
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med., 18 May 2022
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.802686
 Zepei Feng1†
Zepei Feng1† Jinwei Zhang2†
Jinwei Zhang2† Weilong Tan3
Weilong Tan3 Chunhui Wang3
Chunhui Wang3 Qiong Chen3
Qiong Chen3 Chao Shen1
Chao Shen1 Haozhi Fan4
Haozhi Fan4 Yun Zhang1
Yun Zhang1 Peng Huang1*
Peng Huang1* Ming Yue5*
Ming Yue5*Background: With the development of direct-acting antiviral agents (DAAs), the research on kidney transplantation from Hepatitis C virus (HCV)-viremic donors to HCV-negative recipients has grown. The objective of this comprehensive analysis was to evaluate the efficacy and safety of DAAs in kidney transplantation from HCV-viremic donors to negative recipients.
Methods: Multiple databases were searched for a systematic and comprehensive up to March 2022. The primary outcomes included the percentage of sustained virological response at week 12 after the end of treatment (SVR12), adverse events (AEs; any grade), and severe adverse events (SAEs) as the endpoints. Publication bias was examined by using the funnel plots and Egger's test.
Results: In total, 16 studies with 454 subjects were included in the study and the pooled estimate of SVR12, AEs, and SAEs rates were 100.0% (95% CI: 99.2-100.0), 1.9%(95%CI: 0.0-4.9), and 0.0% (95%CI: 0.0-1.5). Subgroup analysis showed that pooled SVR12 rates were 100.0% (95%CI: 99.6-100.0) for genotype (GT)1a and 96.3% (95%CI: 83.3-100.0) for GT2; 100.0% (95%CI: 98.9-100.0) for DAAs treatments; and 100.0% (95%CI: 98.2-100.0) for prophylaxis subgroup. Egger's tests showed that no publication bias was found in this study.
Conclusion: This comprehensive analysis showed the high efficacy and safety of DAAs in kidney transplantation from HCV-viremic donors to HCV-negative recipients.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=246541.
Hepatitis C virus (HCV) infection affects around 180 million individuals worldwide, of which around 71 million people develop chronic HCV infections (1, 2). HCV may develop cirrhosis, hepatocellular carcinoma, and liver-related deaths (3), and 40% of the infected population may have extrahepatic manifestations due to HCV such as kidney injury, insulin resistance, and accelerated atherosclerosis (4). Increased HCV transmission on the heels of dramatic increases in opioid use among young adults.
A high rate of HCV infection was found in patients with chronic kidney disease (CKD). Kidney transplantation is the ultimate treatment for patients suffering from end-stage renal disease (ESRD) but is nevertheless limited by donor shortages (5). In the US, increased HCV transmission is occurring owing to the ongoing opioid epidemic, and opioid abuse and overdose have led to an increased supply of HCV-positive kidneys (6, 7). Meanwhile, the prevalence of ESRD has been rising, with around 95,000 candidates are on a waiting list to get a kidney transplant in America (8). According to the official data from the Organ Procurement and Transplantation Network (OPTN), the health of most candidates is gradually deteriorating. Some patients were more likely to die during the waiting time for a kidney, such as patients ≥ 60 years (9). The gap between the number of kidney transplant patients and the number of available organs will gradually enlarge. Actually, a kidney from HCV-positive donors has long remained an underutilized resource in America so far. Every year about 500 HCV-positive kidneys are discarded (10). The high-discard rate of HCV-positive kidneys was a matter of great concern because these kidneys are often younger and have fewer complications than common donors and with a lower kidney donor profile index (KDPI) (11–13). Such discard was largely driven by the previous clinical practice that HCV+ kidneys were provided only for HCV+ recipients, primarily because interferon (IFN)-based therapies were the main method of HCV, which were limited treatment options with poor efficacy and intolerable side effects (14, 15).
Compared with IFN-based therapies, direct-acting antiviral agents (DAAs) are extremely effective and well-tolerated in the general population with an advantageous side-effect profile, with virologic cure rates achieving 99% even among transplant recipients of the solid organs (16–18). The high efficacy and favorable safety of generic DAAs in real-world clinical practice have been evaluated by several international cohorts for all six major HCV genotypes (19, 20). DAAs broaden the range of patients on the transplant waiting list to allow the transplantation of organs from HCV-viremic donors into negative recipients. Various approaches should be taken into account to prevent the consequences of HCV in recipients of kidneys from HCV+ donors.
Some published literature has studied the safety and feasibility of transplanting organs from HCV-viremic donors. For the argument in the transplant community regarding the effects of using preemptive or post-transplantation treatment with DAAs, further research is needed to provide evidence. Our post-hoc analysis aims to evaluate the efficacy and safety of DAAs in kidney transplantation from HCV-viremic donors (D+) to negative recipients (R–).
