- 1Department of Nephrology, Dialysis and Renal Transplantation, Fondazione IRCCS Ca' Granda Ospedale Policlinico, Milan, Italy
- 2Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy
- 3Renal Research Laboratory Fondazione IRCCS Ca' Granda Ospedale Policlinico, Milan, Italy
- 4Department of Nephrology, Clinical and Translational Sciences, Università degli Studi della Campania L.Vanvitelli, Naples, Italy
- 5Department of General Surgery, Renal Transplantation, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy
Renal transplantation (RTx) allows us to obtain the resolution of the uremic status but is not frequently able to solve all the metabolic complications present during end-stage renal disease. Mineral and bone disorders (MBDs) are frequent since the early stages of chronic kidney disease (CKD) and strongly influence the morbidity and mortality of patients with CKD. Some mineral metabolism (MM) alterations can persist in patients with RTx (RTx-p), as well as in the presence of complete renal function recovery. In those patients, anomalies of calcium, phosphorus, parathormone, fibroblast growth factor 23, and vitamin D such as bone and vessels are frequent and related to both pre-RTx and post-RTx specific factors. Many treatments are present for the management of post-RTx MBD. Despite that, the guidelines that can give clear directives in MBD treatment of RTx-p are still missed. For the future, to obtain an ever-greater individualisation of therapy, an increase of the evidence, the specificity of international guidelines, and more uniform management of these anomalies worldwide should be expected. In this review, the major factors related to post-renal transplant MBD (post-RTx-MBD), the main mineral metabolism biochemical anomalies, and the principal treatment for post-RTx MBD will be reported.
Introduction
Renal transplantation (RTx) is the best therapy for chronic kidney disease (CKD). In this context, RTx permits us to obtain the resolution of the uremic status. Unfortunately, RTx is not able to solve all the metabolic complications present during end-stage renal disease (ESRD).
Mineral and bone disorders (MBD) are frequent from the early stages of CKD and strongly influence the morbidity and mortality of CKD patients. In the presence of an RTx, some mineral metabolism (MM) alterations can persist, also in the presence of complete renal function recovery. These anomalies mainly involve calcium (Ca), phosphorus (P), vitamin-D, and parathormone (PTH), and are responsible for post-renal transplant MBD (post-RTx-MBD). Similar to CKD, also in RTx patients (RTx-p), the MBD can lead to several bone changes in quality and density, thus, determining an important increase in cardiovascular and fracture risk.
In the present review the major factors related to post RTx-MBD, the main MM biochemical anomalies, and the principal treatment for post-RTx MBD will be described.
Mineral and Bone Disorder in RTx
The impact of MBD in patients with RTx is a matter of serious concern. In fact, despite some data reporting a significant bone loss in only 0.1–5.7% of RTx-p, the fracture risk in RTx-p is 5 times higher than in the general population. This risk, present thoughout all the RTx life, is significantly higher during the first 5 years of RTx (1, 2).
The prevalence of MBD in RTx-p could be much higher than previously thought and, therefore, considering the medical complexity of these patients, this process has to be seen as a wide spectrum of risk factors and clinical, biochemical, and histopathologic alterations.
Some data have reported a correlation between fracture events and the risk of mortality. In 2020, Iseri et al. analysed data from the Swedish National Renal Registry of 3,992 first RTx recipients. According to their data, a crude incidence rate of the first episode of major fracture that is present in 279 RTx-p was 13.5/1,000 patient-years, with hip fractures reported in 69 RTx-p in 3.4/1,000 patient-years. The incidence rates were highest during the first 6 months following RTx, and the presence of the first episode of major hip or spine fracture independently predicted an increased all-cause mortality risk (hazard ratio, HR, 1.78) (3).
However, it is important to underline that post-RTx-MBD is not only certainly related to RTx status but is also certainly influenced by the patients in pre-RTx condition.
Pre-transplant Factors Related to RTx-MBD
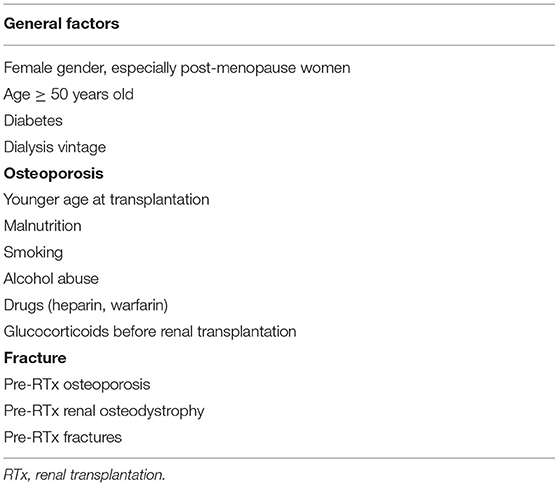
In Table 1, the main pre-RTx factors related to MBD insurgence during transplantation are listed. Among general factors, certainly, the principal modifiable factor is dialysis vintage. In a recent paper published by Sutton et al., several parameters are present in almost 20% of RTx-p, including dialysis vintage, older age, and the presence of severe pre-RTx hyperparathyroidism (HPT) and influence the development of post-RTx HPT. The persistence of this anomaly indicates a pre-RTx MBD of a severe degree (4). This fact is of high relevance and underscores the need to initiate the study for RTx in ESRD as soon as possible, possibly performing living and/or pre-emptive RTx.
An association between bone disease in ESRD and poor nutritional status has been reported in relation to the increase of both intimal and medial vascular calcification and vascular senescence (5). This recent evidence not only increases the cardiovascular and mortality risk in pre-RTx time but also affects the early and long-term RTx outcome (6). This could also explain why some reports indicate that younger age at transplantation is associated with an increase in osteoporosis insurgence risk. This is due to an interplay between the precocious exposure to the uremic toxin and accelerated cellular senescence induced by uremia, and, after RTx, to the administration of glucocorticoid (GC). In fact, from an early age, GC exposure leads to a greater lifetime cumulative GC dose in comparison to patients who are transplanted at an older age. An increased risk of osteoporosis in patients with ESRD has also been related to the long-term use of unfractionated heparin (inhibition of osteoprotegerin and enhancing osteoclastic bone resorption) and warfarin (inhibition of gamma-carboxylation of osteocalcin). All these factors could determine a progressive decrease in bone density (7).
Pre-existing renal osteodystrophy negatively impacts the bone-related and general outcomes in RTx-p (8, 9). Several studies supported this hypothesis such as in the work of Iyer et al., where a cohort of 47 RTx-p glucocorticoids (GCs) was suspended in the first 3 days after RTx. Nevertheless, bone mineral density (BMD) significantly decreased at distal radius at 12 months, while lumbar and hip BMD were not affected (10). This may be due to the persistence of pre-existing renal osteodystrophy, with inappropriately high levels of PTH mainly affecting the compact cortical bone of long bones. In a recent study published by Lee et al., in a multivariate Cox analysis, the pre-RTx osteoporosis and osteopenia were independent risk factors for fracture in 941 RTx-p (HR 11.76 and 5.21, respectively) (11).
