- 1Department of Neurology, The Affiliated Jiangning Hospital With Nanjing Medical University, Nanjing, China
- 2Department of Oncology, The Affiliated Sir Run Run Hospital of Nanjing Medical University, Nanjing, China
Objectives: Previous studies of the associations between white matter hyperintensities (WMH) and chronic kidney disease (CKD) were still conflicting; therefore, our study aimed to conduct a systematic review of all of the available research on this topic and a meta-analysis of the association between WMH and CKD among observational studies.
Setting and Design: Systematic review and meta-analysis.
Outcome Measures: Severity of WMH.
Methods and Participants: All relevant studies in public databases were examined until 15 November 2020. Two independent reviewers assessed all the included studies using the Cross-Sectional/Prevalence Study Quality (CSSQ) scale, and then literature review and meta-analyses were undertaken.
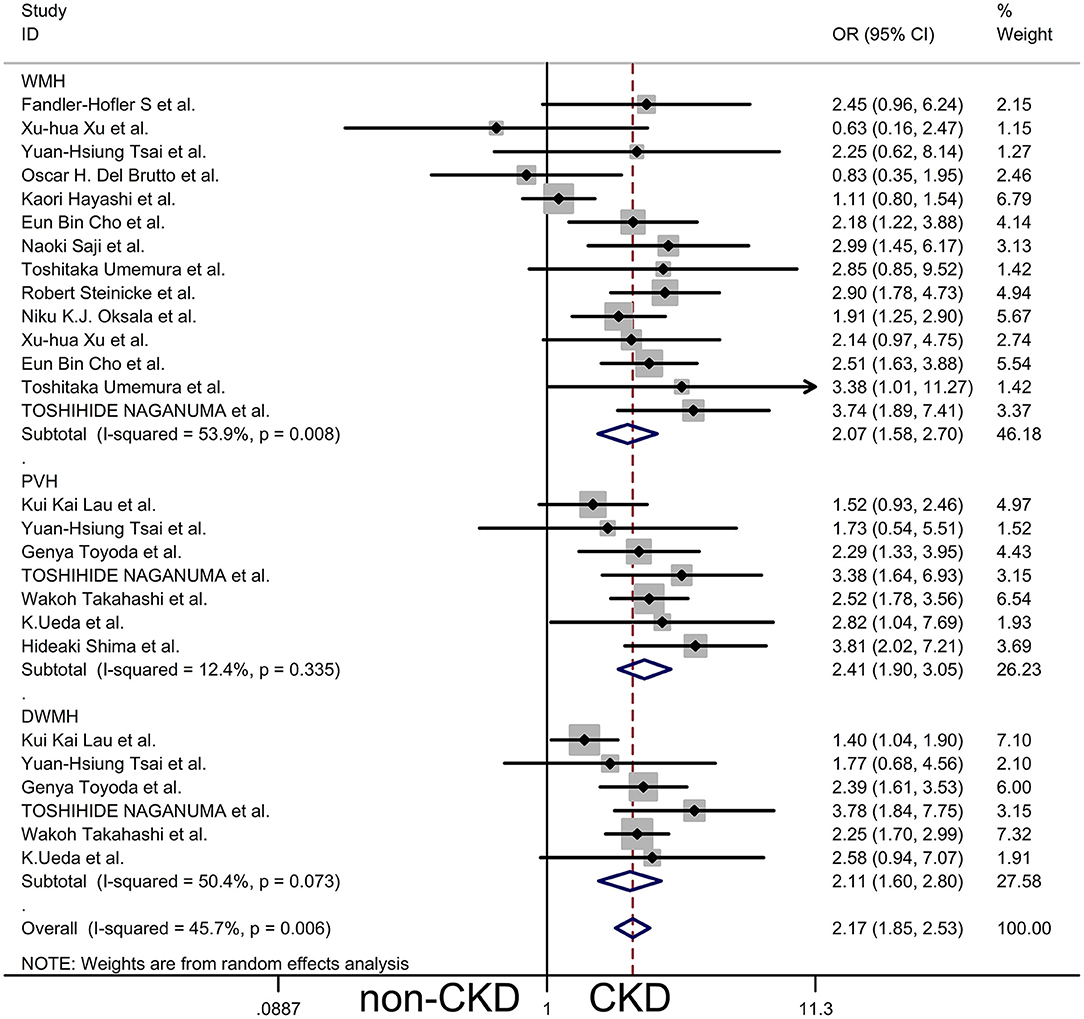
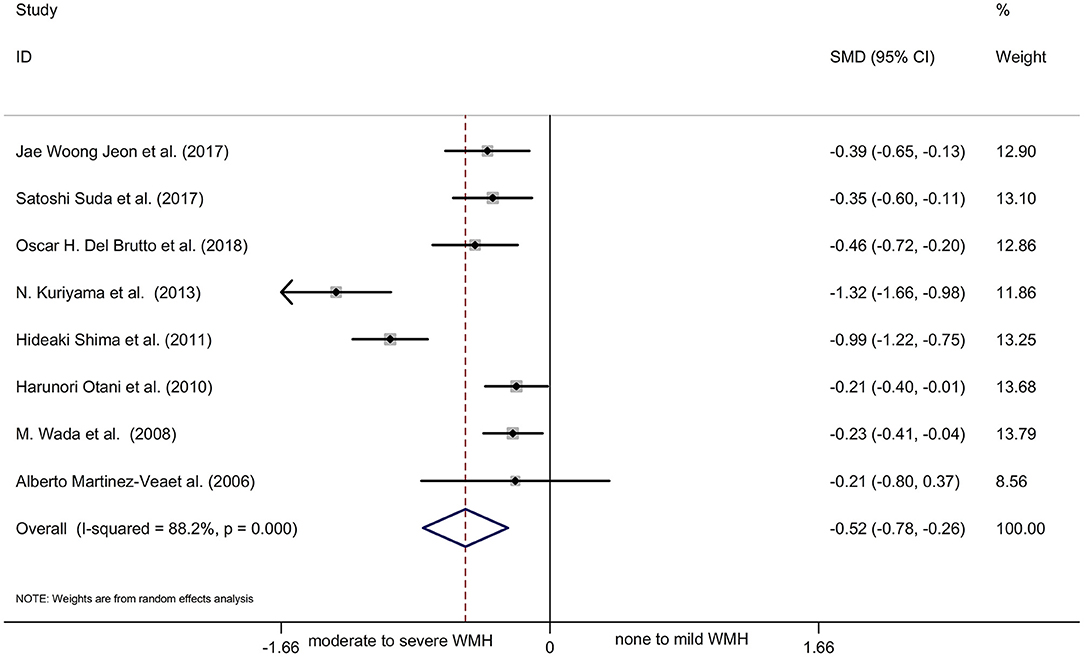
Results: We pooled the odds ratio (OR) for the presence of WMH, periventricular hyperintensities (PVH), and deep subcortical white matter hyperintensities (DWMH) of patients with CKD vs. non-CKD patients by subgroup analysis, and the results obtained were WMH OR 2.07, 95% CI [1.58, 2.70], PVH OR 2.41, 95% CI [1.90, 3.05], and DWMH OR 2.11, 95% CI [1.60, 2.80], respectively. The main outcome showed that patients with CKD were more likely to have WMH in the brain compared to the normal controls. Another meta-analysis showed a statistically significant decline in renal function in patients with moderate to severe WMH compared with those with no to mild WMH.
