- 1Centre of Experimental Medicine and Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
- 2Department of General Medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
- 3Department of Biochemistry, Institute of Science, Banaras Hindu University, Varanasi, India
Fever remains an integral part of acute infectious diseases management, especially for those without effective therapeutics, but the widespread myths about “fevers” and the presence of confusing guidelines from different agencies, which have heightened during the coronavirus disease 2019 (COVID-19) pandemic and are open to alternate interpretation, could deny whole populations the benefits of fever. Guidelines suggesting antipyresis for 37.8–39°C fever are concerning as 39°C boosts the protective heat-shock and immune response (humoral, cell-mediated, and nutritional) whereas ≥40°C initiates/enhances the antiviral responses and restricts high-temperature adapted pathogens, e.g., severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), strains of influenza, and measles. Urgent attention is accordingly needed to address the situation because of the potential public health consequences of the existence of conflicting guidelines in the public domain. We have in this article attempted to restate the benefits of fever in disease resolution, dispel myths, and underline the need for alignment of national treatment guidelines with that of the WHO, to promote appropriate practices and reduce the morbidity and mortality from infectious diseases, such as COVID-19.
Introduction
Fever is integral to our natural defense against acute clinical infections, especially those without effective therapeutics, e.g., common cold, measles, and influenza (1–9). It is an evolutionary conserved adaptive response, at least >400 million years old, protecting us from potentially dangerous pathogens (1, 2, 5–7, 10–12). Kindly note, the term “fever(s)” in the manuscript narrowly applies to the elevation of body temperature in response to an infection, unlike its broader usage in the literature for any temperature elevation. The beneficial role of fever in controlling infectious disease is well recognized and remains part of standard immunology textbooks. Janeway's Immunobiology states “At higher temperatures, bacterial and viral replication is less efficient, whereas the adaptive immune response operates more efficiently” [(13), p.110]. The Kuby Immunology (14) describes “fevers” as “helps to eliminate many temperature-sensitive bacterial strains” (p. 223), “a protective response, as elevated body temperature inhibits replication of some pathogens” (p.323), “decrease microbial viability” (p. 401), etc. Saladin's Anatomy and Physiology, summarizes its role as “Fever is beneficial in that it (1) promotes interferon activity, (2) inhibits the reproduction of bacteria and viruses, and (3) elevates metabolic rate and accelerates tissue repair.” [(15), p.818]. However, the value of fever (≥39°C) in infectious disease resolution and control remains highly underappreciated in the age of antimicrobials and vaccines. The practice of antipyresis has changed tremendously in the last 50–60 years (16–25). It is being recommended for temperatures as low as 37.8–38°C (26, 27).
Recent publications of advisories and guidelines for coronavirus disease 2019 (COVID-19) management (28–31), in disagreement with the existing fever management guidelines from the WHO and various national/local guidelines (32–36) are concerning. Furthermore, there had been a dearth of publications on fever management during the COVID-19 pandemic. As of November 2, 2021, the “PubMed” database search for keyword “COVID-19” fetched 183,837 articles whereas keyword combinations, “COVID-19” AND “Fever”, “COVID-19” AND “Antipyresis”, “COVID-19” AND “Fever” AND “Antipyresis”, and “COVID-19” AND “Fever management” yielded 5,941, 1, 0, and 5 articles, respectively. Out of five articles on COVID-19 fever management, only one (editorial) stressed the need for personalized fever management (37). It prudently suggests ‘targeted temperature management' for patients with low O2 saturation or other life-threatening complications only on the need basis. Retrospective data analysis indicates that fever may increase survival in different COVID-19 situations (38). However, conflicting fever management guidelines approving aggressive antipyresis remain in the public domain (28–31) subjecting public health to infectious diseases, such as COVID-19.
The value of fever has remained well recognized through the ages in different classical treatment systems since the times of ancient physicians, such as Charak and Hippocrates (39–42). The Ayurveda classics, such as “Charak (also Charaka or Caraka) Samhita” (39) and the current naturopathy practice, recognize the role of fever as a facilitator of the elimination of pathogens or toxins (“doshas”) from the body. In modern times, the early 20th-century work of Julius Wagner-Jauregg on neurosyphilis or “dementia paralytica”—an untreatable terminal manifestation of syphilis characterized by progressive paralysis and insanity (43), reestablished and popularized the therapeutic potential of fever. His experimentation with different fever-inducing agents, e.g., tuberculin, streptococci, and malaria parasites, identified malarial parasite inoculation as the most satisfactory method for treatment (44). For this monumental work, he received the Nobel Prize in 1927. Wagner hypothesized the curative potential of fever based upon the reduced occurrence of “dementia paralytica” in malaria-endemic regions and the reported spontaneous curing in isolated cases after a febrile illness (45). Analogous reduced COVID-19 mortality has been observed in malaria and tuberculosis endemic regions of the world (46, 47). The populations' genetic constitution and the cellular immunity-boosting effect of these infections have been proposed as protective determinants (46, 48). The reduced likelihood of aggressive antipyresis practice in these poor regions could have been another favorable determinant as temperatures ≥39°C inhibited severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication (49).
We have attempted in this manuscript to further restate the important role of fever in infectious disease resolution, and dispel the basis for fever paranoia. In addition, we discuss the need to harmonize the many publicly available guidelines on fever management with that of the WHO, particularly concerning the management of COVID-19.
Role of Fever in Response to Infectious Diseases
Fever plays multiple roles in infectious disease control (1–25). During acute infections, the brain-controlled incremental elevation of body temperature up to 39–42°C is seen over several days with intervening normothermia (no fever). Literally, each temperature elevation increases the body's thermotolerance—preparing it for the next higher temperature exposure possibly required to stop the pathogen replication and further enhance the immune response/functions.
Fever of about 39°C boosts the production and accumulation of various heat shock proteins (HSPs) and metallothioneins as a part of protective heat shock response (HSR) to prepare the host cells against any potentially damaging higher or ‘supraphysiological temperatures' exposure and inflammatory response (50–54). It helps amicably resolve any threatening stressful situations of thermal, oxidative, and metabolic nature arising from an overwhelming inflammatory response to the persisting infection (55–57). The repeated heat shocks (such as, recurrent fever), estrogens (E2), and cyclopentenone prostaglandins produced in later stages of disease/inflammation enhance HSR. Mechanistic insights about the potential protective role of HSR in COVID-19 have been reviewed recently (58). Incidentally, robust HSR generation is suppressed in most of the COVID-19 comorbid conditions, e.g., cardiovascular diseases, obesity, frailty, diabetes, and metabolic syndromes (58). Protective alterations in essential metal ions levels are made in different internal milieus (nutritional immunity) during the acute-phase response (59–62). For example, reduced serum zinc levels protect the host tissues from oxidative damage and enhance the chemotaxis and immune cells' activity, such as the targeted intracellular pathogen killing (63–65). Zinc supplementation during infections has been correlated with increased mortality in diseases, such as HIV and COVID-19 (66, 67).
Temperature elevation to about 40°C enhances the production of antiviral interferons (IFNs) (~10 times) and various interleukins that promote pathogen clearance and reduce inflammatory damage (1, 54, 68–70). The IFNs help the surrounding uninfected cells attain a viral infection refractory state through the induction of a complex web of host genes that prevent viral infection and replication (68). The IFNs mediated activation of NK cells and macrophages plays a key role in disabling the viral replication cycle by actively seeking and destroying the virus-infected cells (1, 54, 69). Additionally, it dampens the viral infection by inducing innate and intrinsic antiviral responses (71). The HSR and IFNs, together, orchestra a balanced inflammatory response that minimizes the host damage without jeopardizing an aggressive cell-mediated response for pathogen clearance, e.g., activation of cytotoxic activity of NK cells, K cells, T cells, the cytocidal activity of macrophages, and activation of suppressor T cells (1, 13, 14, 52, 54).
