
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 07 February 2022
Sec. Nephrology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.831648
 Yumeng Shi1,2
Yumeng Shi1,2 Lihua Hu3
Lihua Hu3 Minghui Li4
Minghui Li4 Wei Zhou1,2,5
Wei Zhou1,2,5 Tao Wang1,2,5
Tao Wang1,2,5 Lingjuan Zhu1,2,5
Lingjuan Zhu1,2,5 Huihui Bao1,2,5
Huihui Bao1,2,5 Xiaoshu Cheng1,2,5*
Xiaoshu Cheng1,2,5* Ping Li1,2,5*
Ping Li1,2,5*Background: We aim to evaluate the four surrogate markers of insulin resistance (IR), including triglyceride-glucose index (TyG), lipid accumulation product index (LAP), visceral adiposity index (VAI), triglycerides to high-density lipoprotein cholesterol ratio (TG/HDL), on prevalence of chronic kidney disease (CKD) and to examine any possible effect modifiers in Chinese hypertensive patients.
Methods: A total of 13,055 hypertensive participants were included in this cross-sectional study. In addition, average age of the study population was 63.81 ± 9.46 years, and 47.66% of them are men. The primary outcome was CKD, defined as eGFR <60 ml/min/1.73 m2. Multivariate logistic regression analysis and the generalized additive model and a fitted smoothing curve (penalized spline method) were used to examine the association between the surrogate markers of IR and CKD.
Results: Four surrogate markers of IR were independently and positively associated with CKD in a dose-response fashion. The association between four surrogate markers of IR and the prevalence of CKD was examined as a continuous variable per one unit increment and also as a categorical variable using tertiles with the tertiles (T1) as the reference group. In the fully adjusted model, multivariate logistic analyses showed that the per one unit increments of the TyG, LAP, VAI, and TG/HDL ratios were all significantly associated with 42, 31, 67, and 78% higher risk for CKD, respectively. Consistently, the adjusted ORs (95% CI) for CKD were 1.48 (1.21, 1.81), 1.34 (1.06, 1.69), 1.26 (1.03, 1.53), 1.35 (1.12, 1.63) when comparing the highest tertile to the lowest tertile of the TyG, LAP, VAI, and TG/HDL ratios, respectively. The stratification analysis showed that a significant positive correlation between TyG, VAI, and TG/HDL and CKD in patients over 65 years old.
Conclusion: Four surrogate markers of IR were independently and positively correlated with CKD, and LAP was better than the other surrogate markers of IR for predicting CKD. Only among participants aged over 65 years were higher levels of TyG, VAI and TG/HDL found to be closely related to the increased prevalence of CKD.
Chronic kidney disease (CKD), as a major global public health problem, has aroused widespread concern (1–5). The burden of CKD is not limited to the need for renal replacement therapy for end-stage renal disease (ESRD), and cardiovascular events and mortality are strongly affected by renal involvement (6, 7). It is estimated that five million to 10 million people worldwide die annually from kidney diseases such as CKD, renal failure and ESRD (8, 9). In China, the prevalence of CKD is increasing rapidly due to rising risk factors such as diabetes, hypertension, unhealthy diet, inappropriate physical activity and metabolic syndrome (10–12). In addition, compared with other risk factors, the development of nondiabetic CKD has been confirmed to be closely related to hypertension (13). If patients with hypertension are complicated with CKD at the same time, their cardiovascular disability rate and death risk will greatly increase (14). Therefore, a better understanding of the potential risk factors of CKD increase in hypertensive patients may help to prevent CKD and related cardiovascular diseases. Recently, more and more scholars pay attention to the relationship between insulin resistance (IR) and CKD. and put forward that IR can predict the risk of CKD (15–17).
IR is a pathological state in which tissues have a decreased sensitivity to insulin, leading to a compensatory rise in circulating insulin to maintain normal blood glucose levels (18, 19). The gold standard for evaluating IR is the hyperinsulinemic-normoglycemic clamp test (20). However, the hyperinsulinemic-normoglycemic clamp test is rarely performed in the epidemiological investigations of large sample populations because it requires special equipment, which is time-consuming and expensive. Therefore, to determine whether insulin resistance exists in epidemiological investigations, many researchers have developed simple and feasible alternative markers of IR, such as the triglyceride glucose index (TyG) (21), lipid accumulation product index (LAP) (22), visceral adiposity index (VAI) (23) and TG/HDL ratio (24). The majority of studies have studied the risk of CKD caused by single surrogate markers of IR, such as TyG (25), LAP (26), VAI (27–29) and the TG/HDL ratio (30). However, published data on the relationship between all surrogate markers of IR and CKD risk are limited.
