
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 25 April 2022
Sec. Pulmonary Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.816973
 Hong Jiang1,2†
Hong Jiang1,2† Xue-Mei Yang1,2†
Xue-Mei Yang1,2† Cheng-Qiong Wang1,2
Cheng-Qiong Wang1,2 Jiao Xu1,2
Jiao Xu1,2 Jun Huang3
Jun Huang3 Ji-Hong Feng4
Ji-Hong Feng4 Xiao-Fan Chen5
Xiao-Fan Chen5 Kai Chen6
Kai Chen6 Lin Zhan7*
Lin Zhan7* Xue Xiao1,2*
Xue Xiao1,2* Zheng Xiao1,2*
Zheng Xiao1,2*Introduction: The staphylococcal enterotoxin C (SEC), a commercially available bio-product from Staphylococcus aureus (S. aureus), has been widely used to control MPE.
Objectives: We designed and performed a new systematic review (SR) and meta-analysis to clarify the perfusion protocols with SEC, determine their clinical effectiveness and safety, and reveal the indication and optimum usage for achieving the desired responses.
Methodology: All randomized controlled trials (RCTs) about SEC for MPE were collected from electronic databases (from inception until July 2021), and clustered into multiple logical topics. After evaluating their methodological quality, we pooled the data from each topic using the meta-analysis or descriptive analysis, and summarized the evidence quality using the grading of recommendation assessment, development, and evaluation (GRADE) approach.
Results: All 114 studies were clustered into SEC perfusion alone or plus chemical agents. The SEC alone showed a better complete response (CR), a lower pleurodesis failure, and adverse drug reactions (ADRs), and a higher fever than cisplatin (DDP) alone. The SEC and chemical agents developed 10 perfusion protocols. Among them, only SEC and DDP perfusion showed a better CR, a lower failure, disease progression and ADRs, and a higher fever than DDP alone. The SEC (100–200 ng per time, one time a week for one to four times) with DDP (30–40 mg, or 50–60 mg each time) significantly improved clinical responses for patients with moderate to large volume, Karnofsky performance status (KPS) scores ≥40, ≥50, or ≥60, and anticipated survival time (AST) ≥2 or 3 months. Most results were moderate to low quality.
Conclusion: Current pieces of evidence indicate that super-antigen SEC is a pleurodesis agent, which provides an attractive alternative to existing palliative modalities for patients with MPE. Among 10 protocols, the SEC and DDP perfusion is a most commonly used, which shows a significant improvement in clinical responses with low ADRs. These findings also provide a possible indication and optimal usage for SEC and DDP perfusion.
Malignant pleural effusion (MPE) is a common manifestation of malignant tumors and a significant source of cancer morbidity and mortality, which often causes progressive breathlessness, short survival, and poor quality, and requires palliation (1, 2). So far, the pleurodesis has remained the cornerstone of treatment, and the pleurodesis agents include chemical agents (3–5), biologic response modifiers (6, 7), and traditional Chinese medicine injections (TCMIs) (8, 9), etc. As important biologic response modifiers, serial bio-products from Staphylococcus aureus (S. aureus) (10), hemolytic streptococcialpha (11, 12), corynobactum parvum (C. parvum) (13), and streptococcus pyogenes (S. pyogenes) (14) have been used in clinical studies to achieve pleurodesis and control fluid recurrence. Most strikingly, the S. aureus toxins, super-antigens, stimulate a polyclonal T-cell response, and result in massive cytokine production as interleukin 2 (IL-2), tumor necrosis factor α (TNF α), and interferon gamma (IFN γ), which cause pleural inflammation and fibrosis, culminating in pleurodesis (7, 15, 16). In China, the staphylococcal enterotoxin C (SEC) injection (highly agglutinative staphylococcin), a commercially available bio-product from S. aureus (including enterotoxin C, other proteins, and 18 amino acids) had been approved for adjuvant radiotherapy and chemotherapy in patients with malignant tumors (17, 18). Since the 1990s, SEC alone or in combination with other pleurodesis agents has been widely used to control MPE through intrapleural perfusion (10, 19, 20). According to the Cochrane systematic evaluation, two meta-analyses (21, 22) reported that the SEC in combination with chemotherapeutic drugs or cisplatin (DDP) might improve the clinical efficacy with good safety in pleural effusion and ascites. Previous meta-analyses (21, 22) only determined the clinical effectiveness and safety of SEC pluschemotherapeutic drugs or cisplatin (DDP) for MPE. Obviously, they could not systematically determine whether perfusion with SEC alone is better or equal to other agents. If used with other agents, which perfusion protocols can achieve ideal clinical effectiveness remain unclear. Additionally, no evidence determines their indications and optimal dose, treatment frequency, and times. These questions became the main sources for irrational drug use and clinical decision-making failure. Therefore, we further designed and performed a new systematic review (SR) and meta-analysis to (i) clarify the intrapleural perfusion protocols with SEC, (ii) determine their clinical effectiveness and safety, (iii) reveal their indications and optimum usage, and (iv) provide an evidence framework for formulating scientific and reasonable control strategies in MPE.
To clarify the perfusion protocols with SEC and determine their clinical effectiveness and safety, it is obvious that this study had clinical heterogeneity. So, we classified the heterogeneity as significant and potential clinical heterogeneity. On the basis of the principle of evidence classification (23) and our previous experiences (6, 9), we systematically collected and evaluated all available randomized controlled trials (RCTs), implemented topic clustering to obtain serial homogeneous perfusion protocols, and analyzed the data from each protocol using the meta-analysis or descriptive analysis. Then, we implemented a subgroup analysis to deal with the potential heterogeneity for main protocol. Finally, this study provided an evidence framework for developing a treatment strategy in MPE. This new evaluation was defined as a clustered SR and meta-analysis. During implementation, any disagreements were settled by discussion between two independent reviewers, or with a third party (Zheng Xiao). We designed, performed, and reported this analysis, following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (PRISMA 2020 Checklist) (24).
We developed the retrieval strategy using MeSH and free words. The retrieval form was [“Pleural Effusion” (Mesh) OR Pleural Effusions OR hydrothorax OR Pleural Effusion OR Carcinomatous pleurisy OR Cancerous pleurisy OR Malignant pleurisy OR MPE OR MPEs] AND [“Enterotoxin C, staphylococcal” (Supplementary Concept) OR Staph enterotoxin C OR Staphjlo Toxoid Injection OR Staphylococcal Enterotoxin C Injection OR Staph enterotoxin C2 OR SEC2 toxin OR toxin SEC2 OR Staph enterotoxin C3 OR Staph enterotoxin C1 OR SEC1 toxin OR Highly agglutinative staphylococcin OR Gao, jusheng OR Gao jusheng OR Jinpusu]. Hong Jiang and Cheng-Qiong Wang independently searched all published studies about “SEC for MPE” from the electronic databases (from inception until May 2021), such as PubMed, Embase, Web of Science, China National Knowledge Infrastructure Database (CNKI), Chinese Scientific Journals Full-Text Database (VIP), Wanfang Database, China Biological Medicine Database (CBM), and Cochrane Central Register of Controlled Trials (CENTRAL, Issue 7 of 12, July 2021). All ongoing trials were searched from Chinese clinical trial registry (Chi-CTR, http://www.chictr.org.cn), WHO International Clinical Trials Registry Platform (WHO-ICTRP, http://apps.who.int/trialsearch/), and US-clinical trials (https://clinicaltrials.gov/, up to July 2021). Additionally, all SRs/meta-analyses about “SEC for MPE” were evaluated, and all eligible studies from their references were also included. Hong Jiang and Xue-Mei Yang independently collected eligible studies using the pre-designed inclusion and exclusion criteria.
All eligible studies must meet the following criteria. According to the design characteristics of intervention study, all trials were randomized controlled trials (RCTs), which reported at least a “random allocation.” All patients had symptomatic pleural effusion resulting from an underlying malignant process (of any type and stage), which was diagnosed by using a chest imaging, pleural effusion analysis, cytology, or pleural biopsy. The drainage method of pleural fluid was not limited. One month before perfusion, all patients did not receive intrapleural perfusion with any agents. The intervention studied was SEC (National Medical Products Administration in China, GYZZ.S19990010 or S10970071, 10 ng or 250 IU/ml). The experimental groups received the perfusion with SEC alone or plus chemical agents, and the control groups received the pleurodesis agents alone. The primary indicators were clinical responses and survivals, and the secondary were quality of life (QOL) and adverse events. No restriction was set on the research site and follow-up protocols.
The exclusion criteria were as follows: studies about patients receiving both SEC perfusion and systemic chemotherapy; studies about SEC in combinations with other biologic response modifiers, traditional Chinese medicine injections (TCMIs) or hyperthermia; studies about both groups receiving SEC perfusion; and studies without data of primary or secondary indicators.
The clinical responses were measured by using a complete response (CR), pleurodesis failure, and disease progression (DP). Integrating previous criteria (6, 9, 25–27), the CR is defined as a pleural fluid disappeared for more than 1 month, or the lack of accumulation of fluid; the partial response (PR) is a pleural fluid reduced more than 50% for more than 1 month; the no response (NR)/stable disease (SD) is pleural fluid reduced <50% or increased <25% or the pleural fluid recurred but required no further therapy; and the DP is pleural fluid increased more than 25% along with other signs of progression or symptomatic re-accumulation of the effusion, requiring repeat thoracentesis or chest tube. Accordingly, the pleurodesis failure was defined as NR or SD plus DP. The survivals were measured by using an overall survival (OS) rate, progression-free survival (PFS) rate, or hazard ratio (HR) of the OS and PFS.
If the scores increased ten points or higher after perfusion, the QOL was considered as an improvement according to the Karnofsky Performance Status (KPS) Scale (28, 29). The adverse events were measured by using adverse drug reactions (ADRs), SEC-related adverse events, and treatment-related adverse events (TRAEs). According to the World Health Organization (WHO) (30) or Common Terminology Criteria for Adverse Events (CTCAE) standards (31), the ADRs were defined as myelosuppression, neutropenia, thrombocytopenia, anemia, gastrointestinal reactions, hepatorenal dysfunction, and cardiac dysfunction. The SEC-related adverse events were defined as the drug allergy, fever, and others. The TRAEs were defined as treatment-related mortality and thoracentesis-related events, which included the thoracodynia, fever, respiratory failure, pneumothorax, cutaneous emphysema, and catheter-related infection/chest infection, among others.
Jiao Xu and Jun Huang independently extracted data using a pre-designed data extraction form. If without Kaplan–Meier survival curves or other relevant data, we contacted the authors to obtain available survival data. When unavailable, we reconstructed the Kaplan–Meier survival curves into available data using Engauge Digitizer 4.1 (32). The data included the time of publication, the primary tumors, the volume of pleural fluid, and the KPS score, anticipated survival time (AST) and treatment history, the cases of the experimental and control group, the demographic and methodological characteristics, the drainage method, the usages of SEC and pleurodesis agents, the follow-up, the evaluation criteria, and the primary or secondary indicators.
Hong Jiang and Xue-Mei Yang independently evaluated the methodological bias using the Cochrane Handbook for Systematic Reviews of Interventions Version 6.2.0 (33, 34). The risk indexes were the generating methods of random sequence, the allocation concealment, the blind methods, the incomplete outcome data, the selective reporting, and other bias (e.g., whether the baseline was comparable). The risk of each index was rated as “Yes” for a low bias, “No” for a high risk of bias, or “unclear.”
The primary and secondary indexes were described as odds ratios (OR) or HR and their 95% confidence intervals (CI), and the p < 0.05 was considered statistically significant. We clustered the eligible trials into serial homogeneous topics as SEC alone or SEC plus chemical agents, and further analyzed their effectiveness and safety. After resolving significant clinical heterogeneity, we obtained several homogeneous perfusion protocols. For different protocols, the statistical heterogeneity was measured by using a Cochran's χ2−test and I2 statistic. If without statistical heterogeneity (p ≥ 0.1 and I2 ≤ 50%), a fixed-effects model (FEM) was performed to pool the data. If p < 0.1, I2 > 50%, and the results had good uniformity, a random-effects model (REM) was performed. Otherwise, the pool was abandoned, and a forest graph was adopted to describe the results. Following previous guidance (35) and our experiences (6, 9), a subgroup analysis model was developed to reveal the potential heterogeneity between different trials and determine the effects of variables on clinical responses. The variables were patient baselines, usages of SEC or chemical agents, an evaluation criterion, and published time. A univariable random effects meta-regression was performed to reveal the relationship between each variable and clinical response, and a post-hoc multiple regression analysis was performed to adjust their OR. Hong Jiang and Cheng-Qiong Wang independently pooled the data from each protocol using the Review Manager 5.4. If the included trials > 10, a funnel plot and Egger's test were used to reveal the risk of bias between trials using the STATA V.15.0 software (401506209499).