The appearance of increasing magnitude fevers over time, up to 42°C, is commonplace (22, 23, 72). Gradual elevation of body temperature acts as a failsafe mechanism to eliminate those pathogens that have survived/escaped (mutants) lower temperature exposures (73–76). The temperature of ≥ 39°C, suggested for antipyresis by the WHO, is “restrictive,” i.e., inhibits replication/growth, for most pathogens (73). Higher body temperatures facilitate the clearance of pathogens with higher “restrictive temperature,” e.g., 39–40°C reduces SARS-CoV-2's replication by >100-fold (49); 41–42°C reduces poliovirus' replication by ~200-fold (75) while greatly enhances the serum-induced lysis of Gram-negative bacteria (77). Temperature elevation between 35°C and 41.5°C also increases antimicrobials' efficacy by 4–16 times (74) making the treatment of infections/coinfections of inherently lower temperature niches easier (76).
The fever-induced loss of appetite (anorexia) favorably affects infectious disease resolution. The induced processes, such as unfolded protein response, classical starvation regulated responses, and ketogenesis, supposedly augment survival by reducing reactive oxygen species (ROS) mediated neural tissues damage and the inflammatory damage of other organs, such as the cardiovascular system (78–80). The anorexia-associated beneficial changes in physiology speed-up the repair and recovery process without jeopardizing immune system functions. Animal experiments indicate “fasting” in bacterial diseases and “caloric restriction” (light sugar/glucose intake) in viral disease to greatly diminish mortality rates, essentially supporting the classical notion of “feeding the flu (viral) while starving the fever (bacterial)” (81). Though there remains a dearth of human studies, the classical Indian medicine system and Ayurveda treatises, which are some of the oldest existing medical texts and form the basis of treatment (including the home remedies) for the majority in the Indian subcontinent, do recommend oddly similar personalized diet regimens for patients. For example, “Charak Samhita” recommends elaborate personalized feeding strategies that range from levels of fasting to light intake of sugary foods and meat-soups in respiratory tract infections for different durations as per the stage of infection and patient's condition [(39), p. 65; Sukt:139–141, p. 67; Sukt:149–156], while categorically restricts the intake of fatty and heavy food [(39), p. 80; Sukt:272–283]. Moreover, for acute infections, it advocates a delay in recuperative and fever-pacifying interventions till the fever matures (i.e., performed its function; up to 6 days) on the concerns of complicating the recovery [(39), p. 67; Sukt:160 and others], something aligned with modern understanding of the role of fever in disease resolution and the concepts of nutritional immunity (54, 59, 60). A delay of up to 10 days is suggested for weak patients who still have or had a low-grade fever [(39), p. 80; Sukt:272–283]. Such traditional medicine recommendations could be worth testing in different disease models as per the modern concepts to verify their applicability in infectious disease management. Finally, the benefit of fever-induced anorexia and myalgia-precipitated inactivity in reducing host tissue damage and pathogen dissemination (keeping the Ro down) cannot be overlooked.
Fever Phobia: Prevalence, Possible Causes, and Implications
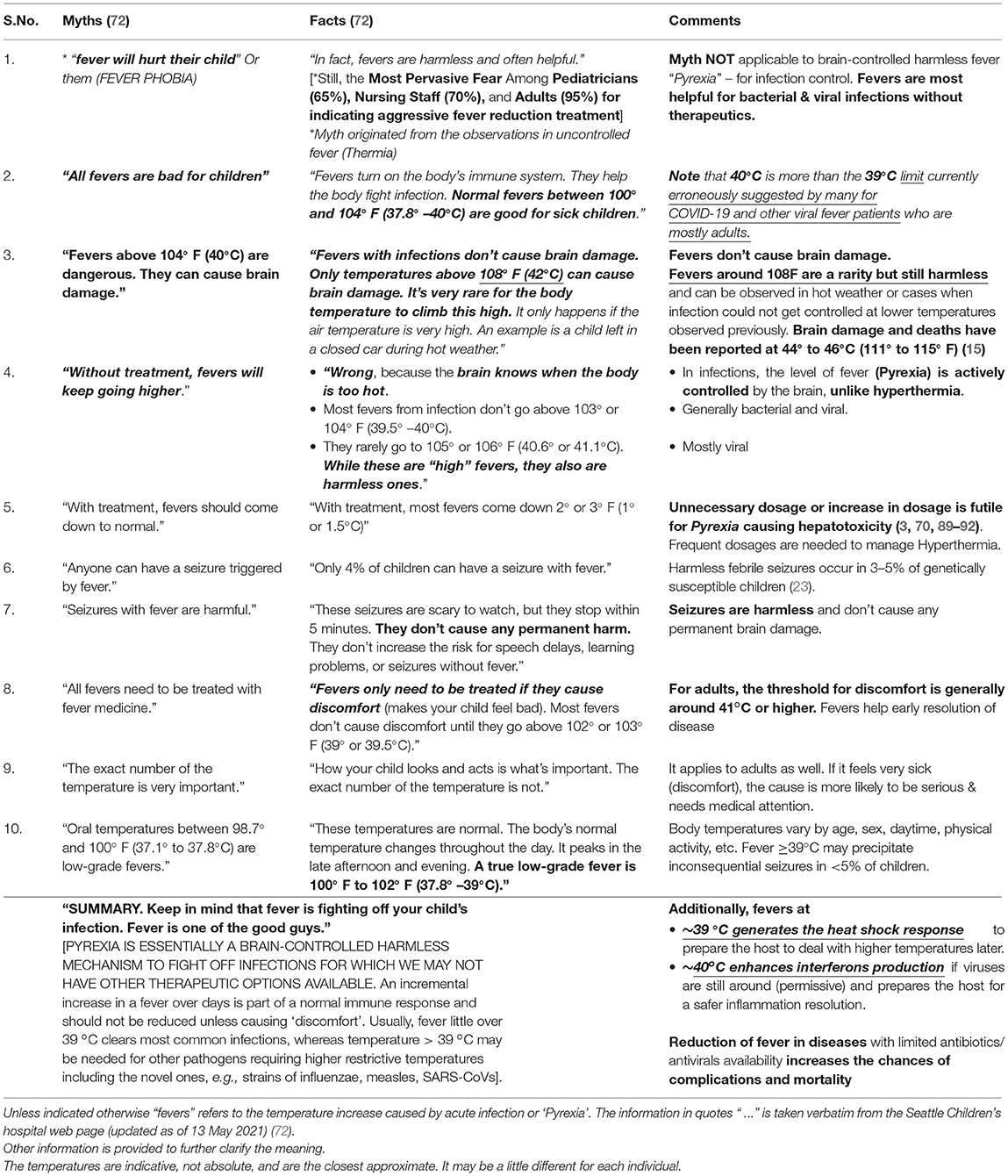
The phobia of fever remains extensive among the general public, nursing staff, and clinicians, despite the body of theoretical and practical/experimental evidence to the contrary (14–22, 36, 72, 82). It has been gradually increasing over the years and promoting the continuation of non-evidence-based irrational management practices (16–25, 72, 82). A rapid increase in unawareness of the role of fever in infections and fever phobia was observed from the 1980s to 2010 among clinicians (12–65%) (3, 22, 23), nursing staff (70%) (83, 84) and parents (60% to 90–95%) in the United States (3, 19, 22–25, 83) and other countries (21, 24, 25, 42). Considering the potential impact of unnecessary antipyretics usage on public health (3, 5–7, 11, 12, 70), the WHO and various national agencies have made efforts to increase the awareness among the stakeholders to improve fever management practices (32–36).