Post-transplant Factors Related to RTx-MBD
In the following paragraphs, the main post-RTx factors related to RTx-MBD will be discussed (Figure 1). In particular, we will focus on the alterations in biochemical parameters, which is a crucial point in the evaluation of MBD in RTx-p, and then, we will discuss the problems related to bone disease and vascular calcification (VC). It is important to note that, before a therapeutic setting, all of these three points should be individually characterised.
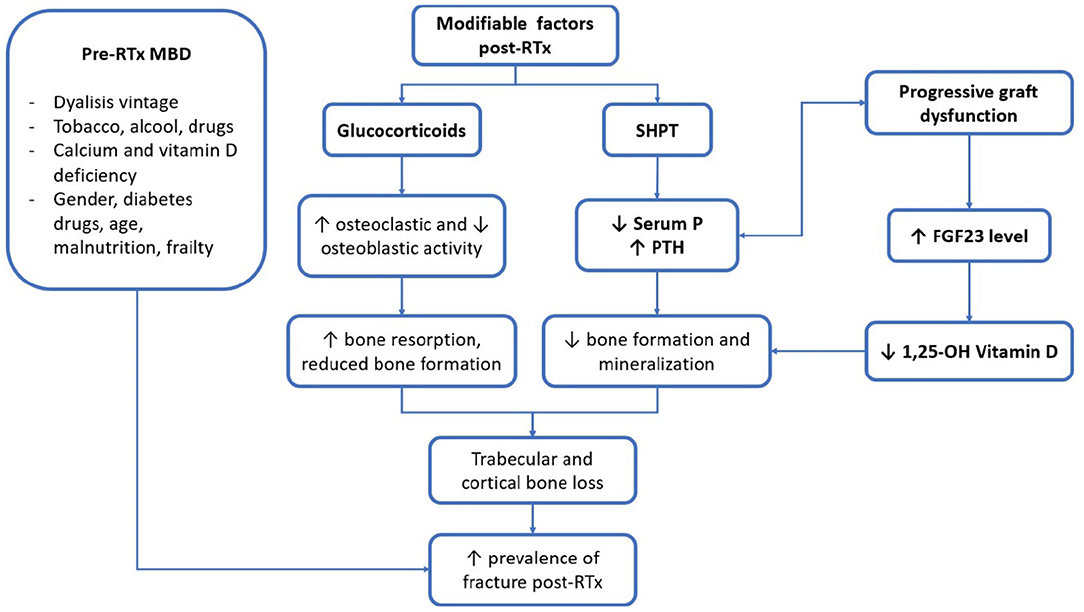
Figure 1. Main clinical and biochemical factors of patients with renal transplantation (RTx-p) mineral metabolism. RTx, renal transplantation; SHPT, secondary hyperparathyroidism; P, phosphorus; PTH, parathormone; FGF23, fibroblast growth factor 23; 1, 25-OH vitamin D, 1, 25 hydroxylated vitamin D.
Biochemical Parameters
Biochemical parameters of MM are subject to important modifications after RTx. In Figure 2, the main factors and their interrelationship in influencing RTx-MBD development are represented. Despite their correlation with mortality for cardiovascular and all-causes events, it is still debated whether these factors may influence the outcome of graft (12). This subject needs to be better clarified by future interventional studies.
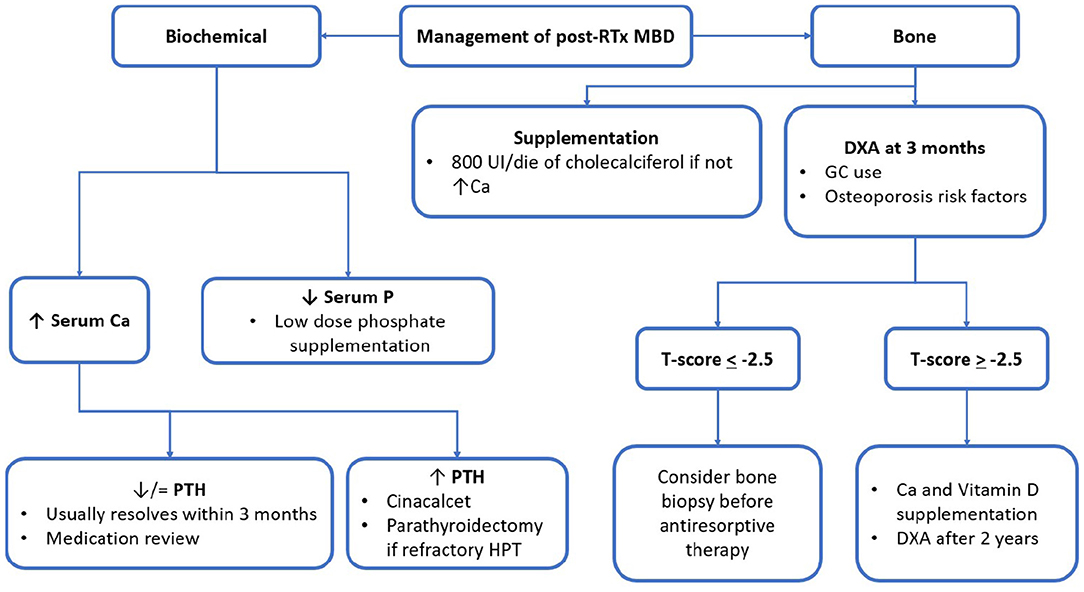
Figure 2. post-RTx MBD monitoring and management algorithm. RTx, renal transplantation; MBD, mineral bone disorder; GC, glucocorticoid; UI, international unit; eGFR, estimated glomerular filtration rate; Ca, calcium; P, phosphorus; PTH, parathormone; ABD, adynamic bone disease; DXA, dual-energy absorptiometry.
Calcium
Usually, serum calcium has a biphasic trend after RTx with an early initial decrease which is probably due to the significant fall in PTH seen after RTx. After that, calcium levels tend to rise, following the increased production of 1,25-OH vitamin D by a healthier kidney and the frequent persistence of HPT. The prevalence of hypercalcemia (hyper-Ca) is reported to be around 10–15% and will reach its maximum in 3 to 6 months after RTx (13). In our experience, a condition of hyper-Ca, defined for Ca levels > 10.4 mg/dl, is present in almost 25% of RTx-p during the first year of RTx. In most cases, hyper-Ca is related to high PTH levels. This condition can be associated with interstitial microcalcifications and potentially impact negatively on graft outcome (4). A work by Thomas et al. showed a negative impact of high calcium level on estimated glomerular filtration rate (eGFR) decrease, especially if hyper-Ca is associated with low P levels (14).
Phosphate
The serum phosphate levels markedly decrease in about 50% of patients after RTx. In our experience, P < 2.4 mg/dl is present in about 50% of RTx-p, after the first month of RTx. A similar condition is present in 15 and 12% of RTx-p after 6 and 12 months of RTx, respectively. Hypo-phosphatemia (Hypo-P) has several causes in RTx-p. First of all, it reflects an improvement in kidney function, which involves a better tubular sensitivity and responsiveness to PTH and fibroblast growth factor-23 (FGF-23) action, which is, sometimes, associated with inappropriately high PTH levels (7). In addition, it is important to remember the effect of some immunosuppressive drugs, such as calcineurin inhibitors (CNIs), that are associated with tubule toxicity and can be the principal reason for the development of Hypo-P in these patients (15).