Our protocol was performed following the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA). This study was registered in the PROSPERO database (CRD42020133457). Two independent reviewers used multiple databases including PubMed, Embase, and Web of Science for a systematic and comprehensive search, which was last updated in March 2022 without language restrictions. The search strategy included (kidney transplant OR renal transplantation OR renal transplantations OR transplantations, renal OR transplantation, renal OR grafting, kidney OR kidney grafting OR transplantation, kidney OR kidney transplantations OR transplantations, kidney) AND (antiviral agents OR agents, antiviral OR antivirals OR antiviral drugs OR drugs, antiviral OR DAA OR direct acting antivirals OR direct acting antiviral) AND (Hepacivirus OR Hepaciviruses OR Hepatitis C-like viruses OR Hepatitis C-like Viruses OR Hepatitis C virus OR Hepatitis C viruses OR HCV). We also manually searched the reference cited in included articles and other relevant systematic reviews for additional appropriate studies to improve the search sensitivity.
Studies were included in the qualitative analysis if they met all the following criteria: (1) recipients were HCV RNA negative at the time of transplant with no evidence of HCV infection by RT-PCR, (2) donors were HCV RNA positive, (3) SVR12 (sustained virological response 12 weeks after the end of treatment) could be measured, and (4) treatment with DAAs (including pre- and post-transplant DAAs therapy).
Studies were excluded if they met any of the following criteria: (1) recipients and/or donors were infected with HIV/HBV, (2) studies without SVR12 data, (3) studies without HCV RNA load, (4) case reports, conferences, meta-analyses, editorials, or reviews, and (5) cost-effectiveness, pharmacokinetics, or pharmacodynamics studies.
The primary outcome was the percentage of sustained virological response at week 12 after the end of treatment (SVR12). SVR12 was defined as plasma HCV RNA < the lower limit of quantification at follow-up of 12 weeks after the end of treatment, which included post-transplant DAAs therapy (post kidney transplantation as positive HCV NAT tests) and pre-transplant DAAs therapy (before virus testing even during transplantation). The secondary outcomes, the percentages of HCV transmission from donors to recipients, were added for pre-transplant DAAs therapies. HCV transmission was defined as positive for HCV RNA in the post-transplant recipient. Stratified by the initiation time of DAAs therapy, HCV transmission rate was divided into the before renal transplant (RT) group and the after RT group for subgroup analysis. The incidence and intensity of adverse events (AEs) and serious adverse events (SAEs) were used to assess safety. Only studies reported the percentages of HCV transmission and AEs/SAEs were analyzed for the secondary outcomes and safety.
Study selection and data extraction were conducted independently by two researchers (ZPF and JWZ). Study selection was performed following the pre-designed inclusion and exclusion criteria. Studies were initially reviewed by titles and abstracts, and then potentially eligible studies were identified and screened again by full texts. The extracted data included the following: the name of the first authors, year, region, study type, publication type, single center or multi-center studies (setting), DAA regimens, the initiation time of DAAs therapy (drug node), duration of DAAs therapy (duration), sample size, the demographics of donors and recipients, waiting time for a kidney transplant (waiting list time), HCV genotype (GT), serum creatinine, glomerular filtration rate (GFR), study outcomes (SVR12, HCV transmission), and safety (AEs/SAEs). When the information or evidence was imprecise, or the opinions of two reviewers were not uniform, the full texts were accessed and discussions were made with a third reviewer.
The quality of included non-randomized studies was assessed by the Methodological Index for Non-Randomized Studies (MINORS), which consists of eight methodological items for non-comparative studies. Each item was scored from 0 to 2, and 16 is the ideal score for non-comparative studies, indicating the highest study quality for non-randomized interventional studies, whereas 24 is the ideal score for comparative studies. The quality of observational studies was assessed using the Newcastle–Ottawa quality assessment scale (NOS) with a total score of nine. Low quality was scored as 0-5 points, moderate quality as 6-7 points, and high quality as 8-9 points. Two investigators independently assessed the quality of each included study.
Effect sizes were collected as pooled event incidences with corresponding 95% CI using the inverse variance method. In the case of 0 or 100% events, we estimated the incidence rate by using the Freeman–Tukey double arcsine transformation. The Cochran Q-statistics and I2 statistics were used to assess the heterogeneity across the included studies. The random-effect model (DerSimonian–Laird Method) was used in case of considerable heterogeneity, which was defined as I2 ≥ 50%; the fixed effects model (Inverse–Variance Method) was used when I2 <50%. Subgroup analysis was used to understand the potential sources of heterogeneity. Publication bias was examined by using the funnel plot and Egger's test. If there is a publication bias, the Duval and Tweedie nonparametric trim and fill analysis will be performed to account for publication bias. The sensitivity analysis was conducted to explore the effect of each study on effect sizes. All the statistical tests were two-sided, with a P-value < 0.05 considered to be statistically significant. All the statistical analyses were performed using the R version 4.0.4.
The initial search identified 3,568 eligible studies. After deleting 1,980 duplicate studies, the titles and abstracts of 1,460 articles were screened. A total of 124 articles were selected for full-text reading and 112 articles were excluded for different reasons. Eventually, 16 articles (21–36) were included in this study (Figure 1). The 16 studies were published during 2018–2022, including 15 full articles and 1 letter, which were conducted in 3 regions: the USA (14 articles), Germany (1 article), and China (1 article). In total, 9 studies conduct DAAs therapies after kidney transplantation when the recipients detected positive HCV RNA; 7 studies started DAAs prophylaxis therapies before or during the transplantation. The total number of study recipients was 454 and the donors were 394. The characteristics of these 16 studies were described in Tables 1, 2.

