- 1Section of Neurosurgery, Department of Surgery, College of Medicine, National Cheng Kung University Hospital, National Cheng Kung University, Tainan, Taiwan
- 2College of Medicine, Institute of Basic Medical Sciences, National Cheng Kung University, Tainan, Taiwan
- 3Department of Cell Biology and Anatomy, College of Medicine, National Cheng Kung University, Tainan, Taiwan
Background: The incidence of brain metastasis from colorectal cancer (CRC) increases along with the greater survival rate for CRC because of the advances in therapeutic modalities. Local treatment strategies for brain metastasis include surgical resection and radiotherapy. Nevertheless, given the incongruent literature, the optimal therapeutic approach remains to be investigated. This study aims to systematically compare the real-world survival outcome of surgical resection and radiotherapy in patients with brain metastasis from CRC.
Methods: Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines (PROSPERO, ID: CRD42021240200), the Cochrane Library, Embase, and Medline were searched from the inception of the database to August 2021. Meta-analyses were conducted with results pooled using hazard ratios with corresponding 95% CIs to evaluate the overall survival (OS) following local treatment for brain metastasis from CRC. Summary effects were evaluated using a series of random-effect models.
Results: In this review, 17 retrospective studies comprising 1,438 participants were included. In comparison with radiotherapy, the OS of patients who received brain metastasectomy was generally longer (HR, 0.53; 95% CI, 0.47–0.60). Extracerebral metastases (HR, 1.58; 95% CI, 1.34–1.86) and multiple brain metastases (HR, 1.38; 95% CI, 1.10–1.72) were associated with worse survival outcomes.
Conclusions: For patients with brain metastasis from CRC, the current real-world evidence demonstrated the survival benefit of aggressive neurosurgical management in suitable patients. Additionally, patients with extracerebral metastases and multiple brain metastases had worse survival outcomes.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=240200.
Introduction
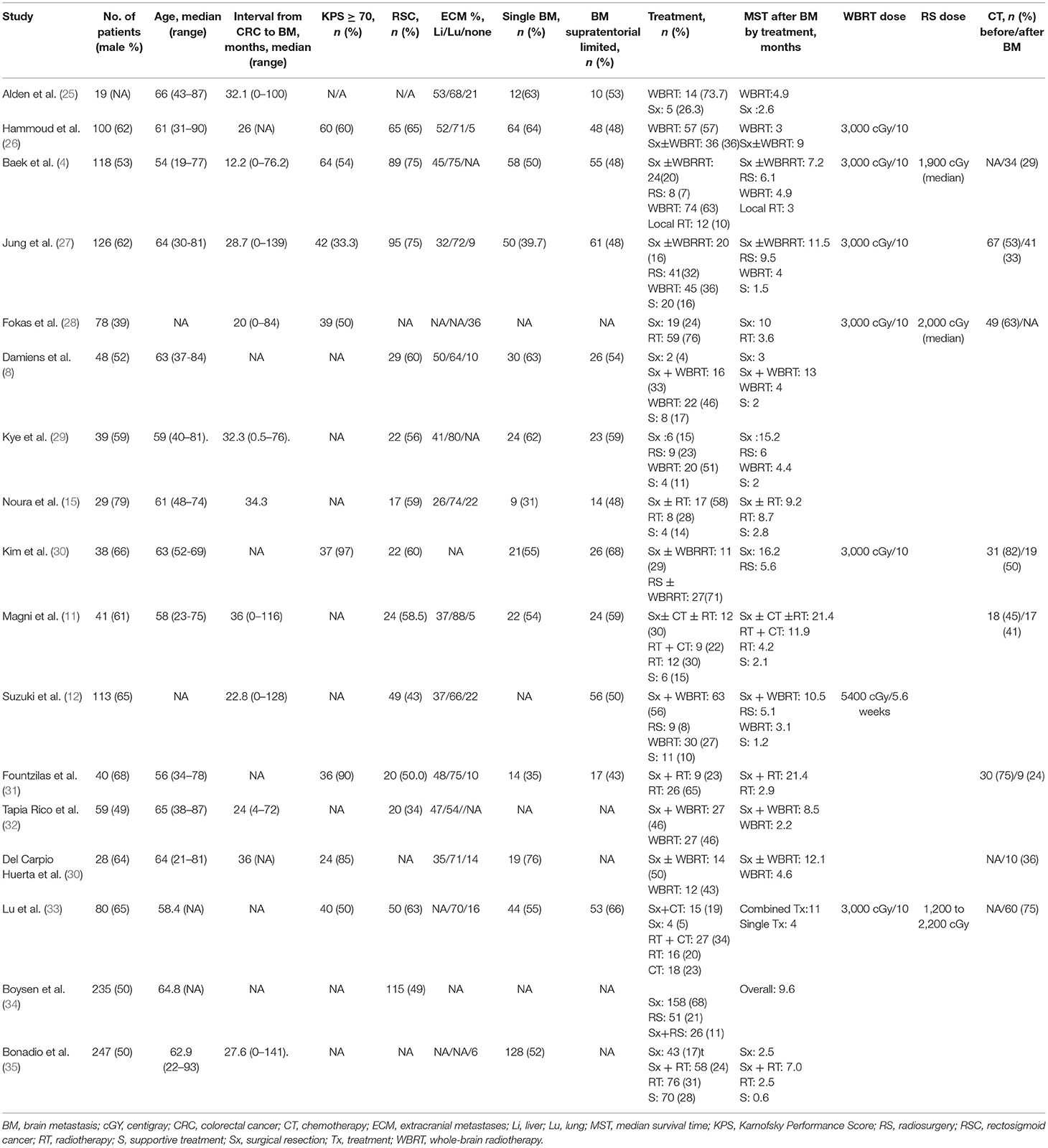
With advances in therapeutic modalities, the survival rate in patients with colorectal cancer (CRC) is greater than before (1). Since the blood–brain barrier impedes the penetration of most chemotherapeutic and biologic agents, the development of brain metastases (BM) increases along with survival, wherein up to 13% of patients with CRC have various forms of BM (1, 2). Regarding the time interval from primary CRC to BM, most BMs are found to be metachronous, whereas other BMs are synchronous (3, 4). Supratentorial BM is more frequent than that in the posterior fossa, and solitary metastases account for 40–60 % of the cases (5–7). Most patients have poor outcomes despite aggressive treatments, including surgery, radiotherapy, radiosurgery, and systemic therapy. The reported median survival time (MST) after the diagnosis of BM varies from 2.5 to 87 months in the literature (6, 7).