Some potential underlying causes promoting the current notion of treating every “fever” along with possible implications are briefly discussed below:
1. Inability to differentiate fever by type and terminology mess: The inability to differentiate the common “hyperpyrexia” from “hyperthermia” is widespread. The former beneficial one results from the brain-controlled incremental increase in temperature during infection, while the latter concerning one results from uncontrolled temperature elevation on the failure of thermoregulatory mechanism as observed in heat shock (15). The pervasive confusion partly stems from the liberal usage of terms “fever,” “pyrexia,” and “Hyperthermia” in literature for a range of temperatures above 37°C. In the literature, the term “hyperpyrexia” has been used for anesthesia-driven uncontrolled temperature elevation as well (85). It makes the situation chaotic, as any temperature elevation could be construed as “hyperthermia” so naturally “dangerous” and “fit for treatment”. The coining and consistent usage of the precise terms for different fever types is required. Reserving the usage of “hyperpyrexia” or more explanatory term “pathogen-hyperpyrexia” for the fevers of infectious disease origin while “hyperthermia” for non-brain-controlled fever is suggested to reduce the prevalent confusion among clinicians and public concerning their management. In clinical practice, many a time it is difficult to know the origin of a fever, so the alleviation of symptoms takes precedence as “why take chances” when antimicrobials and antipyretics are readily available (86, 87). However, the prescription of antipyretics for diseases without effective therapeutics makes it a risky proposition (e.g., COVID-19, influenzae, and measles).
2. Fear of Brain Damage From High Fever: The widely ingrained fear of brain damage including among physicians perceptibly originates from the experience of seizures and writhing observed around 39°C in 2–5% children <5-year-old (23, 32, 70, 72), largely ignoring the hard evidence that in almost last one century no one died or had brain damage from the normal course of fever during infections, except an often-quoted anecdotal report from the 1950s (88). Still up to 65% of physicians considered fever as harmful and 90% believed that febrile convulsions could cause brain damage (20, 22, 23) even though temperatures up to 42°C (108°F) are safe in infections [(22, 23, 72); as shown in Table 1]. Most cases of irreversible brain damage or death have occurred from fevers that reached 44–46°C (111–115°F) [(15), p. 819]. So, the fear of brain damage from 40°C fever could be valid for “hyperthermia” but unfounded for “pathogen-hyperpyrexia”.
3. Most dangerous pathogens get restricted at 39°C: Antipyresis at ≥39°C has been mostly inconsequential in clinical practice since most pathogens cannot replicate above this temperature (73). However, for infections caused by high temperature adapted (>39°C) pathogens, the effect of antipyresis on patients could range from inconsequential to disastrous depending upon their genetic makeup, the virulence of the pathogen, prevailing protective immunity/previous exposure, health status, age, comorbidities, etc. (as shown in the Section below).
4. “No evidence of ill effects of fever reduction” in clinical practice and equating “no evidence of harm” with “evidence of no harm.” Since the 1950s, progressively, most acute diseases have been controlled by vaccines. Availability of antibiotics/antivirals has made fever redundant for common infections. Many times, physicians make the oversight of equating “no evidence of harm” with “evidence of no harm” in prescriptions. Though largely understandable in their practice, these are fatal flaws when dealing with novel diseases. For example, seemingly “harmless” antipyresis in infections without any effective therapeutics could allow rapid pathogen growth, deny timely immune enhancement, and increase the vulnerability to adverse outcomes from future oxidative stress, inflammatory damage, etc. Additionally, normothermia promoted mobility would allow wider dissemination of pathogens as modeled for influenza (93).
5. Need of More Context Clarifying Remarks in Medical Textbooks: The under-appreciation of the role of fever in disease resolution and perpetuation of myths among medical students and practitioners could be also partially stemming from extant remarks present in many widely used medical textbooks that can be easily taken out of the context. For example, Harrison's Principles of Internal Medicine, [(94), p.104] under “The Decision To Treat Fever” states “treatment of fever and its symptoms does no harm and does not slow the resolution of common viral and bacterial infections.” The statement is perfectly all right. However, many could err to equate “common” with “all” infections in their practice where effective therapeutics may not be “commonly” available. They may miss the immediate context, i.e., “Most fevers are associated with self-limited infections, such as common viral diseases.” Generalization of something applicable to “common” and “self-limiting diseases” to “all diseases and conditions” could have fatal consequences in those rare or novel infections that lack the effective therapeutics or the therapeutics are not being co-administered. Inclusion of more context clarifying texts in the reference textbooks could help the message get better understood as fever has an unquestionably essential role in infectious disease resolution (1–9).
6. Lack of clear guidelines and the legal framework for the protection of patients and physicians during pandemics: Pandemics require clear, concise, evidence-based, precise guidelines along with a foolproof legal framework that ensure the protection of patients and physicians (95). During the COVID-19 pandemic, the fear of inviting the wrath of government agencies or litigation on not following the treatment guidelines (96, 97) could have significantly increased the antipyretics prescriptions even for uncertified conditions. Many times, the detection of fever would have caused treatment delays and incorrect patient management under different clinical contexts (98). The lack of universally agreed-on recommendations and legal safety/protection systems for physicians and patients would be adversely affecting the existing healthcare systems, including the increased morbidity, disability, and mortality from unrelated conditions that required direct contact, e.g., elective surgeries and preventive ocular surgeries (99–101). Despite the potential, the fear of government action on mandated treatment guidelines non-pursuance would be also adversely affecting the application of therapy options and fever management practices from established traditional medicine systems (e.g., Ayurveda, Chinese, and Korean), further aggravating the management of infectious diseases (102–104). The prospection/evaluation and potential application/repurposing of the available antiviral medications and vaccines from modern medicine could be affected (48, 105–107).
Adverse Effects of Antipyresis on the Body's Response to Infection
Suppression of initial low-grade fever that prepares host to minimize the damage from a future surge of cytokines besides keeping the infection down, would logically increase the risk of being exposed to sudden overwhelming cytokine storm and experience adverse complications due to host bodies' non-preparedness, i.e., reduced capacity to resolve the ensuing damaging inflammation (4–7, 13, 14, 58). The induced normothermia blunts the host's HSR and essentially its capacity to suitably respond to resolve inflammation triggered in any infection. Fever-induced HSR response through HSF1 controls the expression of proinflammatory cytokines, such as IL-6-one of the purported culprits behind cytokine storm and increased mortality in diseases precipitating acute respiratory distress syndromes (ARDSs) (1, 55, 56, 108–110).
The intact HSR circuitry along with HSF-1 that may be variously deficient in different backgrounds is required for appropriate cytokine response generation, protection from inflammation (55–57), and IgG response (111). Strikingly, most conditions currently associated with higher mortality in COVID-19 and other respiratory tract viral infections have increased prevalence of reduced and/or defective HSR, e.g., aging (112), diabetes (both types 1 and 2) (113, 114), ARDS (115), sepsis (116, 117), renal failure (118), cigarette smoking (119), chronic obstructive lung disease (120). Unnecessary antipyresis would be more problematic for these sections of the population.