Figure 1. The flow diagram of literature screening and following the preferred reporting items of systematic reviews and meta-analyses (PRISMA).
Supplementary Tables S1, S2 showed the quality assessment scores. In total, 13 non-randomized studies were assessed by MINORS. Scores between 0 and 4 correspond to a high risk of bias, scores between 5 and 10 correspond to a moderate risk of bias, and scores between 11 and 16 correspond to a low risk of bias (38). Among them, a median MINORS score was 12 (range: 11–17), which showed a low risk of bias. In three observational studies assessed by using NOS, one was of high quality, and two were of low quality.
All 16 studies (454 cases) reporting SVR12 rates of DAAs in HCV-negative recipients received HCV+ kidneys. The pooled estimations of the SVR12 rate from the fixed-effect model was 100.0% (95%CI: 99.2-100.0, I2= 0.0%, P = 0.97) (Figure 2).
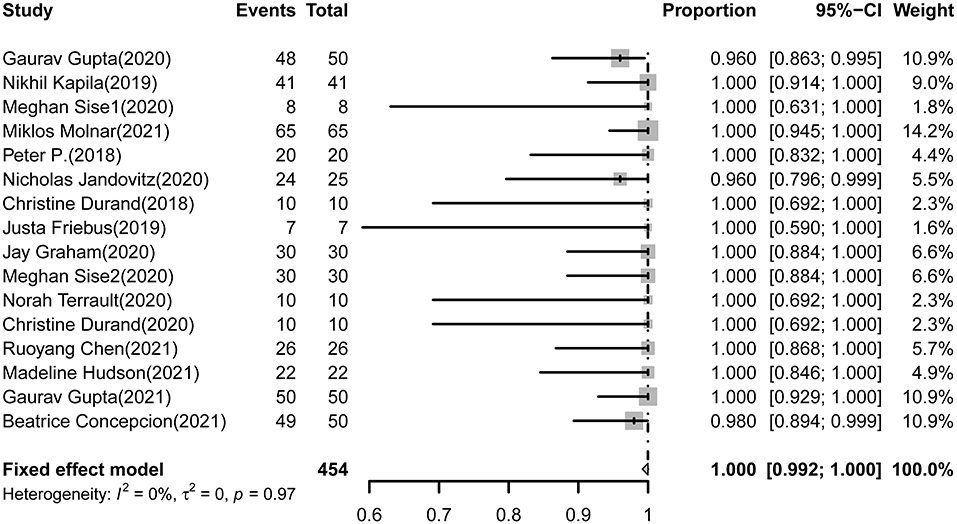
Figure 2. Forest plot of pooled SVR rates of DAAs treatment for HCV-negative recipients received HCV+ kidneys.
In total, seven DAAs prophylaxis studies reported the rate of HCV transmission rates from donors to recipients. Among 184 recipients included in the analysis, the HCV transmission rate was 33.1% (95%CI: 7.8-64.4) and a substantial level of heterogeneity was observed (I2= 94.0%, P < 0.01) (Table 6).
Based on settings, protocols, GTs, regimens and durations, subgroup analysis was conducted as detailed in Table 3. The rate of SVR 12 was 100.0% (95%CI: 95.9-100.0) in multi-center studies and 100.0% (95%CI: 99.0-100.00) in single-center studies. Nearly, 100.0% of patients in both groups achieved SVR12, 95%CI: 98.9-100.0 for post-transplant subgroup, and 95%CI: 98.2-100.0 for pre-transplant subgroup. In total, 14 studies that included 289 patients recorded GTs data for subgroup analysis. In GTs subgroups, the SVR12 rates in GT1, GT1a, GT1b, GT2, GT3, GT1a/3, and GT4 were 100.0% (95%CI: 77.2-100.0), 100.0% (95%CI: 99.6-100.0), 100.0% (95%CI: 81.1-100.0), 96.3% (95%CI:83.3-100.0), 100.0% (95%CI: 98.9-100.0), 100.0% (95%CI: 21.3-100.0), and 100.0% (95%CI: 21.3-100.0), respectively. By different DAAs regimens, the patients' SVR12 rate was 100.0% (95%CI: 100.0-100.0) in glecaprevir/pibrentasvir (GLE/PIB) subgroup, was 100.0% (95%CI: 100.0-100.0) in sofosbuvir/velpatasvir ± ribavirin (SOF/VEL ± RBV) subgroup, was 100.0% (95%CI: 94.2-100.0) in elbasvir/grazoprevir ± ribavirin (EBR/GZR ± RBV) subgroup, and was 99.0% (95%CI: 86.5-100.0) in ledipasvir/sofosbuvir ± ribavirin (LDV/SOF ± RBV) subgroup.