Conclusions: The findings indicated that patients with CKD were more likely to experience WMH than demographically matched controls. On the other hand, patients with moderate to severe WMH in the brain had poor renal function more frequently than those with no to mild WMH.
Introduction
Chronic kidney disease (CKD) is defined as abnormalities of the kidney structure or function present for >3 months (1). The prevalence and incidence of CKD have increased in recent years (2). CKD is associated with increased all-cause and cardiovascular mortality and a group of large vessel diseases, such as hypertension, arterial stiffness, and ischemic heart disease (3). There is an inverse relationship between CVD risk and glomerular filtration rate (GFR), independent of age, sex, and other risk factors (4, 5).
Many studies have found that patients with poor renal function are not only associated with large vessel disease but also with a higher prevalence of white matter hyperintensities (WMH), lacunes, and cerebral microbleeds (CMBs), especially the total burden of cerebral small vessel disease (cSVD) (6–8). At the same time, previous studies have shown that the overall burden of cSVD had a strong link with cognitive impairment and significantly affected the quality of life, exhibited poor health literacy, showed low adherence to medication, and showed greater morbidity and mortality rates in patients with CKD (3, 9, 10). However, as a primary marker of cSVD, previous studies on the associations between WMH and CKD are still conflicting (11–13). Currently, most reviews have described the relationship between CKD and cSVD, and few systematic reviews and meta-analyses have focused explicitly on the relationship between CKD and WMH. Therefore, our study aimed to conduct a systematic review of all the available studies on this topic and a meta-analysis of the association between WMH and CKD among observational studies.
Materials and Methods
As this study was a systematic review of published studies, ethical approval was exempted by the Institutional Review Board of the Office of Research Administration, the Affiliated Jiangning Hospital of Nanjing Medical University, and informed patient consent was not required.
Search Strategy
We collected the relevant clinical data from the literature published until 15 November 2020. All available studies in PubMed, Embase, Web of Science, the Cochrane Library, and Google Scholar were examined. This review focused on observational studies of the association between white matter hyperintensities and chronic kidney disease. We used the following medical subject headings and terms, without language restrictions, in conjunction with a highly sensitive search strategy: (chronic kidney disease) OR (CKD) OR (kidney function) OR (kidney failure) OR (renal disease) OR (renal insufficiency) OR (renal failure) OR [glomerular filtration rate (GFR)] OR [estimated glomerular filtration rate (eGFR)] OR (creatinine) OR (albuminuria) OR (microalbuminuria) OR (macroalbuminuria) OR (proteinuria) OR (kidney injury) AND (white matter hyperintensity) OR (white matter lesions) OR (white matter disease) OR (cerebral small vessel disease) OR (leukoencephalopathy) OR (leukoaraiosis) OR (demyelination of white matter).
The retrieval strategy was adjusted accordingly to different databases due to the dissimilarities of retrieval rules. Where there were multiple publications from the same study population, the most correlative article that reported sufficient data for the analysis was selected.
Inclusion and Exclusion Criteria
Studies fulfilling all of the following criteria were included: (1) observational studies, including cross-sectional, longitudinal, cohort, and case-control studies; (2) studies involving patients 18 years or older, with evidence of kidney injury for at least three months; (3) studies of the association between CKD and WMH as the primary or secondary outcome; and (4) the results of studies presented as continuous or two-class data or those with insufficient data to calculate these statistics. The exclusion criteria were as follows: (1) studies on special kidney injury or white matter lesions, such as hereditary renal disease, demyelinating encephalopathy, metabolic encephalopathy, and toxic encephalopathy and (2) studies such as case reports, letters, and review articles, which were dismissed after the screening. The titles and abstracts of all the relevant items were screened to determine their eligibility for inclusion. The full articles were then checked for further eligibility as per the detailed inclusion criteria.