The induced normothermia is known to increase viral shedding and prolong recovery (121, 122), increase mortality from pneumonia (123–126), and reduce the efficacy of antibiotics (73, 74). The individuals unable to generate appropriate HSR would be prone to adverse outcomes from unnecessary antipyresis. Meta-analysis of animal studies had indicated an increased pooled odds ratio (OR) of 1.34 for influenza mortality with the antipyretics use (127), whereas hyperthermia preconditioning indicated enhanced survival in various relevant conditions, such as sepsis (128), stroke (129), myocardial infarction (130), and hepatic ischemia (131).
Divergence of Emergent Fever Management Guidelines
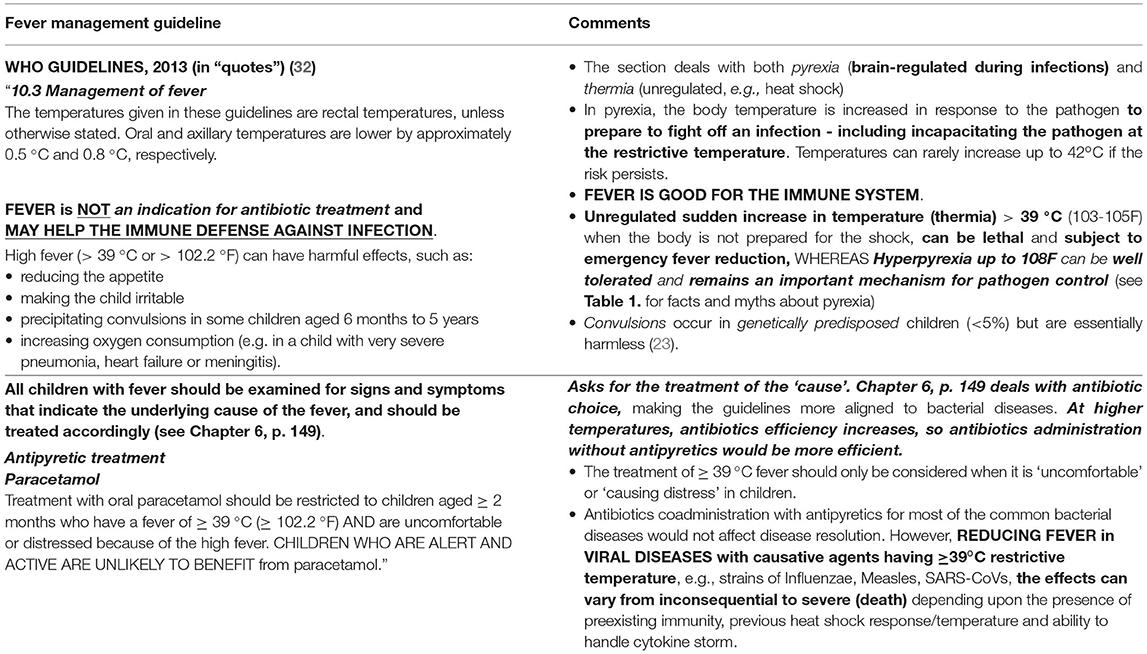
The current WHO guidelines for fever management explicitly stipulate that febrile illness of ≥39°C (≥102.2°F) presented with defined acute complications or discomfort could be considered for fever reduction (32). Furthermore, the treatment should focus on the cause of the fever rather than the fever itself. Refer to Table 2A for an excerpt from guideline (32, Chapter 10: Management of Fever; p.305) that restricts antipyresis to “children uncomfortable or distressed because of fever” and mentions it may not benefit otherwise active and alert children besides compromising the immune defense. Additionally, the fever reduction guidelines are part of “Supportive Care” (32) for “the most vulnerable”, i.e., ~5% of children <5-year-old showing complications/discomfort and genetically predisposed to febrile seizures/convulsions. The fever-reduction consideration should weigh the potential benefit being derived, as the commonly observed frightening seizures/convulsions are inconsequential to children's wellbeing (23, 32, 70, 72). However, measures must be taken to reduce the hazard of accidental choking, self-injury, etc., during febrile seizures in such predisposed children.
It may be opportune to upwardly revise the temperature range for antipyresis consideration. Soon after the publication of the first guideline by the WHO in 2000 (132), a meta-analysis published in the bulletin of the WHO (70) identified 41°C as “normal febrile range,” highlighted the continued practice of antipyresis as “parents and health professionals routinely treat fever in young children” despite the clear-cut realizations of “fever helps survival during infection, and that antipyresis increases mortality” in many diseases and the “potential for hepatotoxicity” and “overdosage” (70, 89–92). Moreover, it indicates “the WHO recommendations for the management of fever in children include the use of paracetamol for children with fever of ≥39°C” despite “insufficient data, however, support this recommendation” and suggest “We recommend that health professionals should not be encouraged to give antipyretics routinely to febrile children. Treatment should only be given to those children in obvious discomfort or those with known painful conditions” (70). The revised fever reduction guidelines were published by the WHO in 2013 to increase awareness among healthcare providers and parents, and to improve the adherence to appropriate practices (32) (as shown in Table 2A). However, the rational use of antipyresis may have further decreased during the COVID-19 pandemic with the publication of confusing and contradicting guidelines by different national agencies suggesting anything from 37.8 to 39°C fit for antipyresis (26–31). A few illustrative guideline examples are given in Table 2B.
What More Could Be Done?
The existing guidelines for fever management in the public domain (26–31) should be clarified and made explicit in stating the dangers of unnecessary fever reduction and emphasizing the necessity of fever in resolving infectious diseases. Inclusion of explanatory myth dispelling statements suitable for the public could help allay the fear of pathogen-hyperpyrexia and increase the awareness about its positive benefits as demonstrated (72). The extant guidelines may be either made strictly for the guidance of medical practitioners or must explicitly state the meanings of “uncomfortable,” “fit for treatment,” and “fever that needs treatment.” The guidelines should consider stating the “danger signs” when emergency medical attention is desired and identify the small minorities who are at greater risk of complications from higher temperatures, e.g., pregnant women (3–4 weeks, ≥39°C), children <5-year-old, frail, and deficient HSR individuals (refer to Sections above).
Public awareness should be increased about the essential role of the routine gradual raising of fever up to 39–42°C in promoting various protective (self-preserving) and offensive (pathogen clearance) measures for an amicable resolution of acute infectious diseases, e.g., immunity stimulation; HSR and anti-oxidative system activation to protect tissues from ensuing inflammatory response; antiviral response activation and enhancement; pathogen growth restriction/killing and clearance through nutrient deprivation, increased chemotaxis, phagocytosis, and reactive radical formation. The 39–42°C fever is desirable in the resolution of infections caused by the novel or high temperature adapted pathogens (restrictive temperature: 40–42°C) with limited treatment options, e.g., SARS-CoV-2, strains of Influenzae, measles, and pneumococcus.
Conclusion
The potential for fever mismanagement during the COVID-19 pandemic has further increased with the publication of numerous confusing guidelines. Unless corrective actions are taken immediately, it would further aggravate the situation causing increased morbidity and mortality from different infectious diseases including the common seasonal viral diseases and COVID-19. Unnecessary antipyresis could promote increased pathogen transmission rates, complications, hospitalization, and associated deaths. The awareness campaigns to dispel the myths and misconceptions surrounding fever, and promotion of evidence-based fever management practices should be undertaken for the public good.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
SS conceptualized and wrote the manuscript. RS and DK contributed to critical inputs and wrote and refined the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
SS acknowledges the funding support from the Institute of Eminence (IoE) seed grant, Banaras Hindu University to his laboratory. The critical insights and inputs provided on the topic by Dr. Manish Singh, MD (Pathology), Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India, during the development of the theme and writing of the manuscript have been crucial. The reviewers are also deeply acknowledged for their constructive contributions. We would also like to acknowledge the contribution of many authors in the relevant subject areas whose articles could not be cited/covered in the current manuscript due to space constraints. A preprint version of the article titled WHO Fever Management Guidelines: Challenges in Harnessing the Benefits During Covid-19 Pandemic is available at https://www.preprints.org/manuscript/202107.0367/v1.
