- 1The Cornea Institute, KVC Campus, LV Prasad Eye Institute, Vijayawada, India
- 2The Cornea Institute, KAR Campus, LV Prasad Eye Institute, Hyderabad, India
- 3Prof. Brien Holden Eye Research Centre (BHERC), LV Prasad Eye Institute, Hyderabad, Telangana, India
Limbal stem cell deficiency (LSCD) can cause significant corneal vascularization and scarring and often results in serious visual morbidity. An early and accurate diagnosis can help prevent the same with a timely and appropriate intervention. This review aims to provide an understanding of the different diagnostic tools and presents an algorithmic approach to the management based on a comprehensive clinical examination. Although the diagnosis of LSCD usually relies on the clinical findings, they can be subjective and non-specific. In such cases, using an investigative modality offers an objective method of confirming the diagnosis. Several diagnostic tools have been described in literature, each having its own advantages and limitations. Impression cytology and in vivo confocal microscopy (IVCM) aid in the diagnosis of LSCD by detecting the presence of goblet cells. With immunohistochemistry, impression cytology can help in confirming the corneal or conjunctival source of epithelium. Both IVCM and anterior segment optical coherence tomography can help supplement the diagnosis of LSCD by characterizing the corneal and limbal epithelial changes. Once the diagnosis is established, one of various surgical techniques can be adopted for the treatment of LSCD. These surgeries aim to provide a new source of corneal epithelial stem cells and help in restoring the stability of the ocular surface. The choice of procedure depends on several factors including the involvement of the ocular adnexa, presence of systemic co-morbidities, status of the fellow eye and the comfort level of the surgeon. In LSCD with wet ocular surfaces, autologous and allogeneic limbal stem cell transplantation is preferred in unilateral and bilateral cases, respectively. Another approach in bilateral LSCD with wet ocular surfaces is the use of an autologous stem cell source of a different epithelial lineage, like oral or nasal mucosa. In eyes with bilateral LSCD with significant adnexal issues, a keratoprosthesis is the only viable option. This review provides an overview on the diagnosis and treatment of LSCD, which will help the clinician choose the best option amongst all the therapeutic modalities currently available and gives a clinical perspective on customizing the treatment for each individual case.
Introduction
The corneal epithelium is essential for the maintenance of the anatomic integrity and physiological functioning of the transparent cornea. The maintenance of the corneal surface is ensured by the constant turnover of the corneal epithelium from the limbal epithelial stem cells (LESC) (1, 2). These LESC straddle the junction between the cornea and the conjunctiva and reside in the basal epithelial layer of the limbus. The microenvironment surrounding the LESC within the palisades of Vogt, is responsible for ensuring the viability and efficacy of the stem cells. The LESC prevent the migration of the conjunctival epithelial cells over the corneal surface and in the presence of a dysfunction of the LESC themselves or the surrounding niche, there occurs conjunctivalization of the cornea.
Limbal stem cell deficiency (LSCD) can stem from numerous etiologies, resulting in serious visual morbidity (3, 4). And so, early diagnosis of this entity is essential in order to institute the appropriate therapy in a timely manner. Also, the need for diagnosing LSCD is even more essential when a keratoplasty is planned as the graft is unlikely to fair well if the LSCD is not corrected in advance. Although the diagnosis of LSCD is still primarily a clinical one, there are several diseases that can mimic its clinical picture (5, 6). In such scenarios, the clinician can choose from an array of diagnostic tests aimed at detecting LSCD. Similarly, numerous therapeutic options are available in management of LSCD and the choice of one intervention over the other depends upon the severity of ocular and adnexal involvement. This review aims to provide an understanding of the various tools in the diagnostic armamentarium of LSCD in the context of their advantages and limitations. It also endeavors to crystallize the clinical approach to a case of LSCD based on the laterality, severity, and resources available.
Etiology
Pathologies that affect the LESC or their supporting niche can cause LSCD (3). These can be classified as per Table 1. Understanding the underlying primary disease process often provides an added perspective into the management of LSCD. Several conditions such as chemical or thermal ocular burns, Stevens-Johnson syndrome (SJS), etc. are one-time insults and usually the treatment approaches are limited to the sequalae that ensue (7). On the other hand, in autoimmune disorders such as mucous membrane pemphigoid (MMP), there is a constant disruption of the systemic and ocular milieu occurring via inflammatory mediators (8). In such cases, addressing the LSCD in isolation invariably has very poor outcomes and so it must be done in conjunction with the management of the systemic pathology. Furthermore, in case of congenital causes of LSCD, treatment options include specific gene targeted therapy which is possible only if a particular type of limbal stem cell transplant (LSCT) is performed. Therefore, it is essential for the treating physician to know the primary disease process in order to make an informed decision and choose the appropriate therapeutic modality on a case-to-case basis.
Clinical Features
Symptoms
Patients with LSCD present with non-specific symptoms such as ocular redness, discomfort, pain, watering, and photophobia. When the disease is severe enough to involve the visual axis, the complaints extend to blurring or decreased vision (2, 7).
Signs
The diagnosis of LSCD is primarily clinical but needs to be confirmed by one or more objective methods. The clinical findings vary depending upon the severity of the disease. In early cases of LSCD, there may be focal areas of the corneal epithelium which take up the characteristic stippled staining pattern (7). There is loss of clarity within the epithelium, creating a lackluster appearance. The limbal palisades of Vogt, which are usually most easily visible superiorly and inferiorly, may be difficult to discern or may become flattened (Figure 1). With the progression of the disease there occurs conjunctivalization of the cornea and superficial corneal vascularization (Figure 2) (7, 8). Due to patches of irregular epithelial thinning, a whorl pattern is noted which is better picked up as areas of pooling up of fluorescein(Figure 3). These zones also exhibit late staining (7, 8). A sharp demarcation between the abnormal and normal corneal epithelium may also be seen in cases of sectoral involvement (7–9). Epithelial instability is a hallmark of the disease process which manifests as repeated breakdown of the epithelium and in advanced cases this can progress to form a persistent epithelial defect (PED) (7). Recurrent episodes of PEDs can affect the underlying stroma leading to scarring or sterile melts in non-resolving cases (7).
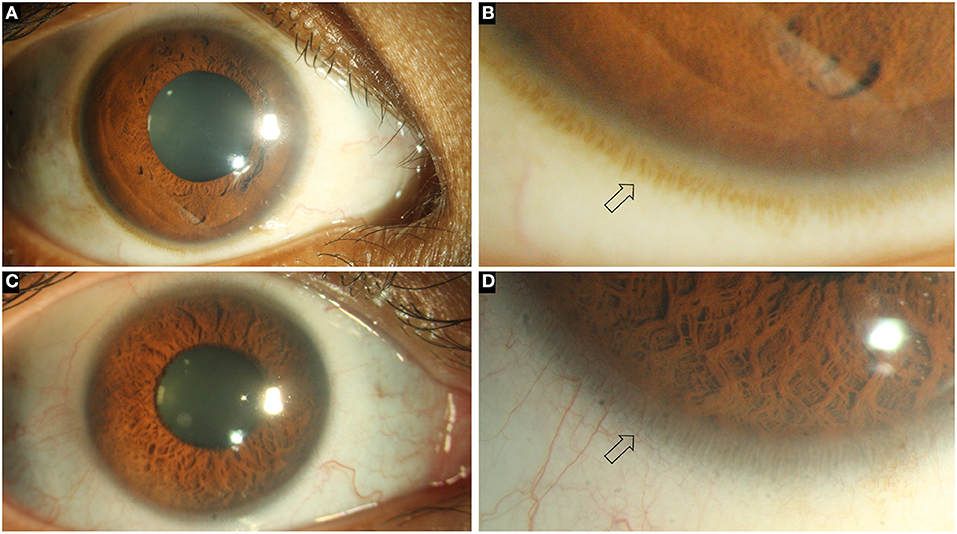
Figure 1. Collage of images depicting the normal ocular surface and limbus (arrows) in pigmented (A, B) and hypopigmented (C, D) eyes.
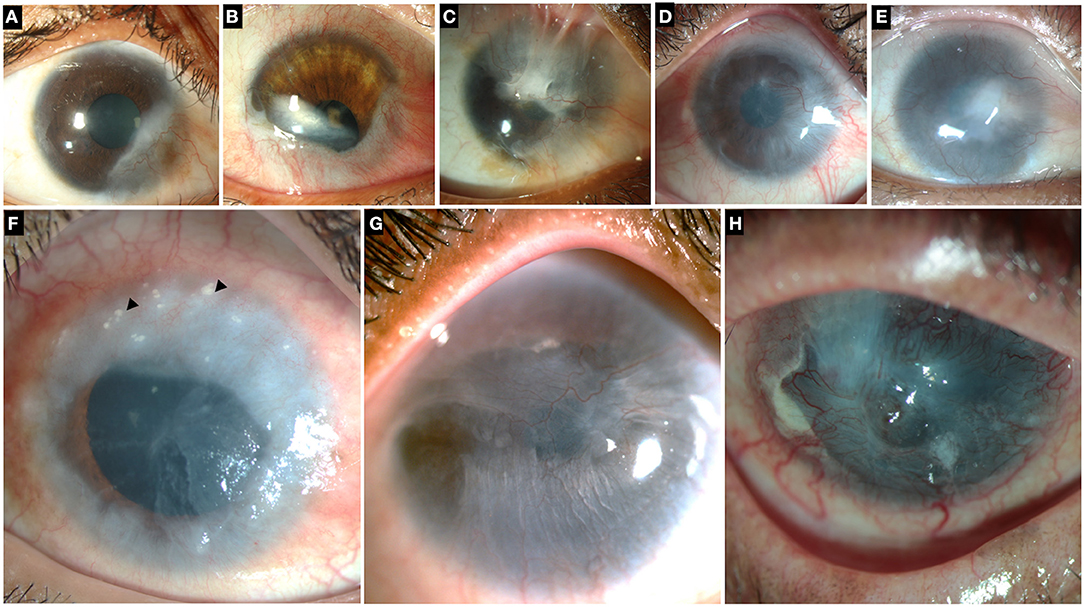
Figure 2. Collage of images illustrating different grades and etiologies of limbal stem cell deficiency (LSCD). Top row: LSCD due to chemical injury which is partial and sparing the visual axis (A), involving the visual axis (B,C). (D,E) Total LSCD in chemical injury. (F) LSCD in chronic vernal keratoconjunctivitis. Superior cornea shows Horner-Trantas dots (black arrowheads). (G) LSCD in Epidermolysis Bullosa (H) LSCD in mucous membrane pemphigoid.
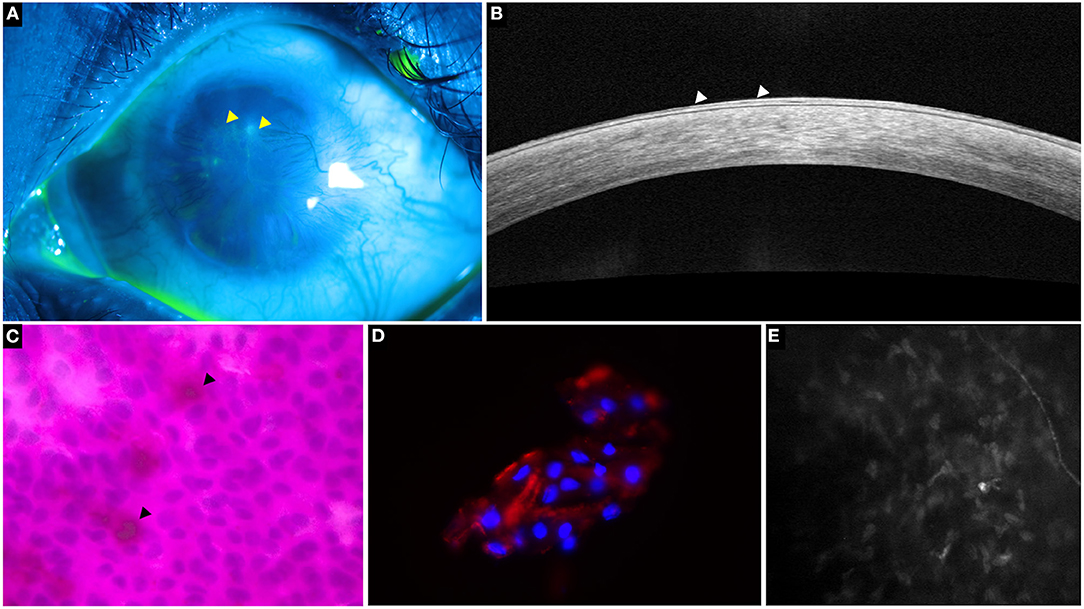
Figure 3. A representative collage of various diagnostic modalities in limbal stem cell deficiency (LSCD). (A) Fluorescein-stained image showing characteristic stippled staining (yellow arrowheads). (B) Optical coherence tomography line scan showing hyperreflective epithelium indicative of LSCD (white arrowheads). (C) Impression cytology depicting Periodic acid-Schiff positive goblet cells (black arrowheads) and CK19 positive cells on immunohistochemistry (D,E) in vivo confocal microscopy showing decreased sub-basal nerve density.
