- State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangdong Provincial Key Laboratory of Ophthalmology and Visual Science, Guangdong Provincial Clinical Research Center for Ocular Diseases, Guangzhou, China
Purpose: To compare intraocular pressure (IOP) values obtained using Goldmann applanation tonometry (IOPGAT) and non-contact tonometry (IOPNCT) in a non-pathologic high myopia population.
Methods: A total of 720 eyes from 720 Chinese adults with non-pathologic high myopia were enrolled in this cross-sectional study. Demographic and ocular characteristics, including axial length, refractive error, central corneal thickness (CCT), and corneal curvature (CC) were recorded. Each patient was successively treated with IOPNCT and IOPGAT. Univariate and multivariable linear regression analyses were conducted to detect factors associated with IOPNCT and IOPGAT, as well as the measurement difference between the two devices (IOPNCT−GAT).
Results: In this non-pathologic high myopia population, the mean IOPNCT and IOPGAT values were 17.60 ± 2.76 mmHg and 13.85 ± 2.43 mmHg, respectively. The IOP measurements of the two devices were significantly correlated (r = 0.681, P < 0.001), however, IOPNCT overestimated IOPGAT with a mean difference of 3.75 mmHg (95% confidence interval: 3.60–3.91 mmHg). In multivariate regression, IOPNCT was significantly associated with body mass index (standardized β = 0.075, p = 0.033), systolic blood pressure (SBP) (standardized β = 0.170, p < 0.001), and CCT (standardized β = 0.526, p < 0.001). As for IOPGAT, only SBP (standardized β = 0.162, p < 0.001), CCT (standardized β = 0.259, p < 0.001), and CC (standardized β = 0.156, p < 0.001) were significantly correlated. The mean IOPNCT−GAT difference increased with younger age (standardized β = −0.134, p < 0.001), higher body mass index (standardized β = 0.091, p = 0.009), higher SBP (standardized β = 0.074, p = 0.027), thicker CCT (standardized β = 0.506, p < 0.001), and lower IOPGAT (standardized β = −0.409, p < 0.001).
Conclusion: In the non-pathologic high myopia population, IOPNCT overestimated IOPGAT at 3.75 ± 2.10 mmHg. This study suggests that the difference between the values obtained by the two devices, and their respective influencing factors, should be considered in the clinical evaluation and management of highly myopic populations.
Introduction
High myopia is an extreme form of myopia, mainly characterized by excessive axial elongation and various pathological ocular lesions (1). A growing body of evidence suggests that high myopia is closely related to the occurrence of glaucoma (2–7). As intraocular pressure (IOP) is the most important target and the only treatable factor in glaucoma, accurate and reliable measurement as well as monitoring of IOP are essential in highly myopic populations.
Many types of devices have been proposed for IOP measurement, such as Goldmann applanation tonometry (GAT), non-contact tonometry (NCT), ICare rebound tonometer, and dynamic contour tonometer, each of which has advantages and disadvantages (8). The GAT is widely regarded as the gold standard for IOP measurement due to its accuracy and excellent reproducibility, while NCT is most widely used in outpatients and in ocular hypertension screening because of its non-invasive and convenient nature (9, 10). However, all measurements are influenced by the structure and biomechanical properties of the cornea, such as the cornea thickness and hysteresis, as well as systemic factors such as systolic pressure (11–13).
Due to excessive elongation of axial length (AL) in high myopia, which is accompanied by changes in scleral and corneal structures and their biomechanics, IOP values obtained may vary among different tonometers. Previous studies have reported the distribution of IOP values in high myopia populations, ranging from 9 to 27 mmHg, as measured by the GAT or NCT (14, 15). Comparative studies on IOP measurement with the NCT and GAT in high myopia populations are limited, and results from different measurements may not be comparable.
The present study aimed to evaluate the difference in IOP measurements obtained with the NCT and GAT in a population with non-pathologic high myopia, and the demographic and ocular characteristics that affect these measurements. The present findings may inform a more comprehensive and reliable management of IOP in highly myopic patients.
Materials and Methods
Study Participants
This prospective cross-sectional study was conducted at the Zhongshan Ophthalmic Center (ZOC), Sun Yat-sen University, Guangzhou. Participants were recruited from a registry cohort study on the natural history of myopic neuropathy, which started from June 2019 to June 2021 (ClinicalTrials.gov identifier: NCT04302220) (16). This study was approved by the Ethics Review Committee of the ZOC, and the study procedure conformed to the Declaration of Helsinki. Informed consent was obtained from all the participants prior to enrollment.
High myopia adults aged 18–65 years were recruited, as previously reported (16). Briefly, patients were eligible for this study if either the left or right eye presented with myopic spherical equivalence (SE) of ≤ -6 diopters or AL of ≥26.5 mm, best corrected visual acuity (BCVA) of ≥6/12, and myopic maculopathy category 0 or 1 [based on the International Photographic Classification and Grading System for Myopic Maculopathy (17)]. The right eye was chosen for analysis if both eyes met the inclusion criteria.
The exclusion criteria were as follows: (1) patients with severe systemic diseases such as malignant tumors, (2) a history of ocular surgery or laser treatment, and (3) ocular infection diseases that could not be measured by the GAT, such as corneal ulcers.
Demographic and Ocular Characteristics
In this study, blood pressure was measured twice using an automated blood pressure apparatus (Omron Healthcare Ltd., Japan), and the average value was recorded. Body mass index (BMI) was calculated as body weight (kg) divided by height (m) squared. Demographic and clinical characteristics such as age, sex, and other medical history data were collected through interviews.