For pre-transplant DAAs therapy group and post-transplant DAAs therapy group, we conducted subgroup analysis by different GTs and DAAs. In DAAs prophylaxis studies, the SVR12 rates in GT1a, GT1b, GT2, and GT3, were 100.0% (95%CI: 96.3-100.0), 100.0% (95%CI: 21.3-100.0), 95.0% (95%CI: 51.2-100.0), and 100.0% (95%CI: 91.3-100.0), respectively. By different DAAs regimens, the patients' SVR12 rates using GLE/PIB, SOF/VEL ± RBV, and EBR/GZR ± RBV were 100.0% (95%CI: 100.0-100.0), 99.6% (97.1-100.0), and 99.0% (95%CI: 83.4-100.0), respectively (Table 4).
In post-transplant studies, the SVR12 rates in GT1a, GT1b, GT2, and GT3, were 100.0% (95%CI: 98.5-100.0), 100.0% (95%CI: 77.5-100.0), 100.0% (95%CI: 83.9-100.0), and 100.0% (95%CI: 96.9-100.0), respectively. By different DAAs regimens, the patients' SVR12 rates using LDV/SOF ± RBV, SOF/VEL ± RBV, and GLE/PIB were 99.0% (95%CI: 86.5-100.0), 100.0% (95%CI: 97.1-100.0), and 100.0% (95%CI: 98.7-100.0), respectively (Table 5).
Stratified by drug nodes, the HCV transmission rate was 4.5% (95%CI: 0.1-13.1) in the before RT subgroup and was 68.4% (95%CI: 50.0-84.5) in the after RT subgroup. The subgroup analysis slightly reduced the heterogeneity (before RT: I2= 62.0%; after RT: I2= 39.0%, respectively), and a significant difference was observed between the two subgroups (P < 0.01) (Table 6).
In total, nine studies reported AEs, and ten studies reported SAEs related to DAAs therapy. The pooled rates of AEs and SAEs were 1.9% (95%CI: 0.0–4.9) and 0.0% (95%CI: 0.0–1.5), respectively. The main SAEs were fibrosing cholestatic hepatitis recorded in two studies.
The funnel plot for the pooled estimations of the SVR12 rate was asymmetrical (Supplementary Figure S2). The results of Egger's test showed no publication bias in this study (t = −0.92, P = 0.37) (Supplementary Figure S1). Furthermore, the sensitivity analysis of the SVR12 rate revealed that all effect sizes did not depend on a single study (Supplementary Figure S3).
This meta-analysis of the clinical trials aims to evaluate the efficacy and safety of direct-acting antivirals in kidney transplantation from HCV-viremic donors to HCV-negative recipients. These results indicated that DAAs therapy for patients with kidney transplantations of HCV NAT+ donors can achieve high SVR12 rates, regardless of sex, age, settings, protocols, GTs, regimens, and durations. There are a few numbers SAEs related to the HCV protocol. In summary, kidneys from HCV NAT+ donors can be safely transplanted into HCV-negative recipients following DAA therapy, which is an effective and secure retreatment option to expand the donor pool and decrease organ discard.
In this study, the pooled SVR12 was100.0% (95%CI: 99.2-100.0), which showed that DAA regimens were highly effective. The results were similar to a recent study by Yang et al. (39). In terms of different DAA regimens, no significant differences were found in subgroups, which is also similar to several published studies of DAA regimens. All the DAA mentioned in the studies are recommended regimens by the American Association for the study of liver diseases (AASLDs) (40). For GTs, there is not only a high SVR12 rate but also no difference in a subgroup analysis of DAA regimens. The SVR12 rate of the GT3 subgroup is also high (100.0%), although HCV GT3-infected patients have been traditionally described as difficult-to-treat than other GTs (41). The development of DAAs allows it possible to use all GTs of the donor's kidneys instead of discarding them, the highly SVR12 rate can provide evidence for the use of HCV+ kidneys.
A low rate of HCV transmission was found in this meta-analysis, with significant heterogeneity (P < 0.01). On the one hand, the small sample size limited the result and the gap between the patient number in each study was large. Therefore, more evidence is needed to confirm. A clinical study reported a heart and lung transplantation from HCV-viremia donors, in which HCV-negative recipients received SOF/VEL therapy from HCV-viremia donors, beginning within a few hours after transplantation. In the study, a total of 42 of 44 recipients (95%) had a detectable Hepatitis C viral load immediately after transplantation (42). Therefore, we inferred that the timing to begin DAAs therapy will also affect HCV spread. On the other hand, in the subgroup analysis by drug node, heterogeneity was slightly reduced (before RT: I2= 62.0%; after RT: I2= 39.0%, respectively), and a significant difference was observed between two subgroups (P < 0.01). In addition, the detectable viremia of recipients after kidney transplantations in all studies was transience and the viral loads were steadily decreased, which was suggestive of the presence of residual viral RNA from donor kidney, rather than active infection and replication, consistently with the previous study that viral loads of donors were significantly associated with viremia in the organ recipients. It is possible to administer DAAs prior to transplant to block the viral transmission and replication in the kidney recipients. Although the transmission rate is not very low, DAAs can further reduce the later infection rate and the viral loads after transplantation. Considering the cost-effectiveness, insurance coverage for DAA drugs might frequently be denied due to a presumed lack of “medical necessity” and the high costs of DAAs have led public and private insurers to restrict access to these medications (43, 44). Thus, a prophylaxis therapy that mitigates the need for a full course of DAA therapy might make this method more appealing to the patients and providers.
