
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Med. , 18 February 2022
Sec. Nuclear Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.795925
This article is part of the Research Topic Clinical Impact of Technological Innovations in Nuclear Medicine View all 16 articles
 Nguyen K. Tram1†
Nguyen K. Tram1† Ting-Heng Chou1†
Ting-Heng Chou1† Surina Patel1
Surina Patel1 Laila N. Ettefagh1
Laila N. Ettefagh1 Michael R. Go2
Michael R. Go2 Said A. Atway3
Said A. Atway3 Mitchel R. Stacy1,2*
Mitchel R. Stacy1,2*Charcot neuropathic osteoarthropathy (CN) is a serious and potentially limb-threatening complication for patients with diabetes mellitus and peripheral arterial disease. In recent decades, nuclear medicine-based approaches have been used for non-invasive detection of CN; however, to date, a positron emission tomography (PET) radionuclide specifically focused on targeted imaging of active bone remodeling has not been explored or validated for patients with CN. The radionuclide 18F-sodium fluoride (NaF) has historically been used as a bone imaging probe due to its high sensitivity for targeting hydroxyapatite and bone turnover, but has not been applied in the context of CN. Therefore, the present study focused on novel application of 18F-NaF PET/computed tomography (CT) imaging to three clinical cases of CN to evaluate active bone remodeling at various time courses of CN. PET/CT imaging in all 3 cases demonstrated focal uptake of 18F-NaF in the bones of the feet afflicted with CN, with bone retention of 18F-NaF persisting for up to 5 years following surgical reconstruction of the foot in two cases. On a group level, 18F-NaF bone uptake in the CN foot was significantly higher compared to the healthy, non-CN foot (p = 0.039). 18F-NaF PET/CT imaging may provide a non-invasive tool for monitoring active bone remodeling in the setting of CN, thereby offering novel opportunities for tracking disease progression and improving treatment and surgical intervention.
Charcot neuropathic osteoarthropathy (CN) is a condition that can impair quality of life and increase risk of limb loss in patients with diabetes mellitus (DM) and peripheral arterial disease (PAD) (1, 2). CN is characterized by local inflammation in the early phase of the condition, followed by degeneration of the bone architecture and ulceration of soft tissues in the foot and ankle in the later phases (3). If left untreated, CN can result in major disruption of normal skeletal structure that can cause significant loss of function and increased morbidity (4). Current treatment for early phases of CN consists of offloading the affected foot using a total contact cast (5), while common surgical interventions for later stages of CN include exostectomy, application of fixators, and minor or major amputations. The overall rate of amputation in patients with CN and diabetes is 3.3–11 per 1,000 patients, with 70–84% of these patients having a preceding ulceration (6). Patients with CN and diabetes undergoing amputation also have a high mortality rate that is ~70% in the first 5 years after amputation (7).
Imaging techniques such as radiography and magnetic resonance imaging (MRI) are often used to evaluate CN. However, x-ray imaging possesses low sensitivity and specificity for diagnosing the early stages of CN (8) while MRI can poorly differentiate between CN and osteomyelitis, which often exist concurrently (9). The nuclear imaging-based approaches of scintigraphy and single photon emission computed tomography (SPECT) are commonly used to detect the early stages of inflammation that precede bone morphology changes related to CN and ultimately assist with early diagnosis of CN. One traditional scintigraphy/SPECT radionuclide used for diagnosing CN is technetium-99m (99mTc)-methylene diphosphonate (MDP), a bone targeted radionuclide which possesses excellent sensitivity for diagnosing CN and can assist with differentiating between osteomyelitis and CN (10). Another scintigraphy- and SPECT-based approach that has been used for imaging of CN includes the use of 99mTc-MDP bone imaging with indium-111 (111In)-labeled white blood cells (WBC) or 99mTc-WBC (11–13), which has been shown to be useful for distinguishing between soft tissue vs. bone infection in patients with CN. While these imaging methods have proven useful, the current gold standard for differentiating between foot infection vs. CN remains dual-isotope scintigraphy/SPECT imaging with 99mTc-sulfur colloid and 111In-WBC, which possesses the best accuracy for detecting CN (14–16).