All subjects underwent a comprehensive ophthalmic examination at the ZOC Clinical Research Center, including BCVA assessment, refractive error assessment with an autorefractor (KR-800, Topcon, Japan); slit lamp bio-microscopy (BQ-900, Haag-Streit, Switzerland), IOP measurement by both the NCT (mputeCT-1 Corized Tonometer, Topcon Ltd., Topcon) and GAT (Haag-Streit, Koniz, Switzerland); AL, central corneal thickness (CCT), and corneal curvature (CC) values were obtained using an IOL Master (IOL Master 700, Carl Zeiss Meditec, Germany); digital stereo fundus photography (Nonmyd WX3D, KOWA, Japan) was performed. All measurements were performed by well-trained technicians.
IOP Measurement
IOP was measured in all participants between 9:00 a.m. and 11:00 a.m. to minimize the influence of IOP circadian variations. Each participant was asked to calm down for at least 5 min before the measurement. The NCT assessment was performed 15 min before the GAT assessment; the assessments were performed three times at 1-min intervals. Both instruments were periodically calibrated according to the manufacturer's guidelines, and the operations were conducted strictly following the manufacturer's instructions. During the GAT measurement, 0.5% proparacaine hydrochloride eye drops (Alcon, Fort Worth, TX, USA) were used for topical anesthesia, and fluorescein strips (Liaoning Meizilin Pharmaceutical Co., Ltd., China) were gently applied to the palpebral conjunctiva for corneal staining. The IOP measurement with the GAT was performed using a slit lamp mounted applanation tonometer and performed by the same experienced ophthalmologist twice, and the average values were recorded.
Statistical Analysis
In descriptive analysis, continuous variables were summarized as mean ± standard deviation and range, and categorical variables were presented as frequency and proportion. The Bland-Altman analysis was performed to evaluate the agreement between IOPNCT and IOPGAT (18). The mean difference and limits of agreement (LOA) were calculated to quantify the extent of bias between the two measurements. Pearson's coefficient was used to assess the correlation between IOPNCT and IOPGAT. Linear regression analyses were conducted to determine the factors associated with IOPNCT, IOPGAT, and IOPNCT−GAT. Variables with p-values of <0.1 in univariable linear regression were included in multivariable linear regression. All multivariable models were adjusted for age. Statistical significance was set at p-values of <0.05. Statistical analyses were performed using Stata 16 software (Stata Corp., T.X., USA).
Results
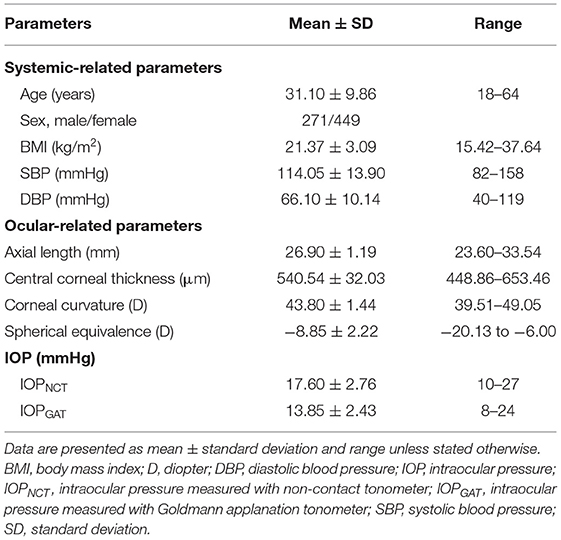
Participants' Characteristics
A total of 812 subjects were enrolled in this study; subsequently, 22 patients with a history of ocular surgery or laser treatment, 10 patients with ocular trauma or infection that could not complete a GAT measurement, 9 patients with severe systemic diseases, 34 patients using ocular hypotensive agents, and 17 patients with missing values of SE or IOL master measurement were excluded. Finally, a total of 720 subjects (720 eyes) with non-pathologic high myopia were included in the analysis. Table 1 presents the clinical characteristics of the participants. The mean age was 31.10 ± 9.86 years (range 18 to 64 years), and 271 (37.64%) participants were male. The average AL, SE, CCT, and CC values were 26.90 ± 1.19 mm,−8.85 ± 2.22 diopter, 540.54 ± 32.03 μm, and 43.80 ± 1.44 diopter, respectively.
Difference Between IOPNCT and IOPGAT Measurements
The average IOPNCT and IOPGAT values were 17.60 ± 2.76 mmHg and 13.85 ± 2.43 mmHg, respectively (Table 1). IOPNCT and IOPGAT values were significantly correlated (r = 0.681, P < 0.001) in linear regression analysis (Figure 1). The Bland-Altman scatter plot showed that the mean difference between the two measurements was 3.75 mmHg, with LOA in the range of −0.35 to 7.86 mmHg. Only 5.14% (37/720) of IOPNCT−GAT data points fell outside the LOA range (Figure 2).
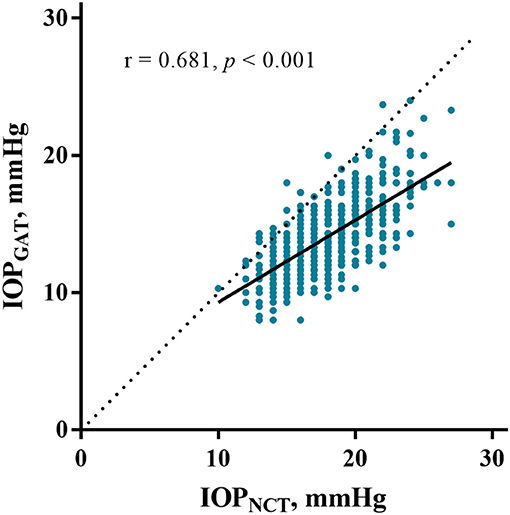
Figure 1. Correlation between IOPNCT and IOPGAT. The scatter plot and regression line (solid line) show comparisons between IOPNCT and IOPGAT values in eyes with high myopia. The dotted line represents the line of identity, and r indicates Pearson's correlation coefficient. IOPNCT, intraocular pressure measured with non-contact tonometer; IOPGAT, intraocular pressure measured with Goldmann applanation tonometer.
