- Department of General Intensive Care Unit, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
Background: Intensive care unit-acquired weakness (ICU-AW) is common in critical illness patients and is well described. Extracorporeal membrane oxygenation (ECMO) is used as a life-saving method and patients with ECMO support often suffer more risk factors of ICU-AW. However, information on the frequency and clinical characteristics of ICU-AW in patients with ECMO support is lacking. Our study aims to clarify the frequency and characteristics of ICU-AW in ECMO patients.
Methods: We conducted a retrospective study, ICU-AW was diagnosed when patients were discharged with a Medical Research Council (MRC) sum score <48. Clinical information was collected from the case report forms. Univariable analysis, LASSO regression analysis, and logistic regression analysis were used to analyze the clinical data of individuals.
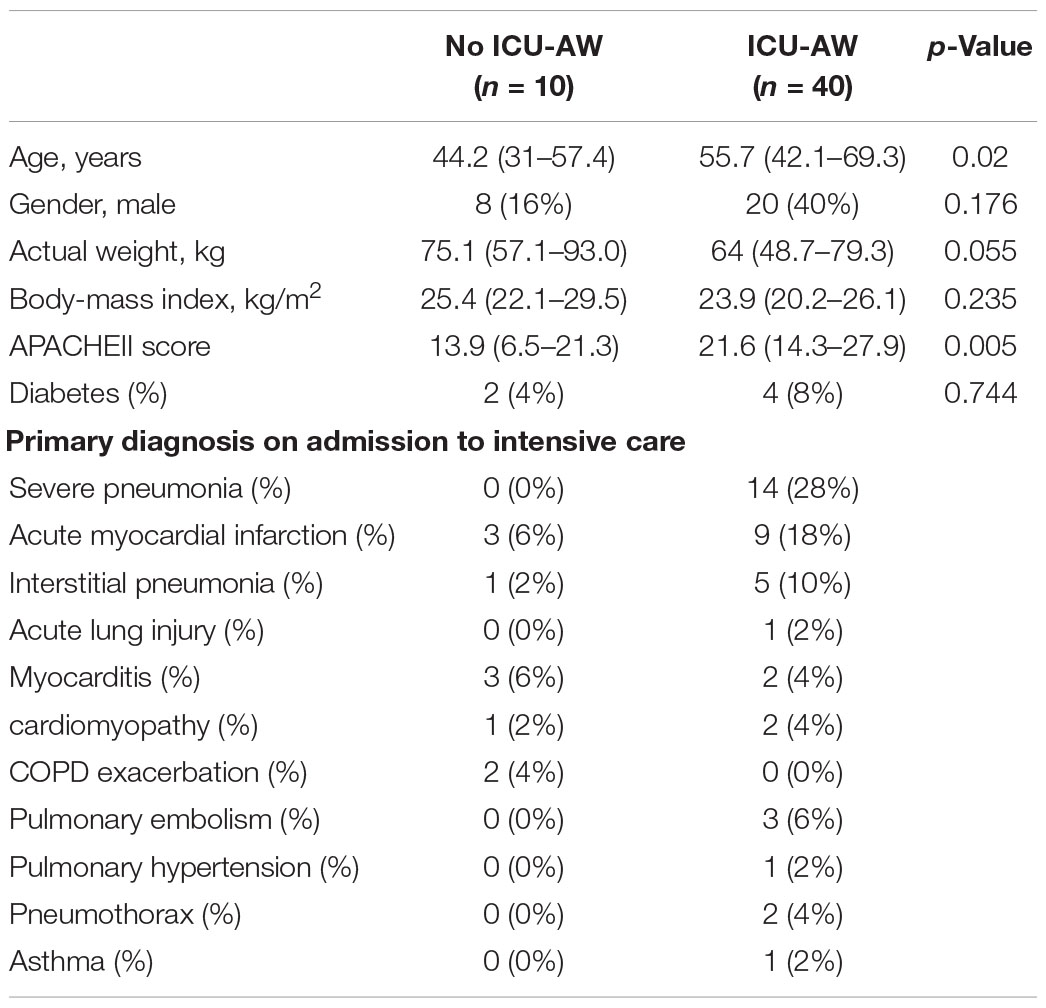
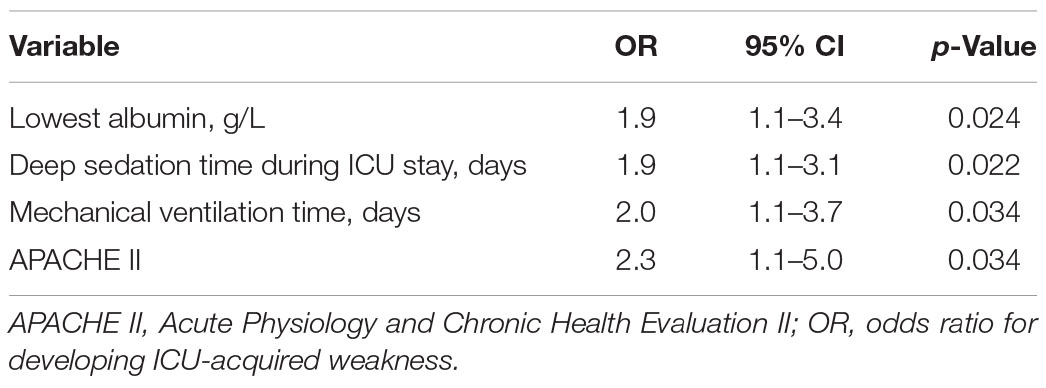
Results: In ECMO population, 40 (80%) patients diagnosed with ICU-AW. On univariable analysis, the ICU-AW group had higher Acute Physiology and Chronic Health Evaluation II (APACHE II) [13.9 (6.5–21.3) versus 21.1 (14.3–27.9), p = 0.005], longer deep sedation time [2 (0–7) versus 6.5 (3–11), p = 0.005], longer mechanical ventilation time [6.8 (2.6–9.3) versus 14.3 (6.6–19.3), p = 0.008], lower lowest albumin [26.7 (23.8–29.5) versus 22.1 (18.5–25.7), p < 0.001]. The LASSO analysis showed mechanical ventilation time, deep sedation time, deep sedation time during ECMO operation, APACHE II, and lowest albumin level were independent predictors of ICU-AW. To investigate whether ICU-AW occurs more frequently in the ECMO population, we performed a 1:1 matching with patients without ECMO and found there was no difference in the incidence of ICU-AW between the two groups. Logistic regression analysis of combined cohorts showed lowest albumin odds ratio (OR: 1.9, p = 0.024), deep sedation time (OR: 1.9, p = 0.022), mechanical ventilation time (OR: 2.0, p = 0.034), and APACHE II (OR: 2.3, p = 0.034) were independent risk factors of ICU-AW, but not ECMO.