References
1. Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. (2015) 15:335–49. doi: 10.1038/nri3843
2. Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. The adaptive value of fever. Infect Dis Clin North Am. (1996) 10:1–20. doi: 10.1016/S0891-5520(05)70282-8
3. Plaisance KI, Mackowiak PA. Antipyretic therapy physiologic rationale, diagnostic implications, and clinical consequences. Intern Med. (2000) 160:449–56. doi: 10.1001/archinte.160.4.449
4. Kluger MJ. The adaptive value of fever. In: Mackowiak PA, edtors. Fever: basic mechanisms and management. New York, NY: Raven Press Ltd. (1991). p. 105–124.
5. Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science. (1975) 188:166–8. doi: 10.1126/science.188.4184.166
6. Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes Infect. (2000) 2:1891–904. doi: 10.1016/S1286-4579(00)01337-X
7. Hasday JD, Singh IS. Fever and the heat shock response: distinct, partially overlapping processes. Cell Stress Chaperones. (2000) 5:471–80. doi: 10.1379/1466-1268(2000)005<0471:fathsr>2.0.co
8. Blatteis CM. Fever: pathological or physiological, injurious or beneficial? J Thermal Biol. (2003) 28:1–13. doi: 10.1016/S0306-4565(02)00034-7
9. Simon HB. Hyperthermia, fever, and fever of undetermined origin. In: Dale DC, editor. Infectious diseases: the clinician‘s guide to diagnosis, treatment, and prevention. New York, NY: WebMD Professional Publishing. (2003).
10. Carmichael LE, Barnes FD, Percy DH. Temperature as a factor in resistance of young puppies to canine herpesvirus. J Infect Dis. (1969) 120:669–78. doi: 10.1093/infdis/120.6.669
11. Shann F. Antipyretics in severe sepsis. Lancet. (1995) 345:338. doi: 10.1016/S0140-6736(95)90337-2
12. Bernheim HA, Kluger MJ. Fever: effect of drug-induced antipyresis on survival. Science. (1976) 193:237–9. doi: 10.1126/science.935867
14. Punt J, Stranford SA, Jones PP, Owen JA. Kuby Immunology. 8th Edn. New York, NY: W. H. Freeman and Company. (2019).
15. Saladin KS, Gan CA, Cushman HN. Anatomy & Physiology: the unity of form and function. Eighth edition. New York, NY, USA: McGraw-Hill Education. (2018).
16. Schmitt BD. Fever phobia. Misconceptions of parents about fevers. Am J Dis Children. (1980) 134:176–81. doi: 10.1001/archpedi.1980.02130140050015
17. May A, Bauchner H. Fever phobia: the pediatrician's contribution. Pediatrics. (1992) 90:851–4. doi: 10.1542/peds.90.6.851
18. Blumenthal I. What parents think of fever. Fam Pract. (1998) 15:513–8. doi: 10.1093/fampra/15.6.513
19. Crocetti M, Moghbeli N, Serwint J. Fever phobia revisited: have parental misconceptions about fever changed in 20 years? Pediatrics. (2001) 107:1241–6. doi: 10.1542/peds.107.6.1241
20. Demir F, Sekreter O. Knowledge, attitudes and misconceptions of primary care physicians regarding fever in children: a cross sectional study. Ital J Pediatr. (2012) 38:40. doi: 10.1186/1824-7288-38-40
21. Zyoud SH, Al-Jabi SW, Sweileh WM, Nabulsi MM, Tubaila MF, Awang R, et al. Beliefs and practices regarding childhood fever among parents: a cross-sectional study from Palestine. BMC Pediatr. (2013) 13:66. doi: 10.1186/1471-2431-13-66
22. El-Radhi AS. Why is the evidence not affecting the practice of fever management? Arch Dis Child. (2008) 93:918–20. doi: 10.1136/adc.2008.139949
23. El-Radhi AS. Fever management: Evidence vs current practice. World J Clin Pediatr. (2012) 1:29–33. doi: 10.5409/wjcp.v1.i4.29
24. Silva HD, Silva SD, Weerasekera K. P385 Primary caregivers knowledge on home management of childhood fever. Archives of Disease in Childhood. (2019) 104:A310–1. doi: 10.1136/archdischild-2019-epa.731
25. Concilla A, Kovacik R, Kobilis J, Stobart-Gallagher M. A. Survey of caregivers' knowledge on detection and management of pediatric fever. Cureus. (2021) 13:e14222. doi: 10.7759/cureus.14222
26. National Health Service (NHS). Inform. Fever in Adults. Available online at: https://www.nhsinform.scot/illnesses-and-conditions/infections-and-poisoning/fever-in-adults (accessed May 5, 2021).
27. Centers for Disease Control and Prevention (CDC) USA. Definitions of Symptoms for Reportable Illnesses. Available online at: https://www.cdc.gov/quarantine/air/reporting-deaths-illness/definitions-symptoms-reportable-illnesses.html (accessed May 14, 2021).
28. National Health Service (NHS). NHS inform. Illness and Conditions. Infections and poisioning. Coronavirus (COVID-19): General Advice & Self-care advice. NHS Scotland. Available online at: https://www.nhsinform.scot/illnesses-and-conditions/infections-and-poisoning/coronavirus-covid-19/caring-for-a-cough-or-fever/coronavirus-covid-19-self-care-advice and https://www.nhsinform.scot/illnesses-and-conditions/infections-and-poisoning/coronavirus-covid-19/coronavirus-covid-19-general-advice (accessed May 14, 2021).
29. COVID-19 Treatment Guidelines Panel. Coronavirus Disease (2019). (COVID-19) Treatment Guidelines. National Institutes of Health. Available online at: https://www.covid19treatmentguidelines.nih.gov/. (accessed May 16, 2021).
30. National Institutes of Health. COVID-19 Treatment Guidelines. Outpatient Management of Acute COVID-19. National Institutes of Health. Available online at: https://www.covid19treatmentguidelines.nih.gov/outpatient-management/ (accessed April 21, 2021).
31. Indian Council of Medical Research (ICMR). FAQs for Patients With Hypertension, Diabetes and Heart Diseases in view of Coronavirus/COVID-19 Pandemic. Indian Council of Medical Research, New Delhi, India. Available online at: https://www.icmr.gov.in/pdf/covid/faqs/FAQs_English_24032020.pdf (accessed: May 16, 2021).
32. Guidelines Review Committee World Health Organization. Pocket book of Hospital Care for Children: Guidelines for the Management of Common Childhood Illnesses – 2nd ed. Geneva: World Health Organization. (2013). Available online at: https://www.who.int/publications/i/item/978-92-4-154837-3 and http://apps.who.int/iris/bitstream/10665/81170/1/9789241548373_eng.pdf (accessed April 28, 2021).
33. National Institute for Health Care Excellence (NICE). Fever in Under 5s: Assessment and Initial Management, Clinical Guideline (CG160). Department of Health and Social Care, National Health Service (NHS), London (UK). (2013). Available online at: https://www.nice.org.uk/guidance/cg160.