The methodological quality and over-estimation to clinical effectiveness and security were core factors affecting the robustness of results. So, the implementation process strictly followed the principle of underestimating effectiveness and safety. We defined the trial as a poor quality when at least one item was considered a high risk. The trial was defined as an over or underestimation when the result was significant difference, and beneficial to SEC perfusion. A sensitivity analysis model was developed to evaluate the robustness (6). Before and after rejecting all the trials with poor quality and over-estimation, if the result had good uniformity, the outcome was good robustness. Otherwise, the outcome was poor.
Through integrating the Grading of Recommendation Assessment, Development and Evaluation (GRADE) approach (36) and the results of publication bias and sensitivity analysis, a modified model was developed to summarize the evidence quality as a “high,” “moderate,” “low,” or “very low” (6, 9) (Appendix 1). The quality was downgraded in five domains as methodological quality, heterogeneity, indirectness, imprecision, and publication bias. Cheng-Qiong Wang and Xiao-Fan Chen summarized the evidence quality and further generated the absolute estimates of effect using the GRADE profiler.
After implementing retrieval strategies, we identified 1,729 records and no ongoing trials. After removing the duplicates, we included 833 records. After reading abstracts and removing irrelevant studies, we collected 250 full texts. After evaluating full texts and removing the ineligible, we collected 114 studies (19, 20, 37–148) and two meta-analyses (21, 22). After evaluating the meta-analyses, we collected 17 studies (19, 20, 48–50, 56, 59, 63, 69, 73, 77, 89, 109, 121, 133, 141, 144) from their references. Finally, we collected 114 studies, which were clustered into SEC alone with 35 trials (38, 40, 41, 46–48, 51, 52, 55, 56, 58, 60, 62, 64, 68, 73–75, 77, 79, 83, 86–88, 115, 118, 122, 125, 130, 134, 139, 140, 143, 146, 147) and SEC plus chemical agents with 99 trials (19, 20, 37–54, 56, 57, 59, 61–67, 69–73, 75–85, 87, 89–114, 116–121, 123, 124, 126–129, 131–133, 135–139, 141–145, 148). The retrieval results, screening process, and important exclusions are listed in Figure 1 and Appendix 2.
In all, we included 114 studies, which were clustered into intrapleural perfusion with SEC alone and SEC-plus chemical agents. About SEC perfusion alone, the 35 trials reported 10 pleurodesis agents, which formed nine comparisons between SEC and DDP (38, 40, 41, 46–48, 51, 52, 56, 62, 64, 68, 73–75, 77, 79, 83, 86, 87, 115, 118, 125, 130, 134, 139, 140, 143, 147), carboplatin (CBP), mitomycin-C (MMC), interleukin-2 (IL-2), mycobacteria, sapylin, recombinant modified human tumor necrosis factor (rmhTNF), elemene or lentinan (Table 1). Among them, 29 trials with 1,547 patients evaluated the comparisons of clinical effectiveness and safety between SEC and DDP. Patient ages were ranged from 20 to 86 years, and 606 and 344 cases were male and female, respectively. The experimental groups with 776 cases were administered with SEC through intrapleural perfusion, and the controls with 771 cases were administered with DDP alone. The SEC was used with 80 ng (8 ml, 2,000 IU) to 400 ng (40 ml, 10,000 IU) per time, one time or two times a week, and lasting one to eight times. The DDP was 40 to 100 mg per time. Only one to five trials reported other comparisons.
About SEC plus chemical agents, the 99 trials (19, 20, 37–54, 56, 57, 59, 61–67, 69–73, 75–85, 87, 89–114, 116–121, 123, 124, 126–129, 131–133, 135–139, 141–145, 148) reported the SEC and 10 agents, which developed 13 protocols as SEC plus DDP (19, 20, 37–53, 56, 57, 59, 61–66, 69–73, 75, 77–79, 81–85, 87, 89–93, 95, 98, 100–102, 104, 105, 107–110, 112, 114, 116–121, 123, 126–129, 133, 136, 137, 139, 143, 145, 148), CBP, nedaplatin (NDP), bleomycin (BLM), 5-fluorouracil (5-FU), etoposide (VP-16), mitoxantrone (MTZ), adriamycin (ADM), docetaxel, MMC, or other agents (Table 1). Seventy-nine trials involving 4,924 patients reported the SEC and DDP perfusion. Patient ages were ranged from 20 to 90 years, and 2,523 and 1,547 cases were male and female, respectively. The combination with SEC and DDP perfusion was administered in experimental groups with 2,539 patients, and the DDP alone was administered in controls with 2,385 patients. The SEC was used with 80 ng (8 ml, 2,000 IU) to 400 ng (40 ml, 10,000 IU) per time, one time or two times a week, and lasting one to eight times. The DDP was 30–100 mg per time. Only one to four trials reported other protocols.
On the whole, 82 studies involved patients with miscellaneous tumors as lung, breast, and ovarian cancers, among others, and 32 only involved lung cancer (40, 46–48, 51, 56, 62, 64, 73, 74, 77, 86, 140). Only some studies completely reported the patients' baselines as the volume of pleural fluid, KPS score, AST, and treatment history. Fifty studies performed perfusion after draining pleural fluid using IPCs. At 2–16 weeks after perfusion, most studies evaluated the clinical responses using Ostrowskimj criterion, and QOL using a KPS scale, and only one study reported the survivals. One hundred and seven studies (19, 20, 37, 39–47, 49–60, 62, 64–98, 100–133, 135–143, 145–148) reported the adverse event. But most trials only reported ADRs using an unclear criterion and ignored the TRAEs and the SEC-related adverse events.
Of 114 studies, only 11 reported the generating methods of random sequence using a number table (40, 43, 53, 58, 76, 106), coin toss (54, 67, 91), or draw (46, 135). Only three studies implemented allocation concealment using an envelope (75, 79, 86). No studies provided the detailed information about the blind methods. All the studies had complete follow-up. Seventy-seven studies had a selective reporting for ADRs (19, 20, 37, 38, 41–45, 47–49, 51, 52, 56, 57, 61, 63–66, 68–70, 72, 75, 76, 78–81, 84, 88, 89, 91, 93, 94, 97–100, 102, 104, 107, 108, 110, 112–118, 120, 122–131, 133–138, 141, 143–148). Thirty-five studies had an unclear comparability for baselines. The risk of methodological bias is shown in Figure 2.
In SEC perfusion alone, 35 trials reported nine comparisons (Table 2; Figure 3A). The Cochran's χ2-test and I2 statistic only found a minimal heterogeneity of CR (I2 = 4%) and failure (I2 = 42%) in SEC vs. DDP; we pooled the OR using a FEM. Compared with DDP or IL-2 alone, the results of meta-analyses determined that SEC alone showed a better CR [OR = 1.69, 95% CI (1.33, 2.15), p < 0.0001; OR = 1.73, 95% CI (1.03, 2.88), p = 0.04] and a lower failure [OR = 0.59, 95% CI (0.48, 0.73), p < 0.00001; OR = 0.32, 95% CI (0.19, 0.53), p < 0.00001] (Table 2; Figure 3A). In addition, only one trial reported that SEC alone was superior to CBP, and equivalent to MMC, mycobacteria, sapylin, rmhTNF, elemene, or lentinan alone (Table 2; Supplementary Figures S1, S2).
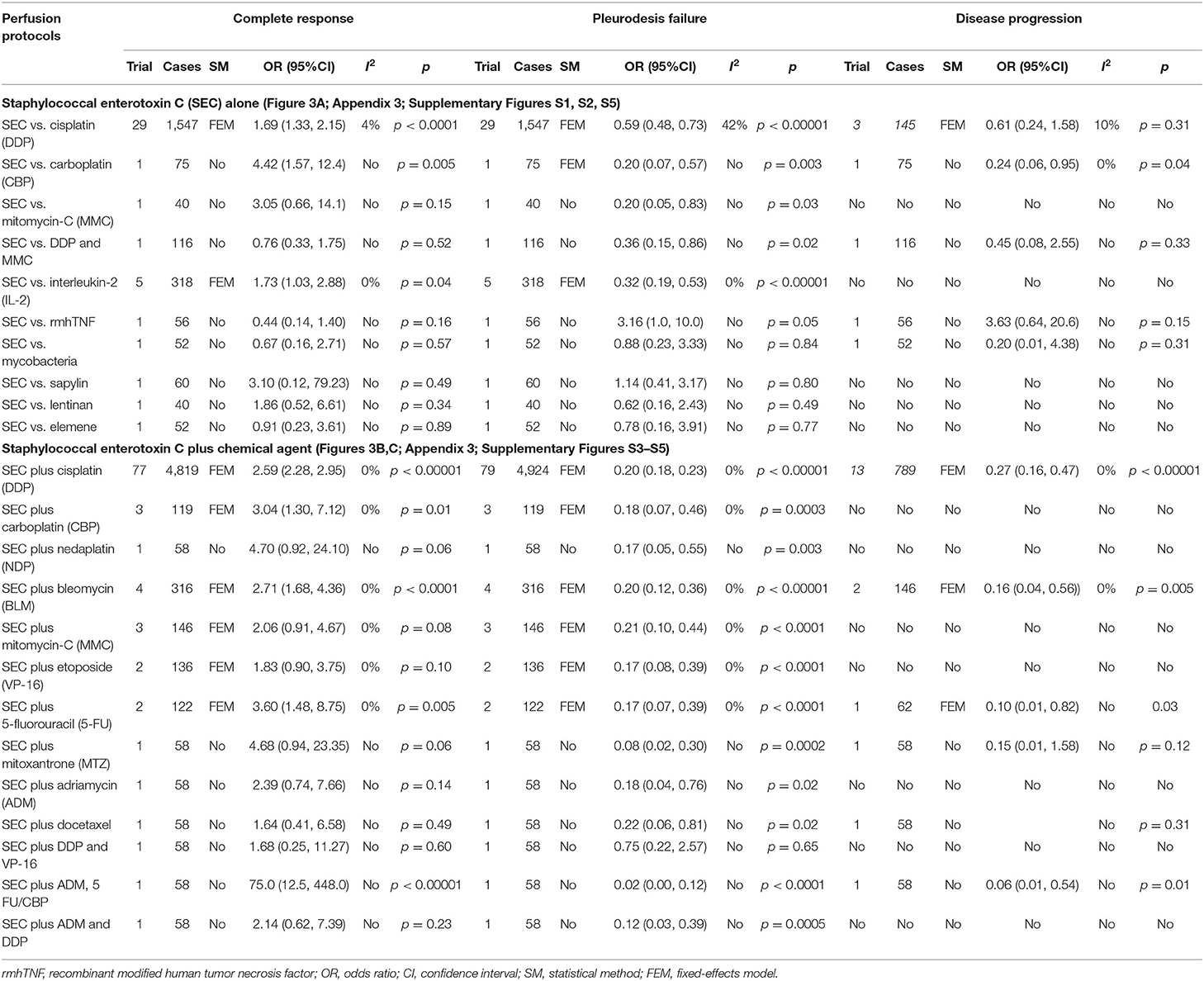
Table 2. The clinical responses (Figures 3A–C; Appendix 3; Supplementary Figures S1–S5).