Concomitant extracerebral metastases (ECM) develop in most cases of BM, predominantly in the lungs and liver (8). Aggressive surgical metastasectomy of the hepatic and pulmonary metastases from CRC is a standard treatment strategy with benefits to survival outcome (9, 10). However, the efficacy of surgical resection of BM remains uncertain in patients with primary CRC. Although some studies have reported a longer overall survival (OS) in patients who underwent neurosurgical resection than in those receiving whole-brain radiation (WBRT) (11–13), discrepancies were found in the results of observational studies (14, 15). Moreover, the impact of newly developed multimodal BM-directed therapeutic approaches, such as stereotaxic radiosurgery (RS) and combined surgery with RS, was addressed in only a few studies (16). Although some retrospective studies aimed to identify outcome predictors and optimize treatment strategies, the relatively low incidence and the paucity of available comparisons between treatment modalities hindered the establishment of a treatment consensus for colorectal cancer with brain metastasis (CRC BM), which remains in a case-by-case fashion currently.
Given the incongruent literature, the optimal therapeutic approach for CRC patients with BM remains to be investigated.
To assess the efficacy of treatment strategies for CRC BM in the literature, including surgical resection, or radiotherapy, we performed a comprehensive review, as well as meta-analysis, of published literature.
Methods
The present systematic review and meta-analysis were based on the Cochrane Handbook for Systematic Reviews and Interventions (17). The results were reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines (Supplementary Methods 1, 2). This study was registered on the online platform PROSPERO (ID: CRD42021240200). The Cochrane Library (United Kingdom), Embase (Netherlands), Medline (United States) electronic databases were searched from the inception of the database until August 2021. Two independent investigators (YC and CEW) executed the search to identify relevant studies for inclusion. Discrepancies were resolved by a senior reviewer consultant (JSL) or by consensus. The exhibiting details of the search are presented in Supplementary Method 3.
Eligibility Criteria
The English-language articles with the following criteria were included: (1) randomized controlled trials and prospective or retrospective cohort studies, except conference abstracts, letters to the editor, case reports, editorials, and review articles; (2) the studies of adults with CRC BM; (3) the studies reporting comparative survival outcome of surgical resection vs. radiotherapy and using OS as an endpoint. Studies with insufficient data to calculate the hazard ratio (HR) to estimate the treatment effect were excluded. In cases of duplicate studies with an accumulating number of patients or different follow-up periods, only the one with the longest follow-up duration was included.
Data Extraction
Two investigators (YC and CEW) independently extracted the following data from eligible studies: first author's last name, year of publication, characteristics of the patients, and treatment strategies for patients with BM from CRC.
Quality Assessment
Two investigators (YC and CEW) independently completed a critical appraisal of the included literature using the Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) (18) tools. Additionally, a senior reviewer (JSL) addressed any item on which assessors did not reach the consensus.
Statistical Analysis
Statistical analyses were performed using the functions available in the metafor package (19) within the R Studio software, United States, (Method 4). Survival outcomes after BM were obtained by extracting the HR directly from each reference. When studies did not report the HR but was rather presented as the Kaplan–Meier survival curves, the estimated HR from these curves were obtained through a well-established method (20) using a calculation spreadsheet developed by Tierney et al. (21).