Abnormalities in P metabolism have been associated with a decrease in osteoblast activity and defective bone mineralization. Post-RTx Hypo-P can have a detrimental effect on bone mineralization and might contribute to impaired osteoblast genesis and early osteoblast apoptosis, which lead to osteoporosis insurgence and progression. In this case, it seems that higher PTH levels exert protective effects on bone by preserving osteoblast survival (16).
PTH and FGF23
Within the first 12 months post-RTx, an initial decrease in the PTH levels is usually found (17). Of note, in up to 50% of cases, as well as in the presence of a well-functioning RTx, a persistent increase of PTH can be present (18). In our experience, the prevalence of high levels of PTH, considering the level of eGFR, in a cohort of 600 RTx-p at 24 months after RTx is around 48%. These data are almost in line with those reported in the literature (19). The impact of persistent HPT on graft outcome is still debated. In a study involving 1,609 RTx-p, persistent HPT was associated with the worse graft survival (10). Nevertheless, the optimal PTH value to achieve in RTx-p is still a matter of debate. The problem of secondary and tertiary HPT in RTx has been deeply presented in a recent paper published by our group (20).
The FGF-23 levels are high in RTx-p during the first 6 months after RTx and are significant in the development and maintenance of low P levels. At the moment, some recent evidence has reported a relationship in RTx-p of FGF-23 levels of RTx with anaemia status and cardiovascular risk (21, 22).
In any case, regarding the RTx-p, the FGF-23 utility in clinical practise is still debated.
Vitamin D
Native vitamin D (25-OH-VitD) is frequently below the sufficient levels in RTx-p. This fact reflects the pre-RTx status but is also related to some RTx specific factors. The evaluation of 25-OH-VitD and active vitamin D form (1-25-OH-VitD) is important in RTx-p. Increased availability of 1-25-OH-VitD might contribute to the development of hyper-Ca by the induction of renal 1-α-OH-ase activity (23). Of note, low levels of 25-OH-VitD in RTx-p are very frequent especially in the early phases of KTx (24). The persistence of low levels of 25-OH-VitD is determined by the need to reduce the sunlight exposition by also using dermatological creams with high protection and, frequently, by low therapeutic compliance. Vitamin D deficiency can have an important role in post-RTx's secondary HPT insurgence (25, 26). Also, in this case, immunosuppressive therapy has a role. GCs induce enzymes related to vitamin D catabolism and increase PTH and FGF23 levels (27). Also, CNIs appear to be inversely associated with vitamin D levels, maybe through inhibition of the 25-OH-ase activity of CYP3A4 by these drugs and downregulation of vitamin-D receptor (VDR) (28, 29).
Recently, it has been reported that low levels of 25-OH-VitD might be associated with worse graft outcomes, thus, promoting rejection episodes, possibly through the immunomodulatory mechanism exerted on the immune system (30). In any case, the direct and independent effect of 25-OH-VitD on graft outcome is still a matter of debate (26, 31–33).
Bone Disease
The most prevalent type of bone alteration in RTx-p is still debated. In this context, the bone biopsy might be considered the gold standard to obtain a precise bone evaluation in these patients. Unfortunately, at the moment, the indications and assessments of bone biopsy are difficult in clinical practise, and bone biopsy is performed only for research purposes. In a cohort of 20 RTx-p, adynamic bone disease (ABD) was the main lesion seen on biopsy specimen (34), while in a different group of 57 RTx-p, the main finding was osteitis fibrosa (35). This evident difference between these two studies may reflect the time in which the bone biopsy was performed, suggesting that RTx bone disease is a long-term evolving process. Recently, two other studies showed the same conflicting results. Neves et al. evaluated histomorphometric parameters in their cohort 2 years after RTx and the results revealed a mineralization defect and a cellular pattern consistent with HPT, with higher osteoid, osteoblastic, osteoclastic, and resorption surface. This pattern was, in part, attributable to hypovitaminosis D (5). On the contrary, Satu et al. conducted a study on 27 RTx-p, performing a bone biopsy at baseline and 2 years after RTx. They found in their cohort a marked decrease in bone turnover and an increase in abnormal bone mineralization. Moreover, they observed a significant decline in osteoblasts and osteoclasts activity and, thus, of bone formation rate. This happened in parallel with a sharp rise in Ca, 25-OH-VitD, and 1-25-OH-VitD, and a decrease in P, PTH, and osteocalcin levels. The abrupt lowering of serum PTH, combined with the fall in serum P levels, could be responsible for these changes in bone metabolism, although, the reason why higher vitamin D levels don't prevent abnormal mineralization remains unexplained (36).
In a very recent study by Jorgensen et al., performed in 97 RTx-p and followed up for 12 months after RTx, interesting results on this matter were shown. The histomorphometric study performed in this cohort showed an overall decrease in skeletal remodelling and bone turnover after RTx. Nevertheless, during the first year of RTx disorders of bone formation linked to HPT, such as excessive bone resorption and marrow fibrosis, were markedly decreased on histologic samples (37). Furthermore, in patients for whom it was possible to obtain dynamic histomorphometric data, similar findings were obtained with no significant changes in bone volume. This follows some evidence in literature where the bone undergoes an initial slowing in turnover rate, which lays the foundations for the subsequent increment in bone mass, mineralization, and overall health (1, 38). Cumulative steroid dosage was directly correlated with slower bone turnover and mineralization. Even if the wider use of steroid minimisation protocols has reduced the impact of GC on bone quality and density after-RTx, it appears that the negative effect of GC is exerted even with low exposure, with the most evident consequences on hip-MBD (37). Bone mineralization showed to be greatly influenced also by phosphate levels. The severity and the duration of hypophosphatemia negatively affected bone mineralization (37, 39). Since several factors that influence bone health, in a positive and a negative manner, come into play in the post-RTx period, it is readably understandable why there has been such great variability in the findings regarding post-RTx BMD.
Another important result highlighted in this study is the reliability of the dual-energy x-ray absorptiometry (DEXA) in evaluating bone mineralization. In this cohort of patients, DEXA findings were suggestive for substantial stability of MBD, especially in the axial skeleton. The main changes, found in a minority of the cohort, are a decrease in MBD at the distal radius and an increase in bone mass at the hip (37). These findings are in accordance with histomorphometric and biochemical findings, thus, corroborating the diagnostic and prognostic value of DEXA in the follow-up of RTx-BMD. Moreover, recent studies have highlighted that DEXA is reliable not only in assessing MBD but also in predicting patient's fracture risk, thus, giving the clinician a rapid and exhaustive overview of the bone status (40, 41).
Moreover, one of the most important findings of this study was the observation that some bone turnover markers (not subjected to significative renal clearance) were reliable markers in predicting the changes in bone histomorphometry. The most important markers are bone alkaline phosphatasis (BALP) (which reflects osteoblast activity), TRAP5b (which reflects osteoclasts number), and PINP (a fragment of pro-collagen laid down during bone deposition) (37, 42). These findings are of major importance because there is an urgent need for a non-invasive method to precisely assess bone status and activity. There is already some literature regarding these novel biomarkers, but more dedicated trials are needed to clarify whether these molecules could effectively be used as a “liquid biopsy” in the near future (43).