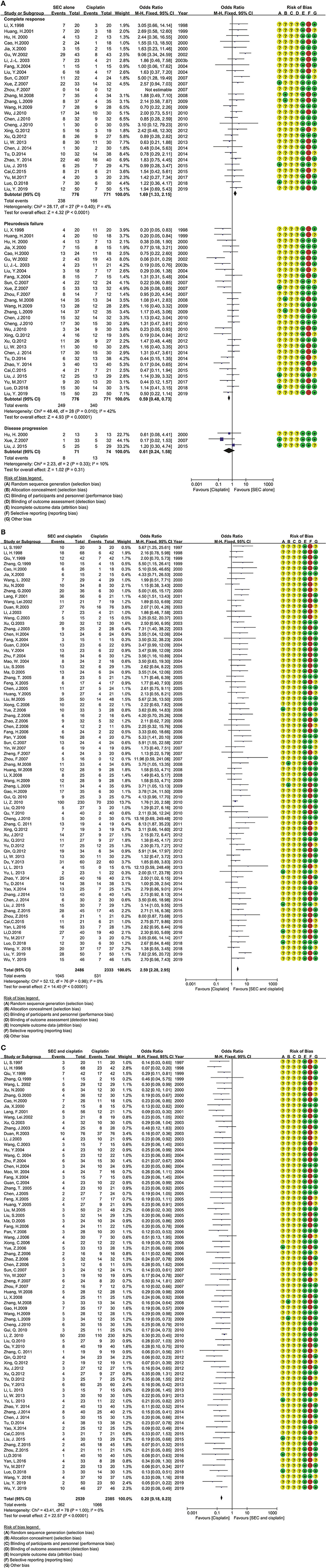
Figure 3. The analysis of clinical responses between the two groups. (A) The clinical responses between SEC and DDP alone. SEC, staphylococcal enterotoxin C; DDP, cisplatin; CI, confidence interval. (B) The complete response in SEC and cisplatin perfusion. SEC, staphylococcal enterotoxin C; DDP, cisplatin; CI, confidence interval. (C) The pleurodesis failure in SEC and cisplatin perfusion. SEC, staphylococcal enterotoxin C; DDP, cisplatin; CI, confidence interval.
In SEC-plus chemical agents, the 99 trials reported ten protocols as SEC plus DDP, CBP, NDP, BLM, MMC, 5-FU, VP-16, MTZ, ADM, or docetaxel (Table 2; Figures 3B,C; Supplementary Figure S5). The Cochran's χ2-test and I2 statistic found no heterogeneity; we pooled the OR using a FEM. Compared with chemical agents alone, the results determined that the SEC plus DDP, BLM or 5-FU significantly improved the CR [OR = 2.59, 95% CI (2.28, 2.95), p < 0.00001; OR = 2.71, 95% CI (1.68, 4.36), p < 0.0001; OR = 3.60, 95% CI (1.48, 8.75), p = 0.005], decreased the failure [OR = 0.20, 95% CI (0.18, 0.23), p < 0.00001; OR = 0.20, 95% CI (0.12, 0.36), p < 0.00001; OR = 0.17, 95% CI (0.08, 0.39), p < 0.0001], and disease progression [OR = 0.27, 95% CI (0.16, 0.47), p < 0.00001; OR = 0.16, 95% CI (0.04, 0.56), p = 0.005; OR = 0.10, 95% CI (0.01, 0.82), p = 0.03]. The SEC plus CBP only improved the CR [OR = 3.04, 95% CI (1.30, 7.12), p = 0.01] and decreased the failure [OR = 0.18, 95% CI (0.07, 0.46), p = 0.0003]. No statistical difference was found between other comparisons.
Only one trial reported the OS rate (Figure 4). Compared with DDP alone, the statistical analysis showed that the SEC and DDP perfusion significantly improved the 0.5-year OS rate [OR = 8.00, 95% CI (1.59–40.33), p = 0.01] and 1 year OS rate [OR = 5.33, 95% CI (1.71–16.62), p = 0.004].
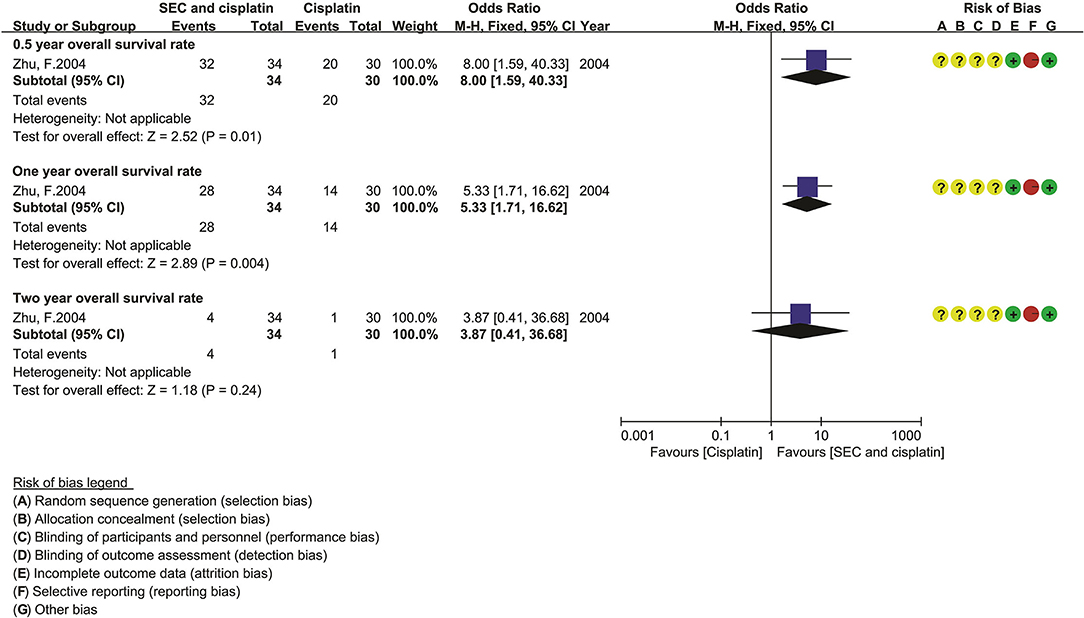
Figure 4. The overall survival of SEC and cisplatin. SEC, staphylococcal enterotoxin C; CI, confidence interval.
Eight trials containing 443 patients reported the QOL in SEC alone, and 31 containing 2,067 patients reported the QOL in SEC and DDP perfusion, and limited trials reported other nine protocols. The Cochran's χ2-test and I2 statistic only found a minimal heterogeneity in SEC vs. DDP (I2 = 38%). The OR was pooled by using a FEM. Compared with DDP alone, the meta-analysis result determined that the SEC alone or/and DDP perfusion significantly improved the QOL [OR = 9.93 95% CI (6.24–15.80), p < 0.00001, and OR = 4.51, 95% CI (3.70–5.50), p < 0.00001] (Figure 5).
Twenty-six trials reported the adverse events in SEC alone (40, 41, 46, 47, 51, 52, 56, 62, 64, 68, 73–75, 77, 79, 83, 86, 87, 115, 118, 125, 130, 139, 140, 143, 147), and 75 reported the adverse events in SEC and DDP perfusion (19, 20, 37, 39–47, 49–53, 56, 57, 59, 62, 64–66, 69–73, 75, 77–79, 81–85, 87, 89–93, 95, 98, 100–102, 104, 105, 107–110, 112, 114, 116–121, 123, 126–129, 133, 136, 137, 139, 143, 145, 148). Limited trials reported others. In SEC alone, the Cochran's χ2-test and I2 statistic only found a statistical heterogeneity in gastrointestinal reaction (I2 = 52%) and minimal heterogeneity in myelosuppression (I2 = 19%), leukopenia (I2 = 8%), and fever (I2 = 29%) (Table 3; Appendix 4; Supplementary Figures S6–S12); we pooled the data of gastrointestinal reaction using a REM, and other data using a FEM. Compared with DDP alone, the results determined that the SEC alone showed lower myelosuppression [OR = 0.19, 95% CI (0.07–0.53), p = 0.002], leukopenia [OR = 0.11, 95% CI (0.05–0.23), p < 0.00001], gastrointestinal reaction [OR = 0.12, 95% CI (0.06–0.26), p < 0.00001], hepatic dysfunction [OR = 0.22, 95% CI (0.05–0.94), p = 0.04], renal dysfunction [OR = 0.13, 95% CI (0.04–0.46), p = 0.002], and a higher fever [OR = 6.66, 95% CI (4.30–10.32), p < 0.00001]. However, the results revealed no statistical differences in cardiac dysfunction and thoracodynia. Additionally, most trials ignored the thoracentesis or SEC-related adverse events.
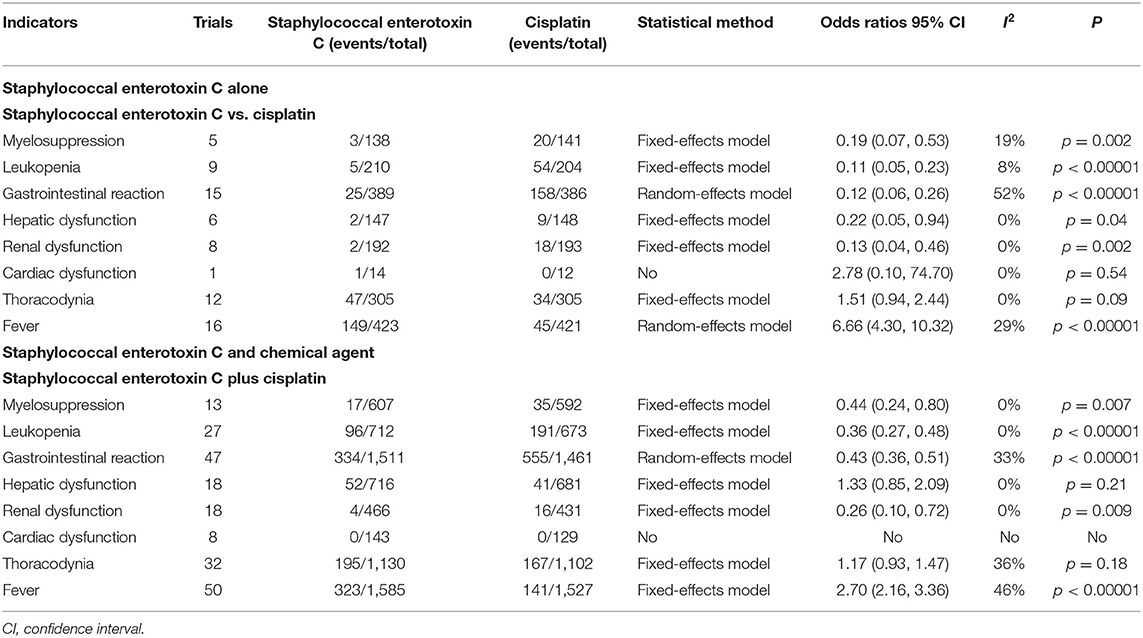
Table 3. Meta-analysis results of adverse events (Appendix 4; Supplementary Figures S6–S12).
In SEC and DDP perfusion, the Cochran's χ2-test and I2-statistic only found a minimal heterogeneity in gastrointestinal reaction (I2 = 33%), thoracodynia (I2 = 36%), and fever (I2 = 46%) (Table 3; Appendix 4; Supplementary Figures S6–S12); we pooled all the data using a FEM. Compared with DDP alone, the results determined that the perfusion protocol showed a low incidence rate of myelosuppression [OR = 0.44, 95% CI (0.24–0.80), p = 0.007], leukopenia [OR = 0.36, 95% CI (0.27–0.48), p < 0.00001], gastrointestinal reaction [OR = 0.43, 95% CI (0.36–0.51), p < 0.00001], renal dysfunction [OR = 0.26, 95% CI (0.10–0.72), p = 0.009], and a high fever [OR = 2.70, 95% CI (2.16–3.36), p < 0.00001], and no difference in thoracodynia and hepatic dysfunction. Additionally, six trials reported no cardiotoxicity, and most ignored the thoracentesis or SEC-related adverse events.
Only the SEC and DDP perfusion protocol included enough trials. So, a subgroup analysis was performed to reveal their potential clinical heterogeneity and determine the effects of variables on clinical responses. The tumors included miscellaneous tumors and lung cancer. The subgroup analysis revealed that the SEC and DDP perfusion significantly improved the CR with a low failure in patients with both conditions (Table 4a; Supplementary Figures S14, S16). The pleural fluid was small to large volume, moderate to large or large; the KPS scores were ≥40, ≥50, or ≥60; the AST was ≥2 or 3 months; and the treatment history was primary treatment or unclear. The perfusion could significantly improve the clinical responses in MPE with moderate to large (Table 4b; Supplementary Figures S18, S20), KPS score ≥40, ≥50, or ≥60 (Table 4c; Supplementary Figures S22, S24), AST ≥ 2 or 3 months (Table 4e; Supplementary Figures S30, S32), and primary treatment (Table 4d; Supplementary Figures S26, S28).