Diagnostic Investigations
In cases of severe ocular burns or advanced cicatricial conjunctivitis following SJS, the diagnosis of LSCD can be straightforward. However, in several cases the clinical presentation is subtle and establishing the diagnosis may be challenging. In such cases the ancillary tests mentioned below help supplementing the diagnosis. In addition to confirming the diagnosis, these tests may facilitate the quantification of the disease and provide an understanding of its progression. They also help to confirm the epithelial phenotype following a stem cell transplant and in monitoring the postoperative recovery (10–13).
Impression Cytology
This test involves sampling of the superficial epithelial cells of the ocular surface and subjecting them to histopathological and immunohistochemistry tests. The sample can be obtained from the cornea or the conjunctiva and is usually acquired using a nitrocellulose or cellulose acetate filter paper (14). Although the test typically acquires the superficial corneal and conjunctival cells, repeated sampling in a particular area will facilitate access to the deeper layers as well (14). Following a standardized sampling technique is recommended as this will affect the quantity and quality of tissue obtained (7, 9, 14). Ensuring that the ocular surface is not too wet and that the pore size of the paper is adequate to collect the epithelial cells will also help in improving the yield (9, 15).
Histopathology
The cytology specimen procured undergoes histopathological processing with various stains such as hematoxylin and eosin (H&E), Giemsa, Periodic acid-Schiff, etc (14). These stains detect the presence of goblets cells which indicates the invasion of conjunctival epithelial cells over the surface of the cornea (14). Although the detection of goblet cells is considered the sine qua non of LSCD (Figure 3), its absence does not imply a healthy limbus. Also, there may be a decrease in the concentration of goblet cells due to the underlying disease process itself as is the case in SJS (16, 17). As mentioned earlier the sensitivity of the test is largely dependent on the sampling procedure. And so, assessment of the epithelial cells which are also concurrently sampled can enhance the detection rate of LSCD. However, the differentiation of corneal from conjunctival epithelial cells is not possible with the routine stains used and requires immunohistochemistry.
Immunohistochemistry
Several markers have been investigated and of these cytokeratin 12 has been found to be specific for the mature corneal epithelium (7, 18). Although cytokeratin 3 was also considered to be cornea specific, recent studies have found this marker in the conjunctiva also (19, 20). Cytokeratin 7, 13 and 19 are markers which are specifically expressed in conjunctival epithelial cells while mucin 5AC(MUC5AC) is used for the detection of goblet cells (Figure 3) (18, 20–22). However, negative MUC5AC staining has been noted despite positive conjunctival marker staining, signifying the low sensitivity of this marker (18). This fallacy has been subverted with the use of reverse transcriptase polymerase chain reaction test for the detection of MUC5AC which increases the test sensitivity to 98% (23).
Obtaining normal corneal cells through impression cytology is challenging because of the inherent adherence of the cells to each other and the underlying basement membrane. This is in contrast to the conjunctival cells which freely desquamate and so, the presence of an abundance of cellularity can itself indicate the presence of conjunctival cells (18, 20) Since conjunctivalization of the cornea is considered a hallmark of LSCD, the confirmation of conjunctival epithelial cells from a corneal cytology specimen has been deemed sufficient to diagnosis LSCD (Figure 3) (20). The subsequent presence of the cytokeratin 12 marker is used to quantify the disease which is considered mild or partial if the corneal marker can still be detected (20). The degree of the fluorescence exhibited by these markers has also been used to quantify the severity of the disease (19, 24).
In-Vivo Confocal Microscopy (IVCM)
IVCM is a non-invasive tool that provides an in vivo picture of the microstructures within the cornea. Of the various parameters measured by the device, presence of goblet cells, the basal epithelial measurements of the cornea and limbus along with the changes of the sub-basal nerve plexus are used in the diagnosis of LSCD (Figure 3).
Goblet Cells
The presence of goblet cells in a corneal IVCM scan is confirmatory of the diagnosis of LSCD. The detection rate of goblet cells with IVCM closely correlates with that of impression cytology (25). However, as mentioned previously, several factors may affect the detection of goblet cells in a case of LSCD and with an IVCM this is further confounded by the small area that is scanned. Also, the described morphology of a goblet cell is variable with descriptions of both a hypo and hyper-reflective cytoplasm (26–28). Thus, although the detection of goblet cells is feasible with an IVCM, the test has low sensitivity.
Corneal and Limbal Epithelial Changes
A decrease in basal cell density (BCD) with an increase in the size of the cells is noted in patients with LSCD (29–31). This decrease corresponds with the severity of the disease and in advanced cases, there is significant alteration in the morphology of the cells with an increased number of visible hyperreflective cell nuclei (31, 32). Deng et al. found that a BCD value of <7930 cells/mm2 for basal cell density diagnosed LSCD with a 95.5% sensitivity and 100% specificity (31). In cases of partial LSCD, the epithelium in the clinically normal areas maintains the normal pattern on IVCM although there is often an increase in the number of dendritic cells in the underlying stroma (25, 33, 34). A clear demarcation is noted at the junction between the corneal and conjunctival epithelial cells as the two have very distinct morphological features on IVCM (33). Corneal basal cells have a dark cytoplasm with well-defined borders and are much smaller than the conjunctival cells. Intraepithelial cystic lesions with surrounding goblet cells have also been described in cases of LSCD (33). Overall thinning of the epithelium is seen in LSCD (35). A similar pattern of change is noted in the limbal epithelium as well with a decreased BCD which correlates with disease severity (34–36). In cases of partial LSCD, the clinically unaffected areas also exhibit the same changes indicating a pre-clinical method of detection of LSCD (34, 36).
Corneal Nerves Changes
A progressive decrease in the density of the sub-basal plexus of nerves is noted with increasing severity of the disease until a complete nerve drop out occurs (Figure 3) (29, 34, 37). Additionally, several other changes have also been reported which include decreased branch length, increased angulation of branching, increased tortuosity, etc (31, 37). A cut off for sub-basal nerve density of 53 nerves/mm2 resulted in an 87% sensitivity and 91.7% specificity for the diagnosis of LSCD (31). Caro-Magdaleno et al. found that the sub basal nerve density had an inverse association with conjunctivalization and a value of <17,215 μm/mm2 diagnosed LSCD with a 95.5% sensitivity and specificity of 90.6% (38).
Anterior Segment Optical Coherence Tomography (AS-OCT)
AS-OCT is a non-invasive imaging tool that has low operator dependence and yields repeatable results. It has been used to augment the diagnosis of LSCD with its corneal and limbal epithelial measurements. Additionally, with the help of image processing software, the reflectivity from these measurements have been quantified. The role of the angiography feature of OCT for detecting LSCD has also been investigated.
Epithelial Changes
Similar to the IVCM findings, a decrease in both the corneal and limbal epithelial thickness has been observed with AS-OCT in eyes with LSCD (Figure 3) (30, 39). Although epithelial thinning is not specific to LSCD and is seen in disease entities such as keratoconus, dry eye, etc.; the degree to which the thinning occurs is different. A 20–30% thinning has been reported in eyes with LSCD, while in other disorders the thinning is <10% (35, 39, 40). Liang et al. proposed a new parameter measured as a mean of the central epithelial thickness and thickness measured at two points, 1 mm on either side of the central thickness (39). Values <46.6 μm for this parameter were considered diagnostic for LSCD with a sensitivity and specificity of 61.7% and 100% respectively (39).
In addition to measuring the limbal epithelium, the OCT can also provide an in vivo visualization of the palisades of Vogt. This is possible even in eyes where the palisades are not visualized clinically (41). Although the IVCM can also image the palisades, the image procurement takes time and requires a skilled and experienced operator whereas the process is much simpler in case of an OCT. Also, as seen with IVCM, in eyes with partial LSCD the thinning of the limbal epithelium is similar in the affected and unaffected areas (39). This epithelial thickness correlates with the presence of the palisades with significant thinning manifesting when the palisades are absent (42). Volumetric scans of the limbus provide a three dimensional image which can further help quantify the severity of LSCD (43, 44).
Scans from an AS-OCT can be subjected to image processing and thus the epithelial and stromal reflectivity is derived. Varma et al. found the epithelial reflectivity value to be a better indicator of the presence of LSCD than stromal reflectivity (45). They also studied the ratio of these two reflectivities (ES ratio) and proposed a cut off 1.29 to be diagnostic of LSCD with good sensitivity and specificity. Furthermore, a reversal of this ratio following SLET was noted by Kate et al. (12). However the values at the end of one year follow up did not reach the ES ratio seen in normal eyes (12).
OCT Angiography (OCT-A)
The use of the angiography feature of the OCT has been explored in quantifying the changes seen in the limbal vasculature as well as in corneal neovascularization (Figure 4) (46, 47). A progressive increase in the density of vascularization and its extent into the cornea has been reported with increasing severity of LSCD (48). Also, OCT-A has been used to differentiate true LSCD from its mimickers which also have corneal vascularization. A significant reduction in vascular density is noted after segmentation of the superficial layers in non-LSCD cases as in these eyes the vessels are usually located within the deep stromal layers (45). When this superficial vascular density values are >0.38, the diagnosis of LSCD can be confirmed with a sensitivity and specificity of 97.9% and 73.8% respectively (45).
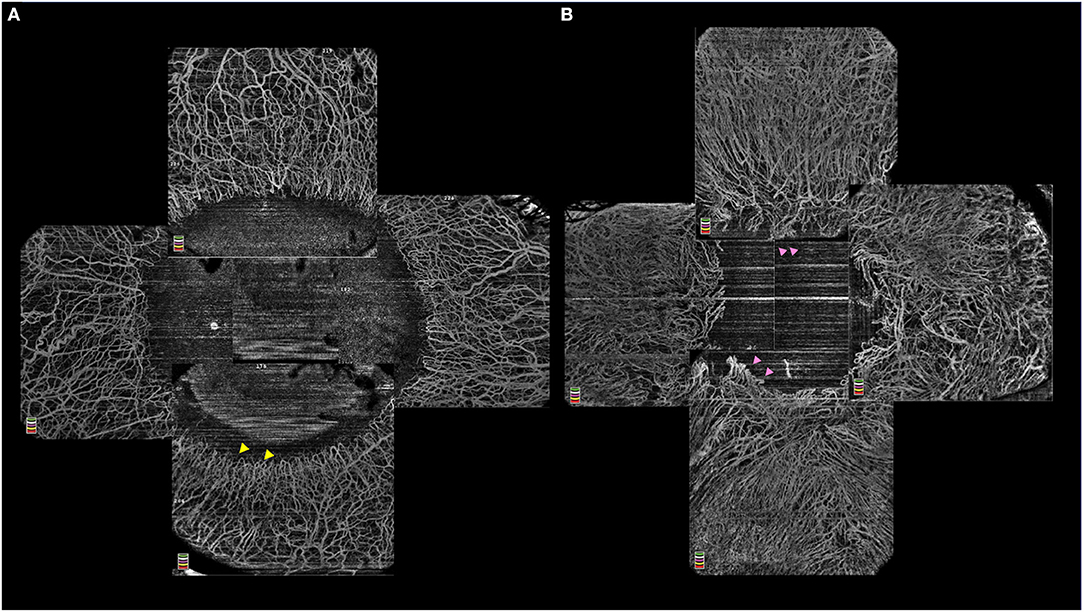
Figure 4. (A) Optical coherence tomography-angiography (OCT-A) illustrating a normal limbal vasculature with hairpin looped limbal vessels (yellow arrowheads) and surrounding normal perilimbal conjunctival and episcleral vessels. (B) OCT-A in in limbal stem cell deficiency with vascular invasion of the peripheral corneal and distortion of the annular ring of hairpin looped limbal vessels (pink arrowheads).
