- 1Anesthesia and Critical Care Department, Hamadan University of Medical Sciences, Hamadan, Iran
- 2Department of Family and Community Medicine, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
Background: Spinal anesthesia (SPA) is the most common type of anesthesia administered for cesarean section. The main aim of this study was to evaluate the effect of aspiration of CSF (0.2 mL) immediately after SPA with hyperbaric 0.5% bupivacaine on the extent of sensory and motor block.
Methods: In this clinical trial, 60 women at ≥37 weeks of gestation and aged between 18 and 46 years, candidate for cesarean delivery under spinal anesthesia were randomly allocated into two equal groups (n = 30). Group A (CSF-aspiration group) received the spinal anesthesia with 10 mg of hyperbaric 0.5% bupivacaine with aspiration of 0.2 ml of CSF. Group B (no-CSF-aspiration group) received only 10 mg of 0.5% hyperbaric bupivacaine. Pin-prick analgesia and motor block were tested during the induction.
Results: The mean maximum level of analgesia was T6 in each group. Although the mean time to reach the maximum level of anesthesia (4.43 ± 5.14 vs. 2.76 ± 2.04, P = 0.107) and to reach T10 level (50.56 ± 11.51 vs. 49.10 ± 13.68, P = 0.665) in the CSF-aspiration group is longer than the non-CSF-aspiration group, but this differences were not significant. There were no significant between-group differences regarding sensory and motor block quality (P = 0.389) or failed SPA (four cases in CSF-aspiration group vs. two cases in no-CSF-aspiration group, P = 0.389). The incidence of bradycardia, hypotension, headache, vomiting and nausea were similar in both groups (P > 0.05). In addition, the difference in hemodynamic parameters between the two groups over times was not statistically significant.
Conclusion: Our finding indicated that the aspiration of 0.2 ml of CSF after injection of spinal anesthesia with hyperbaric 0.5% bupivacaine does not seem to affect the extent of sensory and motor block, success rate, or outcome after SPA in cesarean section.
Clinical Trial Registration: [https://www.irct.ir/search/result?query=IRCT2012091501 0841N25], identifier [IRCT20120915010841N25].
Introduction
Cesarean delivery (C-section) is one of the methods to termination of pregnancy that is performed under general or regional anesthesia (1). General anesthesia has become less commonly used in recent decades due to the widespread utilization of regional anesthesia methods and the understanding that these techniques can be provided even in an emergency situation (2). Despite recent devices that facilitate endotracheal intubation and clinical algorithms, guiding anesthesiologists still facing significant challenging scenarios, risks, and complications of general anesthesia for both mother and neonate(s) at the time of delivery (3).
Today, the pervasive method in cesarean section is regional anesthesia, which is usually a safer option than general anesthesia (4). Spinal anesthesia (SPA), epidural anesthesia, and combined spinal-epidural anesthesia (CSE) are the three regional anesthetic techniques available for cesarean delivery (5, 6). Regional anesthesia especially SPA has been favored as the best choice for elective cesarean delivery due to its faster onset of action, simpler technique, more complete sensory and motor block, greater maternal comfort, infant safety, and less risk of aspiration of gastric content (7, 8). This method is safe and effective, but it is not a 100% successful method. Hypotension (9), post-dural puncture headache (PDPH) (10), bradycardia (11), nerve damage (12), nausea and vomiting (13), high level of anesthesia and failure in SPA are the most common complications (14).
Failed spinal anesthesia can be defined as partial or incomplete spinal block within 15–20 min after injection and requiring supplemental analgesia or conversion to general anesthesia (15, 16). Evidence showed that the incidence of failure SPA is between 1 and 17% (17, 18). History of previous anesthesia, obesity, dry tap of cerebrospinal fluid (CSF), bloody CSF, miscalculation of the dose, maldistribution of the local anesthetic, multiple lumbar puncture attempts, use of the L4/L5 interspace and technical errors were shown to significantly associate with failed spinal anesthesia (14, 19, 20).
The control of anesthetic level with both hyperbaric and isobaric bupivacaine that used for spinal block is always difficult. In previous studies, the effect of different volumes, dosages, and concentrations on the spread of sensory and motor blocks has been investigated and has shown wide variability (21–24). Among these factors, the effect of CSF aspiration injection on the distribution of local anesthesia remains controversial and there is little information about its effectiveness in quantitative terms (25–27). Therefore, we conducted this study to evaluate the effect of aspiration of 0.2 mL of CSF immediately after injection of 2.5 ml hyperbaric 0.5% bupivacaine in subarachnoid space on the extent of sensory and motor block level in cesarean section under spinal anesthesia.
Materials and Methods
Study Design
This prospective, randomized, double blind, controlled clinical trial study was conducted in Fatemiyeh Hospital in Hamadan, Iran, between 2019 and 2020. The protocol study was reviewed and approved by the Ethics Committees of Hamadan University of Medical Sciences (IR.UMSHA.REC.1399.630). This study Registered at Iranian Registry of Clinical Trials (IRCT20120915010841N25). Written informed consent were obtained from each patient. The study was conducted in accordance with the Declaration of Helsinki and subsequent (28).
Participants
American Society of Anesthesiologists (ASA) physical status I–II term nulliparous women at ≥37 weeks of gestation with a singleton pregnancy and aged between 18 and 46 years, who presented for cesarean delivery under spinal anesthesia were included in this study. Any patients not willing to participate in this study, presence of any contraindication for SPA including with spinal deformities, inability to maintain the required body position during needle puncture, elevated intracranial pressure, localized infection at the site of needle insertion, severe allergies to local anesthetics, neurological disorders, and acute comorbidities such as severe hypotension (mean arterial pressure <50 mm/Hg), cardiovascular diseases (ejection fraction <30%), liver diseases (liver enzyme levels 1.5 times higher than normal levels), and renal diseases (creatine >1.5 mg/dl) were excluded from the study.
Sample Size
The sample size of this study was calculated based on previous studies was conducted by Bjurström et al. (27), showed that the reduction of CSF volume by 10 mL increased the extent of sensory anesthesia (mean thoracic level 4.3 ± 2.4 vs. 7.1 ± 2.6, P < 0.001). We used formula for difference in proportions between two groups, and concerning the Type I error (α) set as two-sided 5% (Z1–α/2 = 1.96), type II error (β) set as 20% (Z1–β = 0.84) and power of 80%. A sample size of 60 patients, 30 in each arm, is sufficient to detect a clinically important difference between two groups.
μ1: Mean of thoracic level before intervention (4.3).
μ2: Mean of thoracic level after intervention (7.1).
Sd1: standard deviation of the thoracic level before intervention (2.4).
Sd2: standard deviation of the thoracic level after intervention (2.6).
Randomization and Blinding
Women at more than 37 weeks of gestation with a singleton pregnancy who met the all criteria for inclusion were randomly assigned to the Group A or B based on block randomization. Group A (CSF-aspiration group) underwent spinal anesthesia with hyperbaric 0.5% bupivacaine and immediately afterward received aspiration of 0.2 mL of CSF. Group B (no-CSF-aspiration group) received only spinal anesthesia with hyperbaric 0.5% bupivacaine. Block randomization was performed using sealed envelope technique and computer generated random numbers by Random Allocation Software (RAS; Informer Technologies, Inc., Madrid, Spain). In addition, the researchers and nurses who completed the data collection forms were blinded to the group allocated of patients.
Anesthesia Procedure and Data Collection
Before the administration of anesthesia drug, ringer serum (10 ml/kg) was injected into a suitable peripheral cubital vein. In sitting position, spinal puncture was applied in lumber (L3-L4 or L4-L5 region) including 2.5 ml hyperbaric 0.5% bupivacaine using a 25-G Quincke pencil point spinal needle. In group A (CSF-aspiration group), at the end of the anesthesia drug injection, 0.2 mL of CSF was re-aspirated and re-injected into the subarachnoid space. In group B (no-CSF-aspiration group), after the injection of the anesthesia drug without CSF aspiration, the spinal needle was removed immediately after the injection and the patients were turned to the supine position.