However, the initiation time of DAAs therapy in HCV-negative recipients is controversial (45, 46). Our study results indicate that there are no significant differences between the pre-transplant DAAs therapy group and the post-transplant DAAs therapy group. Both groups get a high SVR12 rate. In the process of organ transplantation, it is feasible to suffer from viral active infection and replication due to the intense immunosuppression (47). The immunological risks include acute hepatitis, fibrosing cholestatic hepatitis, acute/chronic rejection, etc. The initiation of DAAs regimens administered very early would likely mitigate some of these risks. On the other hand, there are novel combinations of DAAs with pan-genotypic activity by the development of DAAs, which were licensed even for patients with eGFR <30 ml/min/1.73 m2. In November 2019, the U.S. Food and Drug Administration (FDA) amended the package inserts for sofosbuvir-containing regimens to allow use in patients with renal disease, including those with an eGFR ≤ 30 ml/min (40). It is possible to use DAAs therapy during the perioperative period with low-kidney function. For the pharmacokinetics of DAAs, a study reported that sofosbuvir's AUCs were higher in the subjects with mild, moderate, and severe renal impairment in patients with renal function impairment, whereas its active metabolite, GS-331007 AUCs were also higher (48). Furthermore, sofosbuvir and GS-331007 AUC were 28 and 1,280% higher when sofosbuvir was dosed 1 h prior to hemodialysis compared with 60 and 2,070% higher when sofosbuvir was dosed 1 h after hemodialysis, respectively (37). Comparing ESRD and healthy participants, geometric mean ratios (GMRs) for EBR and GZR AUC were 0.99 (0.75-1.30) and 0.83 (0.56-1.22) on hemodialysis (HD) days (49). The aforementioned reasons provide some theoretical basis for the implementation of the prophylaxis protocol. Although the sample size of prophylaxis was small (n = 7), which caused poor accuracy and reliability so more prophylaxis cases should be included to obtain enough evidence, the results of our research also support the scientific hypothesis, which can be used as reference evidence for the treatment plan in the future.
Among all the included studies, patients who failed to achieve SVR12 were reported in only three studies. Two patients with treatment failure in the first study were found to have resistance-associated substitutions (RASs) in the NS3/4A or NS5A region, not at the time of baseline (21). RASs were produced by the error-prone replication of HCV that could decrease the efficacy of the DAAs regimens (50). In the second study, a very high viral load was detected in one recipient at SVR12, with a mixed HCV 1a and 2b subtypes infection (25). This patient received an HCV+ kidney with 2b subtype, but was treated with LDV/SOF based for the HCV 1a genotype, which did not respond to 2b subtype. In the third study, one HCV 1a-infected recipient, treated with LDV/SOF and without viral resistance testing, neither achieved SVR-4 nor SVR12 with unknown cause, but eventually obtained SVR12 after retreating with sofosbuvir/velpatasvir/voxilaprevir (36). From the aforementioned studies, it is essential to accurately capture the HCV genotyping and RASs information of donors and recipients to make sure that patients can benefit from the best treatment for their condition.
According to the studies that reported renal function at 6 months or 12 months post-transplant, all the patients had normal levels of the glomerular filtration rate or creatinine, indicating that the recent outcomes of kidney transplantation were relatively good. The observations were consistent with a recent study by Potluri et al., who showed no significant difference in the outcomes (12-month eGFR post-transplant) between HCV+ kidneys recipients and HCV- kidneys, respectively (51).
There are several limitations to this study. First, for the ethical and medical considerations, most of these included medication studies were not designed as randomized controlled trials, and thus, making the relative risk for the various subgroups could not be evaluated. Second, the most included studies were from America, potentially limiting the choice of DAAs regimens and our results' applicability to the rest of the world. Third, subgroup analyses were not conducted because of inadequate data on kidneys recipients.
Nevertheless, our comprehensive analysis exhibited several strengths. First, this meta-analysis was the first to evaluate the efficacy and safety of DAAs in kidney transplantation from HCV-viremic donors (D+) to negative recipients (R–). We screened 12 studies including 306 individuals, which allowed us to accurately assess the pooled SVR12 rates, viral transmission, and SAEs rates of populations who received HCV-positive kidneys. Given the low heterogeneity shown in the most included studies, we are confident that the results in this study are reliable and can provide a reference for clinicians.