Hazard ratios (HRs) from the included studies were pooled through the inverse variance method using the random-effects model with the DerSimonian and Laird method (22) adopted for heterogeneity estimation. Furthermore, we aimed to alleviate the statistical and conceptual heterogeneity of our meta-analysis by performing subgroup analysis. For studies reporting multivariate risk factors or prognostic factors for OS, we also acquired HRs and pooled them in the meta-analysis.
The effect sizes were presented with their corresponding 95% CIs. Heterogeneity was assessed using I2 statistics proposed by Higgins and Thompson (23, 24), with I2 < 25%, 25% < I2 < 50%, and I2 > 50%, thereby indicating low, moderate, and high heterogeneity, respectively.
Publication Bias
For meta-analyses including more than 10 studies, we used a funnel plot to detect publication bias. The Egger's test was performed to indicate significant asymmetrical distribution with P < 0.05.
Results
Study Selection
Our search strategy identified 5,261 references from the Cochrane Library, Embase, and Medline electronic databases. After screening the titles and abstracts, we excluded duplicates (n = 696) and irrelevant references (n = 4,565). The remaining 59 studies were retrieved for a full-text review, 17 of which were considered eligible for qualitative and quantitative syntheses (Figure 1).
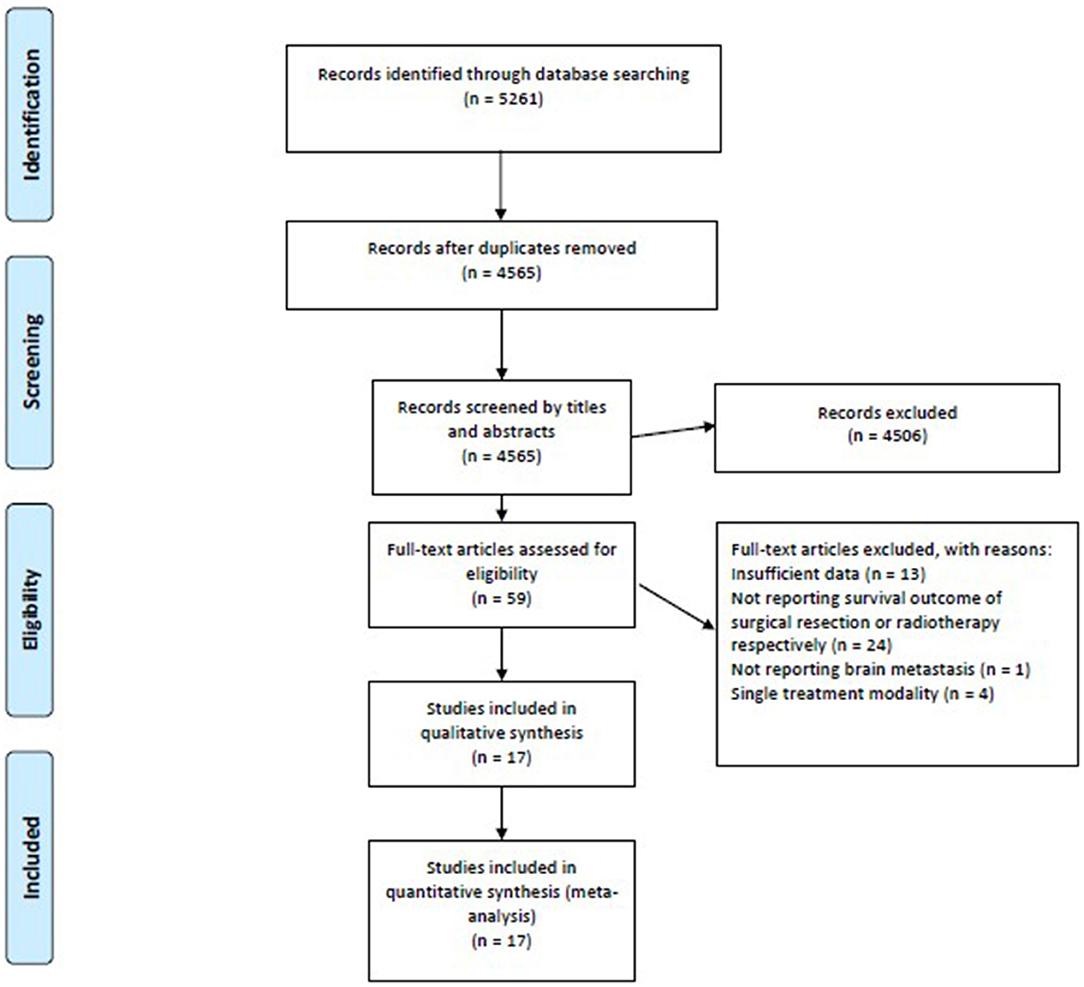
Figure 1. The PRISMA flow diagram demonstrates a total of 5,261 potential references were extracted initially, and the meta-analysis included 17 studies meeting the eligibility criteria. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Supplementary Table 1 summarizes the excluded articles after a full-text review.
