
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 28 March 2022
Sec. Ophthalmology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.808409
This article is part of the Research TopicClinical Application and Development of Ocular ImagingView all 47 articles
Purpose: The aim of this study was to investigate changes in the retinal and choroidal thickness between high myopic amblyopia (HMA), low myopia (LM), moderate myopia (MM), high myopia (HM), and normal group (NG) using a spectral-domain optical coherence tomography (SD-OCT).
Materials and Methods: A total of 75 Chinese children (128 eyes; mean age 10.5 years) were recruited. Retinal thickness (RT) and choroidal thickness (CT) were measured at different locations including subfoveal (SF), and at 0.5 mm/1.0 mm/1.5 mm/2.0 mm/2.5 mm/3.0 mm to the fovea in superior, nasal, inferior, and temporal sectors using enhanced depth imaging (EDI) system of SD-OCT. Axial length (AL), best-corrected visual acuity (BCVA), and refraction errors were also collected.
Results: No significant differences were found in subfoveal retinal thickness (SFRT). Moreover, a significantly thinner subfoveal choroidal thickness (SFCT) was found in HMA compared to NG, LM, and MM, but not compared to HM. RT at 0.5 mm to fovea, HMA was significantly thinner compared to LM and MM in the three sectors (superior, inferior, and temporal). Nevertheless, no significant differences were found compared to NG and HM. CT at 0.5 mm to fovea, HMA was the significantly thinnest in all four sectors compared to NG, LM, and MM. RT at 1.0 mm/1.5 mm/2.0 mm/2.5 mm/3.0 mm to fovea, HMA was thinner compared to NG, LM, and MM. CT at 1.0 mm/1.5 mm/2.0 mm/2.5 mm/3.0 mm to fovea, HMA was thinner compared to NG, LM, and MM. At the superior and inferior sectors, HMA showed to be statistically thinner compared with HM. Moreover, SFCT in the HMA, HM, and NG were negatively correlated with AL.
Conclusions: Thinner retina and choroidal tissue appear to be related to HMA, and thus can be used as useful parameters for discovering the underlying mechanisms of the disease.
Amblyopia is a neuro-developmental disorder of the visual cortex, caused by visual deprivation or abnormal binocular interactions, including unilateral strabismus, no corrective anisometropia, and high refractive error (1). Amblyopia is the most common cause of vision loss in children and adolescents, with an estimated incidence of 1–3.5% worldwide (2). Early detection and treatment offer the best outcome. If not detected and treated early in life, amblyopia can cause a permanent loss of vision (3). Compared to unilateral amblyopia, such as anisometropic amblyopia and strabismic amblyopia that offer good treatment outcomes especially at younger age (4), refractive correction of amblyopia combined with high myopia (HM) is a difficult clinical problem. Currently, there is no satisfactory surgical procedure for the correction of high myopic amblyopia (HMA).
It has been reported that the pathogenesis of amblyopia may involve various levels of visual pathways, such as the visual cortex, lateral geniculate nucleus, retina, and optic nerve (5, 6). Meanwhile, retinal abnormalities mainly include retinal photoreceptor cells, retinal pigment epithelium (RPE), retinal nerve fiber layer, and optic nerve abnormalities (5–7). Choroid is an important tissue structure in the eyeball, located in the outer layer of the retina, which provides nutrients and oxygen to the retinal pigment cells and the optic nerve (8). Previous studies have suggested that changes in retinal and choroidal thickness may be involved in amblyopia. However, the results are disputed (9, 10).
With the development of optical coherence tomography (OCT) technology, it has become possible to measure and analyze the choroidal thickness (CT) (11). Over the recent years, many studies have investigated the changes in CT in various eye diseases. Pachychoroid is a relatively new concept describing a phenotype characterized by attenuation of the choriocapillaris overlying dilated choroidal veins, and progressive retinal pigment epithelium dysfunction and neovascularization are also present (12). Pachychoroid disease spectrum includes (12–14) central serous chorioretinopathy (CSC) (12–15), pachychoroid neovasculopathy (PNV) (12–14), peripapillary pachychoroid syndrome (PPS) (12–14), polypoidal choroidal vasculopathy (PCV) (12–14, 16) /aneurysmal type 1 neovascularization (AT1) (12–14), focal choroidal excavation (FCE) (12–14), and pachychoroid pigment epitheliopathy (PPE) (12–14, 17). These images and data suggest that choroid has an important role in the occurrence and development of ophthalmic diseases. Similarly, choroid is closely related to axial length (AL) and refractive error. Myopia is associated with a higher spherical equivalent (SE), a longer AL, and thinner CT (18). Teberik et al. (19) have reported that subfoveal choroidal thickness (SFCT) was significantly thinner in the HM group than the healthy subjects (p < 0.001). However, according to our knowledge, there are no studies that have examined the retinal thickness (RT) and CT in HMA.