Quality Assessment
The risk of bias was assessed by two independent reviewers using the Cross-Sectional/Prevalence Study Quality (CSSQ) scale recommended by the Agency for Healthcare Research and Quality (AHRQ). Two authors assessed the methodological quality of all the selected studies. The CSSQ contains the following eleven items in the form of questions: (1) define the source of information (survey, record review); (2) list the inclusion and exclusion criteria for exposed and unexposed participants (cases and controls) or refer to previous publications; (3) indicate the time period used for identifying patients; (4) indicate whether participants were consecutive, if not population-based; (5) indicate if the evaluators of the subjective components of the study were masked to other aspects of the status of the participants; (6) describe any assessment undertaken for quality assurance purposes (e.g., test/retest of primary outcome measurements); (7) explain any patient exclusion from the analysis; (8) describe how confounding data was assessed and/or controlled; (9) if applicable, explain how missing data was handled in the analysis; (10) summarize patient response rates and the completeness of the data collection; and (11) clarify what follow-up, if any, was expected and the percentage of patients for which incomplete data or follow-up was obtained. Each item was responded to with “yes,” “no,” or “unclear.” When any item of the CSSQ was not reported, a zero score was allocated. The total scores ranged from 0 to 11 (with 11 being the highest level of quality). Additionally, two reviewers assessed the studies independently based on the inclusion criteria.
Data Extraction
We used a standardized data form in duplicate to collect the following descriptive information: surname and initials of the first author, the year of publication or submission, number of participants, study design, and population characteristics (age, sex (male/female), evaluation of kidney dysfunction, and white matter hyperintensities). We extracted or calculated the number of white matter hyperintensity signals in patients with or without renal insufficiency. For eGFR, the mean and standard deviation were extracted to calculate effect sizes.
Statistical Analysis
For all studies, only the baseline data were used. The meta-analysis was divided into two parts. First, we calculated the combined OR value for the combined analysis of white matter hyperintensity in patients with or without renal insufficiency. For eGFR, the pooled mean and standard deviation were calculated for meta-analysis. The two meta-analyses were performed using the fixed-effects method or the random-effects method, depending on the absence or presence of significant heterogeneity. Because this review included only observational studies, a greater level of heterogeneity was expected. We used a funnel plot to check for publication bias, and sensitivity and subgroup analyses were undertaken to analyze the source of heterogeneity. The statistical heterogeneity among trials was assessed using the I2 index, with a p < 0.10 or I2 value > 50% being considered heterogeneous. A subgroup analysis was conducted to analyze heterogeneity. All statistical comparisons were two-tailed, and a p < 0.05 was considered statistically significant. All analyses were performed using STATA software (version 12.0).
Literature Search
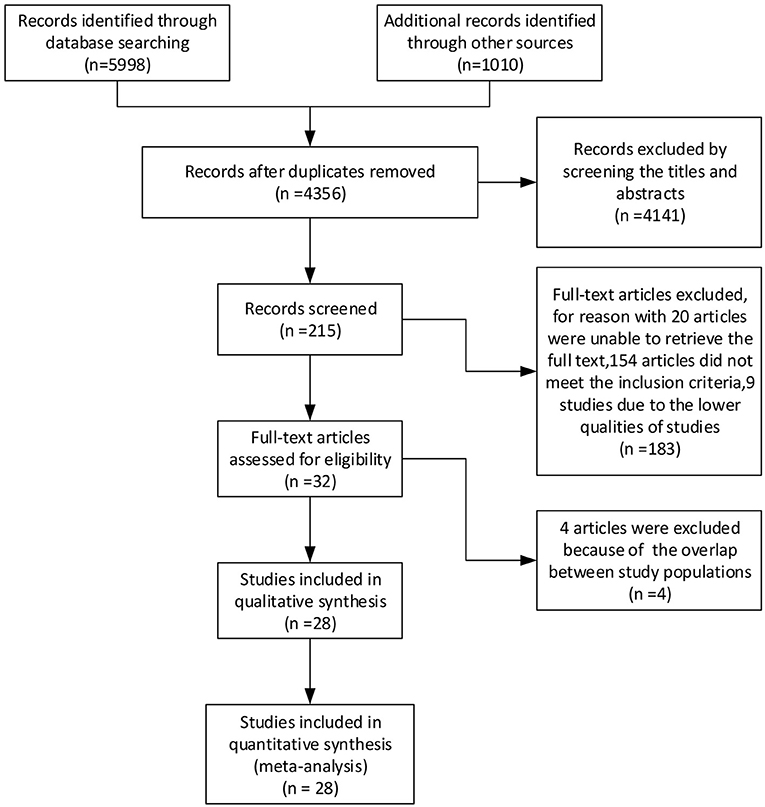
The literature search yielded 7,008 articles, including 3,565 articles retrieved by PubMed, 1,524 articles from Embase, 35 clinical trials from the Cochrane Library, 874 articles from Web of Science, and 1,010 articles from Google Scholar, up until 18 October 2020. After screening the titles and abstracts and removing duplicate articles, 215 articles were included. The full text of these 215 articles were then searched for further analysis, and 183 were excluded because we could not retrieve the full text of 20 articles, 154 articles did not meet the inclusion criteria, and nine studies were of poor quality. Finally, four more articles were excluded because of the potential overlap between study populations. After analysis, 28 studies were included in the review. A flow diagram of the search strategy is provided in Figure 1.
Results
Description of Included Studies
The characteristics of the 28 studies are presented in Table 1. There were a total of 19,420 participants across 21 cross-sectional studies, four cohort studies, two case-control studies, and one longitudinal study. The mean age of the participants ranged from 44.5 to 79.0 years, and the participants were primarily normal individuals who came for health checkups; patients with stroke, those with type 2 diabetes, and those with CKD; local community participants; and voluntary participants; among others. In most studies, the number of male participants was more than female participants, except in eight studies. In three studies, the proportion of female participants was significantly higher than male participants (24, 35, 36). In the remaining five studies, the proportion of female participants was slightly higher than that of male participants. Glomerular filtration rate (GFR) <60 ml/(min·1.73m2) was used as the evaluation standard of CKD in most studies. The other evaluation criteria of CKD included urine protein, abnormally elevated urinary albumin-creatinine ratio (UACR), and serum creatinine levels. Unfortunately, in the study of Naganuma et al. (31), we could not obtain specific information for CKD evaluation. The Fazekas score is widely used to evaluate the severity of WMH, including periventricular WMH (PVWMH) and deep WMH (DWMH), as the scale showed good reliability in the intra-rater reliability analysis. Moreover, white matter lesion (WML) volume and other scales, such as a 10-point scale and Schmidt scale, have been used for measurements of WMH in some studies. Model adjustments generally included age, sex, arterial hypertension, dyslipidemia, diabetes mellitus, smoking, and obesity.