Conclusion: The ICU-AW was common with a prevalence of 80% in the ECMO population. Mechanical ventilation time, deep sedation time, deep sedation time during ECMO operation, APACHE II, and lowest albumin level were risk factors of ICU-AW in ECMO population. The ECMO wasn’t an independent risk factor of ICU-AW.
Introduction
Extracorporeal membrane oxygenation (ECMO) is increasingly being used worldwide in recent years as a rescue therapy for critically ill patients (1). Though ECMO has almost become standard care as a therapy in critically ill patients when less invasive measures have failed, it is potentially associated with serious complications, such as bleeding, infection, acute kidney injury, and neuromuscular complications, which should be considered and weighted (2).
According to the previous studies, intensive care unit-acquired weakness (ICU-AW) can occur in critically ill patients just several days after their ICU admission and muscle loss can exceed 10% during the first week in ICU (3, 4). Muscle strength and mass reduction are common and 43% of critically ill patients have suffered muscle strength decrease (3, 4). It leads to difficulty in weaning off mechanical ventilation, which in turn increases the risk of ICU-AW and diagram dysfunction (5, 6). Moreover, ICU-AW increases the long-term and short-term mortality rates and decreases the life quality when discharged hospital (7).
The ICU-AW is a usual complication in critically ill patients with a prevalence of 43% (interquartile range 25–75%) (4, 8). Patients with ECMO support always suffer more risk factors of ICU-AW such as prolonged mechanical ventilation duration, deep sedation and paralyzed, and long-time of immobility, they are more likely to suffer ICU-AW. Thus, we need to pay more attention to distinguish those who are more vulnerable to muscle weakness and prevent the occurrence of ICU-AW.
Few studies to review the prevalence and clinical characteristics of ICU-AW in patients with ECMO support. The goal of our study is to clarify the clinical characteristics and frequency of ICU-AW in ECMO support individuals. We herein collect clinical information of our patients and analyze their clinical characteristics, hoping to provide some clinical references for our colleagues.
Materials and Methods
Study Design and Setting
We conducted a retrospective study of critically ill patients who used ECMO during their ICU stay. Patients were recruited from the general ICU of the second affiliated hospital of Zhejiang University, between March 2017 and March 2020.
Patients
Patients who had received ECMO therapy during ICU stay were eligible for inclusion criteria in the study. Exclusion criteria were patients who used ECMO to bridge to lung transplantation, experienced cardiac heart arrest, less than 18 years old, had been proven or suspected neurological impairment, using ECMO less than 24 h, had severe head or spinal cord injury and pregnant woman.
we performed a 1:1 matching of each ECMO patient with no ECMO patient who is a statistical twin in terms of APACHE II (intervals of 2 points), SOFA (intervals of 2 points), sex, age group (in 5 years intervals), BMI group, Charlson Comorbidity Index (intervals of 2 points), non-frail before ICU. The 2 of ECMO patients were in the surgical ICU and 48 patients in the medical ICU and 2 surgical ICU patients with ECMO support were matched with 2 surgical patients without ECMO support. A total of 48 ECMO patients in the medical ICU were also matched to corresponding medical ICU patients without ECMO support.
Limb Muscle Strength
Muscle strength was assessed by physical and occupational therapists on hospital discharge. The strength of muscle of included patients was assessed by Medical Research Council (MRC) score, using a scale from 0 to 5. ICU-AW was diagnosed when a patient is awake and attentive who had a muscle strength sum score <48 out of a maximal score of 60.
Processes
Following enrollment, demographic data were collected which included gender, age, BMI score, SOFA score, APACHE II, and reasons for admitted to ICU. Therapy details were recorded as well, such as time and depth of sedation (such as deep sedation time and light sedation time), nutrition and glycemic control condition, ventilator settings and duration, drug details, and rehabilitation information. The RASS score was collected from the case reports. The days of light sedation and deep sedation time were collected from the case reports, light sedation was defined as the RASS score between 1 and −2 when using sedative drugs, deep sedation was defined as the deep sedation was defined as RASS score ≤−3 when using sedative drugs (9). The actual calories and protein intake were recorded from the case report form in patients who used enteral nutrition, excepting 1 patient in ICU-AW group used parenteral nutrition. The goal calories were 25–30 kcal/kg and the goal protein intake was 1.2–2.0 g/kg. ECMO settings (such as types of ECMO, rotating speed, gas speed, blood speed, and fraction of inspiration O2) and ventilator settings (ventilator mode) were collected 1 day after the ECMO initiation. Complications during ECMO support were also recorded from the case report form and inspection report.
Statistical Analysis
Continuous variables were expressed as median (median ± interquartile range) or mean (mean standard deviation). Categorical variables were expressed as frequency. Statistical analysis was performed using SPSS, tests exercised were Pearson’s test, Fishers’ exact test, non-parametric test, and logistic regression analysis. Use paired T-tests, paired non-parametric tests, and paired Chi-square analysis in the analysis of paired samples. Statistical analyses were performed with SPSS version 23. The R statistical software version 4.1.3 was used to perform the statistical analyses. The LASSO regression analysis was operated with the “glmnet” package. The p-value < 0.05 indicated statistical significance.