The aim of this study was to investigate changes in the retina and choroid from among HMA, low myopia (LM), moderate myopia (MM), HM, and normal group (NG) using spectral-domain optical coherence tomography (SD-OCT).
This study was approved by the local ethics committee of Guangzhou hospital of integrated traditional and western medicine and was in accordance with the principles of the Declaration of Helsinki. All subjects and their guardians provided written informed consent.
A total of 75 Chinese children (128 eyes) were recruited from the department of ophthalmology at Guangzhou hospital of integrated traditional and western medicine, between July 2014 and November 2014 (Table 1). The subjects were aged between 4 and 15 years. All participants underwent a comprehensive ophthalmic examination, including visual acuity (VA), slit-lamp biomicroscopy, cycloplegic refraction, dilated fundus examination with indirect ophthalmoscopy, and A-scan ultrasound biometry for measuring AL. In addition, each participant had no history of other ocular diseases, and a detailed medical history was also obtained.
Visual acuity was measured using LogMAR E chart, and refractive (spherical equivalent, SE) error was determined using retinoscopy with cyclopentolate 1%. All subjects had astigmatism ≤1.0 D without strabismus. For NG (22 eyes), VA should be ≥0.6 for children aged 4 years, ≥0.8 for children aged 5–6 years, and 1.0 or better for children aged 7 years and above. The SE was between −0.5 D and −3.0 D for LM, between −3.0 D and −6.0 D for MM, and more than 6.0 D for HM. Amblyopia is usually classified as BCVA should be < 0.5 for children aged 4, < 0.6 for children aged 5–6 < 0.8 for children aged 7 and above, and an interocular difference in SE of less than 2.0 D in the same child.
All subjects with dilated pupils were examined using enhanced depth imaging (EDI) system of SD-OCT (Heidelberg Engineering, Heidelberg, Germany). All imaging data were collected by a single skilled technician during the day, but not at the same time due to randomness of the subjects' visits. Each image was averaged for 100 scans using the automatic averaging and eye tracking characteristics. The RT was measured from the outer portion of the hyperreflective line corresponding to the internal limiting membrane (ILM) to the retinal pigment epithelium (RPE) (Figure 1). CT was measured from the outer portion of the hyperreflective line corresponding to RPE to the inner surface of the sclera (Figure 2). These RT and CT were measured at the subfoveal and at 500 μm intervals from the fovea to 3 mm superior, nasal, inferior, and temporal sectors (20).