Along with scintigraphy and SPECT imaging methods, PET imaging with fluorine-18 (18F)-fluorodeoxyglucose (FDG) has emerged in recent years for evaluating the inflammatory origins of CN (17). Multimodality imaging studies have demonstrated superior accuracy of 18F-FDG PET vs. MRI in the diagnosis of CN lesions (17, 18) and higher specificity of PET/CT than MRI for diagnosing osteomyelitis in patients with chronic CN (19). Additionally, 18F-FDG PET/CT imaging has shown potential for monitoring of serial changes in inflammation in patients with CN (20).
While SPECT- and PET-based imaging approaches have shown promise for evaluating CN, a PET imaging method that specifically targets active bone remodeling in the setting of CN has not been investigated, which would offer considerable advantages over current SPECT methods by providing improved image spatial resolution and quantification. 18F-sodium fluoride (NaF) has historically been used since the 1960s as a radionuclide for targeting bone remodeling due to its high affinity for hydroxyapatite, the mineral form of calcium apatite (21). 18F-NaF has also been used for other indications, such as low back pain (22), brown tumors in hyperparathyroidism (23), and bone metastases (24). However, to date, 18F-NaF has not been studied in the context of CN. Additionally, while several imaging approaches have been used to identify early onset CN (stage 0) (25), targeted bone imaging in patients with CN following surgical intervention remains understudied. Therefore, the purpose of this study was to evaluate the utility of 18F-NaF PET/CT imaging as a tool for non-invasively characterizing active bone remodeling in a series of patients with CN who were at various time points following surgical reconstruction of the foot.
Three patients with CN, type 2 diabetes mellitus (DM), and peripheral arterial disease (PAD) were prospectively enrolled into an ongoing study evaluating the prognostic value of nuclear imaging techniques in patients with PAD (https://clinicaltrials.gov, NCT03622359) (26). As an additional component of this study, PET/CT imaging was performed 75 min after intravenous injection of 18F-NaF (375.6 ± 10.9 MBq) to evaluate active remodeling of the bones in the feet. All patients underwent PET imaging using a commercially available scanner (Discovery PET/CT 690, GE Healthcare). A low-dose CT scan of the feet was also acquired to guide manual image segmentation of the bones in the feet and ankles, and for PET image attenuation correction. All PET data was converted to standardized uptake values (SUVs) following correction for injected dose, patient body weight, attenuation, and radionuclide decay.
Semiautomated segmentation of the bones of the ankle and foot from CT images for each limb was performed using commercially available image analysis software (PMOD Technologies LLC, Zürich, Switzerland). First, a volume of interest (VOI) was drawn around the foot. Second, within the foot VOI, any tissues with Hounsfield units (HUs) equal to or >100 HU were classified as bone, based on our own experience evaluating common HU values for bones of the feet in our clinical sample. Following segmentation of bones using this approach, the segmentation was further evaluated for accuracy and manually corrected on a slice-by-slice basis, as needed.
For PET image analysis, the average target-to-background ratio (TBRavg) of each foot was calculated using the SUVs acquired for each leg, with the average SUV across all bones of the feet representing the target value while a small (5 mm3) piece of the cortical bone of the tibia represented the background value. A paired t-test was used to compare the TBR between the diseased foot and the healthy foot. A p < 0.05 was considered statistically significant. All statistical analysis was performed using Prism v9 for macOS (GraphPad Software, San Diego, CA, USA).
Patient 1 initially presented with a chronic left foot wound deep to the level of bone. MRI was consistent with osteomyelitis of the navicular bone as well as the medial and middle cuneiform bones. The patient also had a history of CN due to type 2 DM. Conservative treatment options were exhausted, and the patient underwent surgical intervention. External fixation was applied to the medial foot, which consisted of a Biomet Mini-Rail and four half pins across the mid-tarsal joint (Figure 1A) that were then removed 2 months later. Five years after surgery, radiography demonstrated stable bone structures (Figure 1B). PET/CT images were also acquired 5 years after surgery and revealed focal uptake of 18F-NaF within the bones of the foot with CN, which suggested ongoing bone remodeling 5 years post-surgical reconstruction (Figures 1C,D).