Results
Over 3 years of the study period, 85 patients who had used ECMO were assessed for eligibility. Among the 85 patients, 35 patients presented exclusion criteria and 50 patients were included in the final analysis (Figure 1). A total of 40 (80%) patients were diagnosed with ICU-AW when discharged.
Baseline Characteristics of the Study Population
Baseline characteristics of the population were described in Table 1. VV-ECMO and VA-ECMO were most commonly used, there were 22 patients used VV-ECMO support and 27 patients used VA-ECMO support. Severe pneumonia and acute myocardial infarction were most common reasons for the ICU admission. Notably, APACHE II was comparable between 2 groups, patients with ICU-AW had higher APACHE II [13.9 (6.5–21.3) versus 21.1 (14.3–27.9), p = 0.005] compared with no ICU-AW.
Therapy Details During Intensive Care Unit Stay
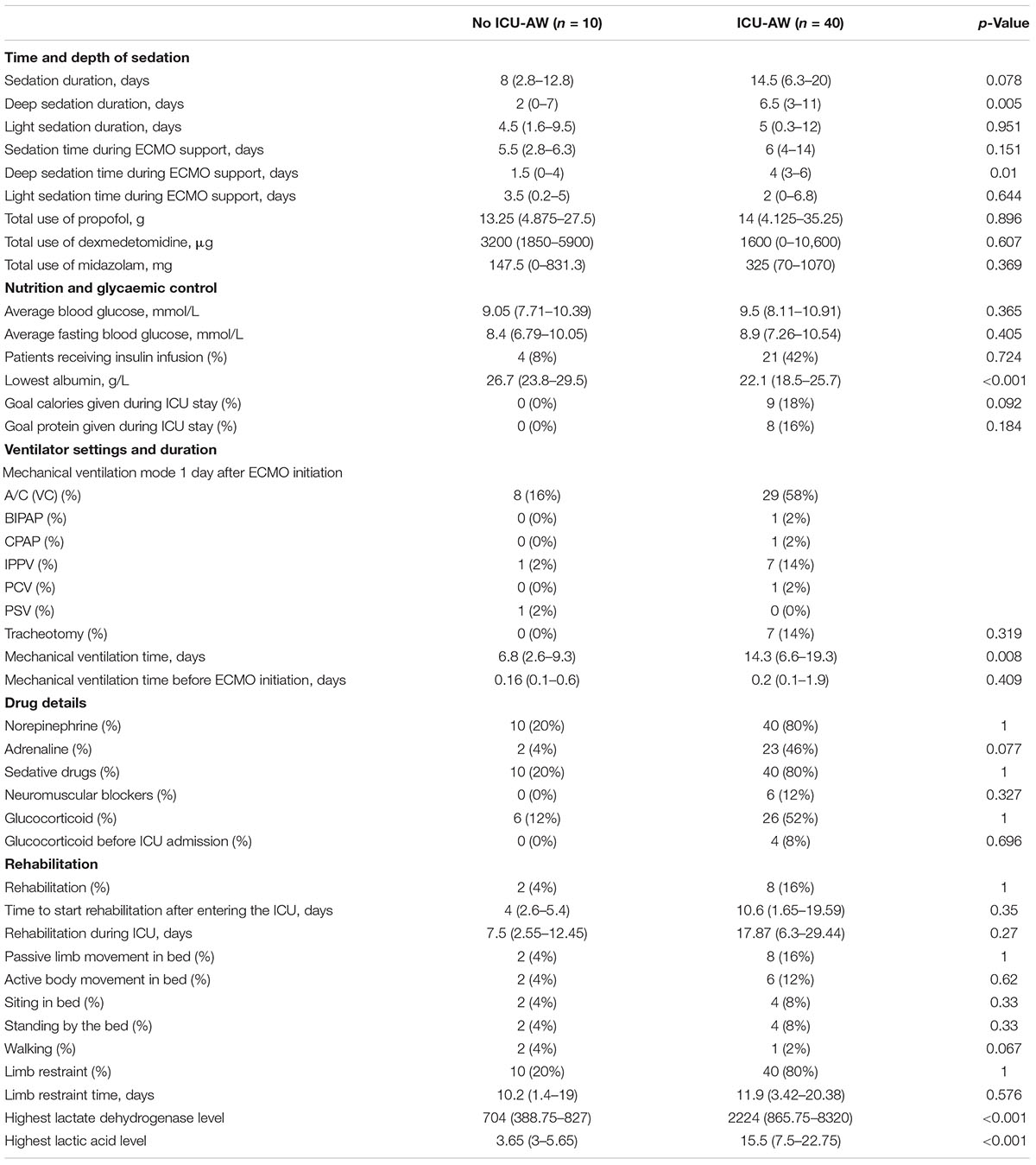
Table 2 demonstrated the therapy details of the study population. The deep sedation time during ICU stay differed between 2 groups [2 (0–7) versus 6.5 (3–11), p = 0.005]. Meanwhile, the deep sedation time during ECMO support was comparable between 2 groups [1.5 (0–4) versus 4 (3–6), p = 0.01]. The ICU-AW group underwent sedation time at a median of 14.5 days whereas the no ICU-AW group underwent a median time of 8 days, but did not differ significantly in 2 groups. The lowest albumin levels differed between 2 groups [26.7 (23.8–29.5) versus 22.1 (18.5–25.7), p < 0.001]. However, the average glucose levels and insulin infusion were similar between 2 groups, including the percentage of patients reached goal calories and protein intake.
Patients in the ICU-AW group experienced longer ventilation time during ICU stay [6.8 (2.6–9.3) versus 14.3 (6.6–19.3), p = 0.008]. Characteristics of mechanical ventilation did not differ significantly between 2 groups, such as the mode, settings of ventilator, and the percentage of tracheotomy. Mechanical ventilation duration before ECMO initiation did not statistically significant between ICU-AW and no ICU-AW group.