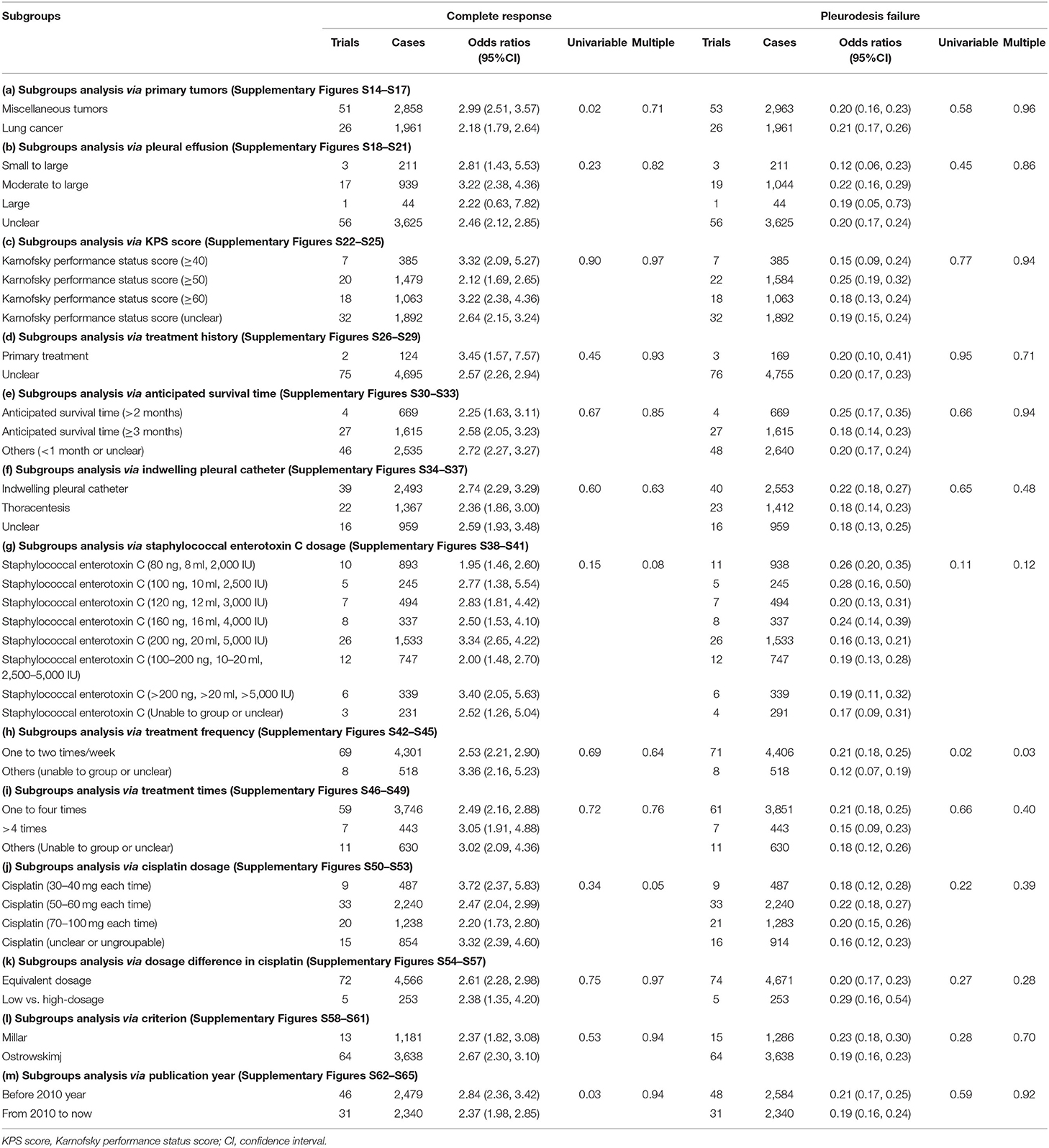
Table 4. Subgroups and meta-regression analysis (Appendix 5; Supplementary Figures S14–S65).
The SEC was mainly used with 100 ng (10 ml, 2,500 IU) to 200 ng (20 ml, 5,000 IU) per time, one time or two times a week, and lasting one to four times. The dosages of DDP were categorized into 30–100 mg per time. In combinations with DDP (30–40 mg, 50–60 mg, and 70–100 mg per time), mainly 50–60 mg per time, SEC could significantly improve the clinical responses (Tables 4g–j; Supplementary Figures S38, S40, S42, S44, S46, S48, S50, S52). Moreover, there were dosage differences between two groups. Like high dosage DDP, the SEC with low-dosage also significantly improved a similar response (Table 4k; Supplementary Figures S54, S57). The drainage was IPC or thoracentesis; the criterion was Ostrowskimj or Millar, and the publication year was before or after 2010 year. The perfusion achieved above effects under these conditions (Tables 4f,l,m; Supplementary Figures S34, S36, S58, S60, S62, S64). But the univariable meta-regression only revealed a correlation between tumor type and CR (p = 0.02), and between treatment frequency and pleurodesis failure (p = 0.02). The multiple meta-regression analysis further determined that the treatment frequency was associated with pleurodesis failure (Table 4).
In perfusion with SEC alone, more than ten trials were included for CR, pleurodesis failure, gastrointestinal reactions, thoracodynia, and fever. The funnel plot and Egger's test showed a publication bias in failure (P > |t| = 0.00001, Coef = −4.31, 95% CI −6.52 to −2.11), gastrointestinal reactions (P > |t| = 0.009, Coef = −2.6495%, CI −4.50 to −0.77), and the trials underestimated them. No publication bias was found in other outcomes, which were objectively reported (Table 5; Supplementary Figures S67, S68). In perfusion with SEC and DDP, more than 10 trials were included for CR, pleurodesis failure, disease progression, quality of life, myelosuppression, gastrointestinal reactions, leukopenia, thoracodynia, and fever. A publication bias was found in CR (P > |t| = 0.00001, Coef = 0.99, 95% CI, 0.50–1.49), failure (P > |t| = 0.004, Coef = −0.8, 95% CI, −1.33 to −0.26), gastrointestinal reactions (P > |t| = 0.03, Coef = −1.03, 95% CI, −1.95 to −0.11), and fever (P > |t| = 0.00001, Coef = 1.593, 95% CI, 0.77–2.40); the trials underestimated the failure and gastrointestinal reactions, and overestimated the CR and fever. No publication bias was found in others, which were objectively reported (Table 5; Supplementary Figures S71, S72, S77, S79).
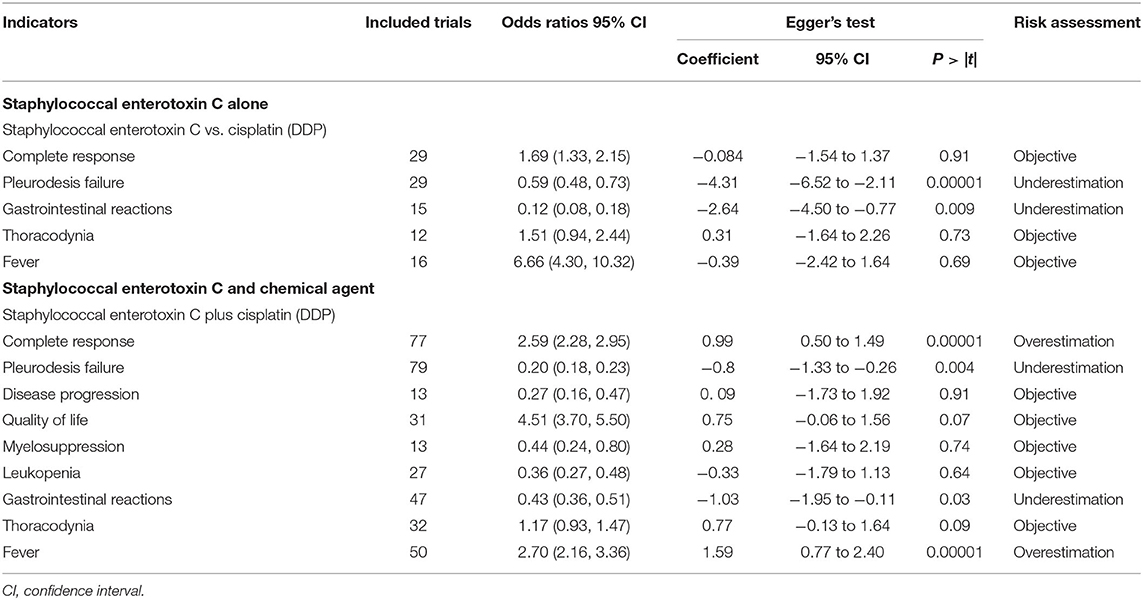
Table 5. Publication bias risk (Appendix 6; Supplementary Figures S66–S79).
In perfusion with SEC alone, all indicators involved poor and over- or under-estimated trials. In SEC vs. DDP/IL-2, the OR of CR, failure, QOL, and neutropenia had poor robustness before and after removing the poor and over- or underestimation, and others had good robustness (Table 6). In SEC and chemical agent perfusion, all indicators involved poor and over- or underestimated trials. In SEC and DDP perfusion, the OR of disease progression, myelosuppression, and nephrotoxicity was poor robustness before and after removing the poor and underestimation. In SEC with BLM, 5-FU or MMC, the OR of CR, failure, and disease progression were poor robustness before and after removing the poor and over- or underestimation, and others had good robustness (Table 6).
In methodology, 21 poor trials were involved in SEC perfusion alone (38, 41, 47, 48, 51, 52, 56, 64, 68, 75, 79, 88, 115, 118, 122, 125, 130, 134, 143, 146, 147). In SEC vs. DDP/IL-2, the OR of CR, failure, QOL, and neutropenia had poor robustness. Therefore, we downgraded the quality with two grades. While others had robustness, we downgraded the quality one grade. The statistical heterogeneity was found for CR, failure, QOL, and neutropenia in SEC vs. DDP, and, for CR and failure in SEC vs. IL-2, the sensitivity analysis showed poor robustness. The sample size for disease progression, QOL and hepatotoxicity was lower than 300 subjects. A publication bias was found in failure and gastrointestinal reactions, and the failure had poor robustness. So, we downgraded their quality one grade. Finally, we summarized a “moderate” quality for gastrointestinal reactions, nephrotoxicity, thoracodynia, and fever in SEC vs. DDP, and a “low” to “very low” for others (Table 7).
Sixty-eight poor trials were involved in SEC and chemical agent perfusion (19, 20, 37, 38, 41–45, 47–49, 51, 52, 56, 57, 61, 63–66, 69, 70, 72, 75, 76, 78–81, 84, 89, 91, 93, 94, 97–100, 102, 104, 107, 108, 110, 112–114, 116–118, 120, 123, 124, 126–129, 131, 133, 135–138, 141, 143–145, 148). In SEC and DDP perfusion, the poor robustness was found for the OR of disease progression, myelosuppression, and nephrotoxicity. In SEC and BLM, MMC or 5-FU perfusion, the poor robustness was found in CR, failure, and disease progression. And we downgraded their quality with two grades. While others had robustness, we downgraded the quality with one grade. For SEC and DDP perfusion, the statistical heterogeneity was found in gastrointestinal reaction, fever, and thoracodynia, which had robustness. A publication bias was found in CR, failure, gastrointestinal reactions, and fever, which had robustness, and we did not downgrade the quality. For SEC and DDP perfusion, the samples were lower than 300 subjects in thrombocytopenia. For SEC plus CBP, BLM, 5-FU, MMC or VP-16, the samples were lower than 300 subjects in CR and failure. So, we downgraded the quality one grade. Finally, we summarized a “moderate” for CR, failure, QOL, neutropenia, gastrointestinal reactions, hepatotoxicity, thoracodynia, and fever in SEC and DDP perfusion, and a “low” to “very low” for others (Table 7).
