- Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Objective: The aim of this study was to evaluate the potential of metagenomic next-generation sequencing (mNGS) for the diagnosis of pneumocystis pneumonia (PCP) in patients with non-human immunodeficiency virus-infection and to discuss the clinical characteristics and identify prognostic factors associated with patients with non-HIV PCP.
Methods: Forty-six patients with PCP who were admitted in respiratory intensive care unit (ICU) between May 2018 and May 2020 were retrospectively reviewed. The subjects were divided into survivor and non-survivor groups according to the patients' outcome. Conventional methods and mNGS for detecting Pneumocystis jirovecii (P. jirovecii) were analyzed. The patients' demographics, comorbidities, laboratory parameters, and treatments were compared and evaluated in both groups to identify risk factors for mortality by using univariate and multivariate logistic regression.
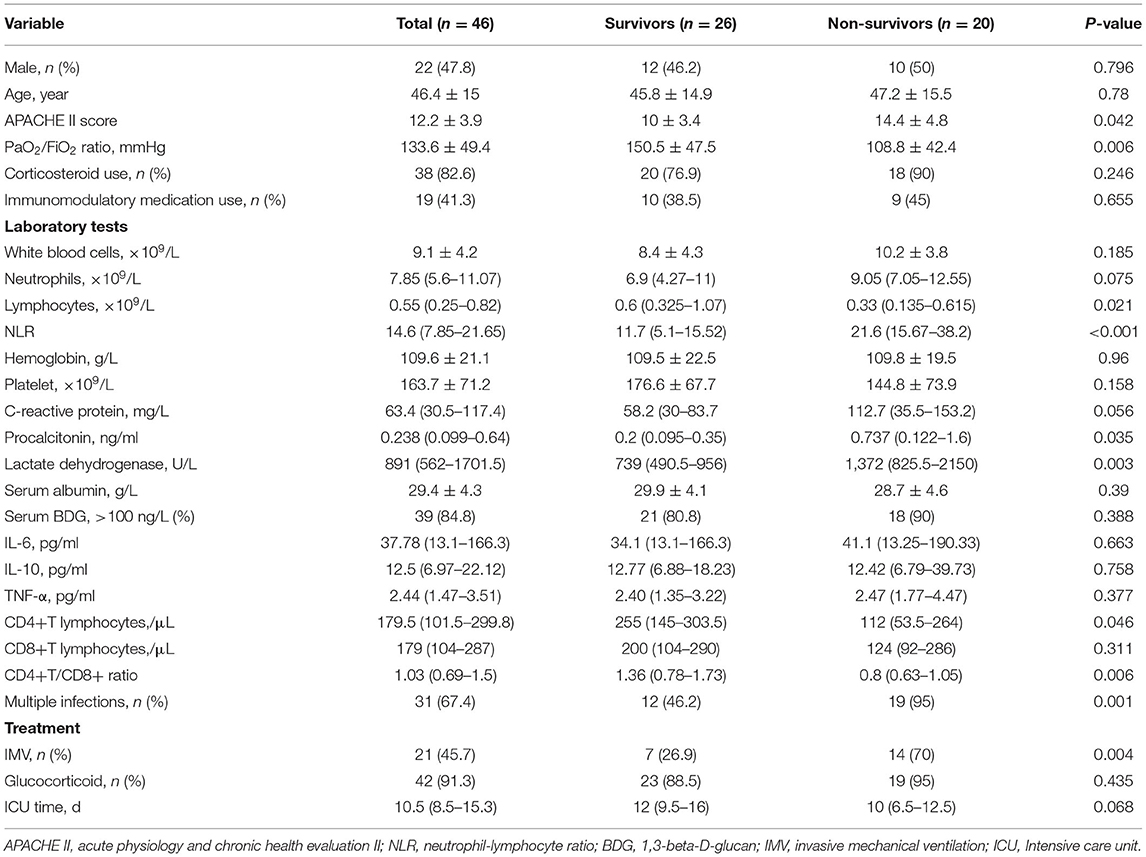
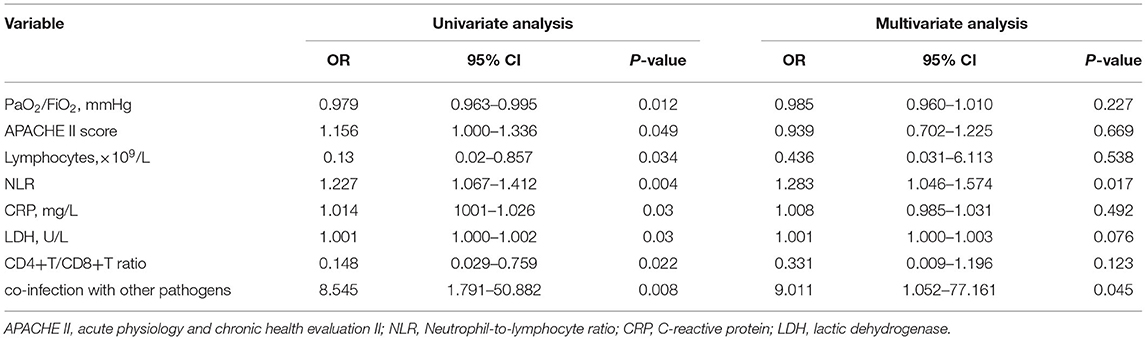
Results: Metagenomic next-generation sequencing (mNGS) showed a satisfying diagnostic performance of 100% positive of detecting P. jirovecii from bronchoalveolar lavage (BAL) specimens in forty-six patients with non-HIV PCP, compared to only 15.2% for Gomori Methenamine silver (GMS) staining and 84.8% for Serum 1,3-beta-D-glucan (BDG). Among them, the mean age was 46.4-year-old (range 18–79-year-old) and mortality rate was 43.5%. The dominant underlying conditions were connective tissue diseases (34.8%), autoimmune kidney diseases (30.4%), followed by hematologic malignancies (10.9%), and solid organ transplantation (6.5%). A total of 38 cases (82.6%) received glucocorticoid and 19 cases (41.3%) used immunosuppressant within 3 months before diagnosed PCP. Multiple infections were very common, over two thirds' cases had mixed infections. Compared with survivors, non-survivors had a higher acute physiology and chronic health evaluation II (APACHE II) score (14.4 ± 4.8 vs. 10 ± 3.4), Procalcitonin (PCT) [ng/ml: 0.737 (0.122–1.6) vs. 0.23 (0.095–0.35)], lactic dehydrogenase (LDH) [U/L: 1372 (825.5–2150) vs. 739 (490.5–956)], and neutrophil-lymphocyte ratio (NLR) [21.6 (15.67–38.2) vs. 11.75 (5.1–15.52)], but had a lower PaO2/FiO2 ratio (mmHg:108.8 ± 42.4 vs. 150.5 ± 47.5), lymphocytes [×109/L: 0.33 (0.135–0.615) vs. 0.69 (0.325–1.07)] and CD4+ T cells [cell/μl: 112 (53.5–264) vs. 255 (145–303.5)], all P < 0.05. Furthermore, we found non-survivors' PaO2/FiO2 ratio of day 3 and day 7 had not improved when compared with that of day one, and platelet level and NLR became worse. Multivariate analysis showed that other pathogens' co-infection (OR = 9.011, 95% CI was 1.052–77.161, P = 0.045) and NLR (OR = 1.283, 95% CI was 1.046–1.547, P = 0.017) were the independent risk factors of poor prognosis.