The objective of the present study was to investigate whether the four surrogate markers of IR are associated with the prevalence of CKD and to examine any possible effect modifiers in Chinese hypertensive patients based on data from a large observational study, the China H-type Hypertension Registry Study (CHHRS).
The research program was approved by the Ethics Commission of Anhui Medical University's Institute of Biomedicine (No. CH1059). Written informed consent was formally obtained from all participants.
The data are from the CHHRS Study (Registration number: ChiCTR1800017274) in rural areas of southern China. Briefly, CHHRS is an ongoing real-world, observational registry study conducted from March 2018 to August 2018 in Wuyuan, Jiangxi Province, China. The inclusion criterion was hypertensive patients over 18 years old, The exclusion criteria were as follows: (1) unable to sign informed consent due to psychological or nervous system damage and (2) inability to be followed up due to a relocation plan in the short term. The details on the trial design and methods have been described in previous publications (31).
Baseline data collection was completed by 14268 participants. After excluding subjects with nonhypertension (n = 34), missing VAI data (n = 12), using glucose-lowering medications (n = 754) and using lipid-lowering medications (n = 413), 13,055 participants were analyzed in the current study (Figure 1).
With the help of trained researchers, all participants completed a questionnaire survey on socioeconomic and demographic factors, lifestyle habits, history of disease, and drugs used in the past 2 weeks. In this standardized questionnaire, smoking habits and drinking habits were classified variables, and their classifications were never and present, respectively. The education level was classified according to less than junior college or at least junior college. According to the participants' personal evaluation, physical activities were classified as mild, moderate or vigorous.
At the same time, the baseline data also included anthropometric indicators such as height, weight and waist circumference (WC). Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, with the participants wearing lightweight clothing and without shoes. WC was measured to the nearest 5 mm directly touching the participant's skin using cloth tape. Body mass index (BMI), in kg/m2, was calculated as the weight divided by the height squared. After resting for at least 10 min, an electronic sphygmomanometer (Omron; Dalian, China) was used to measure blood pressure (BP) in the sitting position, and the average of the three blood pressures was used in the final analysis. Hypertension was defined as seated resting systolic blood pressure (SBP) ≥ 140 mm Hg or diastolic blood pressure (DBP) ≥ 90 mm Hg at screening visits or on the use of antihypertensive medications.
After 12 h of fasting, blood samples were collected from the anterior cubital vein between 8 and 10 a.m. Homocysteine (Hcy), fasting glucose, serum lipid [total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C)], serum uric acid, serum creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and serum γ-glutamyltransferase (GGT) were assayed in a single laboratory (Biaojia Biotechnology Laboratory, Shenzhen, China) on an automatic clinical analyzer (Beckman Coulter). The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (32) was used to calculate the estimated glomerular filtration rate (eGFR). The formulas for eGFR are as follows: males with creatinine ≤ 0.9 mg/dl: eGFR (ml/min/1.73 m2) = 141 × (creatinine/0.9)−0.411 × 0.933age; males with creatinine > 0.9 mg/dl: eGFR (ml/min/1.73 m2) = 141 × (creatinine/0.9)−1.209 × 0.933age; females with creatinine ≤ 0.7 mg/dl: eGFR (ml/min/1.73 m2) = 144 × (creatinine/0.7)−0.329 × 0.933age; and females with creatinine > 0.7 mg/dl: eGFR (ml/min/1.73 m2) = 144 × (creatinine/0.7)−1.209 × 0.933 age.
The TyG index was calculated as ln[fasting triglycerides (mg/dl) × fasting glucose (mg/dl)/2] (33). LAP was determined from WC (cm) plus TG (mmol/L) for men [(WC-65) × TG] and women [(WC-58) × TG] (34). VAI was calculated using the following sex-specific equations, where the units for WC, BMI, and TG and HDL are cm kg/m2, and mmol/L: males: VAI = [WC/[39.68 + (1.88 × BMI)]) × (TG/1.03) × (1.31/HDL); females: VAI = [WC/[36.58 + (1.89 × BMI)]) × (TG/0.81) × (1.52/HDL) (35). The TG/HDL ratios were calculated as TG divided by HDL. The diagnostic criteria for CKD were eGFR <60 ml/min/1.73 m2 (36).
P < 0.05 with two tails was statistically significant. All statistical analyses were performed using the statistical package R (http://www.R-project.org, The R Foundation) and Empower Stats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA).
Continuous variables are presented as the means ± standard deviations (SDs) or medians (interquartile ranges) (IQRs), and categorical variables are presented as numbers (percentages). A t-test of students' normally distributed data, the Mann–Whitney test of nonparametric nonnormally distributed data and χ2 test of classified data were used to compare the differences in data characteristics with or without CKD.