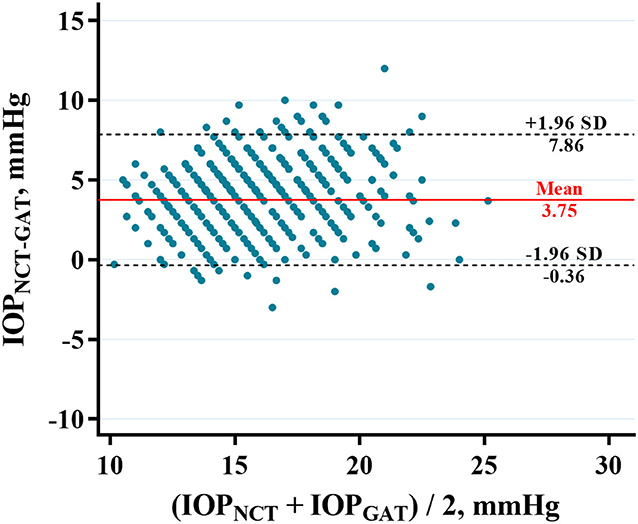
Figure 2. Bland-Altman plot showing the agreement between IOPNCT and IOPGAT in highly myopic eyes. X axis, mean of IOPNCT and IOPGAT values. Y axis, the difference between IOPNCT and IOPGAT values. The two black dashed lines indicate mean difference ± 1.96 standard deviation between IOPNCT and IOPGAT (−0.36–7.86 mmHg). The red line indicates the mean difference in IOP (3.75 mmHg). IOPNCT, intraocular pressure measured with non-contact tonometer; IOPGAT, intraocular pressure measured with Goldmann applanation tonometer.
Factors Associated With IOPNCT and IOPGAT
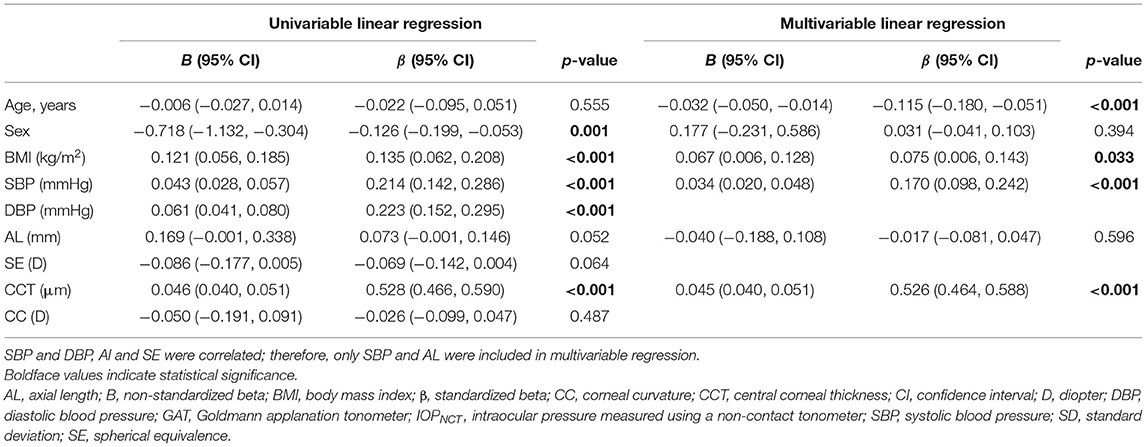
Univariate linear regression revealed that IOPNCT was strongly associated with sex, BMI, SBP, and CCT. Multivariable regression analysis, which included IOPNCT as the dependent variable, and age, sex, BMI, SBP, AL, and CCT as independent variables, showed that age (standardized β = −0.115, p < 0.001) was negatively associated with IOPNCT, while BMI (standardized β = 0.075, p = 0.033), SBP (standardized β = 0.170, p < 0.001), and CCT (standardized β = 0.526, p < 0.001) were positively correlated with IOPNCT (Table 2). The related regression plots (Figure 3) showed that the R2 values of SBP and CCT on IOPNCT were 0.047 and 0.279, respectively.
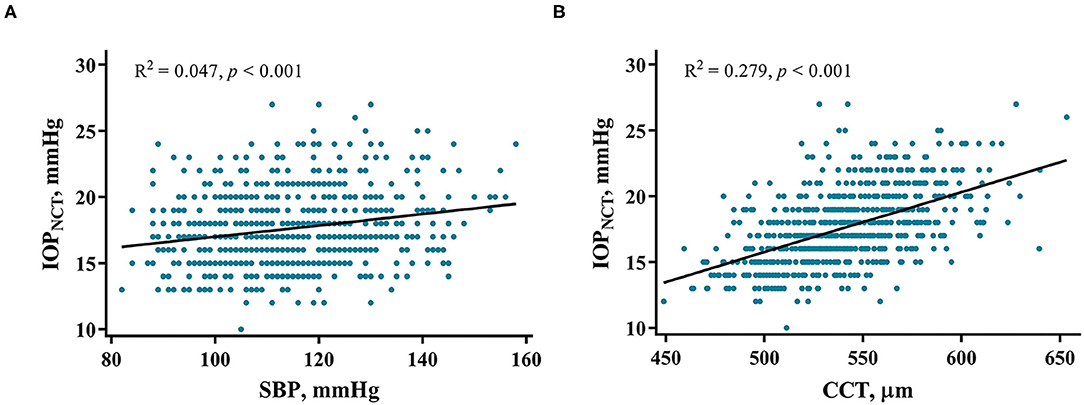
Figure 3. Correlations between IOPNCT values and risk factors. Regression plots show systolic blood pressure (SBP) (A) and central corneal thickness (CCT) (B) effect on IOPNCT with R2 = 0.047 and 0.279, respectively. The full lines indicate regression lines. IOPNCT, intraocular pressure measured with non-contact tonometer.
