first study that informs optimisation of access and uptake of HIV self-testing among migrants, especially those who cannot easily access Medicare-subsidised health services.
Unlike Australian-born GBMSM and men from RHCA countries, the cost of an HIV test was the most important attribute for GBMSM from non-RHCA countries. This finding is consistent with previous studies that find that promoting free or reduced-cost HIV services among undertested people who have constrained access to healthcare improves their engagement in the HIV cascade (23, 53–56). In Australia, free HIV testing services are available through state government-funded sexual health centres and community-based health services, regardless of their Medicare status (21, 22). Raising awareness among migrants of services that offer free HIV care in Australia may help improve testing uptake. But, it is worth noting that free or low-cost services are not uniformly available across the country and tend to be concentrated in capital cities and regional centres.
Furthermore, given the preferences of all GBMSM, an ideal HIV testing kit would be a saliva-based test that has high accuracy, a short window period and allows them to test by themselves. However, albeit with a general preference for oral-fluid tests, currently there is no oral-fluid rapid test for self-testing in Australia that is regulatory approved. In addition, the oral self-tests are likely to be less sensitive than blood-based self-tests and all self-tests for HIV, regardless of whether it is saliva or blood-based test, have reduced sensitivity during the window period (57, 58). To date, the Australian Therapeutic Goods Administration (TGA) has approved only one HIVST that uses a blood sample (59). The availability of blood-based HIVST may appeal to those who value accuracy the most but may not be the kit those who prefer an easy-to-use and painless test want to purchase. Our study showed that oral testing is the preferred option for migrants from non-RHCA countries, especially older men born in South East Asia countries. Consequently, to reach this group of migrants, it is important to have different types of test kits available in the market to give them more choice.
Consistent with previous Australian research, our findings suggest that HIVST is acceptable to migrant men (60). In addition, compared to Australian GBMSM, a higher proportion of migrant men had previously used HIVST, and a higher percentage (about a quarter of men) reported that they had delayed HIV testing because HIVST was not available. The Atomo HIV self-test kit was approved for marketing in Australia at the end of 2018. The initial regulation restricted the sale of HIVST kits to online purchasing or through approved health organisations (59). Our findings from latent class models show that over half of non-RHCA migrants most preferred to get the HIVST kits through pharmacies, which could be ideal for those who do not want to make a medical appointment or wait for long periods before testing. Our results support the recent decision of Australian TGA to allow HIVST to be sold in pharmacies at the end of 2021. Further research is needed to fully understand the impact of changes in the accessibility of HIVST on its uptake.
In addition, migrants from the non-RHCA country group also showed a stronger preference for accessing the kits in medical clinics. In our study, nearly two-thirds of the men from the non-RHCA country group were born in Southeast Asia and Sub-Saharan Africa. Studies have shown that migrants from Sub-Saharan Africa and Asia often prefer some level of assistance to perform HIVST when they conduct their first or second test, as support from healthcare workers may ensure the accuracy of results (61–63). The majority of men in our study had not previously used an HIVST kit, which may explain a preference for assisted HIVST to ensure correct use, at least for the first time. Further studies are needed to understand the factors affecting migrant GBMSM from Asia and Africa to identify and respond to their specific needs and better tailor HIVST interventions to this group.
The strength of this study is its exploration of HIV testing preferences among migrant GBMSM compared with Australian-born GBMSM. Until this study, the availability of preference data from this underserved population was limited, mainly due to the small sample size of previous DCE studies and a lack of targeted recruitment of migrant GBMSM (46). Another strength is that we used the established methodology of DCE to quantitatively measure preference heterogeneity (64). There are a few limitations that should be noted. First, all surveys were presented in English, which might affect the generalizability of the findings to non-English speaking migrants. Language barriers could potentially impede migrants' access to healthcare in facilities (65). Our findings that migrants from the non-RHCA group preferred accessing HIVST in a clinic may not apply to migrants who do not speak English. Second, we did not assess men's residency status in Australia but used the eligibility for RHCA as a proxy for access to subsidised healthcare in Australia, similar to previous studies (23). Some men from the non-RHCA country group may have access to subsided healthcare after receiving permanent residency in Australia. If this is so, our result may underestimate the role of Medicare in determining the preferences of migrant men towards the cost of HIV testing services. Conversely, it is also possible that men from the RHCA eligible group, who are young and healthy, may not be aware of the scheme and therefore not register for temporary access to Medicare. Future research on migrants could include visa status and Medicare status to better understand differences among this population group.