Because LAP, VAI and TG/HDL had skewed distributions, they were transformed into normal distributions by lg-transformation. If the estimated effect changed by ≥10% alone, the variables called traditional risk factors and potential confounding factors of chronic kidney disease were selected (37). Logistic regression analyses were performed to assess the association between four surrogate markers of IR (TyG, LAP, VAI and the TG/HDL-C ratios) and CKD. The association between four surrogate markers of IR and the prevalence of CKD was examined as a continuous variable per one increment and as a categorical variable using tertiles with the tertiles (T1) as the reference group. In the case of the logistic regression analysis of the ORs of CKD, the three models constructed were as follows: Model 1 was adjusted for age and sex; Model 2 was adjusted for age, sex, BMI, education, and physical activity; and Model 3 was adjusted for age, sex, BMI, education, physical activity, current smoking, current drinking, SBP, DBP, pulse, Hcy, AST, GGT, LDL-C, antihypertensive drugs, antiplatelet drugs, and self-reported diabetes. To examine the significant associations between four surrogate markers of insulin resistance (IR) and CKD, a generalized additive model and a fitted smoothing curve (penalized spline method) were used to further explore the shape of their dose–response relations.
Tests for interaction were performed using a likelihood ratio test to compare models with and without interaction terms. Further stratified analyses by subgroups, including sex (male or female), age (<65 or ≥65 years), BMI (<25 or ≥25 kg/m2), current smoking (no or yes), current drinking (no or yes), physical activity (mild, moderate, vigorous), serum LDL-C (<2.6 or ≥2.6 mmol/L), SBP (<140, 140–159, ≥160 mm Hg), and self-reported diabetes (no or yes), were also explored by multivariable logistic regression models to test for consistency of results.
A total of 13,055 patients with hypertension were enrolled in the study. In addition, the average age of the study population was 63.81 ± 9.46 years, and 47.66% of them were men. There were 1,218 (9.32%) participants with CKD and 1,775 (13.60%) participants with diabetes.