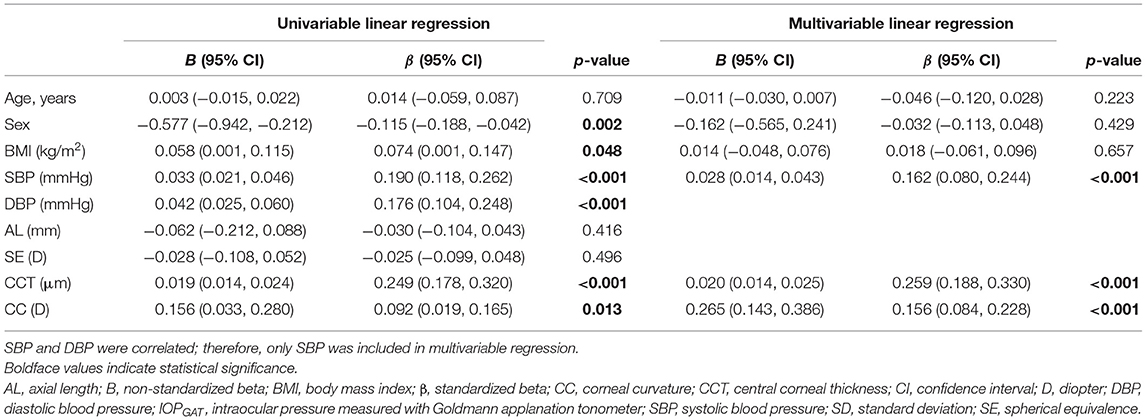
Univariate linear regression showed that IOPGAT was significantly positively associated with sex, BMI, SBP, CCT, and CC (Table 3). Multivariable regression confirmed the results of univariate linear regression and revealed that only SBP (standardized β = 0.162, p < 0.001), CCT (standardized β = 0.259, p < 0.001), and CC (standardized β = 0.156, p < 0.001) were significantly associated with IOPGAT. However, age, sex, and BMI were not associated with the IOPGAT. The related regression plots (Figure 4) showed that the R2 values of SBP, CCT, and CC on GAT were 0.036, 0.062, and 0.009, respectively.
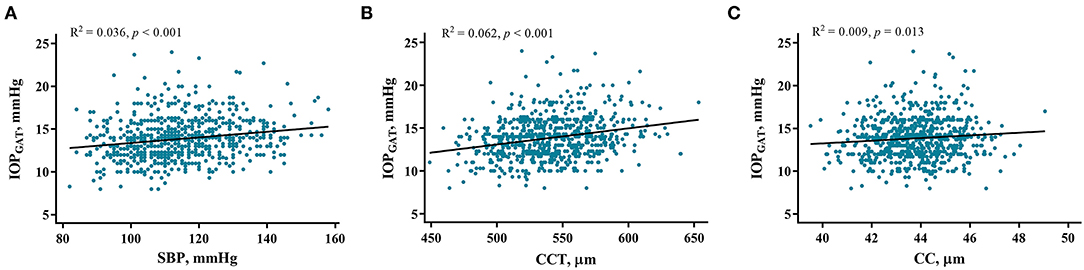
Figure 4. Correlations between IOPGAT values and risk factors. Regression plots show systolic blood pressure (SBP) (A), central corneal thickness (CCT) (B), and corneal curvature (CC) (C) effect on IOPGAT with R2 = 0.036, 0.062, and 0.009, respectively. The full lines indicate regression lines. IOPGAT, intraocular pressure measured with Goldmann applanation tonometer.
Factors Affecting IOPNCT-GAT
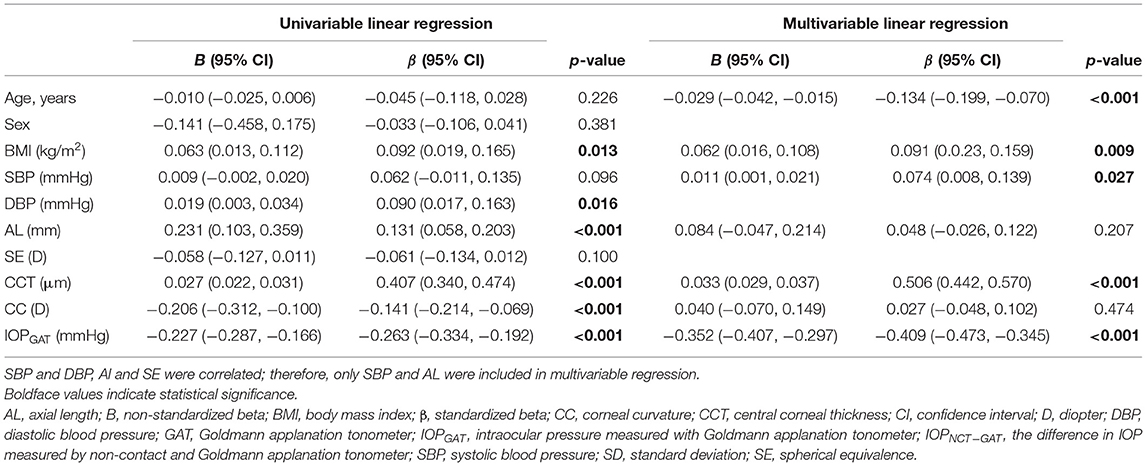
IOPNCT−GAT values can be influenced by specific systemic and ocular factors. In the high myopia group, univariate linear regression showed that IOPNCT−GAT was positively associated with BMI, AL, and CCT, and negatively associated with CC and IOPGAT (Table 4). Multivariable regression confirmed that IOPNCT−GAT was positively associated with BMI (standardized β = 0.091, p = 0.009), SBP (standardized β = 0.074, p = 0.027), and CCT (standardized β = 0.506, p < 0.001), and negatively associated with age (standardized β = −0.134, p < 0.001) and IOPGAT (standardized β = −0.409, p < 0.001). The related regression plots (Figure 5) showed that the R2 values of CCT and IOPGAT on IOPNCT−GAT were 0.167 and 0.069, respectively.