Variables assessed included demographic such as age and clinical variables included cesarean reasons, gravida, hemodynamic parameters, duration of surgery, spinal anesthesia time, time to reach maximum level of anesthesia, time to reach the tenth thoracic vertebra (T10) level, sensory and motor block quality, maximum level sensory block according to thoracic vertebra, high spinal, failed SPA, sensory level in recovery and SPA-related complications (such as hypotension, PDPH, bradycardia, nausea, and vomiting) were recorded for each patients. All parameters were noted by an anesthesiologist and nurses blinded to the group allocation.
Standard monitoring including electrocardiography, pulse oximetry and non-invasive blood pressure (NIBP) monitoring was performed for each patient. Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rates (HR) were measured and recorded using an X162 monitor (Saadat Co., Tehran, Iran) at baseline (Time 0) and immediately after spinal anesthesia (T1) and then every 2 min and up to 10-min (T2–T6), and then every 5 min up to 30-min (T7–T10), and then every 10 min up to 60-min (T11–T13) min after infusion of an anesthesia drug. So that each of the hemodynamic parameters from the time before the injection of anesthesia to 1 h after the injection of anesthesia was recorded 14 times for each patient. Any fall in the SBP below 90 mmHg or a fall in MAP of more than 20% from baseline value was taken as hypotension. Hypotension and bradycardia (heart rate less than 60 beats per minute), managed with intravenous 10, 20, or 30 mg of ephedrine and 0.5 or 1 mg atropine, respectively. The amount of ephedrine and atropine used were also recorded.
Maximum level of sensory block, was evaluated by a pin-prick method using a 25-gauge needle and time to reach maximum level of anesthesia and time to reach T10 level was recorded for each patients. Quality of sensory block was evaluated by the visual analog scale (VAS); scores were recorded by making a handwritten mark on a 10-cm line that represents a continuum between “excellent” and “poor” (29). VAS scores described postoperative excellent quality as none (0), mild (<3), moderate (3–6), or poor (7–10). Quality of motor block was evaluated by the Bromage Scale (30). The modified Bromage Scale was used: 0 = no motor block; 1 = able to flex knee free movement of feet, unable to raise extended leg (partial motor block); 2 = free movement of feet only (almost complete motor block); 3 = unable to move hips, knees, feet (complete motor block).
Sedation was assessed by Ramsay scale, it divides a patient’s level of sedation into six categories ranging from severe agitation to deep coma; 1 = anxious and agitated or restless or both; 2 = co-operative, oriented and tranquil; 3 = responding to commands only; 4 = brisk response to light glabellar tap or loud auditory stimulus; 5 = sluggish response to light glabellar tap or loud auditory stimulus; 6 = No response to stimulus (31).
If, within 20 min of the initial spinal anesthesia, no signs of sensory or motor block are observed and confirmed by the anesthesiologist to initiate surgery, the patient will be a candidate for second SPA or general anesthesia (GA) and will be registered as a spinal failure in the questionnaire. Also, in cases where the level of anesthesia is more than the required amount for cesarean section (above T4) supportive measures are taken for the mother and she was recorded in the questionnaire as high spinal. In addition, neonatal Apgar score in minutes 1 and 5 were checked and recorded.
Statistical Analysis
The Shapiro–Wilk test was conducted to test whether the data were normally distributed. Descriptive baseline characteristics for two group comparisons were tabulated as the mean ± standard deviation (SD) or as percentages. A chi-square or Fisher’s exact test was performed for comparisons between two groups of categorical data. Continuous data were statistically analyzed using a t-test or Mann-Whitney U test. Primary efficacy data were examined using an intention-to-treat analysis. Using a general linear model, hemodynamic changes and complications between the two groups were compared using a repeated measurement ANOVA test, with the baseline values (age, cause of cesarean, and gravida) used as covariates in the model. The assumption of sphericity was addressed by Mauchly’s test of sphericity, and when the assumption was not satisfied, the Greenhouse–Geisser correction of P-value were utilized. To assess the effect of intervention, the analysis of covariance (ANCOVA) was used after controlling for baseline measures and confounders in a two-step hierarchical model. For the ordinal primary outcome, the ordinal regressions were utilized after controlling for baseline measures and confounders in a two-step hierarchical model. Statistical analysis was carried out using SPSS software (ver. 21) (SPSS Inc., Chicago, IL, United States). In all analyses, P-values less than 0.05 were considered as significant.
Results
Participants of Study
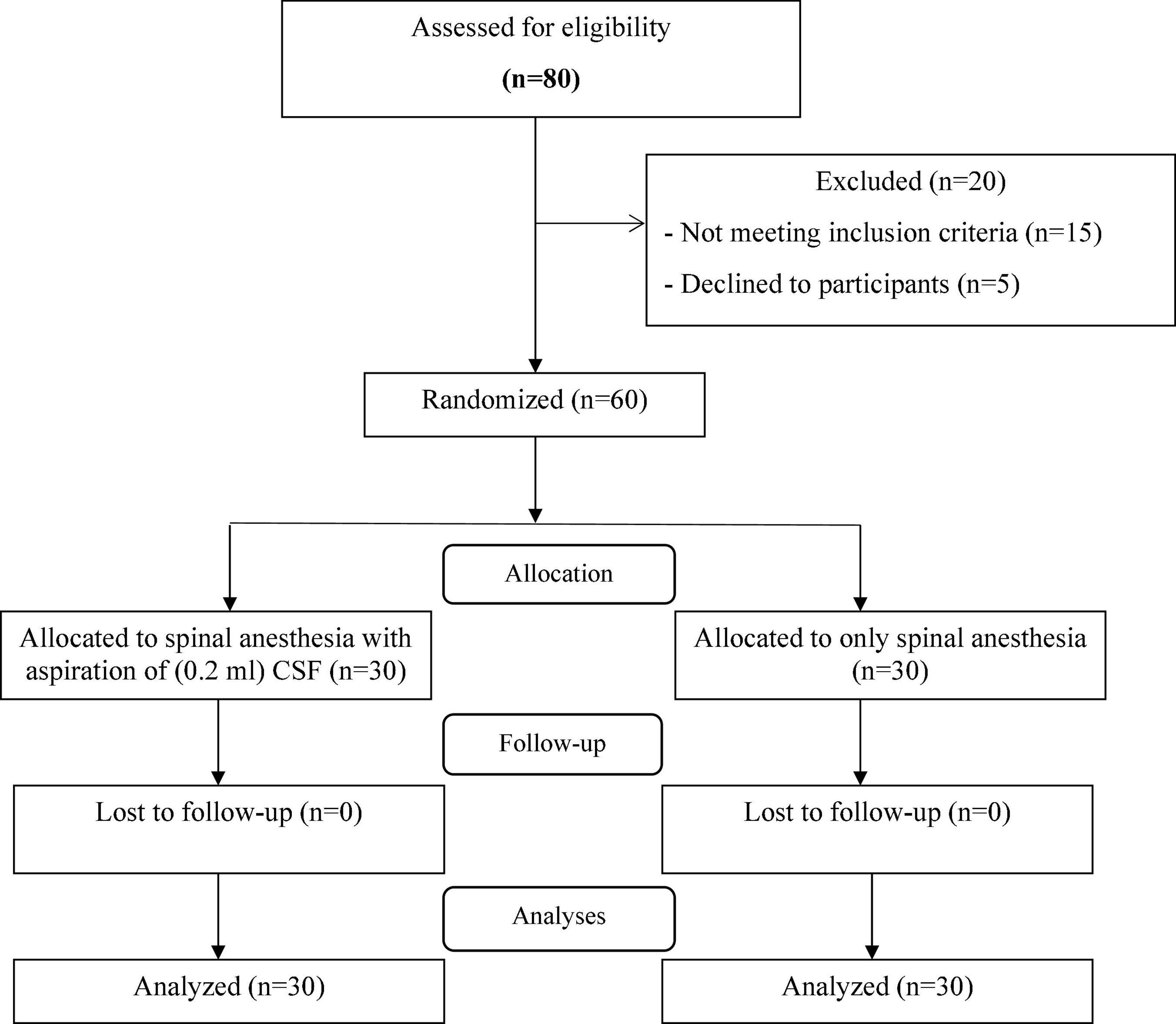
A total of sixty parturient women were enrolled in the study. The enrollment flow chart of patients is presented in Figure 1. Eighty physical status I–II ASA term nulliparous women at ≥37 weeks of gestation with a singleton pregnancy and aged between 18 and 46 years, in Fatemiyeh Hospital in Hamadan, Iran, screened for eligibility criteria. Out of 80 cases, 60 patients met the inclusion criteria and randomly assigned into two groups with 30 patients in each group; CSF-aspiration group (received spinal anesthesia with 10 mg of hyperbaric 0.5% bupivacaine with aspiration of 0.2 ml of CSF immediately afterward) and no-CSF-aspiration group (received only spinal anesthesia with 10 mg of hyperbaric 0.5% bupivacaine). During the intervention and follow-up stages, no patient was excluded from the study and finally 30 patients in each group were analyzed.