Conclusion: mNGS is a very sensitive diagnostic tool for identifying P. jirovecii in patients who are non-HIV immunocompromised. PCP in patients who are non-HIV infected is associated with a high rate of multiple infections and severe condition. Mixed infection and elevation of NLR were the independent risk factors of poor prognosis.
Introduction
Pneumocystis pneumonia (PCP) is an opportunistic pulmonary fungal infection caused by Pneumocystis jirovecii (P. jirovecii) in immunocompromised populations. And it is very common among patients with HIV/AIDS. Especially, patients with HIV/AIDS with a low CD4 count (CD4 counts <200/μl) are at the highest risk of PCP (1, 2). With the widespread use of antiretroviral and PCP prophylaxis therapy, the incidence and mortality of PCP in patients with HIV/AIDS has gradually declined and it is around 10–20% nowadays (3, 4). However, the emerging number of conditions associated with immunosuppression has led to its continuing appearance in non-HIV patient population. The incidence of PCP is gradually increasing among patients who are non-HIV immunocompromised such as those receiving chronic corticosteroid therapies, with hematological or solid malignancies, transplant recipients and those who receive immunomodulatory or biological therapy (5, 6). Moreover, in the non-HIV-infected population, PCP is often associated with a higher rate of progressing to respiratory failure which occurs within a short time and causes a mortality rate of 30–60% (7). The growing incidence and high mortality of pneumocystis infection in the non-HIV patients suggest the need of more attention, including earlier diagnosis and treatment.
Despite the growing prevalence of PCP in immunocompromised individuals, establishing a microbiological diagnosis remains a challenge in this vulnerable population. Because the Inability to culture pneumocystis and non-specific clinical manifestations, together with relatively lower burden of organisms in HIV-uninfected patients (4, 8), conventional microbiological methods usually have an inadequate performance of finding P. jirovecii, and it may present false negative results of conventional tests (9). Metagenomics next-generation sequencing (mNGS) is a molecular technology of nucleic acid sequencing with high-throughput capacity and unbiased pathogen detection in a single assay, which has been considered a promising microbial identification technology in infectious diseases (10). Recently, several studies have shown its advantage in detecting a wide range of pathogens from different clinical specimens (11–13). However, the study about the utility of mNGS of bronchoalveolar lavage (BAL) fluid specimens for the diagnosis of PCP in non-HIV patients remains rare.