According to CKD, the clinical and demographic characteristics of the participants are presented in Table 1. Participants with CKD were older and more often male with self-reported diabetes; they had higher levels of Hcy and lower levels of physical activity, BMI, DBP, LDL-C, ALT, LAP, and VAI. They were also less frequently current drinkers and more frequently using antihypertensive drugs and antiplatelet drugs (all P < 0.05). There was no significant difference in smoking, education, SBP, GGT, pulse, TyG, AST, or TG/HDL ratio among patients with or without CKD (all P > 0.05).
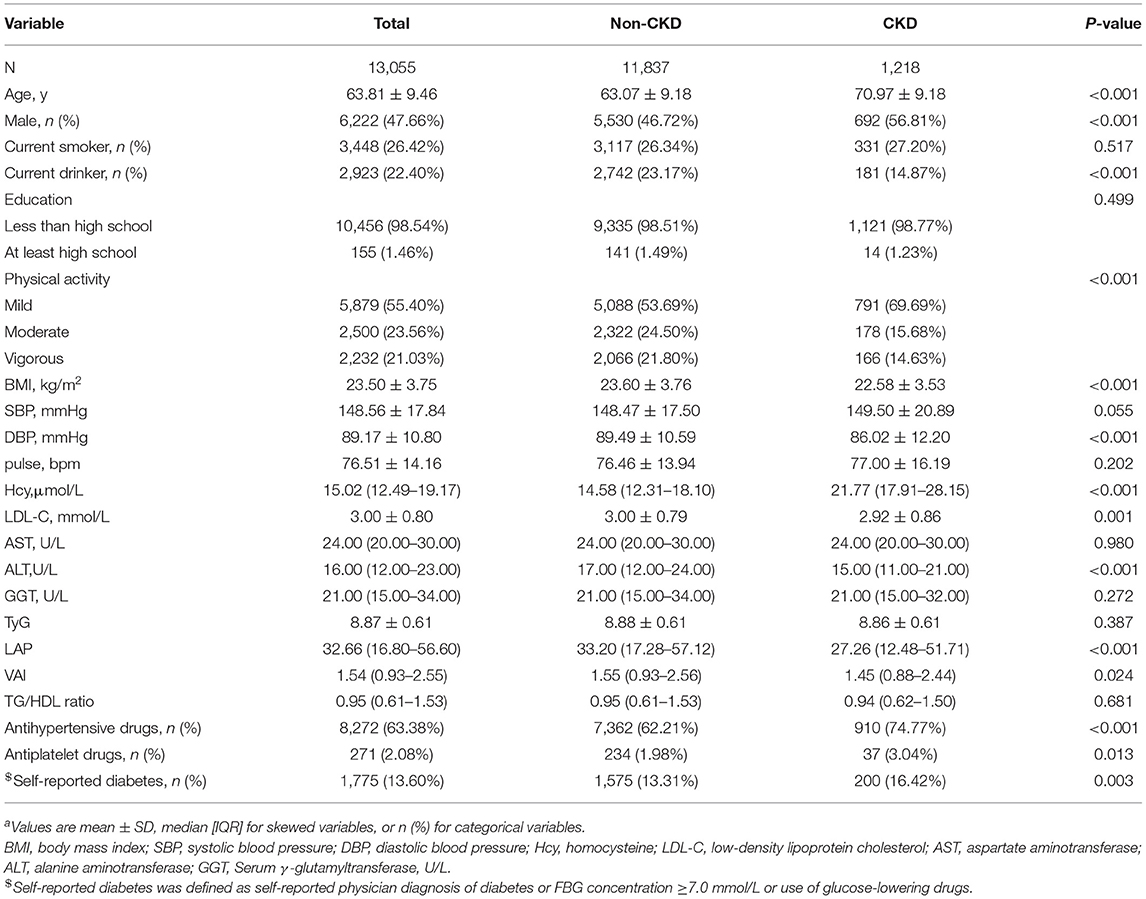
Table 1. Baseline characteristics of study participantsa.
Multiple logistic regression analyses were used to assess the association between surrogate markers of IR and CKD. Table 2 shows that the ORs and 95% CIs for CKD showed a significant gradual increase at higher levels of TyG, LAP, VAI and TG/HDL in a dose-dependent manner (all P for trend < 0.05). The per one increment of the TyG, LAP, VAI and TG/HDL levels was significantly associated with 42, 31, 67 and 78% higher risks for CKD, respectively. After full adjustment, hypertension subjects in the top tertiles of TyG had 1.48-fold increased odds of prevalent CKD relative to those in the bottom tertiles of TyG (OR: 1.48, 95% CI: 1.21–1.81), and the ORs of prevalent CKD increased by a factor of 1.34 for individuals in the highest tertiles of the LAP group (OR: 1.34, 95% CI: 1.06–1.69) compared with the reference group. Compared with the reference group, the TG/HDL-C level in the highest group increased (OR: 1.35, 95% CI: 1.12–1.63), while the smallest OR for the highest VAI tertiles with regard to CKD was 1.26 (95% CI: 1.03–1.53). Further analysis using fitted curves confirmed the dose–response association between the four surrogate markers of IR and the prevalence of CKD and showed that the association between the four surrogate markers of IR and the prevalence of CKD was linearly positive (Figure 2). Moreover, LAP was better than the other surrogate markers of IR for predicting CKD (Supplementary Figure 3, Supplementary Table 1).
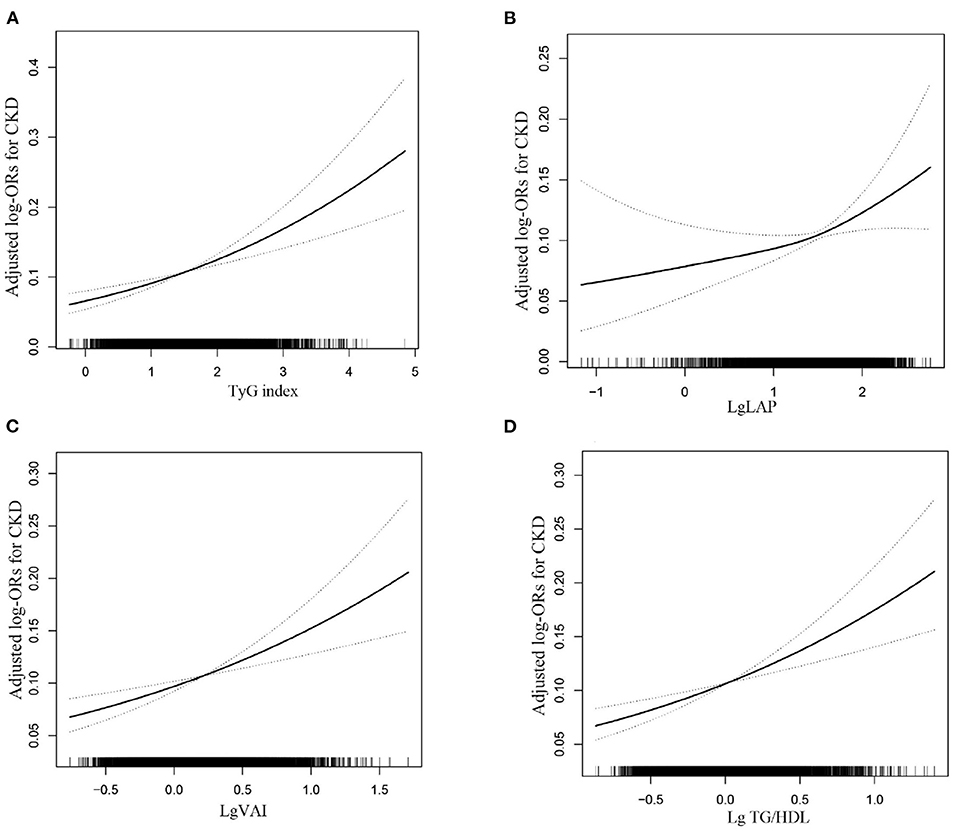
Figure 2. The association between the TyG (A), LAP (B) and VAI (C) and TG/HDL (D) level and the risk of CKD. The solid line and dashed line represent the estimated values and their corresponding 95% confidence interval, respectively. The adjustment factors included age, sex, BMI, education, physical activity, current smoking, current drinking, SBP, DBP, pulse, Hcy, AST, GGT, LDL-C, Antihypertensive drugs, Antiplatelet drugs, self-reported diabetes.