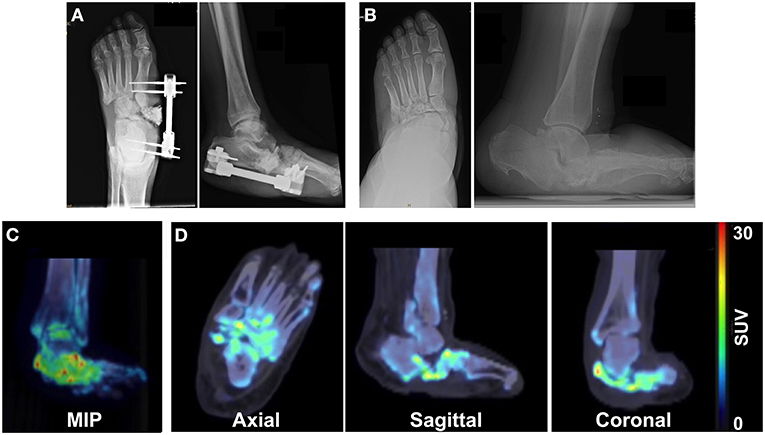
Figure 1. Multimodality imaging evaluation of a 65-year old male patient with a history of Charcot foot, type 2 DM, and PAD. X-rays acquired at (A) the time of external fixation and (B) 5 years after surgery reveal the architecture of the foot. (C) Maximum intensity projection (MIP) images and (D) axial, sagittal, and coronal 18F-NaF PET/CT images of the foot 5 years after surgery demonstrate focal increased uptake of 18F-NaF in the bones of the foot, indicating ongoing physiological remodeling of the afflicted foot 5 years after surgical reconstruction.
Patient 2 originally presented with a Charcot deformity of the right foot with chronic ulceration to the medial aspect of the foot. Following successful wound healing, the patient elected to have surgical intervention to reconstruct the foot and excise the prominent navicular bone (Figure 2A). After complete removal of the navicular bone, a medial column BioMet Advanced Locking Plate System plate was fixated across the talo-medial cuneiform joint and across the first tarsometatarsal joints using a total of three non-locking and four locking screws. Five years after surgery, x-rays demonstrated stable bone architecture with partial fusion at the midfoot architecture (Figure 2B). However, PET/CT images also acquired 5 years post-surgery revealed increased focal uptake of 18F-NaF in the bones of the right foot, thus indicating an ongoing process of bone remodeling (Figures 2C,D).
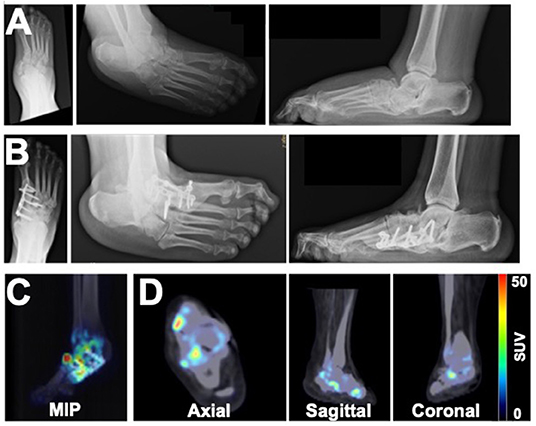
Figure 2. Imaging assessment of a 49-year old female patient with a history of right foot Charcot deformity. X-rays were acquired at (A) the time of navicular bone removal and (B) 5 years after surgical reconstruction of the foot. (C) MIP and (D) PET/CT images acquired 5 years after surgery revealed heterogeneous boney uptake of 18F-NaF and suggested potential for ongoing remodeling of the foot.
Patient 3 presented with a chronic wound on the plantar aspect of the right third digit as well as semi-rigid hammertoe contractures of digits 2 and 3 of the right foot, with the third digit ultimately undergoing amputation. The patient also had a history of Charcot joint of the right ankle and had intramedullary fixation applied to stabilize the ankle. X-rays acquired 9 months after surgical fixation showed stable bone structures without progression of the CN deformity (Figure 3A). PET/CT imaging at 9 months after surgical intervention revealed increased focal uptake of 18F-NaF at the level of the right ankle (Figures 3B,C), suggesting ongoing bone remodeling at the level of prior surgery.