Twenty-eight studies met the inclusion criteria, including twenty-one cross-sectional studies, four cohort studies, two case-control studies, and one longitudinal study. Eleven studies had more than 500 participants. The number of participants in eight studies exceeded 1,000, and only two studies had <100 participants. In general, most of the included studies showed that chronic kidney disease was associated with increased severity of WMH; furthermore, the severity of kidney dysfunction was positively correlated with the severity of WMH (11, 16, 17, 21, 23–26, 28–30, 33–38). However, there was no significant correlation between GFR level and WMH severity in three studies (19, 20, 27). In the cross-sectional study by Lei Yang and colleagues (14), 993 patients with acute lacunar infarction aged 25–95 years were enrolled to assess kidney function and the severity of PVWMH and DWMH. They found that CKD was independently associated with moderate-severe PVWMH in patients with acute lacunar infarction but not with DWMH. Similarly, the study performed by Zong et al. demonstrated that renal dysfunction (eGFR) was independently associated with the severity of PVH but not of subcortical DWMH (SDWMH) in patients with acute ischemic stroke (22). However, another cross-sectional study with 142 patients with acute hypertensive intracerebral hemorrhage (ICH) arrived at controversial conclusions: In patients with hypertensive ICH, the prevalence of CKD was associated with the prevalence of cerebral microbleeds (CMB) and DWMH but not PVWMH. A large cross-sectional study with 959 patients with ischemic stroke in China showed that renal impairment was independently associated with microbleed, white matter hyperintensity, and global SVD burden in individuals aged below 60 but not in those aged 60 and above. However, another community-based study of older adult individuals in Japan, with an average age ranging from 65.2 to 70.5 years, demonstrated that patients with worse kidney function tended to have more lacunar infarcts and higher grades of white matter lesions. In addition, the mean grades of WMLs or the mean numbers of lacunar infarctions were greater in patients with albuminuria than in those without albuminuria.
Meta-Analyses
Most of the studies we included were divided into two groups according to whether there was CKD or not; therefore, we performed a meta-analysis of the presence of WMH in CKD vs. non-CKD (12 cross-sectional studies, two cohort studies, one case-control study, and one longitudinal study). The remaining studies were used as a reference for the meta-analysis outcome, and baseline data were extracted from all studies for meta-analysis. We pooled ORs for the presence of WMH, PVH, and DWMH of CKD vs. non-CKD by subgroup analysis, and a random-effects model was used to compare the differences (Figure 2). The results showed that compared to patients without CKD, the overall risk of WMH in CKD patients was OR 2.17, 95% CI [1.85, 2.53], z = 9.74, p = 0.000. However, the results showed bias because a few studies were included in the meta-analysis more than once. Consequently, the results of the subgroup analysis were closer to those of the general population, WMH OR 2.07, 95% CI [1.58, 2.70], z = 5.35, p = 0.000, PVH OR 2.41, 95% CI [1.90, 3.05], z = 7.30, p = 0.000, DWMH OR 2.11, 95% CI [1.60, 2.80], z = 5.22, p = 0.000. The main outcome showed that patients with CKD were more likely to have WMH in the brain compared to the normal controls, as shown by the outcomes in Figure 2. Although there was a statistically significant difference in WMH between patients with CKD and those without CKD, this difference showed significant heterogeneity, and the results of I2 and P-values in the three subgroups were WMH I2 = 59.3%, p = 0.008; PVH I2 = 12.4%, p = 0.335; and DWMH I2 = 50.4%, p = 0.073. This was probably due to the different methods of assessing CKD and WMH, the different demographic characteristics, and the different complications in each study.
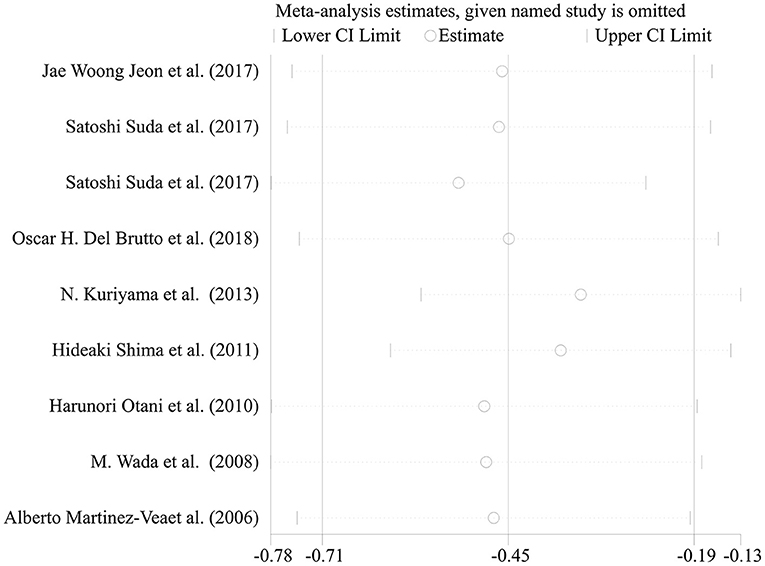
Most of the remaining studies were grouped according to the severity of WMH with the evaluation of the Fazekas scale; grade 0–1 was considered none-to-mild, and grades 2–3 were considered moderate-to-severe WMH. Due to the diverse grouping methods, some results were combined with the mean and standard deviation before the meta-analysis. The following formulae were used to calculate the combined mean and standard deviation: the combined average X = (x1*n1 + x2*n2)/(n1+n2), where x and n are the number and average of their respective groups, and the combined variance S2 = ((n1-1)*S12 + (N2−1)*S22)/(n1+N2-1), where S is the standard deviation of each group and S2 is the variance of each group. The six cross-sectional studies, one cohort study, and one case-control study were pooled and analyzed using a random-effects model. This analysis showed a statistically significant decline in renal function in patients with moderate-to-severe WMH than in those with none-to-mild WMH (Figure 3: SMD = −0.52, 95% CI [-0.78,−0.26], z =3.89, p = 0.000, df = 7). The results showed significant heterogeneity because the I2 and P-values were 88.2 and 0.000, respectively (Figure 3). Therefore, sensitivity analysis with a random-effects model was used to explore the sources of heterogeneities (Figure 4). As shown in Figure 4, the sensitivity analysis results comparing none-to-mild WMH vs. moderate-to-severe WMH demonstrated no significant difference after the removal of each study one by one.