A stratified analysis of potential covariates was conducted in this survey. When participants were stratified by sex (male or female), BMI (<25 or ≥25 kg/m2), current smoking (no or yes), current drinking (no or yes), hypertension (yes or no), physical activity (mild, moderate, vigorous), serum LDL cholesterol (<2.6 or ≥2.6 mmol/L), SBP (<140, 140–159, ≥160 mm Hg), and self-reported diabetes (no or yes), the association of four surrogate markers of IR and the prevalence of CKD remained consistent (all P for interactions > 0.05; see Additional file 1: Supplementary Figures 1, 2). Nevertheless, there was a significant interaction between subgroups stratified by age (<65 or ≥65 years) between TyG, VAI, and TG/HDL and the prevalence of CKD, and a strong positive correlation between TyG, VAI, and TG/HDL and the prevalence of CKD was observed in elderly individuals over 65 years old (Supplementary Figures 1, 2).
This large-scale cross-sectional survey among Chinese hypertensive participants ≥18 years old provides comprehensive estimates of the effects of four surrogate markers of IR on CKD in China. After adjusting for confounders, including demographic characteristics, lifestyle habits, previous medical diagnoses, drugs used, and biochemical indicators, TyG, LAP, VAI, and TG/HDL were independently and positively correlated with the prevalence of CKD. Moreover, LAP was better than the other surrogate markers of IR for predicting CKD. However, only TyG, VAI, and TG/HDL were positively correlated with the prevalence of CKD in patients over 65 years old.
In line with previous studies (25–28, 30), TyG, LAP, VAI, and TG/HDL were independently and positively correlated with the prevalence of CKD. However, in previous studies, these indicators were regarded as obesity-related indicators, and only one or two indicators were discussed in relation to CKD. Moreover, most of the above studies were conducted in healthy people and found gender differences between them. Dai et al. (27). conducted a cross-sectional study with 11,192 participants in northern China to study the relationship between two new obesity indicators (VAI and LAP) and CKD. The results showed that high levels of VAI and LAP were closely related to a higher risk of CKD but only in women, and compared with waist circumference and BMI, VAI and LAP had a better ability to predict CKD. However, studies by Seong et al. (26) found different results. A cross-sectional study based on the data from the Korean Nutrition Survey included 4,947 adults over the age of 20 and showed that CKD risk was positively correlated with the LAP index and VAI index only in men but not in women in the fully adjusted model (26). These differences in research results may be due to the different ages of the women in the study population. The women in the study by Seong et al. were mostly premenopausal women, and estrogen has an obvious protective effect on the kidney (38), so it can resist kidney damage caused by increases in LAP and VAI levels. The purpose of the second study of the China Health and Retirement Longitudinal Study (CHARLS) was to explore the relationship between TG/HDL and the risk of renal function decline. The results showed that TG/HDL was an independent risk factor for renal function decline (30). A cohort study conducted by Okamura et al. (25) included 6,026 males and 5,686 females. The aim of cohort study was to observe the influence of the TyG index on incident CKD. The conclusion of this study was that the TyG index can predict the risk of CKD in both men and women.