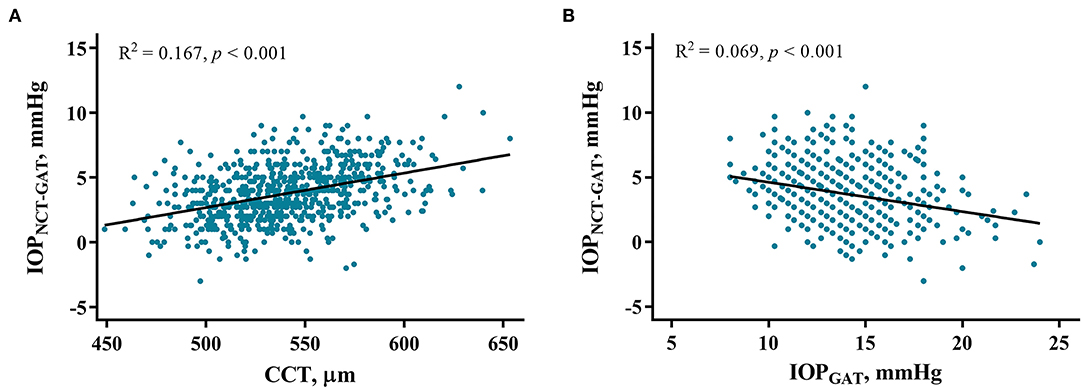
Figure 5. Correlations between IOPNCT−GAT values and risk factors. Regression plots show central corneal thickness (CCT) (A) and IOPGAT value (B) effect on IOPNCT−GAT with R2 = 0.167 and 0.069, respectively. The full lines indicate regression lines. IOPGAT, intraocular pressure measured with Goldmann applanation tonometer; IOPNCT−GAT, the difference of intraocular pressure measured with non-contact and Goldmann applanation tonometer.
Discussion
To the best of our knowledge, this is the first study to comprehensively compare the differences between the GAT and NCT measurements and factors that affect them in a non-pathological high myopia population. Among the high myopia participants with a mean age of 31.10 years and IOP of <30 mmHg, although the IOP measurements of the two devices were significantly correlated (r = 0.681, P < 0.001), IOPNCT overestimated IOPGAT by 3.75 ± 2.10 mmHg. SBP and CCT were the main factors influencing both measurements. Younger age, higher BMI, higher SBP, thicker CCT, and lower IOPGAT significantly broadened the difference in IOPNCT−GAT.
In this cross-sectional study, the mean IOPNCT and IOPGAT values were 17.60 and 13.85 mmHg, respectively. Li et al. showed that IOPGAT was 15.1 ± 2.4 mmHg in highly myopic population with a mean age of 22.8 years (14), and a Spanish study suggested that IOPGAT was 15.54 ± 2.78 mmHg in a population with a mean age of 33.8 years (19); these values were slightly higher than those in the present study. Studies regarding IOPNCT in the high myopia population are limited (20). In normal young subjects, IOPNCT usually overestimates IOPGAT by 1–2 mmHg (21–23). Compared with that in our study, the mean difference between IOPNCT and IOPGAT in the present study was greater, which might be related to the different measuring principles and corneal biomechanics in high myopia populations.
The GAT is based on the area of flattened cornea (approximately 7.35 mm2), which is converted in mmHg following the Imbert-Fick law (24). The pneumatic system of the NCT generates a puff of air and flattens the central cornea (approximately 10.17 mm2), and the time required for applanation is measured and converted to the IOP value (25). We believe that the flattening of the corneal area by the NCT is larger than that by the GAT, and air flattening is more sensitive to ocular surface conditions and corneal structural properties. In our study, the Bland-Altman consistency analysis revealed that the mean difference between the two measurements was 3.75 mmHg, and the 95% upper LOA was 7.86 mmHg. Although there was a correlation between the values obtained by the two devices, their measurement difference was not acceptable from a clinical point of view, as it can affect the correct assessment of IOP, especially in those high myopia patients with a greater probability of combining glaucoma. In general, IOPNCT cannot simply substitute IOPGAT in patients with high myopia.
IOP measurements are affected by various factors. The present study demonstrated that CCT and SBP are the most important factors that can significantly affect IOPNCT, IOPGAT, and IOPNCT−GAT. The effect of CCT was expected, which is in line with most other reports (14, 26–30). Because the slope estimate in NCT is slightly steeper than that in GAT (Figures 3, 4), CCT had a greater influence on IOPNCT than on IOPGAT. Thus, it is easy to understand that IOPNCT−GAT increases as the CCT increases. This finding is in good agreement with those of other studies (26, 28, 29).
Blood pressure is another risk factor that has been widely investigated in the context of IOP measurements. Higher SBP may increase aqueous humor drainage by increasing capillary pressure and decreasing outflow by elevating episcleral venous pressure (31). The Japanese Kumejima Study, the European Prospective Investigation into Cancer-Norfolk Eye Study, and the Northern Ireland Cohort for the Longitudinal Study of Aging (NICOLA) reported that higher SBP was a strong determinant of higher IOP (32–34); this finding is consistent with that of the present study. Clinicians should consider the potential effects of CCT and SBP on both types of IOP measurements.
We reported that younger age and higher BMI were significantly correlated with higher IOPNCT but not IOPGAT, which is in line with previous studies (13, 14, 31, 32, 35–37). However, the Anyang Childhood Eye Study and NICOLA Study found that IOP increased with older age (34, 38). It has been proposed that both corneal hysteresis and corneal resistance factor values decrease with aging (39). In high myopia populations, corneal hysteresis was reported to be lower than that in normal subjects (40–42). Overall, age-dependent changes in corneal biomechanical properties as well as changes in high myopia may account for our results.