34. Indian Council of Medical Research (ICMR). ‘Management of Acute Fever' in ‘Treatment Guidelines for Antimicrobial Use in Common Syndromes'. 2nd edition. Indian Council of Medical Research, Department of Health Research, New Delhi, India. (2019) p. 10, Available online at: https://main.icmr.nic.in/sites/default/files/guidelines/Treatment_Guidelines_2019_Final.pdf
35. NSW Health. Infants and Children: Acute Management of Fever. NSW Health. North Sydney, N.S.W: NSW Dept. of Health, 2nd ed. (2010).
36. SA Health. Management of Fever Without Focus in Children (excluding neonates). Clinical Guideline. Adelaide, S.A.: Dept. of Health, Government of South Australia. (2013).
37. Peluso L, Abella BS, Ferrer R, Kucher N, Sunde K, Taccone FS. Fever management in COVID-19 patients. Minerva Anestesiol. (2021) 87:1–3. doi: 10.23736/S0375-9393.20.15195-2
38. Drewry AM, Hotchkiss R. & Kulstad, E. Response to “Body temperature correlates with mortality in COVID-19 patients”. Crit Care. (2020) 24:460. doi: 10.1186/s13054-020-03186-w
39. Sharma PV. Carak-Samhita. 4th Edition, (1998). Ch.II. Chikithsasthanam, Chap. 3. Varanasi, India: Chaukhamba Orientalia. p.52-85
40. Simpson WM. Artificial fever therapy of syphilis and gonococcic infections. Br J Vener Dis. (1936) 12:133–66. doi: 10.1136/sti.12.3.133
41. Hippocrates, Chadwick J, Mann WN. The medical works of Hippocrates. Oxford: Blackwell Scientific Publications. (1950) p. 204.
42. Young PJ, Saxena MK, Beasley RW. Fever and antipyresis in infection. Med J Aust. (2011) 195:458–9. doi: 10.5694/mja11.10502
43. Solomon HC, Kopp I. Fever therapy. N Engl J Med. (1937) 217:805–14. doi: 10.1056/NEJM193711182172101
44. Karamanou M, Liappas I, Antoniou Ch, Androutsos G, Lykouras E. Julius Wagner-Jauregg (1857-1940): Introducing fever therapy in the treatment of neurosyphilis. Psychiatriki. (2013). 24:208–12. Available online at: https://www.psychiatriki-journal.gr/documents/psychiatry/24.3-EN-2013-208.pdf
45. Austin SC, Stolley PD, Lasky T. The history of malariotherapy for neurosyphilis. Modern parallels. JAMA. (1992) 268:516–9. doi: 10.1001/jama.1992.03490040092031
46. Napoli PE, Nioi M. Global spread of coronavirus disease 2019 and malaria: an epidemiological paradox in the early stage of a pandemic. J Clin Med. (2020) 9:1138. doi: 10.3390/jcm9041138
47. Singh S. BCG Vaccines May Not Reduce Covid-19 Mortality Rates. medRxiv. (2020). doi: 10.1101/2020.04.11.20062232
48. Singh S, Maurya RP, Singh RK. “Trained Immunity” From Mycobacterium Spp. Exposure or BCG Vaccination and COVID-19 Outcomes. PloS Pathog. (2020) 16:e1008969. doi: 10.1371/journal.ppat.1008969
49. Herder V, Dee K, Wojtus JK, Goldfarb D, Rozario C, Gu Q, et al. Elevated temperature inhibits SARS-CoV-2 replication in respiratory epithelium independently of the induction of IFN-mediated innate immune defences. bioRxiv. (2020). doi: 10.1101/2020.12.04.411389
50. Pockley AG, Calderwood SK, Santoro MG. (Eds). Prokaryotic and Eukaryotic Heat Shock Proteins in Infectious Disease. Netherlands: Springer. (2010). doi: 10.1007/978-90-481-2976-8
51. Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. (2010) 11:545–55. doi: 10.1038/nrm2938
52. Hasday JD, Thompson C, Singh IS. Fever, immunity, and molecular adaptations. Compr Physiol. (2014) 4:109–48. doi: 10.1002/cphy.c130019
53. Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. (2010) 11:515–28. doi: 10.1038/nrm2918
54. LeGrand EK, Alcock J. Turning up the heat: immune brinksmanship in the acute-phase response. Q Rev Biol. (2012) 87:3–18. doi: 10.1086/663946
55. Anckar J, Sistonen L. Regulation of HSF1 Function in the Heat Stress Response: Implications in Aging and Disease. Annu Rev Biochem. (2011) 80:1089–115. doi: 10.1146/annurev-biochem-060809-095203
56. Takii R, Inouye S, Fujimoto M, Nakamura T, Shinkawa T, Prakasam R, et al. Heat shock transcription factor 1 inhibits expression of IL-6 through activating transcription factor 3. J Immunol. (2010) 184:1041–8. doi: 10.4049/jimmunol.0902579
57. Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, et al. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. (1999) 18:5943–52. doi: 10.1093/emboj/18.21.5943
58. Heck TG, Ludwig MS, Frizzo MN, Rasia-Filho AA, Homem de. Bittencourt PI. Suppressed anti-inflammatory heat shock response in high-risk COVID-19 patients: lessons from basic research (inclusive bats), light on conceivable therapies. Clin Sci (Lond). (2020) 134:1991–2017. doi: 10.1042/CS20200596
59. Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. (2012) 10:525–37. doi: 10.1038/nrmicro2836
60. Wang C, Zhang R, Wei X, Lv M, Jiang Z. Metalloimmunology: The metal ion-controlled immunity. Adv Immunol. (2020) 145:187–241. doi: 10.1016/bs.ai.2019.11.007
61. Haase H, Schomburg L. You'd Better Zinc—Trace Element Homeostasis in Infection and Inflammation. Nutrients. (2019) 11:2078. doi: 10.3390/nu11092078
62. Healy C, Munoz-Wolf N, Strydom J, Faherty L, Williams NC, Kenny S, et al. Nutritional immunity: the impact of metals on lung immune cells and the airway microbiome during chronic respiratory disease. Respir Res. (2021) 22:133. doi: 10.1186/s12931-021-01722-y
63. Lonergan ZR, Skaar EP. Nutrient zinc at the host–pathogen interface. Trends Biochem Sci. (2019) 44:1041–56. doi: 10.1016/j.tibs.2019.06.010
64. Scott A Read, Stephanie Obeid, Chantelle Ahlenstiel, Golo Ahlenstiel, The role of zinc in antiviral immunity. Advances in Nutrition. (2019) 10:696–710. doi: 10.1093/advances/nmz013
65. Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. (2017) 9:624. doi: 10.3390/nu9060624
66. Freiberg MS, Cheng DM, Gnatienko N, Blokhina E, Coleman SM, Doyle MF, et al. Effect of zinc supplementation vs placebo on mortality risk and HIV disease progression among hiv-positive adults with heavy alcohol use: a randomized clinical trial. JAMA Netw Open. (2020) 3:e204330. doi: 10.1001/jamanetworkopen.2020.4330
67. Singh S, Diwaker A, Singh BP, Singh RK. Nutritional immunity, zinc sufficiency, and COVID-19 mortality in socially similar european populations. Front Immunol. (2021) 12:699389. doi: 10.3389/fimmu.2021.699389
68. Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol. (2014) 32:513–45. doi: 10.1146/annurev-immunol-032713-120231
69. Roberts NJ Jr. Impact of temperature elevation on immunologic defenses. Rev Infect Dis. (1991) 13:462–72. doi: 10.1093/clinids/13.3.462
70. Russell FM, Shann F, Curtis N, Mulholland K. Policy and Practice: Evidence on the use of paracetamol in febrile children. Bulletin of the World Health Organization. (2003). 81:367–74. World Health Organization. Available online at: https://apps.who.int/iris/bitstream/handle/10665/71985/bulletin_2003_81%285%29_367-372.pdf?sequence=1&isAllowed=y (accessed May 20, 2021)
71. Yan N, Chen Z. Intrinsic antiviral immunity. Nat Immunol. (2012) 13:214–22. doi: 10.1038/ni.2229
72. Seattle Children's Hospital. Fever - Myths Versus Facts. Seattle, WA. Available online at: https://www.seattlechildrens.org/conditions/a-z/fever-myths-versus-facts/
73. Mackowiak PA. Direct effects of hyperthermia on pathogenic microorganisms: Teleologic implications with regard to fever. Rev Infect Dis. (1981) 3:508–20. doi: 10.1093/clinids/3.3.508
74. Mackowiak PA, Marling-Cason M, Cohen RL. Effects of temperature on antimicrobial susceptibility of bacteria. J Infect Dis. (1982) 145:550–3. doi: 10.1093/infdis/145.4.550
75. Lwoff A. From protozoa to bacteria and viruses. Fifty years with microbes (André Lwoff). Annu Rev Microbiol. (1971) 25:1–26. doi: 10.1146/annurev.mi.25.100171.000245
76. Mourtzoukou EG, Falagas ME. Exposure to cold and respiratory tract infections. Int J Tuberc Lung Dis. (2007) 11:938–43.