Regarding osteoporosis, the use of GCs remains the key risk factor for the development of this type of bone disease. An early post-RTx time is characterised by a rapid loss of bone mass that mainly affects trabecular bone due to intensive GC regimens (44). The GCs inhibit osteoblast proliferation and differentiation and stimulate apoptosis of both osteoblasts and osteoclasts. Moreover, they have indirect effects on the skeleton, inhibiting the synthesis of testosterone, oestrogen, and adrenal androgens. Lowering steroids exposure can reduce bone loss and should be especially considered for young patients, and, when possible, in RTx-p with severe and documented pre-RTx bone disease (45). Various studies have demonstrated that both early and late GC withdrawal can improve patients' bone health (46). Lastly, GCs have shown, in vitro, their capacity to enhance bone response to PTH (47). As underlined in two consecutive studies by Evenepoel et al. and Jorgensen et al., the GC cumulative dosage reduction of the latest immunosuppressive protocols had a great beneficial impact on bone health, favouring bone mineralization and reducing bone loss. Steroid minimisation protocols were associated with stable MBD after RTx, with significantly present bone loss only in the distal radius area (37, 38, 48). However, it must be remembered that even low doses of GC hamper bone metabolism, reducing bone formation and mineralization, and independently increasing the fracture risk (34, 49). Therefore, it is of foremost importance to aim for GC minimisation strategy, whenever possible, because of the great impact that this particular therapeutic approach could have on different aspects of patients' quality of life. A strong debate is present concerning the groups of patients, in which an early GC withdrawal is recommended. In our Unit in Milan, an accurate discussion for any case is made; the GS withdrawal is usually prescribed in presence of young recipients, as well as in patients with basal nephropathy with a low risk of recurrence (f.i. ADPKD) and, if possible, in patients with strong pre-RTx diabetes.
Vascular Calcifications
As previously described, the development of vascular calcifications (VC) is a dynamic and actively regulated process. By the time a patient requires RTx, endothelial dysfunction and atherosclerosis are common, with 50–60% of patients having detectable vascular calcifications (50). Despite RTx and amelioration of kidney function, calcification of the thoracic aorta and coronary arteries progresses around 4 and 11% per year, respectively (51). Increasing evidence is emerging regarding the influence of bone health and metabolism and vascular calcifications even in RTx-p. In a work performed by our group, the presence of aortic calcification was found in more than 60% of cases, and osteoprotegerin levels were inversely associated with VC prevalence and progression 12 months after RTx. A recent study by Sotomayor et al. performed in 678 RTx-p found that the prevalence of VC was 9% in patients with normal BMD, 11% in patients with osteopenia, and 25% in patients with osteoporosis. Moreover, higher BMD was associated with a lower risk of VC, independent of age, gender, body mass index, eGFR, and immunosuppressive therapy (52).
At present, several studies have reported an association between VC and mortality (53, 54). In a study by Lewis et al. in patients with RTx and kidney-pancreas transplantation, a significant association between VC and mortality was found. However, no correlation was reported with graft outcome (55). In another study performed by our group, a significant progression of coronary calcifications in a 5-year follow-up has been demonstrated. Both the presence of coronary calcifications and the progression were associated with cardiac events and death (56).
Management of the Post-Transplant Bone Mineral Disorder
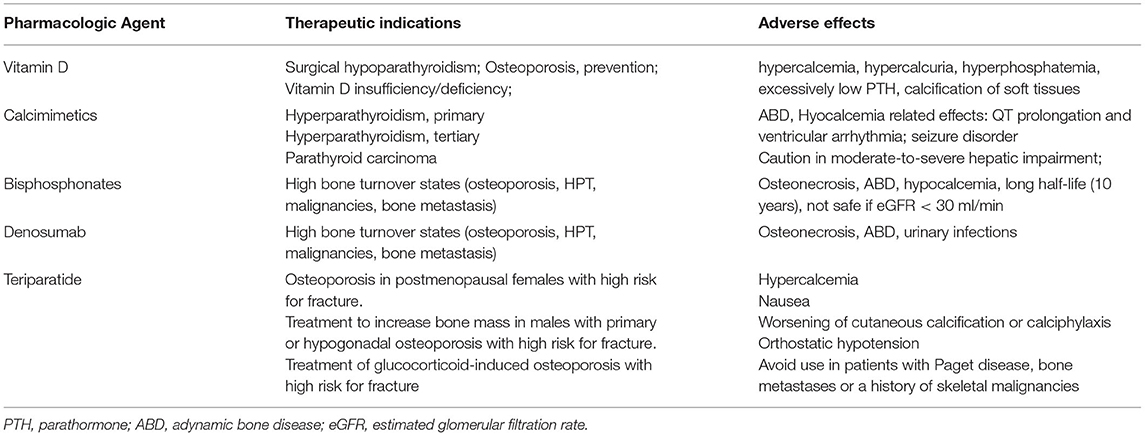
Despite the approach to post-RTx MBD being postulated on KDIGO guidelines, levels of recommendations are still not satisfying enough (45, 57). Thus, there is a need for stronger evidence to make it possible for researchers to delineate firmer guidelines for clinical practise. The following figure summarised the main principles for MBD treatment during RTx (Figure 2). In the following part, the review will focus on the pharmacologic agents of major interest in clinical practise. To help the reader, Tables 2, 3 were reports of the key readings about MBD post-RTx and the main therapeutic agents that have been reported.
Vitamin D Supplementation
Vitamin D supplementation can lower bone loss both in the femoral and lumbar regions, but nowadays, evidence regarding the eventual effect on fracture rate is still lacking. Moreover, vitamin D has well-known pleiotropic effects on the renin-angiotensin system and immune-modulatory power on T and dendritic cells, virtually influencing graft function and outcome (58). Data on the effect of vitamin D supplementation on post-RTx MBD are controversial. Recent evidence has found that the administration of 25,000 UI of cholecalciferol was effective in reducing PTH levels, but not in ameliorating MBD (59, 60). However, in other studies, when vitamin D was administered in combination with calcium supplementation, preservation of BMD in various sites was observed. In particular, one report indicated that vitamin D 400 UI/die, combined with 600 mg/die of oral calcium, was associated at 12 months with a reduction in lumbar spine, femoral neck, and total hip bone loss (61). A possible dosage of 25-OH-VitD might be 400 UI/die in RTx-p with CKD G1-3T (62). Nevertheless, there has been little research investigating the impact of Vitamin D levels and supplementation on fractures incidence. A recent retrospective observational trial found out that higher supplementation of Vitamin D levels was associated with a reduction in vitamin deficiency and a pronounced reduction in fracture rate, from 9.1 to 3.1% (63). All these studies suggest that supplementation of vitamin D, both active and inactive forms could ameliorate post-RTx MBD, but more research on this topic is needed to better delineate the effects of vitamin D on bone homeostasis in RTx-p.
Paracalcitol has shown similar effects to native vitamin D but was linked to a higher risk of hypercalcemia (64). A randomised study found a protective effect of paricalcitol on bone remodelling, with a reduction in bone alkaline phosphatasis (bALP) and osteocalcin in association with improvements in lumbar and spine BMD (65).
