
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 07 April 2022
Sec. Ophthalmology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.820370
This article is part of the Research Topic Clinical Application and Development of Ocular Imaging View all 47 articles
Purpose: As the human immunodeficiency virus (HIV) pandemic is far from over, whether there are subclinical macular changes in HIV-positive patients is something that should not be overlooked. We aimed to apply optical coherence tomography angiography (OCTA) to assess the macular structure and microvasculature changes in patients with HIV without infectious retinopathy.
Methods: HIV-positive and -negative participants were included and classified into three groups: HIV-negative, HIV-positive, and HIV-positive with microvasculopathy. OCTA parameters regarding macular structure and microvasculature were analyzed.
Results: Compared with the HIV-negative group, the superficial retinal vessel density (VD) in the parafovea sectors and the whole Early Treatment of Diabetic Retinopathy Study (ETDRS) grid and the choroidal vascularity index (CVI) in the whole ETDRS grid were significantly decreased in the HIV-positive and HIV-positive with microvasculopathy groups (p < 0.05). No differences were found in OCTA parameters between the HIV-positive and HIV-positive with microvasculopathy groups. Retinal, retinal nerve fiber layer-ganglion cell layer-inner plexiform layer (RNFL-GCL-IPL), RNFL, GCL-IPL, and INL thickness showed a negative association with the duration of HIV diagnosis or antiretroviral therapy (ART) (all p < 0.05). All OCTA microvasculature parameters showed no association with HIV-related clinical variables (all p > 0.05).
Conclusions: Subclinical macular changes existed in HIV-infected patients without clinical infectious retinopathy. Substructures from inner retinal layers might be associated with HIV infection or ART duration.
The human immunodeficiency virus (HIV) pandemic has lasted four decades. Although remarkable achievements have been witnessed in it, it is still far from over (1). Since universal recommendations in the mid-90s, the “treat-all” policy on antiretroviral therapy (ART) greatly contributed toward reducing the visual morbidity and mortality of HIV infection (2–5). However, a higher risk of non-AIDS comorbidities, such as cardiovascular diseases, bone disease, renal and hepatic dysfunction, and neurological disease and cancers should be considered (3, 4).
There were hypotheses about accelerated neuroretinal degeneration because of persistent HIV infection and ART or other factors (6). For patients with HIV infection without ocular opportunistic infections, subtle macular or peripapillary changes were confirmed by various previous studies (7–9). Some results or associations between these structural changes and clinical features were inconsistent (9–11), which might be due to differences in research design and detection machinery.
Optical coherence tomography angiography (OCTA) is a non-invasive instrument possessing an advantage to image retinal and choroidal blood flow and obtain quantitative thickness measurements (12, 13). Besides the wide applications of OCTA in various fundus diseases (13–17) and many systemic diseases (18–20), this non-invasive technology had been proved to be feasible for detecting microvasculopathy in patients with HIV/AIDS with and without clinical retinal diseases (21, 22).
We sought to apply OCTA to assess the macular structure and microvasculature changes in patients with HIV, while there were no clinical infectious retinopathy or only asymptomatic cotton wool spots.
This prospective cohort study was conducted between August 2019, and October 2019, and approved by the ethics committee of Beijing Youan Hospital, Capital Medical University.
Patients with or without HIV infection were initially included. Patients with a history of diabetes mellitus and cardiovascular and cerebrovascular diseases were excluded. After a routine ocular examination of the anterior segment and fundus, eyes without visible ocular abnormalities or with HIV microvasculopathy were also included. HIV microvasculopathy was diagnosed as asymptomatic cotton wool spots by ophthalmoscopy, which could coexist with small intraretinal hemorrhages, microaneurysms, and telangiectasia (23, 24). The HIV-infected group in this study was defined as having no visible ocular abnormalities in patients with HIV infection. Participants were classified into three groups: HIV-negative, HIV-positive, and HIV-positive with microvasculopathy. Only the right eye was selected. The left eye was recruited when the right eye was substandard. Eyes with a history of intraocular surgery, ocular trauma, amblyopia, glaucoma, and other ocular diseases were excluded. The study was reviewed and approved by the ethics committee of Beijing Youan Hospital, Capital Medical University (LL-2018-150-K). Written informed consent was obtained from all participants.