Classification
Several classifications have been proposed to grade the severity of LSCD (1, 2, 31, 49). These are based on corneal epithelial thinning, fluorescein staining patterns, presence of neovascularization, fibrovascular pannus, etc. The grading proposed by the Limbal Stem Cell Working Group has divided the corneal involvement into three groups depending on involvement of the central 5 mm of the cornea and these groups have further been subcategorized based on the percentage of limbal involvement (7). These gradations which are based on corneal findings help understand disease severity and assess progression. This is particularly helpful for uniform and standardized documentation for research and monitoring progression or response to therapy. However, the classification does not include adnexal involvement, and this is vital in the decision-making process for the management of these eyes. Hence, classification systems that incorporate the eyelid and conjunctival changes in addition to the corneal ones may better help in delivering appropriate therapy based on the composite disease severity (50).
Management
The management of LSCD includes several surgical and non-surgical options and for each patient the treatment plan has to be tailored to suit the involved eye. However, LSCD rarely occurs in isolation and so the concurrent management of the systemic and ocular comorbidities is vital and often has to precede the surgical management of the disease. This includes systemic immunosuppression in cases of MMP, ocular anti-inflammatory therapy in cases of vernal keratoconjunctivitis, SJS, etc. A component of aqueous deficiency dry eye (ADDE) is usually present in most of these eyes and addressing the same with preservative free lubricants, punctal occlusion, etc. will aid in stabilizing the tear film prior to the surgical intervention.
Several of the comorbidities present with LSCD also require surgical intervention and the sequence of these surgeries often determines the final functional outcome. Ideally, lid and other adnexal issues are addressed prior to the stem cell deficiency. In the presence of significant corneal scarring there is often need for a keratoplasty for visual rehabilitation (Figure 5). Although LSCT contributes to stromal remodeling and eventually a decrease in the density of the scar is noted, the degree to which this happens may vary. And so, several of these cases ultimately require a partial or full thickness corneal transplantation to restore an optically clear visual axis.
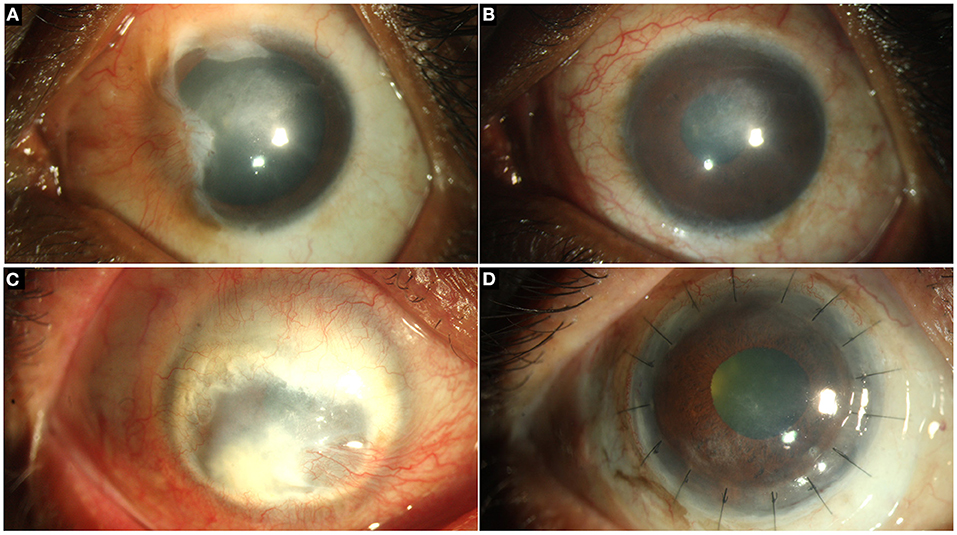
Figure 5. (A) Partial limbal stem cell deficiency (LSCD) following chemical injury managed with conjunctival limbal autograft (CLAu). (B) Restoration of a stable ocular surface is noted. (C) Total LSCD with leucomatous corneal scarring. (D) Reestablishment of an optically clear visual axis and a stable corneal epithelium with deep anterior lamellar keratoplasty and CLAu.
The management of LSCD can be surgical or non-surgical depending upon the severity of damage to the LESC and the underlying pathology. Based on the clinical presentation, an algorithmic approach can be considered in most of the cases of LSCD (Figure 6).
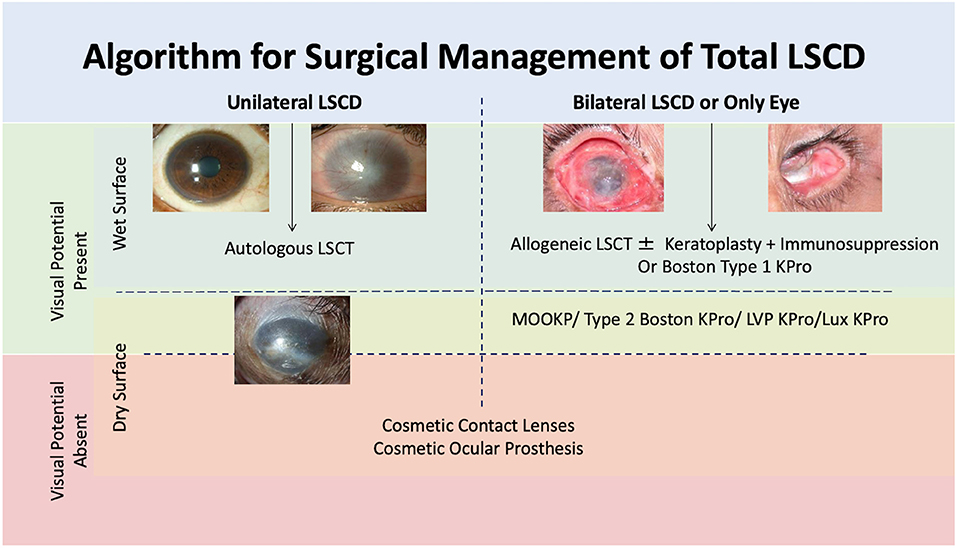
Figure 6. Algorithmic approach of management of limbal stem cell deficiency (LSCD). LSCT: limbal stem cell transplantation, KPro: keratoprosthesis, MOOKP: modified osteo-odontokeratoprosthesis.
Partial LSCD
In cases of partial LSCD, the decision of surgical intervention is dictated by the involvement of the visual axis (Figures 2A-C). If the visual axis is affected, a surgical therapy is required is most cases. However, if the axis is clear, the patient can be followed up at regular intervals to determine if the disease is progressive or stationary. In case of the former, again the eye will require a surgical procedure while in case of the latter the same can be deferred.
Non-surgical Intervention
Eyes with partial LSCD with sparing of the visual axis and documented non-progression of the disease can be observed with regular follow ups. These cases can be visually rehabilitated with glasses or with rigid contact lenses when significant irregular astigmatism is present. Scleral lenses with large vaults are particularly beneficial in such eyes as they provide a fluid layer which addresses the dry eye component in addition to improving the visual acuity (51–53). Lenses which vault over the limbus are preferred as mechanical compression and trauma to the limbal epithelium is prevented (53). Optimizing the fit of the lenses in eyes with LSCD is vital as the resultant hypoxia in eyes with a compromised fit can exacerbate the severity of the LSCD (54).
Surgical Intervention
When partial LSCD is progressive or involving the visual axis, a surgical procedure is usually carried out to correct the same. The choice of procedure depends upon the involvement of the fellow eye. In unilateral cases an autologous LSCT is preferred where the LESC can be harvested from the contralateral eye or from the uninvolved areas of the same eye. In a comparative series with 70 patients, the outcome in eyes where the LESC were harvested from the same eye was similar to the outcome of eyes with stem cells from the contralateral eye (55). In bilateral cases also an autologous LSCT can be considered if the involved areas are limited to 3-4 clock hours in both eyes (56). Several studies have described the use of an amniotic membrane (AM) alone in the treatment of partial LSCD (57–62). Most of these reports have combined a superficial keratectomy to remove the conjunctival epithelium prior to placing the AM. Although the initial corneal epithelialization rates are good, the ability of the AM to maintain a stable epithelial surface in the long run is poor (58–61, 63). And so, an AM can be used for the temporary restoration of the ocular surface, until a LSCT can be performed. The use of only conjunctival autografts (CAG) has also been described in the treatment of partial LSCD. Shanbhag et al. found a better anatomical success rate with CAG when compared to LSCT in eyes with partial unilateral LSCD (64). Following the treatment of the LSCD, these patients may eventually require rigid contact lenses for visual rehabilitation.
Total LSCD
In eyes with total LSCD, the initial step to determine the therapeutic approach would be to assess the presence of visual potential (Figure 6). In eyes with no visual potential, no further intervention is carried out unless there is a need to restore cosmesis in which case a contact lens trial is given, or an ocular prosthesis is implanted. In the presence of visual potential, the status of the fellow eye determines the next course of treatment.
Unilateral Total LSCD
In unilateral cases, if the surrounding adnexa is relatively uninvolved and the ocular surface is wet with a fairly clear corneal stroma, an autologous LSCT is performed. If there are significant cicatricial changes of the conjunctiva, a combined or staged procedure with a conjunctival autograft (in unilateral cases) or mucous membrane graft (in bilateral cases) can be planned (65). Similarly if a lamellar or penetrating keratoplasty (LK or PK) is planned for visual rehabilitation, it can carried out as a one or two step procedure (66–69). Although the grafts maintain clarity in the initial postoperative period after a combined procedure, the rate of rejection is usually higher in these cases and so a staged procedure is preferred (67–70). Whenever possible a LK is favored over a PK as the former lacks a transplanted endothelium and so is associated with lower rates of rejection.
Bilateral Total LSCD
The treatment algorithm for bilateral cases is similar to that of unilateral cases (71). If no dry eye is detected and the conjunctiva and lids are relatively uninvolved, then an allogeneic LSCT is the chosen procedure. In the presence of significant symblephara with adnexal pathologies the choice of LSCT over keratoprosthesis (KPro) depends upon the surgeon's preference. The former will require multiple procedures to correct the co-morbid pathologies before the LSCD is addressed. Systemic immunosuppression will also be necessary in view of the allogeneic nature of the transplant. A keratoprosthesis will circumvent these issues and offers a one-step procedure with early visual rehabilitation (72). Nevertheless, this technique is associated with several serious sight threatening complications such as glaucoma, retinal detachment, implant extrusion, endophthalmitis, etc (73–75). Thus, KPros are usually reserved for eyes with end stage corneal pathologies or in eyes where prior LSCTs have failed (76).
There are different types of KPros and the choice of one KPro over the other is determined by the presence or absence of ADDE. Table 2 lists different types of KPros that have been utilized in the management of LSCD. If the surface is wet, a Boston KPro type 1 or Aurolab KPro (auroKPro) is carried out and if the eye has ADDE, then a Boston KPro type 2, LV Prasad KPro (LVP KPro) or modified osteo-odontokeratoprosthesis (MOOKP) is performed (Figure 7). The Boston KPro type 1 is the most commonly used prosthesis and has an optical cylinder with a skirt of donor cornea (Figures 7A,B). It has good visual outcomes and retention rates especially in eyes with non-autoimmune underlying diseases (74, 75, 77, 92–94) Since the cost of the device is a major inhibitory factor for its use, the auroKPro, its cheaper alternative is a more viable option in low resource settings. Both prosthesis have similar outcomes in terms of visual function, retention rates, and other secondary complications (95, 96).