Study Characteristics
As shown in Table 1, a total of 17 retrospective cohort studies (4, 8, 11–13, 15, 25–35), involving 1,438 patients with CRC BM were included. Among the 17 studies, 11 (4, 8, 12, 13, 25–27, 29, 30, 32, 34) studies clearly pointed out the radiation modalities with either WBRT or RS, whereas the remaining 6 (11, 15, 28, 31, 33, 35) did not.
Quality Assessment of Included Studies
Supplementary Table 2 demonstrates the summary of ROBINS-I of the included studies. No critical risk of bias was detected. Ten studies were assessed as serious risk of bias, and seven studies as the moderate risk of bias.
Survival Outcome
Regarding the survival outcome of treatment modalities for CRC BM, we compared the OS of patients receiving metastasectomy with those treated with radiotherapy. The surgical group exhibited better survival outcomes than the radiotherapy group, and no statistical heterogeneity was detected (HR, 0.53; 95% CI, 0.47–0.6; I2 = 0%) (Figure 2).
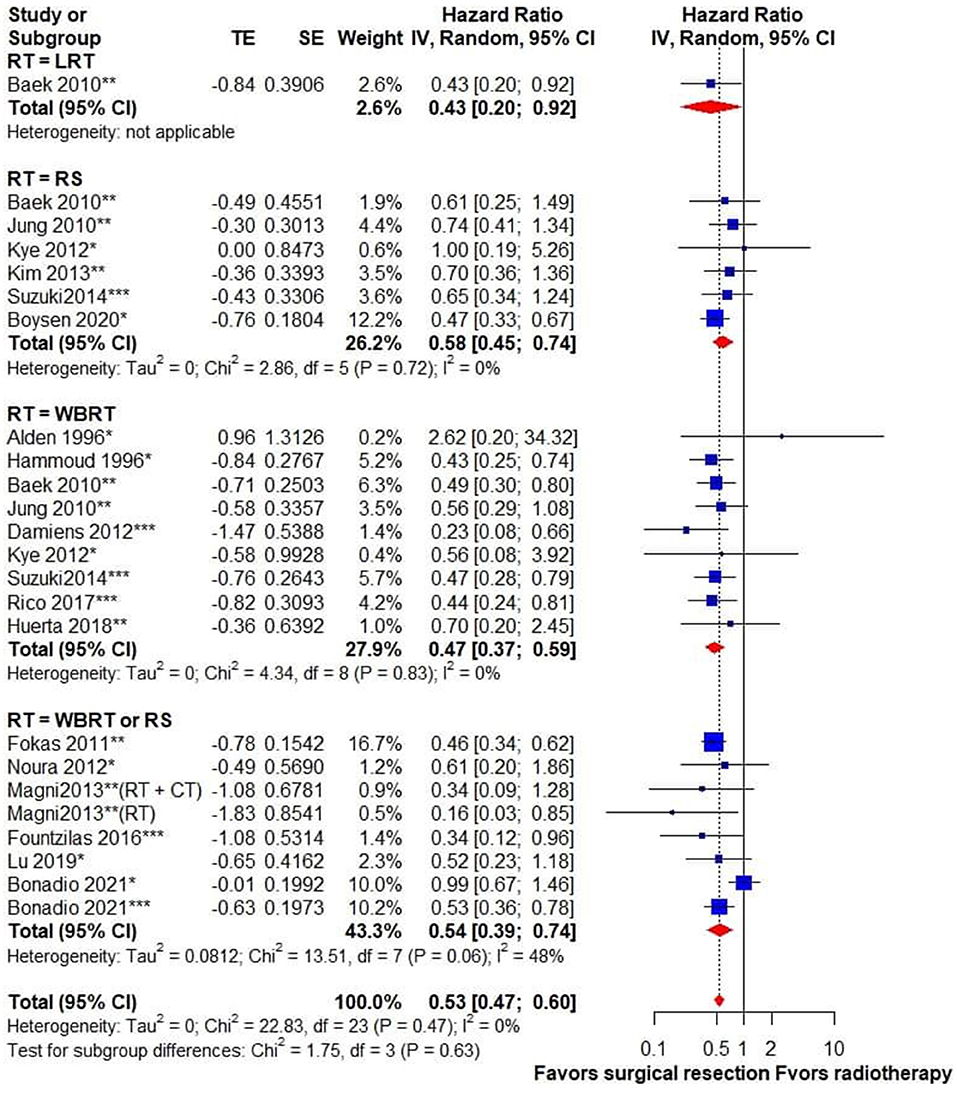
Figure 2. Forest plot for overall survival in patients receiving surgical resection vs. radiotherapy for brain metastasis from colorectal cancer. Pooled HR with 95% CI was calculated under random-effects models. HR, hazard ratio; CI, confidence interval; CT, chemotherapy; LRT, local radiotherapy; RT, radiotherapy; RS, radiosurgery; WBRT, whole-brain radiotherapy. *Surgical resection alone, **surgical resection ± RT, ***surgical resection + RT.