The main ocular examinations included slit-lamp biomicroscopy for anterior segment, fundus imaging by Optos Daytona, and axial length by Zeiss IOL Master 500.
HIV infection was identified by self-reporting and a previous positive test. For patients with HIV infection, critical HIV-related parameters were collected, including the duration of infection, duration of ART, CD4/CD8, nadir CD4 counts, and blood HIV-RNA.
The OCT device (VG200; SVision Imaging, Ltd., Luoyang, China) used for macular examination was the same as described in previous studies (21, 25). It is a swept-source (SS) OCT with a central wavelength of 1,050 nm (990–1,100 nm full width) and a scanning rate of 200,000 A-scans per second. With automatical pupil focusing and OCT focusing, both 3 × 3 mm and 6 × 6 mm scan patterns centering on the fovea were conducted in this study. Rescan was conducted if the image quality was dissatisfied with apparent flaws. Images with a high signal strength index (SSI) were further evaluated, while images with flow projection artifacts or camera artifacts were excluded.
OCTA parameters were automatically calculated. For segmentation errors, manual corrections can be used to fix them. Macular sectors were performed according to the Early Treatment of Diabetic Retinopathy Study (ETDRS) grid, which was also described in our previous study (21). Within the ETDRS grid, parameters from the inner circle were further classified into the central fovea and parafovea (temporal, superior, nasal, and inferior); while parameters from the outer circle were excluded because of an incomplete edge in the 6 × 6 mm scanning area.
OCTA parameters were stratified into macular microvasculature and macular structural parameters. Microvasculature parameters included the foveal avascular zone (FAZ) from a 3 × 3 mm scan pattern, superficial retinal vessel density (VD), inner retinal VD, and choroidal vascularity index (CVI) from a 6 × 6 mm scan pattern, which also contained quantitative measurements of retinal and choroidal thicknesses. Besides, there were detailed measurements of the retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), RNFL, GCL-IPL, and INL from the inner retinal layer, as well as photoreceptor-retinal pigment epithelium (PR-RPE). The classification of each OCTA parameter was also described in our previous study (21).
SPSS 25.0 software was used for all statistical analyses. Mann-Whitney test was used for HIV-related variables between HIV-positive and HIV-positive with microvasculopathy groups. Analysis of variance and chi-square test were used for continuous normal distributed and categorical variables, respectively. Differences in macular parameters between groups were tested by one-way ANOVA and Bonferroni tests. Multivariable linear regression analysis was performed between clinical variables and each OCTA parameter in the whole ETDRS grid. p < 0.05 was considered statistically significant.
After excluding participants who did not meet the inclusion criteria, this study included 36 controls without HIV infection (29 males), 46 patients with HIV infection (40 males), and 20 patients with HIV infection with microvasculopathy (19 males). Age, gender, axial length (AL), and signal strength index was not significantly different between the groups (p > 0.05). The duration of HIV infection and blood HIV-RNA were not different between the HIV-positive and HIV-positive with microvasculopathy groups (p = 0.061 and 0.052, respectively). The ART duration and CD4/CD8 and CD4 levels in the HIV-positive with microvasculopathy group were significantly different from those in the HIV-positive group (p = 0.006, 0.001, and p < 0.001, respectively) (Table 1).
All OCTA parameters were automatically calculated. All recruited eyes from each group showed no segmentation errors, and no manual correction was used in this study. Microvascular and structural OCTA parameters from the groups are shown in Supplementary Table 1. For macular microvascular variables, compared with the HIV-negative group, superficial retinal VD in all parafovea and the whole ETDRS grid were significantly decreased in the HIV-positive group and HIV-positive with microvasculopathy groups (p < 0.05), while CVI in the whole ETDRS grid was also significantly decreased in these two groups (p < 0.001) (Table 2; Figure 1).