In the present study, we describe the use of mNGS of BAL fluids for detecting P. jirovecii and discuss the characteristics and risk factors of the outcome of non-HIV immunocompromised patients with PCP.
Materials and Methods
Study Participants
We retrospectively collected a total of 46 immunocompromised patients diagnosed with PCP by mNGS test of BAL in our respiratory intensive care unit (ICU) from May 2018 to May 2020. This study was approved by the ethical committee of the first affiliated hospital of Zhengzhou University (The ethics approval number was 2020-KY-521), and informed consents were signed by patients or surrogates. Patients who met the following criteria were diagnosed with PCP: (1) Clinical symptoms (cough, fever, or shortness of breath) and laboratory parameters [1,3-beta-D-glucan (BDG) test and lactic dehydrogenase (LDH)] relevant to PCP; (2) Imaging findings compatible with PCP (present bilateral interstitial, ground-glass opacity and alveolar infiltrates in perihilar areas); (3) Identifying the genetic sequences of P. jirovecii by mNGS of BAL specimens. All patients enrolled were defined as HIV-negative, with one or more of the following immunosuppressive host conditions: receiving corticosteroid therapy within 3 months; active malignancy and/or receiving cancer chemotherapy; solid organ transplantation; hematological malignancy; receiving biologic immune modulators and/or immunosuppressive therapy. Patients with the following conditions were excluded: (1) Age < 18 years old; (2) HIV infection; (3) Length of stay in ICU < 24 h; (4) Incomplete medical record.
Microbiological Investigations
All patients had bronchoscopy performed, and BAL specimens were obtained. Each BAL specimen was divided into aliquots for both conventional microbiological test and mNGS test. Some specimens were sent to the Vision Medical Co., Ltd. (China) for the mNGS analysis, and the processes of nucleic acid extraction, library construction, and high-throughput sequencing were performed, in turn. Finally, bioinformatics analysis and pathogen data interpretation were performed, all of which were already noted in detail in previous studies (14, 15).
At the same time, conventional tests were also conducted for microbiological analysis, including bacterial/fungal smear and culture, acid-fast staining, P. jirovecii smear [Gomori Methenamine silver (GMS) staining], and real-time PCR including cytomegalovirus (CMV), influenza virus. Real-time PCR was performed with a commercial CMV assay using MagNA Pure LC instrument (Roche Molecular Biochemicals, Indianapolis, IN). Positive control and negative control were included in all assays. The lower limit of detection of the assay was estimated to be <50 copies/ml (as per the instruction of the manufacturer). DNA integrity of the samples was confirmed by the presence of internal control DNA. The mixed infection was defined as the isolation of more than one pathogenic species. Furthermore, there was a consensus of clinically significant pathogens reached by physicians based on the comprehensive analysis of conventional and mNGS results, clinical features, and laboratory findings.
Clinical Data Collection
Clinical parameters of each patient were acquired through review of electronic medical records. We recorded patient data regarding demographics, underlying diseases, use of immunosuppressant, laboratory test results, acute physiology and chronic health evaluation II (APACHE II) score, the time of ICU stay, and the outcome of the patient.
Statistical Analysis
For continuous variables, normally distributed variables were reported as the mean and standard deviation and as the median and interquartile range (IQR) if they had a skewed distribution. Categorical variables were expressed as frequencies and percentages. Continuous variables were compared using the t-test or Mann-Whitney U test where suited. Categorical variables were compared using the chi-squared or Fisher's test, and repeated-measures analysis of variance was used when comparing differences between continuous variables over time. Logistic regression models were used to identify the risk factors of poor outcome. Covariates with a P < 0.05 in the univariate analysis were included in the final model of multivariable logistic regression. All statistical analyses were performed using the SPSS software version 23.0 (IBM, Armonk, NY, USA). P < 0.05 were considered significant, and all tests were 2-tailed.
Results
Characteristics of Study Subjects and Detection of P. jirovecii in BAL Specimens by mNGS
Forty-six patients were enrolled during the study period. Among them, the mean age was 46.4 (18–79) years old and 22 (47.8%) of the patients were male. The mean age and gender compositions of survival and non-survival groups were similar. The underlying diseases of PCP patients were as follows: connect tissue diseases (34.8%), autoimmune kidney disease (30.4%), hematological malignancies (10.9%), and kidney transplant recipients (6.5%). As expected, PCP patients had various immunosuppressive conditions: Within 3 months prior the PCP diagnosis, 38 patients (82.6%) received steroids for inflammatory and/or autoimmune diseases, 19 cases (41.3%) took more than one immunomodulatory drug. However, none of them received PCP prophylaxis therapy (As shown in Table 1).