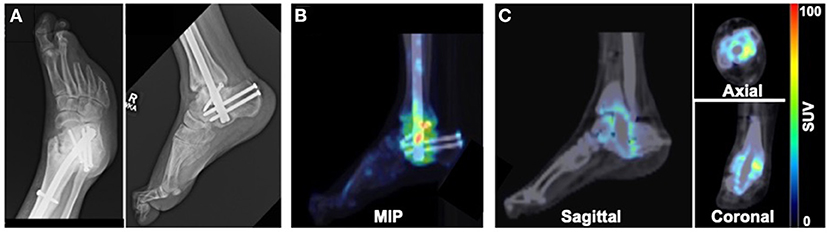
Figure 3. Imaging evaluation of 40-year old male patient with history of Charcot joint of the right ankle. (A) X-rays acquired 9 months after surgical reconstruction revealed stable bone architecture. (B) MIP and (C) PET/CT images acquired 9 months after surgery demonstrated focal uptake of 18F-NaF in the region of the surgically reconstructed ankle impacted by CN, suggesting ongoing remodeling of the bones of the ankle.
Segmentation of the bones of the ankle and feet was achieved using our semiautomated CT image analysis approach (Figure 4A). Quantitative PET/CT image analysis demonstrated significantly higher 18F-NaF uptake (i.e., TBR) in the foot afflicted by CN compared to the healthy foot (CN foot: 1.64 ± 0.30 vs. healthy foot: 1.12 ± 0.35; p = 0.039) (Figure 4B).
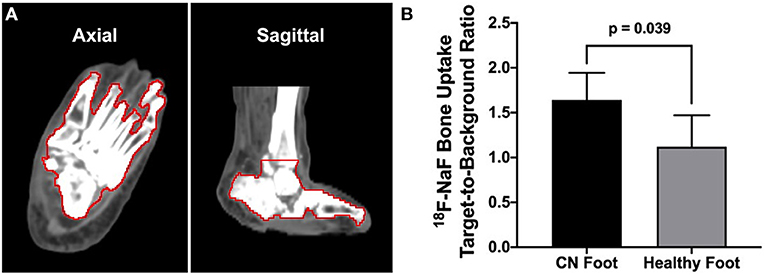
Figure 4. CT image-guided segmentation of the bones of the foot and quantitative 18F-NaF PET/CT image analysis. (A) Semiautomated segmentation of the bones of the foot and ankle (bone VOI outlined in red). (B) Quantitative analysis of PET/CT imaging demonstrated significantly higher bone uptake of 18F-NaF in the CN foot compared to the healthy non-CN foot. N = three patients. Values represent means ± SD.
The series of cases in the present study represent the first clinical application of 18F-NaF PET/CT imaging for assessing active bone remodeling in patients with CN. PET/CT imaging demonstrated increased retention of 18F-NaF in the lower extremity bones impacted by CN as early as 9 months and as late as 5 years following surgical reconstruction of the foot/ankle, thus suggesting that CN is a persistent condition characterized by active bone turnover that may not be fully suppressed for years after surgical intervention. Additionally, these initial cases reveal the potential of 18F-NaF PET/CT imaging for non-invasively detecting the active process of CN. By comparison, standard of care x-rays of the feet/ankles could not identify the continued neuroarthropathic process following surgical reconstruction.
Prior studies using nuclear medicine imaging approaches have primarily focused on assessing the inflammatory origins of CN or differentiating between soft tissue infection vs. osteomyelitis in the feet of patients with CN (25). In recent years, PET/CT imaging with 18F-FDG has emerged as a quantitative imaging approach for non-invasively evaluating the inflammatory origins of CN; however, 18F-FDG does not provide insight into the active process of bone remodeling. While 99mTc-MDP does provide insight into active remodeling of bone, 18F-NaF has significantly more bone absorption than MDP. Additionally, PET imaging is more quantitative in nature and possesses higher spatial resolution than scintigraphy or SPECT, thereby offering potential advantages over conventional approaches for the evaluation of bone remodeling in patients with CN. Furthermore, due to its well-established use as a bone perfusion imaging radionuclide (21), future studies performing dynamic PET imaging at the time of 18F-NaF administration may provide additional functional assessment of the feet in patients with CN. The present investigation is the first imaging study to evaluate active bone remodeling in patients with CN using a quantitative PET/CT imaging method. This proof-of-concept report reveals that 18F-NaF PET/CT imaging may serve as a non-invasive biomarker for monitoring ongoing bone remodeling for months or years following surgical reconstruction of the foot in patients with CN. Additional work is needed to understand the potential role of 18F-NaF PET/CT imaging in the diagnosis and treatment planning for patients with CN.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board at Nationwide Children's Hospital. The patients/participants provided their written informed consent to participate in this study.