In China, the staphylococcal enterotoxin C (SEC), a super-antigen, has been used to control the MPE in the 1990s. To clarify the intrapleural perfusion protocols with SEC, determine their clinical effectiveness and safety, and reveal their indications and optimum usage, we integrated the previous meta-analyses (21, 22), supplemented 97 studies (37–47, 51–55, 57, 58, 60–62, 64–68, 70–72, 74–76, 78–88, 90–108, 110–120, 122–132, 134–140, 142, 143, 145–148), and implemented a clustered SR/meta-analysis. This new analysis found that the perfusion protocols were mainly SEC alone or plus chemical agents, which showed significant clinical heterogeneity. So, we implemented topic clustering to obtain serial homogeneous protocols, and analyzed the data from each protocol using the meta-analysis or descriptive analysis. In SEC perfusion alone, 10 pleurodesis agents formed nine comparisons. The results of meta-analysis determined that the SEC perfusion alone could show a better CR and QOL, a lower pleurodesis failure, hematotoxicity, gastrointestinal reactions and hepatorenal toxicity, and a higher fever than DDP alone. And it also showed better responses than IL-2 alone. But most results had “low to very low” quality. In addition, limited trials showed that it might obtain similar responses to bio-products as mycobacteria (88), sapylin (52) or rmhTNF (46), and TCMIs as elemene (60) or lentinan (58). Many studies (7, 10, 15) had reported that treatment with staphylococcal super-antigenic products could result in massive cytokine production (IL-2, TNF α, and IFN γ), which plays a crucial role in the initiation and maintenance of pleural inflammation and pleural space obliteration. In addition, the bio-products from hemolytic streptococcialpha (11, 12), corynobactum parvum (13), and streptococcus pyogenes (14) have been used in clinical studies to achieve pleurodesis and control fluid recurrence. These results indicate that the super-antigen SEC is a pleurodesis agent, which induces pleural inflammation and achieves pleurodesis (Figure 6). This analysis further revealed that the SEC and 10 agents developed 30 perfusion protocols. The results determined that only the SEC and DDP perfusion could significantly improve the CR and QOL with a low failure, disease progression, hematotoxicity, gastrointestinal reactions, and hepatorenal toxicity, but with a high fever. Enough trials were included, and most results had “moderate” quality. Other protocols only included one to four trials, and the results had a “low to very low” quality. The related SR/meta-analyses reported that the biologic response modifiers, as Rh-Endostatin, lentinan or IL-2 with DDP perfusion (6, 9, 150) also showed a clinical benefit rate in MPE. These results indicate that among 13 protocols, the SEC and DDP perfusion is a commonly used protocol, which shows a significant improvement in clinical responses with low ADRs (Figure 6).
Among 13 protocols, only the SEC and DDP perfusion included enough trials. The potential clinical heterogeneity still exists in baseline characteristics, interventions, and evaluation criteria between different trials. Different from previous studies (21, 22), we implemented a subgroup analysis to deal with the potential heterogeneity. Further subgroup analysis revealed that the SEC and DDP perfusion could improve clinical responses in both patients with lung cancer and miscellaneous tumors. It also improved clinical responses in patients with moderate to large volume, KPS scores ≥40, ≥50, or ≥60, AST ≥2 or 3 months or primary treatment. However, only two to seven trials were included for treatment conditions such as KPS score (≥40), AST (≥2) or primary treatment. The univariable meta-regression revealed only a positive correlation between the tumor type and CR. So, we adjusted the treatment conditions as moderate to large volume, KPS scores ≥50 or ≥60, or AST ≥3 months, and no restriction on the tumor type. The relevant SR/meta-analyses (6, 9) reported that the Rh-endostatin or lentinan and DDP infusion could also improve the clinical responses under these conditions. So, we believe that bio-products perfusion may have similar treatment conditions, and a moderate to large fluid, KPS scores ≥50 or ≥60, and AST ≥3 months is a possible indication for SEC and DDP perfusion. The rational drug use is another key to affect clinical effectiveness and safety. Previous SR/meta-analyses (6, 9) reported that, in combination with Rh-endostatin/lentinan, the DDP perfusion was mainly used with 30–60 mg per time. This analysis found that the SEC was used with 80 ng (8 ml, 2,000 IU) to 400 ng (40 ml, 10,000 IU) per time, one time or two times a week and lasting one to four times, and the DDP was used with 30–100 mg per time. Fifty-eight trials reported the dosage of SEC as mainly 100 ng (10 ml, 2,500 IU) to 200 ng (20 ml, 5,000 IU), and 42 trials reported the DDP as 30–60 mg per time. The subgroup analysis revealed that, under these conditions, the SEC and DDP perfusion could improve the clinical responses, and the SEC with low-dosage obtained similar responses to high dosage. The results indicate that the SEC combined with DDP can reduce the dosage of DDP. Finally, the subgroup analysis found that drainage methods, evaluation criteria, or the publication year showed no impact on clinical responses. However, the univariable meta-regression and multivariate regression analysis only revealed a positive correlation between the pleurodesis failure and treatment frequency. Based on the principle of cost to effectiveness, we believe that the SEC (100–200 ng per time, one or two times a week and lasting one to four times) and DDP (30–40 mg or 50–60 mg each time) are possible optimal usage for achieving an ideal response (Figure 6).
In this study, we developed a clustered SR/meta-analysis, and some potential shortcomings were inevitable. During the implementation, we followed the strategy of underestimating effectiveness and security. We tested the robustness of the results in an extreme condition, developed a modified model to summarize the evidence quality, and actively reduced the quality of all the results. We only retrieved the Chinese and English databases, which existed potential retrieval bias. In 114 studies, most had unclear or high risk of methodological bias. Only some studies completely reported the baseline information, such as fluid volume, treatment history, functional status, and expected survival. Most selectively reported the ADRs and ignored the TRAEs, treatment-related death, overall mortality, and hospital stay. Two criteria were used to evaluate the clinical effectiveness and safety. In subgroup analysis, the univariate or multivariate regression analysis only found a sporadic correlation between clinical responses and tumor type or treatment frequency. These potential shortcomings might lead to an unfair evaluation for SEC in controlling MPE. In SEC perfusion alone, only one to five trials were included for other eight comparisons; most results had “low to very low” quality, and the network meta-analysis could not be performed. Therefore, the current evidence could not determine which does better between SEC and other bio-products or TCMIs. In SEC and chemical agents, only one trial supported that the SEC and DDP perfusion might improve the overall survival. Two to four trials for SEC plus CBP, BLM, 5-FU, MMC or VP-16, and the outcomes had “low to very low” quality. So, the current evidence could not demonstrate their clinical effectiveness, safety levels, indications, and optimal usage.
This clustered SR/meta-analysis found that the perfusion protocols were mainly SEC alone or plus chemical agents, which showed obvious complexity and diversity. The super-antigen SEC is a pleurodesis agent, which provides an attractive alternative to existing palliative modalities for patients with MPE. But the relationship between the SEC and others and which pleurodesis agent does better need to be further confirmed by new trials or network meta-analysis. Among 13 SEC plus chemical agent protocols, only the SEC and DDP perfusion could significantly improve the clinical responses with low ADRs. These findings provide a main perfusion protocol for controlling MPE, which have clinical significance for improving decision-making, preventing recurrence, and improving clinical response and a prognosis. But only one trial reported that the SEC and DDP perfusion could improve overall survival. Most studies selectively reported the ADRs, and ignored the TRAEs, which might lead to an unfair evaluation for its long-term survival and security. Compared with previous meta-analyses [21, 22], this analysis successfully implemented topic clustering to solve the complex problems, analyzed the data from each protocol using the meta-analysis or descriptive analysis, and provided serial systematic and complete pieces of evidence for treatment strategy using the TPs alone or plus chemical agents to control MPE, which will also provide theoretical and technical references for evaluating similar biological products. In addition, the included trials reported that the dosage of SEC was 80–400 ng per time, and the DDP was 30–100 mg per time, which might be main reasons for irrational drug use and clinical decision-making failure. The subgroup analyses further found that, under the conditions, as moderate to large volume, KPS scores ≥50 or ≥60, or AST ≥3 months, the SEC (100–200 ng per time, one time or two times a week and lasting one to four times) and DDP (30–40 mg or 50–60 mg each time) are possible optimal usage for achieving an ideal response. All these provide a possible indication and optimal usage for SEC and DDP perfusion. Compared with traditional analysis (21, 22), this analysis performed a subgroup analysis to analyze the potential heterogeneity and found serial indirect results, which further provide an indication and optimal usage for an optimal control strategy, which is of clinical significance to formulate the optimal perfusion protocol, reject the unreasonable, and control medical expenses. But these conclusions came from indirect evidence. So, these conclusions need be further confirmed by using direct evidence.
Current pieces of evidence indicate that the super-antigen SEC is a pleurodesis agent, which provides an attractive alternative to existing palliative modalities for patients with MPE. Among 13 perfusion protocols, the SEC and DDP perfusion is a most commonly used, which shows a significant improvement in clinical responses and QOL with low chemical drugs-related adverse events. For this protocol, the possible indications are moderate to large volume, KPS score (≥50), and AST (≥3 months). The SEC (100–200 ng per time, one time a week for one to four times) with DDP (30–40 mg, or 50–60 mg each time) is optimum usage for achieving an ideal response. Finally, we hope that this analysis provides a valuable evidence framework for an optimal control strategy of using SEC in MPE.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Conception and design by ZX, XX, and LZ. Development of methodology by ZX, X-FC, and C-QW. Literature search and statistical analysis by HJ and C-QW. Article selection and assessment of methodological bias risk by HJ and X-MY. Data extraction by JX and JH. GRADE assessment by C-QW and X-FC. Preparing the manuscript draft by HJ, X-MY, and ZX. Review and revision of the manuscript by KC, J-HF, and LZ. Study supervision by ZX and XX. All authors contributed to the article and approved the submitted version.
This work was funded by a High-Level Innovative Talent Program in Guizhou (No. fzc 120171001), and special funds for Science and Technology Research Into Traditional Chinese and National Medicine in Guizhou (No. QZYY 2017-084).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.816973/full#supplementary-material
5-FU, 5-fluorouracil; ADM, adriamycin; ADRs, adverse drug reactions; AST, anticipated survival time; BLM, bleomycin; CBM, China biological medicine database; CBP, carboplatin; CENTRAL, Cochrane central register of controlled trials; CI, confidence intervals; CNKI, China National Knowledge Infrastructure Database; Coef, coefficient; CR, complete response; CTCAE, common terminology criteria for adverse events; DDP, cisplatin; DP, disease progression; FEM, fixed-effects model; GRADE approach, grades of recommendation assessment, development, and evaluation approach; HR, hazard ratio; IL-2, interleukin 2; IFN γ, interferon gamma; IPCs, indwelling pleural catheters; IU, international unit; KPS, Karnofsky performance status; LBP, lobaplatin; MMC, mitomycin-C; MPE, malignant pleural effusion; MTZ, mitoxantrone; MU, million units; NDP, nedaplatin; NMPA, National Medical Products Administration; NR, no response; NSCLC, non-small cell lung cancer; OR, odds ratios; OS, overall survival; PFS, progression-free survival; PR, partial response; PRISMA guidelines, preferred reporting items for systematic reviews and meta-analyses guidelines; PT, primary treatment; QOL, quality of life; RCTs, randomized controlled trials; rmhTNF, recombinant modified human tumor necrosis factor; RT, retreatment; REM, random-effects model; SM, statistical method; SEC, staphylococcal enterotoxin C; SD, stable disease; SR, systematic review; TCMIs, traditional Chinese medicine injections; TNF α, tumor necrosis factor α; TH, treatment history; TRAEs, thoracentesis-related adverse events; VIP, Chinese Scientific Journals Full-Text Database; VP-16, etoposide; WHO, World Health Organization.