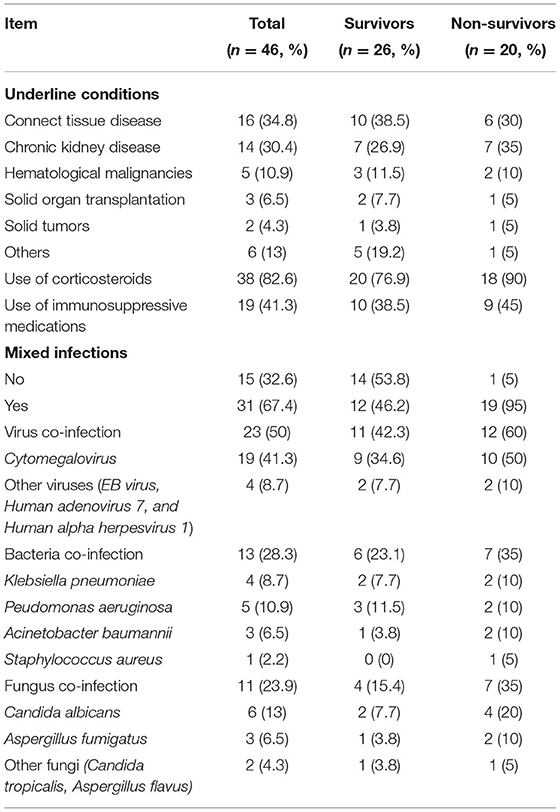
Table 1. Underlying and co-infection conditions of non-HIV immunocompromised pneumocystis pneumonia (PCP) patients.
Metagenomics next-generation sequencing (mNGS) and GMS staining of BAL specimens were performed in each patient. The mNGS test of forty-six patients' specimens all found P. jirovecii (sequence reads 12–214,898), and the result of GMS staining of BAL specimens showed that P. jirovecii was identified only in 7 cases (15.2%). Additionally, sputum specimens were obtained from 18 patients (39.1%); however, all showed negative results of P. jirovecii. BDG was widely used as a serologic biomarker of PCP. In this study, Serum BDG testing showed that 84.8% of patients had a positive result (Serum BDG > 100 ng/L).
Treatments and Outcome
All patients were treated with trimethoprim-sulfamethoxazole or combined with caspofungin. Meanwhile, 42 patients (93.5%) received adjuvant corticosteroid therapy. As for oxygen support, 21 patients needed invasive mechanical ventilation, the remaining patients were oxygenated by non-invasive mechanical ventilation and high-flow nasal oxygen treatment. The non-survival group had a higher rate of receiving invasive mechanical ventilation when compared with the survival group (70% vs. 26.9%, P = 0.004).
Multi-infections were very common among PCP patients, and about 2/3 of patients had mixed infections. Among them, the most commonly detected co-infection pathogen was CMV. A total of 19 cases were co-infected with CMV, but there was no significant difference on the incidence between the two groups. Although patients with multi-infections were prescribed with relevant anti-bacterial or anti-fungal, antiviral agents, patients with multiple infections still had higher risk of poor outcomes than those without. Non-survivors had a significantly higher rate of mixed infection (95% vs. 46.2%, P = 0.001), which was also shown to be an independent risk factor of death in multivariate logistic analysis. The hospital mortality rate of the patients was 43.5%. The length of stay in the ICU of non-survivors was shorter than those of the survivors, but no statistical significance was observed (As shown in Table 2).
Prognostic Factors by Univariate Analysis
As shown in Table 2, compared with survivals, non-survivals appeared to have higher white blood cell count, neutrophil count and serum C-reactive protein, but no significance was observed between the two groups. All PCP patients showed lymphopenia with a median lymphocyte count of 0.55 × 109/L in peripheral blood and had significantly high LDH levels. Median serum levels of LDH (739 vs. 1,372 U/L) and neutrophil-to-lymphocyte ratio (NLR) (11.75 vs. 21.6) remarkably increased in both cohorts. Compared with survivors, non-survivors had a significantly higher APACHE II score within 24 h while admitted in the ICU, higher NLR ratio, PCT and LDH level, but had a lower PaO2/FiO2 ratio, lower lymphocytes, CD4+T cells and CD4+/CD8+ ratio; all P < 0.05. There was no significant difference in comparison of the cytokine' levels (IL-6, IL-10, and TNF-α) between the two groups.
Multivariate Analysis
Covariates with a P < 0.05 in the univariate analysis were included in the final model of multivariable logistic regression, and multivariate analysis showed that multiple infection and NLR were independent risk factors for non-HIV PCP patients that resulted in death (OR 1.283; 95% CI 1.046–1.574; OR 9.011; 95% CI 1.052–77.161; respectively) (Shown in Table 3).