NT, T-HC, and MS were involved in the conception and design of the study. T-HC collected and organized the data. SP and LE assisted with patient enrollment and chart review. NT analyzed the imaging data and wrote the initial draft of the manuscript. MG, SA, and MS critically reviewed and assisted in the preparation of the manuscript. All authors made critical contributions to the manuscript, approved the final version of the manuscript, and took responsibility for the findings of the study.
This work was supported by National Institutes of Health award R01 HL135103.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cates NK, Elmarsafi T, Akbari CM, Tefera E, Evans KK, Steinberg JS, et al. Complications of Charcot reconstruction in patients with peripheral arterial disease. J Foot Ankle Surg. (2021) 60:941–5. doi: 10.1053/j.jfas.2019.08.039
2. Bandeira MA, Dos Santos ALG, Woo K, Gamba MA, de Gouveia Santos VLC. Incidence and predictive factors for amputations derived from Charcot's neuroarthropathy in persons with diabetes. Int J Low Extrem Wounds. (2021). doi: 10.1177/15347346211025893. [Epub ahead of print].
3. Dodd A, Daniels TR. Charcot neuroarthropathy of the foot and ankle. J Bone Joint Surg Am. (2018) 100:696–711. doi: 10.2106/JBJS.17.00785
4. Ha J, Hester T, Foley R, Reichert ILH, Vas PRJ, Ahluwalia R, et al. Charcot foot reconstruction outcomes: a systematic review. J Clin Orthop Trauma. (2020) 11:357–68. doi: 10.1016/j.jcot.2020.03.025
5. Rosskopf AB, Loupatatzis C, Pfirrmann CWA, Boni T, Berli MC. The Charcot foot: a pictorial review. Insights Imaging. (2019) 10:77. doi: 10.1186/s13244-019-0768-9
6. Evans KK, Attinger CE, Al-Attar A, Salgado C, Chu CK, Mardini S, et al. The importance of limb preservation in the diabetic population. J Diabetes Complications. (2011) 25:227–31. doi: 10.1016/j.jdiacomp.2011.02.001
7. McCabe CJ, Stevenson RC, Dolan AM. Evaluation of a diabetic foot screening and protection programme. Diabet Med. (1998) 15:80–4. doi: 10.1002/(SICI)1096-9136(199801)15:1<80::AID-DIA517>3.0.CO;2-K
8. Ergen FB, Sanverdi SE, Oznur A. Charcot foot in diabetes and an update on imaging. Diabet Foot Ankle. (2013) 4:21884. doi: 10.3402/dfa.v4i0.21884
9. Ledermann HP, Morrison WB. Differential diagnosis of pedal osteomyelitis and diabetic neuroarthropathy: MR imaging. Semin Musculoskelet Radiol. (2005) 9:272–83. doi: 10.1055/s-2005-921945
10. Peterson N, Widnall J, Evans P, Jackson G, Platt S. Diagnostic imaging of diabetic foot disorders. Foot Ankle Int. (2017) 38:86–95. doi: 10.1177/1071100716672660
11. Crerand S, Dolan M, Laing P, Bird M, Smith ML, Klenerman L. Diagnosis of osteomyelitis in neuropathic foot ulcers. J Bone Joint Surg Br. (1996) 78:51–5. doi: 10.1302/0301-620X.78B1.0780051
12. Schauwecker DS, Park HM, Burt RW, Mock BH, Wellman HN. Combined bone scintigraphy and indium-111 leukocyte scans in neuropathic foot disease. J Nucl Med. (1988) 29:1651–5.
13. Poirier JY, Garin E, Derrien C, Devillers A, Moisan A, Bourguet P, et al. Diagnosis of osteomyelitis in the diabetic foot with a 99mTc-HMPAO leucocyte scintigraphy combined with a 99mTc-MDP bone scintigraphy. Diabetes Metab. (2002) 28:485–90.
14. Palestro CJ, Love C, Tronco GG, Tomas MB, Rini JN. Combined labeled leukocyte and technetium 99m sulfur colloid bone marrow imaging for diagnosing musculoskeletal infection. Radiographics. (2006) 26:859–U228. doi: 10.1148/rg.263055139
15. Palestro CJ, Mehta HH, Patel M, Freeman SJ, Harrington WN, Tomas MB, et al. Marrow versus infection in the Charcot joint: indium-111 leukocyte and technetium-99m sulfur colloid scintigraphy. J Nucl Med. (1998) 39:346–50.