Baseline Demographic and Clinical Characteristics
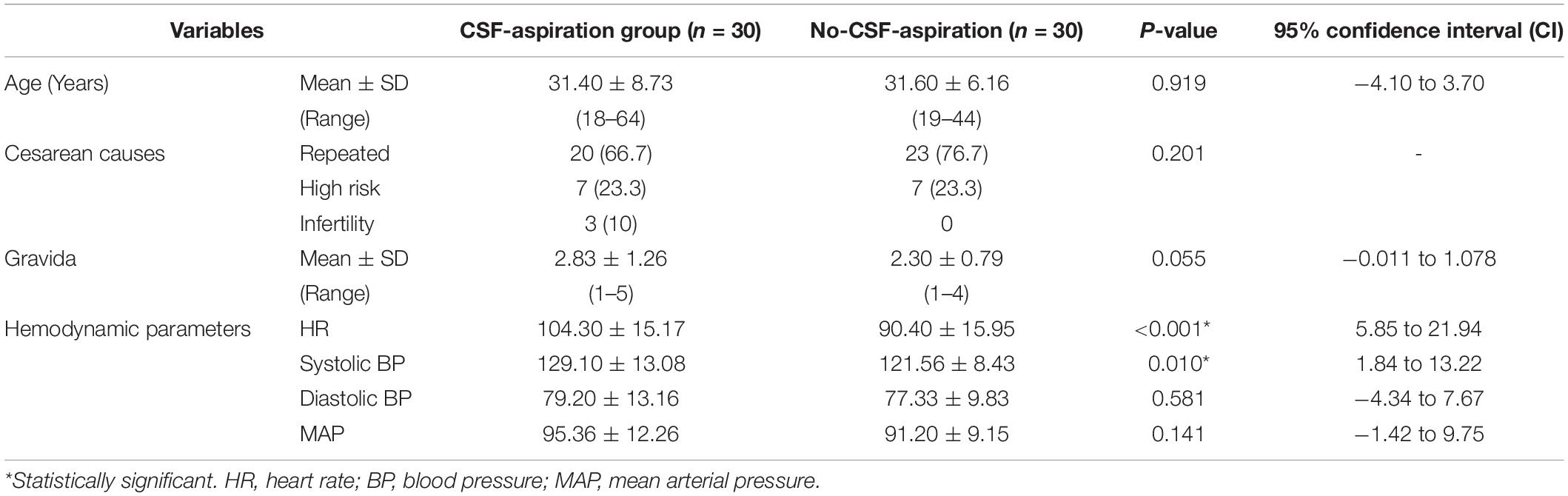
Table 1 shows the baseline demographic and clinical characteristics of the study participants in two groups of study. There were no statistically significant differences in baseline demographic and clinical characteristics of parturient such as; age (P = 0.919), cause of cesarean (P = 0.201) and gravida (P = 0.055). In terms of hemodynamic parameters, statistical significant differences was observed between HR (P < 0.001) and systolic BP (P = 0.01) in the CSF-aspiration and no-CSF-aspiration groups.
Spinal Anesthesia Characteristics and Outcomes
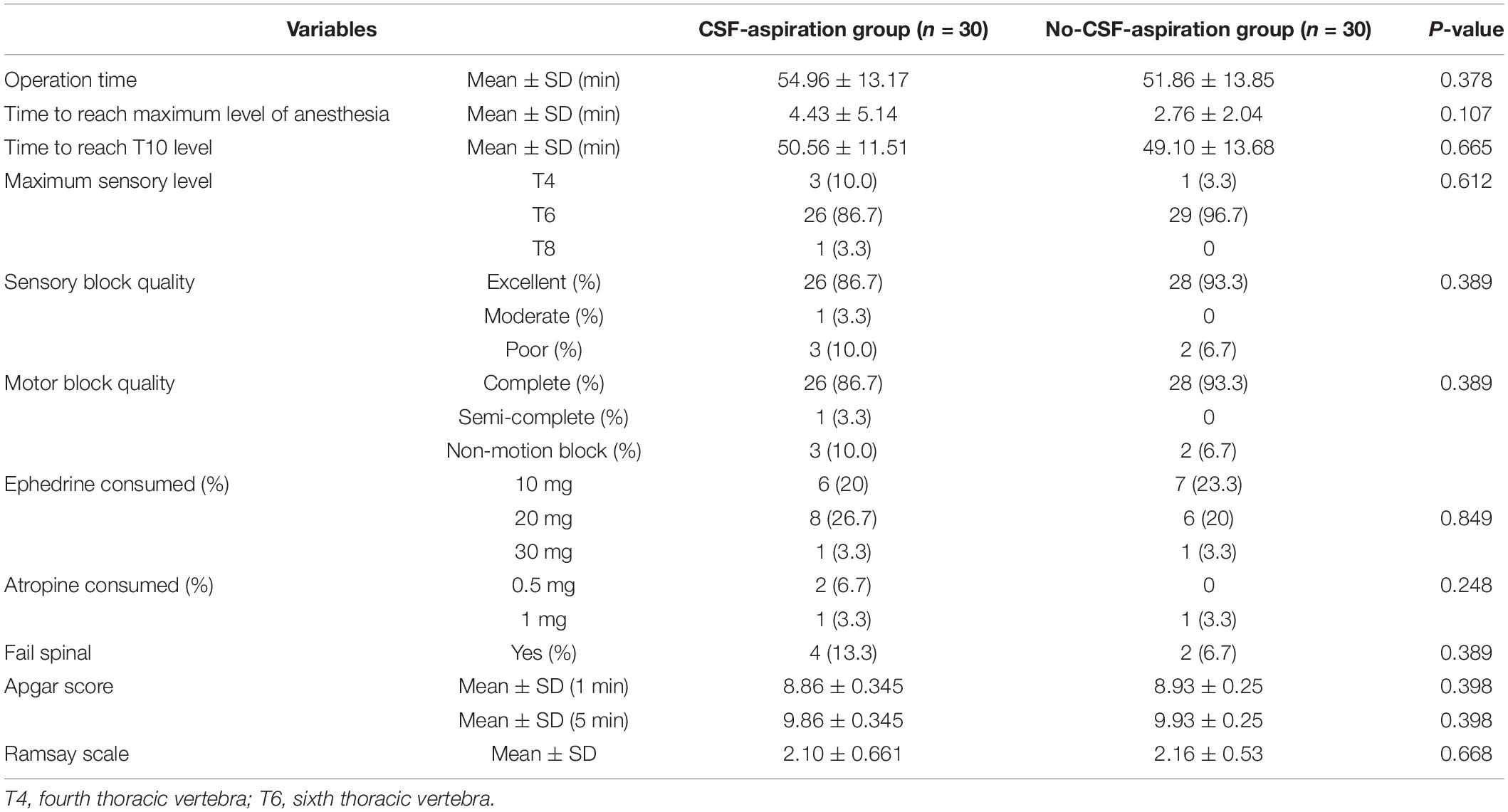
Comparison of spinal anesthesia characteristics and outcomes in two groups of study are presented in Table 2. According to the results, there were no significant differences between the two groups with respect to sensory and motor block quality (P = 0.398). Excellent and complete quality of sensory and motor blocks was observed in more than 80% of patients, respectively.