Calcimimetics
Cinacalcet is a positive allosteric modulator of calcium-sensing receptor (CaSr). In a study concerning Hyper-Ca and secondary HPT, performed on 114 RTx-p, cinacalcet effectively reduced PTH and calcium and determined an increase in serum phosphorus without any adverse effects on graft function compared to placebo (66). Recently, Bernador et al. performed a multicentre study that involved 20 paediatric RTx-p with SHPT. This work showed a significant and linear relationship between cinacalcet dose and therapy duration, PTH and calcium reduction, and phosphorus increase. Most importantly, no significant impact on eGFR was found (67). A recent study performed by Hyang et al., performed on 9 RTx-p with tertiary HPT showed conflicting results. Cinacalcet was able to normalise serum calcium and phosphorus levels but failed to normalise PTH in those patients who had very high baseline PTH levels (68). This may imply that more studies are needed to ascertain if cinacalcet could be a definitive and effective alternative to parathyroidectomy in RTx-p. Moreover, in other studies, it has been observed that cinacalcet had no positive effects on BMD even if it suppresses PTH. This might be due to the concomitant sensitisation of calcium-sensing receptors in the bone, which may counteract benefits deriving from lower PTH levels (69). An important side effect of cinacalcet is that of inducing hypercalciuria and consequent nephrocalcinosis, even if a small study involving 34 RTx-p found no lesions of this kind in biopsies conducted at 3 and 12 months after RTx (70). Moreover, the effect of cinacalcet on bone histomorphometry has not been extensively studied yet.
Antiresorptive Agents
Bisphosphonates and denosumab are common agents widely used in osteoporosis. They have to be prescribed carefully in RTx-p, especially in patients who are at risk of ABD, and, thus, a bone biopsy could be considered before the start of these agents (45).
Bisphosphonates bind strongly to the mineralized bone, inhibiting osteoclasts' action and inducing apoptosis of these cells. Their half-life is particularly long, about 10 years. About 50% of the drug is not taken up by the bone and is cleared by the kidney via glomerular filtration and tubular secretion. Thus, the safety of these drugs strongly depends on kidney function. For this reason, they are considered safe if used in presence of an eGFR > 30 ml/min (45).
Most of the studies conducted on bisphosphonates in RTx-p showed preservation of lumbar spine and femoral neck BMD in the early post-RTx period. Furthermore, ABD has not been a widely observed phenomenon. In a study conducted by Coco et al., pamidronate, combined with calcium and calcitriol supplementation, led to the preservation of vertebral BMD, but increased the low turnover rate on bone biopsy (71). A a few years later the same group studied the effects of risedronate on BMD. They found that this drug was not associated with an increased risk of ABD but did not affect the preservation of BMD either (56). This may reflect different drug power, with second-generation bisphosphonates (pamidronate), having a stronger enzymatic inhibition ability than the third generation (risendronate).
A series of studies, in which vitamin D was part of the standard care or where there was a reduced GCs load, failed to show bisphosphonates' benefits on BMD (72). This suggests that nowadays, considering the widespread use of vitamin D and low GCs exposure, bisphosphonates could be reserved only for patients who are at high-risk.
Denosumab is a humanised monoclonal antibody directed against the receptor activator of the Nf-kB ligand. It reduces bone resorption, significantly increases BMD, and decreases the risk of fracture in women with osteoporosis (73).
Its efficacy has also been proven in patients with severe impairment of kidney function (74). Different to bisphosphonates, Denosumab is not cleared, so it is an attractive therapy in CKD and RTx-p. Recently, interesting studies have emerged about its use in RTx-p. Bonani et al. performed a randomised case-control trial on the denosumab effect on a cohort of 90 RTx-p. They administered the drug twice a year for the first two years of RTx. They found that treatment with denosumab was associated with increased BMD at 12 months in both lumbar spine and hip; while at 6 months, only the lumbar spine showed significant benefits on BMD. Moreover, bone turnover decreased significantly in the denosumab group, whereas it remained unchanged in the control group. Infections were significantly more frequent in the denosumab group, and they were mainly of bacterial aetiology, with UTI being the most frequent. Viral infections showed no difference between the two groups. Hypocalcaemia was more frequent in the denosumab group (75). A study performed by our group on 32 RTx-p showed similar results. After 12 months of denosumab therapy, there was a significant improvement of T score both at vertebral and femoral neck sites. The improvement was so significant that caused a reduction in the number of patients affected by osteoporosis, because many patients shifted to osteopenia class, according to T-score. No significant relationship between denosumab and hypocalcaemia and UTI was found in our cohort (76). A recent meta-analysis confirmed the results observed in these studies. Denosumab was associated with increased BMD at 12 months at the lumbar spine and femoral neck. Hypocalcaemia was a frequent adverse effect, but all reported episodes were mild and asymptomatic, requiring only Ca and vitamin D supplementation. Infections were confirmed to be another frequent complication following denosumab administration; in particular, it was confirmed an increased frequency of diarrhoea and UTI. However, no differences in RTx function and rejection were found (77).
Teriparatide
This anabolic agent can improve BMD in RTx-p with GC-induced osteoporosis. However, in a study conducted on 26 RTx-p with a FU time of 6 months, patients who received teriparatide did not show improvement of bone density in the lumbar spine and distal radius; still, there was a stabilisation of BMD in the femoral neck (78). Although teriparatide could be useful for patients with osteoporosis and ABD, given the cost of the drug, more RCTs are needed to delineate solid indications for its use in RTx-p.
Conclusions
The RTx is characterised by several mineral and bone anomalies, resulting in loss of bone density, increased risk of fracture, and potentially increased risk of mortality. The correct treatment of this condition is strictly determined by a deep and careful evaluation of biochemical anomalies and of the pre- and post-RTx MBD-related factors (f.i. immunosuppression, anticoagulant therapy, etc.) present in any specific case. The disorders might be sometimes difficult to be treated, but fortunately, at the moment, many different therapeutic options are available. In the future, to obtain an ever-greater individualisation of therapy, an increase of the evidence and the specificity of international guidelines and more uniform management of these anomalies worldwide should be expected.