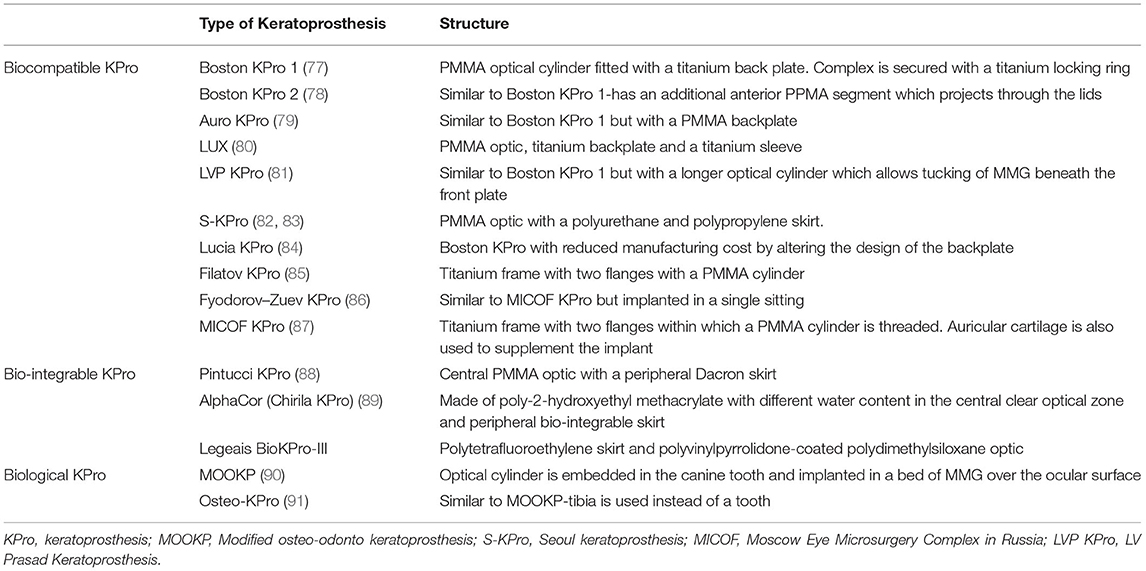
Table 2. Brief description of various KPros employed in the management of limbal stem cell deficiency.
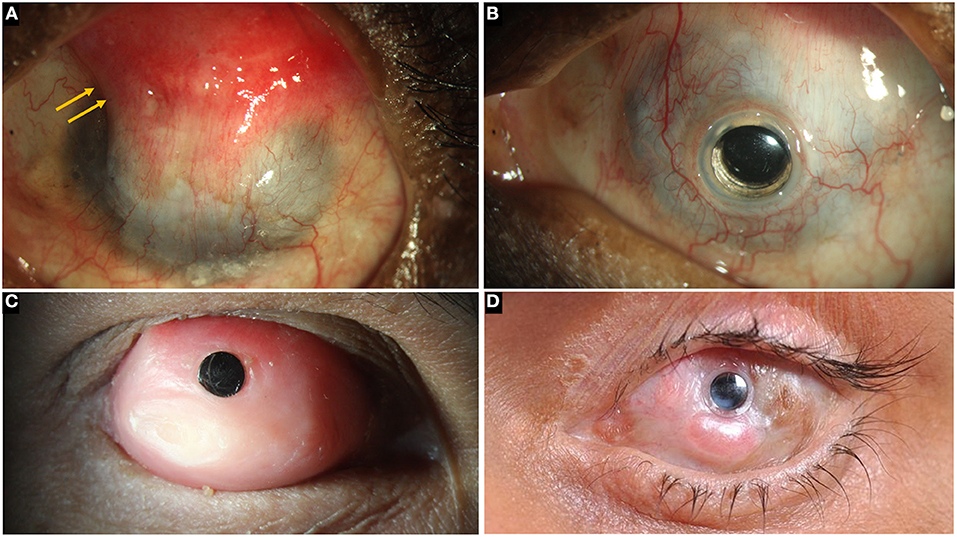
Figure 7. (A) Left eye in a case of bilateral total limbal stem cell deficiency (LSCD) with a wet surface due to Stevens-Johnson syndrome (SJS). A superior conjunctival hooding (yellow arrows) was carried out previously for microbial keratitis with a corneal perforation. (B) A Boston keratoprosthesis in the same eye. (C) Modified osteo-odontokeratoprosthesis in an eye with total LSCD and a dry ocular surface. (D) LVP KPro in an eye with SJS.
In case of dry eyes or dermalised ocular surfaces with lid changes, both Boston KPro type 2 and the MOOKP have good functional and anatomical outcomes (90, 97–99). The former is similar to its type 1 counterpart and has a longer cylinder which is exteriorized through lid while the latter has a cylinder embedded in an osteo-dental lamina (Figure 7C). However, the surgical procedure for both devices is cumbersome, time consuming and has a steep learning curve. The LVP KPro, which is similar to the Boston KPro with a longer optical cylinder, is implanted as a two staged procedure under a mucous membrane graft used to reconstruct the ocular surface (Figure 7D) (78, 100). Its anatomical outcomes are better than those of Boston KPro type 2 but they are not superior than those of MOOKP (78). Table 3 compares the outcomes of the most commonly used KPros in LSCD.
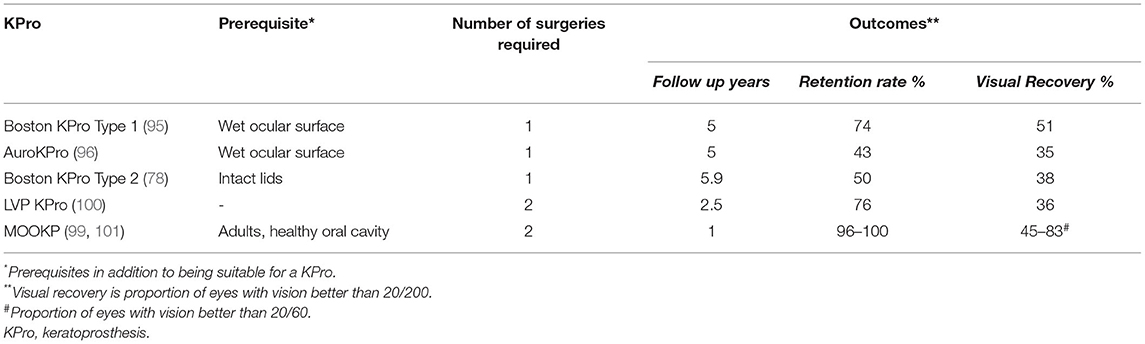
Table 3. Comparison of the most commonly used keratoprosthesis in the management of limbal stem cell deficiency.
Transplantation of cultivated oral mucosal epithelium (COMET) is another alternative in eyes with bilateral LSCD where labial or buccal epithelial cells are cultured on an AM and transplanted over the cornea. Studies have reported a stable ocular surface following the procedure however there is a higher risk of persistent epithelial defects, corneal neovascularization and graft rejection when compared to LSCT (81, 102–104). And so, an allogeneic LSCT is considered superior to and is favored over COMET despite the latter being an autologous transplant with no requirement for systemic immunosuppression (104). In a series comparing the outcomes of cell based therapies (CLET, CLAL, COMET) vs. Boston KPro type 1 in cases of bilateral LSCD without ADDE, the KPro group was found to have the best functional outcome at the end of five years (68, 71). However, a recent meta-analysis revealed that in patients undergoing LSCT, nearly 61% maintained a vision of at least 20/200 at end of 2.5 years which is similar to the 64% of patients who had the same vision in the KPro group (105).
Various modifications of the COMET procedure have been proposed which alter the type of carriers used to transfer the cultivated cells. These include the AM, fibrin glue and temperature sensitive polymers. In case of the latter, the polymer is stable at 37°C, however when the temperature drops to 30°C, the cultivated epithelial sheet detaches spontaneously (106, 107). This is in contrast to traditional methods where a carrier or enzymatic detachment is required. Furthermore, biomaterial free sheets have also been used, wherein the cultivated sheet is directly transplanted from the culture plate onto the eye without a carrier for the cells (108, 109). Establishment of a well epithelialized surface have been reported with the use of the same and these outcomes were found to be better than those of COMET with the use of AM as a substrate (108, 109).
As an alternative to cultivation of oral mucosal epithelial cells, which requires the necessary infrastructure, direct transplantation of the oral mucosa has also been described for the management of LSCD (110, 111). The graft is transplanted directly over the limbal area and can re-establish a stable surface and cause regression of neovascularization (110, 111). An additional benefit that the mucosal graft has over conventional LSCT is that adnexal pathologies such as lid margin keratinization or symblephara can be addressed with the same harvested tissue. As the procedure is autologous, no systemic immunosuppression is required. A similar approach has also been reported with the use of nasal mucosal grafts which primarily aim to replenish the goblet cells in the ocular surface (112).
Technique of LSCT
Types
There are two chief types of LSCT: allogeneic and autologous. These can be further divided into different types based on the anatomical source of the graft which includes conjunctival limbal auto or allograft (CLAu and CLAL), allogeneic keratolimbal allograft (KLAL) or pure limbal tissues as in cases of auto and allogeneic cultivated or simple limbal epithelial transplants (CLET and SLET). In cases of allogeneic LSCT, the donor can be a cadaveric or a living related donor. In pure limbal transplants, once the limbal lenticule is harvested it can be directly transplanted as in SLET where the proliferation of epithelial cells occurs in vivo over the corneal surface. Alternatively, the biopsied tissue can be cultivated in vitro and then transplanted as a sheet of epithelium as in case of CLET.
Choice of Procedure
As mentioned previously autologous procedures are performed in unilateral cases while allogeneic transplants are reserved for bilateral LSCD (Figure 8). The major difference between the two lies in the need for long term systemic immunosuppression for allogeneic LSCT. A combination of corticosteroids and steroid sparing agents are usually given initially, and the patients are then maintained only on the steroid sparing immunosuppressive agent (113, 114) Most of these medications are both expensive and associated with a side effect profile necessitating regular systemic monitoring (113, 114).
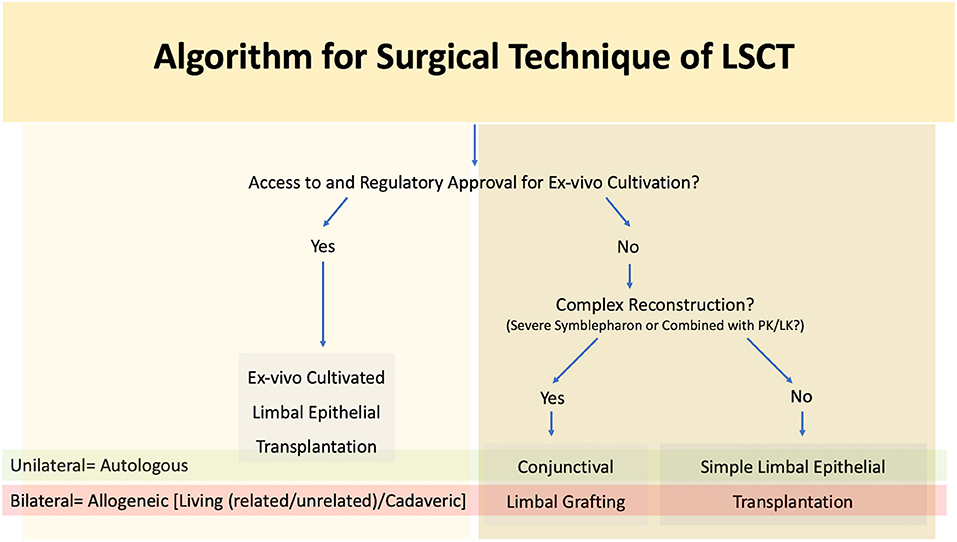
Figure 8. Algorithm for surgical technique of limbal stem cell transplantation (LSCT) PK, penetrating keratoplasty; LK, lamellar keratoplasty.
The choice of procedure is often determined by the extent of involvement of the surrounding adnexa. A limbal transplant (SLET/CLET for autologous cases, SLET/CLET/KLAL for allogeneic cases) is preferred for LSCD in wet eyes without significant adnexal involvement (Figure 9). Access to a laboratory facility with regulatory approval is required for the practice of cultivated stem cells. CLAu or CLAL is preferred in cases where concurrent correction of cicatricial conjunctival changes is also required as seen in eyes with significant symblephara adjacent to a partial LSCD (Figure 5). The graft can be harvested from the same eye or fellow eye, depending upon the amount of healthy residual limbus. In the traditional CLAu, a large limbal graft is usually harvested (4-6 clock hours) which can result in an iatrogenic LSCD. To avoid this complication, a mini-CLAu with only 1-2 clock hours of limbal tissue is a viable substitute (66, 115). Alternatively conjunctival tissue can be harvested separately as a CAG along with a pure limbal transplant (CLET/SLET). This combination is usually adopted in eyes with total LSCD and symblephara. Tables 4, 5 detail the relative advantages and disadvantages of each of the LSCT procedures.