In multivariable analysis, higher CC was a risk factor for increased IOPGAT but not for IOPNCT. This result is consistent with that of a previous study (43). Theoretically, a steeper cornea may require greater flattening and deformation to reach a standard contact area; thus, more pressure and higher IOPGAT readings were generated. However, other studies reported that CC affected IOP measurement with dynamic contour tonometry but not with GAT (44, 45). At present, the influence of CC on IOP measurements is inconclusive, and further research is needed to elucidate the reasons for this discordance. Findings on the association between myopia and IOP have also been inconsistent (3, 14, 37, 46). Due to the strong association between AL and SE, and given that SE is affected by more factors than AL, only AL was incorporated into multivariable analysis. However, there was no significant association between AL and IOP in our study; although AL was longer in patients with high myopia, it did not affect the IOP readings. Differences in mechanical strain of the sclera and organizational compliance may explain these findings (37).
Moreover, the present findings suggest that IOPNVT−GAT values increased as IOPGAT values decreased (Table 4), indicating that with the lowering of IOP, the measurement accuracy of IOPNCT decreased. This finding is consistent with that of a previous study, which reported that NCT overestimated IOP in the lower value range and underestimated IOP in the higher value range (22). However, some studies reported opposite findings, suggesting that IOPNCT values were approximately equal to IOPGAT values in the group with low IOP, while they were overestimated in that with high IOP (10, 23). Larger sample sizes are required to validate these results.
Our study has several strengths. First, this was a large cross-sectional study of non-pathologic high myopia patients. Second, the variables of interest included demographic characteristics, AL, SE, CCT, and CC values. Third, we used the NCT and GAT, which are most commonly used in clinical practice, and we examined factors that affect the accuracy of their measurements. The present results highlight the following: (1) CCT and SBP have a strong effect on IOPNCT, IOPGAT, and IOPNCT−GAT measurements in high myopia patients; (2) younger age and higher BMI values contributed to higher IOPNCT and IOPNCT−GAT; (3) CC positively affected IOPGAT measurement; (4) IOPNCT−GAT increased in the higher IOPGAT range; and (5) sex and excessive AL in high myopia patients were not associated with IOP measurements.
There are some limitations to this study. First, this cross-sectional study examined correlations among the relevant factors; however, no causal inferences can be made from the presented findings. Further longitudinal studies are required to confirm these findings. Second, corneal biomechanical properties were not accounted for, including corneal hysteresis and corneal resistance factor, which may greatly affect IOP measurements. Other risk factors, such as smoking and drinking, should also be included in future studies. Third, a control group of age-matched healthy subjects should be included in the analysis. It remains unclear whether the present findings are unique to patients with high myopia. Fourth, the range of IOPGAT in our subjects was 8 to 24 mmHg, and the number of patients with higher IOP values was relatively small. High myopia patients with higher IOP levels may be underrepresented in our sample. Studies involving larger samples with a wide range of IOP values or population-based study are required to provide reliable evidence on IOP measurements obtained with different methods in patients with high myopia.
In conclusion, we believe that IOPNCT cannot simply substitute IOPGAT in high myopia populations, in particular, in patients with thicker CCT and lower IOP values, which are the main factors that broaden the difference in IOPNCT−GAT. Moreover, the difference between IOPNCT and IOPGAT and the factors that affect it, including demographic and ocular characteristics, should be considered when evaluating the IOP values. Further studies involving more participants and accounting for corneal biomechanical properties are needed to determine the reliability of different IOP measurements in highly myopic patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Review Committee of the Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
PW: design and data screening and manuscript drafting. PW, YS, FL, XG, and WC: acquisition, analysis, and interpretation of data. ZW: statistical analysis. MC, YP, and YL: collected and measured data. XZ and SC: study concept and design, project supervision, and manuscript revision. All authors discussed the results and approved the submitted version.
Funding
This study was supported by the High-level Hospital Construction Project, Zhongshan Ophthalmic Center, Sun Yat-sen University (303020104) and the Science and Technology Program of Guangzhou, China (202102010209).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the ZOC Clinical Research Center for providing the platform and all the staff for data collection.
References
1. Jonas JB, Wang YX, Dong L, Panda-Jonas S. High myopia and glaucoma-like optic neuropathy. Asia Pac J Ophthalmol. (2020) 9:234–8. doi: 10.1097/APO.0000000000000288
2. Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the blue mountains eye study. Ophthalmology. (1999) 106:2010–5. doi: 10.1016/S0161-6420(99)90416-5