Dynamic Changes of Laboratory Parameters Between Survival and Non-survival PCP Patients
Figure 1 showed the survivor and death groups' comparison on the dynamic changes of several laboratory parameters on day 1, day 3, and day 7 in ICU. Apparently, PaO2/FiO2 ratio of survival patients was improved gradually. PaO2/FiO2 ratios on day 3 and 7 were obviously higher than day 1. However, non-survivors hadn't gained PaO2/FiO2 ratio's elevation, and comparison of the tendency of PaO2/FiO2 ratio between the two groups also showed significant difference. In addition, the lymphocyte level had increased gradually and tended to be normal. C-reactive protein (CRP) and LDH levels decreased among survivors, all of which presented converse change in non-survivors. Furthermore, non-survivors had a significant decrement of platelets and an obvious rise of NLR ratio, and the repeated-measures analysis of these variance showed significant difference; all P < 0.05.
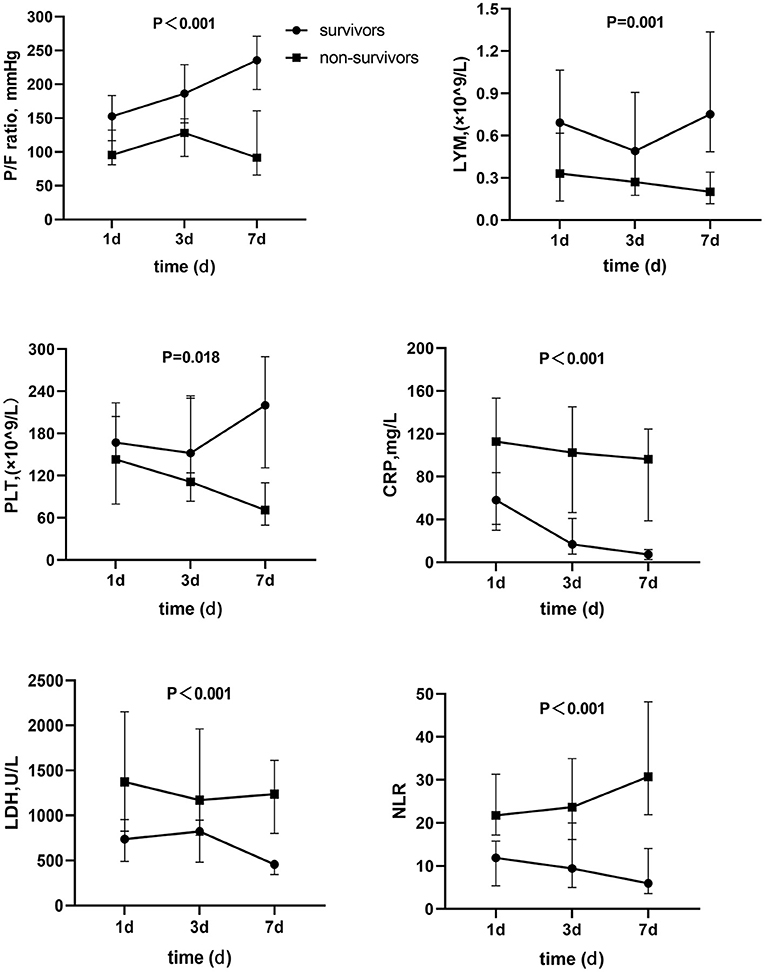
Figure 1. Dynamic change of parameters between survivors and non-survivors. P/F ratio, PaO2/PiO2 ratio; LYM, Lymphocyte; PLT, Platelet; CRP, C reactive protein; LDH, Lactate dehydrogenase; NLR, Neutrophil-to-lymphocyte ratio.
Discussion
With a growing incidence among immunocompromised populations, PCP became a more common opportunistic infection which can be life-threatening. In this retrospective study of 46 immunocompromised patients in ICU settings, all patients were diagnosed with PCP infection based on mNGS of BAL fluids, and none of the subjects had received any PCP prophylaxis. Meanwhile, mixed infections were very common among PCP patients. The hospital mortality rate was 43.5%. Multivariate analysis showed that mixed infection and NLR were independent risk factors of poor prognosis.
Conventional method via direct staining and microscopy for the detection of P. jirovecii lacks sensitivity. Furthermore, the organism cannot be cultured in a laboratory (7, 16). In the present study, mNGS which is a burgeoning microbial detection method confirmed the diagnosis of PCP among these patients. Genetic sequences of P. jirovecii were founded by mNGS from the BAL specimen of each patient while GMS staining only showed 15.2% positive results. Compared with the inadequate sensitivity of conventional methods (9), we found that mNGS had an excellent capability for the diagnosis of PCP and also had advantages in identifying co-pathogens in PCP patients with mixed infections. Again, data from several recent studies supported its advantages in detecting opportunistic pathogens and mixed infections, especially in detecting uncultivable pathogens, such as P. jirovecii (17–19). More importantly, in a retrospective study of evaluating the utility of mNGS for the diagnosis of PCP, their research data revealed that the sensitivity of mNGS was 100% in non-HIV infected PCP patients, which was dramatically higher than GMS staining and serum BDG (20).