In summary, our study indicates that low-cost and high performing self-testing kits might improve HIV testing among migrant GBMSM in Australia, especially those from non-RHCA countries. Our study highlights differences in HIV self-testing kit distribution preferences that can inform future HIVST implementation projects targeting migrants. However, until now, no HIVST kit distribution strategies have thus far been tailor-designed to reach migrant GBMSM in Australia. Providing targeted testing services and supports for migrant men is essential to get newly arrived men tested shortly after arrival to prevent onward transmission in this population. These findings emphasise the importance of expanding the availability and ease of access to multiple types of HIVST kits in Australia to optimise the uptake of HIVST among migrant men.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
This study obtained ethical approval from the New South Wales South Eastern Sydney Local District Human Research Ethics Committee (17/147) and Alfred Health Human Research Ethics Committee (486/17). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
The study was designed by YZ, JO, RL, DS, KS, MJ, AH, KJ, BB, MH, JK, and RG. YZ, JO, VW, RG, and FT-P were involved in data analysis and interpretation. YZ and JO contributed to the writing of the manuscript. All authors contributed to the interpretation results, provided advice of the draft, and approved the final draft of submission.
Funding
This study was funded by the Australian National Health and Medical Research Council (Grant Nos. APP1104781 and 568971). EC and JO are supported by an Australian National Health and Medical Research Council (NHMRC) Emerging Leadership Investigator Grant (GNT1172873 and GNT1193955, respectively). CF was supported by an NHMRC Leadership Investigator Grant (GNT1172900).
Author Disclaimer
The contents in this article are those of the authors alone and do not necessarily reflect the view of the World Health Organisation.
Conflict of Interest
AH was employed by Thorne Harbour Health. KJ was employed by ACON.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all PUSH study participants and staff at the recruitment sites.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.839479/full#supplementary-material
References
2. Zimmerman C, Kiss L, Hossain M. Migration and health: a framework for 21st century policy-making. PLoS Med. (2011) 8:e1001034. doi: 10.1371/journal.pmed.1001034
3. Abubakar I, Aldridge RW, Devakumar D, Orcutt M, Burns R, Barreto ML, et al. The UCL-lancet commission on migration and health: the health of a world on the move. Lancet. (2018) 392:2606–54. doi: 10.1016/S0140-6736(18)32114-7
4. Australian Bureau of Statistics. Statistics on Australia's International Migration, Internal Migration (Interstate and Intrastate), and the Population by Country of Birth. Canberra, ACT (2021). Available online at: https://www.abs.gov.au/statistics/people/population/migration-australia/latest-release (accessed April 23, 2021).
5. Mullens AB, Kelly J, Debattista J, Phillips TM, Gu Z, Siggins F. Exploring HIV risks, testing and prevention among sub-Saharan African community members in Australia. Int J Equity Health. (2018) 17:62. doi: 10.1186/s12939-018-0772-6
6. Kirby Institute. Annual Report Sydney. Sydney, NSW: Australia: Kirby institute, UNSW Sydney (2018).
7. Alvarez-del Arco D, Monge S, Azcoaga A, Rio I, Hernando V, Gonzalez C, et al. HIV testing and counselling for migrant populations living in high-income countries: a systematic review. Eur J Public Health. (2013) 23:1039–45. doi: 10.1093/eurpub/cks130
8. Weine SM, Kashuba AB. Labor migration and HIV risk: a systematic review of the literature. AIDS Behav. (2012) 16:1605–21. doi: 10.1007/s10461-012-0183-4
9. Kirby Institute. HIV Knowledge, Risk Behaviour and Testing: A Community Survey in People From Culturally and Linguistically Diverse (CALD) Backgrounds in NSW, Australia. Sydney, NSW: The Kirby Institute, UNSW Sydney (2016).
10. Kickbusch I, Pelikan JM, Apfel F, Tsouros AD. Health Literacy: The Solid Facts. Copenhagen: WHO Regional Office for Europe (2013).