Author Contributions
PMo, CA, DM, MC, AC, EF, SM, PMe, and GC actively contributed to the manuscript by writing individual sections of the final article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brandenburg VM, Politt D, Ketteler M, Fassbender WJ, Heussen N, Westenfeld R, et al. Early rapid loss followed by long-term consolidation characterizes the development of lumbar bone mineral density after kidney transplantation. Transplantation. (2004) 77:1566–71. doi: 10.1097/01.TP.0000131990.13277.28
2. Nikkel LE, Hollenbeak CS, Fox EJ, Uemura T, Ghahramani N. Risk of fractures after renal transplantation in the United States. Transplantation. (2009) 87:1846–51. doi: 10.1097/TP.0b013e3181a6bbda
3. Iseri K, Carrero JJ, Evans M, Felländer-Tsai L, Berg HE, Runesson B, et al. Fractures after kidney transplantation: Incidence, predictors, and association with mortality. Bone. (2020) 140:115554. doi: 10.1016/j.bone.2020.115554
4. Sutton W, Chen X, Patel P, Karzai S, Prescott JD, Segev DL, et al. Prevalence and risk factors for tertiary hyperparathyroidism in kidney transplant recipients. Surgery. (2022) 171:69–76. doi: 10.1016/j.surg.2021.03.067
5. Hobson S, Arefin S, Kublickiene K, Shiels PG, Stenvinkel P. Senescent Cells in Early Vascular Ageing and Bone Disease of Chronic Kidney Disease-A Novel Target for Treatment. Toxins (Basel). (2019) 11:82. doi: 10.3390/toxins11020082
6. Dai L, Qureshi AR, Witasp A, Lindholm B, Stenvinkel P. Early vascular ageing and cellular senescence in chronic kidney disease. Comput Struct Biotechnol J. (2019) 17:721–9. doi: 10.1016/j.csbj.2019.06.015
7. Signorelli SS, Scuto S, Marino E, Giusti M, Xourafa A, Gaudio A. Anticoagulants and Osteoporosis. Int J Mol Sci. (2019) 20:5275. doi: 10.3390/ijms20215275
8. Malluche HH, Monier-Faugere M-C, Herberth J. Bone disease after renal transplantation. Nat Rev Nephrol. (2010) 6:32–40. doi: 10.1038/nrneph.2009.192
9. Neves CL, dos Reis LM, Batista DG, Custodio MR, Graciolli FG, Martin RC, et al. Persistence of bone and mineral disorders 2 years after successful kidney transplantation. Transplantation. (2013) 96:290–6. doi: 10.1097/TP.0b013e3182985468
10. Iyer SP, Nikkel LE, Nishiyama KK, Dworakowski E, Cremers S, Zhang C, et al. Kidney transplantation with early corticosteroid withdrawal: paradoxical effects at the central and peripheral skeleton. J Am Soc Nephrol. (2014) 25:1331–41. doi: 10.1681/ASN.2013080851
11. Lee ES, Lim JH, Cho JH, Jung HY, Choi JY, Park SH, et al. KNOW-KT Study Group. Pretransplant osteoporosis and osteopenia are risk factors for fractures after kidney transplantation. Transplant Proc. (2019) 51:2704–9. doi: 10.1016/j.transproceed.2019.03.071
12. Alfieri C, Mattinzoli D, Messa P. Tertiary and postrenal transplantation hyperparathyroidism. Endocrinol Metab Clin North Am. (2021) 50:649–62. doi: 10.1016/j.ecl.2021.08.004
13. Wolf M, Weir MR, Kopyt N, Mannon RB, Von Visger J, Deng H, et al. A prospective cohort study of mineral metabolism after kidney transplantation. Transplantation. (2016) 100:184–93. doi: 10.1097/TP.0000000000000823
14. Hiemstra TF, Brown AJD, Chaudhry AN, Walsh M. Association of calcium, phosphate and parathyroid hormone with renal allograft function: a retrospective cohort study. Am J Nephrol. (2013) 37:339–45. doi: 10.1159/000348376
15. Chevarria J, Sexton DJ, Murray SL, Adeel CE, O'Kelly P, Williams YE, et al. Calcium and phosphate levels after kidney transplantation and long-term patient and allograft survival. Clin Kidney J. (2021) 14:1106–13. doi: 10.1093/ckj/sfaa061
16. Rojas E, Carlini RG, Clesca P, Arminio A, Suniaga O, De Elguezabal K, et al. The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney Int. (2003) 63:1915–23. doi: 10.1046/j.1523-1755.2003.00938.x
17. Reinhardt W, Bartelworth H, Jockenhovel F, Schmidt-Gayk H, Witzke O, Wagner K, et al. Sequential changes of biochemical bone parameters after kidney transplantation. Nephrol Dial Transplant. (1998) 13:436–442. doi: 10.1093/oxfordjournals.ndt.a027843
18. Muirhead N, Zaltman JS, Gill JS, Churchill DN, Poulin-Costello M, Mann V, et al. Hypercalcemia in renal transplant patients: prevalence and management in Canadian transplant practice. Clin Transplant. (2014) 28:161–5. doi: 10.1111/ctr.12291
19. Lou I, Foley D, Odorico SK, Leverson G, Schneider DF, Sippel R, et al. How well does renal transplantation cure hyperparathyroidism? Ann Surg. (2015) 262:653–9. doi: 10.1097/SLA.0000000000001431
20. Messa P, Alfieri CM. Secondary and Tertiary Hyperparathyroidism. Front Horm Res. (2019) 51:91–108. doi: 10.1159/000491041
21. Algul Durak B, Karakan MS Sr. Circulating Fibroblast Growth Factor-23 is Associated with Cardiovascular Prognosis and Graft Function in Renal Transplant Recipients. Cureus. (2020) 12:e7140. doi: 10.7759/cureus.7140
22. Baloglu I, Ozer H, Ozturk Y, Erdur MF, Tonbul HZ, Turkmen K. The relationship between FGF23 and anemia in HD and renal transplant patients. Int Urol Nephrol. (2021). doi: 10.1007/s11255-021-02982-9. [Epub ahead of print].
23. Saha HH, Salmela KT, Ahonen PJ, Pietilä KO, Mörsky PJ, Mustonen JT, et al. Sequential changes in vitamin D and calcium metabolism after successful renal transplantation. Scand J Urol Nephrol. (1994) 28:21–7. doi: 10.3109/00365599409180465
24. Stavroulopoulos A, Cassidy MJ, Porter CJ, Hosking DJ, Roe SD. Vitamin D status in renal transplant recipients. Am J Transplant. (2007) 7:2546–52. doi: 10.1111/j.1600-6143.2007.01978.x
25. Alshayeb HM, Josephson MA, Sprague SM. CKD-mineral and bone disorder management in kidney transplant recipients. Am J Kidney Dis. (2013) 61:310–25. doi: 10.1053/j.ajkd.2012.07.022
26. McGregor R, Li G, Penny H, Lombardi G, Afzali B, Goldsmith DJ. Vitamin D in renal transplantation - from biological mechanisms to clinical benefits. Am J Transplant. (2014) 14:1259–70. doi: 10.1111/ajt.12738
27. Cianciolo G, Galassi A, Capelli I, Angelini M, La Manna G, Cozzolino M. Vitamin D in kidney transplant recipients: mechanisms and therapy. Am J Nephrol. (2016) 43:397–407. doi: 10.1159/000446863
28. Filipov JJ, Zlatkov BK, Dimitrov EP, Svinarov D. Relationship between vitamin D status and immunosuppressive therapy in kidney transplant recipients. Biotechnol Equip. (2015) 29:331–5. doi: 10.1080/13102818.2014.995415
29. Burkhalter F, Schaub S, Dickenmann M. Preserved circannual rhythm of vitamin D in kidney transplant patients. Swiss Med Wkly. (2012) 142:w13672. doi: 10.4414/smw.2012.13672
30. Lee JR, Dadhania D, August P, Lee JB, Suthanthiran M, Muthukumar T. Circulating levels of 25-hydroxyvitamin D and acute cellular rejection in kidney allograft recipients. Transplantation. (2014) 98:292–9. doi: 10.1097/TP.0000000000000055
31. Doi Y, Hamano T, Ichimaru N, Tomida K, Obi Y, Fujii N, et al. Serum phosphate levels modify the impact of parathyroid hormone levels on renal outcomes in kidney transplant recipients. Sci Rep. (2020) 10:13766. doi: 10.1038/s41598-020-70709-4
32. Thiem U, Heinze G, Segel R, Perkmann T, Kainberger F, Muhlbacher F, et al. VITA-D: Cholecalciferol substitution in vitaminDdeficient kidney transplant recipients: A randomized, placebo-controlled study to evaluate the posttransplant outcome. Trials. (2009) 10:36. doi: 10.1186/1745-6215-10-36
33. Obi Y, Ichimaru N, Sakaguchi Y, Iwadoh K, Ishii D, Sakai K, et al. Correcting anemia and native vitamin D supplementation in kidney transplant recipients: a multicenter, 2 × 2 factorial, open-label, randomized clinical trial. Transpl Int. (2021) 34:1212–25. doi: 10.1111/tri.13885
34. Cruz EAS, Lugon JR, Jorgetti V, Draibe SA, Carvalho AB. Histologic evolution of bone disease 6 months after successful kidney transplantation. Am J Kidney Dis. (2004) 44:747–56. doi: 10.1016/S0272-6386(04)00955-2
35. Lehmann G. Ott, Stein G, Steiner T, Wolf G. Renal osteodystrophy after successful renal transplantation: a histomorphometric analysis in 57 patients. Transplant Proc. (2007) 39:3153–8. doi: 10.1016/j.transproceed.2007.10.001
36. Keronen S, Martola L, Finne P, Burton IS, Kröger H, Honkanen E. Changes in bone histomorphometry after kidney transplantation. Clin J Am Soc Nephrology. (2019) 14:894–903. doi: 10.2215/CJN.09950818
37. Jørgensen HS, Behets G, Bammens B, Claes K, Meijers B, Naesens M, et al. (n.d.). Natural History of Bone Disease following Kidney Transplantation.