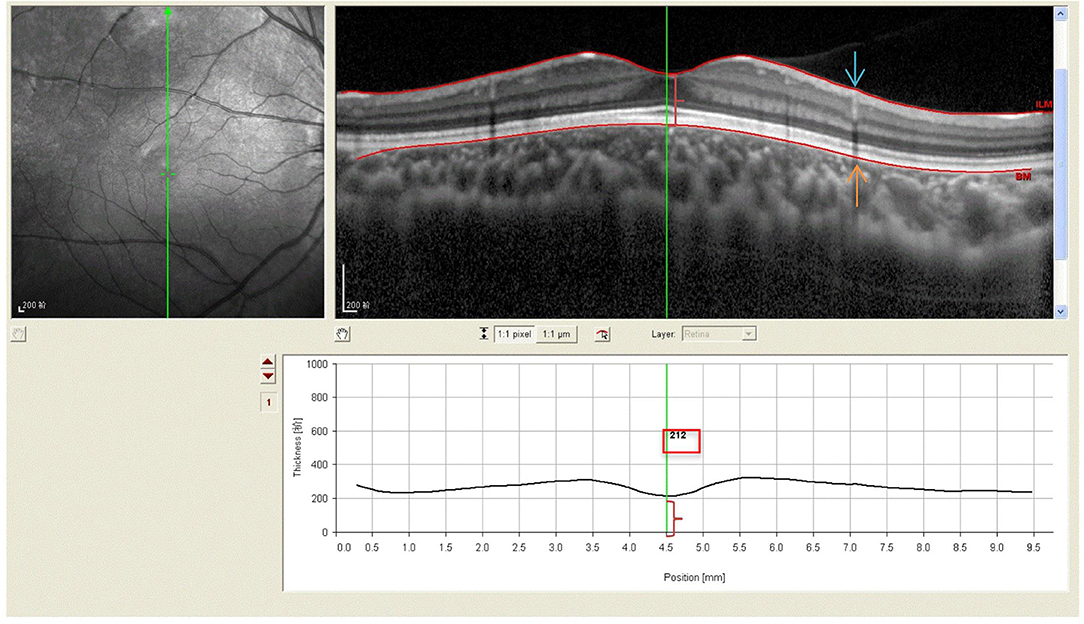
Figure 1. Spectral-domain optical coherence tomography (SD-OCT) scans showing retinal thickness of high myopic amblyopic eyes. (Top left) Fundus image showing vertical scan lines go through the fovea in 6-year-old subject. (Top right) The retinal thickness (red brace) was defined from ILM (redline, blue arrow) to the basal aspect of the RPE (redline, yellow arrow). (Bottom) The retinal thickness 212 μm (red brace, red boxes) was measured vertically at the subfoveal by Heidelberg own software, and at 500 μm intervals from the fovea to 3 mm superior, inferior.
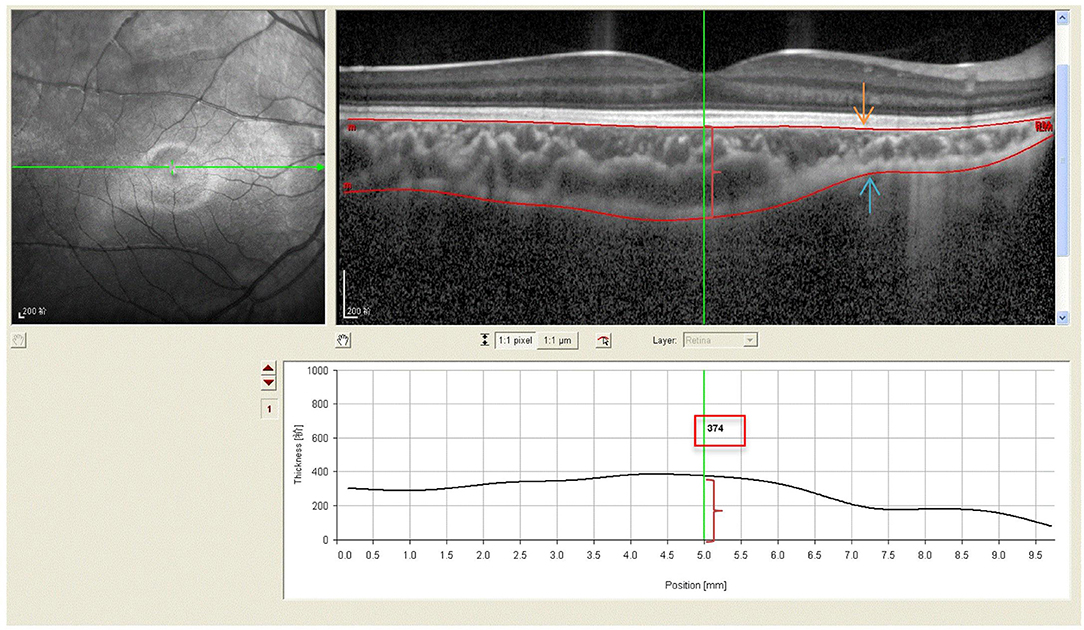
Figure 2. Spectral-domain optical coherence tomography (SD-OCT) scans showing choroidal thickness of high myopic amblyopic eyes. (Top left) Fundus image showing horizontal scan lines go through the fovea in 6-year-old subject. (Top right) The choroidal thickness (red brace) was defined from the basal aspect of the RPE (redline,yellow arrow) to the outer border of the choroid (redline, blue arrow). (Bottom) The choroidal thickness 374 μm (red brace, red boxes) was measured vertically at the subfoveal by Heidelberg own software, and at 500 μm intervals from the fovea to 3 mm nasal, temporal.