11. Hernando V, Alvarez-del Arco D, Alejos B, Monge S, Amato-Gauci AJ, Noori T, et al. HIV infection in migrant populations in the European union and European economic area in 2007-2012: an epidemic on the move. J Acquir Immune Defic Syndr. (2015) 70:204–11. doi: 10.1097/QAI.0000000000000717
12. Rice B, Elford J, Yin Z, Croxford S, Brown A, Delpech V. Trends in HIV diagnoses, HIV care, and uptake of antiretroviral therapy among heterosexual adults in England, Wales, and Northern Ireland. Sex Transm Dis. (2014) 41:257–65. doi: 10.1097/OLQ.0000000000000111
13. Xiridou M, van Veen M, Prins M, Coutinho R. How patterns of migration can influence the heterosexual transmission of HIV in The Netherlands. Sex Transm Infect. (2011) 87:289–91. doi: 10.1136/sti.2010.048512
14. Patel PG, Keen P, McManus H, Duck T, Callander D, Selvey C, et al. Increased targeted HIV testing and reduced undiagnosed HIV infections among gay and bisexual men. HIV Med. (2021) 22:605–16. doi: 10.1111/hiv.13102
15. Kirby Institute,. National Update on HIV, Viral Hepatitis Sexually Transmissible Infections in Australia. Kirb (2020). Available online at: https://data.kirby.unsw.edu.au/hiv (accessed May 27, 2021).
16. Gray RT, Wilson DP, Guy RJ, Stoove M, Hellard ME, Prestage GP, et al. Undiagnosed HIV infections among gay and bisexual men increasingly contribute to new infections in Australia. J Int AIDS Soc. (2018) 21:e25104. doi: 10.1002/jia2.25104
17. Telfer BSC, Bowden V, Charman M, Darnell J, Clarke K, Sheppeard V, et al. Predictors of Late Diagnosis for People Newly Diagnosed With HIV Infection in NSW. Sydney, NSW: Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (2017).
18. Asante A, Körner H, Kippax S. Understanding late HIV Diagnosis Among People From Culturally and Linguistically Diverse Backgrounds. Sydney, NSW: National Centre in HIV Social Research, The University of New South Wales (2009).
19. Blackshaw LCD, Chow EPF, Varma R, Healey L, Templeton DJ, Basu A, et al. Characteristics of recently arrived Asian men who have sex with men diagnosed with HIV through sexual health services in Melbourne and Sydney. Aust N Z J Public Health. (2019) 43:424–8. doi: 10.1111/1753-6405.12926
20. Australian Government,. Reciprocal Health Care Agreements. (2019). Available online at: https://www.servicesaustralia.gov.au/individuals/services/medicare/reciprocal-health-care-agreements (accessed October 1, 2021).
21. Walia AM, Fairley CK, Bradshaw CS, Chen MY, Chow EPF. Disparities in characteristics in accessing public Australian sexual health services between medicare-eligible and medicare-ineligible men who have sex with men. Aust N Z J Public Health. (2020) 44:363–8. doi: 10.1111/1753-6405.13029
22. NSW Government Health Department. Sexual Health Check-up. NSW Government Health Department. Available online at: https://www.health.nsw.gov.au/sexualhealth/Pages/sexual-health-check-up.aspx
23. Marukutira T, Gray RT, Douglass C, El-Hayek C, Moreira C, Asselin J, et al. Gaps in the HIV diagnosis and care cascade for migrants in Australia, 2013-2017: a cross-sectional study. PLoS Med. (2020) 17:e1003044. doi: 10.1371/journal.pmed.1003044
24. Marukutira T, Gunaratnam P, Douglass C, Jamil MS, McGregor S, Guy R, et al. Trends in late and advanced HIV diagnoses among migrants in Australia; implications for progress on fast-track targets: a retrospective observational study. Medicine. (2020) 99:e19289. doi: 10.1097/MD.0000000000019289
25. Herrmann S, Wardrop J, John M, Gaudieri S, Lucas M, Mallal S, et al. The impact of visa status and medicare eligibility on people diagnosed with HIV in Western Australia: a qualitative report. Sex Health. (2012) 9:407–13. doi: 10.1071/SH11181