Publication Bias
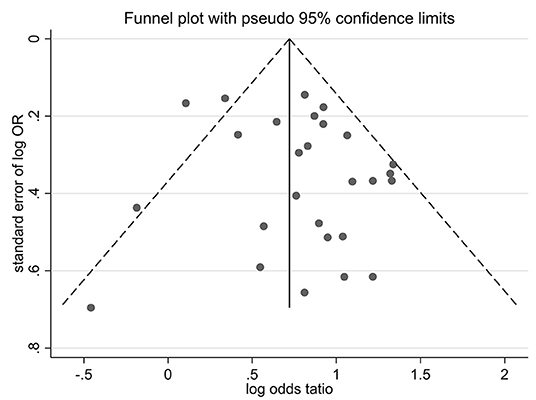
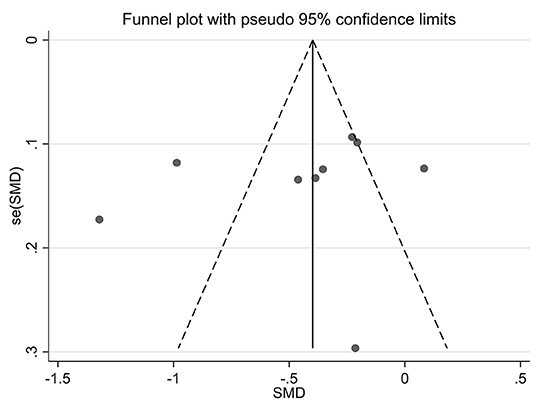
Funnel plots of effect size vs. standard error were created using STATA software (version 12.0) to investigate possible publication bias. No significant asymmetry was observed in the comparative funnel plots between patients with CKD and non-CKD patients (Begg's test for the effects: Kendall's score (P-Q) = 87, Std. Dev. of score = 47.97, z = 1.81, p = 0.07, Figure 5). Similarly, we did not find significant statistical asymmetry in the funnel plots comparing none-to-mild WMH with moderate-to-severe WMH (Begg's test for the effects: Kendall's score (P-Q) = −12, Std. Dev. of score = 9.59, z = −1.25, p = 0.21, Figure 6).
Discussion
Chronic kidney disease is a global public health issue worldwide. The complications caused by CKD, especially abnormalities in brain structure, have increased the attention it receives as the risk factors for CKD, such as hypertension, diabetes, and obesity have increased along with a longer life span of patients with CKD (2). The progress of magnetic resonance imaging (MRI) techniques has brought many new perspectives for assessing brain structure and function in patients with CKD (39, 40). WMHs are more frequent (up to 70% in patients requiring dialysis) in patients with CKD than in those without CKD or even in the early stage of CKD, suggesting that structural alterations begin early in the CKD disease process (36, 41). The white matter plays an important role in coordinating interactions between different regions of and in the normal functioningof the brain, and impaired white matter integrity appears to be a primary contributor to cognitive decline in patients with CKD (42–45).
The review included 19,420 participants and revealed that most previous observational studies were related to this topic. The results showed that patients with CKD had a higher risk of WMH than those without. In addition, the results of subgroup analysis showed that the severity of PVH and DWMH presented significant differences in patients with or without CKD. Another meta-analysis that compared renal function in patients with moderate-to-severe WMH with those with none-to-mild WMH showed that the patients with moderate-to-severe WMH presented a more serious decrease in kidney function.
The mechanism for the impairment of WMH in patients with CKD is thought to be associated with cerebrovascular disease, systemic inflammation, cerebral hypoperfusion, oxidative stress, disturbances of small blood vessels, breakdown of the blood-brain barrier (BBB), glial activation, loss of oligodendrocytes, and demyelination caused by chronic diffuse hypoperfusion (46–48). Cerebrovascular disease is a significant risk factor for the development of WMH in patients with CKD. The vascular risk factors relevant to the impairment of WMH in the brain likely include hypertension, diabetes, hyperlipidemia, elevated homocysteine, and cigarette smoking (49, 50). Hypertension was a primary vascular risk factor, as reported in previous studies. Mean arterial pressure was associated with white matter hyperintensity volume even in the absence of associations between changes in the brain tissue and tonometry measures (51, 52). The kidney and the brain are often involved simultaneously when vascular risk factors are present. The principal reason is that the kidney and the brain share unique susceptibilities to vascular injury since the vascular regulation of the microvasculatures of the two organs is anatomically and functionally similar. Moreover, the arteries of the kidney and brain are exposed to high pressure, and they have to maintain a strong vascular tone to provide a large pressure gradient over a short distance; therefore, hypertensive vascular damage occurs first and most severely in such strained vessels (52, 53).
In conclusion, the systematic review and meta-analysis findings indicate that patients with CKD are more likely to experience WMHs (including PVHs and DWMHs) than demographically matched controls. On the other hand, patients with moderate-to-severe WMH in the brain have poor renal function more frequently than those with no to mild WMH.
Study Strengths and Limitations
▸ A strength of this systematic review is that it analyzes the association between CKD and WMH concerning almost all the relevant observational studies available in the public domain and meta-analyses is that it was carried out from the perspective of CKD and WMH.