In both the ICU-AW and no ICU-AW populations, there were few people participating in rehabilitation, with 2 (4%) patients in no ICU-AW participating in rehabilitation training and 8 (16%) patients in ICU-AW patients participating in rehabilitation training. In the two groups, the time to start rehabilitation and the total time of rehabilitation treatment were not differed significantly, as well as the content of rehabilitation training.
In our analysis, the use of adrenaline during ICU stay was more common in ICU-AW group than in the no ICU-AW group, 26 patients with ICU-AW received adrenaline compared with 2 of patients with no ICU-AW, but they were not statistically significant. Other risk factors such as steroid use and the use of neuromuscular blockade agents did not differ significantly in our analysis. Moreover, there was no difference whether patients participate in rehabilitation exercises.
Extracorporeal Membrane Oxygenation Management
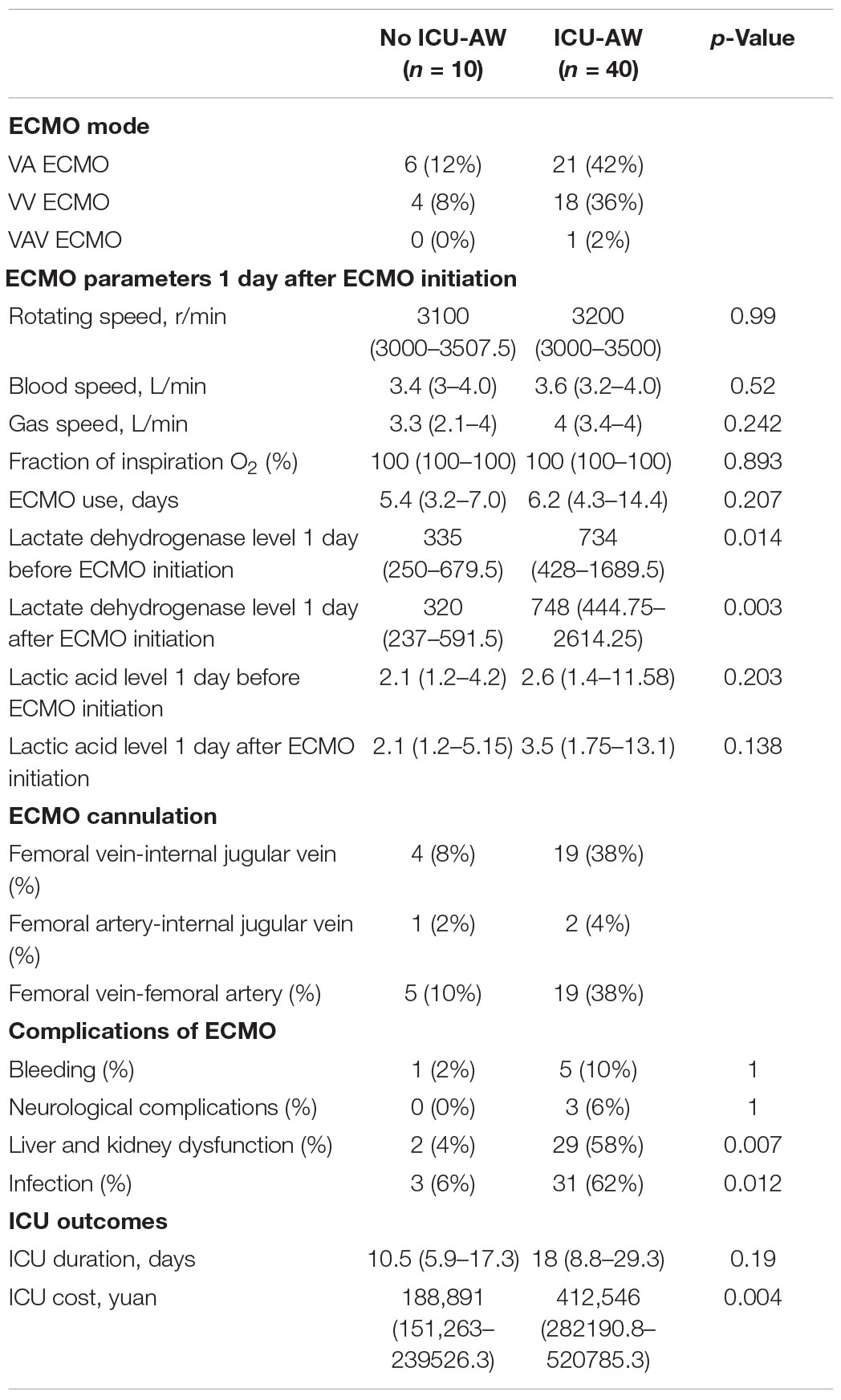
Extracorporeal membrane oxygenation parameters such as types of ECMO, rotating speed, gas speed, blood speed, fraction of inspiration O2, cannulation, and complications during ECMO operation were showed in Table 3. They were not comparable except the occurrence of infection and liver and kidney dysfunction during ECMO support. As compared to the no ICU-AW group, the ICU-AW group had higher percentage of infection during ECMO support [3 (6%) versus 31 (62%), p = 0.012] and liver and kidney dysfunction [2 (4%) versus 29 (58%), p = 0.007]. The length of ECMO duration appeared no significant difference in our research. Moreover, the ICU-AW group suffers greater financial burden.
LASSO Regression Analysis of Intensive Care Unit-Acquired Weakness
According to the results of the univariate analysis, we selected mechanical ventilation time, mechanical ventilation during ECMO operation, deep sedation time, deep sedation time during ECMO operation, liver and kidney dysfunction, infection during ECMO operation, APACHE II, SOFA, lowest albumin, and ECMO duration into the LASSO regression analysis. The five potential predictors with non-zero coefficients in LASSO analysis were selected, which included the duration of mechanical ventilation, duration of deep sedation, deep sedation time during ECMO operation, APACHE II, and lowest albumin level (Figure 2).