26. MacLachlan JH, Cowie BC. Bridging the access gap: medicare ineligibility in people living with chronic hepatitis B. Intern Med J. (2019) 49:122–5. doi: 10.1111/imj.14175
27. Sibanda EL, McCoy SI. Secondary distribution of HIV self-tests improves coverage. Lancet HIV. (2020) 7:e732–3. doi: 10.1016/S2352-3018(20)30237-X
28. Bil JP, Prins M, Fakoya I, Volny-Anne A, Burns F, Zuure FR. Usage of purchased self-tests for HIV infections among migrants living in the UK, France and the Netherlands: a cross-sectional study. Sex Transm Infect. (2019) 95:629–32. doi: 10.1136/sextrans-2018-053583
29. Jamil MS, Prestage G, Fairley CK, Grulich AE, Smith KS, Chen M, et al. Effect of availability of HIV self-testing on HIV testing frequency in gay and bisexual men at high risk of infection (FORTH): a waiting-list randomised controlled trial. Lancet HIV. (2017) 4:e241–50. doi: 10.1016/S2352-3018(17)30023-1
30. Katz DA, Golden MR, Hughes JP, Farquhar C, Stekler JD. HIV self-testing increases HIV testing frequency in high-risk men who have sex with men: a randomized controlled trial. J Acquir Immune Defic Syndr. (2018) 78:505–12. doi: 10.1097/QAI.0000000000001709
31. MacGowan RJ, Chavez PR, Borkowf CB, Owen SM, Purcell DW, Mermin JH, et al. Effect of internet-distributed HIV self-tests on hiv diagnosis and behavioral outcomes in men who have sex with men: a randomized clinical trial. JAMA Intern Med. (2020) 180:117–25. doi: 10.1001/jamainternmed.2019.5222
32. Zhang C, Koniak-Griffin D, Qian HZ, Goldsamt LA, Wang H, Brecht ML, et al. Impact of providing free HIV self-testing kits on frequency of testing among men who have sex with men and their sexual partners in China: a randomized controlled trial. PLoS Med. (2020) 17:e1003365. doi: 10.1371/journal.pmed.1003365
33. Hoagland B, Torres TS, Bezerra DRB, Benedetti M, Pimenta C, Veloso VG, et al. High acceptability of PrEP teleconsultation and HIV self-testing among PrEP users during the COVID-19 pandemic in Brazil. Braz J Infect Dis. (2021) 25:101037. doi: 10.1016/j.bjid.2020.11.002
34. Jiang H, Xie Y, Xiong Y, Zhou Y, Lin K, Yan Y, et al. HIV self-testing partially filled the HIV testing gap among men who have sex with men in China during the COVID-19 pandemic: results from an online survey. J Int AIDS Soc. (2021) 24:e25737. doi: 10.1002/jia2.25737
35. Maatouk I, Nakib ME, Assi M, Farah P, Makso B, Nakib CE, et al. Community-led HIV self-testing for men who have sex with men in Lebanon: lessons learned and impact of COVID-19. Health Res Policy Syst. (2021) 19 (Suppl. 1):50. doi: 10.1186/s12961-021-00709-x
36. Ontario HIV Treatment Network. HIV Self-Testing in High-Income Settings: Acceptability, Potential Benefits and Harms, Issues Related to Linkage to Care, Interventions to Increase HIV Self-Testing. Toronto, ON: Ontario HIV Treatment Network (2019).
37. Xiao W, Yan L, Chen L, Fu G, Yang H, Yang C, et al. Sexual network distribution of HIV self-testing kits: findings from the process evaluation of an intervention for men who have sex with men in China. PLoS ONE. (2020) 15:e0232094. doi: 10.1371/journal.pone.0232094
38. Eshun-Wilson I, Jamil MS, W TC, Glidden DV, Cheryl J, Noelle T, et al. A systematic review and network meta-analyses to assess the effectiveness of HIV self-testing distribution strategies. Clin Infect Dis. (2021) 73:e1018–28. doi: 10.1093/cid/ciab029
39. World Health Organization. How to Conduct a Discrete Choice Experiment for Health Workforce Recruitment and Retention in Remote and Rural Areas: A User Guide With Case Studies. Geneva: WHO (2012).