All data were analyzed with an analysis software program (SPSS 18.0; SPSS, Inc., Chicago, IL). All data were reported as mean ± standard deviation (SD), with a 95% confidence interval (CI). Analysis of variance (ANOVA) was used to analyze differences in retinal and choroidal thickness among normal, LM, MM, and HMA. Pearson correlation was used to analyze the relationship between subfoveal RT (SFRT), SFCT, and axial length, and gender. A value of p < 0.05 was considered statistically significant.
A total of 75 Chinese children (128 eyes) were recruited in this study, including 22 NG eyes, 37 LM eyes, 22 MM eyes, 22 HM eyes, and 25 HMA eyes. The mean age of the participants was 10.5 years (range, 4–15 years), and there were 45 males (60%) and 30 females (40%). With reference to RT, no significant differences in subfoveal region were observed between HMA and the other four groups (all p > 0.05) (Tables 2, 3). However, at 0.5 mm, RT in the superior, inferior, and temporal sectors were thinner in the HMA patients compared with the LM and MM groups (p < 0.05), while no significant differences were observed compared to the NG and HM group (p > 0.05). At 1.0 mm, RT was significantly thinner in all four sectors in HMA compared to the LM and MM groups (p < 0.05). Meanwhile, compared to the NG, RT was significantly thinner in the inferior, nasal, and temporal sectors (p = 0.000) in HMA, while no significant differences were observed for the superior sector. In addition, in HMA, RT was thinner in inferior sector compared to the HM group (p < 0.05). At 1.5 mm (except in the superior sector) and at 2.0 mm (except in the temporal sector) no significant differences were found between HMA and other groups, and the other measurement points were the thinnest compared with the other four groups (p < 0.05). At 2.5 mm, no difference in the RT of the inferior and temporal sectors were found between HM and MM groups (p > 0.05). At 3.0mm, HMA showed statistically significant thinning compared with NG, LM, and MM in all four directions (P <0.05), and there was also significant thinning compared with HM in inferior(P < 0.05).
The CT at the subfoveal and 0.5 mm/1.0 mm/1.5 mm/2.0 mm/2.5 mm/3.0 mm in the four directions (superior/nasal/inferior/temporal) was significantly thinner in HMA compared with NG, LM, and MM groups (p = 0.000) (Tables 2, 4). In addition, compared with HM, CT in HMA was also significantly thinner at 1.0 mm/1.5 mm/2.0 mm/2.5 mm/3.0 mm in superior and inferior sectors (p < 0.05); nevertheless, there was no significant difference in the nasal and temporal sectors (p > 0.05).
As shown in Tables 5, 6, in the five groups, SFRT and SFCT were not correlated with age and sex, and there was no significant correlation between SFRT and AL (Table 7). SFCT in HMA, HM, and NG was negatively correlated with AL. In addition, the SFCT in HMA (r = −0.531; p = 0.013) showed a correlation with AL for the subfoveal location, similar to NG (r = −0.538; p = 0.010). Compared with LM (r = −0.334; p = 0.050), MM (r = −0.353; P = 0.108), and HM (r = −0.483; p = 0.036), SFCT in HMA (r = −0.531; p = 0.013) showed a negative correlation with AL (Figure 3, Table 8).
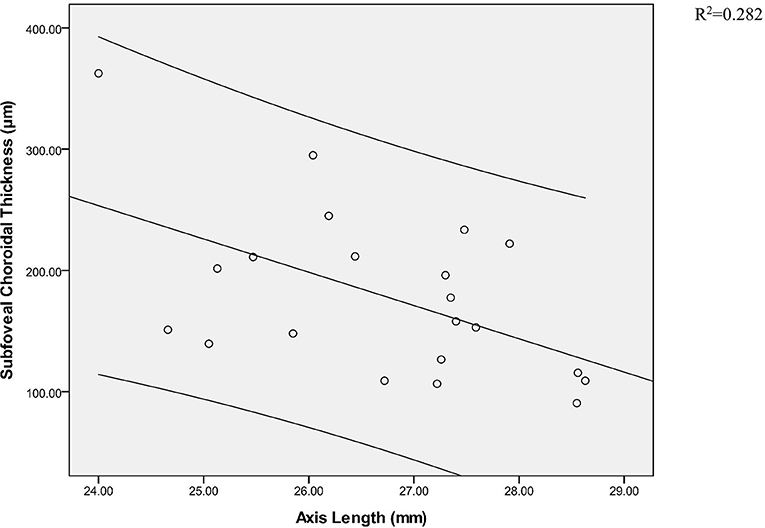
Figure 3. Pearson correlation analysis of subfoveal choroidal thickness and axial axis in high myopic amblyopic eyes.