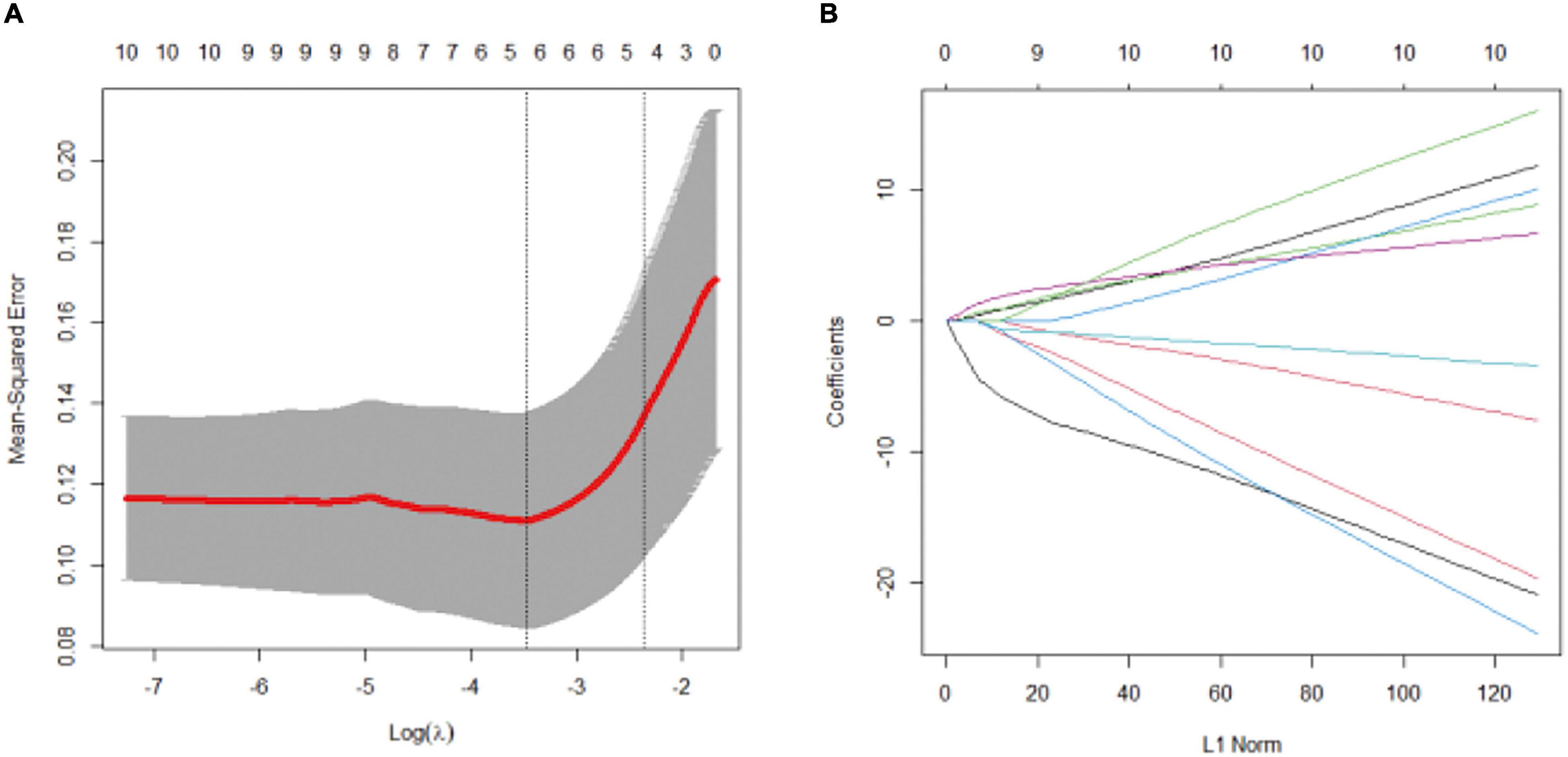
Figure 2. LASSO regression analysis. Variable selection by LASSO binary logistic regression model. (A) Selection of tuning parameter (λ) in the LASSO regression using 10-fold cross-validation via minimum criteria. five variables with non-zero coefficients were selected by optimal lambda. (B) LASSO coefficient profiles for clinical features.
Comparison of the Occurrence of Intensive Care Unit-Acquired Weakness and Clinical Characteristics Between Patients With Extracorporeal Membrane Oxygenation Support and Without Extracorporeal Membrane Oxygenation Support
To explore whether ICU-AW occurs more frequently in the ECMO population than in the no ECMO population, we performed 1:1 matching of each ECMO patients with no ECMO patients. We found 35 patients in the no ECMO group suffered ICU-AW, 40 patients with ECMO suffered ICU-AW, but there were no significant differences between the two groups. However, we found lower lowest albumin level [23 (19.1–26.9) versus 24.58 (20.8–18.3), p = 0.025], higher average blood glucose level [9.7 (8.6–10.5) versus 8.2 (7.2–9.9), p = 0.017], longer duration of sedation [11.5 (5.8–19.3) versus 7.5 (4–14.3), p = 0.014], especially deep sedation time [6 (3–9.3) versus 3.5 (0.75–5), p < 0.001], and more hospitalization costs [363,231 (198,673–477,706) versus 123,768 (67,453–230,473), p < 0.001] in ECMO patients (Table 4).