This comprehensive analysis showed the high efficacy and safety of DAAs in kidney transplantation from HCV-positive donors to HCV-negative recipients. DAAs therapy should be given early to reduce the risk of HCV infection post-transplant. The findings of this study may help to expand the donor pool and shorten the waiting time for kidney transplantation.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
ZPF, PH, MY, and JWZ participated in the design of the study. ZPF, JWZ, CHW, YZ, and CS took charge of literature retrieval, data collection and quality control. ZPF, WT, and HZF performed the statistical analysis. QC, YZ, and MY contributed to analysis. ZPF, JWZ, and PH wrote the paper. All authors read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (81773499), Key Project of Natural Science Foundation of Yunnan Province (2019FA005), Science Foundation for Distinguished Young Scholars of Jiangsu Province (BK20190106), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.802686/full#supplementary-material
2. Spearman CW, Dusheiko GM, Hellard M, Sonderup M. Hepatitis C. Lancet. (2019) 394:1451–66. doi: 10.1016/S0140-6736(19)32320-7
3. [Guidelines for the prevention and treatment of hepatitis C (2019 Version)]. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chin J Hepatol. (2019) 27:962–79. doi: 10.3760/cma.j.issn.1007-3418.2019.12.008
4. Morales JM, Fabrizi F. Hepatitis C and its impact on renal transplantation. Nat Rev Nephrol. (2015) 11:172–82. doi: 10.1038/nrneph.2015.5
5. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. (1999) 341:1725–30. doi: 10.1056/NEJM199912023412303
6. Chute DF, Sise ME. Effect of the opioid crisis on the donor pool for kidney transplantation: an analysis of national kidney deceased donor trends from 2010-2016. Am J Nephrol. (2018) 47:84–93. doi: 10.1159/000486516
7. Durand CM, Bowring MG, Thomas AG, Kucirka LM, Massie AB, Cameron A, et al. The drug overdose epidemic and deceased-donor transplantation in the united states: a national registry study. Annals Intern Med. (2018) 168:702–11. doi: 10.7326/M17-2451
8. Kobashigawa J, Dadhania D, Bhorade S, Adey D, Berger J, Bhat G, et al. Report from the American Society of Transplantation on Frailty in solid organ transplantation. Am J Transplant. (2019) 19:984–94. doi: 10.1111/ajt.15198
9. Schold J, Srinivas TR, Sehgal AR, Meier-Kriesche HU. Half of kidney transplant candidates who are older than 60 years now placed on the waiting list will die before receiving a deceased-donor transplant. Clin J Am Soc Nephrol. (2009) 4:1239–45. doi: 10.2215/CJN.01280209
10. Reese PP, Abt PL, Blumberg EA, Goldberg DS. Transplanting hepatitis C-positive kidneys. N Engl J Med. (2015) 373:303–5. doi: 10.1056/NEJMp1505074
11. Sibulesky L, Kling CE, Blosser C, Johnson CK, Limaye AP, Bakthavatsalam R, et al. Are we underestimating the quality of aviremic hepatitis C-positive kidneys? Time to reconsider. Am J Transpl. (2018) 18:2465–72. doi: 10.1111/ajt.14701
12. Bowring MG, Holscher CM, Zhou S, Massie AB, Garonzik-Wang J, Kucirka LM, et al. Turn down for what? Patient outcomes associated with declining increased infectious risk kidneys. Am J Transpl. (2018) 18:617–24. doi: 10.1111/ajt.14577
13. Bowring MG, Kucirka LM, Massie AB, Ishaque T, Bae S, Shaffer AA, et al. Changes in utilization and discard of HCV antibody-positive deceased donor kidneys in the era of direct-acting antiviral therapy. Transplantation. (2018) 102:2088–95. doi: 10.1097/TP.0000000000002323
14. Carbognin SJ, Solomon NM, Yeo FE, Swanson SJ, Bohen EM, Koff JM, et al. Acute renal allograft rejection following pegylated Ifn-alpha treatment for chronic HCV in a repeat allograft recipient on hemodialysis: a case report. Am J Transpl. (2006) 6:1746–51. doi: 10.1111/j.1600-6143.2006.01374.x
15. Rostaing L, Izopet J, Baron E, Duffaut M, Puel J, Durand D. Treatment of chronic hepatitis C with recombinant interferon alpha in kidney transplant recipients. Transplantation. (1995) 59:1426–31. doi: 10.1097/00007890-199505270-00012
16. Younossi ZM, Stepanova M, Sulkowski M, Foster GR, Reau N, Mangia A, et al. Ribavirin-free regimen with sofosbuvir and velpatasvir is associated with high efficacy and improvement of patient-reported outcomes in patients with genotypes 2 and 3 chronic hepatitis C: results from Astral-2 and−3 Clinical Trials. Clin Infect Dis. (2016) 63:1042–8. doi: 10.1093/cid/ciw496
17. Hepatitis C Guidance 2018 Update: Aasld-Idsa recommendations for testing managing and treating hepatitis C virus infection. Clin Infect Dis. (2018) 67:1477–92. doi: 10.1093/cid/ciy585
18. Chute DF, Chung RT, Sise ME. Direct-acting antiviral therapy for hepatitis C virus infection in the kidney transplant recipient. Kidney Int. (2018) 93:560–7. doi: 10.1016/j.kint.2017.10.024
19. Hill A, Khwairakpam G, Wang J, Golovin S, Dragunova J, Smith R, et al. High sustained virological response rates using imported generic direct acting antiviral treatment for hepatitis C. J Virus Erad. (2017) 3:200–3. doi: 10.1016/S2055-6640(20)30324-1
20. Omar H, El Akel W, Elbaz T, El Kassas M, Elsaeed K, El Shazly H, et al. Generic daclatasvir plus sofosbuvir, with or without ribavirin, in treatment of chronic hepatitis C: real-world results from 18 378 patients in Egypt. Aliment Pharmacol Therapeut. (2018) 47:421–31. doi: 10.1111/apt.14428