▸ The main limitation of the present study is that the meta-analysis is based mostly on cross-sectional studies because most relevant studies are cross-sectional. Additionally, we did not compare the severity of PVH and DWMH because of the different pathogenesis.
▸ Another limitation is that we used the raw data to compare differences, which may be one of the sources of heterogeneity in this study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
X-MC and J-RL contributed to the conception, design, and manuscript revision. C-YY, LW, and J-YJ contributed to the selection process and screening of trials included in this metaanalysis. QD and C-SW contributed to data extraction and risk of bias assessment. C-SW and C-YY contributed to the data analysis. RZ and X-RY were involved in the writing of the paper. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the clinical medical science and technology development fund of Jiangsu University (JLY2021153), Nanjing Medical Science and technique Development Foundation (YKK20203), and the Project of Development Research of Kangda College in Nanjing Medical University (KD2019KYJJYB047).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.770184/full#supplementary-material
Abbreviations
CKD, chronic kidney disease; WMH, white matter hyperintensities; CMBs, cerebral microbleeds; cSVD, cerebral small vessel disease; CSSQ, Cross-Sectional/Prevalence Study Quality scale; AHRQ, Agency Healthcare Research and Quality; PVH, periventricular WMH; DWMH, deep subcortical WMH; DWM, deep white matter; GFR, glomerular filtration rate; eGFR, estimated glomerular filtration rate; ACR, albumin-creatinine ratio; UACR, urinary albumin-creatinine ratio; WMLs, white matter lesions; DWLs, deep white matter lesions; HD, hemodialysis patients; KDIGO, Kidney Disease: Improving Global Outcomes; CCr, Calculated creatinine clearance; AIS, acute ischemic stroke; TIA, transient ischemic attack; AAs, African Americans; DM, diabetes medications; ICH, intracerebral hemorrhage; CRP, C-reactive protein; BNP, brain natriuretic peptide; BMI, Body Mass Index; CHD, Coronary heart disease; NIHSS, National Institutes of Health Stroke Scale; AF, atrial fibrillation; HF, heart failure; MI, myocardial infarction; PAD, peripheral artery disease; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; IMT, intima-media thickness; ACE, angiotensin-converting enzyme; LVM, Left ventricular mass; SMD, standardized mean difference; CI, confidence interval; OR, odds ratio; BBB, blood–brain barrier.
References
1. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. (2013) 158:825–30. doi: 10.7326/0003-4819-158-11-201306040-00007
2. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. (2013) 382:260–72. doi: 10.1016/S0140-6736(13)60687-X
3. Miglinas M, Cesniene U, Janusaite MM, Vinikovas A. Cerebrovascular disease and cognition in chronic kidney disease patients. Front Cardiovasc Med. (2020) 7:96. doi: 10.3389/fcvm.2020.00096
4. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey A S, de Jong P E, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. (2010) 375:2073–81. doi: 10.1016/S0140-6736(10)60674-5
5. Hill NR, Fatoba ST, Oke JL, Hirst J A, O'Callaghan C A, Lasserson D S, et al. Global Prevalence of Chronic Kidney Disease – A Systematic Review and Meta-Analysis. PLoS ONE. (2016) 11:e158765. doi: 10.1371/journal.pone.0158765
6. Miwa K, Tanaka M, Okazaki S, Furukado S, Yagita Y, Sakaguchi M, et al. Chronic kidney disease is associated with the risk of dementia in patients with vascular risk factors independently of cerebral small vessel disease. Stroke. (2014) 45. doi: 10.1161/str.45.suppl_1.108
7. Yamaguchi S, Bokura H, Nagai A, Oguro H, Onoda K, Kobayashi S, et al. Association of decreased kidney function with silent brain lesions in healthy elderly. Stroke. (2010) 41:e367.
8. Vemuri P, Knopman DS, Jack CJ, Lundt E S, Weigand S D, Zuk S M, et al. Association of kidney function biomarkers with brain MRI findings: the BRINK study. J Alzheimers Dis. (2017) 55:1069–82. doi: 10.3233/JAD-160834
9. Jimenez-Balado J, Riba-Llena I, Pizarro J, Palasi A, Penalba A, Ramirez C, et al. Kidney function changes and their relation with the progression of cerebral small vessel disease and cognitive decline. J Neurol Sci. (2020) 409. doi: 10.1016/j.jns.2019.116635
10. Bos D, Wolters FJ, Darweesh S, Vernooij M W, de Wolf F, Ikram M A, et al. Cerebral small vessel disease and the risk of dementia: A systematic review and meta-analysis of population-based evidence. Alzheimers Dement. (2018) 14:1482–92. doi: 10.1016/j.jalz.2018.04.007
11. Del BO, Mera RM. Understanding the direction of the relationship between white matter hyperintensities of vascular origin, sleep quality, and chronic kidney disease-Results from the Atahualpa Project. Clin Neurol Neurosurg. (2018) 165:10–4. doi: 10.1016/j.clineuro.2017.12.019
12. Xu XH, Ye XH, Cai JS, Gao T, Zhao G H, Zhang W J, et al. Association of renal dysfunction with remote diffusion-weighted imaging lesions and total burden of cerebral small vessel disease in patients with primary intracerebral hemorrhage. Front Aging Neurosci. (2018) 10:171. doi: 10.3389/fnagi.2018.00171