However, our study did not find any sex differences between the above markers of IR and CKD but rather found significant differences between TyG, VAI, and TG/HDL and CKD observed in different age groups. Compared with TyG, VAI and TG/HDL, LAP had a better ability to predict CKD. A strong positive correlation between TyG, VAI, and TG/HDL and the prevalence of CKD can be observed in elderly individuals over 65 years old. Although the LAP index was positively correlated with CKD in patients over 65 years old, its positive correlation was not significant. Age is an important factor for determining the prevalence of CKD; moreover, animal experiments show higher insulin levels with increasing age (39), so the subgroup results appear. The mechanism of CKD caused by IR can be explained from the following aspects: inflammation, oxidative stress and metabolic acidosis. First, Shimobayashi et al. (40) demonstrated that IR induced adipose tissue inflammation by inhibiting the insulin signaling pathway and increasing monocyte chemoattractant protein one production. M2 macrophages activated by adipose tissue inflammation produce and release proinflammatory cytokines, such as interleukin-6 and tumor necrosis factor-α. Inflammatory factors such as interleukin-6 and tumor necrosis factor-α can cause dysfunction of the glomerular endothelium, which leads to CKD (41, 42). Second, oxidative stress and inflammation impair the activation of nuclear factor erythroid-2-related factor-2, which protects the kidney from tissue damage (43). Finally, metabolic acidosis increases renal plasma flow and glomerular filtration rate to discharge excessive acid load, leading to impaired renal function (44).
There are also some limitations to this study. First, this is a cross-sectional investigation, and we only analyzed the correlation between four surrogate markers of IR and CKD without attempting to identify the causality or mechanisms. Second, although a large number of possible confounding factors were adjusted in the multivariate regression analysis, there may still be unidentified residual confounding variables. Third, the current study mainly focused on a Chinese hypertension population, so the associations identified here should also be examined in another population before generalization. The main advantages of this study are that it explored the relationship between four surrogate markers of IR and CKD in Chinese hypertensive patients for the first time and found that age can modify the relationship between them and that VAI and the TG/HDL-C ratio were better than the other surrogate markers of IR for predicting CKD.
In summary, a substudy of the Chinese Type H Hypertension Registration Study demonstrated that four surrogate markers of IR were independently and positively correlated with CKD, and the LAP was better than the other surrogate markers of IR for predicting CKD. Only among participants aged over 65 years were higher levels of TyG, VAI and TG/HDL found to be closely related to the increased prevalence of CKD. These four surrogate markers of IR can be easily determined in the primary health care environment. Hypertensive patients with these higher indicators should receive additional screening and preventive intervention for CKD.
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
The studies involving human participants were reviewed and approved by Ethics Commission of Anhui Medical University' Institute of Biomedicine (No. CH1059). The patients/participants provided their written informed consent to participate in this study.
YS participated in the literature search, data analysis, data interpretation, and wrote the manuscript. LH extracted and collected data. LH, ML, WZ, TW, LZ, and HB conceived of the study and participated in its design and coordination. PL and XC participated in the study design and provided critical revision. All authors read and approved the final manuscript.
This work was supported by the establishment and application of big data platform for clinical and scientific research management of hypertension in Jiangxi province (20172BCB22027), the National Natural Science Foundation of China (81760049), the Jiangxi Science and Technology Innovation Platform Project (20165BCD41005), the National Key R&D Program of China (2018YFC1312902), and the Key Project of Education Department of Jiangxi Province (GJJ170013).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank all of the study participants and staff for their valuable.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.831648/full#supplementary-material
IR, Insulin resistance; CKD, chronic kidney disease; TyG, triglyceride-glucose index; LAP, lipid accumulation product index; VAI, visceral adiposity index; TG/HDL ratio, triglycerides to high-density lipoprotein cholesterol ratio; CHHRS, China H-type Hypertension Registry Study; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; Hcy, homocysteine; LDL-C, low-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, Serum γ -glutamyltransferase, U/L.