21. Gupta G, Yakubu I, Bhati CS, Zhang Y, Kang L, Patterson JA, et al. Ultra-short duration direct acting antiviral prophylaxis to prevent virus transmission from hepatitis C viremic donors to hepatitis C negative kidney transplant recipients. Am J Transpl. (2020) 20:739–51. doi: 10.1111/ajt.15664
22. Kapila N, Menon KVN, Al-Khalloufi K, Vanatta JM, Murgas C, Reino D, et al. Hepatitis C virus nat-positive solid organ allografts transplanted into hepatitis C virus-negative recipients: a real-world experience. Hepatology. (2020) 72:32–41. doi: 10.1002/hep.31011
23. Sise ME, Strohbehn IA, Chute DF, Gustafson J, Van Deerlin VM, Smith JR, et al. Preemptive treatment with elbasvir and grazoprevir for hepatitis C-viremic donor to uninfected recipient kidney transplantation. Kidney Int Rep. (2020) 5:459–67. doi: 10.1016/j.ekir.2020.01.001
24. Reese PP, Abt PL, Blumberg EA, Van Deerlin VM, Bloom RD, Potluri VS, et al. Twelve-month outcomes after transplant of hepatitis C-infected kidneys into uninfected recipients: a single-group trial. Annals Internal Med. (2018) 169:273–81. doi: 10.7326/M18-0749
25. Jandovitz N, Nair V, Grodstein E, Molmenti E, Fahmy A, Abate M, et al. Hepatitis C-positive donor to negative recipient kidney transplantation: a real-world experience. Transplant Infect Dis. (2021) 23:e13540. doi: 10.1111/tid.13540
26. Molnar MZ, Azhar A, Tsujita M, Talwar M, Balaraman V, Bhalla A, et al. Transplantation of kidneys from hepatitis C virus-infected donors to hepatitis c virus-negative recipients: one-year kidney allograft outcomes. Am J Kidney Dis. (2021) 77:739–47.e1. doi: 10.1053/j.ajkd.2020.10.017
27. Durand CM, Bowring MG, Brown DM, Chattergoon MA, Massaccesi G, Bair N, et al. Direct-acting antiviral prophylaxis in kidney transplantation from hepatitis C virus-infected donors to noninfected recipients: an open-label nonrandomized trial. Annals Internal Med. (2018) 168:533–40. doi: 10.7326/M17-2871
28. Friebus-Kardash J, Gäckler A, Kribben A, Witzke O, Wedemeyer H, Treckmann J, et al. Successful early sofosbuvir-based antiviral treatment after transplantation of kidneys from HCV-viremic donors into HCV-negative recipients. Transplant Infect Dis. (2019) 21:e13146. doi: 10.1111/tid.13146
29. Graham JA, Torabi J, Ajaimy M, Akalin E, Liriano LE, Azzi Y, et al. Transplantation of viral-positive hepatitis C-positive kidneys into uninfected recipients offers an opportunity to increase organ access. Clin Transpl. (2020) 34:e13833. doi: 10.1111/ctr.13833
30. Sise ME, Goldberg DS, Kort JJ, Schaubel DE, Alloway RR, Durand CM, et al. Multicenter study to transplant hepatitis C-infected kidneys (mythic): an open-label study of combined glecaprevir and pibrentasvir to treat recipients of transplanted kidneys from deceased donors with hepatitis C virus infection. J Am Soc Nephrol. (2020) 31:2678–87. doi: 10.1681/ASN.2020050686
31. Terrault NA, Burton J, Ghobrial M, Verna E, Bayer J, Klein C, et al. Prospective multicenter study of early antiviral therapy in liver and kidney transplant recipients of HCV-viremic donors. Hepatology. (2021) 73:2110–23. doi: 10.1002/hep.31551
32. Durand CM, Barnaba B, Yu S, Brown DM, Chattergoon MA, Bair N, et al. Four-week direct-acting antiviral prophylaxis for kidney transplantation from hepatitis C-viremic donors to hepatitis C-negative recipients: an open-label nonrandomized study. Annals Internal Med. (2021) 174:137–8. doi: 10.7326/M20-1468
33. Chen R, Li D, Zhang M, Yuan X. Sofosbuvir/velpatasvir prophylaxis for 12 weeks in hepatitis C virus (Hcv)-negative recipients receiving kidney transplantation from Hcv-positive donors. Annals Transpl. (2021) 26:e933313. doi: 10.12659/AOT.933313
34. Hudson MR, Webb AR, Logan AT, Silverman A, Brueckner AJ. Outcomes of hepatitis C virus nucleic acid testing positive donors in aviremic recipients with delayed direct-acting antiviral initiation. Clin Transpl. (2021) 35:e14386. doi: 10.1111/ctr.14386
35. Gupta G, Yakubu I, Zhang Y, Kimball P, Kang L, Mitchell K, et al. Outcomes of short-duration antiviral prophylaxis for hepatitis C positive donor kidney transplants. Am J Transpl. (2021) 21:3734–42. doi: 10.1111/ajt.16747
36. Concepcion BP, Binari LA, Schaefer H, Rega S, Feurer I, Shawar S, et al. Kidney transplantation from hepatitis C viremic deceased donors to aviremic recipients in a real-world setting. Transpl Direct. (2021) 7:e761. doi: 10.1097/TXD.0000000000001217
37. Smolders EJ, Jansen AME, Ter Horst PGJ, Rockstroh J, Back DJ, Burger DM. Viral Hepatitis C therapy: pharmacokinetic and pharmacodynamic considerations: a 2019 update. Clin Pharmacokinetics. (2019) 58:1237–63. doi: 10.1007/s40262-019-00774-0
38. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
39. Yang H, Hu X, Pu L, Ren S, Feng Y. Efficacy and safety of direct-acting antiviral-based treatment in hepatitis C Virus infected patients with chronic renal function impairment: an updated systemic review and meta-analysis. Nephrology. (2020) 25:829–38. doi: 10.1111/nep.13704
40. Aasld (American Association for the Study of Liver Diseases) and Idsa (Infectious Disease Society of America). Hcv Guidance: Recommendations for Testing, Managing, and Treating Hepatitis, C. (2021).