77. Osawa E. Muschel, L H. Studies relating to the serum resistance of certain Gram-negative bacteria. J Exp Med. (1964) 119:41–51. doi: 10.1084/jem.119.1.41
78. Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. (2013) 339:211–4. doi: 10.1126/science.1227166
79. Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. (2015) 21:263–9. doi: 10.1038/nm.3804
80. Liao S, Tang Y, Yue X, Gao R, Yao W, Zhou Y, et al. β-Hydroxybutyrate Mitigated Heart Failure with Preserved Ejection Fraction by Increasing Treg Cells via Nox2/GSK-3β. J Inflamm Res. (2021) 14:4697–706. doi: 10.2147/JIR.S331320
81. Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell. (2016) 166:1512–1525.e12. doi: 10.1016/j.cell.2016.07.026
82. Martins M, Abecasis F. Healthcare professionals approach paediatric fever in significantly different ways and fever phobia is not just limited to parents. Acta Paediatr. (2016) 105:829–33. doi: 10.1111/apa.13406
83. Thomas V, Riegel B, Andrea J, Murray P, Gerhart A, Gocka I. National survey of pediatric fever management practices among emergency department nurses. J Emerg Nurs. (1994) 20:505–10.
84. Kiekkas P, Konstantinou E, Psychogiou KS, Tsampoula I, Stefanopoulos N, Bakalis N. Nursing personnel's attitudes towards fever and antipyresis of adult patients: cross-sectional survey. J Clin Nurs. (2014) 23:2949–57. doi: 10.1111/jocn.12551
85. Ellis FR. Malignant hyperpyrexia. Arch Dis Child. (1984). 59:1013–5. doi: 10.1136/adc.59.11.1013
86. McIntyre J. Management of fever in children. Arch Dis Child. (2011). 96:1173–4. doi: 10.1136/archdischild-2011-301094
87. Section Section on Clinical Pharmacology and Therapeutics; Committee on Drugs, Sullivan JE, Farrar HC. Fever and antipyretic use in children. Pediatrics. (2011) 127:580–7. doi: 10.1542/peds.2010-3852
88. Ekholm E, Niemineva K. On convulsions in early childhood and their prognosis; an investigation with follow-up examinations of patients treated for convulsions at the Children's Clinic of Helsinki University. Acta Paediatr. (1950) 39:481–501. doi: 10.1111/j.1651-2227.1950.tb08545.x
89. Heubi JE, Barbacci MB, Zimmerman HJ. Therapeutic misadventures with acetaminophen: hepatoxicity after multiple doses in children. J Pediatr. (1998) 132:22–7. doi: 10.1016/S0022-3476(98)70479-2
90. Kearns GL, Leeder JS, Wasserman GS. Acetaminophen overdose with therapeutic intent. J Pediatr. (1998) 132:5–8. doi: 10.1016/S0022-3476(98)70476-7
91. Rivera-Penera T, Gugig R, Davis J, McDiarmid S, Vargas J, Rosenthal P, et al. Outcome of acetaminophen overdose in pediatric patients and factors contributing to hepatotoxicity. J Pediatr. (1997) 130:300–4. doi: 10.1016/S0022-3476(97)70359-7
92. Penna AC, Dawson KP, Penna CM. Is prescribing paracetamol 'pro re nata' acceptable? J Paediatr Child Health. (1993) 29:104–6.6. doi: 10.1111/j.1440-1754.1993.tb00460.x
93. Earn DJ, Andrews PW, Bolker BM. Population-level effects of suppressing fever. Proc Biol Sci. (2014) 281:20132570. doi: 10.1098/rspb.2013.2570
94. Dinarello CA, Porat R. In: Fauci AS, Kasper DL, Longo DL, Braunwald E, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 20th edn. New York, NY: McGraw-Hill. (2019).
95. AMA. Sustainability: Liability Protections for Health Care Professionals During COVID-19. Available online at: https://www.ama-assn.org/practice-management/sustainability/liability-protections-health-care-professionals-during-covid-19 (acessed November 02, 2021).