Subgroup Analysis
To identify the impact of different radiation modalities on the survival outcome, a subgroup analysis of surgery vs. WBRT and RS was performed. It indicated that surgery was associated with superior survival outcome when compared with either WBRT (HR, 0.47; 95% CI, 0.37–0.59; I2 = 0%) or RS (HR, 0.58; 95% CI, 0.45–0.74; I2 = 0%). Subgroup analysis based on univariate or adjusted HRs showed no statistical subgroup differences (Supplementary Figure 1). We also performed a subgroup analysis by classifying studies published within 5 years (after 2016) or more than 5 years ago (before 2016), and there was no subgroup difference (Supplementary Figure 2). Subgroup analysis based on different sample sizes showed similar results in studies with different case numbers (Supplementary Figure 3).
Prognostic Factors
The pooled result of prognostic factors from the included studies indicated that patients with ECM (HR, 1.58; 95% CI, 1.34–1.86; I2 = 10%) and multiple BM (HR, 1.38; 95% CI, 1.1–1.72; I2 = 56%) had worse survival outcome (Supplementary Figures 4, 5). Whether BM developed metachronously or synchronously was not associated with the survival outcome (HR, 2.05; 95% CI, 0.89–4.77; I2 = 0%) and the primary CRC location (rectum or colon) had little impact on the OS (HR, 0.8; 95% CI, 0.35–1.82; I2 = 89%) (Supplementary Figures 6, 7).
Publication Bias
The visually symmetrical funnel plot with Egger's test result (p = 0.85) indicated no potential publication bias (Supplementary Figures 8, 9).
Discussion
Key Findings
This is the first meta-analysis to compare different treatment modalities for CRC BM in terms of survival outcome. Our findings suggested that patients undergoing surgical resection of BM were associated with longer survival. Moreover, these findings would be reinforced by the large sample size and low heterogeneity across the included studies.
Surgical Resection vs. Radiotherapy
Brain metastasis was considered an end-stage disease because of its poor survival. Given the inevitable craniotomy-related surgical risks and the advance in radiotherapeutic techniques, a large group of patients with CRC BM opts for non-surgical radiation modalities as local treatment.
Neurological improvement after WBRT has been reported in BM from various types of cancer (36). However, the majority of reported MST after WBRT for CRC BM was <5 months (3, 4, 6, 12, 16, 25, 26, 28, 32). In contrast, the MST after BM has been reported to be over 10 months in patients treated surgically (6, 7, 12, 13, 28). Since metastatic CRC harbors a unique survival benefit from aggressive metastasectomy in hepatic and pulmonary metastases, we drew attention to the potential benefit of brain metastasectomy in CRC. The result of the pooled analyses provides quantitative evidence indicating that patients who received surgical resection of BM were associated with longer survival compared with those receiving WBRT.
The recent advances in radiosurgical modalities in recent years including Gamma Knife or CyberKnife, which can precisely deliver high-dose radiation to lesions <2.5 cm, can be applied to single as well as multiple lesions simultaneously (37, 38). Therefore, RS has drawn attention in the treatment of CRC BM and was proposed as either an alternative or an add-on to WBRT (4, 27, 30). However, incongruent results were reported. While Matsunaga et al. (39). have reported the benefit of RS for the suppression of local tumor growth in their single-arm study, the survival time in patients treated with RS was not longer than those receiving metastasectomy in other studies (12, 30, 34). Through a more robust statistical method, our analysis indicated that patients undergoing surgical resection of BM exhibited better survival outcomes than RS.
The current surgical indications of elective BM resection, including stable systemic disease, Karnofsky performance score (KPS) of >70, and surgically accessible lesion (40), were largely based on the previous observations in patients with primary lung cancer, breast cancer, and renal cell carcinoma. These indications may not reflect the real applicability in CRC BM, and more patients could potentially benefit from surgical resection. Further investigation using well-designed prospective studies may provide clearer indications for surgical resection of CRC BM.
Although the survival benefit of BM metastasectomy was observed in our analysis, this beneficial effect may be potentially associated with the adjuvant radiotherapy administered following surgical resection. The surgical group contained patients receiving radiotherapy in combination with surgery in several studies (8, 11, 27, 28, 30, 32). Recurrence in the surgical bed is common following resection alone for BM; therefore, several trials (41, 42) reported the potential benefit of postoperative radiotherapy with WBRT or RS. However, it should be noted that only a portion of the studies explicitly described whether patients in the surgical group received adjuvant radiotherapy.