40. McFadden D. Conditional logit analysis of qualitative choice behavior. Front Econometr. (1973) 24:151–8.
41. Determann D, Gyrd-Hansen D, de Wit GA, de Bekker-Grob EW, Steyerberg EW, Lambooij MS, et al. Designing unforced choice experiments to inform health care decision making: implications of using opt-out, neither, or status quo alternatives in discrete choice experiments. Med Decis Making. (2019) 39:681–92. doi: 10.1177/0272989X19862275
42. Pan SW, Durvasula M, Ong JJ, Liu C, Tang W, Fu H, et al. No place like home? Disentangling preferences for HIV testing locations and services among men who have sex with men in China. AIDS Behav. (2019) 23:847–59. doi: 10.1007/s10461-018-2366-0
43. Sibanda EL, d'Elbee M, Maringwa G, Ruhode N, Tumushime M, Madanhire C, et al. Applying user preferences to optimize the contribution of HIV self-testing to reaching the “first 90” target of UNAIDS fast-track strategy: results from discrete choice experiments in Zimbabwe. J Int AIDS Soc. (2019) 22 (Suppl. 1):e25245. doi: 10.1002/jia2.25245
44. Ostermann J, Njau B, Mtuy T, Brown DS, Muhlbacher A, Thielman N. One size does not fit all: HIV testing preferences differ among high-risk groups in Northern Tanzania. AIDS Care. (2015) 27:595–603. doi: 10.1080/09540121.2014.998612
45. Ostermann J, Njau B, Brown DS, Muhlbacher A, Thielman N. Heterogeneous HIV testing preferences in an urban setting in Tanzania: results from a discrete choice experiment. PLoS ONE. (2014) 9:e92100. doi: 10.1371/journal.pone.0092100
46. Sharma M, Ong JJ, Celum C, Terris-Prestholt F. Heterogeneity in individual preferences for HIV testing: a systematic literature review of discrete choice experiments. EClinicalMedicine. (2020) 29–30:100653. doi: 10.1016/j.eclinm.2020.100653
47. Ong JJ, De Abreu Lourenco R, Street D, Smith K, Jamil MS, Terris-Prestholt F, et al. The preferred qualities of human immunodeficiency virus testing and self-testing among men who have sex with men: a discrete choice experiment. Value Health. (2020) 23:870–9. doi: 10.1016/j.jval.2020.04.1826
48. Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CG, Prior T, Marshall DA, Cunningham C, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. (2016) 19:300–15. doi: 10.1016/j.jval.2016.04.004
49. Hole AR. Modelling heterogeneity in patients' preferences for the attributes of a general practitioner appointment. J Health Econ. (2008) 27:1078–94. doi: 10.1016/j.jhealeco.2007.11.006
50. Sawleshwarkar S, Harrison C, Britt H, Mindel A. Determinants of HIV testing. Sex Transm Infect. (2011) 87:426–32. doi: 10.1136/sti.2011.049601
51. Chan C, Broady TR, Bavinton BR, Mao L, Prestage GP, Holt M. Assessing the HIV prevention needs of young gay and bisexual men in the PrEP Era: an analysis of trends in Australian behavioural surveillance, 2014-2018. AIDS Behav. (2020) 24:2382–6. doi: 10.1007/s10461-020-02797-2
52. Blondell SJ, Kitter B, Griffin MP, Durham J. Barriers and facilitators to HIV testing in migrants in high-income countries: a systematic review. AIDS Behav. (2015) 19:2012–24. doi: 10.1007/s10461-015-1095-x
53. MacGibbon J, Lea T, Ellard J, Murphy D, Kolstee J, Power C, et al. Access to subsidized health care affects HIV Pre-exposure prophylaxis (PrEP) uptake among gay and bisexual men in Australia: results of national surveys 2013-2019. J Acquir Immune Defic Syndr. (2021) 86:430–5. doi: 10.1097/QAI.0000000000002572
54. Gray C, Lobo R, Narciso L, Oudih E, Gunaratnam P, Thorpe R, et al. Why I can't, won't or don't test for HIV: insights from Australian migrants born in Sub-Saharan Africa, Southeast Asia and Northeast Asia. Int J Environ Res Public Health. (2019) 16:1034. doi: 10.3390/ijerph16061034
55. Mutch AJ, Lui CW, Dean J, Mao L, Lemoire J, Debattista J, et al. Increasing HIV testing among hard-to-reach groups: examination of RAPID, a community-based testing service in Queensland, Australia. BMC Health Serv Res. (2017) 17:310. doi: 10.1186/s12913-017-2249-5
56. Gray RT, Watson J, Cogle AJ, Smith DE, Hoy JF, Bastian LA, et al. Funding antiretroviral treatment for HIV-positive temporary residents in Australia prevents transmission and is inexpensive. Sex Health. (2018) 15:13–9. doi: 10.1071/SH16237
57. Rosenberg NE, Kamanga G, Phiri S, Nsona D, Pettifor A, Rutstein SE, et al. Detection of acute HIV infection: a field evaluation of the determine(R) HIV-1/2 Ag/Ab combo test. J Infect Dis. (2012) 205:528–34. doi: 10.1093/infdis/jir789
58. Pavie J, Rachline A, Loze B, Niedbalski L, Delaugerre C, Laforgerie E, et al. Sensitivity of five rapid HIV tests on oral fluid or finger-stick whole blood: a real-time comparison in a healthcare setting. PLoS ONE. (2010) 5:e11581. doi: 10.1371/journal.pone.0011581
59. Atomo. HIV 1&2 Tests. Atomo (2019). Available online at: http://atomodiagnostics.com/hiv-tests/#HIV_Self_Test
60. Cogle A, Petoumenos K. Medicare Ineligible PLHIV in Australia: An Analysis of New Data With Recommendations for Systemic Improvements. Sydney, NSW: National Association of People With HIV Australia (2019).