High myopic amblyopia is a special type of amblyopia that has received little attention. In this study, we used EDI-OCT to evaluate the mean RT and CT in different refractive eyes.
Previous studies have reported that RT showed variations by sex and age, and CT can be significantly influenced by age and AL in normal eyes (21, 22). Nevertheless, in this study, we found no correlation between SF RT/SFCT and age (Tables 5, 6) in NG, as well as those with LM, MM, or HMA. In addition, no significant correlation between SFRT and AL was observed between the groups (Table 7). However, SFCT was negatively correlated with AL in NG, HM, and HMA (Table 8). Furthermore, after examining the RT of 0.5 mm to fovea, HMA was the significantly thinnest compared to LM and MM in three sectors (superior, inferior, and temporal); nevertheless, no significant differences were found compared to HM. In addition, no statistical difference at SFRT was found between HMA and other groups. Our data was not consistent with previous reports. Pang et al. (10) examined 31 amblyopic eyes with HM and found significant changes in macular thickness (MT) between amblyopic eyes and normal fellow eyes. Moreover, similar data were reported by Araki et al. (23), who measured 46 amblyopic eyes (31 with myopic anisometropia and 15 with hyperopic anisometropia). Average MT was calculated in the center, inner, and outer macular regions, and the normal eyes did not have SE grading, which might explain the difference in their results.
Although the area of 1 mm diameter range around the fovea is the main imaging area for central vision, our results suggested that the structure of the retina cannot fully reflect its function, and we should look at the problem as a whole rather than locally. In this study, we found that at 1.0 mm/1.5 mm/2.0 mm/2.5 mm/3.0 mm to fovea, HMA has thinner RT in all four sectors compared to NG, LW, and MM, except for two points. RT of HMA was thinner than HM at each measurement point in the four directions, and was more significant in the inferior, with statistical difference. This may be due to particularity of HMA, in which retinal structure itself is different. It might also be that the retinal tissue structure becomes thinner as the AL increases. According to our current study, there was no difference between HMA and HM on AL, while the treatment effect was poor in HMA. BCVA was below normal, which induced the question whether reducing RT to a certain extent would affect VA.
Yoon et al. (24) and Dickmann et al. (25) have investigated different types of amblyopia and found no difference in MT between the amblyopic and normal fellow eyes. In addition, Park and his team (26) reveal differences between amblyopic and fellow eyes in the thickness of some retinal layers, including a notable difference in the ganglion cell layer plus inner plexiform layer. Although, these studies included subjects with hyperopic amblyopia, whereas in the current study, we examined subjects with myopia and myopic amblyopia. In this study, participants with HMA were compared to HM, spherical equivalent (SE) refractive errors of these eyes was less than −6.0 D and no significant difference in AL was found between these two groups. Nonetheless, RT at most of the measurement points was statistically thinner (especially in the inferior sector) in the HMA compared to HM.
Furthermore, we found that HMA has thinner CT compared to NG, LW, and MM. In addition, at 1.0 mm/1.5 mm/2.0 mm/2.5 mm/3.0 mm in superior and inferior sectors, HMA showed statistically thinner CT compared with HM, while no significant difference between HMA and HM was observed at subfoveal and 1.0 mm/1.5 mm/2.0 mm/2.5 mm/3.0 mm in nasal and temporal sectors. Spaide et al. (11) used EDI technique to examine the SFCT in 17 normal subjects (average age was 33.4 years) and found that SFCT was 318 μm in right eyes and 335 μm in left eyes. Moreover, Margolis and Spaide (20) reported an SFCT of 287 μm in 30 subjects with an average age of 50.4 years, where nasal thickness rapidly decreased, thus reaching a minimum mean of only 145 μm at 3 mm nasal to the fovea. Ding et al. (27) have reported that SFCT was 261.93 μm in 210 volunteers (mean age, 49.73 years). Our present study showed that SFCT was 330.20 μm in 19 normal Chinese children subjects (22 eyes) with an average age of 10.18 ± 2.63 years, 262.68 μm at 3 mm of temporal sector, and 302.09 μm at 3 mm of nasal sector. Our results differed from previous studies in that our subjects were significantly younger than the average age of the study population, and choroid thickness thins with age.