1. Psallidas I, Kalomenidis I, Porcel JM, Robinson BW, Stathopoulos TG. Malignant pleural effusion: from bench to bedside. Eur Respir Rev. (2016) 25:189–98. doi: 10.1183/16000617.0019-2016
2. Thomas R, Roy B, Maldonado F, Lee GYC. Management of malignant pleural effusions-what is new. Semin Respir Crit Care Med. (2019) 40:323–39. doi: 10.1055/s-0039-1698285
3. Dipper A, Jones HE, Bhatnagar R, Preston NJ, Maskell N, Clive OA. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev. (2020) 4:Cd010529. doi: 10.1002/14651858.CD010529.pub3
4. Feller-Kopman DJ, Reddy CB, DeCamp MM, Diekemper RL, Gould MK, Henry T, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. (2018) 198:839–49. doi: 10.1164/rccm.201807-1415ST
5. Shafiq M, Feller-Kopman D. Management of malignant pleural effusions. Clin Chest Med. (2020) 41:259–67. doi: 10.1016/j.ccm.2020.02.009
6. Wang CQ, Huang XR, He M, Zheng XT, Jiang H, Chen Q, et al. Intrapleural administration with Rh-endostatin and chemical irritants in the control of malignant pleural effusion: a systematic review and meta-analysis. Front Oncol. (2021) 11:649999. doi: 10.3389/fonc.2021.649999
7. Shivaee A, Sedighi M, Imani Fooladi AA. Staphylococcal enterotoxins as good candidates for cancer immunotherapy: a systematic review. Ann Ig. (2020) 32:648–63. doi: 10.7416/ai.2019.2386
8. Xiang G, Haiying L, Jiangye W, Xueming G, Wenxiao C. Evaluation of efficacy and safety for Brucea javanica oil emulsion in the control of the malignant pleural effusions via thoracic perfusion. BMC Cancer. (2018) 18:411. doi: 10.1186/s12885-018-4328-3
9. Xiao Z, Jiang Y, Chen XF, Wang CQ, Zheng XT, Xu WH, et al. Intrathoracic infusion therapy with Lentinan and chemical irritants for malignant pleural effusion: a systematic review and meta-analysis of 65 randomized controlled trials. Phytomedicine. (2020) 76:153260. doi: 10.1016/j.phymed.2020.153260
10. Ren S, Terman DS, Bohach G, Silvers A, Hansen C, Colt H, et al. Intrapleural staphylococcal superantigen induces resolution of malignant pleural effusions and a survival benefit in non-small cell lung cancer. Chest. (2004) 126:1529–39. doi: 10.1378/chest.126.5.1529
11. Zhao Y, Liang C, Xing D. The short-term efficacy of Mannan peptide combined with chemotherapeutic drug thoracic perfusion in the treatment of malignant pleural effusion. Guide China Med. (2014) 12:232–3. doi: 10.15912/j.cnki.gocm.2014.34.178
12. Wu M, Chen X, Ming B, Liu S. Efficacy and safety of Mannatide combined with cisplatin in the treatment of malignant pleural effusion. Chin J Med Guide. (2014) 16:1488–90. doi: 10.3969/j.issn.1009-0959.2014.12.023
13. Millar JW, Hunter AM, Horne WN. Intrapleural immunotherapy with Corynebacterium parvum in recurrent malignant pleural effusions. Thorax. (1980) 35:856–8. doi: 10.1136/thx.35.11.856
14. Kishi K, Homma S, Sakamoto S, Kawabata M, Tsuboi E, Nakata K, et al. Efficacious pleurodesis with OK-432 and doxorubicin against malignant pleural effusions. Eur Respir J. (2004) 24:263–6. doi: 10.1183/09031936.04.00137403
15. Psallidas I, Stathopoulos TG. Staphylococcus aureus bio-products: new biological roles for a pleurodesis agent. Respirology. (2014) 19:948–9. doi: 10.1111/resp.12354
16. Yang SB, Li TL, Chen X, An YF, Zhao CQ, Wen JB, et al. Staphylococcal enterotoxin B-derived haptens promote sensitization. Cell Mol Immunol. (2013) 10:78–83. doi: 10.1038/cmi.2012.32
17. Zhou P, Liang P, Dong B, Yu X, Han X, Wang Y, et al. Long-term results of a phase II clinical trial of superantigen therapy with staphylococcal enterotoxin C after microwave ablation in hepatocellular carcinoma. Int J Hyperthermia. (2011) 27:132–9. doi: 10.3109/02656736.2010.506670
18. Zhang J, He G, Chen J, Qiu S. Observation on the short-term efficacy of highly agglutinative staphylococcin combined with chemotherapy in the treatment of advanced primary liver cancer. Chin J Clin Oncol. (2000) 27:861–2. doi: 10.3969/j.issn.1000-8179.2000.11.027
19. Qiu Y, Han G, Ma S. Observation of the curative effect of high-agglomerated staphylococcus and cisplatin on malignant pleural fluid. Sichuan J Cancer Contr. (1999) 12:28–30.
20. Li S, Wu Y, Wang X. Observation on the clinical efficacy of combined intrapleural injection of high-aggregating Staphylococcus aureus and cisplatin in the treatment of malignant pleural effusion (analysis of 40 cases). J Pract Oncol. (1997) 12:171–2.
21. Liu J, Zhao H, Liu X. The efficacy of highly agglutinative staphylococcin combined with chemotherapeutic drugs in the treatment of malignant pleural and ascites: a meta analysis. Shenyang Army Med. (2001) 14:556–7.
22. Liu Y, Zhang X, Huang L. Meta -analysis of the treatment efficacy of staphylococcin plus cisplatin versus cisplatin monotherapy in malignant pleural effusion and malignant intraperitoneal effusion. J Mod Oncol. (2016) 24:3479–84. doi: 10.3969/j.issn.1672-4992.2016.21.037
23. Chen Y, Li Y, Du L, Wang L, Wen J, Yang X. Evolution of levels of evidence and strength of recommendations in medical research. Chin J Evid Based Med. (2008) 8:127–33. doi: 10.3969/j.issn.1672-2531.2008.02.012
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
25. Emad A, Rezaian RG. Treatment of malignant pleural effusions with a combination of bleomycin and tetracycline. A comparison of bleomycin or tetracycline alone versus a combination of bleomycin and tetracycline. Cancer. (1996) 78:2498–501. doi: 10.1002/(SICI)1097-0142(19961215)78:12<2498::AID-CNCR8>3.0.CO;2-G
26. Zaloznik AJ, Oswald SG, Langin M. Intrapleural tetracycline in malignant pleural effusions. A randomized study Cancer. (1983) 51:752–5. doi: 10.1002/1097-0142(19830215)51:4<752::AID-CNCR2820510434>3.0.CO;2-7
27. Keeratichananont W, Kaewdech A, Keeratichananont S. Efficacy and safety profile of autologous blood versus talc pleurodesis for malignant pleural effusion: a randomized controlled trial. Ther Adv Respir Dis. (2018) 12:1753466618816625. doi: 10.1177/1753466618816625
28. Yates JW, Chalmer B, McKegney PF. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. (1980) 45:2220–4. doi: 10.1002/1097-0142(19800415)45:8<2220::AID-CNCR2820450835>3.0.CO;2-Q
30. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. (1981) 47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::AID-CNCR2820470134>3.0.CO;2-6
31. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v30: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. (2003) 13:176–81. doi: 10.1016/S1053-4296(03)00031-6
32. Guyot P, Ades AE, Ouwens MJ, Welton JN. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2012) 12:9. doi: 10.1186/1471-2288-12-9
33. Higgins JPT, Thomas J, Chandler J, Cumpston MLT, Page MJ, Welch WV, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane (2021). Available online at: http://www.training.cochrane.org/handbook (accessed February 2021).
34. Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng TX. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. (2020) 7:7. doi: 10.1186/s40779-020-00238-8
35. Sun X, Briel M, Walter SD, Guyatt HG. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ. (2010) 340:c117. doi: 10.1136/bmj.c117
36. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
37. Wu Y. Efficacy of intrapleural injection of different drugs in the treatment of pleural effusion of lung cancer. Contemp Med. (2019) 25:16–8. doi: 10.3969/j.issn.1009-4393.2019.26.007
38. Liu Y. Comparative observation on the efficacy of intrapleural injection of different drugs in treating pleural effusion. J Aero Med. (2019) 30:148–50. doi: 10.3969/j.issn.2095-1434.2019.02.009
39. Wang Y. The clinical effect of Gaojusheng combined with cisplatin in the treatment of malignant pleural effusion. Guide China Med. (2018) 16:126–7. doi: 10.15912/j.cnki.gocm.2018.02.109
40. Luo D, Guo L, Wang Y, Wu Z, Chu J, Zhao B. Chest cavity injection of different drugs in the treatment of lung adenocarcinoma pleural effusion. Pract J Cancer. (2018) 33:1890–2. doi: 10.3969/j.issn.1001-5930.2018.11.044
41. Yu M, Sheng Y. Comparison of the curative effect of intrapleural injection of different drugs in the treatment of lung cancer pleural effusion. J Med Theory Pract. (2017) 30:1304–5. doi: 10.19381/j.issn.1001-7585.2017.09.026
42. Yan L. Comparative analysis of intrathoracic injections of different drugs to treat lung cancer pleural effusion. Yiyao Qianyan. (2016) 6:21–2.
43. Li D. Clinical observation on the treatment of pleural effusion caused by lung cancer with high aggregate staphylococcus and cisplatin. China Health Care Nutr. (2016) 26:209–10.
44. Zhou Z. Observation of the clinical effect of Staphylococcus aureus combined with cisplatin in the treatment of lung cancer pleural effusion. China Pract Med. (2015) 10:155–6. doi: 10.14163/j.cnki.11-5547/r.2015.26.110
45. Zhang Z, Meng Y, Meng C, Ma M, Hu R. Treatment of 45 cases of pleural effusion caused by lung cancer with high aggregate staphylococcus and cisplatin. China Pharm. (2015) 24:69–70.
46. Liu J, Song W, You T, Huang J. Clinical observation of highly agglutinative staphlococcin, tumor necorsis factor and Cisplatin in the treatment of malignant pleural effusion caused by lung adenocarcinoma. China Med Herald. (2015) 12:86–9.
47. Cai C. Application of intrapleural injection of different drugs in treatment of pleural effusion caused by lung cancer. World Latest Med Inform. (2015) 15:95–95. doi: 10.3969/j.issn.1671-3141.2015.34.081
48. Zhao Y. Comparative observation on the efficacy of intrapleural injection of different drugs in the treatment of lung cancer pleural effusion. Yiyao Qianyan. (2014) 4:208–208. doi: 10.3969/j.issn.2095-1752.2014.12.227
49. Zhang J. Observation and nursing of intrapleural injection of different drugs in treating malignant pleural effusion. Nurs Pract Res. (2014) 11:65–6. doi: 10.3969/j.issn.1672-9676.2014.10.035
50. Yao X. Therapeutic effect of cisplatin combined with Staphylococcin on malignant pleural effusion. Chin Pract J Rural Doct. (2014) 21:51–2. doi: 10.3969/j.issn.1672-7185.2014.18.031
51. Tu D, Zhang Q, Hong J, Chen Y, Yang Q. Comparative observation on the efficacy of intrapleural injection of different drugs in treating pleural effusion. Hebei Med. (2014) 20:296–8. doi: 10.3969/j.issn.1006-6233.2014.02.048
52. Chen J. Progress in local treatment of malignant pleural effusion and choice of drugs. China Foreign Med Treatment. (2014) 33:112–3. doi: 10.16662/j.cnki.1674-0742.2014.02.107
53. Yu L, Wang Y. Clinical study of Gaojusheng combined with cisplatin in the treatment of pleural effusion caused by lung cancer. China Health Care Nutr. (2013) 23:2963–4. doi: 10.3969/j.issn.1004-7484(x).2013.06.114
54. Xu T, Zhang T, Gao H, Zhou J, Zheng Y. Docetaxel combined with highly agglutinative staphylococcin local treatment observation of lung cancer with malignant pleural effusion. Med Inform. (2013) 26:73–4.
55. Shi D. Clinical analysis of high-aggregating Staphylococcus aureus plus cisplatin combined with chemotherapy for malignant pleural effusion. Guide China Med. (2013) 11:12. doi: 10.15912/j.cnki.gocm.2013.24.56
56. Li W. Efficacy of high-aggregation Staphylococcus aureus combined with cisplatin in the treatment of pleural effusion caused by lung cancer. China Health Care Nutr. (2013) 23:4857–8. doi: 10.3969/j.issn.1004-7484(s).2013.09.077
57. Li L, Qian X. Clinical effect of highly agglutinative staphylococcin combined with cisplatin in treatment of malignant pleural effusion. J Mod Oncol. (2013) 21:1762–4. doi: 10.3969/j.issn.1672-4992.2013.08.31
58. Gao H, Zhang X, Yang J. Comparison of Lentinan and Staphylococcin aureus in the treatment of lung cancer with pleural effusion. Chin J Med Guide. (2013) 15:285–7.