1. Lin B, Shao L, Luo Q, Ou-yang L, Zhou F, Du B, et al. Prevalence of chronic kidney disease and its association with metabolic diseases: a cross-sectional survey in Zhejiang province, Eastern China. BMC Nephrol. (2014) 15:36 doi: 10.1186/1471-2369-15-36
2. Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. (2012) 379:815–822 doi: 10.1016/S0140-6736(12)60033-6
3. Zelnick LR, Weiss NS, Kestenbaum BR, Robinson-Cohen C, Heagerty PJ, Tuttle K, et al. Diabetes and CKD in the United States Population, 2009-2014. Clin J Am Soc Nephrol. (2017) 12:1984–90 doi: 10.2215/CJN.03700417
4. Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. (2017) 13:104–14 doi: 10.1038/nrneph.2016.163
5. Xu H, Kuja-Halkola R, Chen X, Magnusson P, Svensson P, Carrero JJ. Higher body mass index is associated with incident diabetes and chronic kidney disease independent of genetic confounding. Kidney Int. (2019) 95:1225–33 doi: 10.1016/j.kint.2018.12.019
6. Nugent RA, Fathima SF, Feigl AB, Chyung D. The burden of chronic kidney disease on developing nations: a 21st century challenge in global health. Nephron Clin Pract. (2011) 118:c269–77 doi: 10.1159/000321382
7. Bello AK, Nwankwo E, El Nahas AM. Prevention of chronic kidney disease: a global challenge. Kidney Int Suppl. (2005) 98:S11–17. doi: 10.1111/j.1523-1755.2005.09802.x
8. Global regional and national life expectancy all-cause mortality and and cause-specific mortality for 249 causes of death 1980-2015: 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. (2016) 388:1459–544. doi: 10.1016/S0140-6736(16)31012-1
9. Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, et al. International society of nephrology's 0by25 initiative for acute kidney injury. (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. (2015) 385:2616–43. doi: 10.1016/S0140-6736(15)60126-X
10. Chen J. Epidemiology of hypertension and chronic kidney disease in China. Curr Opin Nephrol Hypertens. (2010) 19:278–82 doi: 10.1097/MNH.0b013e328337f921
11. Liu M, Liu SW, Wang LJ, Bai YM, Zeng XY, Guo HB, et al. Burden of diabetes, hyperglycaemia in China from to 2016: findings from the 1990 to 2016 global burden of disease study. Diabetes Metab. (2019) 45:286–93. doi: 10.1016/j.diabet.2018.08.008
12. Zhang L, Zuo L, Wang F, Wang M, Wang S, Liu L, et al. Metabolic syndrome and chronic kidney disease in a Chinese population aged 40 years and older. Mayo Clin Proc. (2007) 82:822–7 doi: 10.4065/82.7.822
13. Levey AS. Clinical practice. Nondiabetic kidney disease. N Engl J Med. (2002) 347:1505–11 doi: 10.1056/NEJMcp013462
14. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. (2004) 351:1296–305. doi: 10.1056/NEJMoa041031
15. de Boer IH, Mehrotra R. Insulin resistance in chronic kidney disease: a step closer to effective evaluation and treatment. Kidney Int. (2014) 86:243–5. doi: 10.1038/ki.2014.123
16. Schrauben SJ, Jepson C, Hsu JY, Wilson FP, Zhang X, Lash JP, et al. Insulin resistance and chronic kidney disease progression, cardiovascular events, and death: findings from the chronic renal insufficiency cohort study. BMC Nephrol. (2019) 20:60 doi: 10.1186/s12882-019-1220-6
17. Chen J, Muntner P, Hamm LL, Fonseca V, Batuman V, Whelton PK, et al. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol. (2003) 14:469–77. doi: 10.1097/01.ASN.0000046029.53933.09
18. Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. (2005) 16:2134–40. doi: 10.1681/ASN.2005010106
19. Nakashima A, Kato K, Ohkido I, Yokoo T. Role and treatment of insulin resistance in patients with chronic kidney disease: a review. Nutrients. (2021) 13:4349. doi: 10.3390/nu13124349
20. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. (2008) 294:E15–26. doi: 10.1152/ajpendo.00645.2007
21. Kang B, Yang Y, Lee EY, Yang HK, Kim HS, Lim SY, et al. Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents. Int J Obes. (2017) 41:789–92. doi: 10.1038/ijo.2017.14
22. Xia C, Li R, Zhang S, Gong L, Ren W, Wang Z, et al. Lipid accumulation product is a powerful index for recognizing insulin resistance in non-diabetic individuals. Eur J Clin Nutr. (2012) 66:1035–8. doi: 10.1038/ejcn.2012.83
23. Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. (2016) 11:e0149731. doi: 10.1371/journal.pone.0149731
24. McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease. Am J Cardiol. (2005) 96:399–404. doi: 10.1016/j.amjcard.2005.03.085
25. Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Triglyceride-glucose index is a predictor of incident chronic kidney disease: a population-based longitudinal study. Clin Exp Nephrol. (2019) 23:948–55. doi: 10.1007/s10157-019-01729-2