The maximal level of anesthesia was almost identical T6 in each group. Though the mean ± SD (min) time taken to reach the maximal anesthesia level (4.43 ± 5.14 vs. 2.76 ± 2.04) and also time to reach T10 level (50.56 ± 11.51 vs. 49.10 ± 13.68) were obviously longer in the CSF-aspiration group than in the no-CSF-aspiration group. However, this differences were not reach to statistically significant, (P = 0.107 and P = 0.665). In addition, according to ANCOVA adjusted for age, cesarean reasons and gravida, time to reach the maximum anesthesia level and also time to reach T10 level was not significant difference in two group of study (Table 3).
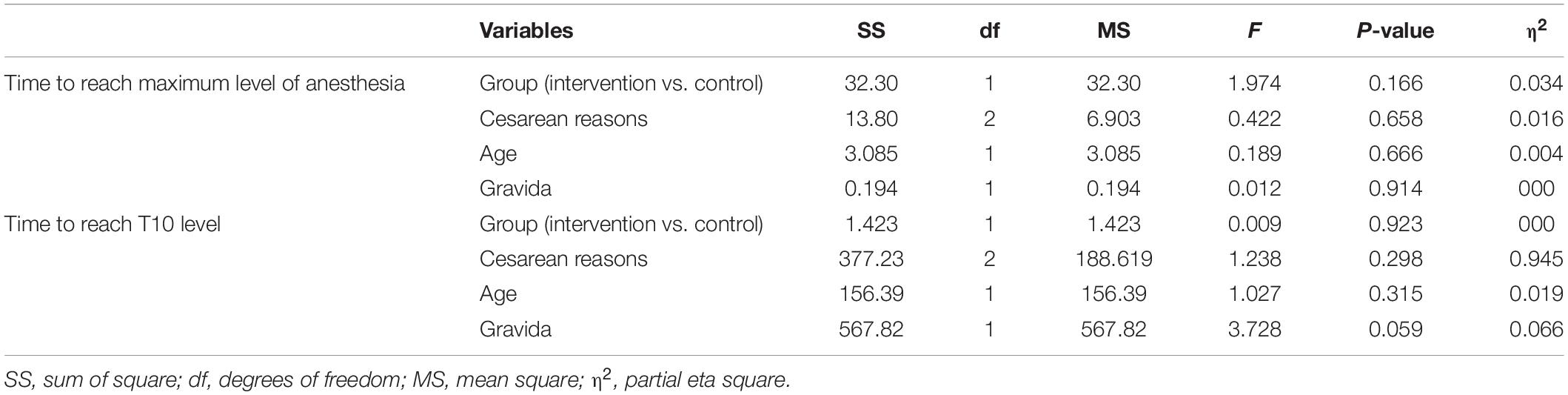
Table 3. Univariate effect of collected variables on time to reach maximum level of anesthesia and time reach T10 level.
Four patients (13.3%) in the CSF-aspiration group (intervention group) and two patients (6.7%) in the no-CSF-aspiration group experienced failed SPA. There was no significant difference between groups regarding incidence of failed SPA (P = 0.389). According to Table 2, the mean ± SD of Apgar showed no significant difference between the two groups in the first minute (8.86 ± 0.345 vs. 8.93 ± 0.25) and 5 min (9.86 ± 0.345 vs. 9.93 ± 0.25) based on the t-test (P = 0.398). In addition, no significant difference was observed between the two groups in terms of using ephedrine and atropine (P < 0.05).
Changes in Hemodynamic Parameters Over Time
Time trends of hemodynamic parameters in two groups of study are presented in Table 4. Hemodynamic parameters (systolic BP, diastolic BP, MAP, and HR) were recorded at baseline and immediately after spinal anesthesia and then every 2 min up to 10-min, and then every 5 min up to 30-min (T7-T10), and then every 10 min up to 60-min after infusion of an anesthesia drug. Figure 2A shows the mean values for changes of systolic BP in each group over times. At baseline the systolic BP was significantly higher in the CSF-aspiration group compared to the no-CSF-aspiration group (129.1 ± 13.1 vs. 121.5 ± 8.43, P < 0.01), while the difference was not statistically significant between other times (P > 0.05). In within group, time effect on SBP was statistically significant in each group (a within-subject difference or time effect) (P < 0.001). As shown in Figure 2B, there was a statistically significant time trend (a within-subject difference or time effect) for diastolic BP in each group (P < 0.001). However, the trend in changes in diastolic BP levels was not statistically significant between two groups (group × time interaction or an interaction effect) (P = 0.518). Figures 3A,B shows the mean values for changes of MAP and HR in each group over times, respectively. There was a statistically significant time trend (a within-subject difference or time effect) for MAP in both groups (P < 0.001). However, the trend in changes in MAP levels was not statistically significant between two groups (group × time interaction or an interaction effect) (P = 0.324). At baseline the HR was significantly higher in the CSF-aspiration group compared to the no-CSF-aspiration group (104.3 ± 15.1 vs. 90.4 ± 15.9, P = 0.01), while the difference was not statistically significant between other times (P > 0.05). In within group, time effect on HR was statistically significant only in the CSF-aspiration group (P < 0.002).