61. Wulandari LPL, Kaldor J, Guy R. Uptake and acceptability of assisted and unassisted HIV self-testing among men who purchase sex in brothels in Indonesia: a pilot intervention study. BMC Public Health. (2020) 20:730. doi: 10.1186/s12889-020-08812-4
62. Blondell SJ, Debattista J, Griffin MP, Durham J. I think they might just go to the doctor': qualitatively examining the (un)acceptability of newer HIV testing approaches among Vietnamese-born migrants in greater-Brisbane, Queensland, Australia. Sex Health. (2021) 18:50–7. doi: 10.1071/SH20064
63. Wong HT, Prankumar SK, Prihaswan P, Kao SC, Chen T, Wark T, et al. Notes From the Field: Challenges, Concerns and Opportunities in Relationship Building and Health Promotion Among Gay Asian International Students in Australia. Sydney, NSW: HIV Australia (2019).
64. Zhou M, Thayer WM, Bridges JFP. Using latent class analysis to model preference heterogeneity in health: a systematic review. Pharmacoeconomics. (2018) 36:175–87. doi: 10.1007/s40273-017-0575-4
Keywords: discrete choice experiment (DCE), migrants, men who have sex with men—MSM, HIV testing, HIV self-testing (HIVST), health preference research
Citation: Zhang Y, Wiseman V, Applegate TL, Lourenco RDA, Street DJ, Smith K, Jamil MS, Terris-Prestholt F, Fairley CK, McNulty A, Hynes A, Johnson K, Chow EPF, Bavinton BR, Grulich A, Stoove M, Holt M, Kaldor J, Guy R and Ong JJ (2022) Preferences for HIV Testing Services and HIV Self-Testing Distribution Among Migrant Gay, Bisexual, and Other Men Who Have Sex With Men in Australia. Front. Med. 9:839479. doi: 10.3389/fmed.2022.839479
Received: 20 December 2021; Accepted: 16 March 2022;
Published: 19 April 2022.
Edited by:
Segundo Ramos León Sandoval, San Juan Bautista Private University, PeruReviewed by:
Rayner Kay Jin Tan, University of North Carolina Project-China, ChinaSusan Graham, University of Washington, United States
Copyright © 2022 Zhang, Wiseman, Applegate, Lourenco, Street, Smith, Jamil, Terris-Prestholt, Fairley, McNulty, Hynes, Johnson, Chow, Bavinton, Grulich, Stoove, Holt, Kaldor, Guy and Ong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ye Zhang, eXpoYW5nQGtpcmJ5LnVuc3cuZWR1LmF1
†These authors have contributed equally to this work and share senior authorship
 Ye Zhang
Ye Zhang Virginia Wiseman1,2
Virginia Wiseman1,2 Muhammad S. Jamil
Muhammad S. Jamil Anna McNulty
Anna McNulty Eric P. F. Chow
Eric P. F. Chow Benjamin R. Bavinton
Benjamin R. Bavinton Martin Holt
Martin Holt John Kaldor
John Kaldor