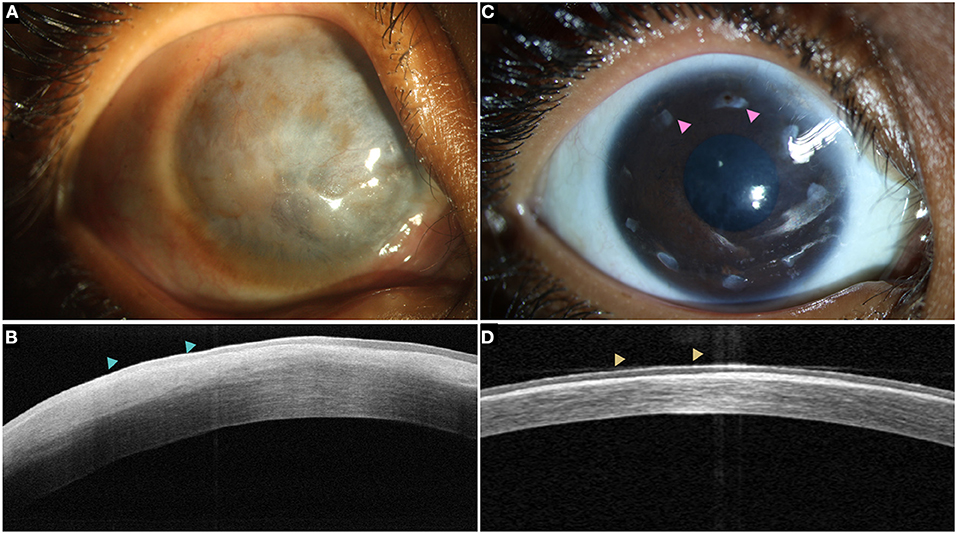
Figure 9. (A) Total LSCD with a thick pannus in a case of chronic vernal keratoconjunctivitis with hyperreflective epithelium (blue arrowheads) on the optical coherence tomography (OCT) line scan (B). (C) A stable ocular surface is observed following allogeneic simple epithelial limbal transplantation. The intact limbal tissues are also visible (pink arrowheads). (D) Restoration of epithelium with a normal reflectivity is noted on the OCT scan (yellow arrowheads).
Comparison of Outcomes
In a systematic review of 1023 eyes, SLET and CLAu were found to have better outcomes than CLET in cases of unilateral LSCD (116). A similar result was seen in a recent meta-analysis where SLET was found to have better functional outcomes when compared to CLET (117). The overall performance of autologous procedures has been deemed to be better than that of allogeneic procedures with the latter having a failure rate of up to 40% (105). The former group of procedures also have a higher percentage of patients with a 2 line improvement in visual acuity following surgery (105).
Ganger et al. found CLET and KLAL to have similar anatomical outcomes, but KLAL fared better than CLET in terms of functional outcomes (117). The cumulative success of KLAL from a systematic review was found to be 63% with 69% of cases having vision better than 20/200 (118). A recent series on allogeneic SLET reported a success rate of 83% and more than 60% of the cases had an improvement in vision which was >20/60 (119). And so, in the context of the expensive nature of CLET with its need for a laboratory set up, KLAL and allogeneic SLET are perhaps the more feasible options in cases of bilateral LSCD. However more studies are required on the long-term outcomes of allogeneic SLET to determine its benefits over other allogeneic procedures. Table 6 compares the outcomes of different modalities of stem cell transplants.
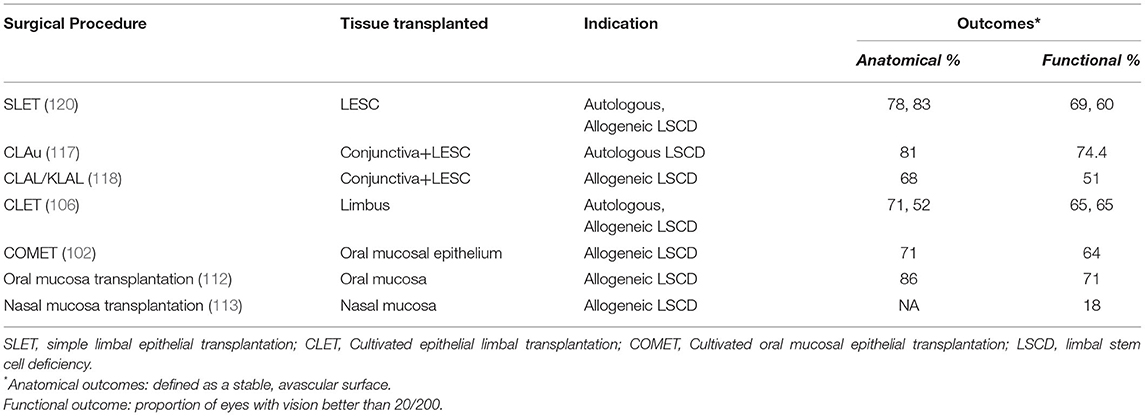
Table 6. Comparison of indications and outcomes of different surgical modalities of management of limbal stem cell deficiency.
Recent Advances
The search for new therapies for LSCD is always ongoing because of the need for treatment modalities that do not have the risk of rejection, require immunosuppression, etc. And the epitome of such endeavors would be to arrive at a medical therapy for LSCD. One such intervention was identified serendipitously during the treatment of patients with ocular surface neoplasia with interferon α-2b and retinoic acid (120). These cases had partial LSCD which responded to the topical medications. The rationale proposed for the same was that retinoic acid improves corneal wound healing and promotes proliferation of transient amplifying cells while interferon α-2b mediates the healing through its anti-inflammatory function, specifically on macrophages (120).
Another novel technique in the treatment of total LSCD is the amnion-assisted conjunctival epithelial redirection (ACER) which involves the placement of an amniotic membrane over the cornea and limbal explants. The edges of the membrane are tucked under the free edges of the recessed conjunctiva and as a result of this, the conjunctival cells migrate over the membrane (121). This allows the limbal explants under the membrane to proliferate over the surface of the cornea unhindered. Establishment of a stable ocular surface has been reported following this procedure. The use of a modified version of this procedure has also been described for partial LSCD with good outcomes (122).
Novel prosthetic devices such as the Lux and CorNeat keratoprosthesis are being developed as alternatives to LSCT. The former is similar to a traditional Boston KPro with a polymethylmethacrylate cylinder and a titanium backplate (123). This prothesis does not rely on the presence of intact lids which is required for Boston KPro type 2 and has better cosmesis than a MOOKP. Thus the Lux KPro is a viable option for eyes with dry ocular surfaces and LSCD, with good functional vision and retention rates (123). The long term outcomes with this device are awaited. The CorNeat is a true corneal prosthetic device and is structurally different from other KPros. This synthetic cornea has a central PMMA optic and a surrounding porous skirt made of polyurethane fibers (80). The skirt is implanted beneath the conjunctiva where it integrates with the surrounding tissue. Animal models with the CorNeat KPro have shown good retention of the implant while results of human trials are awaited (80).
The use of stems cells obtained from sources other than the LESC is another interesting avenue being explored in the management of LSCD. Of these, limbal mesenchymal stem cells have been best studied and have an established role in corneal wound healing, scar remodeling and angiogenesis (124–127). Its role as a therapeutic option for LSCD is being investigated with a recent clinical trial suggesting that they are as efficacious as CLET in restoring a stable ocular surface (128). Other stem cells that are being studied include those from hair follicles, dental pulp, embryonic stem cells, etc (129–133). Their exact utility and efficacy in LSCD is yet to be determined.
Summary
This review presents an overview of the different diagnostic tests and management modalities in LSCD in order to provide a clinical perspective which will help the physician determine the best course of therapy in cases with LSCD. An in-depth write-up on the pathophysiology of stem cell deficiency is beyond the scope of this review. The diagnosis of limbal stem cell deficiency is often made based on clinical features but can be supplemented by several investigative tools especially when faced with challenging case scenarios. Although both impression cytology and IVCM can confirm the diagnosis of LSCD the expense of the equipment involved, and the skilled personnel required often restrict their use. AS-OCT is a more commonly available device and has several measurable parameters which can be used in the diagnosis of LSCD. However more studies are required to determine the exact diagnostic cut offs. The interpretation of the results of any of these tests must be made in the context of the clinical picture to arrive at the correct diagnosis. Additionally, these investigative modalities have also been used to monitor the response to LSCT and to confirm the restoration of a corneal epithelial phenotype (10, 134–136). Using a combination of clinical and one or more diagnostic tests, a standardized method of validating the outcomes of LSCT can be established.
A comprehensive approach is usually required for the management of LSCD with simultaneous treatment of comorbid ocular and systemic pathologies. Autologous LSCT for unilateral LSCD and allogeneic LSCT for bilateral cases, in the absence of dry eye, are the preferred modalities of therapy which render a stable ocular surface and good visual outcomes. A KPro is favored in more complex cases and provides a rapid visual recovery. The exact choice of procedure is ultimately dependent upon the status of the adnexa, the resources available and the expertise of the surgeon.
Author Contributions
AK contributed to the collection of resources, original draft preparation, and revisions of the manuscript. SB contributed to the conceptualization, methodology, supervision, revision, and editing of the manuscript. Both authors contributed to the article and approved the submitted version.
Funding
Hyderabad Eye Research Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dua HS, Joseph A, Shanmuganathan VA, Jones RE. Stem cell differentiation and the effects of deficiency. Eye (Lond). (2003) 17:877–85. doi: 10.1038/sj.eye.6700573
2. Sacchetti M, Lambiase A, Cortes M, Sgrulletta R, Bonini S, Merlo D, et al. Clinical and cytological findings in limbal stem cell deficiency. Graefes Arch Clin Exp Ophthalmol. (2005) 243:870–6. doi: 10.1007/s00417-005-1159-0
3. Vazirani J, Nair D, Shanbhag S, Wurity S, Ranjan A, Sangwan V. Limbal stem cell deficiency-demography and underlying causes. Am J Ophthalmol. (2018) 188:99–103. doi: 10.1016/j.ajo.2018.01.020
4. Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. (2010) 363:147–55. doi: 10.1056/NEJMoa0905955
5. Le Q, Samson CM, Deng SX. A case of corneal neovascularization misdiagnosed as total limbal stem cell deficiency. Cornea. (2018) 37:1067–70. doi: 10.1097/ICO.0000000000001631
6. Chan E, Le Q, Codriansky A, Hong J, Xu J, Deng SX. Existence of normal limbal epithelium in eyes with clinical signs of total limbal stem cell deficiency. Cornea. (2016) 35:1483–7. doi: 10.1097/ICO.0000000000000914
7. Deng SX, Borderie V, Chan CC, Dana R, Figueiredo FC, Gomes JAP, et al. Global consensus on the definition, classification, diagnosis and staging of limbal stem cell deficiency. Cornea. (2019) 38:364–75. doi: 10.1097/ICO.0000000000001820
8. Taurone S, Spoletini M, Ralli M, Gobbi P, Artico M, Imre L, et al. Ocular mucous membrane pemphigoid: a review. Immunol Res. (2019) 67:280–9. doi: 10.1007/s12026-019-09087-7
9. Sejpal K, Bakhtiari P, Deng SX. Presentation, diagnosis and management of limbal stem cell deficiency. Middle East Afr J Ophthalmol. (2013) 20:5–10. doi: 10.4103/0974-9233.106381
10. Le Q, Xu J, Deng SX. The diagnosis of limbal stem cell deficiency. Ocul Surf. (2018) 16:58–69. doi: 10.1016/j.jtos.2017.11.002
11. Prabhasawat P, Chirapapaisan C, Ngowyutagon P, Ekpo P, Tangpagasit W, Lekhanont K, et al. Efficacy and outcome of simple limbal epithelial transplantation for limbal stem cell deficiency verified by epithelial phenotypes integrated with clinical evaluation. Ocul Surf. (2021) 22:27–37. doi: 10.1016/j.jtos.2021.06.012
12. Pauklin M, Kakkassery V, Steuhl K-P, Meller D. Expression of membrane-associated mucins in limbal stem cell deficiency and after transplantation of cultivated limbal epithelium. Curr Eye Res. (2009) 34:221–30. doi: 10.1080/02713680802699408
13. Kate A, Mudgil T, Basu S. Longitudinal changes in corneal epithelial thickness and reflectivity following simple limbal epithelial transplantation: an optical coherence tomography-based study. Curr Eye Res. (2022) 47:336–42. doi: 10.1080/02713683.2021.1988985
14. Pauklin M, Steuhl K-P, Meller D. Characterization of the corneal surface in limbal stem cell deficiency and after transplantation of cultivated limbal epithelium. Ophthalmology. (2009) 116:1048–56. doi: 10.1016/j.ophtha.2009.01.005
15. Singh R, Joseph A, Umapathy T, Tint NL, Dua HS. Impression cytology of the ocular surface. Br J Ophthalmol. (2005) 89:1655–9. doi: 10.1136/bjo.2005.073916
16. Vadrevu VL, Fullard RJ. Enhancements to the conjunctival impression cytology technique and examples of applications in a clinico-biochemical study of dry eye. CLAO J. (1994) 20:59–63.