13. Cho EB, Shin HY, Park SE, Chun P, Jang H R, Yang J J, et al. Albuminuria, cerebrovascular disease and cortical atrophy: among cognitively normal elderly individuals. Sci Rep. (2016) 6:20692. doi: 10.1038/srep20692
14. Yang L, Yu L, Kong Y, Zhang X, Li Y, Yang S, et al. Relationship between white matter hyperintensities and chronic kidney disease in patients with acute lacunar stroke. Neurol Sci. (2020) 41:3307–13. doi: 10.1007/s10072-020-04397-3
15. Fandler-Hofler S, Enzinger C, Kneihsl M, Pinter D, Eppinger S, Obermayer-Pietsch B, et al. Early renal dysfunction and fibroblast growth factor-23 in patients with small vessel disease-related stroke. Sci Rep. (2019) 9:15410. doi: 10.1038/s41598-019-51965-5
16. Yeh YC, Kuo YT, Huang MF, Hwang S J, Tsai J C, Kuo M C, et al. Association of brain white matter lesions and atrophy with cognitive function in chronic kidney disease. Int J Geriatr Psychiatry. (2019) 34:1826–32. doi: 10.1002/gps.5198
17. Lau KK, Tsang A, Teo KC, Li H L, Chu L K, Li J, et al. Age-specific associations of renal impairment and cerebral small vessel disease burden in chinese with ischaemic stroke. J Stroke Cerebrovasc Dis. (2019) 28:1274–80. doi: 10.1016/j.jstrokecerebrovasdis.2019.01.018
18. Tsai Y, Lee M, Lin L, Chang S, Weng H, Yang J, et al. Association of chronic kidney disease with small vessel disease in patients with hypertensive intracerebral hemorrhage. Front Neurol. (2018) 9:284. doi: 10.3389/fneur.2018.00284
19. Suda S, Kanamaru T, Okubo S, Aoki J, Shimoyama T, Suzuki K, et al. Urinary albumin-to-creatinine ratio is associated with white matter lesions severity in first-ever stroke patients. J Neurol Sci. (2017) 373:258–62. doi: 10.1016/j.jns.2017.01.011
20. Hayashi K, Takayama M, Kanda T, Kashiwagi K, Hishikawa A, Iwao Y, et al. Association of kidney dysfunction with asymptomatic cerebrovascular abnormalities in a Japanese population with health checkups. Circ J. (2017) 81:1191–7. doi: 10.1253/circj.CJ-17-0140
21. Jeon JW, Jeong HS, Choi DE, Ham Y R, Na K R, Lee K W, et al. Prognostic relationships between microbleed, lacunar infarction, white matter lesion, and renal dysfunction in acute ischemic stroke survivors. J Stroke Cerebrovasc Dis. (2017) 26:385–92. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.037
22. Zong L, Yao M, Ni J, Zhou L, Yuan J, Peng B, et al. Kidney function is associated with severity of white matter hyperintensity in patients with acute ischemic stroke/TIA. BMC Neurol. (2016) 16:193. doi: 10.1186/s12883-016-0714-0
23. Sink KM, Divers J, Whitlow CT, Palmer N D, Smith S C, Xu J, et al. Cerebral structural changes in diabetic kidney disease: African American-Diabetes Heart Study MIND. Diabetes Care. (2015) 38:206–12. doi: 10.2337/dc14-1231
24. Toyoda G, Bokura H, Mitaki S, Onoda K, Oguro H, Nagai A, et al. Association of mild kidney dysfunction with silent brain lesions in neurologically normal subjects. Cerebrovasc Dis Extra. (2015) 5:22–7. doi: 10.1159/000373916
25. Saji N, Sato T, Sakuta K, Aoki J, Kobayashi K, Matsumoto N, et al. Chronic kidney disease is an independent predictor of adverse clinical outcomes in patients with recent small subcortical infarcts. Cerebrovasc Dis Extra. (2014) 4:174–81. doi: 10.1159/000365565
26. Kuriyama N, Mizuno T, Ohshima Y, Yamada K, Ozaki E, Shigeta M, et al. Intracranial deep white matter lesions (DWLs) are associated with chronic kidney disease (CKD) and cognitive impairment: a 5-year follow-up magnetic resonance imaging (MRI) study. Arch Gerontol Geriatr. (2013) 56:55–60. doi: 10.1016/j.archger.2011.11.009
27. Umemura T, Kawamura T, Umegaki H, Kawano N, Mashita S, Sakakibara T, et al. Association of chronic kidney disease and cerebral small vessel disease with cognitive impairment in elderly patients with type 2 diabetes. Dement Geriatr Cogn Dis Extra. (2013) 3:212–22. doi: 10.1159/000351424
28. Steinicke R, Gaertner B, Grittner U, Schmidt W, Dichgans M, Heuschmann P U, et al. Kidney function and white matter disease in young stroke patients: analysis of the stroke in young fabry patients study population. Stroke. (2012) 43:2382–8. doi: 10.1161/STROKEAHA.111.645713
29. Takahashi W, Tsukamoto Y, Takizawa S, Kawada S, Takagi S. Relationship between chronic kidney disease and white matter hyperintensities on magnetic resonance imaging. J Stroke Cerebrovasc Dis. (2012) 21:18–23. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.015
30. Naganuma T, Takemoto Y, Shoji T, Shima H, Ishimura E, Okamura M, et al. Factors associated with cerebral white matter hyperintensities in haemodialysis patients. Nephrology (Carlton). (2012) 17:561–8. doi: 10.1111/j.1440-1797.2012.01596.x
31. Shima H, Ishimura E, Naganuma T, Ichii M, Yamasaki T, Mori K, et al. Decreased kidney function is a significant factor associated with silent cerebral infarction and periventricular hyperintensities. Blood Press Res. (2011) 34:430–8. doi: 10.1159/000328722
32. Ueda K, Watanabe Y, Katsumata T, Kaneko T, Otori T, Utsumi K, et al. Carotid intima-media thickness and cerebral white matter lesions are more advanced in acute ischemic stroke patients with renal dysfunction. Clin Nephrol. (2011) 76:290–5. doi: 10.5414/CN106932