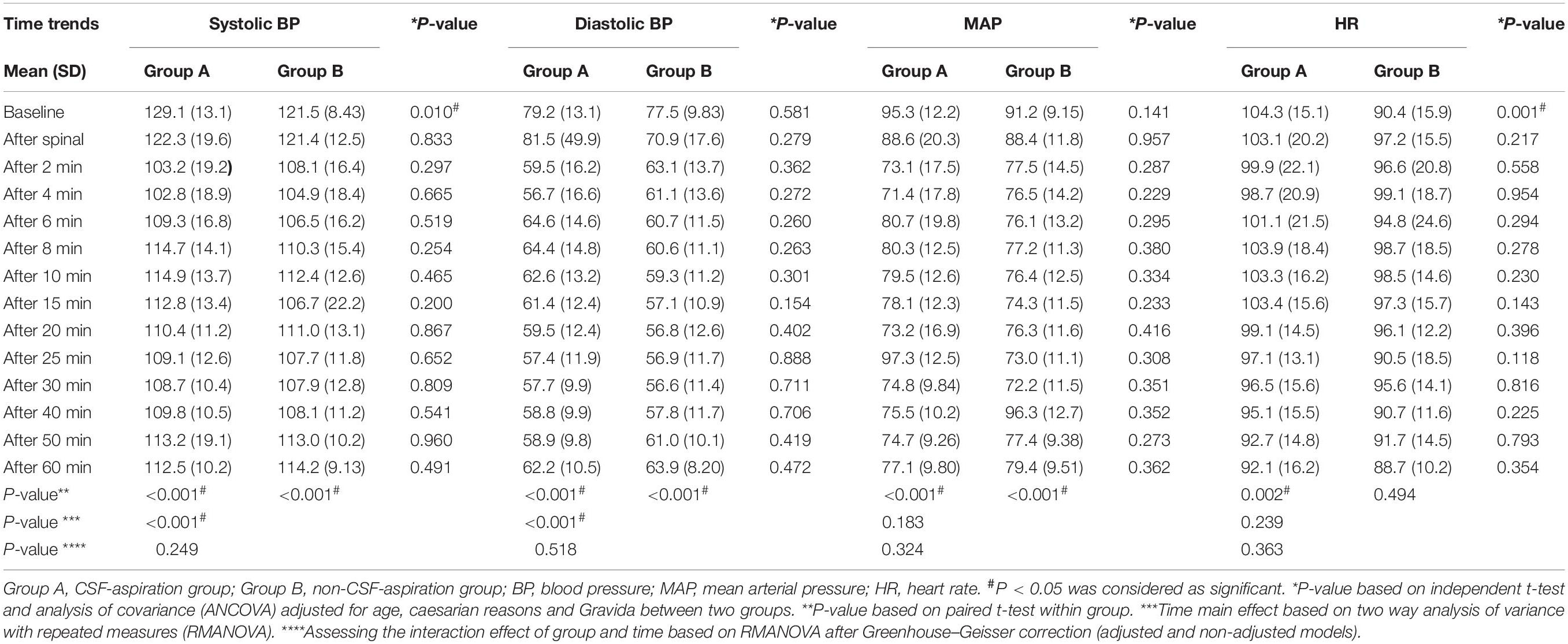
Table 4. Comparison of hemodynamic parameters in the intervention and control groups according to time trends.
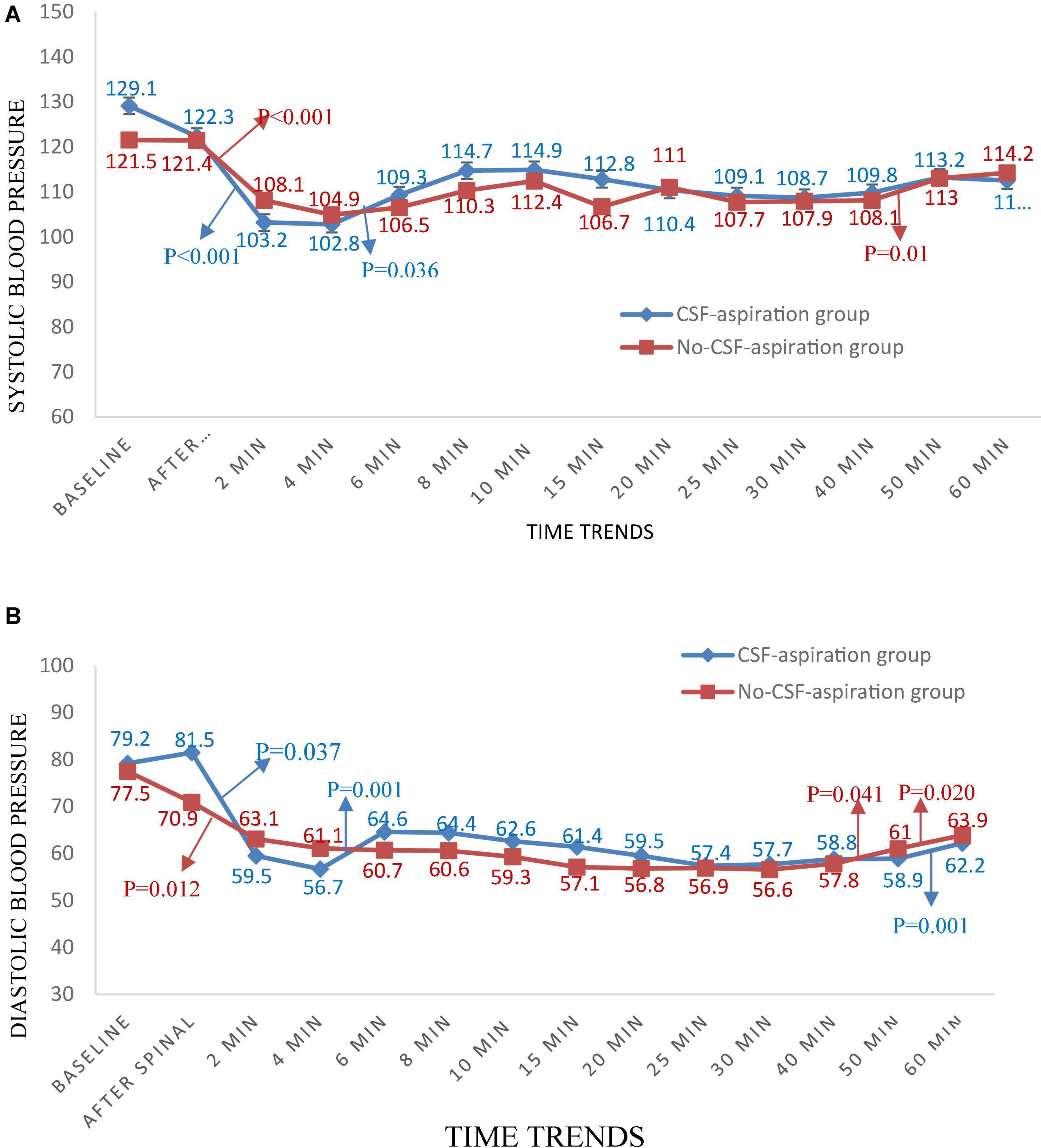
Figure 2. Changes (A) systolic and (B) diastolic blood pressure in two groups of study over times, *P-values shows statistically significant between two times within groups.