Prognostic Factors
From the pooled results, we observed that the presence of ECM may be associated with poor survival outcomes. A recent study (43) focusing on the relevance of extracranial metastatic patterns and the survival outcome of patients with CRC BM showed that patients with lung metastasis lived longer than those with liver metastasis, and concurrent liver and lung metastasis were associated with the worst survival outcome. However, only five studies (4, 12, 28, 30, 35) reported the survival outcome of patients with and without ECM, and the survival of patients with different metastatic patterns was not reported in the included studies.
The other significant factor predicting poor survival outcome was multiple BM. We believed that the presence of multiple metastases may affect the choice of local treatment for BM; however, the presence of multiple BM is not a contraindication for metastasectomy (44). Unfortunately, the criteria to determine surgery or radiotherapy for patients with CRC BM were not reported in studies included in our meta-analysis. In reality, the treatment choice for surgical resection or radiotherapy for CRC BM may not only be influenced by the number of metastases but also by the anatomic location, surgically accessibility, the condition of patients, and the experience of surgeons. The effect of the potential allocation bias could not be clearly clarified in our study and should be addressed in future studies.
Comparison With the Previous Synthesis
In a recently published systematic review (45), Müller et al. included 86 articles to investigate the incidence, symptoms, diagnosis, treatment, and prognosis of CRC BM. Although it provided a comprehensive perspective regarding CRC BM, the inclusion criteria were not clearly defined in their method. In contrast, we emphasized the current evidence on local treatment modalities. As a result, we focused on studies comparing the treatment outcomes and included fewer studies than the aforementioned review. Although the authors summarized predictors for poor survival in CRC BM, such as advanced age, low KPS, ECM, multiple BM, and elevated carcinoembryonic antigen, direct comparison among therapeutic approaches was still omitted. Our meta-analysis is the first one focusing on comparative survival outcomes of patients with CRC BM receiving different local treatment modalities.
Limitations
The present meta-analysis has limitations. First, there were several studies not included in our review due to insufficient data for survival outcomes based on different treatments. Second, all included studies were retrospective cohorts and the detailed baseline characteristics in each treatment group were limited; therefore, the bias due to confounders would not be well-adjusted. Third, the paucity of information on adjuvant radiotherapy in the surgical group hindered an accurate assessment of the treatment modalities. Fourth, our results supported the survival benefit of surgical resection compared with radiotherapy for BM; however, the selection criteria for a patient of surgery or radiotherapy were not detailed in most of the studies included in our meta-analysis.
Last, the lack of details regarding the chemotherapeutic agents in the included studies limited our assessment of their effect. Similarly, although Fountzilas et al. (31) reported survival benefits of patients receiving biologic agents after BM, no relevant data in other included studies were reported. Thus, it is difficult to value the effect of chemotherapeutic and biologic agents for patients with CRC BM treated with surgical resection or radiotherapy.
Conclusions
This systematic review and meta-analysis involving patients with CRC BM have demonstrated the benefit of aggressive neurosurgical management in suitable patients. Additionally, identifying prognosticators including ECM and multiple BM would aid in the treatment and decision-making in these patients. Considering the potential limitations, further prospective studies are warranted.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
YC and C-EW contributed to study conceptualization, data curation, statistical analysis, data interpretation, drafting of the manuscript, and has full access to all the data in the study. P-HL contributed to statistical analysis, data interpretation, and critical review of the manuscript. C-CH contributed to data interpretation and critical review of the manuscript. J-SL contributed to study conceptualization, data interpretation, critical review of the manuscript, and is responsible for the integrity and accuracy of data analysis. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to Sheng-Hsiang Lin for providing the statistical consulting services from the Biostatistics Consulting Center, National Cheng Kung University Hospital.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.768896/full#supplementary-material