3. Wong TY, Klein BE, Klein R, Knudtson M, Lee KE. Refractive errors, intraocular pressure, and glaucoma in a white population. Ophthalmology. (2003) 110:211–7. doi: 10.1016/s0161-6420(02)01260-5
4. Kuzin AA, Varma R, Reddy HS, Torres M, Azen SP. Los Angeles Latino Eye Study Group. Ocular biometry and open-angle glaucoma: the Los Angeles latino eye study. Ophthalmology. (2010) 117:1713–9. doi: 10.1016/j.ophtha.2010.01.035
5. Marcus MW, de Vries MM, Junoy Montolio FGJ, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. (2011) 118:1989–94.e2. doi: 10.1016/j.ophtha.2011.03.012
6. Pan CW, Cheung CY, Aung T, Cheung CM, Zheng YF, Wu RY, et al. Differential associations of myopia with major age-related eye diseases: the singapore Indian eye study. Ophthalmology. (2013) 120:284–91. doi: 10.1016/j.ophtha.2012.07.065
7. Shen L, Melles RB, Metlapally R, Barcellos L, Schaefer C, Risch N, et al. The association of refractive error with glaucoma in a multiethnic population. Ophthalmology. (2016) 123:92–101. doi: 10.1016/j.ophtha.2015.07.002
8. Brusini P, Salvetat ML, Zeppieri M. How to measure intraocular pressure: An updated review of various tonometers. J Clin Med. (2021) 10:3860. doi: 10.3390/jcm10173860
9. Sakaue Y, Ueda J, Seki M, Tanaka T, Togano T, Yoshino T, et al. Evaluation of the new digital Goldmann applanation tonometer for measuring intraocular pressure. J Ophthalmol. (2014) 2014:461681. doi: 10.1155/2014/461681
10. Stock RA, Ströher C, Sampaio RR, Mergener RA, Bonamigo EL. A comparative study between the Goldmann applanation tonometer and the non-contact air-puff tonometer (Huvitz HNT 7000) in normal eyes. Clin Ophthalmol. (2021) 15:445–51. doi: 10.2147/OPTH.S294710
11. Broman AT, Congdon NG, Bandeen-Roche K, Quigley HA. Influence of corneal structure, corneal responsiveness, and other ocular parameters on tonometric measurement of intraocular pressure. J Glaucoma. (2007) 16:581–8. doi: 10.1097/IJG.0b013e3180640f40
12. Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement. J Cataract Refr Surg J Cataract Refract Surg. (2005) 31:146–55. doi: 10.1016/j.jcrs.2004.09.031
13. Kawase K, Tomidokoro A, Araie M, Iwase A, Yamamoto T. Tajimi Study Group, Japan Glaucoma Society. Ocular and systemic factors related to intraocular pressure in Japanese adults: The Tajimi study. Br J Ophthalmol. (2008) 92:1175–9. doi: 10.1136/bjo.2007.128819
14. Li Z, Li S, Liu R, Scheetz J, Xiao O, Zhang J, et al. Distribution of intraocular pressure and related risk factors in a highly myopic Chinese population: An observational, cross-sectional study. Clin Exp Optom. (2021) 104:767–72. doi: 10.1080/08164622.2021.1878817
15. Cagatay HH, Ekinci M, Yazar Z, Gokce G, Ceylan E. Comprasion of ICare rebound tonometer and Goldmann applanation tonometer in high myopia. Scientific World Journal. (2014) 2014:869460. doi: 10.1155/2014/869460
16. Song Y, Wang W, Lin F, Chen S, Jin L, Li F, et al. Natural history of glaucomatous optic neuropathy in highly myopic Chinese: Study protocol for a registry cohort study. BMJ Open. (2020) 10:e039183. doi: 10.1136/bmjopen-2020-039183
17. Ohno-Matsui K, Kawasaki R, Jonas JB, Cheung CMG, Saw SM, Verhoeven VJM, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. (2015) 159:877–83.e7. doi: 10.1016/j.ajo.2015.01.022
18. Martin Bland J, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. (1986) 327:307–10. doi: 10.1016/S0140-6736(86)90837-8
19. Del Buey MA, Lavilla L, Ascaso FJ, Lanchares E, Huerva V, Cristóbal JA. Assessment of corneal biomechanical properties and intraocular pressure in myopic Spanish healthy population. J Ophthalmol. (2014) 2014:905129. doi: 10.1155/2014/905129
20. Lee R, Chang RT, Wong IY, Lai JS, Lee JW, Singh K. Assessment of corneal biomechanical parameters in myopes and emmetropes using the Corvis ST. Clin Exp Optom. (2016) 99:157–62. doi: 10.1111/cxo.12341
21. Jorge J, González-Méijome JM, Queirós A, Fernandes P, Diaz-Rey JA. A comparison of the NCT Reichert R7 with Goldmann applanation tonometry and the Reichert ocular response analyzer. Ophthalmic Physiol Opt. (2011) 31:174–9. doi: 10.1111/j.1475-1313.2010.00817.x
22. Mansoori T, Balakrishna N. Effect of central corneal thickness on intraocular pressure and comparison of Topcon CT-80 non-contact tonometry with Goldmann applanation tonometry. Clin Exp Optom. (2018) 101:206–12. doi: 10.1111/cxo.12620
23. Chen M, Zhang L, Xu J, Chen X, Gu Y, Ren Y, et al. Comparability of three intraocular pressure measurement: Icare pro rebound, non-contact and Goldmann applanation tonometry in different IOP group. BMC Ophthalmol. (2019) 19:225. doi: 10.1186/s12886-019-1236-5
24. Aziz K, Friedman DS. Tonometers-which one should I use? Eye. (2018) 32:931–7. doi: 10.1038/s41433-018-0040-4
25. Shields MB. The non-contact tonometer. Its value and limitations. Surv Ophthalmol. (1980) 24:211–9. doi: 10.1016/0039-6257(80)90042-9
26. Pelit A, Altan-Yaycioglu R, Pelit A, Akova YA. Effect of corneal thickness on intraocular pressure measurements with the Pascal dynamic contour, Canon TX-10 non-contact and Goldmann applanation tonometers in healthy subjects. Clin Exp Optom. (2009) 92:14–8. doi: 10.1111/j.1444-0938.2008.00299.x
27. Ito K, Tawara A, Kubota T, Harada Y. IOP measured by dynamic contour tonometry correlates with IOP measured by Goldmann applanation tonometry and non-contact tonometry in Japanese individuals. J Glaucoma. (2012) 21:35–40. doi: 10.1097/IJG.0b013e31820275b4