With reference to the hyperopic amblyopi a, a large number of previous articles (28–32) have reported that SFCT with hyperopic anisometropic amblyopia is significantly thicker compared to the fellow eye and the age-matched controls. Yet, only a few studies have suggested that CT is related to the occurrence of amblyopia (29, 31, 32). Xu and colleagues (31) have suggested that SFCT in amblyopic eyes negatively correlated with AL, but did not correlate with SE, VA, or age. However, Xu et al. (31) did not describe the RT. Moreover, Araki et al. (23) found that in the strabismic amblyopia group, there was no significant difference in the mRNFL, GCL+IPL, GCC thicknesses and CT (subfovea, center 1 mm, nasal and inferior of the inner ring, nasal of the outer ring, and center 6 mm) among the amblyopic, fellow, and control eyes."
In this study, obviously thinner CT was observed at 1.0 mm/1.5 mm/2.0 mm/2.5 mm/3.0 mm in the superior and inferior sectors of HMA compared with HM. In addition, the CT was thinner in the inferior sector compared to the superior one. At the same time, at the 0.5 mm area outside the fovea in the inferior sector, HMA had thinner RT compared to HM, while no significant difference in SFCT and SFRT were observed between the two groups. This was a novel finding that was not consistent with previous research. Consequently, our data suggested that CT thinning is associated with RT thinning in HMA, and amblyopia influences RT and CT. Thus, thinner choroid supplies less blood to the outer retina, resulting in thinning of the retina. In our current study, we used different measurements, which is why the obtained data do not adequately reflect the areas. Therefore, future studies should use SD-OCT to measure RT and CT in four directions in HMA.
In the present study, changes in the RT and CT in HMA were the most remarkable in inferior area, except for the 0.5 mm measurement point. However, this still does not explain why HMA occurs.
Previous reports have disputed about CT before and after treatment for amblyopia. Araki et al. (33) have reported that there were no significant changes in SFCT, center 1 mm CT, or center 6 mm CT before and after treatment in the amblyopic and fellow eyes. In addition, Liu et al. (34) have used a meta-analysis and reported that the SFCT in unilateral amblyopia was thicker than that in the fellow and control eyes. In the current study, we did not observe the CT before and after the treatment of HMA, which should be addressed in future studies. On the other hand, Pang and his team (35) have reported that contrast sensitivity function at the middle and higher frequencies was reduced in the amblyopic eyes associated with myopic anisometropia compared to the fellow eyes with high myopia. Therefore, further studies on visual function are also necessary.
This study has the following limitations: First, it is difficult to match HMA and HM in the same subject. If HMA and HM can be identical with different eyes of the subject, the errors caused by individual differences can be excluded. Second, there were fewer subjects in the study. Third, no MRI was performed to rule out visual abnormalities.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of Guangzhou Hospital of Integrated Traditional and Western Medicine. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This study was supported by Guangzhou Huadu District Science and Technology Project of China (14-HDWS-026).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
HMA, high myopic amblyopia; LM, low myopia; MM, moderate myopia; HM, high myopia; NG, normal group; SD-OCT, spectral-domain optical coherence tomography; RT, retinal thickness; CT, choroidal thickness; EDI, enhanced depth imaging; AL, axial length; BCVA, best-corrected visual acuity; SF, subfoveal; MT, macular thickness.