41. Goossens N, Negro F. Is genotype 3 of the hepatitis C virus the New Villain? Hepatology. (2014) 59:2403–12. doi: 10.1002/hep.26905
42. Woolley AE, Singh SK, Goldberg HJ, Mallidi HR, Givertz MM, Mehra MR, et al. Heart and lung transplants from Hcv-infected donors to uninfected recipients. N Engl J Med. (2019) 380:1606–17. doi: 10.1056/NEJMoa1812406
43. Gowda C, Lott S, Grigorian M, Carbonari DM, Saine ME, Trooskin S, et al. Absolute insurer denial of direct-acting antiviral therapy for hepatitis C: a national specialty pharmacy cohort study. Open Forum Infect Dis. (2018) 5:ofy076. doi: 10.1093/ofid/ofy076
44. Marshall AD, Pawlotsky JM, Lazarus JV, Aghemo A, Dore GJ, Grebely J. The removal of daa restrictions in europe - one step closer to eliminating Hcv as a major public health threat. J Hepatol. (2018) 69:1188–96. doi: 10.1016/j.jhep.2018.06.016
45. Burton JR Jr, Terrault NA, Goldberg DS, Bloom RD, Gilroy R, Heimbach JK, et al. Liver and kidney recipient selection of hepatitis C virus viremic donors: meeting consensus report from the 2019 controversies in transplantation. Transplantation. (2020) 104:476–81. doi: 10.1097/TP.0000000000003014
46. Verna EC, Tsapepas D, Emond JC, Brown RS Jr, Mohan S. Utilization of hepatitis C Virus (Hcv)-viremic organs for Hcv negative recipients: is practice speeding past the evidence? Hepatology. (2020) 71:4–7. doi: 10.1002/hep.30933
47. Fabrizi F, Cerutti R, Alfieri CM, Messa P. Updated view on kidney transplant from Hcv-infected donors and daas. Pharmaceutics. (2021) 13:496. doi: 10.3390/pharmaceutics13040496
48. German P, Mathias A, Brainard D, Kearney BP. Clinical pharmacokinetics and pharmacodynamics of ledipasvir/sofosbuvir, a fixed-dose combination tablet for the treatment of hepatitis C. Clin Pharmacokinetics. (2016) 55:1337–51. doi: 10.1007/s40262-016-0397-0
49. Caro L, Wenning L, Feng HP, Guo Z, Du L, Bhagunde P, et al. Pharmacokinetics of elbasvir and grazoprevir in subjects with end-stage renal disease or severe renal impairment. Eur J Clin Pharmacol. (2019) 75:665–75. doi: 10.1007/s00228-018-2585-3
50. Sarrazin C, Dvory-Sobol H, Svarovskaia ES, Doehle BP, Pang PS, Chuang SM, et al. Prevalence of resistance-associated substitutions in Hcv Ns5a, Ns5b, or Ns3 and outcomes of treatment with ledipasvir and sofosbuvir. Gastroenterology. (2016) 151:501–12.e1. doi: 10.1053/j.gastro.2016.06.002
Keywords: antiviral agents, HCV-viremia, Hepatitis C, kidney donors, kidney transplant, meta-analysis
Citation: Feng Z, Zhang J, Tan W, Wang C, Chen Q, Shen C, Fan H, Zhang Y, Huang P and Yue M (2022) Efficacy and Safety of Direct-Acting Antivirals in Kidney Transplantation From HCV-Viremic Donors to Negative Recipients: A Meta-Analysis. Front. Med. 9:802686. doi: 10.3389/fmed.2022.802686
Received: 04 November 2021; Accepted: 05 April 2022;
Published: 18 May 2022.
Edited by:
Monica Catarina Botelho, Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA), PortugalReviewed by:
Xinyu Sheng, Zhejiang Hospital, ChinaCopyright © 2022 Feng, Zhang, Tan, Wang, Chen, Shen, Fan, Zhang, Huang and Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Huang, aHVhbmdwZW5nQG5qbXUuZWR1LmNu; Ming Yue, eXVlbWluZ0Buam11LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.