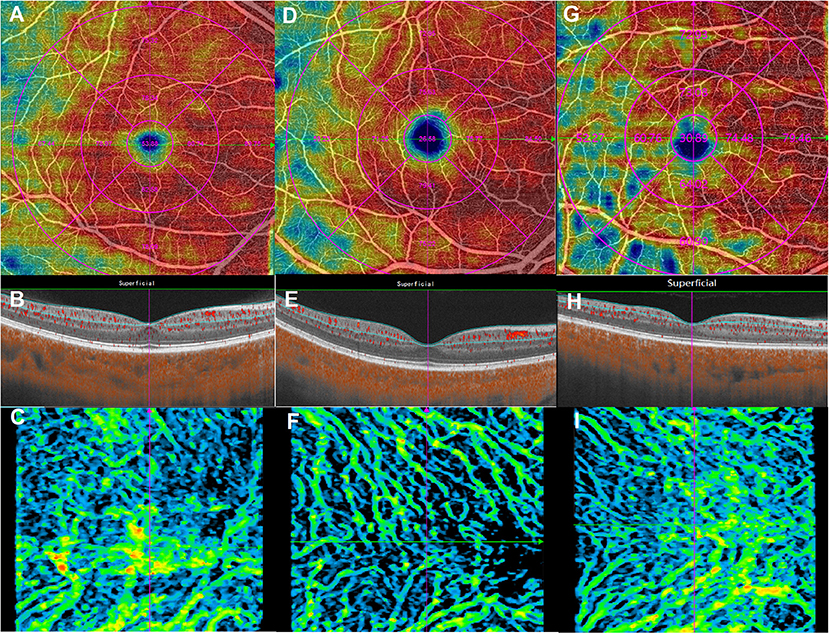
Figure 1. Macular microvasculature images from the 6 × 6 mm scan model: en face optical coherence tomography angiography images of the superficial retinal vessel density (upper), B-scans (middle), and choroidal vascularity index (lower). (A–C) are from the same eye in the human immunodeficiency virus (HIV)-negative group; (D–F) are from the same eye in the HIV-positive group; (G–I) are from the same eye in the HIV-positive with microvasculopathy group.
For macular structural variables, retinal thickness, choroidal thickness, RNFL-GCL-IPL thickness, RNFL thickness, and GCL-IPL thickness from all macular quadrants showed no intergroup differences (p > 0.05). However, the HIV microvasculopathy group showed a significantly higher INL thickness and lower PR-RPE thickness than those of the HIV-negative group (p = 0.021 and 0.023, respectively) (Supplementary Table 2).
Duration of HIV diagnosis and ART duration were strongly related variants, which were divided into two different multivariable linear regression models (Tables 3, 4, respectively). For patients with HIV infection, the thickness of retinal, RNFL-GCL-IPL, RNFL, GCL-IPL, and INL showed a strong negative association with the duration of HIV infection or ART duration (all p < 0.05). All OCTA microvasculature parameters (FAZ, superficial retinal VD, inner retinal VD, and CVI) showed no association with HIV-related clinical variables, including CD4, CD4/CD8, blood HIV-RNA, duration of HIV, and duration of ART (p > 0.05) (Supplementary Table 3).

Table 3. Multivariable linear regression analysis between macular structural parameters in the entire Early Treatment of Diabetic Retinopathy Study grid and systemic variables in all patients with human immunodeficiency virus infection (model one).
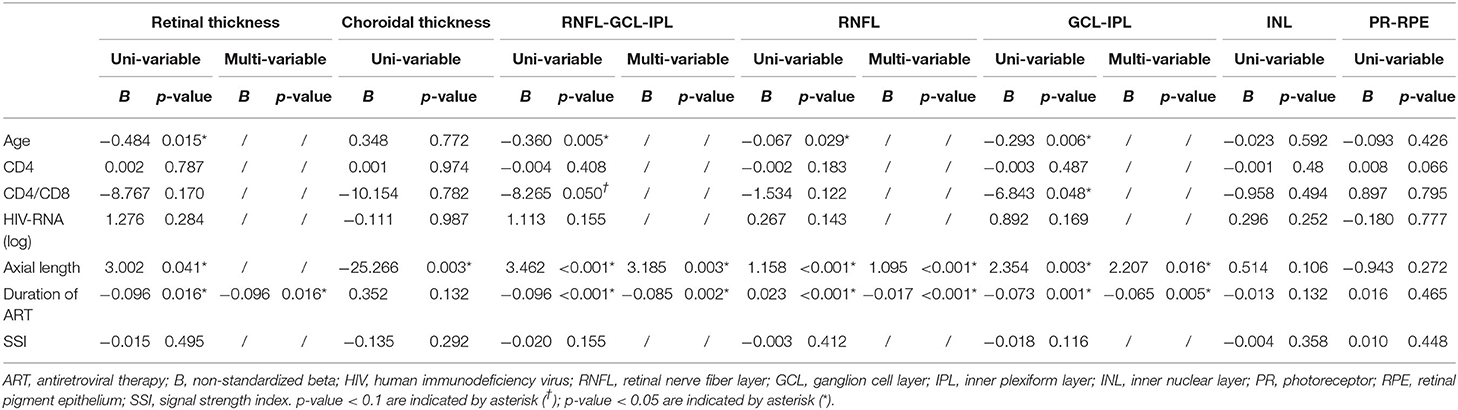
Table 4. Multivariable linear regression analysis between macular structural parameters in the entire Early Treatment of Diabetic Retinopathy Study grid and systemic variables in all patients with human immunodeficiency virus infection (model two).