References
1. Nieder C, Spanne O, Mehta MP, Grosu AL, Geinitz H. Presentation, patterns of care, and survival in patients with brain metastases: what has changed in the last 20 years? Cancer. (2011) 117:2505–12. doi: 10.1002/cncr.25707
2. Christensen TD, Spindler KL, Palshof JA, Nielsen DL. Systematic review: brain metastases from colorectal cancer–incidence and patient characteristics. BMC Cancer. (2016) 16:260. doi: 10.1186/s12885-016-2290-5
3. Byrne BE, Geddes T, Welsh FK, John TG, Chandrakumaran K, Rees M. The incidence and outcome of brain metastases after liver resection for colorectal cancer metastases. Colorectal Dis. (2012) 14:721–6. doi: 10.1111/j.1463-1318.2011.02762.x
4. Baek JY, Kang MH, Hong YS, Kim TW, Kim DY, Oh JH, et al. Characteristics and prognosis of patients with colorectal cancer-associated brain metastases in the era of modern systemic chemotherapy. J Neurooncol. (2011) 104:745–53. doi: 10.1007/s11060-011-0539-z
5. Nozawa H, Ishihara S, Kawai K, Sasaki K, Murono K, Otani K, et al. Brain metastasis from colorectal cancer: predictors and treatment outcomes. Oncology. (2017) 93:309–14. doi: 10.1159/000478661
6. Farnell GF, Buckner JC, Cascino TL, O'Connell MJ, Schomberg PJ, Suman V. Brain metastases from colorectal carcinoma: the long term survivors. Cancer. (1996) 78:711–6. doi: 10.1002/(SICI)1097-0142(19960815)78:4<711::AID-CNCR3>3.0.CO;2-H
7. Ko FC, Liu JM, Chen WS, Chiang JK, Lin TC, Lin JK. Risk and patterns of brain metastases in colorectal cancer: 27-year experience. Dis Colon Rectum. (1999) 42:1467–71. doi: 10.1007/BF02235049
8. Damiens K, Ayoub JP, Lemieux B, Aubin F, Saliba W, Campea MP, et al. Clinical features and course of brain metastases in colorectal cancer: an experience from a single institution. Curr Oncol. (2012) 19:254–8. doi: 10.3747/co.19.1048
9. Shimizu K, Ohtaki Y, Okumura T, Boku N, Horio H, Takenoyama M, et al. Outcomes and prognostic factors after pulmonary metastasectomy in patients with colorectal cancer with previously resected hepatic metastases. J Thorac Cardiovasc Surg. (2019) 157:2049–57.e2041. doi: 10.1016/j.jtcvs.2018.12.075
10. Ampollini L, Gnetti L, Goldoni M, Viani L, Faedda E, Campanini N, et al. Pulmonary metastasectomy for colorectal cancer: analysis of prognostic factors affecting survival. J Thorac Dis. (2017) 9:s1282–90. doi: 10.21037/jtd.2017.07.100
11. Magni E, Santoro L, Ravenda PS, Leonardi MC, Bonomo G, Monfardini L, et al. Brain metastases from colorectal cancer: main clinical factors conditioning outcome. Int J Colorectal Dis. (2014) 29:201–8. doi: 10.1007/s00384-013-1781-y
12. Suzuki Y, Yamaguchi T, Matsumoto H, Nakano D, Honda G, Shinoura N, et al. Prognostic factors and treatment effects in patients with curatively resected brain metastasis from colorectal cancer. Dis Colon Rectum. (2014) 57:56–63. doi: 10.1097/01.dcr.0000436998.30504.98
13. Del Carpio Huerta L, Virgili Manrique AC, Szafranska J, Martin-Richard M, Paez Lopez-Bravo D, Sebio Garcia A, et al. Brain metastases in colorectal cancer: prognostic factors and survival analysis. Int J Colorectal Dis. (2018) 33:1517–23. doi: 10.1007/s00384-018-3107-6
14. Onodera H, Nagayama S, Tachibana T, Fujimoto A, Imamura M. Brain metastasis from colorectal cancer. Int J Colorectal Dis. (2005) 20:57–61. doi: 10.1007/s00384-004-0631-3
15. Noura S, Ohue M, Shingai T, Fujiwara A, Imada S, Sueda T, et al. Brain metastasis from colorectal cancer: prognostic factors and survival. J Surg Oncol. (2012) 106:144–8. doi: 10.1002/jso.23055
16. Nieder C, Pawinski A, Balteskard L. Colorectal cancer metastatic to the brain: time trends in presentation and outcome. Oncology. (2009) 76:369–74. doi: 10.1159/000210026
17. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane (2021). Available online at: www.training.cochrane.org/handbook (accessed August 31, 2021).
18. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
19. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. (2010) 36:1–48. doi: 10.18637/jss.v036.i03
20. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17:2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8
21. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
22. Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird's methodology to perform multivariate random effects meta-analyses. Stat Med. (2010) 29:1282–97. doi: 10.1002/sim.3602
23. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
24. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
25. Alden TD, Gianino JW, Saclarides TJ. Brain metastases from colorectal cancer. Dis Colon Rectum. (1996) 39:541–5. doi: 10.1007/BF02058708
26. H Hammoud MA, McCutcheon IE, Elsouki R, Schoppa D, Patt YZ. Colorectal carcinoma and brain metastasis: distribution, treatment, and survival. Ann Surg Oncol. (1996) 3:453–63. doi: 10.1007/BF02305763