Previous studies showed that PaO2/FiO2 ratio and invasive mechanical ventilation were the influence factors of PCP prognosis (21, 22). Consistent with these studies, we also found that non-survivors had a higher rate of invasive mechanical ventilation and lower PaO2/FiO2 ratio level. Moreover, non-survivors had almost gained no PaO2/FiO2 ratio's elevation during the first 7 days in ICU while PaO2/FiO2 ratio of survivors was improved gradually and was obviously higher than those of the non-survivors.
The elevation of LDH was shown to be correlated with PCP patients' poor outcome (23) in the study of Schimdt et al. Meanwhile, Yoshida et al. found that hypoproteinemia also affects prognosis among PCP patients with inflammatory bowel disease (24). By comparing the dynamic change of laboratory parameters (PaO2/FiO2 ratio, LDH and NLR, and so on) on day 1, 3, and 7 after admission in the ICU, our study demonstrated a significant different trend between survivors and non-survivors. Briefly, the non-survival group showed an increase in NLR and a decrement in platelets gradually, but gained no improvement in PaO2/FiO2 ratio. At the same time, the increase of CRP and LDH, which would indicate the ineffective response to treatment and the high risk of poor prognosis, was also significantly higher than that of the survivors' group. Similarly, the research on risk factors of short-term outcome in PCP patients with HIV infection showed that over 90% of patients had an abnormal test of CRP, ESR, PaO2, LDH, and KL-6 (25). In the present study, several laboratory parameters of PCP patients with poor prognosis got worse over the first 7 days in the ICU. Therefore, identifying the sickest patients early and taking the appropriate measures in a timely manner would improve the patient's outcome.
Because of depressive immune status, together with lower lymphocytes and T lymphocyte subsets than normal level, except PCP infection, these patients also had high risks of multiple infections occurring. The research of Huang et al. (26) of PCP patients with acute respiratory failure showed that occurrence of sepsis shock, ventilation related pneumonia, and CMV infection in PCP patients were 36.6, 43.9, and 58.5%, respectively. The result of another study about non-HIV PCP patients with CMV co-infection (27) indicated that fungus co-infection was a risk factor of CMV happening on PCP patients. Our study still found that mixed infections were very common in over 2/3 of patients with PCP, and the occurrence of mixed infection was also associated with poor prognosis. Multiple infections therefore need intense attention among immunocompromised PCP patients. Multilogistic regression analysis also showed NLR was a predictor of poor outcome. The elevation of NLR may be correlated with mixed infection and progress of the disease. The parameters of neutrophil and lymphocyte can be gained easily from regular daily blood test, so NLR would be a convenient and effective indicator which can be used to assess the patients' condition and prognosticate an outcome dynamically. However, there still need to be more prospective research to confirm its accuracy.
There is a need to mention that none of the patients in this study had received prophylaxis with sulfamethoxazole-trimethoprim, which would contribute to an occurrence of PCP in these patients. Almost similarly, the prophylaxis rates were all <20% in several previous studies on patients with PCP and non-HIV infection (21, 23, 28). Importantly, PCP prevention therapy can significantly reduce the morbidity and mortality of patients with PCP and HIV or patients with PCP but without HIV infection (29). There were already several clinical practice guidelines issued which suggest that the kidney transplant recipients and hematological malignancy patients should conduct PCP prophylaxis (30, 31). It is important that physicians should realize that non-HIV immunocompromised patients are also at risk of PCP and that rapid diagnosis and early initiation of treatment can lead to better prognosis. More attention is still needed to be paid on PCP prophylaxis among those non-HIV-infection populations with immunosuppression.
Our study had several limitations. First, the subjects were recruited from a single medical center, and the number of study participants was relatively small. Second, this was a retrospective study, some biases (such as selective bias) may influence the accuracy of our research outcome. Third, despite the combined use of clinical symptoms, radiographic findings, and mNGS test for PCP diagnosis, the possibility of including patients with P. jirovecii colonization cannot be completely eliminated. Finally, the study participants were all enrolled from ICU, so the patients' condition was critical and severely ill. Therefore, our research outcome may be inapplicable for mild patients. Further studies are still needed among patients with PCP.
In conclusion, mNGS is a very sensitive diagnostic tool for identifying P. jirovecii inpatients who are non-HIV immunocompromised. PCP in patients who are non-HIV-infected is associated with a high rate of multiple infections and severe condition. Mixed infection and NLR were the independent risk factors of poor prognosis.