Macular damages in retinochoroid structure and microvasculature were identified by OCTA in patients with HIV infection who were free of infectious retinopathy. Both superficial retinal VD and CVI were significantly decreased in the HIV-positive group and HIV-positive with microvasculopathy groups. INL thickness was increased in the HIV microvasculopathy group. Macular substructures from the inner retinal layer, including RNFL-GCL-IPL, RNFL, GCL-IPL, and INL thickness, were associated with the duration of HIV diagnosis or ART.
HIV microvasculopathy manifested as asymptomatic cotton wool spots (CWSs), which could also be called “HIV retinopathy” or “noninfectious retinopathy” (23, 24). These benign lesions were first described by Holland in 1982 (26). The pathophysiology of HIV microvasculopathy is complicated and ambiguous. CWSs in the imaging of OCTA were accompanied by microvasculature changes, including in non-perfused areas in the periphery of the CWSs (27). A degenerative retinal process after CWSs resolution was identified in patients with HIV retinopathy (28). However, none of the macular parameters showed differences between the HIV infection and HIV microvasculopathy groups. First, this could be attributed to the 3-mm diameter inner circle of the ETDRS chart. All OCTA parameters were captured in the fovea and parafoveal area, while CWSs were located in areas rich in RNFL. Second, the mean duration from HIV diagnosis (4 months in the HIV infection group and 1 month in the HIV microvasculopathy group) is very short for significant changes to have occurred. Macular structure and microvasculature changes after CWSs resolution deserve further investigation.
The HIV epidemic is still a challenge in China (29). Ongoing support and care for patients with HIV are still needed in the future, especially for patients beyond viral suppression (30, 31). Various studies had tried to explore retinal vasculature changes in patients with HIV infection with retinal vascular calibers measurements by fundus photographs (32). Studies about retinochoroid microvasculature using OCTA were limited. A previous study found retinal microvasculature changes, including decreased macular VD and perfusion density, in patients with HIV infection (33), similar to our findings. Although OCTA parameters might be different between various OCTA devices and algorithms, the device with a wavelength of 1,050 nm in this study should have its ascendancy over other OCTA devices with shorter wavelengths (34). We also observed decreased retinochoroid microvasculature parameters in the HIV infection group or HIV microvasculopathy group, including superficial retinal VD and CVI. Macular microvasculature parameters showed no association with HIV diagnosis or ART duration, which faithfully reflected that a short duration from HIV diagnosis or ART was not enough to changes microvasculature.
Retinal structural assessments in patients with HIV infection using OCT had been conducted by various studies (35–37). There were hypotheses about the presence of accelerated neuroretinal degenerations in these HIV-positive patients (6), which were in line with the reduced macular structure and microvasculature in our study. The persistence of inflammatory state in patients living with HIV are associated with non-AIDS morbidities, such as cardiovascular diseases and neoplastic diseases (38). Whether these neuroretinal degenerations were associated with chronic inflammation and persistent HIV reservoirs (3) deserves further studies. Besides, despite effective HIV suppression, the comorbidities and aging of patients with HIV infection were still challenges. Increased mortality and comorbidity were associated with risk factors in certain populations, such as smoking, obesity, drug and alcohol abuse, and drug toxicity (39, 40), which could also be the potential risk for these macular changes in this study.
In this study, various substructures from the inner retinal layers showed a marked negative association with the duration of HIV infection or ART, indicating a degeneration tendency from the persistent HIV infection or ART. It provided a valuable clue for further studies about the relationships between retinal vascular changes and systemic vascular diseases in HIV-positive patients. Such presumed changes could help to monitor or predict systemic vascular diseases in these patients.