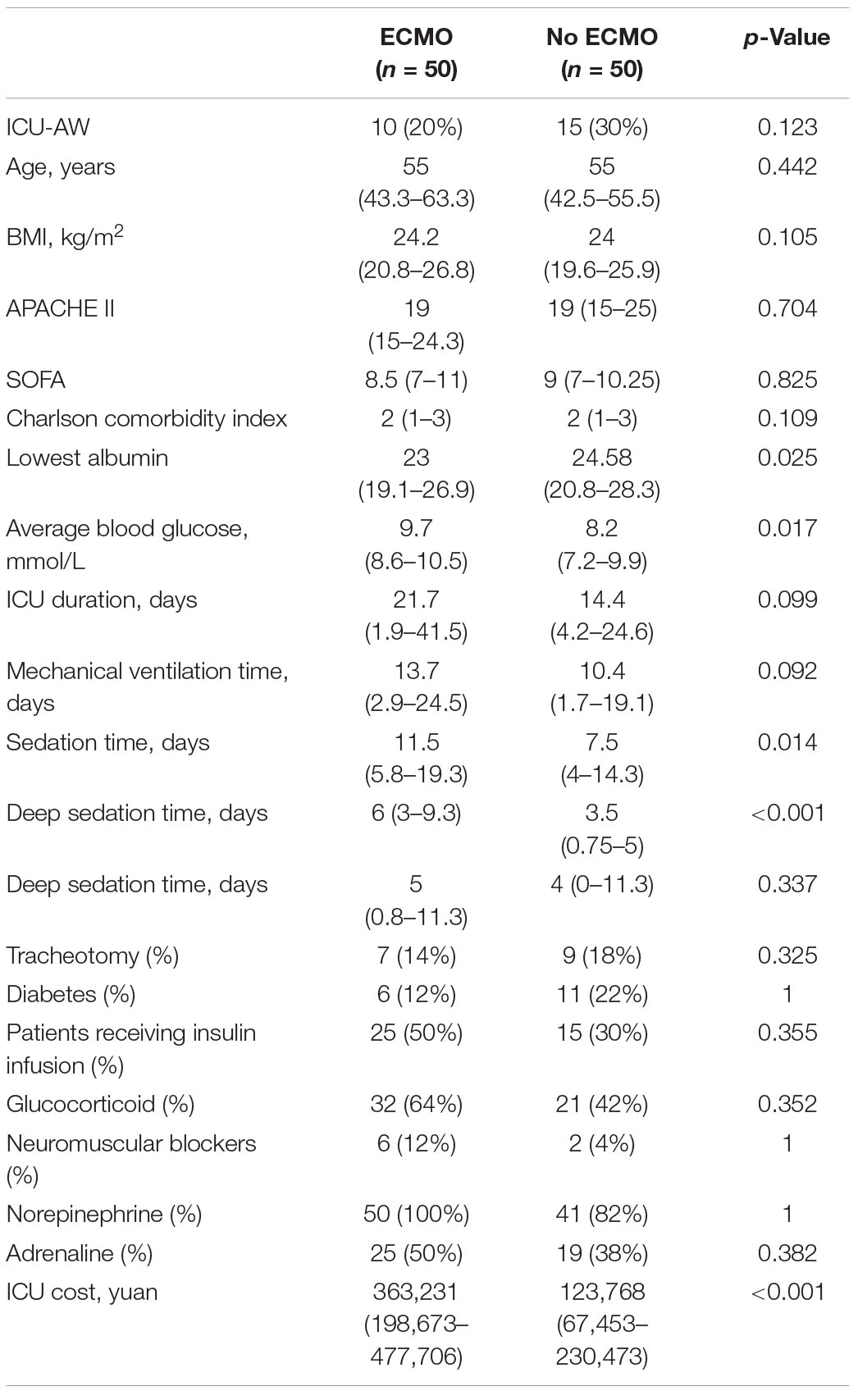
Table 4. Comparison of the occurrence of ICU-AW and clinical characteristics between patients with ECMO support and without ECMO support.
Logistic Regression Analysis for Predictors of Intensive Care Unit-Acquired Weakness in Combined Cohorts
In the combined cohorts, the clinical characteristics of ICU-AW and no ICU-AW patients were shown in Table 5. In order to further investigate the risk factors of ICU-AW and explore whether ECMO is an independent risk factor of ICU-AW. We performed a LASSO regression analysis and included the use of ECMO, age, APACHE II, SOFA, minimum albumin, average blood glucose, mechanical ventilation time, deep sedation time, sedation time, hospitalization time, and the use of norepinephrine in LASSO regression model (Supplementary Figure 1). The four related variables were selected for the logistic regression analysis, such as APACHE II, minimum albumin, mechanical ventilation time, and deep sedation time. We further performed logistic regression analysis on these four variables and found that they were independent risk factors for the occurrence of ICU-AW. According to logistic regression analysis of combined cohorts, lowest albumin odds ratio (OR: 1.9, p = 0.024), deep sedation time (OR: 1.9, p = 0.022), mechanical ventilation time (OR: 2.0, p = 0.034), and APACHE II (OR: 2.3, p = 0.034) were independent risk factors of ICU-AW, but not ECMO (Table 6).
Discussion
With the advancements in modern intensive care medicine, mortality of critically ill patients has decreased, however, at the cost of a growing incidence of ICU-AW (10). ICU-AW is known to have detrimental effects on both short-term and long-term clinical outcomes, identifying those who are at high risk of developing ICU-AW is important (7, 11). In our research, we find that the duration of mechanical ventilation, duration of deep sedation, deep sedation time during ECMO operation, APACHE II, and lowest albumin level were independent predictors of ICU-AW in patient with ECMO support.
Our study initially found that ECMO was not an independent risk factor for ICU-AW. But ECMO patients tend to have longer deep sedation time, and lower lowest albumin levels, which were independent risk factors for ICU-AW (5). According to LASSO regression analysis, long deep sedation time, low albumin, prolonged mechanical ventilation time, and high APACHE II were independent predictors of ICU-AW in ECMO patients. Logistic analysis of the combined cohorts also found that APACHE II, minimum albumin, mechanical ventilation time, and deep sedation time are independent risk factors for the occurrence of ICU-AW. These results suggested that deep sedation time, mechanical ventilation time, and albumin levels play a very important role in the occurrence of ICU-AW.