59. Du Y. Clinical observation on the treatment of pleural effusion caused by lung cancer with high aggregate staphylococcus and cisplatin. Chin J Clin Oncol Rehab. (2013) 20:987–9. doi: 10.13455/j.cnki.cjcor.2013.09.033
60. Zhou L, Ye W, Zhang W, Pan H. Radiofrequency hyperthermia combined with intraplearal infusion of Elemene emulsion or staphylococcin in the treatment of malignant pleural effusion. Chin J Clin Med. (2012) 19:25–6. doi: 10.3969/j.issn.1008-6358.2012.01.010
61. Yu D, Xiao Q. Experience in the treatment of 50 cases of lung cancer with malignant pleural effusion. National Med Front China. (2012) 49:18. doi: 10.3969/j.issn.1673-5552.2012.13.0031
62. Xu Q. Observation of the clinical effect of high-aggregating Staphylococcus aureus combined with cisplatin in the treatment of pleural effusion caused by lung cancer. China Foreign Med Treat. (2012) 31:89–90. doi: 10.3969/j.issn.1674-0742.2012.34.059
63. Xu J. Efficacy of high-aggregation Staphylococcus aureus combined with cisplatin in the treatment of pleural effusion caused by lung cancer. Strait Pharm J. (2012) 24:191–2. doi: 10.3969/j.issn.1006-3765.2012.10.102
64. Xing Q, Li J, Chen H. Curative effect of intrathoracic injection of various medicines on pleural effusion caused by lung cancer. J Clin Pulm Med. (2012) 17:1423–4. doi: 10.3969/j.issn.1009-6663.2012.08.031
65. Qin Q, Zhang H. Observation of the curative effect of cisplatin combined with aureus in the treatment of malignant pleural effusion. Inner Mongolia Med J. (2012) 44:113–4. doi: 10.16096/j.cnki.nmgyxzz.2012.s8.108
66. Zhang C, Li M. Clinical study of high polymer combined with cisplatin in the treatment of malignant pleural effusion. China Mod Doct. (2011) 120:133. doi: 10.3969/j.issn.1673-9701.2011.03.070
67. Xu T, Gao H, Zhang T, Yang B. Clinical obersavation of highly agglutinative staphylococcin combined with nedaplatin for malignant pleural effusion as local treatment. Chin J Cancer Prev Treat. (2010) 17:1229–30. doi: 10.16073/j.cnki.cjcpt.2010.15.022
68. Wu J, Deng C, Dai P. Central venous catheter closed drainage combined with pleural injection of aureus in the treatment of malignant pleural effusion. Guide China Med. (2010) 8:91–3. doi: 10.3969/j.issn.1671-8194.2010.35.069
69. Qu Y, Liu T, Cao Y, Zhu L. The therapeutic effects of highly agglutinative staphylococcin combined with cisplatin in the treatment of patients with malignant pleural effusion. J Basic Clin Oncol. (2010) 23:413–4. doi: 10.3969/j.issn.1673-5412.2010.05.019
70. Liu Q. Curative effect observation of highly agglutinative staphylococcin plus cisplatin in treatment of malignant hydrothorax. Lab Med Clin. (2010) 07:1700–1. doi: 10.3969/j.issn.1672-9455.2010.16.018
71. Li Z, Man H. Efficacy of cisplatin combined with staphylococcin aureus in pleural effusion of lung cancer treated with intrathoracic perfusion. Med Innov China. (2010) 07:45–6. doi: 10.3969/j.issn.1674-4985.2010.26.025
72. Gui Q. Efficacy analysis of the treatment of malignant pleural effusion with Engerfi and cisplatin. Chin J Misdiagn. (2010) 10:2839–40.
73. Cheng J, An Y, Zhang X, Wang Y, Liu W, Wang Y, et al. Clinical observation on treatment of malignant hydrothorax with locally administered highly agglutinative staphylococcin and cisplatin. China Pharm. (2010) 21:1130–1.
74. Chen J, Li G, Zu J, Zhu Y, Wang H. Analysis of the treatment of malignant pleural effusion with cisplatin combined with high agglutination staphylococcal. Zhejiang Clin Med J. (2010) 12:1204–5. doi: 10.3969/j.issn.1008-7664.2010.11.018
75. Zhang L, Chen Q. Clinical observation of Gaojusheng combined with cisplatin in the treatment of malignant pleural effusion. Chin J Cancer Prev Treat. (2009) 16:1517. doi: 10.16073/j.cnki.cjcpt.2009.19.020
76. Yuan X, Wang D, Shi Y, Xu C, Wang D, Tian Z. Clinical observation of 60 cases of malignant pleural effusion treated by minimally invasive insertion of central venous catheter. J Chin Physician. (2009) 11:1234–5. doi: 10.3760/cma.j.issn.1008-1372.2009.09.036
77. Wang H, Wang Y, Su L, Chen J, Fu M, Sun G. Clinical observation on the treatment of 84 cases of pleural effusion caused by lung cancer. Chongqing Med. (2009) 38:66–7. doi: 10.3969/j.issn.1671-8348.2009.01.030
78. Gao H, Qiu F, Ma W, Li B. Clinical observation on treatment of malignant pleural effusion with cisplatin and golden staphylococcus. Mod Pract Med. (2009) 830:832. doi: 10.3969/j.issn.1671-0800.2009.08.017
79. Zhang M, Wang J, Liu H, Ning L. Clinical observation of Gaojusheng combined with cisplatin in the treatment of malignant pleural effusion. Chin J Clin Oncol. (2008) 35:499–500. doi: 10.3969/j.issn.1000-8179.2008.09.007
80. Mo W. Clinical study of malignant pleural effusion treated by intrapleural perfusion of staphylococcal enterotoxin combined with bleomycin. Mod J ITCWM. (2008) 17:1787–1788, 1791. doi: 10.3969/j.issn.1008-8849.2008.12.002
81. Li X, Zhang K, Wang Z, Du J, Wang H, Guo L. Observation of therapeutic effect of gaojusheng combined with cisplatin on malignant pleural effusion. Chin J Coal Ind Med. (2008) 11:550–1. doi: 10.3969/j.issn.1007-9564.2008.04.063
82. Huang W, Wang H. Observation on the clinical effect of Gaojusheng combined with cisplatin in the treatment of lung cancer pleural effusion. Chin J Clin Med Res. (2008) 4:8–9.
83. Zhou F, Bi Y, Zhang T, Rao X. Treatment of malignant pleural effusion using dleomysin and engefei intrapleurally infusion. J Pract Med. (2007) 23:973–5. doi: 10.3969/j.issn.1006-5725.2007.07.015
84. Zheng F. Clinical observation of Gaojusheng combined with cisplatin in the treatment of malignant pleural effusion. Chin J Postgrad Med. (2007) 30:45–45. doi: 10.3760/cma.j.issn.1673-4904.2007.z1.072
85. Yin W, Tao M. Efficacy of high-aggregation Staphylococcus aureus combined with intrapleural injection of cisplatin to treat 38 cases of malignant pleural effusion. Chin Med Sci Health. (2007) 4:3–4.
86. Xue Z, Wu Y, Zhuang Y. Treatment of 65 cases of metastatic pleural effusion with lung cancer by drainage and perfusion with high-poly health. Chin J Clin Oncol. (2007) 34:890–1. doi: 10.3969/j.issn.1000-8179.2007.15.017
87. Sun C, Wang L. Observation of curative effect of minimally invasive catheter placement and intrapleural injection of high-aggregate Staphylococcus aureus in the treatment of non-small cell lung cancer with malignant pleural effusion. Chin J Lung Cancer. (2007) 10:61–3. doi: 10.3779/j.issn.1009-3419.2007.01.16
88. Kuang H, Xiao P, Chen H, Sheng Q, Li C, Deng Z, et al. Clinical observation of Mycobacteria pleural injection in the treatment of malignant pleural effusion. J Clin Pulm Med. (2007) 12:1306–7. doi: 10.3969/j.issn.1009-6663.2007.12.006
89. Zhao Z, Li K, Lu W, Wang J. Clinical observation of Gaojusheng combined with cisplatin in the treatment of malignant pleural effusion. Pract Clin J ITCWM. (2006) 6:19–20. doi: 10.3969/j.issn.1671-4040.2006.06.013
90. Zhang Z. Observation on the effect of combined treatment of cisplatin and high-aggregating Staphylococcus aureus on malignant pleural effusion. Med J Commun. (2006) 20:721–721. doi: 10.3969/j.issn.1006-2440.2006.06.052
91. Yue Z, Bai W. Observation of curative effect of Gaojusheng combined with cisplatin in local treatment of malignant pleural effusion. J Pract Oncol. (2006) 20:410–1. doi: 10.3969/j.issn.1002-3070.2006.05.019
92. Xiong C, Liu S. Efficacy of Engefei combined with cisplatin in the treatment of malignant pleural effusion. Guangxi Med J. (2006) 28:1392–4. doi: 10.3969/j.issn.0253-4304.2006.09.039
93. Wang J, Wang Y, Zhao J. Observation on the curative effect of pleural drainage and injection of high-aggregating Staphylococcus aureus for malignant pleural effusion. J Mod Oncol. (2006) 14:1400–1. doi: 10.3969/j.issn.1672-4992.2006.11.029
94. Shen S, Jiang X, Fang P, Chen D, Chen S. Treatment of malignant pleural effusion using dleomysin and En-Ge-Fei Intrapleurally Infusion. Clin J Med Officers. (2006) 34:20–2. doi: 10.3969/j.issn.1671-3826.2006.01.008
95. Pan Y, Tang Y, Zheng Z, Xu X. Clinical observation of highly agglutinative staphylococc combined with cisplatin in treatment of malignant hydrothorax. China J Mod Med. (2006) 16:410–2. doi: 10.3969/j.issn.1005-8982.2006.03.026
96. Jiang D, Liu W, Yan M, Zhang H, Chang W. Observation of therapeutic effect of Gaojusheng combined with carboplatin on malignant pleural effusion. Chin J Misdiagn. (2006) 6:2703–4. doi: 10.3969/j.issn.1009-6647.2006.14.034
97. Huang D, Sun S, He Z. Clinical observation on the treatment of malignant pleural effusion by intrapleural injection of high polymer Staphylococcus aureus. J Nongken Med. (2006) 28:431–2. doi: 10.3969/j.issn.1008-1127.2006.06.010
98. Fang H, Zhang L, Yu H. Observation of curative effect of Gaojusheng combined with cisplatin on malignant pleural effusion. Clin Med. (2006) 26:64–5. doi: 10.3969/j.issn.1003-3548.2006.02.037
99. Ding H, Xie Y, Nie S. Clinical effects of highly agglutinated staphylococcin on malignant pleural effusion. J Clin Exp Med. (2006) 5:919–919. doi: 10.3969/j.issn.1671-4695.2006.07.044
100. Chen Z, Le H, Gu L, Jing W. Efficacy analysis of Ngefei combined with cisplatin on malignant pleural effusion. Chin J Hosp Pharm. (2006) 26:195–6. doi: 10.3321/j.issn:1001-5213.2006.02.038
101. Zhang T, Hu X. Treatment of malignant pleural effusion by highly agglutinative staphylococcin combined with cisplatin. J Clin Pulm Med. (2005) 10:415–6. doi: 10.3969/j.issn.1009-6663.2005.04.003
102. Ma D, Yang W, Lu W, Cai J. Treatment of malignant pleural effusion with Gaojusheng and cisplatin perfusion. J Chin Pract Diagn Therapy. (2005) 19:919–20. doi: 10.3969/j.issn.1674-3474.2005.12.040
103. Liu Z. Clinical observation of Gaojusheng combined with VP-16 in the treatment of lung cancer complicated with pleural effusion. Chin J Compos Clin Med. (2005) 7:42–42.