38. Evenepoel P, Claes K, Meijers B, Laurent MR, Bammens B, Naesens M, et al. Natural history of mineral metabolism, bone turnover and bone mineral density in de novo renal transplant recipients treated with a steroid minimization immunosuppressive protocol. Nephrol Dial Transplant. (2020) 35:697–705. doi: 10.1093/ndt/gfy306
39. Bhadada SK, Rao SD. Role of phosphate in biomineralization. Calcif Tissue Int. (2021) 108:32–40. doi: 10.1007/s00223-020-00729-9
40. Iimori S, Mori Y, Akita W, Kuyama T, Takada S, Asai T, et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients–a single-center cohort study. Nephrol Dial Transplant. (2012) 27:345–51. doi: 10.1093/ndt/gfr317
41. Akaberi S, Simonsen O, Lindergård B, Nyberg G. Can DXA predict fractures in renal transplant patients? Am J Transplant. (2008) 8:2647–51. doi: 10.1111/j.1600-6143.2008.02423.x
42. Evenepoel P, Cavalier E, D'Haese PC. Bone biomarkers in de novo renal transplant recipients. Clin Chim Acta. (2020) 501:179–85. doi: 10.1016/j.cca.2019.10.035
43. Soeiro EMD, Castro L, Menezes R, Elias RM, dos Reis LM, Jorgetti V, et al. Association of parathormone and alkaline phosphatase with bone turnover and mineralization in children with CKD on dialysis: effect of age, gender, and race. Pediatric Nephrol. (2020) 35:1297–305. doi: 10.1007/s00467-020-04499-2
44. Monier-Faugere MC, Mawad H, Qi Q, Friedler RM, Malluche HH. High prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. J Am SocNephrol. (2000) 11:1093–9. doi: 10.1681/ASN.V1161093
45. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group: KDIGO clinical practice guideline for the diagnosis evaluation prevention, and treatment of Chronic Kidney Disease-Mineral and BoneDisorder (CKD-MBD). Kidney Int Suppl. (2009) 113: S1–S130. doi: 10.1038/ki.2009.188
46. Farmer CKT, Hampson G, Abbs IC, Hilton RM, Koffman CG, Fogelman I, et al. Late low-dose steroid withdrawal in renal transplant recipients increases bone formation and bone mineral density. Am J Transplant. (2006) 6:2929–36. doi: 10.1111/j.1600-6143.2006.01557.x
47. Chen TL, Feldman D. Glucocorticoid receptors and actions in subpopulations of cultured rat bone cells. Mechanism of dexamethasone potentiation of parathyroid hormone-stimulated cyclic AMP production. J Clin Investig. (1979) 63:750–8. doi: 10.1172/JCI109359
48. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. (2017) 7:1–59. doi: 10.1016/j.kisu.2017.04.001
49. van Staa TP, Leufkens HGM, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. (2002) 13:777–87. doi: 10.1007/s001980200108
50. Chau K, Martinez G, Elder GJ. Vascular calcification in patients undergoing kidney and simultaneous pancreas-kidney transplantation. Nephrology (Carlton). (2014) 19:275–81 doi: 10.1111/nep.12212
51. Marechal C, Coche E, Goffin E, Dragean A, Schlieper G, Nguyen P, et al. Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. Am J Kidney Dis. (2012) 59:258–69. doi: 10.1053/j.ajkd.2011.07.019
52. Sotomayor CG, Benjamens S, Gomes-Neto AW, Pol RA, Groothof D, Te Velde-Keyzer CA, et al. Bone Mineral Density and Aortic Calcification: Evidence for a Bone-vascular Axis After Kidney Transplantation. Transplantation. (2021) 105:231–9 doi: 10.1097/TP.0000000000003226
53. Claes KJ, Heye S, Bammens B, Kuypers DR, Meijers B, Naesens M, et al. Aortic calcifications and arterial stiffness as predictors of cardiovascular events in incident renal transplant recipients. Transpl Int. (2013) 26:973–81. doi: 10.1111/tri.12151
54. Disthabanchong S, Vipattawat K, Phakdeekitcharoen B, Kitiyakara C, Sumethkul V. Abdominal aorta and pelvic artery calcifications on plain radiographs may predict mortality in chronic kidney disease, hemodialysis and renal transplantation. Int Urol Nephrol. (2018) 50:355–64. doi: 10.1007/s11255-017-1758-9
55. Lewis JR, Wong G, Taverniti A, Vucak-Dzumhur M, Elder GJ. Association between Aortic Calcification, Cardiovascular Events, and Mortality in Kidney and Pancreas-Kidney Transplant Recipients. Am J Nephrol. (2019) 50:177–86. doi: 10.1159/000502328
56. Alfieri C, Forzenigo L, Tripodi F, Meneghini M, Regalia A, Cresseri D, et al. Long-term evaluation of coronary artery calcifications in kidney transplanted patients: a follow up of 5 years. Sci Rep. (2019) 9:6869. doi: 10.1038/s41598-019-43216-4
57. Chen W, Bushinsky DA. Chronic kidney disease: KDIGO CKD-MBD guideline update: evolution in the face of uncertainty. Nat Rev Nephrol. (2017) 13:600–2. doi: 10.1038/nrneph.2017.118
58. Bienaime' F, Girard D, Anglicheau D, Canaud G, Souberbielle JC, Kreis H, et al. Vitamin D status and outcomes after renal transplantation. J Am Soc Nephrol. (2013) 24:831–41. doi: 10.1681/ASN.2012060614
59. Wissing KM, Broeders N, Moreno-Reyes R, Gervy C, Stallenberg B, Abramowicz DA. controlled study of vitamin D3 to prevent bone loss in renal-transplant patients receiving low doses of steroids. Transplantation. (2005) 79:108–15. doi: 10.1097/01.TP.0000149322.70295.A5
60. Torres A, Garcia S, Gomez A, Gonzalez A, Barrios Y, Concepcion MT, et al. Treatment with intermittent calcitriol and calcium reduces bone loss after renal transplantation. Kidney Int. (2004) 65:705–12. doi: 10.1111/j.1523-1755.2004.00432.x