Our analysis shows ICU-AW patients received longer deep sedation time, but not light sedation time. Critically ill patients often receive sedative medications in the ICU, particularly when receiving mechanical ventilation or other invasive intubation, to decrease oxygen consumption, prevent the removal of the tubes and make patients to facilitate treatments and synchrony with the mechanical ventilator (12, 13). However, such deep sedation also makes patients immobile for significantly long periods of time (14). And this kind of deep and continuous sedation with subsequent immobilization can increase the risks of ICU-AW as well as prolonged mechanical ventilation time, which are associated with increased mortality and other negative consequences (9, 15, 16). Work by others finds that daily interruption of sedative infusions can decrease the mechanical ventilation duration and the length of ICU stay (17). Deep sedation in early mechanical ventilation time has been found to be associated with prolonged mechanical ventilation time and increased mortality (9). Therefore, it is of great significance to prevent ICU-AW in critically ill patients and avoid excessive sedation and choose appropriate sedation strategies for different diseases.
In our analysis, we found that ICU-AW patients had a longer mechanical ventilation time compared with no ICU-AW patients and it was independently associated with the occurrence of ICU-AW. Prolonged mechanical ventilation time is an independent risk factor of ICU-AW (11). Because of ventilator−induced diaphragmatic weakness and injury and longtime of immobility, ICU-AW is more likely to happen in patients who received long period of mechanical ventilation, leading to subsequent weaning difficulties and prolonged duration of mechanical ventilation, which can, in turn, increase the mechanical ventilation period and ICU-AW (11). The lengthy periods of ventilatory support contributed to a grip strength decrease in ICU patients, regardless of illness severity (18). ECMO provides important life support in critically ill patients, it improves oxygenation and facilitates protective and ultraprotective mechanical ventilation, minimizing ventilation-induced lung injury (18). Our research showed that the ICU-AW group had longer mechanical ventilation time among patients using ECMO support. Therefore, reducing the duration of mechanical ventilation and early extubation may help to reduce the risk of ICU-AW.
Our analysis shows that ICU-AW patients have lower lowest albumin levels and ICU-AW patients are older than no ICU-AW patients. This indicates that ICU-AW patients have worse nutritional conditions. Other research has also demonstrated that age is associated with the occurrence of ICU-AW (5). We found a higher incidence of ICU-AW in older patients, but it does not show a significant difference in the regression analysis. Early rehabilitation therapy has been shown to be an important mean of reducing the occurrence of ICU-AW, but there are no differences in the ICU-AW and no ICU-AW populations in terms of participation in rehabilitation, initiation and duration of rehabilitation, and patterns of rehabilitation in our experiments (19–22). It might be due to the very small number of people involved in rehabilitation and the fact that we started rehabilitation late in most patients. Other factors such as the use of steroids, the use of neuromuscular blocking agents, glucocorticoids, and rehabilitation didn’t show significantly different in our research, which may partly due to the small number of patients who receive these treatments.
Current treatments of ICU-AW mainly aimed to reduce the risk factors of ICU-AW, such as early rehabilitation, reducing the use of sedative drugs and reduce mechanical duration. However, these treatment methods have limited the outcomes and ICU-AW remains to be a serious clinical problem (5). There were several novel studies showed different methods to which may help to prevent the occurrence of ICU-AW. For example, recently reported clinical research found that obese patients were less likely to have ICU-AW (23). In animal models, ketone diester was found to attenuate skeletal muscle atrophy and inflammation-induced catabolism, which demonstrates the anti-catabolic effects of ketone bodies in muscle atrophy (24). A clinical study also revealed that the ketogenic diet has the potential to improve neurological outcomes for patients with various traumatic injuries (25).
There are some limitations with our research. First, our major limitation is the small number of included patients and this study is based on one center, this might introduce bias and may, therefore, limit the clinical application. Secondly, the dataset is examined retrospectively, which limits the comparison to other studies. Our study primary revealed the frequency and the clinical characteristics of ICU-AW in ECMO support patients. We hope more research to further identify the clinical characteristics and risk factors of ICU-AW in patients with ECMO support, to help in early recognition and treatment of ICU-AW.