Data Availability Statement
The data presented in the study are deposited in the NCBI repository. Accession numbers, SRA: SRP356529, Bioproject: PRJNA782857.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Committee of The First Affiliated Hospital of Zhengzhou University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JD and JG participated in the research design, data analysis, and writing of the paper. QL and MS participated in data analysis and revising of the paper. YL and YT participated in the improving and revising of the paper. LX provided substantial advice in designing the study and assisting in the division of labor, writing, and revising the paper. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (82074212) and Key Medical Technologies R&D Program of Henan Province (SB201901036).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis. (2017) 17:e334–43. doi: 10.1016/S1473-3099(17)30303-1
2. Izadi M, Jafari NJ, Sadraei J, Poornaki AM, Rezavand B, Zarrinfar H, et al. The prevalence of Pneumocystis jiroveci in bronchoalveolar lavage specimens of lung transplant recipients examined by the nested PCR. Jundishapur J Microbiol. (2014) 7:e13518. doi: 10.5812/jjm.13518
3. Thomas CF Jr., Limper AH. Pneumocystis pneumonia. N Engl J Med. (2004) 350:2487–98. doi: 10.1056/NEJMra032588
4. Fishman JA. Pneumocystis jiroveci. Semin Respir Crit Care Med. (2020) 41:141–57. doi: 10.1055/s-0039-3399559
5. Pegorie M, Denning DW, Welfare W. Estimating the burden of invasive and serious fungal disease in the United Kingdom. J Infect. (2017) 74:60–71. doi: 10.1016/j.jinf.2016.10.005
6. Reid AB, Chen SC, Worth LJ. Pneumocystis jirovecii pneumonia in non-HIV-infected patients: new risks and diagnostic tools. Curr Opin Infect Dis. (2011) 24:534–44. doi: 10.1097/QCO.0b013e32834cac17
7. Tasaka S. Recent advances in the diagnosis and management of pneumocystis pneumonia. Tuberc Respir Dis. (2020) 83:132–40. doi: 10.4046/trd.2020.0015
8. Limper AH, Offord KP, Smith TF, Martin WJ II. Pneumocystis carinii pneumonia. Differences in lung parasite number inflammation in patients with without AIDS. Am Rev Respir Dis. (1989) 140:1204–9. doi: 10.1164/ajrccm/140.5.1204
9. Procop GW, Haddad S, Quinn J, Wilson ML, Henshaw NG, Reller LB, et al. Detection of Pneumocystis jiroveci in respiratory specimens by four staining methods. J Clin Microbiol. (2004) 42:3333–5. doi: 10.1128/JCM.42.7.3333-3335.2004
10. Goldberg B, Sichtig H, Geyer C, Ledeboer N, Weinstock GM. Making the leap from research laboratory to clinic: challenges and opportunities for next-generation sequencing in infectious disease diagnostics. mBio. (2015) 6:e01888–15. doi: 10.1128/mBio.01888-15
11. Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. (2019) 20:341–55. doi: 10.1038/s41576-019-0113-7
12. Han D, Li R, Shi J, Tan P, Zhang R, Li J. Liquid biopsy for infectious diseases: a focus on microbial cell-free DNA sequencing. Theranostics. (2020) 10:5501–13. doi: 10.7150/thno.45554
13. Wu X, Li Y, Zhang M, Li M, Zhang R, Lu X, et al. Etiology of severe community-acquired pneumonia in adults based on metagenomic next-generation sequencing: a prospective multicenter study. Infect Dis Ther. (2020) 9:1003–15. doi: 10.1007/s40121-020-00353-y
14. Zhan Y, Xu T, He F, Guan WJ, Li Z, Li S, et al. Clinical evaluation of a metagenomics-based assay for pneumonia management. Front Microbiol. (2021) 12:751073. doi: 10.3389/fmicb.2021.751073
15. Miao Q, Ma Y, Wang Q, Pan J, Zhang Y, Jin W, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. (2018) 67:S231–40. doi: 10.1093/cid/ciy693
16. Chen J, He T, Li X, Wang X, Peng L, Ma L. Metagenomic next-generation sequencing in diagnosis of a case of Pneumocystis jirovecii pneumonia in a kidney transplant recipient and literature review. Infect Drug Resist. (2020) 13:2829–36. doi: 10.2147/IDR.S257587
17. Xie Y, Du J, Jin W, Teng X, Cheng R, Huang P, et al. Next generation sequencing for diagnosis of severe pneumonia: China, 2010-2018. J Infect. (2019) 78:158–69. doi: 10.1016/j.jinf.2018.09.004
18. Wilson MR, Sample HA, Zorn KC, Arevalo S, Yu G, Neuhaus J, et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med. (2019) 380:2327–40. doi: 10.1056/NEJMoa1803396