27. Jung M, Ahn JB, Chang JH, Suh CO, Hong S, Roh JK, et al. Brain metastases from colorectal carcinoma: prognostic factors and outcome. J Neurooncol. (2011) 101:49–55. doi: 10.1007/s11060-010-0214-9
28. Fokas E, Henzel M, Hamm K, Surber G, Kleinert G, Engenhart-Cabillic R. Multidisciplinary treatment of brain metastases derived from colorectal cancer incorporating stereotactic radiosurgery: analysis of 78 patients. Clin Colorectal Cancer. (2011) 10:121–5. doi: 10.1016/j.clcc.2011.03.009
29. Kye BH, Kim HJ, Kang WK, Cho HM, Hong YK, Oh ST. Brain metastases from colorectal cancer: the role of surgical resection in selected patients. Colorectal Dis. (2012) 14:e378–385. doi: 10.1111/j.1463-1318.2012.02962.x
30. Kim HJ, Huh JW, Jung TY, Kim IY, Kim HR, Jung S, et al. Clinical outcome with gamma-knife surgery or surgery for brain metastases from colorectal cancer. J Clin Neurosci. (2013) 20:1417–21. doi: 10.1016/j.jocn.2012.12.020
31. Fountzilas C, Chang K, Hernandez B, Michalek J, Crownover R, Floyd J, et al. Clinical characteristics and treatment outcomes of patients with colorectal cancer who develop brain metastasis: a single institution experience. J Gastrointest Oncol. (2017) 8:55–63. doi: 10.21037/jgo.2016.12.11
32. Tapia Rico G, Price TJ, Karapetis C, Piantadosi C, Padbury R, Roy A, et al. Brain metastasis in advanced colorectal cancer: results from the South Australian metastatic colorectal cancer (SAmCRC) registry. Cancer Biol Med. (2017) 14:371–6. doi: 10.20892/j.issn.2095-3941.2017.0068
33. Lu X, Cai Y, Xia L, Ju H, Zhao X. Treatment modalities and relative survival in patients with brain metastasis from colorectal cancer. Biosci Trends. (2019) 13:182–8. doi: 10.5582/bst.2019.01044
34. Boysen AK, Ording AG, Astradsson A, Høyer M, Spindler KL. Metastasis directed treatment of brain metastases from colorectal cancer–a Danish population-based cohort study. Acta Oncol. (2020) 59:1118–22. doi: 10.1080/0284186X.2020.1769861
35. Bonadio RC, Freitas GF, Batista DN, Moreira OAN, Dias CAR, Castria TB, et al. Epidemiology and outcomes of patients with brain metastases from colorectal cancer-who are these patients? Clin Colorectal Cancer. (2021) 20:e195–200. doi: 10.1016/j.clcc.2021.04.002
36. Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. (2006) 24:1295–304. doi: 10.1200/JCO.2005.04.6185
37. Kocher M, Wittig A, Piroth MD, Treuer H, Seegenschmiedt H, Ruge M, et al. Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO working group on stereotactic radiotherapy. Strahlenther Onkol. (2014) 190:521–32. doi: 10.1007/s00066-014-0648-7
38. Nieder C, Grosu AL, Gaspar LE. Stereotactic radiosurgery (SRS) for brain metastases: a systematic review. Radiat Oncol. (2014) 9:155. doi: 10.1186/1748-717X-9-155
39. Matsunaga S, Shuto T, Inomori S, Fujino H, Yamamoto I. Gamma knife radiosurgery for intracranial haemangioblastomas. Acta Neurochir. (2007) 149:1007–13 doi: 10.1007/s00701-007-1274-2
40. Soffietti R, Rudā R, Mutani R. Management of brain metastases. J Neurol. (2002) 249:1357–69. doi: 10.1007/s00415-002-0870-6
41. Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. (2017) 18:1049–60. doi: 10.1016/S1470-2045(17)30441-2
42. Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. (1998) 280:1485–9. doi: 10.1001/jama.280.17.1485
43. Thurmaier J, Heinemann V, Engel J, Schubert-Fritschle G, Wiedemann M, Nüssler NC, et al. Patients with colorectal cancer and brain metastasis: the relevance of extracranial metastatic patterns predicting time intervals to first occurrence of intracranial metastasis and survival. Int J Cancer. (2021) 148:1919–27. doi: 10.1002/ijc.33364
44. Paek SH, Audu PB, Sperling MR, Cho J, Andrews DW. Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery. (2005) 56:1021–34. doi: 10.1227/01.NEU.0000158321.90608.BE
Keywords: metastasectomy, radiotherapy, surgical resection, colorectal cancer, brain metastases
Citation: Chang Y, Wong C-E, Lee P-H, Huang C-C and Lee J-S (2022) Survival Outcome of Surgical Resection vs. Radiotherapy in Brain Metastasis From Colorectal Cancer: A Meta-Analysis. Front. Med. 9:768896. doi: 10.3389/fmed.2022.768896
Received: 01 September 2021; Accepted: 24 January 2022;
Published: 08 March 2022.
Edited by:
Michel Gonzalez, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Stefan Sponholz, Agaplesion Markus Krankenhaus, GermanyLuis Schiappacasse, Centre Hospitalier Universitaire Vaudois (CHUV), Switzerland
Copyright © 2022 Chang, Wong, Lee, Huang and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jung-Shun Lee, bnNsZWUxMjE4QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yu Chang1†
Yu Chang1† Chia-En Wong
Chia-En Wong Po-Hsuan Lee
Po-Hsuan Lee Chi-Chen Huang
Chi-Chen Huang Jung-Shun Lee
Jung-Shun Lee