Conclusion
The ICU-AW was common with a prevalence of 80% in ECMO population. Duration of mechanical ventilation, deep sedation time, deep sedation time during ECMO operation, APACHE II, and lowest albumin level were risk factors of ICU-AW in ECMO population. ECMO was not an independent risk factor of ICU-AW.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Second Affiliated Hospital of Zhejiang University. Written informed consent from the participants or their legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
XC designed the research, analyzed the characteristics of the data, and drafted the manuscript. XL helped in designing the research. XL, XX, and YZ collected the clinical data and helped check the manuscript. MH supervised the research process, provided the clinical reference, and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation (No. 81801940 YZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks for all the staff of ICU for providing useful reference and study details of the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.792201/full#supplementary-material
References
1. The ReVA Research Network and the PROVE Network Investigators, Serpa Neto A, Schmidt M, Azevedo LCP, Bein T, Brochard L, et al. Associations between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: a pooled individual patient data analysis: mechanical ventilation during ECMO. Intensive Care Med. (2016) 42:1672–84. doi: 10.1007/s00134-016-4507-0
2. White A, Fan E. What is ECMO? Am J Respir Crit Care Med. (2016) 193:9–10. doi: 10.1164/rccm.1936P9
3. Dres M, Dubé B-P, Mayaux J, Delemazure J, Reuter D, Brochard L, et al. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med. (2017) 195:10. doi: 10.1164/rccm.201602-0367OC
4. Fan E, Cheek F, Chlan L, Gosselink R, Hart N, Herridge MS, et al. An official American thoracic society clinical practice guideline: the diagnosis of intensive care unit–acquired weakness in adults. Am J Respir Crit Care Med. (2014) 190:1437–46. doi: 10.1164/rccm.201411-2011ST
5. Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med. (2020) 46:637–53. doi: 10.1007/s00134-020-05944-4
6. Combes A, Schmidt M, Hodgson CL, Fan E, Ferguson ND, Fraser JF, et al. Extracorporeal life support for adults with acute respiratory distress syndrome. Intensive Care Med. (2020) 46:2464–76. doi: 10.1007/s00134-020-06290-1
7. Van Aerde N, Meersseman P, Debaveye Y, Wilmer A, Gunst J, Casaer MP, et al. Five-year impact of ICU-acquired neuromuscular complications: a prospective, observational study. Intensive Care Med. (2020) 46:1184–93. doi: 10.1007/s00134-020-05927-5
8. Harnisch L-O, Riech S, Mueller M, Gramueller V, Quintel M, Moerer O. Longtime neurologic outcome of extracorporeal membrane oxygenation and non extracorporeal membrane oxygenation acute respiratory distress syndrome survivors. JCM. (2019) 8:1020. doi: 10.3390/jcm8071020
9. The Sedation Practice in Intensive Care Evaluation (SPICE) Study Group investigators, Shehabi Y, Chan L, Kadiman S, Alias A, Ismail WN, et al. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med. (2013) 39:910–8. doi: 10.1007/s00134-013-2830-2
10. Friedrich O, Reid MB, Van den Berghe G, Vanhorebeek I, Hermans G, Rich MM, et al. The sick and the weak: neuropathies/myopathies in the critically ill. Physiol Rev. (2015) 95:1025–109. doi: 10.1152/physrev.00028.2014
11. Thille AW, Boissier F, Muller M, Levrat A, Bourdin G, Rosselli S, et al. Role of ICU-acquired weakness on extubation outcome among patients at high risk of reintubation. Crit Care. (2020) 24:86. doi: 10.1186/s13054-020-2807-9
12. Mart MF, Pun BT, Pandharipande P, Jackson JC, Ely EW. ICU survivorship—the relationship of delirium, sedation, dementia, and acquired weakness. Crit Care Med. (2021) 49:1227–40. doi: 10.1097/CCM.0000000000005125
13. Patel SB, Kress JP. Sedation and analgesia in the mechanically ventilated patient. Am J Respir Crit Care Med. (2012) 185:486–97. doi: 10.1164/rccm.201102-0273CI
14. Weinert CR, Calvin AD. Epidemiology of sedation and sedation adequacy for mechanically ventilated patients in a medical and surgical intensive care unit*. Crit Care Med. (2007) 35:393–401. doi: 10.1097/01.CCM.0000254339.18639.1D
15. Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous IV sedation is associated with prolongation of mechanical ventilation. Chest. (1998) 114:541–8. doi: 10.1378/chest.114.2.541
16. Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. (2012) 186:724–31. doi: 10.1164/rccm.201203-0522OC
17. Kress JP. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. (2000) 342:1471–7. doi: 10.1056/NEJM200005183422002
18. Sanchez ML. Mechanical ventilation in patients subjected to extracorporeal membrane oxygenation (ECMO). Med Intensiva. (2017) 41:491–6. doi: 10.1016/j.medin.2016.12.007
19. Scheffenbichler L, Teja B, Scheffenbichler F, Blobner M, Houle T, Eikermann M, et al. Influence of the acuity of patients’ illness on effectiveness of early, goal-directed mobilization in the intensive care unit: a post hoc analysis. Crit Care. (2020) 24:663. doi: 10.1186/s13054-020-03346-y
20. Scheffenbichler FT, Teja B, Wongtangman K, Mazwi N, Waak K, Schaller SJ, et al. Effects of the level and duration of mobilization therapy in the surgical ICU on the loss of the ability to live independently: an international prospective cohort study. Crit Care Med. (2021) 49:e247–57. doi: 10.1097/CCM.0000000000004808
21. Fuest KE, Lorenz M, Grunow JJ, Weiss B, Mörgeli R, Finkenzeller S, et al. The functional trajectory in frail compared with non-frail critically ill patients during the hospital stay. Front Med. (2021) 8:748812. doi: 10.3389/fmed.2021.748812
22. Schaller SJ, Anstey M, Blobner M, Edrich T, Grabitz SD, Gradwohl-Matis I, et al. International Early SOMS-guided mobilization research initiative. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet. (2016) 388:1377–88. doi: 10.1016/S0140-6736(16)31637-3
23. Goossens C, Weckx R, Derde S, Dufour T, Vander Perre S, Pauwels L, et al. Adipose tissue protects against sepsis-induced muscle weakness in mice: from lipolysis to ketones. Crit Care. (2019) 23:236. doi: 10.1186/s13054-019-2506-6
24. Koutnik AP, Poff AM, Ward NP, DeBlasi JM, Soliven MA, Romero MA, et al. Ketone bodies attenuate wasting in models of atrophy. J Cachexia Sarcopenia Muscle. (2020) 11:973–96. doi: 10.1002/jcsm.12554
Keywords: ECMO, ICU-acquired weakness, mechanical ventilation, ECMO complications, sedation
Citation: Chen X, Lei X, Xu X, Zhou Y and Huang M (2022) Intensive Care Unit-Acquired Weakness in Patients With Extracorporeal Membrane Oxygenation Support: Frequency and Clinical Characteristics. Front. Med. 9:792201. doi: 10.3389/fmed.2022.792201
Received: 09 October 2021; Accepted: 04 April 2022;
Published: 10 May 2022.
Edited by:
Katarzyna Kotfis, Pomeranian Medical University, PolandReviewed by:
Manfred Blobner, Technical University of Munich, GermanyLongxiang Su, Peking Union Medical College Hospital (CAMS), China
Copyright © 2022 Chen, Lei, Xu, Zhou and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Man Huang, aHVhbmdtYW5Aemp1LmVkdS5jbg==
 Xinyi Chen
Xinyi Chen Xiong Lei
Xiong Lei Man Huang
Man Huang