61. Sahin G, Yasar NS, Sirmagul B, Bal C, Yalcin AU. The effect of low-dose cholecalciferol and calcium treatment on posttransplant bone loss in renal transplant patients: a prospective study. Ren Fail. (2008) 30:992–9. doi: 10.1080/08860220802406369
62. Holden RM, Mustafa RA, Alexander RT, Battistella M, Bevilacqua MU, Knoll G, et al. Canadian Society of Nephrology Commentary on the Kidney Disease Improving Global Outcomes 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder. Can J Kidney Health Dis. (2020) 7:2054358120944271. doi: 10.1177/2054358120944271
63. Perrin P, Kiener C, Javier RM, Braun L, Cognard N, Gautier-Vargas G, et al. Recent changes in chronic kidney disease-mineral and bone disorders (CKD-MBD) and associated fractures after kidney transplantation. Transplantation. (2017) 101:1897–1905. doi: 10.1097/TP.0000000000001449
64. Amer H, Griffin MD, Stegall MD, Cosio FG, Park WD, Kremers WK, et al. Oral paricalcitol reduces the prevalence of posttransplant hyperparathyroidism: Results of an open label randomized trial. Am J Transplant. (2013) 13:1576–85. doi: 10.1111/ajt.12227
65. Trillini M, Cortinovis M, Ruggenenti P, Reyes Loaeza J, Courville K, Ferrer-Siles C, et al. Paricalcitol for secondary hyperparathyroidism in renal transplantation. J Am Soc Nephrol. 2015 26:1205–14. doi: 10.1681/ASN.2013111185
66. Evenepoel P, Cooper K, Holdaas H, Messa P, Mourad G, Olgaard K, et al. A randomized study evaluating cinacalcet to treat hypercalcemia in renal transplant recipients with persistent hyperparathyroidism. Am J Transplant. (2014) 14:2545–55. doi: 10.1111/ajt.12911
67. Bernardor J, Schmitt CP, Oh J, Sellier-Leclerc AL, Büscher A, Dello Strologo L, et al. The use of cinacalcet after pediatric renal transplantation: an international CERTAIN Registry analysis. Pediatric Nephrol. (2020) 35:1707–18. doi: 10.1007/s00467-020-04558-8
68. Jo HA, Han KH, So YK, Jun H, Han SY. Effect of Cinacalcet in Kidney Transplant Patients With Hyperparathyroidism. Transplant Proc. (2019) 51:1397–401. doi: 10.1016/j.transproceed.2019.01.141
69. Peacock M, Bolognese MA, Borofsky M, Scumpia S, Sterling LR, Cheng S, et al. Cinacalcet treatment of primary hyperparathyroidism: Biochemical and bone densitometric outcomes in a five-year study. J Clin Endocrinol Metab. (2009) 94:4860–7. doi: 10.1210/jc.2009-1472
70. Courbebaisse M, Diet C, Timsit MO, Mamzer MF, Thervet E, Noel LH, et al. Effects of cinacalcet in renal transplant patients with hyperparathyroidism. Am J Nephrol. (2012) 35:341–8. doi: 10.1159/000337526
71. Coco M, Glicklich D, Faugere MC, Burris L, Bognar I, Durkin P, et al. Prevention of bone loss in renal transplant recipients: a prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol. (2003) 14:2669–76. doi: 10.1097/01.ASN.0000087092.53894.80
72. Smerud KT, Dolgos S, Olsen IC, A° sberg A, Sagedal S, Reisæter AV, et al. A 1-year randomized, doubleblind, placebo-controlled study of intravenous ibandronate on bone loss following renal transplantation. Am J Transplant. (2012) 12:3316–25. doi: 10.1111/j.1600-6143.2012.04233.x
73. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. (2009) 361:756–65. doi: 10.1056/NEJMoa0809493
74. Jamal SA, Ljunggren O, Stehman-Breen C, Cummings SR, McClung MR, Goemaere S, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res. (2011) 26:1829–35. doi: 10.1002/jbmr.403
75. Bonani M, Frey D, Brockmann J, Fehr T, Mueller TF, Saleh L, et al. Effect of twice-yearly denosumab on prevention of bone mineral density loss in de novo kidney transplant recipients: a randomized controlled trial. Am J Transplant. (2016) 16:1882–91. doi: 10.1111/ajt.13692
76. Alfieri C, Binda V, Malvica S, Cresseri D, Campise M, Gandolfo MT, et al. Bone Effect and Safety of One-Year Denosumab Therapy in a Cohort of Renal Transplanted Patients: An Observational Monocentric Study. J Clin Med. (2021) 10:1989. doi: 10.3390/jcm10091989
77. Thongprayoon C, Acharya P, Aeddula NR, Torres-Ortiz A, Bathini T, Sharma K, et al. Effects of denosumab on bone metabolism and bone mineral density in kidney transplant patients: a systematic review and meta-analysis. Arch Osteoporos. (2019) 14:35. doi: 10.1007/s11657-019-0587-0
Keywords: mineral disorders, bone disorders, renal transplantation, graft outcome, CKD-MBD treatment
Citation: Molinari P, Alfieri CM, Mattinzoli D, Campise M, Cervesato A, Malvica S, Favi E, Messa P and Castellano G (2022) Bone and Mineral Disorder in Renal Transplant Patients: Overview of Pathology, Clinical, and Therapeutic Aspects. Front. Med. 9:821884. doi: 10.3389/fmed.2022.821884
Received: 24 November 2021; Accepted: 08 February 2022;
Published: 10 March 2022.
Edited by:
Pasquale Esposito, University of Genoa, ItalyReviewed by:
Andrea Galassi, Santi Paolo e Carlo Hospital, ItalyMaría Azancot, Vall d'Hebron University Hospital, Spain
Copyright © 2022 Molinari, Alfieri, Mattinzoli, Campise, Cervesato, Malvica, Favi, Messa and Castellano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlo Maria Alfieri, Y2FybG8uYWxmaWVyaTFAZ21haWwuY29t; Y2FybG8uYWxmaWVyaUB1bmltaS5pdA==
†These authors have contributed equally to this work
 Paolo Molinari1†
Paolo Molinari1† Carlo Maria Alfieri
Carlo Maria Alfieri Piergiorgio Messa
Piergiorgio Messa