1. Levi DM. Rethinking amblyopia 2020. Vision Res. (2020) 176:118–29. doi: 10.1016/j.visres.2020.07.014
2. Holmes JM, Clarke MP. Amblyopia. J Lancet. (2006) 367:1343–51. doi: 10.1016/S0140-6736(06)68581-4
4. Caputo R, Frosini R, De LC, Campa L, Del Magro EF, Secci J. Factors influencing severity of and recovery from anisometropic amblyopia. J Strabismus. (2007) 15:209–14. doi: 10.1080/09273970701669983
5. Barnes GR, Li X, Thompson B, Singh KD, Dumoulin SO, Hess RF. Decreased gray matter concentration in the lateral geniculate nuclei in human amblyopes. Invest Ophthalmol Vis Sci. (2010) 51:1432–8. doi: 10.1167/iovs.09-3931
6. von Noorden GKV. Histological studies of the visual system in monkeys with experimental amblyopia. J Invest Ophthalmology. (1973) 12:727–38.
7. Crawford MLJ, Von Noorden GK. Optically induced concomitant strabismus in monkeys. Invest Ophthalmol Vis Sci. (1980) 19:1105–9.
8. Linsenmeier RA, Padnick-Silver L. Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci. (2000) 41:3117–23.
9. Al-Haddad CE, Mollayess GM, Cherfan CG, Jaafar D, Bashshur Z. Retinal nerve fibre layer and macular thickness in amblyopia as measured by spectral-domain optical coherence tomography. Br J Ophthalmol. (2011) 95:1696–9. doi: 10.1136/bjo.2010.195081
10. Pang Y, Goodfellow GW, Allison C, Block S, Frantz KA. A prospective study of macular thickness in amblyopic children with unilateral high myopia. Invest Ophthalmol Vis Sci. (2011) 52:2444–9. doi: 10.1167/iovs.10-5550
11. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. (2008) 146:496–500. doi: 10.1016/j.ajo.2008.05.032
12. Cheung CMG, Lee WK, Koizumi H, Dansingani K, Lai TYY, Freund KB. Pachychoroid disease. Eye. (2019) 33:14–33. doi: 10.1038/s41433-018-0158-4
13. Borooah S, Sim PY, Phatak S, Moraes G, Wu CY, Cheung CMG, et al. Pachychoroid spectrum disease. Acta Ophthalmol. (2021) 99:e806–22. doi: 10.1111/aos.14683
14. Bo Q, Yan Q, Shen M, Song M, Sun M, Yu Y, et al. Appearance of polypoidal lesions in patients with polypoidal choroidal vasculopathy using swept-source optical coherence tomographic angiography. JAMA Ophthalmol. (2019) 137:642–50. doi: 10.1001/jamaophthalmol.2019.0449
15. Chung YR, Kim JW, Kim SW, Lee K. Choroidal thickness in patients with central serous chorioretinopathy: assessment of haller and sattler layers. J Retina. (2016) 36:1652–7. doi: 10.1097/IAE.0000000000000998
16. Padrón-Pérez N, Arias L, Rubio M, Lorenzo D, García-Bru P, Català-Mora J, et al. Changes in choroidal thickness after intravitreal injection of anti-vascular endothelial growth factor in pachychoroid neovasculopathy. Invest Ophthalmol Vis Sci. (2018) 59:1119–24. doi: 10.1167/iovs.17-22144
17. Sakurada Y, Fragiotta S, Leong BCS, Parikh R, Hussnain SA, Freund KB. Relationship between choroidal vascular hyperpermeability, choriocapillaris flow density, and choroidal thickness in eyes with pachychoroid pigment epitheliopathy. Retina. (2020) 40:657–62. doi: 10.1097/IAE.0000000000002635
18. El-Shazly AA, Farweez YA, ElSebaay ME, Elzawahry WMA. Correlation between choroidal thickness and degree of myopia assessed with enhanced depth imaging optical coherence tomography. Eur J Ophthalmol. (2017) 27:577–84. doi: 10.5301/ejo.5000936
19. Teberik K, Kaya M. Retinal and choroidal thickness in patients with high myopia without maculopathy. Pak J Med Sci. (2017) 33:1438–43. doi: 10.12669/pjms.336.13726
20. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. (2009) 147:811–5. doi: 10.1016/j.ajo.2008.12.008