There are limitations to this study. Although objective macular parameters regarding structure and microvasculature were ample in this study, data about visual function were not involved, such as color vision, contrast sensitivity, multifocal electroretinography, and visual fields. As HIV infection is turning into a type of chronic disease, the presence of many possible systemic confounders could contribute to observed changes. Further trials with longitudinal data and large sample size are warranted.
With precise structure and microvasculature measurements, OCTA revealed decreased superficial retinal VD and CVI in patients with HIV infection with microvasculopathy or clinically normal fundus. HIV infection or ART duration could influence the inner retinal layer substructures. The application of OCTA was meaningful in the ocular observation of patients with HIV infection.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee of Beijing Youan Hospital, Capital Medical University (LL-2018-150-K). The patients/participants provided their written informed consent to participate in this study.
K-FD and W-BW contributed to the conception of the protocol. K-FD and CC obtained the dataset. K-FD, X-JH, L-YX, and W-JK analyzed the data. K-FD and X-JH contributed to writing the first draft of the manuscript and to the revision and editing of the present version of the manuscript. H-WD and W-BW commented on the manuscript and gave final approval of the version to be published. All authors contributed to the article and approved the submitted version.
This study was supported by the Scientific Research Project of Beijing Youan Hospital, CCMU, 2018 (YNKTQN20180201), the National Science and Technology Major Project of China during the 13th Five-Year Plan Period (2017ZX10201101), and the Beijing Excellent Talent Plan (2018000021223ZK04).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.820370/full#supplementary-material
HIV, Human immunodeficiency virus; OCTA, Optical coherence tomography angiography; VD, Vessel density; ETDRS, Early Treatment of Diabetic Retinopathy Study; CVI, Choroidal vascularity index; INL, Inner nuclear layer; RNFL, Retinal nerve fiber layer; GCL, Ganglion cell layer; IPL, Inner plexiform layer; ART, Antiretroviral therapy; FAZ, Foveal avascular zone; PR-RPE, Photoreceptor-retinal pigment epithelium; AL, Axial length; CWS, Cotton wool spot.
1. Beyrer C. A pandemic anniversary: 40 years of HIV/AIDS. Lancet. (2021) 397:2142–3. doi: 10.1016/S0140-6736(21)01167-3
2. Zhang J, Huang X, Tang W, Chu Z, Hu Q, Liu J, et al. Rapid clinical progression and its correlates among acute HIV infected men who have sex with men in china: findings from a 5-year multicenter prospective cohort study. Front Immunol. (2021) 12:712802. doi: 10.3389/fimmu.2021.712802
3. Ghosn J, Taiwo B, Seedat S, Autran B, Katlama C. HIV. Lancet. (2018) 392:685–97. doi: 10.1016/S0140-6736(18)31311-4
4. Deeks SG, Overbaugh J, Phillips A, Buchbinder S. HIV infection. Nat Rev Dis Primers. (2015) 1:15035. doi: 10.1038/nrdp.2015.35
5. Goldberg DE, Smithen LM, Angelilli A, Freeman WR. HIV-associated retinopathy in the HAART era. Retina. (2005) 25:633–49, 682–3. doi: 10.1097/00006982-200507000-00015
6. Demirkaya N, Wit F, Schlingemann R, Verbraak F. Neuroretinal degeneration in HIV patients without opportunistic ocular infections in the cART era. AIDS Patient Care STDs. (2015) 29:519–32. doi: 10.1089/apc.2015.0091
7. Kozak I, Bartsch DU, Cheng L, McCutchan A, Weinreb RN, Freeman WR. Scanning laser polarimetry demonstration of retinal nerve fiber layer damage in human immunodeficiency virus-positive patients without infectious retinitis. Retina. (2007) 27:1267–73. doi: 10.1097/IAE.0b013e31806463fb