104. Liu S, Qian W, Shen W. Clinical observation on treatment of malignant pleural effusion with minimally invasive tube drainage and perfusion of high agglutination staphylococcus and cisplatin. J Clin Med Pract. (2005) 9:24–5. doi: 10.3969/j.issn.1672-2353.2005.08.010
105. Liu M, Liu H, Chen Y, Wang D, Cai Y. The treatment of Engefei combined with cisplatin for malignant pleural effusion. Clin Med. (2005) 25:27–8. doi: 10.3969/j.issn.1003-3548.2005.12.013
106. Liang J, Zhang R, Lan J. Clinic study on treatment of malignant thoracic hydrops by carboplatin combined with HAS. J Med Forum. (2005) 26:34–35, 37. doi: 10.3969/j.issn.1672-3422.2005.19.016
107. Huang Y., Zhendong, Chen Z, Liang J. Observation of the therapeutic effect of cisplatin combined with biological response modifier in the treatment of malignant pleural effusion of lung cancer. Guangxi Med J. (2005) 27:109–10. doi: 10.3969/j.issn.0253-4304.2005.01.062
108. Feng X, Teng B, Zhao Y. Treatment of 34 cases of senile malignant pleural effusion with microtubule drainage and local chemotherapy. Chin J Mod Clin Med. (2005) 3:2496–7.
109. Chen J, Cheng X. Clinical observation on treating malignant pleural effussion with both gaojusheng and cisplatin. Jiangxi J TCM. (2005) 36:19–20. doi: 10.3969/j.issn.0411-9584.2005.08.012
110. Zhu F, Wang Y, Li G. Treatment of 34 cases of malignant pleural effusion by injecting cisplatin combined with high agglutination Staphylococcus aureus. Cancer Res Clin. (2004) 16:411–2. doi: 10.3760/cma.j.issn.1006-9801.2004.06.028
111. Xu Z, Zhang Z, Wu R. Gaojusheng combined with intrapleural injection of carboplatin to treat malignant pleural effusion. Med J Liaoning. (2004) 18:156–156. doi: 10.3969/j.issn.1001-1722.2004.03.020
112. Wang C. Observation of the clinical effect of pleural perfusion of Jinpuye combined with cisplatin on malignant pleural effusion. Chin J Cancer Prev Treat. (2004) 11:1284, 1314. doi: 10.3969/j.issn.1673-5269.2004.12.035
113. Tao M, Yin W, Wan H. Clinical study of highly agglutinative staphylococcin combined with mitomycin in the treatment of malignant pleural effusion. Chin J Compos Clin Med. (2004) 6:43–4.
114. Mao W. Highly agglutinative staphylococcin combined with chemotherapy in the treatment of 40 cases of malignant pleural effusion. Chin J Coal Ind Med. (2004) 7:632–632. doi: 10.3969/j.issn.1007-9564.2004.07.033
115. Liu Y, Chang J, Li Y. The comparative analysis of curative effect of HAS and DDP treating malignant pleural effusion (35 example clinical reports). J Shenyang Med Coll. (2004) 6:13. doi: 10.3969/j.issn.1008-2344.2004.01.004
116. Hu Y, Sun B, Yuan D. Clinical observation of natural growth factor plus cisplatin in treatment of advanced neoplasm and malignant pleural effusion. Med J Commun. (2004) 18:493–4. doi: 10.3969/j.issn.1006-2440.2004.05.006
117. Guan C, Yin Z, Ma G, Lu H. Clinical study of high agglutinative staphylococin in treatment of malignant pleural effusion. Cancer Res Prev Treat. (2004) 31:241–2. doi: 10.3971/j.issn.1000-8578.2004.04.021
118. Fang X. The effect of Gaojusheng combined with cisplatin in the treatment of malignant pleural effusion. Cancer Res Clin. (2004) 16:58–9. doi: 10.3760/cma.j.issn.1006-9801.2004.01.031
119. Chen H, Lu G, Chen W. Clinical effect of Gaojusheng combined with cisplatin in the treatment of malignant pleural effusion. Guangdong Med J. (2004) 25:591–2. doi: 10.3969/j.issn.1001-9448.2004.05.064
120. Zhang J, Wu Y, Liu L. The treatment of malignant pleural effusion with Gaojusheng combined with chemotherapy drugs. J Med Forum. (2003) 24:44–5. doi: 10.3969/j.issn.1672-3422.2003.07.026
121. Xu Q, Luo K, Qin H. Observation on the curative effect of low-dose cisplatin combined with high-aggregation Staphylococcus aureus in the treatment of 62 cases of malignant pleural effusion. Chongqing Med. (2003) 32:1659–59, 1661. doi: 10.3969/j.issn.1671-8348.2003.12.088
122. Wang Y, Geng J, Zhang L. 40 cases of malignant pleural effusion treated by intrapleural injection of Gaojusheng. Hebei Med J. (2003) 25:109. doi: 10.3969/j.issn.1002-7386.2003.02.014
123. Wang C, Zhou Y. Analysis of the short-term curative effect of high-aggregating Staphylococcus aureus and cisplatin in the treatment of malignant pleural effusion. China New Med. (2003) 2:90–1.
124. Sun W, Lai Y. Treatment of malignant pleural effusion with high-aggregation Staphylococcus aureus and 5-fluorouracil. Int Med Heal Guid News. (2003) 9:84–5. doi: 10.3760/cma.j.issn.1007-1245.2003.18.053
125. Li J-L. Clinical observation of local pleural cavity perfusion with gaojusheng for treatment of malignant pleural effusion. Harbin Med J. (2003) 23:12–3.
126. Li J, Yang Q, Zeng R, Zou H. Clinical observation on the treatment of 44 cases of malignant pleural effusion with high agglutination staphylococcus and cisplatin. J Mod Oncol. (2003) 11:126–126. doi: 10.3969/j.issn.1672-4992.2003.02.020
127. Duan R, Tian G, Zhang G, Wang T. Clinical observation on the treatment of 152 cases of malignant pleural effusion with high polymers and cisplatin. Chin J Coal Ind Med. (2003) 6:163–163. doi: 10.3969/j.issn.1007-9564.2003.02.064
128. Wang L, Sun M, Fang S. Clinical observation on the treatment of malignant pleural effusion with high aggregate staphylococcus and cisplatin. Jilin Med J. (2002) 23:178–178. doi: 10.3969/j.issn.1004-0412.2002.03.025
129. Wang L. Observation and nursing care of malignant pleural effusion treated with high aggregate staphylococcus and cisplatin. Chin J Pract Med. (2002) 29:61–2. doi: 10.3760/cma.j.issn.1674-4756.2002.08.067
130. Gu W, Du J, Ding Y. Observation of therapeutic effect of Jinpu liquid on malignant pleural effusion. Clin Med. (2002) 22:4–4. doi: 10.3969/j.issn.1003-3548.2002.05.003
131. Zhang L. Efficacy analysis of high-dose high-aggregating Staphylococcus aureus combined with intrapleural injection in the treatment of malignant pleural effusion. Chin J Coal Ind Med. (2001) 4:546–7. doi: 10.3969/j.issn.1007-9564.2001.07.055
132. Tian C. Observation of the short-term curative effect of Gaojusheng combined with intrapleural injection of VP-16 in the treatment of lung cancer complicated with malignant pleural effusion. J Basic Clin Oncol. (2001) 14:284–5. doi: 10.3969/j.issn.1673-5412.2001.04.024
133. Lang F, Zhao M, Zhao M, Wang Z, Jiang Q. Observtion of the result of highly agglutinative staphylococcin and cisplatin in treating of malignant pleural feeusion. China J Cancer Prev Treat. (2001) 8:256–8. doi: 10.3969/j.issn.1673-5269.2001.z1.073
134. Huang H, Sun N, Wang D. Observation on 38 cases of malignant pleural effusion treated by high agglutination staphylococcus and cisplatin. Flight Surgeon. (2001) 29:210–210.
135. Zhang S. Treatment of malignant pleural effusion with highly agglutinative staphylococcin and mitomycin. Tianjin Med J. (2000) 28:500–1. doi: 10.3969/j.issn.0253-9896.2000.08.024
136. Zhang G, Li Y, Gao X, Qi Y. Analysis of curative effect of intrapleural injection of fenbesu and cisplatin in the treatment of cancerous pleural effusion. Shandong Med J. (2000) 40:55–6. doi: 10.3969/j.issn.1002-266X.2000.05.049
137. Xu N, Meng X. Observation of therapeutic effect on 64 cases of highly agglutinative staphylococcin plus cisplatin in treatment of malignant pleural effusion. J Shanxi Coll Tradit Chin Med. (2000) 1:38–9.
138. Tang Z. The effectt of high dose of HASL instillation intrapleural in malignent pleural effusion. Cancer Res Prev Treat. (2000) 27:218–9. doi: 10.3971/j.issn.1000-8578.2000.03.024
139. Jia X, Ding S, Wang Z. Clinical study of intrapleural injection of golden staphylococcus l in the treatment of cancerous pleural effusion. J Chin Oncol. (2000) 6:147–8.
140. Hu H, Jiang G. Observation of therapeutic effect of high polymer Staphylococcus aureus on pleural effusion of lung cancer. P Clin Med. (2000) 9:530–1. doi: 10.3969/j.issn.1671-8631.2000.07.024
141. Fu J. Treatment of 55 cases of cancerous pleural effusion with highly agglutinative staphylococcin. China Pharm. (2000) 9:40–1. doi: 10.3969/j.issn.1006-4931.2000.10.033
142. Chen Y, Chen Z, Zhang M. Observation of curative effect of high-agglomerative staphylococcus and bleomycin in the treatment of cancerous pleural effusion. Mil Med J Southeast China. (2000) 2:27–8.
143. Cao H. Clinical observation on treatment of malignant pleural effusion with high aggregate staphylococcus and cisplatin. Chin Gen Pract. (2000) 3:410–410. doi: 10.3969/j.issn.1007-9572.2000.05.043
144. Zhang Y. Observation on the effect of high-aggregating Staphylococcus aureus combined with mitoxantrone in the treatment of lung pleural effusion. West China J Pharmaceut Sci. (1999) 14:200–200. doi: 10.3969/j.issn.1006-0103.1999.03.025
145. Zhang Q, Zhou J, Tan Q. Observation of the curative effect of injecting high-aggregating staphylocust and cisplatin on malignant pleural effusion after closed thoracic drainage. J Nantong Univ (Med Sci). (1999) 19:66–66.
146. Gao Z, Zhang W, Mao A, Wang Y, Zhang L. The treatment of carcinomatous pleural effusion by cannula perfusi0n of biological products. Chin J Coal Ind Med. (1999) 2:9–10.
147. Li X. Local biological treatment of malignant pleural effusion. J Pract Oncol. (1998) 12:154–6.
148. Li H, Yang F. Analysis of 68 cases of malignant pleural effusion treated with high aggregate staphylococcus. Qingdao Med J. (1998) 30:9–9.
149. Huang W, Wang H. Observation on the clinical effect of Gaojusheng combined with cisplatin in the treatment of lung cancer pleural effusion. Chin J Clin Med Res. (2008) 4:8–9.
Keywords: malignant pleural effusion (MPE), staphylococcal enterotoxin C (SEC), intrapleural infusion, pleurodesis agent, clustered systematic review, meta-analysis
Citation: Jiang H, Yang X-M, Wang C-Q, Xu J, Huang J, Feng J-H, Chen X-F, Chen K, Zhan L, Xiao X and Xiao Z (2022) Intrapleural Perfusion With Staphylococcal Enterotoxin C for Malignant Pleural Effusion: A Clustered Systematic Review and Meta-Analysis. Front. Med. 9:816973. doi: 10.3389/fmed.2022.816973
Received: 16 December 2021; Accepted: 21 March 2022;
Published: 25 April 2022.
Edited by:
Konstantinos Kostikas, University of Ioannina, GreeceReviewed by:
Petros Bakakos, Athens Chest Hospital Sotiria, GreeceCopyright © 2022 Jiang, Yang, Wang, Xu, Huang, Feng, Chen, Chen, Zhan, Xiao and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Xiao, enk0MjZmQDE2My5jb20=; Xue Xiao, eHhlbGxlbkAxNjMuY29t; Lin Zhan, emhhbmxpbjMwMEBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.