26. Seong JM, Lee JH, Gi MY, Son YH, Moon AE, Park CE, et al. Gender difference in the association of chronic kidney disease with visceral adiposity index and lipid accumulation product index in Korean adults: Korean national health and nutrition examination survey. Int Urol Nephrol. (2021) 53:1417–25. doi: 10.1007/s11255-020-02735-0
27. Dai D, Chang Y, Chen Y, Chen S, Yu S, Guo X, et al. Visceral adiposity index and lipid accumulation product index: two alternate body indices to identify chronic kidney disease among the rural population in Northeast China. Int J Environ Res Public Health. (2016) 13:1231. doi: 10.3390/ijerph13121231
28. Xu X, Zhao Y, Zhao Z, Zhu S, Liu X, Zhou C, et al. Correlation of visceral adiposity index with chronic kidney disease in the People's Republic of China: to rediscover the new clinical potential of an old indicator for visceral obesity. Ther Clin Risk Manag. (2016) 12:489–94. doi: 10.2147/TCRM.S96340
29. Xiao H, Xiong C, Shao X, Gao P, Chen H, Ning J, et al. Visceral adiposity index and chronic kidney disease in a non-diabetic population: a cross-sectional study. Diabetes Metab Syndr Obes. (2020) 13:257–65. doi: 10.2147/DMSO.S231656
30. Lv S, Zhang H, Chen J, Shen Z, Zhu C, Gu Y, et al. The effect of triglycerides to high-density lipoprotein cholesterol ratio on the reduction of renal function: findings from China health and retirement longitudinal study. (CHARLS). Lipids Health Dis. (2021) 20:110. doi: 10.1186/s12944-021-01542-5
31. Li M, Zhan A, Huang X, Hu L, Zhou W, Wang T, et al. Positive association between triglyceride glucose index and arterial stiffness in hypertensive patients: the China H-type hypertension registry study. Cardiovasc Diabetol. (2020) 19:139. doi: 10.1186/s12933-020-01124-2
32. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
33. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. (2008) 6:299–304 doi: 10.1089/met.2008.0034
34. Ioachimescu AG, Brennan DM, Hoar BM, Hoogwerf BJ. The lipid accumulation product and all-cause mortality in patients at high cardiovascular risk: a PreCIS database study. Obesity. (2010) 18:1836–44 doi: 10.1038/oby.2009.453
35. Amato MC, Giordano C. Visceral adiposity index: an indicator of adipose tissue dysfunction. Int J Endocrinol. (2014) 2014:730827. doi: 10.1155/2014/730827
36. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation classification and stratification. Am J Kidney Dis. (2002) 39:S1–266.
37. Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. (1989) 79:340–9. doi: 10.2105/AJPH.79.3.340
38. Ikeda M, Swide T, Vayl A, Lahm T, Anderson S, Hutchens MP. Estrogen administered after cardiac arrest and cardiopulmonary resuscitation ameliorates acute kidney injury in a sex- and age-specific manner. Crit Care. (2015) 19:332. doi: 10.1186/s13054-015-1049-8
39. Templeman NM, Flibotte S, Chik J, Sinha S, Lim GE, Foster LJ, et al. Reduced circulating insulin enhances insulin sensitivity in old mice and extends lifespan. Cell Rep. (2017) 20:451–63. doi: 10.1016/j.celrep.2017.06.048
40. Shimobayashi M, Albert V, Woelnerhanssen B, Frei IC, Weissenberger D, Meyer-Gerspach AC, et al. Insulin resistance causes inflammation in adipose tissue. J Clin Invest. (2018) 128:1538–50. doi: 10.1172/JCI96139
41. Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. (2007) 27:2576–81. doi: 10.1161/ATVBAHA.107.153080
42. Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, et al. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. (2006) 99:69–77. doi: 10.1161/01.RES.0000229685.37402.80
43. Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol. (2010) 298:F662–71. doi: 10.1152/ajprenal.00421.2009
Keywords: chronic kidney disease, lipid accumulation product index, triglyceride-glucose index, triglycerides to high-density lipoprotein cholesterol ratio, visceral adiposity index
Citation: Shi Y, Hu L, Li M, Zhou W, Wang T, Zhu L, Bao H, Cheng X and Li P (2022) Association Between the Surrogate Markers of Insulin Resistance and Chronic Kidney Disease in Chinese Hypertensive Patients. Front. Med. 9:831648. doi: 10.3389/fmed.2022.831648
Received: 08 December 2021; Accepted: 05 January 2022;
Published: 07 February 2022.
Edited by:
Sree Bhushan Raju, Nizam's Institute of Medical Sciences, IndiaReviewed by:
Babak Arjmand, Tehran University of Medical Sciences, IranCopyright © 2022 Shi, Hu, Li, Zhou, Wang, Zhu, Bao, Cheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoshu Cheng, eGlhb3NodW1lbmZhbjEyNkAxNjMuY29t; Ping Li, bGlwaW5nc3lkbmV5QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.