8. Bartsch DU, Kozak I, Grant I, Knudsen VL, Weinreb RN, Lee BR, et al. Retinal nerve fiber and optic disc morphology in patients with human immunodeficiency virus using the heidelberg retina tomography 3. PLoS ONE. (2015) 10:e0133144. doi: 10.1371/journal.pone.0133144
9. Demirkaya N, Wit FWNM, van Den Berg TJTP, Kooij KW, Prins M, Schlingemann RO, et al. HIV-Associated neuroretinal disorder in patients with well-suppressed HIV-infection: a comparative cohort study. Invest Ophthalmol Vis Sci. (2016) 57:1388–97. doi: 10.1167/iovs.15-18537
10. Van Tassel SH, Petrakos P, Marlow E, Mauer E, Singh HK, Demetriades AM. Retinal nerve fiber layer changes based on historic CD4 nadir among HIV positive patients undergoing glaucoma evaluation. Int J Ophthalmol. (2019) 12:789–94. doi: 10.18240/ijo.2019.05.14
11. Cetin EN, Kutlu SS, Parca O, Kutlu M, Pekel G. The thicknesses of choroid, macular segments, peripapillary retinal nerve fiber layer, and retinal vascular caliber in HIV-1-infected patients without infectious retinitis. Retina. (2019) 39:1416–23. doi: 10.1097/IAE.0000000000002146
12. Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y, et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. (2017) 7:42201. doi: 10.1038/srep42201
13. Kashani AH, Chen CL, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, et al. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res. (2017) 60:66–100. doi: 10.1016/j.preteyeres.2017.07.002
14. Waheed NK, Moult EM, Fujimoto JG, Rosenfeld PJ. Optical coherence tomography angiography of dry age-related macular degeneration. Dev Ophthalmol. (2016) 56:91–100. doi: 10.1159/000442784
15. Invernizzi A, Cozzi M, Staurenghi G. Optical coherence tomography and optical coherence tomography angiography in uveitis: a review. Clin Exp Ophthalmol. (2019) 47:357–71. doi: 10.1111/ceo.13470
16. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. (2018) 64:1–55. doi: 10.1016/j.preteyeres.2017.11.003
17. Arrigo A, Corbelli E, Aragona E, Manitto MP, Martina E, Bandello F, et al. Optical coherence tomography and optical coherence tomography angiography evaluation of combined hamartoma of the retina and retinal pigment epithelium. Retina. (2019) 39:1009–15. doi: 10.1097/IAE.0000000000002053
18. Zhang X, Xiao H, Liu C, Liu S, Zhao L, Wang R, et al. Optical coherence tomography angiography reveals distinct retinal structural and microvascular abnormalities in cerebrovascular disease. Front Neurosci. (2020) 14:588515. doi: 10.3389/fnins.2020.588515
19. Hwang TS, Hagag AM, Wang J, Zhang M, Smith A, Wilson DJ, et al. Automated quantification of nonperfusion areas in 3 vascular plexuses with optical coherence tomography angiography in eyes of patients with diabetes. Jama Ophthalmol. (2018) 136:929–36. doi: 10.1001/jamaophthalmol.2018.2257
20. Shah A, Apte RS. Optical coherence tomography angiography: a window into central nervous system neurodegeneration. Trends Mol Med. (2020) 26:892–5. doi: 10.1016/j.molmed.2020.08.003
21. Du KF, Huang XJ, Chen C, Kong WJ, Xie LY, Wei WB. Macular structure and microvasculature changes in aids-related cytomegalovirus retinitis using optical coherence tomography angiography. Front Med. (2021) 8:696447. doi: 10.3389/fmed.2021.696447
22. Collins LF, Shantha JG, Nesper PL, Sheth AN, Fawzi AA, Yeh S, et al. Assessment of retinal microvascular health by optical coherence tomography angiography among persons with HIV. AIDS. (2021) 35:1321–4. doi: 10.1097/QAD.0000000000002890
23. Harkins T. Noninfectious retinopathy. Optom Vis Sci. (1995) 72:302–4. doi: 10.1097/00006324-199505000-00005
24. Finlayson J, Laing RB, Cadwgan A, Green F. HIV retinopathy at seroconversion. Br J Ophthalmol. (1998) 82:1339–40. doi: 10.1136/bjo.82.11.1339a
25. Yang J, Wang E, Yuan M, Chen Y. Three-dimensional choroidal vascularity index in acute central serous chorioretinopathy using swept-source optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. (2020) 258:241–7. doi: 10.1007/s00417-019-04524-7