16. Palestro CJ, Roumanas P, Swyer AJ, Kim CK, Goldsmith SJ. Diagnosis of musculoskeletal infection using combined in-111 labeled leukocyte and Tc-99m Sc marrow imaging. Clin Nucl Med. (1992) 17:269–73. doi: 10.1097/00003072-199204000-00001
17. Basu S, Chryssikos T, Houseni M, Malay DS, Shah J, Zhuang HM, et al. Potential role of FDG PET in the setting of diabetic neuro-osteoarthropathy: can it differentiate uncomplicated Charcot's neuroarthropathy from osteomyelitis and soft-tissue infection? Nucl Med Commun. (2007) 28:465–72. doi: 10.1097/MNM.0b013e328174447f
18. Hopfner S, Krolak C, Kessler S, Tiling R, Brinkbaumer K, Hahn K, et al. Preoperative imaging of Charcot neuroarthropathy in diabetic patients: comparison of ring PET, hybrid PET, and magnetic resonance imaging. Foot Ankle Int. (2004) 25:890–5. doi: 10.1177/107110070402501208
19. Rastogi A, Bhattacharya A, Prakash M, Sharma S, Mittal BR, Khandelwal N, et al. Utility of PET/CT with fluorine-18-fluorodeoxyglucose-labeled autologous leukocytes for diagnosing diabetic foot osteomyelitis in patients with Charcot's neuroarthropathy. Nucl Med Commun. (2016) 37:1253–9. doi: 10.1097/MNM.0000000000000603
20. Ruotolo V, Di Pietro B, Giurato L, Masala S, Meloni M, Schillaci O, et al. A new natural history of charcot foot clinical evolution and final outcome of stage 0 charcot neuroarthropathy in a tertiary referral diabetic foot clinic. Clin Nucl Med. (2013) 38:506–9. doi: 10.1097/RLU.0b013e318292eecb
21. Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med. (2010) 51:1826–9. doi: 10.2967/jnumed.110.077933
22. Mabray MC, Brus-Ramer M, Behr SC, Pampaloni MH, Majumdar S, Dillon WP, et al. (18)F-Sodium fluoride PET-CT hybrid imaging of the lumbar facet joints: tracer uptake and degree of correlation to CT-graded arthropathy. World J Nucl Med. (2016) 15:85–90. doi: 10.4103/1450-1147.174698
23. Graf C, Huellner M, Tschopp O, Bode-Lesniewska B, Schmid C. (18)F-NaF-PET/CT in patients with primary hyperparathyroidism and brown tumors. J Bone Miner Metab. (2020) 38:299–309. doi: 10.1007/s00774-019-01059-z
24. Araz M, Aras G, Kucuk ON. The role of 18F-NaF PET/CT in metastatic bone disease. J Bone Oncol. (2015) 4:92–7. doi: 10.1016/j.jbo.2015.08.002
25. Chou TH, Stacy MR. Clinical applications for radiotracer imaging of lower extremity peripheral arterial disease and critical limb ischemia. Mol Imaging Biol. (2020) 22:245–55. doi: 10.1007/s11307-019-01425-3
26. U.S. National Institutes of Health, U.S. National Library of Medicine Website, ClinicalTrials.gov. Radiotracer-Based Perfusion Imaging of Patients With Peripheral Arterial Disease. (2021). Available online at: https://clinicaltrials.gov/ct2/show/NCT03622359 (accessed December 22, 2021).
Keywords: sodium fluoride, positron emission tomography, charcot, computed tomography, bone remodeling
Citation: Tram NK, Chou T-H, Patel S, Ettefagh LN, Go MR, Atway SA and Stacy MR (2022) Novel Application of 18F-NaF PET/CT Imaging for Evaluation of Active Bone Remodeling in Diabetic Patients With Charcot Neuropathy: A Proof-of-Concept Report. Front. Med. 9:795925. doi: 10.3389/fmed.2022.795925
Received: 15 October 2021; Accepted: 26 January 2022;
Published: 18 February 2022.
Edited by:
Martin Huellner, University Hospital Zürich, SwitzerlandReviewed by:
Debanjali Sinha, Institute of Neurosciences, Kolkata (I-NK), IndiaCopyright © 2022 Tram, Chou, Patel, Ettefagh, Go, Atway and Stacy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mitchel R. Stacy, Mitchel.Stacy@NationwideChildrens.org
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.