28. Lee M, Ahn J. Effects of central corneal stromal thickness and epithelial thickness on intraocular pressure using Goldmann applanation and Non-Contact tonometers. PLoS ONE. (2016) 11:e0151868. doi: 10.1371/journal.pone.0151868
29. Tonnu PA, Ho T, Newson T, El Sheikh A, Sharma K, White E, et al. The influence of central corneal thickness and age on intraocular pressure measured by pneumotonometry, non-contact tonometry, the Tono-Pen XL, and Goldmann applanation tonometry. Br J Ophthalmol. (2005) 89:851–4. doi: 10.1136/bjo.2004.056622
30. Babalola OE, Kehinde AV, Iloegbunam AC, Akinbinu T, Moghalu C, Onuoha I, et al. comparison of the Goldmann applanation and non-contact (Keeler Pulsair EasyEye) tonometers and the effect of central corneal thickness in indigenous African eyes. Ophthalmic Physiol Opt. (2009) 29:182–8. doi: 10.1111/j.1475-1313.2008.00621.x
31. Han X, Yang T, Zhang J, Yu S, Guo X, Yan W, et al. Longitudinal changes in intraocular pressure and association with systemic factors and refractive error: lingtou eye cohort study. BMJ Open. (2018) 8:e019416. doi: 10.1136/bmjopen-2017-019416
32. Tomoyose E, Higa A, Sakai H, Sawaguchi S, Iwase A, Tomidokoro A, et al. Intraocular pressure and related systemic and ocular biometric factors in a population-based study in Japan: the kumejima study. Am J Ophthalmol. (2010) 150:279–86. doi: 10.1016/j.ajo.2010.03.009
33. Foster PJ, Broadway DC, Garway-Heath DF, Yip JLY, Luben R, Hayat S, et al. Intraocular pressure and corneal biomechanics in an adult British population: The EPIC-Norfolk eye study. Invest Ophthalmol Vis Sci. (2011) 52:8179–85. doi: 10.1167/iovs.11-7853
34. McCann P, Hogg R, Wright DM, Chakravarthy U, Peto T, Cruise S, et al. Intraocular pressure and circumpapillary retinal nerve fibre layer thickness in the northern ireland cohort for the longitudinal study of ageing (NICOLA): distributions and associations. Br J Ophthalmol. (2021) 105:948–56. doi: 10.1136/bjophthalmol-2020-316499
35. Sugihara K, Tanito M. Different effects of aging on intraocular pressures measured by three different tonometers. J Clin Med. (2021) 10:4202. doi: 10.3390/jcm10184202
36. Cohen E, Kramer M, Shochat T, Goldberg E, Garty M, Krause I. Relationship between body mass index and intraocular pressure in men and women: a population-based study. J Glaucoma. (2016) 25:e509–13. doi: 10.1097/IJG.0000000000000374
37. Ma D, Wei S, Sun Y, Li SM, An WZ, Hu JP, et al. Distribution of IOP and its relationship with refractive error and other factors: the Anyang University students eye study. Int J Ophthalmol. (2021) 14:554–9. doi: 10.18240/ijo.2021.04.12
38. Li S, Li SM, Wang XL, Kang MT, Liu LR, Li H, et al. Distribution and associations of intraocular pressure in 7- and 12-year-old Chinese children: the anyang childhood eye study. PLoS ONE. (2017) 12:e0181922. doi: 10.1371/journal.pone.0181922
39. Ishii K, Saito K, Kameda T, Oshika T. Elastic hysteresis in human eyes is an age-dependent value. Clin Exp Ophthalmol. (2013) 41:6–11. doi: 10.1111/j.1442-9071.2012.02830.x
40. Shen M, Fan F, Xue A, Wang J, Zhou X, Lu F. Biomechanical properties of the cornea in high myopia. Vision Res. (2008) 48:2167–71. doi: 10.1016/j.visres.2008.06.020
41. Xu S, Xu A, Tao A, Wang J, Fan F, Lu F. Corneal biomechanical properties and intraocular pressure in high myopic anisometropia. Eye Contact Lens. (2010) 36:204–9. doi: 10.1097/ICL.0b013e3181e4a60a
42. Jiang Z, Shen M, Mao G, Chen D, Wang J, Qu J, et al. Association between corneal biomechanical properties and myopia in Chinese subjects. Eye. (2011) 25:1083–9. doi: 10.1038/eye.2011.104
43. Harada Y, Hirose N, Kubota T, Tawara A. The influence of central corneal thickness and corneal curvature radius on the intraocular pressure as measured by different tonometers: Noncontact and Goldmann applanation tonometers. J Glaucoma. (2008) 17:619–25. doi: 10.1097/IJG.0b013e3181634f0f
44. Francis BA, Hsieh A, Lai MY, Chopra V, Pena F, Azen S, et al. Los Angeles latino eye study group. Effects of corneal thickness, corneal curvature, and intraocular pressure level on Goldmann applanation tonometry and dynamic contour tonometry. Ophthalmology. (2007) 114:20–6. doi: 10.1016/j.ophtha.2006.06.047
45. Rask G, Behndig A. Effects of corneal thickness, curvature, astigmatism and direction of gaze on Goldmann applanation tonometry readings. Ophthal Res. (2006) 38:49–55. doi: 10.1159/000089762
Keywords: intraocular pressure, high myopia, non-contact tonometry, Goldmann applanation tonometry, axial length, central corneal thickness, corneal curvature
Citation: Wang P, Song Y, Lin F, Wang Z, Gao X, Cheng W, Chen M, Peng Y, Liu Y, Zhang X and Chen S (2022) Comparison of Non-contact Tonometry and Goldmann Applanation Tonometry Measurements in Non-pathologic High Myopia. Front. Med. 9:819715. doi: 10.3389/fmed.2022.819715
Received: 22 November 2021; Accepted: 10 February 2022;
Published: 03 March 2022.
Edited by:
Ya Xing Wang, Capital Medical University, ChinaReviewed by:
Kui-Fang Du, Capital Medical University, ChinaZhe Pan, Peking University Third Hospital, China
Copyright © 2022 Wang, Song, Lin, Wang, Gao, Cheng, Chen, Peng, Liu, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shida Chen, Y2hlbnNoZDNAbWFpbC5zeXN1LmVkdS5jbg==; Xiulan Zhang, emhhbmd4bDJAbWFpbC5zeXN1LmVkdS5jbg==
 Peiyuan Wang
Peiyuan Wang Yunhe Song
Yunhe Song Xiulan Zhang
Xiulan Zhang Shida Chen
Shida Chen