33. Oksala N K, Salonen T, Strandberg T, Oksala A, Pohjasvaara T, Kaste M, et al. Cerebral small vessel disease and kidney function predict long-term survival in patients with acute stroke. Stroke. (2010) 41:1914–20. doi: 10.1161/STROKEAHA.110.587352
34. Otani H, Kikuya M, Hara A, Terata S, Ohkubo T, Kondo T, et al. Association of kidney dysfunction with silent lacunar infarcts and white matter hyperintensity in the general population: the Ohasama study. Cerebrovasc Dis. (2010) 30:43–50. doi: 10.1159/000313612
35. Weiner D E, Bartolomei K, Scott T, Price L L, Griffith J L, Rosenberg I, et al. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis. (2009) 53:438–47. doi: 10.1053/j.ajkd.2008.08.022
36. Wada M, Nagasawa H, Iseki C, Takahashi Y, Sato H, Arawaka S, et al. Cerebral small vessel disease and chronic kidney disease (CKD): results of a cross-sectional study in community-based Japanese elderly. J Neurol Sci. (2008) 272:36–42. doi: 10.1016/j.jns.2008.04.029
37. Anan F, Masaki T, Iwao T, Eto T, Shimomura T, Umeno Y, et al. The role of microalbuminuria and insulin resistance as significant risk factors for white matter lesions in Japanese type 2 diabetic patients. Curr Med Res Opin. (2008) 24:1561–7. doi: 10.1185/03007990802061818
38. Martinez-Vea A, Salvado E, Bardaji A, Gutierrez C, Ramos A, Garcia C, et al. Silent cerebral white matter lesions and their relationship with vascular risk factors in middle-aged predialysis patients with CKD. Am J Kidney Dis. (2006) 47:241–50. doi: 10.1053/j.ajkd.2005.10.029
39. Shima H, Ishimura E, Naganuma T, Yamazaki T, Kobayashi I, Shidara K, et al. Cerebral microbleeds in predialysis patients with chronic kidney disease. Nephrol Dial Transplant. (2010) 25:1554–9. doi: 10.1093/ndt/gfp694
40. Ikram M A, Vernooij M W, Hofman A, Niessen W J, van der Lugt A, Breteler M M. Kidney function is related to cerebral small vessel disease. Stroke. (2008) 39:55–61. doi: 10.1161/STROKEAHA.107.493494
41. Cho A H, Lee S B, Han S J, Shon Y M, Yang D W, Kim B S. Impaired kidney function and cerebral microbleeds in patients with acute ischemic stroke. Neurology. (2009) 73:1645–8. doi: 10.1212/WNL.0b013e3181c1defa
42. Sedaghat S, Cremers L G, de Groot M, Hoorn E J, Hofman A, van der Lugt A, et al. Kidney function and microstructural integrity of brain white matter. Neurology. (2015) 85:154–61. doi: 10.1212/WNL.0000000000001741
43. Tuladhar A M, van Norden A G, de Laat K F, Zwiers M P, van Dijk E J, Norris D G, et al. White matter integrity in small vessel disease is related to cognition. Neuroimage Clin. (2015) 7:518–24. doi: 10.1016/j.nicl.2015.02.003
44. Liu M, Wu Y, Wu X, Ma X, Yin Y, Fang H, et al. White Matter Microstructure Changes and Cognitive Impairment in the Progression of Chronic Kidney Disease. Front Neurosci. (2020) 14:559117. doi: 10.3389/fnins.2020.559117
45. Eldehni M T, Odudu A, Mcintyre C W. Brain white matter microstructure in end-stage kidney disease, cognitive impairment, and circulatory stress. Hemodial Int. (2019) 23:356–65. doi: 10.1111/hdi.12754
46. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. (2010) 9:689–701. doi: 10.1016/S1474-4422(10)70104-6
47. De Silva TM, Miller A. A cerebral small vessel disease: targeting oxidative stress as a novel therapeutic strategy? Front Pharmacol. (2016) 7:61. doi: 10.3389/fphar.2016.00061
48. Li Q, Yang Y, Reis C, Tao T, Li W, Li X, et al. Cerebral small vessel disease. Cell Transplant. (2018) 27:1711–22. doi: 10.1177/0963689718795148
49. Jackson CA, Hutchison A, Dennis MS, Wardlaw J M, Lindgren A, Norrving B, et al. Differing risk factor profiles of ischemic stroke subtypes: evidence for a distinct lacunar arteriopathy? Stroke. (2010) 41:624–9. doi: 10.1161/STROKEAHA.109.558809
50. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. (2013) 12:483–97. doi: 10.1016/S1474-4422(13)70060-7
51. Tsao CW, Himali JJ, Beiser AS, Larson M G, Decarli C, Vasan R S, et al. Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology. (2016) 86:619–26. doi: 10.1212/WNL.0000000000002368
52. Bronas UG, Puzantian H, Hannan M. Cognitive impairment in chronic kidney disease: vascular milieu and the potential therapeutic role of exercise. Biomed Res Int. (2017) 2017:2726369. doi: 10.1155/2017/2726369
Keywords: chronic kidney disease, cerebral small vessel disease, systematic review, white matter hyperintensities, meta-analysis
Citation: Wei C-S, Yan C-Y, Yu X-R, Wang L, Zhang R, Jiang J-Y, Dai Q, Li J-R and Chen XM (2022) Association Between White Matter Hyperintensities and Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Front. Med. 9:770184. doi: 10.3389/fmed.2022.770184
Received: 07 September 2021; Accepted: 04 April 2022;
Published: 03 May 2022.
Edited by:
Audrey Adji, Victor Chang Cardiac Research Institute, AustraliaReviewed by:
Xiang Cao, Nanjing Drum Tower Hospital, ChinaHui Zhao, Nanjing Drum Tower Hospital, China
Copyright © 2022 Wei, Yan, Yu, Wang, Zhang, Jiang, Dai, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-Rong Li, bGpyeTYxMkAxNjMuY29t; Xue Mei Chen, MTMzNDc4MDg1NzlAMTg5LmNvbQ==
†These authors have contributed equally to this work
 Cun-Sheng Wei1†
Cun-Sheng Wei1† Cai-Yun Yan
Cai-Yun Yan Xue Mei Chen
Xue Mei Chen