17. Kinoshita S, Kiorpes TC, Friend J, Thoft RA. Goblet cell density in ocular surface disease. a better indicator than tear mucin. Arch Ophthalmol. (1983) 101:1284–7. doi: 10.1001/archopht.1983.01040020286025
18. Rivas L, Oroza MA, Perez-Esteban A. Murube-del-Castillo J. Morphological changes in ocular surface in dry eyes and other disorders by impression cytology. Graefes Arch Clin Exp Ophthalmol. (1992) 230:329–34. doi: 10.1007/BF00165940
19. Poli M, Burillon C, Auxenfans C, Rovere M-R, Damour O. Immunocytochemical diagnosis of limbal stem cell deficiency: comparative analysis of current corneal and conjunctival biomarkers. Cornea. (2015) 34:817–23. doi: 10.1097/ICO.0000000000000457
20. Barbaro V, Ferrari S, Fasolo A, Pedrotti E, Marchini G, Sbabo A, et al. Evaluation of ocular surface disorders: a new diagnostic tool based on impression cytology and confocal laser scanning microscopy. Br J Ophthalmol. (2010) 94:926–32. doi: 10.1136/bjo.2009.164152
21. Poli M, Janin H, Justin V, Auxenfans C, Burillon C, Damour O. Keratin 13 immunostaining in corneal impression cytology for the diagnosis of limbal stem cell deficiency. Invest Ophthalmol Vis Sci. (2011) 52:9411–5. doi: 10.1167/iovs.10-7049
22. Ramirez-Miranda A, Nakatsu MN, Zarei-Ghanavati S, Nguyen CV, Deng SX. Keratin 13 is a more specific marker of conjunctival epithelium than keratin 19. Mol Vis. (2011) 17:1652–61.
23. Liang Q, Le Q, Wang L, Cordova D, Baclagon E, Garrido SG, et al. Cytokeratin 13 is a new biomarker for the diagnosis of limbal stem cell deficiency. Cornea. (in press) doi: 10.1097/ICO.0000000000002903
24. García I, Etxebarria J, Merayo-Lloves J, Torras J., Boto-de-Los-Bueis A, Díaz-Valle D, et al. Novel molecular diagnostic system of limbal stem cell deficiency based on MUC5AC transcript detection in corneal epithelium by PCR-reverse dot blot. Invest Ophthalmol Vis Sci. (2013) 54:5643–52. doi: 10.1167/iovs.13-11933
25. Pisella PJ, Brignole F, Debbasch C, Lozato PA, Creuzot-Garcher C, Bara J, et al. Flow cytometric analysis of conjunctival epithelium in ocular rosacea and keratoconjunctivitis sicca. Ophthalmology. (2000) 107:1841–9. doi: 10.1016/s0161-6420(00)00347-x
26. Araújo AL de, Ricardo JR da S, Sakai VN, Barros JN de, Gomes JÁP. Impression cytology and in vivo confocal microscopy in corneas with total limbal stem cell deficiency. Arq Bras Oftalmol. (2013) 76:305–8. doi: 10.1590/s0004-27492013000500011
27. Efron N, Al-Dossari M, Pritchard N. In vivo confocal microscopy of the bulbar conjunctiva. Clin Exp Ophthalmol. (2009) 37:335–44. doi: 10.1111/j.1442-9071.2009.02065.x
28. Hong J, Zhu W, Zhuang H, Xu J, Sun X, Le Q, et al. In vivo confocal microscopy of conjunctival goblet cells in patients with Sjogren's syndrome dry eye. Br J Ophthalmol. (2010) 94:1454–8. doi: 10.1136/bjo.2009.161059
29. Zhu W, Hong J, Zheng T, Le Q, Xu J, Sun X. Age-Related changes of human conjunctiva on in vivo confocal microscopy. Br J Ophthalmol. (2010) 94:1448–53. doi: 10.1136/bjo.2008.155820
30. Bhattacharya P, Edwards K, Harkin D, Schmid KL. Central corneal basal cell density and nerve parameters in ocular surface disease and limbal stem cell deficiency: a review and meta-analysis. Br J Ophthalmol. (2020) 104:1633–9. doi: 10.1136/bjophthalmol-2019-315231
31. Banayan N, Georgeon C, Grieve K, Ghoubay D, Baudouin F, Borderie V. In vivo confocal microscopy and optical coherence tomography as innovative tools for the diagnosis of limbal stem cell deficiency. J Fr Ophtalmol. (2018) 41:e395–406. doi: 10.1016/j.jfo.2018.09.003
32. Deng SX, Sejpal KD, Tang Q, Aldave AJ, Lee OL Yu F. Characterization of limbal stem cell deficiency by in vivo laser scanning confocal microscopy: a microstructural approach. Arch Ophthalmol. (2012) 130:440–5. doi: 10.1001/archophthalmol.2011.378
33. Nubile M, Lanzini M, Miri A, Pocobelli A, Calienno R, Curcio C, et al. In vivo confocal microscopy in diagnosis of limbal stem cell deficiency. Am J Ophthalmol. (2013) 155:220–32. doi: 10.1016/j.ajo.2012.08.017
34. Miri A, Alomar T, Nubile M, Al-Aqaba M, Lanzini M, Fares U, et al. In vivo confocal microscopic findings in patients with limbal stem cell deficiency. Br J Ophthalmol. (2012) 96:523–9. doi: 10.1136/bjophthalmol-2011-300551
35. Chidambaranathan GP, Mathews S, Panigrahi AK, Mascarenhas J, Prajna NV, Muthukkaruppan V. In vivo confocal microscopic analysis of limbal stroma in patients with limbal stem cell Ddficiency. Cornea. (2015) 34:1478–86. doi: 10.1097/ICO.0000000000000593
36. Chan EH, Chen L, Yu F, Deng SX. Epithelial thinning in limbal stem cell deficiency. Am J Ophthalmol. (2015) 160:669–77.e4. doi: 10.1016/j.ajo.2015.06.029
37. Chan EH, Chen L, Rao JY, Yu F, Deng SX. Limbal basal cell density decreases in limbal stem cell deficiency. Am J Ophthalmol. (2015) 160:678-684.e4. doi: 10.1016/j.ajo.2015.06.026
38. Chuephanich P, Supiyaphun C, Aravena C, Bozkurt TK Yu F, Deng SX. Characterization of corneal subbasal nerve plexus in limbal stem cell deficiency. Cornea. (2017) 36:347–52. doi: 10.1097/ICO.0000000000001092
39. Caro-Magdaleno M, Alfaro-Juárez A, Montero-Iruzubieta J, Fernández-Palacín A, Muñoz-Morales A, Castilla-Martino MA, et al. In vivo confocal microscopy indicates an inverse relationship between the sub-basal corneal plexus and the conjunctivalisation in patients with limbal stem cell deficiency. Br J Ophthalmol. (2019) 103:327–31. doi: 10.1136/bjophthalmol-2017-311693
40. Liang Q, Le Q, Cordova DW, Tseng C-H, Deng SX. Corneal epithelial thickness measured using AS-OCT as a diagnostic parameter for limbal stem cell deficiency. Am J Ophthalmol. (2020) 216:132–9. doi: 10.1016/j.ajo.2020.04.006
41. Mehtani A, Agarwal MC, Sharma S, Chaudhary S. Diagnosis of limbal stem cell deficiency based on corneal epithelial thickness measured on anterior segment optical coherence tomography. Indian J Ophthalmol. (2017) 65:1120–6. doi: 10.4103/ijo.IJO_218_17
42. Haagdorens M, Behaegel J, Rozema J, Van Gerwen V, Michiels S, Ní Dhubhghaill S, et al. method for quantifying limbal stem cell niches using OCT imaging. Br J Ophthalmol. (2017) 101:1250–5. doi: 10.1136/bjophthalmol-2016-309549
43. Le Q, Yang Y, Deng SX, Xu J. Correlation between the existence of the palisades of Vogt and limbal epithelial thickness in limbal stem cell deficiency. Clin Exp Ophthalmol. (2017) 45:224–31. doi: 10.1111/ceo.12832
44. Grieve K, Ghoubay D, Georgeon C, Thouvenin O, Bouheraoua N, Paques M, et al. Three-Dimensional structure of the mammalian limbal stem cell niche. Exp Eye Res. (2015) 140:75–84. doi: 10.1016/j.exer.2015.08.003
45. Bizheva K, Hutchings N, Sorbara L, Moayed AA, Simpson T. In vivo volumetric imaging of the human corneo-scleral limbus with spectral domain OCT. Biomed Opt Express. (2011) 2:1794–1702. doi: 10.1364/BOE.2.001794
46. Varma S, Shanbhag SS, Donthineni PR, Mishra DK, Singh V, Basu S. High-Resolution optical coherence tomography angiography characteristics of limbal stem cell deficiency. Diagnostics (Basel). (2021) 11:1130. doi: 10.3390/diagnostics11061130
47. Patel CN, Antony AK, Kommula H, Shah S, Singh V, Basu S. Optical coherence tomography angiography of perilimbal vasculature: validation of a standardised imaging algorithm. Br J Ophthalmol. (2020) 104:404–9. doi: 10.1136/bjophthalmol-2019-314030
48. Oie Y, Nishida K. Evaluation of corneal neovascularization using optical coherence tomography angiography in patients with limbal stem cell deficiency. Cornea. (2017) 36 Suppl 1:S72–5. doi: 10.1097/ICO.0000000000001382
49. Binotti WW, Nosé RM, Koseoglu ND, Dieckmann GM, Kenyon K, Hamrah P. The utility of anterior segment optical coherence tomography angiography for the assessment of limbal stem cell deficiency. Ocul Surf. (2021) 19:94–103. doi: 10.1016/j.jtos.2020.04.007
50. Shortt AJ, Bunce C, Levis HJ, Blows P, Doré CJ, Vernon A, et al. Three-year outcomes of cultured limbal epithelial allografts in aniridia and Stevens-Johnson syndrome evaluated using the clinical outcome assessment in surgical trials assessment tool. Stem Cells Transl Med. (2014) 3:265–75. doi: 10.5966/sctm.2013-0025
51. Sotozono C, Ang LPK, Koizumi N, Higashihara H, Ueta M, Inatomi T, et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology. (2007) 114:1294–302. doi: 10.1016/j.ophtha.2006.10.029
52. Parra AS, Roth BM, Nguyen TM, Wang L, Pflugfelder SC, Al-Mohtaseb Z. Assessment of the Prosthetic Replacement of Ocular Surface Ecosystem (PROSE) scleral lens on visual acuity for corneal irregularity and ocular surface disease. Ocul Surf. (2018) 16:254–8. doi: 10.1016/j.jtos.2018.01.003
53. Wong BM, Garg A, Trinh T, Mimouni M, Ramdass S, Liao J, et al. Diagnoses and outcomes of prosthetic replacement of the ocular surface ecosystem treatment-a Canadian experience. Eye Contact Lens. (2021) 47:394–400. doi: 10.1097/ICL.0000000000000779
54. Harthan JS, Shorter E. Therapeutic uses of scleral contact lenses for ocular surface disease: patient selection and special considerations. OPTO. (2018) 10:65–74. doi: 10.2147/OPTO.S144357
55. Bonnet C, Lee A, Shibayama VP, Tseng C-H, Deng SX. Clinical outcomes and complications of fluid-filled scleral lens devices for the management of limbal stem cell deficiency. Cont Lens Anterior Eye. (in press) 101528. doi: 10.1016/j.clae.2021.101528
56. Vazirani J, Basu S, Kenia H, Ali MH, Kacham S, Mariappan I, et al. Unilateral partial limbal stem cell deficiency: contralateral versus ipsilateral autologous cultivated limbal epithelial transplantation. Am J Ophthalmol. (2014) 157:584–90.e1–2. doi: 10.1016/j.ajo.2013.11.011
57. Sangwan VS, Vemuganti GK, Iftekhar G, Bansal AK, Rao GN. Use of autologous cultured limbal and conjunctival epithelium in a patient with severe bilateral ocular surface disease induced by acid injury: a case report of unique application. Cornea. (2003) 22:478–81. doi: 10.1097/00003226-200307000-00016
58. Anderson DF, Ellies P, Pires RT, Tseng SC. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br J Ophthalmol. (2001) 85:567–75. doi: 10.1136/bjo.85.5.567
59. Gomes JAP, dos Santos MS, Cunha MC, Mascaro VLD, Barros J de N, de Sousa LB. Amniotic membrane transplantation for partial and total limbal stem cell deficiency secondary to chemical burn. Ophthalmology. (2003) 110:466–73. doi: 10.1016/s0161-6420(02)01888-2
60. Kheirkhah A, Casas V, Raju VK, Tseng SCG. Sutureless amniotic membrane transplantation for partial limbal stem cell deficiency. Am J Ophthalmol. (2008) 145:787–94. doi: 10.1016/j.ajo.2008.01.009
61. Konomi K, Satake Y, Shimmura S, Tsubota K, Shimazaki J. Long-Term results of amniotic membrane transplantation for partial limbal deficiency. Cornea. (2013) 32:1110–5. doi: 10.1097/ICO.0b013e31828d06d2
62. Le Q, Deng SX. The application of human amniotic membrane in the surgical management of limbal stem cell deficiency. Ocul Surf. (2019) 17:221–9. doi: 10.1016/j.jtos.2019.01.003
63. Sangwan VS, Matalia HP, Vemuganti GK, Rao GN. Amniotic membrane transplantation for reconstruction of corneal epithelial surface in cases of partial limbal stem cell deficiency. Indian J Ophthalmol. (2004) 52:281–5.