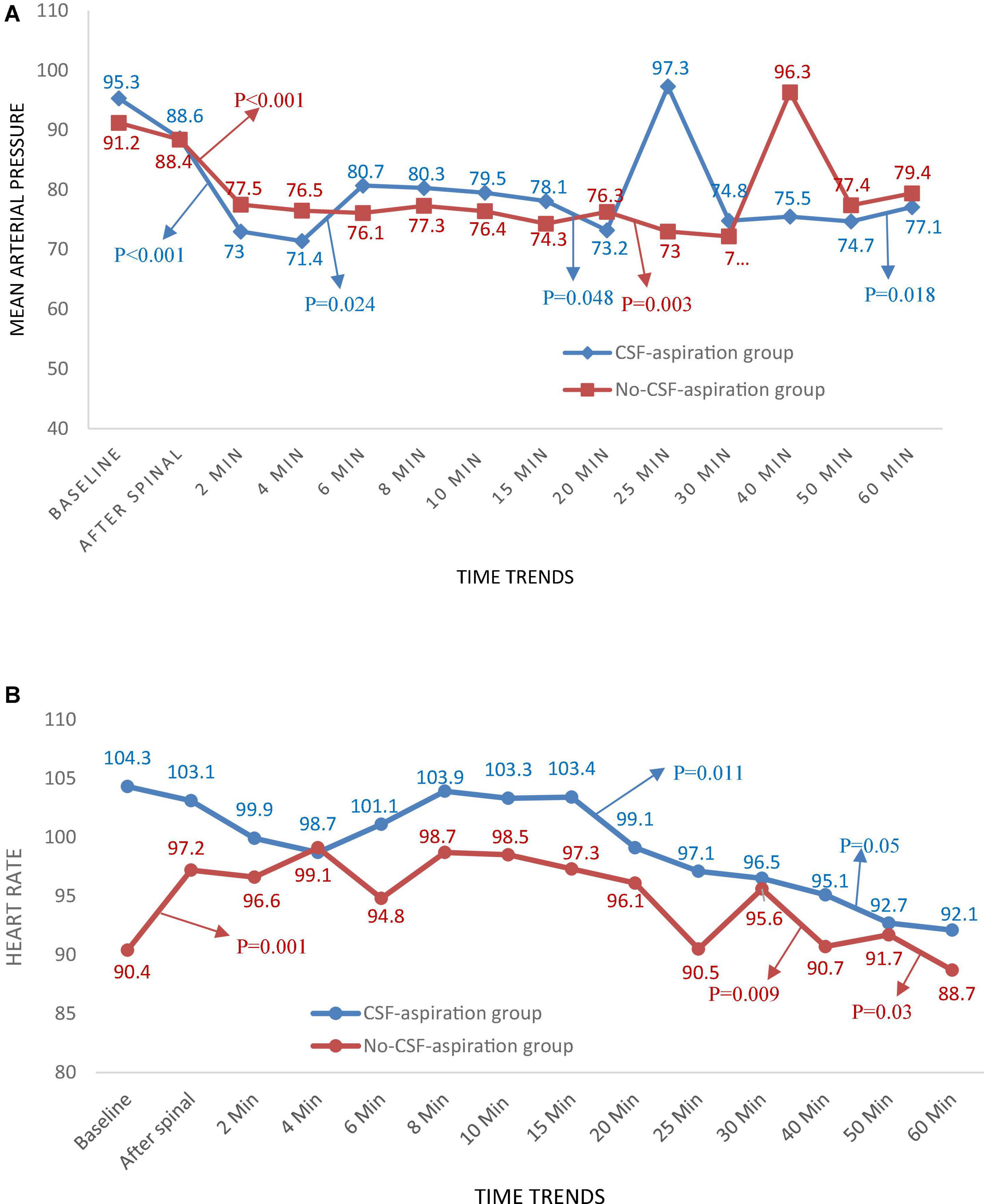
Figure 3. Changes (A) main arterial pressure and (B) heart rate in two groups of study over times, *P-values shows statistically significant between two times within groups.
Spinal Anesthesia-Related Complications
Table 5 shows comparison of SPA-related complications during operation and recovery in two groups of study. According to our findings, hypotension is a common side effect of SPA and it occurred in 17 patients (56.7%) and 14 patients (46.7%) in the CSF-aspiration and non-CSF-aspiration groups, respectively. The frequency distribution of nausea and vomiting in both groups of study was similar (23.3%). There were no significant differences between the two groups with respect to SPA-related complications during operation and recovery (P > 0.05).
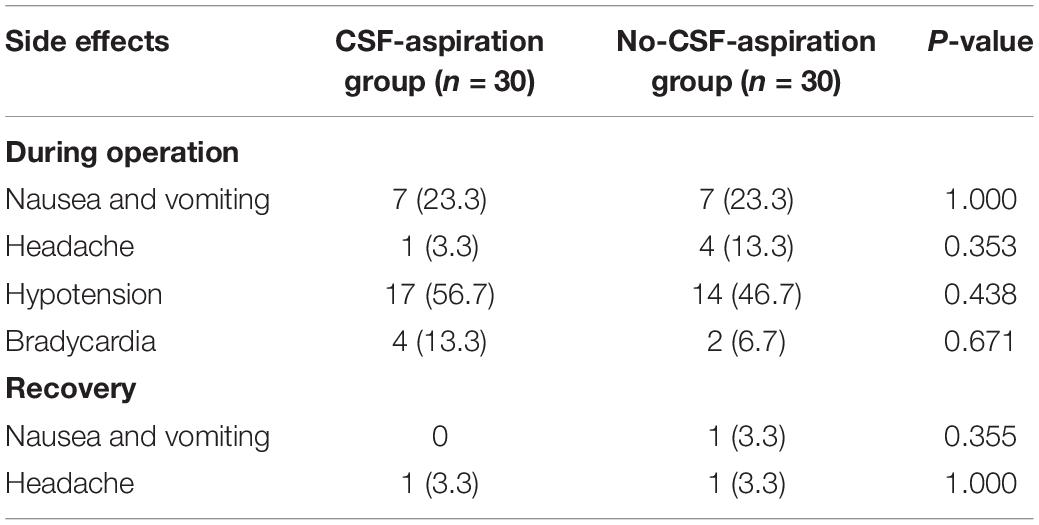
Table 5. Comparison of SPA-related complications during operation and recovery in two groups of study.
Exploratory Correlation Analyses
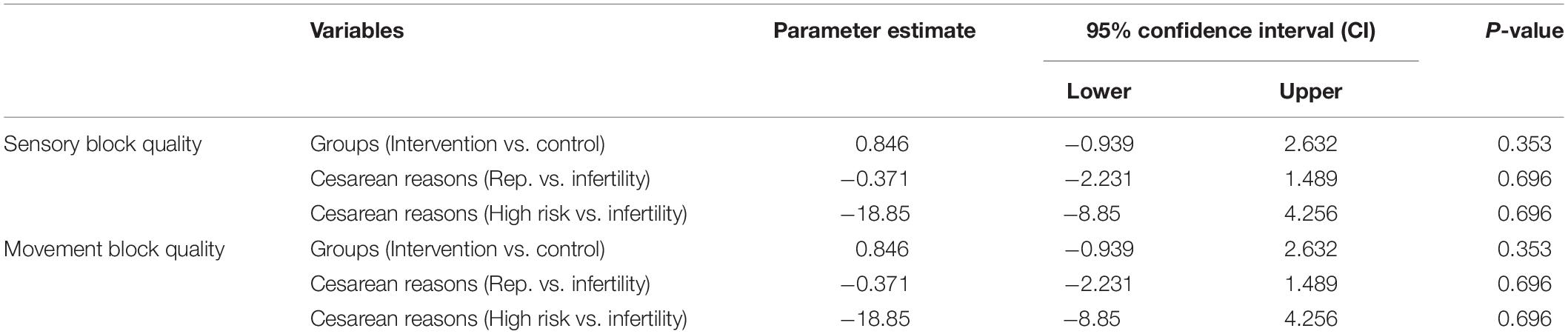
We found no significant relationships between groups (intervention vs. control), cause of cesarean (repeated vs. infertility or high risk vs. infertility), and sensory block quality or motor block quality. The results of ordinal regressions for sensory and movement block quality are shown in Table 6.