19. Pan T, Tan R, Qu H, Weng X, Liu Z, Li M, et al. Next-generation sequencing of the BALF in the diagnosis of community-acquired pneumonia in immunocompromised patients. J Infect. (2019) 79:61–74. doi: 10.1016/j.jinf.2018.11.005
20. Jiang J, Bai L, Yang W, Peng W, An J, Wu Y, et al. Metagenomic next-generation sequencing for the diagnosis of Pneumocystis jirovecii pneumonia in non-HIV-infected patients: a retrospective study. Infect Dis Ther. (2021) 10:1733–45. doi: 10.1007/s40121-021-00482-y
21. Gaborit BJ, Tessoulin B, Lavergne RA, Morio F, Sagan C, Canet E, et al. Outcome and prognostic factors of Pneumocystis jirovecii pneumonia in immunocompromised adults: a prospective observational study. Ann Intensive Care. (2019) 9:131. doi: 10.1186/s13613-019-0604-x
22. Choi JS, Lee SH, Leem AY, Song JH, Kim SY, Chung KS, et al. Pneumocystis jirovecii pneumonia (PCP) PCR-negative conversion predicts prognosis of HIV-negative patients with PCP and acute respiratory failure. PLoS ONE. (2018) 13:e0206231. doi: 10.1371/journal.pone.0206231
23. Schmidt JJ, Lueck C, Ziesing S, Stoll M, Haller H, Gottlieb J, et al. Clinical course, treatment and outcome of Pneumocystis pneumonia in immunocompromised adults: a retrospective analysis over 17 years. Crit Care. (2018) 22:307. doi: 10.1186/s13054-018-2221-8
24. Yoshida A, Kamata N, Yamada A, Yokoyama Y, Omori T, Fujii T, et al. Risk factors for mortality in Pneumocystis jirovecii pneumonia in patients with inflammatory Bowel Disease. Inflamm Intest Dis. (2019) 3:167–72. doi: 10.1159/000495035
25. Li AX, Huang CY, Zhang HW, Zhang T, Wu H, Wang W. Research on risk factors of short-term outcome in AIDS patients with pneumocystis pneumonia. Natl Med J China. (2017) 97:833–7. doi: 10.3760/cma.j.issn.0376-2491.2017.11.008
26. Huang X, Weng L, Yi L, Li M, Feng Y, Tian Y, et al. Acute respiratory failure due to Pneumocystis pneumonia in connective tissue disease patients: clinical manifestation and prognostic factors related to hospital mortality. Zhonghua Jie He He Hu Xi Za Zhi. (2018) 41:196–200. doi: 10.3760/cma.j.issn.1001-0939.2018.03.010
27. Jiang H, Zhang QB, Zhu HD. Analysis of risk factors of non HIV Pneumocystis jirovecii pneumonia patients complicated with cytomegaloviremia. Chin J Emerg Med. (2018) 27:1004–9. doi: 10.3760/cma.j.issn.1671-0282.2018.09.010
28. Dunbar A, Schauwvlieghe A, Algoe S, Hellemond J, Reynders M, Vandecasteele S, et al. Epidemiology of Pneumocystis jirovecii pneumonia and (non-)use of prophylaxis. Front Cell Infect Microbiol. (2020) 10:224. doi: 10.3389/fcimb.2020.00224
29. Lim PL, Zhou JL, Ditangco RA, Law MG, Sirisanthana T, Kumarasamy N, et al. Failure to prescribe Pneumocystis prophylaxis is associated with increased mortality, even in the cART era: results from the Treat Asia HIV observational database. J Int AIDS Soc. (2012) 15:1–9. doi: 10.1186/1758-2652-15-1
30. Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. (2010) 77:299–311. doi: 10.1038/ki.2009.377
31. Maertens J, Marchetti O, Herbrecht R, Cornely OA, Flückiger U, Frêre P, et al. Third European Conference on Infections in Leukemia. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3–2009 update. Bone Marrow Transplant. (2011) 46:709–18. doi: 10.1038/bmt.2010.175
Keywords: immunocompromised, Pneumocystis jirovecii, pneumonia, non-HIV, mNGS
Citation: Duan J, Gao J, Liu Q, Sun M, Liu Y, Tan Y and Xing L (2022) Characteristics and Prognostic Factors of Non-HIV Immunocompromised Patients With Pneumocystis Pneumonia Diagnosed by Metagenomics Next-Generation Sequencing. Front. Med. 9:812698. doi: 10.3389/fmed.2022.812698
Received: 10 November 2021; Accepted: 20 January 2022;
Published: 03 March 2022.
Edited by:
Lei Chen, Shanghai Jiao Tong University School of Medicine, ChinaReviewed by:
Mark Poritz, Idaho Molecular Inc., United StatesHossein Zarrinfar, Mashhad University of Medical Sciences, Iran
Copyright © 2022 Duan, Gao, Liu, Sun, Liu, Tan and Xing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihua Xing, eGluZ2xpaHVhOTUwODhAMTYzLmNvbQ==
 Jiali Duan
Jiali Duan Jing Gao
Jing Gao