64. Sharma N, Mohanty S, Jhanji V, Vajpayee RB. Amniotic membrane transplantation with or without autologous cultivated limbal stem cell transplantation for the management of partial limbal stem cell deficiency. Clin Ophthalmol. (2018) 12:2103–6. doi: 10.2147/OPTH.S181035
65. Shanbhag SS, Chanda S, Donthineni PR, Basu S. Surgical management of unilateral partial limbal stem cell deficiency: conjunctival autografts vs. simple limbal epithelial transplantation. Clin Ophthalmol. (2021) 15:4389–97. doi: 10.2147/OPTH.S338894
66. Shanbhag SS, Tarini S, Kunapuli A, Basu S. Simultaneous surgical management of unilateral limbal stem cell deficiency and symblepharon post chemical burn. BMJ Case Rep. (2020) 13:e237234. doi: 10.1136/bcr-2020-237234
67. Kate A, Basu S. Mini-conjunctival autograft combined with deep anterior lamellar keratoplasty for chronic sequelae of severe unilateral chemical burn: a case report. Int J Surg Case Rep. (2021) 88:106508. doi: 10.1016/j.ijscr.2021.106508
68. Fogla R, Padmanabhan P. Deep anterior lamellar keratoplasty combined with autologous limbal stem cell transplantation in unilateral severe chemical injury. Cornea. (2005) 24:421–5. doi: 10.1097/01.ico.0000151550.51556.2d
69. Deng SX, Kruse F, Gomes JAP, Chan CC, Daya S, Dana R, et al. Global consensus on the management of limbal stem cell deficiency. Cornea. (2020) 39:1291–302. doi: 10.1097/ICO.0000000000002358
70. Omoto M, Shimmura S, Hatou S, Ichihashi Y, Kawakita T, Tsubota K. Simultaneous deep anterior lamellar keratoplasty and limbal allograft in bilateral limbal stem cell deficiency. Jpn J Ophthalmol. (2010) 54:537–43. doi: 10.1007/s10384-010-0879-9
71. Basu S, Mohamed A, Chaurasia S, Sejpal K, Vemuganti GK, Sangwan VS. Clinical outcomes of penetrating keratoplasty after autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol. (2011) 152:917–24.e1. doi: 10.1016/j.ajo.2011.05.019
72. Vazirani J, Mariappan I, Ramamurthy S, Fatima S, Basu S, Sangwan VS. Surgical management of bilateral limbal stem cell deficiency. Ocul Surf. (2016) 14:350–64. doi: 10.1016/j.jtos.2016.02.006
73. Atallah MR, Palioura S, Perez VL, Amescua G. Limbal stem cell transplantation: current perspectives. Clin Ophthalmol. (2016) 10:593–602. doi: 10.2147/OPTH.S83676
74. Goldman DR, Hubschman J-P, Aldave AJ, Chiang A, Huang JS, Bourges J-L, et al. Postoperative posterior segment complications in eyes treated with the Boston type I keratoprosthesis. Retina. (2013) 33:532–41. doi: 10.1097/IAE.0b013e3182641848
75. Lee WB, Shtein RM, Kaufman SC, Deng SX, Rosenblatt MI. Boston keratoprosthesis: outcomes and complications: a report by the American academy of ophthalmology. Ophthalmology. (2015) 122:1504–11. doi: 10.1016/j.ophtha.2015.03.025
76. Greiner MA Li JY, Mannis MJ. Longer-term vision outcomes and complications with the Boston type 1 keratoprosthesis at the university of California, Davis. Ophthalmology. (2011) 118:1543–50. doi: 10.1016/j.ophtha.2010.12.032
77. Shanbhag SS, Saeed HN, Paschalis EI, Chodosh J. Boston keratoprosthesis type 1 for limbal stem cell deficiency after severe chemical corneal injury: a systematic review. Ocul Surf. (2018) 16:272–81. doi: 10.1016/j.jtos.2018.03.007
78. Lee R, Khoueir Z, Tsikata E, Chodosh J, Dohlman CH, Chen TC. Long-Term visual outcomes and complications of Boston keratoprosthesis type II implantation. Ophthalmology. (2017) 124:27–35. doi: 10.1016/j.ophtha.2016.07.011
79. Sharma N, Falera R, Arora T, Agarwal T, Bandivadekar P, Vajpayee RB. Evaluation of a low-cost design keratoprosthesis in end-stage corneal disease: a preliminary study. Br J Ophthalmol. (2016) 100:323–7. doi: 10.1136/bjophthalmol-2015-306982
80. Bakshi SK, Graney J, Paschalis EI, Agarwal S, Basu S, Iyer G, et al. Design and outcomes of a novel keratoprosthesis: addressing unmet needs in end-stage cicatricial corneal blindness. Cornea. (2020) 39:484–90. doi: 10.1097/ICO.0000000000002207
81. Basu S, Sureka S, Shukla R, Sangwan V. Boston type 1 based keratoprosthesis (Auro Kpro) and its modification (LVP Kpro) in chronic Stevens Johnson syndrome. BMJ Case Rep. (2014) 2014:bcr2013202756. doi: 10.1136/bcr-2013-202756
82. Lee JH, Wee WR, Chung ES, Kim HY, Park SH, Kim YH. Development of a newly designed double-fixed Seoul-type keratoprosthesis. Arch Ophthalmol. (2000) 118:1673–8. doi: 10.1001/archopht.118.12.1673
83. Kim MK, Lee SM, Lee JL, Chung TY, Kim YH, Wee WR, et al. Long-Term outcome in ocular intractable surface disease with Seoul-type keratoprosthesis. Cornea. (2007) 26:546–51. doi: 10.1097/ICO.0b013e3180415d35
84. Bakshi SK, Paschalis EI, Graney J, Chodosh J. Lucia and beyond: development of an Affordable Keratoprosthesis. Cornea. (2019) 38:492–7. doi: 10.1097/ICO.0000000000001880
85. Iakymenko S. Forty-five years of keratoprosthesis study and application at the Filatov Institute: a retrospective analysis of 1 060 cases. Int J Ophthalmol. (2013) 6:375–80. doi: 10.3980/j.issn.2222-3959.2013.03.22
86. Ghaffariyeh A, Honarpisheh N, Karkhaneh A, Abudi R, Moroz ZI, Peyman A, et al. Fyodorov-Zuev keratoprosthesis implantation: long-term results in patients with multiple failed corneal grafts. Graefes Arch Clin Exp Ophthalmol. (2011) 249:93–101. doi: 10.1007/s00417-010-1493-8
87. Huang Y, Yu J, Liu L, Du G, Song J, Guo H. Moscow eye microsurgery complex in Russia keratoprosthesis in Beijing. Ophthalmology. (2011) 118:41–6. doi: 10.1016/j.ophtha.2010.05.019
88. Pintucci S, Pintucci F, Cecconi M, Caiazza S. New dacron tissue colonisable keratoprosthesis: clinical experience. Br J Ophthalmol. (1995) 79:825–9. doi: 10.1136/bjo.79.9.825
89. Hicks CR, Crawford GJ, Dart JKG, Grabner G, Holland EJ, Stulting RD, et al. AlphaCor: clinical outcomes. Cornea. (2006) 25:1034–42. doi: 10.1097/01.ico.0000229982.23334.6b
90. Falcinelli G, Falsini B, Taloni M, Colliardo P, Falcinelli G. Modified osteo-odonto-keratoprosthesis for treatment of corneal blindness: long-term anatomical and functional outcomes in 181 cases. Arch Ophthalmol. (2005) 123:1319–29. doi: 10.1001/archopht.123.10.1319
91. Iyer G, Srinivasan B, Agarwal S, Talele D, Rishi E, Rishi P, et al. Keratoprosthesis: current global scenario and a broad Indian perspective. Indian J Ophthalmol. (2018) 66:620–9. doi: 10.4103/ijo.IJO_22_18
92. Hou JH. de la Cruz J, Djalilian AR. Outcomes of Boston keratoprosthesis implantation for failed keratoplasty after keratolimbal allograft. Cornea. (2012) 31:1432–5. doi: 10.1097/ICO.0b013e31823e2ac6
93. Ciolino JB, Belin MW, Todani A, Al-Arfaj K, Rudnisky CJ. Boston keratoprosthesis type 1 study group. retention of the Boston keratoprosthesis type 1: multicenter study results. Ophthalmology. (2013) 120:1195–200. doi: 10.1016/j.ophtha.2012.11.025
94. Sejpal K, Yu F, Aldave AJ. The Boston keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea. (2011) 30:1187–94. doi: 10.1097/ICO.0b013e3182114467
95. Priddy J, Bardan AS, Tawfik HS, Liu C. Systematic review and meta-analysis of the medium- and long-term outcomes of the boston type 1 keratoprosthesis. Cornea. (2019) 38:1465–73. doi: 10.1097/ICO.0000000000002098
96. Shanbhag SS, Senthil S, Mohamed A, Basu S. Outcomes of the Boston type 1 and the aurolab keratoprosthesis in eyes with limbal stem cell deficiency. Br J Ophthalmol. (2021) 105:473–8. doi: 10.1136/bjophthalmol-2020-316369
97. Basu S, Serna-Ojeda JC, Senthil S, Pappuru RR, Bagga B, Sangwan V. The aurolab Keratoprosthesis (KPro) vs. the Boston type I Kpro: 5-year clinical outcomes in 134 cases of bilateral corneal blindness. Am J Ophthalmol. (2019) 205:175–83. doi: 10.1016/j.ajo.2019.03.016
98. Pujari S, Siddique SS, Dohlman CH, Chodosh J. The Boston keratoprosthesis type II: the Massachusetts eye and ear infirmary experience. Cornea. (2011) 30:1298–303. doi: 10.1097/ICO.0b013e318215207c
99. Iyer G, Pillai VS, Srinivasan B, Falcinelli G, Padmanabhan P, Guruswami S, et al. Modified osteo-odonto keratoprosthesis–the Indian experience–results of the first 50 cases. Cornea. (2010) 29:771–6. doi: 10.1097/ICO.0b013e3181ca31fc
100. Basu S, Nagpal R, Serna-Ojeda JC, Bhalekar S, Bagga B, Sangwan V. LVP keratoprosthesis: anatomical and functional outcomes in bilateral end-stage corneal blindness. Br J Ophthalmol. (2018) 103:592–98. doi: 10.1136/bjophthalmol-2017-311649
101. Basu S, Pillai VS, Sangwan VS. Mucosal complications of modified osteo-odonto keratoprosthesis in chronic Stevens-Johnson syndrome. Am J Ophthalmol. (2013) 156:867–73.e2. doi: 10.1016/j.ajo.2013.06.012
102. Cabral JV, Jackson CJ, Utheim TP, Jirsova K. Ex vivo cultivated oral mucosal epithelial cell transplantation for limbal stem cell deficiency: a review. Stem Cell Res Ther. (2020) 11:301. doi: 10.1186/s13287-020-01783-8