Discussion
The main objective of this clinical trial study was to evaluate the effect of CSF aspiration to SPA on extent of sensory and motor block. Our findings indicated that the aspiration of 0.2 ml of CSF after injection of spinal anesthesia with hyperbaric 0.5% bupivacaine had no effect on the extent of sensory and motor block, success rate, or outcome after SPA in cesarean section. The mean maximum level of analgesia was T6 in each group. The excellent quality of sensory block and the complete quality of motor block was high in both groups; 86.7% in the CSF-aspiration group and 93.3% in the no-CSF-aspiration group. Although the mean time to reach the maximum level of anesthesia and to reach T10 level in the CSF-aspiration group is longer than the non-CSF-aspiration group, but this differences were not significant. Hypothetically, it can cause insufficient diffusion and decrease the level of spinal anesthesia or delay the onset of block (26, 32). Cerebrospinal fluid volume seems to be a key determinant of sensory block extent and motor block duration in SPA (33, 34). Given that lumbosacral CSF volume varies considerably between individuals (35), and regional anesthetic is diluted in the CSF before uptake into nerve roots and spinal cord. So, acute reduction in the volume of CSF can be change the effect of SPA (27). To the best of our knowledge, no previous CSF aspiration study in conjunction with SPA has examined acute reduction of CSF volume >1 mL. Pitkänen et al. (26) found no significant difference regarding pin-prick assessed maximum level of sensory block between groups receiving isobaric bupivacaine 15 mg (3 mL) with or without aspiration of 3 mL CSF prior to SPA in 60 elderly (58–77 years) orthopedic or urological patients. Kokki et al. (36) evaluated the effect of 1–3 mL CSF aspiration, equal to the weight-adjusted volume of levobupivacaine SPA, in 186 children aged 10 months to 18 years. No significant difference was found regarding extent of sensory block or duration of motor block, and there were no failed SPAs in the aspiration group. A prospective study was conducted by Cherng et al. (25), to assessed the effect on SPA of the dilution of isobaric 0.5% bupivacaine with CSF. Their findings showed only statistical difference in the time to reach complete motor block, which was shorter in group without CSF aspiration as compared to groups with aspiration. While no differences in onset time and duration of sensory block was observed between group with and without CSF aspiration. In contrast, Jawan and Lee (32), showed that aspiration of 5 mL of CSF before SPA with isobaric bupivacaine (10 mg, 2 mL) led to significantly higher sensory block compared to 3-mL aspiration and no aspiration in 66 patients who undergoing urological procedures. Recently, Bjurström et al. (27) reported that acute reduction of CSF volume by 10 mL prior to SPA with hyperbaric bupivacaine 0.5% (3 mL) leads to a higher thoracic level of sensory block.
In present study, the failure rate of SPA was not significant between two groups of study. However, it was 13.3% in the CSF-aspiration group vs. 6.7% in the no-CSF-aspiration group. Differences in outcomes between groups may be related to CSF aspiration and re-injection, which removes the needle tip from the spinal membrane (dura) and places it in the epidural space. On the other hand, the success of SPA depends on the competence of the anesthetic provider and has proven its effectiveness in skilled hands (37). This block must be performed with great care and method to reach a success rate of almost 100%. SPA in skilled hands is safer, although several factors are thought to affect the SPA such as the presence of anatomical disorders such as kyphoscoliosis, sclerosis, and spinal stenosis following previous intrathecal surgery or chemotherapy obesity and decreased potency of anesthetic drug due to prolonged exposure to light (38–40). The complete failure of spinal anesthesia is generally managed by conversion to general anesthesia or repeating the spinal anesthesia procedure. As assumed that all pregnant patients have a high risk of aspiration and difficulty for intubation, therefore, conversion to general anesthesia is associated with a relatively higher risk of the general population (41).
To our best knowledge, this study was the first CSF aspiration study exploring the effect of CSF aspiration volume <1 ml after injection SPA on the extent of sensory and motor block. The main limitation of the present study is the small sample size. So, further high-quality research with larger sample size is needed to strengthen the evidence base.
Conclusion
The data indicate that the aspiration of 0.2 ml of CSF after injection of spinal anesthesia with hyperbaric 0.5% bupivacaine does not seem to affect the spread, duration, or outcome of SPA in cesarean section.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committees of Hamadan University of Medical Sciences (IR.UMSHA.REC.1399.630). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
FR-B, NM, and FE-A: study concept and design, and critical revision of the manuscript for important intellectual content. FR-B, NM, and ZM: analysis and interpretation of data. ZM: acquisition of data and drafting of the manuscript. ZM and FA: statistical analysis. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ghaffari S, Dehghanpisheh L, Tavakkoli F, Mahmoudi H. The effect of spinal versus general anesthesia on quality of life in women undergoing cesarean delivery on maternal request. Cureus. (2018) 10:e3715. doi: 10.7759/cureus.3715
2. Mhyre JM, Sultan P. General anesthesia for cesarean delivery: occasionally essential but best avoided. Anesthesiology. (2019) 130:864–6. doi: 10.1097/aln.0000000000002708
3. Ring L, Landau R, Delgado C. The current role of general anesthesia for cesarean delivery. Curr Anesthesiol Rep. (2021) 11:18–27. doi: 10.1007/s40140-021-00437-6
4. Iddrisu M, Khan ZH. Anesthesia for cesarean delivery: general or regional anesthesia—a systematic review. Ain Shams J Anesthesiol. (2021) 13:1. doi: 10.1186/s42077-020-00121-7
5. Wahal C, Kumar A, Pyati S. Advances in regional anaesthesia: a review of current practice, newer techniques and outcomes. Indian J Anaesth. (2018) 62:94–102. doi: 10.4103/ija.IJA_433_17
6. Hutton M, Brull R, Macfarlane AJR. Regional anaesthesia and outcomes. BJA Educ. (2018) 18:52–6. doi: 10.1016/j.bjae.2017.10.002
7. Kim WH, Hur M, Park SK, Yoo S, Lim T, Yoon HK, et al. Comparison between general, spinal, epidural, and combined spinal-epidural anesthesia for cesarean delivery: a network meta-analysis. Int J Obstetr Anesth. (2019) 37:5–15. doi: 10.1016/j.ijoa.2018.09.012
8. Kessous R, Weintraub AY, Wiznitzer A, Zlotnik A, Pariente G, Polachek H, et al. Spinal versus general anesthesia in cesarean sections: the effects on postoperative pain perception. Arch Gynecol Obstet. (2012) 286:75–9. doi: 10.1007/s00404-012-2265-y
9. Hofhuizen C, Lemson J, Snoeck M, Scheffer GJ. Spinal anesthesia-induced hypotension is caused by a decrease in stroke volume in elderly patients. Local Reg Anesth. (2019) 12:19–26. doi: 10.2147/lra.s193925
10. Kwak KH. Postdural puncture headache. Korean J Anesthesiol. (2017) 70:136–43. doi: 10.4097/kjae.2017.70.2.136
11. Lesser JB, Sanborn KV, Valskys R, Kuroda M. Severe bradycardia during spinal and epidural anesthesia recorded by an anesthesia information management system. Anesthesiology. (2003) 99:859–66. doi: 10.1097/00000542-200310000-00018
12. Kent CD, Bollag L. Neurological adverse events following regional anesthesia administration. Local Reg Anesth. (2010) 3:115–23. doi: 10.2147/lra.s8177
13. Jelting Y, Klein C, Harlander T, Eberhart L, Roewer N, Kranke P. Preventing nausea and vomiting in women undergoing regional anesthesia for cesarean section: challenges and solutions. Local Reg Anesth. (2017) 10:83–90. doi: 10.2147/lra.s111459
14. Parikh KS, Seetharamaiah S. Approach to failed spinal anaesthesia for caesarean section. Indian J Anaesth. (2018) 62:691–7. doi: 10.4103/ija.IJA_457_18