21. Ooto S, Hangai M, Yoshimura N. Effects of sex and age on the normal retinal and choroidal structures on optical coherence tomography. J Curr Eye Res. (2015) 40:213–25. doi: 10.3109/02713683.2014.952828
22. Wang J, Gao X, Huang W, Wang W, Chen S, Du S, et al. Swept-source optical coherence tomography imaging of macular retinal and choroidal structures in healthy eyes. J BMC Ophthalmology. (2015) 15:122–31. doi: 10.1186/s12886-015-0110-3
23. Araki S, Miki A, Goto K, Yamashita T, Takizawa G, Haruishi K, et al. Macular retinal and choroidal thickness in unilateral amblyopia using swept-source optical coherence tomography. J BMC Ophthalmology. (2017) 17:167–79. doi: 10.1186/s12886-017-0559-3
24. Yoon SW, Park WH, Baek SH, Kong SM. Thicknesses of macular retinal layer and peripapillary retinal nerve fiber layer in patients with hyperopic anisometropic amblyopia. Korean J Ophthalmology. (2005) 19:62–7. doi: 10.3341/kjo.2005.19.1.62
25. Dickmann A, Petroni S, Salerni A, Dell'Omo R, Balestrazzi E. Unilateral amblyopia: An optical coherence tomography study J AAPOS. (2009) 13:148–50. doi: 10.1016/j.jaapos.2008.10.009
26. Park KA, Park DY, Oh SY. Analysis of spectral-domain optical coherence tomography measurements in amblyopia: a pilot study. Br J Ophthalmol. (2011) 95:1700–6. doi: 10.1136/bjo.2010.192765
27. Ding X, Li J, Zeng J, Ma W, Liu R, Li T, et al. Choroidal Thickness in healthy Chinese subjects. Invest Ophthalmol Vis Sci. (2011) 52:9555–60. doi: 10.1167/iovs.11-8076
28. Tenlik A, Güler E, Kulak AE, Totan Y, Dervişogullari MS, Güragaç FB. Evaluation of Choroidal Thickness in Amblyopia Using Enhanced Depth Imaging Optical Coherence Tomography. J Current Eye Res. (2015) 40:1063–7. doi: 10.3109/02713683.2014.971933
29. Aygit ED, Yilmaz I, Ozkaya A, Alkin Z, Gokyigit B, Yazici AT, et al. Choroidal thickness of children's eyes with anisometropic and strabismic amblyopia. J AAPOS. (2015) 19:237–41. doi: 10.1016/j.jaapos.2015.03.013
30. Mori T, Sugano Y, Maruko I, Sekiryu T. Subfoveal choroidal thickness and axial length in preschool children with hyperopic anisometropic amblyopia. J Curr Eye Res. (2015) 40:954–61. doi: 10.3109/02713683.2014.964418
31. Xu J, Zheng J, Yu S, Sun Z, Zheng W, Qu P, et al. Macular choroidal thickness in unilateral amblyopic children. Invest Ophthalmol Vis Sci. (2014) 55:7361–8. doi: 10.1167/iovs.14-14439
32. Kara O, Altintas O, Karaman S, Emre E, Caglar Y. Analysis of choroidal thickness using spectral-domain OCT in children with unilateral amblyopia. J Pediatr Ophthalmol Strabismus. (2015) 52:159–66. doi: 10.3928/01913913-20150311-11
33. Araki S, Miki A, Goto K, Yamashita T, Takizawa G, Haruishi K, et al. Effect of amblyopia treatment on choroidal thickness in hypermetropic anisometropic amblyopia using swept-source optical coherence tomography. J BMC Ophthalmology. (2018) 18:227–32. doi: 10.1186/s12886-018-0894-z
34. Yanli L, Yi D, Kanxing Z. A Meta-Analysis of Choroidal Thickness Changes in Unilateral Amblyopia. J Ophthalmol. (2017) 2017:1–10. doi: 10.1155/2017/4231489
Keywords: myopia, amblyopia, retina thickness, choroid thickness, OCT
Citation: Wan J, Zhang Z and Tian Y (2022) Examination of Macular Retina and Choroidal Thickness in High Myopic Amblyopia Using Spectral-Domain Optical Coherence Tomography. Front. Med. 9:808409. doi: 10.3389/fmed.2022.808409
Received: 03 November 2021; Accepted: 07 February 2022;
Published: 28 March 2022.
Edited by:
Feng Wen, Sun Yat-sen University, ChinaReviewed by:
Zeynep Alkin, Biruni University, TurkeyCopyright © 2022 Wan, Zhang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Tian, MjczMzMyNDcwNkBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.