26. Holland GN, Gottlieb MS, Yee RD, Schanker HM, Pettit TH. Ocular disorders associated with a new severe acquired cellular immunodeficiency syndrome. Am J Ophthalmol. (1982) 93:393–402. doi: 10.1016/0002-9394(82)90127-1
27. Mahdjoubi A, Bousnina Y, Barrande G, Bensmaine F, Chahed S, Ghezzaz A. Features of cotton wool spots in diabetic retinopathy: a spectral-domain optical coherence tomography angiography study. Int Ophthalmol. (2020) 40:1625–40. doi: 10.1007/s10792-020-01330-7
28. Kozak I, Sasik R, Freeman WR, Sprague LJ, Gomez ML, Cheng L, et al. A degenerative retinal process in HIV-associated non-infectious retinopathy. PLoS ONE. (2013) 8:e74712. doi: 10.1371/journal.pone.0074712
29. Zheng S. The growing threat of China's HIV epidemic. Lancet Public Health. (2018) 3:e311. doi: 10.1016/S2468-2667(18)30098-7
30. Williams BG, Granich R. Ending AIDS: myth or reality? Lancet. (2017) 390:357. doi: 10.1016/S0140-6736(17)31832-9
31. Safreed-Harmon K, Anderson J, Azzopardi-Muscat N, Behrens G, D'Arminio MA, Davidovich U, et al. Reorienting health systems to care for people with HIV beyond viral suppression. Lancet Hiv. (2019) 6:e869–77. doi: 10.1016/S2352-3018(19)30334-0
32. Kalyani PS, Fawzi AA, Gangaputra S, van Natta ML, Hubbard LD, Danis RP, et al. Retinal vessel caliber among people with acquired immunodeficiency syndrome: relationships with visual function. Am J Ophthalmol. (2012) 153:428–33.e1. doi: 10.1016/j.ajo.2011.08.027
33. Akmaz B, Akay F, Güven YZ, Kaptan F, Demirdal T. The long-term effect of human immunodeficiency virus infection on retinal microvasculature and the ganglion cell-inner plexiform layer: an OCT angiography study. Graefes Arch Clin Exp Ophthalmol. (2020) 258:1671–6. doi: 10.1007/s00417-020-04749-x
34. Lane M, Moult EM, Novais EA, Louzada RN, Cole ED, Lee B, et al. Visualizing the choriocapillaris under drusen: comparing 1050-nm swept-source versus 840-nm spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. (2016) 57:T585–90. doi: 10.1167/iovs.15-18915
35. Faria E Arantes TE, Garcia CR, Mello PA, Muccioli C. Structural and functional assessment in HIV-infected patients using optical coherence tomography and frequency-doubling technology perimetry. Am J Ophthalmol. (2010) 149:571–6.e2. doi: 10.1016/j.ajo.2009.11.026
36. Kozak I, Bartsch DU, Cheng L, Kosobucki BR, Freeman WR. Objective analysis of retinal damage in HIV-positive patients in the HAART era using OCT. Am J Ophthalmol. (2005) 139:295–301. doi: 10.1016/j.ajo.2004.09.039
37. Moschos MM, Mostrou G, Psimenidou E, Spoulou V, Theodoridou M. Objective analysis of retinal function in HIV-positive children without retinitis using optical coherence tomography. Ocul Immunol Inflamm. (2007) 15:319–23. doi: 10.1080/09273940701375154
38. Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis. (2016) 214(Suppl. 2):S44–50. doi: 10.1093/infdis/jiw275
39. Croxford S, Kitching A, Desai S, Kall M, Edelstein M, Skingsley A, et al. Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort. Lancet Public Health. (2017) 2:e35–46. doi: 10.1016/S2468-2667(16)30020-2
Keywords: HIV, macular, non-infectious, microvasculopathy, microvasculature, optical coherence tomography angiography, structure
Citation: Du K-F, Huang X-J, Chen C, Kong W-J, Xie L-Y, Dong H-W and Wei W-B (2022) Macular Changes Observed on Optical Coherence Tomography Angiography in Patients Infected With Human Immunodeficiency Virus Without Infectious Retinopathy. Front. Med. 9:820370. doi: 10.3389/fmed.2022.820370
Received: 22 November 2021; Accepted: 04 March 2022;
Published: 07 April 2022.
Edited by:
Feng Wen, Sun Yat-sen University, ChinaCopyright © 2022 Du, Huang, Chen, Kong, Xie, Dong and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Wei Dong, aG9uZ3dlaXlhbmtlQDE2My5jb20=; Wen-Bin Wei, d2Vpd2VuYmludHJAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.