15. At A, So O. Failed spinal anaesthesia for caesarean section. J West Afr Coll Surg. (2011) 1:1–17.
16. Ashagrie HE, Ahmed SA, Melesse DY. The incidence and factors associated with failed spinal anesthesia among parturients underwent cesarean section, 2019: a prospective observational study. Int J Surg Open. (2020) 24:47–51. doi: 10.1016/j.ijso.2020.03.009
17. Alabi AA, Adeniyi OV, Adeleke OA, Pillay P, Haffajee MR. Factors associated with failed spinal anaesthesia for Caesarean sections in Mthatha general hospital, Eastern Cape, South Africa. S Afr Fam Pract. (2017) 59:128–32. doi: 10.1080/20786190.2017.1292696
18. Garry M, Davies S. Failure of regional blockade for caesarean section. Int J Obstet Anesth. (2002) 11:9–12. doi: 10.1054/ijoa.2001.0903
19. Colish J, Milne AD, Brousseau P, Uppal V. Factors associated with failure of spinal anesthetic: an 8-year retrospective analysis of patients undergoing elective hip and knee joint arthroplasty. Anesth Analg. (2020) 130:e19–22. doi: 10.1213/ane.0000000000004304
20. Aasvang EK, Laursen MB, Madsen J, Krøigaard M, Solgaard S, Kjaersgaard-Andersen P, et al. Incidence and related factors for intraoperative failed spinal anaesthesia for lower limb arthroplasty. Acta Anaesthesiol Scand. (2018) 62:993–1000. doi: 10.1111/aas.13118
21. Chambers WA, Littlewood DG, Edstrom HH, Scott DB. Spinal anaesthesia with hyperbaric bupivacaine: effects of concentration and volume administered. Br J Anaesth. (1982) 54:75–80. doi: 10.1093/bja/54.1.75
22. Bajwa SJ, Kaur J. Clinical profile of levobupivacaine in regional anesthesia: a systematic review. J Anaesthesiol Clin Pharmacol. (2013) 29:530–9. doi: 10.4103/0970-9185.119172
23. Burlacu CL, Buggy DJ. Update on local anesthetics: focus on levobupivacaine. Ther Clin Risk Manag. (2008) 4:381–92. doi: 10.2147/tcrm.s1433
24. Axelsson KH, Edström HH, Sundberg AE, Widman GB. Spinal anaesthesia with hyperbaric 0.5% bupivacaine: effects of volume. Acta Anaesthesiol Scand. (1982) 26:439–45. doi: 10.1111/j.1399-6576.1982.tb01796.x
25. Cherng YG, Huang HH, Chen TG, Huang CL. The effect of cerebrospinal fluid dilution of isobaric 0.5% bupivacaine used for spinal anaesthesia. Anaesthesia. (1995) 50:906–9. doi: 10.1111/j.1365-2044.1995.tb05863.x
26. Pitkänen M, Tuominen M, Asantila R, Rosenberg PH. Effect of aspiration of cerebro-spinal fluid on spinal anaesthesia with isobaric 0.5% bupivacaine. Acta Anaesthesiol Scand. (1985) 29:590–3. doi: 10.1111/j.1399-6576.1985.tb02260.x
27. Bjurström MF, Mattsson N, Harsten A, Dietz N, Bodelsson M. Acute reduction of cerebrospinal fluid volume prior to spinal anesthesia: implications for sensory block extent. Minerva Anestesiol. (2020) 86:636–44. doi: 10.23736/s0375-9393.20.14138-5
28. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
29. Delgado DA, Lambert BS, Boutris N, McCulloch PC, Robbins AB, Moreno MR, et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev. (2018) 2:e088. doi: 10.5435/JAAOSGlobal-D-17-00088
30. Graham AC, McClure JH. Quantitative assessment of motor block in labouring women receiving epidural analgesia. Anaesthesia. (2001) 56:470–6. doi: 10.1046/j.1365-2044.2001.01524-6.x
31. Rasheed A, Amirah M, Abdallah M, Parameaswari PJ, Issa M, Alharthy A. RAMSAY Sedation scale and Richmond Agitation Sedation Scale (RASS): a cross sectional study. Health Sci J. (2018) 12:604. doi: 10.21767/1791-809X.1000604
32. Jawan B, Lee JH. The effect of removal of cerebrospinal fluid on cephalad spread of spinal analgesia with 0.5% plain bupivacaine. Acta Anaesthesiol Scand. (1990) 34:452–4. doi: 10.1111/j.1399-6576.1990.tb03121.x
33. Carpenter RL, Hogan QH, Liu SS, Crane B, Moore J. Lumbosacral cerebrospinal fluid volume is the primary determinant of sensory block extent and duration during spinal anesthesia. Anesthesiology. (1998) 89:24–9. doi: 10.1097/00000542-199807000-00007
34. Higuchi H, Adachi Y, Kazama T. The influence of lumbosacral cerebrospinal fluid volume on extent and duration of hyperbaric bupivacaine spinal anesthesia: a comparison between seated and lateral decubitus injection positions. Anesth Analg. (2005) 101:555–60. doi: 10.1213/01.ane.0000158465.17547.f1
35. Chazen JL, Dyke JP, Holt RW, Horky L, Pauplis RA, Hesterman JY, et al. Automated segmentation of MR imaging to determine normative central nervous system cerebrospinal fluid volumes in healthy volunteers. Clin Imaging. (2017) 43:132–5. doi: 10.1016/j.clinimag.2017.02.007
36. Kokki M, Heikkinen M, Kumpulainen E, Vähäoja A, Kokki H. Levobupivacaine for spinal anesthesia in children: cerebrospinal fluid aspiration before the injection does not affect the spread or duration of the sensory block. Anesthesiol Pain Med. (2016) 6:e33815. doi: 10.5812/aapm.33815
37. Klimek M, Rossaint R, van de Velde M, Heesen M. Combined spinal-epidural vs. spinal anaesthesia for caesarean section: meta-analysis and trial-sequential analysis. Anaesthesia. (2018) 73:875–88. doi: 10.1111/anae.14210
38. Fettes PDW, Jansson J-R, Wildsmith JAW. Failed spinal anaesthesia: mechanisms, management, and prevention. Br J Anaesth. (2009) 102:739–48. doi: 10.1093/bja/aep096
39. Westphal M, Götz T, Booke M. Failed spinal anaesthesia after intrathecal chemotherapy. Eur J Anaesthesiol. (2005) 22:235–6. doi: 10.1017/s0265021505220409
40. Kumar R, Singh K, Prasad G, Patel N. Repeat spinal anesthesia after a failed spinal block in a pregnant patient with kyphoscoliosis for elective cesarean section. J Obstet Anaesth Crit Care. (2014) 4:84–6. doi: 10.4103/2249-4472.143879
41. Bhar D, RoyBasunia S, Das A, Chhaule S, Mondal SK, Bisai S, et al. Repeat spinal anesthesia in cesarean section: a comparison between 10 mg and 12 mg doses of intrathecal hyperbaric (0.05%) bupivacaine repeated after failed spinal anesthesia: a prospective, parallel group study. Anesth Essays Res. (2016) 10:362–9. doi: 10.4103/0259-1162.172725
Keywords: cerebrospinal fluid, spinal anesthesia, sensory block, motor block, cesarean section
Citation: Manouchehrian N, Miri Z, Esna-Ashari F and Rahimi-Bashar F (2022) Evaluation Effect of Aspiration of 0.2 ml of Cerebrospinal Fluid After Completion of Injection 0.5% Bupivacaine and Reinjection Into Subarachnoid Space on Sensory and Motor Block in Cesarean Section: A Randomized Clinical Trial. Front. Med. 9:816974. doi: 10.3389/fmed.2022.816974
Received: 17 November 2021; Accepted: 04 March 2022;
Published: 25 March 2022.
Edited by:
You Shang, Huazhong University of Science and Technology, ChinaReviewed by:
Pasquale Sansone, University of Campania Luigi Vanvitelli, ItalyAta Mahmoodpoor, Tabriz University of Medical Sciences, Iran
Copyright © 2022 Manouchehrian, Miri, Esna-Ashari and Rahimi-Bashar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farshid Rahimi-Bashar, ZnJfcmFoaW1pYmFzaGFyQHlhaG9vLmNvbQ==
†ORCID: Nahid Manouchehrian, orcid.org/0000-0003-1063-8537; Farzaneh Esna-Ashari, orcid.org/0000-0002-3151-4843; Farshid Rahimi-Bashar, orcid.org/0000-0001-8276-1425
 Nahid Manouchehrian
Nahid Manouchehrian Zahra Miri1
Zahra Miri1 Farshid Rahimi-Bashar
Farshid Rahimi-Bashar