103. Satake Y, Dogru M, Yamane G-Y, Kinoshita S, Tsubota K, Shimazaki J. Barrier function and cytologic features of the ocular surface epithelium after autologous cultivated oral mucosal epithelial transplantation. Arch Ophthalmol. (2008) 126:23–8. doi: 10.1001/archopht.126.1.23
104. Ma DH-K, Kuo M-T, Tsai Y-J, Chen H-CJ, Chen X-L, Wang S-F, et al. Transplantation of cultivated oral mucosal epithelial cells for severe corneal burn. Eye (Lond). (2009) 23:1442–50. doi: 10.1038/eye.2009.60
105. Wang J, Qi X, Dong Y, Cheng J, Zhai H, Zhou Q, et al. Comparison of the efficacy of different cell sources for transplantation in total limbal stem cell deficiency. Graefes Arch Clin Exp Ophthalmol. (2019) 257:1253–63. doi: 10.1007/s00417-019-04316-z
106. Le Q, Chauhan T, Yung M, Tseng C-H, Deng SX. Outcomes of limbal stem cell transplant: a meta-analysis. JAMA Ophthalmol. (2020) 138:660–70. doi: 10.1001/jamaophthalmol.2020.1120
107. Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. (2004) 351:1187–96. doi: 10.1056/NEJMoa040455
108. Burillon C, Huot L, Justin V, Nataf S, Chapuis F, Decullier E, et al. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Invest Ophthalmol Vis Sci. (2012) 53:1325–31. doi: 10.1167/iovs.11-7744
109. Kim YJ, Lee HJ Ryu JS, Kim YH, Jeon S, Oh JY, Choung HK, et al. Prospective clinical trial of corneal reconstruction with biomaterial-free cultured oral mucosal epithelial cell sheets. Cornea. (2018) 37:76–83. doi: 10.1097/ICO.0000000000001409
110. Hirayama M, Satake Y, Higa K, Yamaguchi T, Shimazaki J. Transplantation of cultivated oral mucosal epithelium prepared in fibrin-coated culture dishes. Invest Ophthalmol Vis Sci. (2012) 53:1602–9. doi: 10.1167/iovs.11-7847
111. Liu J, Sheha H, Fu Y, Giegengack M, Tseng SC. Oral mucosal graft with amniotic membrane transplantation for total limbal stem cell deficiency. Am J Ophthalmol. (2011) 152:739-47.e1. doi: 10.1016/j.ajo.2011.03.037
112. Choe HR, Yoon CH, Kim MK. Ocular surface reconstruction using circumferentially trephined autologous oral mucosal graft transplantation in limbal stem cell deficiency. Korean J Ophthalmol. (2019) 33:16–25. doi: 10.3341/kjo.2018.0111
113. Wenkel H, Rummelt V, Naumann GOH. Long term results after autologous nasal mucosal transplantation in severe mucus deficiency syndromes. Br J Ophthalmol. (2000) 84:279–84. doi: 10.1136/bjo.84.3.279
114. Kate A, Basu S. Systemic immunosuppression in Cornea and ocular surface disorders: a ready reckoner for ophthalmologists. Semin Ophthalmol. (2022) 37:330–44. doi: 10.1080/08820538.2021.1966059
115. Serna-Ojeda JC, Basu S, Vazirani J, Garfias Y, Sangwan VS. Systemic immunosuppression for limbal allograft and allogenic limbal epithelial cell transplantation. Med Hypothesis Discov Innov Ophthalmol. (2020) 9:23–32.
116. Kheirkhah A, Raju VK, Tseng SCG. Minimal conjunctival limbal autograft for total limbal stem cell deficiency. Cornea. (2008) 27:730–3. doi: 10.1097/QAI.0b013e31815cea8b
117. Shanbhag SS, Nikpoor N, Rao Donthineni P, Singh V, Chodosh J, Basu S. Autologous limbal stem cell transplantation: a systematic review of clinical outcomes with different surgical techniques. Br J Ophthalmol. (2020) 104:247–53. doi: 10.1136/bjophthalmol-2019-314081
118. Ganger A, Singh A, Kalaivani M, Gupta N, Vanathi M, Mohanty S, et al. Outcomes of surgical interventions for the treatment of limbal stem cell deficiency. Indian J Med Res. (2021) 154:51–61. doi: 10.4103/ijmr.IJMR_1139_18
119. Shanbhag SS, Saeed HN, Paschalis EI, Chodosh J. Keratolimbal allograft for limbal stem cell deficiency after severe corneal chemical injury: a systematic review. Br J Ophthalmol. (2018) 102:1114–21. doi: 10.1136/bjophthalmol-2017-311249
120. Shanbhag SS, Patel CN, Goyal R, Donthineni PR, Singh V, Basu S. Simple limbal epithelial transplantation (SLET): review of indications, surgical technique, mechanism, outcomes, limitations, and impact. Indian J Ophthalmol. (2019) 67:1265–77. doi: 10.4103/ijo.IJO_117_19
121. Tan JC, Tat LT, Coroneo MT. Treatment of partial limbal stem cell deficiency with topical interferon α-2b and retinoic acid. Br J Ophthalmol. (2016) 100:944–8. doi: 10.1136/bjophthalmol-2015-307411
122. Dua HS, Miri A, Elalfy MS, Lencova A, Said DG. Amnion-Assisted conjunctival epithelial redirection in limbal stem cell grafting. Br J Ophthalmol. (2017) 101:913–9. doi: 10.1136/bjophthalmol-2015-307935
123. Han SB, Ibrahim FNIM, Liu Y-C, Mehta JS. Efficacy of modified Amnion-Assisted Conjunctival Epithelial Redirection (ACER) for partial limbal stem cell deficiency. Medicina (Kaunas). (2021) 57:369. doi: 10.3390/medicina57040369
124. Litvin G, Klein I, Litvin Y, Klaiman G, Nyska A. CorNeat KPro: ocular implantation study in rabbits. Cornea. (2021) 40:1165–74. doi: 10.1097/ICO.0000000000002798
125. Al-Jaibaji O, Swioklo S, Connon CJ. Mesenchymal stromal cells for ocular surface repair. Expert Opin Biol Ther. (2019) 19:643–53. doi: 10.1080/14712598.2019.1607836
126. Demirayak B, Yüksel N, Çelik OS, Subaşi C, Duruksu G, Unal ZS, et al. Effect of bone marrow and adipose tissue-derived mesenchymal stem cells on the natural course of corneal scarring after penetrating injury. Exp Eye Res. (2016) 151:227–35. doi: 10.1016/j.exer.2016.08.011
127. Basu S, Hertsenberg AJ, Funderburgh ML, Burrow MK, Mann MM, Du Y, et al. Human limbal biopsy–derived stromal stem cells prevent corneal scarring. Sci Transl Med. (2014) 6:266ra172. doi: 10.1126/scitranslmed.3009644
128. Alio del Barrio JL, Chiesa M, Garagorri N, Garcia-Urquia N, Fernandez-Delgado J, Bataille L, et al. Acellular human corneal matrix sheets seeded with human adipose-derived mesenchymal stem cells integrate functionally in an experimental animal model. Exp Eye Res. (2015) 132:91–100. doi: 10.1016/j.exer.2015.01.020
129. Calonge M, Pérez I, Galindo S, Nieto-Miguel T, López-Paniagua M, Fernández I, et al. proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl Res. (2019) 206:18–40. doi: 10.1016/j.trsl.2018.11.003
130. Meyer-Blazejewska EA, Call MK, Yamanaka O, Liu H, Schlötzer-Schrehardt U, Kruse FE, et al. From hair to cornea: toward the therapeutic use of hair follicle-derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells. (2011) 29:57–66. doi: 10.1002/stem.550
131. Gomes JAP, Geraldes Monteiro B, Melo GB, Smith RL, Cavenaghi Pereira da., Silva M, et al. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest Ophthalmol Vis Sci. (2010) 51:1408–14. doi: 10.1167/iovs.09-4029
132. Kushnerev E, Shawcross SG, Sothirachagan S, Carley F, Brahma A, Yates JM, et al. Regeneration of corneal epithelium with dental pulp stem cells using a contact lens delivery system. Invest Ophthalmol Vis Sci. (2016) 57:5192–9. doi: 10.1167/iovs.15-17953
133. Kumagai Y, Kurokawa MS, Ueno H, Kayama M, Tsubota K, Nakatsuji N, et al. Induction of corneal epithelium-like cells from cynomolgus monkey embryonic stem cells and their experimental transplantation to damaged cornea. Cornea. (2010) 29:432–8. doi: 10.1097/ICO.0b013e3181b9ffcc
134. Ueno H, Kurokawa MS, Kayama M, Homma R, Kumagai Y, Masuda C, et al. Experimental transplantation of corneal epithelium-like cells induced by Pax6 gene transfection of mouse embryonic stem cells. Cornea. (2007) 26:1220–7. doi: 10.1097/ICO.0b013e31814fa814
135. Prabhasawat P, Chirapapaisan C, Jiravarnsirikul A, Ekpo P, Uiprasertkul M, Thamphithak R, et al. Phenotypic characterization of corneal epithelium in long-term follow-up of patients post-autologous cultivated oral mucosal epithelial transplantation. Cornea. (2021) 40:842–50. doi: 10.1097/ICO.0000000000002498
136. Prabhasawat P, Luangaram A, Ekpo P, Lekhanont K, Tangpagasit W, Boonwong C, et al. Epithelial analysis of simple limbal epithelial transplantation in limbal stem cell deficiency by in vivo confocal microscopy and impression cytology. Cell Tissue Bank. (2019) 20:95–108. doi: 10.1007/s10561-018-09746-3
Keywords: Limbal stem cell deficiency (LSCD), simple limbal epithelial transplantation (SLET), limbal stem cell transplantation (LSCT), Keratoprosthesis (KPro), Anterior segment optical coherence tomography (AS-OCT), impression cytology (IC), confocal microscopy, cultivated limbal epithelial transplantation (CLET)
Citation: Kate A and Basu S (2022) A Review of the Diagnosis and Treatment of Limbal Stem Cell Deficiency. Front. Med. 9:836009. doi: 10.3389/fmed.2022.836009
Received: 15 December 2021; Accepted: 03 May 2022;
Published: 25 May 2022.
Edited by:
Jorge L. Alió Del Barrio, Miguel Hernández University of Elche, SpainReviewed by:
Yousef Ahmed Fouad, Ain Shams University, EgyptMee Kum Kim, Seoul National University, South Korea
Copyright © 2022 Kate and Basu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sayan Basu, c2F5YW5iYXN1QGx2cGVpLm9yZw==
 Anahita Kate
Anahita Kate Sayan Basu
Sayan Basu