96. Kumar A, Kapila M, Pankaj R. Medicine and law in the times of COVID-19 pandemic: understanding the interphase. Indian J Crit Care Med. (2020) 24:971–4. doi: 10.5005/jp-journals-10071-23553
97. Dyer C. Covid-19: Doctors' call for legal protection against claims of unlawful killing is rejected. BMJ. (2021) 372:n164. doi: 10.1136/bmj.n164
98. Nioi M, Napoli PE, Finco G, Demontis R, Fossarello M, D'aloja E. Fear of the COVID-19 and medical liability. Insights from a series of 130 consecutives medico-legal claims evaluated in a single institution during SARS-CoV-2-related pandemic. Signa Vitae. (2021) 17:79–85. doi: 10.22514/sv.2021.098
99. Søreide K, Hallet J, Matthews JB, Schnitzbauer AA, Line PD, Lai PBS, et al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br J Surg. (2020) 107:1250–61. doi: 10.1002/bjs.11670
101. Napoli PE, Nioi M. d'Aloja E, Fossarello M. Safety recommendations and medical liability in ocular surgery during the COVID-19 pandemic: an unsolved dilemma. J Clin Med. (2020) 9:1403. doi: 10.3390/jcm9051403
102. Gopalakrishna Pillai GK. Traditional medicine and COVID-19. J Ayurveda Integr Med. (2021) 12:413–4. doi: 10.1016/j.jaim.2021.07.015
103. Girija PLT, Sivan N. Ayurvedic treatment of COVID-19/SARS-CoV-2: A case report. J Ayurveda Integr Med. (2020) 100329. doi: 10.1016/j.jaim.2020.06.001
104. Muthappan S, Ponnaiah M. Time to tread cautiously during public health emergencies: Reactions from traditional and complementary/alternative medical systems to ongoing Coronavirus (COVID-19) outbreak. J Ayurveda Integr Med. (2020). 13:100315. doi: 10.1016/j.jaim.2020.04.004
105. Napoli PE, Mangoni L, Gentile P, Braghiroli M, Fossarello M. A panel of broad-spectrum antivirals in topical ophthalmic medications from the drug repurposing approach during and after the coronavirus disease (2019) Era J Clin Med. (2020) 9:2441. doi: 10.3390/jcm9082441
106. O'Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. (2020) 20:335–7. doi: 10.1038/s41577-020-0337-y
107. Singh S, Kishore D, Singh RK. ‘Trained immunity' from Mycobacterium spp. (environmental or BCG) exposure predicts protection from Coronavirus disease (2019). (COVID-19). medRxiv. (2021). doi: 10.1101/2021.02.11.20233593
108. Schell-Chaple HM, Puntillo KA, Matthay MA, Liu KD, Wiedemann HP, Arroliga AC, et al. Body temperature and mortality in patients with acute respiratory distress syndrome. Am J Crit Care. (2015) 24:15–23. doi: 10.4037/ajcc2015320
109. Schulman CI, Namias N, Doherty J, Manning RJ, Li P, Alhaddad A, et al. The effect of antipyretic therapy upon outcomes in critically ill patients: a randomized, prospective study. Surg Infect. (2005) 6:369–75. doi: 10.1089/sur.2005.6.369
110. Petitjeans F, Leroy S, Pichot C, Geloen A. GhignoneM, Quintin L. Hypothesis: fever control, a niche for alpha-2 agonists in the setting of septic shock and severe acute respiratory distress syndrome? Temperature. (2018) 5:224–56. doi: 10.1080/23328940.2018.1453771
111. Inouye S, Izu H, Takaki E, Suzuki H, Shirai M, Yokota Y, et al. Impaired IgG production in mice deficient for heat shock transcription factor 1. J Biol Chem. (2004) 279:38701–9. doi: 10.1074/jbc.M405986200
112. Murshid A, Eguchi T, Calderwood SK. Stress proteins in aging and life span. Int J Hyperthermia. (2013) 29:442–7. doi: 10.3109/02656736.2013.798873
113. Hooper PL, Hooper JJ. Loss of defense against stress: diabetes and heat shock proteins. Diabetes Technol Ther. (2005) 7:204–8. doi: 10.1089/dia.2005.7.204
114. Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, et al. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. (2002) 51:1102–9. doi: 10.2337/diabetes.51.4.1102
115. Weiss YG, Bouwman A, Gehan B, Schears G, Raj N, Deutschman CS. Cecal ligation and double puncture impairs heat shock protein 70 (HSP-70) expression in the lungs of rats. Shock. (2000) 13:19–23. doi: 10.1097/00024382-200013010-00004
116. Schroeder S, Lindemann C, Hoeft A, Putensen C, Decker D, von Ruecker AA, et al. Impaired inducibility of heat shock protein 70 in peripheral blood lymphocytes of patients with severe sepsis. Crit Care Med. (1999) 27:1080–4. doi: 10.1097/00003246-199906000-00023
117. Duan X, Berthiaume F, Yarmush D, Yarmush ML. Proteomic analysis of altered protein expression in skeletal muscle of rats in a hypermetabolic state induced by burn sepsis. Biochem J. (2006) 397:149–58. doi: 10.1042/BJ20051710
118. Marzec L, Zdrojewski Z, Liberek T, Bryl E, Chmielewski M, Witkowski JM, et al. Expression of Hsp72 protein in chronic kidney disease patients. Scand J Urol Nephrol. (2009) 43:400–8. doi: 10.3109/00365590903089489
119. Cappello F, Di Stefano A, David S, Rappa F, Anzalone R, La Rocca G, et al. Hsp60 and Hsp10 down-regulation predicts bronchial epithelial carcinogenesis in smokers with chronic obstructive pulmonary disease. Cancer. (2006) 107:2417–24. doi: 10.1002/cncr.22265
120. Xie J, Zhao J, Xiao C, Xu Y, Yang S, Ni W. Reduced heat shock protein 70 in airway smooth muscle in patients with chronic obstructive pulmonary disease. Exp Lung Res. (2010) 36:219–26. doi: 10.3109/01902140903349562
121. Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. Increased virus shedding with aspirin treatment of rhinovirus infection. JAMA. (1975) 231:1248–51. doi: 10.1001/jama.1975.03240240018017
122. Doran TF, De Angelis C, Baumgardner RA, Mellits ED. Acetaminophen: more harm than good for chickenpox? J Pediatr. (1989) 114:1045–8. doi: 10.1016/S0022-3476(89)80461-5
123. Jefferies S, Weatherall M, Young P, Eyers S, Beasley R. Systematic review and meta-analysis of the effects of antipyretic medications on mortality in Streptococcus pneumoniae infections. Postgrad Med J. (2012) 88:21–7. doi: 10.1136/postgradmedj-2011-130217
124. Ahkee S, Srinath L, Ramirez J. Community-acquired pneumonia in the elderly: association of mortality with lack of fever and leukocytosis. South Med J. (1997) 90:296–8. doi: 10.1097/00007611-199703000-00006
125. Kuikka A, Sivonen A, Emelianova A, Valtonen VV. Prognostic factors associated with improved outcome of Escherichia coli bacteremia in a Finnish university hospital. Eur J Clin Microbiol Infect Dis. (1997) 16:125–34. doi: 10.1007/BF01709471
126. Kuikka A, Valtonen VV. Factors associated with improved outcome of Pseudomonas aeruginosa bacteremia in a Finnish university hospital. Eur J Clin Microbiol Infect Dis. (1998) 17:701–8. doi: 10.1007/s100960050164
127. Eyers S, Weatherall M, Shirtcliffe P, Perrin K, Beasley R. The effect on mortality of antipyretics in the treatment of influenza infection: systematic review and meta-analysis. J R Soc Med. (2010) 103:403–11. doi: 10.1258/jrsm.2010.090441
128. Güllüoglu BM, Aksoy BS, Ozveri ES, Yüksel M, Demiralp EE, Aktan AO. Optimal timing and temperature for hyperthermic preconditioning in an animal model of fecal peritonitis. J Invest Surg. (2002) 15:117–24. doi: 10.1080/08941930290085877
129. Xu H, Aibiki M, Nagoya J. Neuroprotective effects of hyperthermic preconditioning on infarcted volume after middle cerebral artery occlusion in rats: role of adenosine receptors. Crit Care Med. (2002) 30:1126–30. doi: 10.1097/00003246-200205000-00028
130. Yamashita N, Hoshida S, Taniguchi N, Kuzuya T, Hori M. Whole-body hyperthermia provides biphasic cardioprotection against ischemia/reperfusion injury in the rat. Circulation. (1998) 98:1414–21. doi: 10.1161/01.CIR.98.14.1414
131. Oba M, Suico MA, Morino S, Yano S, Matsuno T, Koga T, et al. Modified mild heat shock modality attenuates hepatic ischemia/reperfusion injury. J Surg Res. (2010) 162:213–20. doi: 10.1016/j.jss.2009.03.093
Keywords: benefits of fever during infection, fever management guidelines, mortality, heat shock, inflammation, antipyresis, COVID-19, respiratory diseases
Citation: Singh S, Kishore D and Singh RK (2022) Potential for Further Mismanagement of Fever During COVID-19 Pandemic: Possible Causes and Impacts. Front. Med. 9:751929. doi: 10.3389/fmed.2022.751929
Received: 02 August 2021; Accepted: 26 January 2022;
Published: 02 March 2022.
Edited by:
Guodong Ding, Shanghai Children's Hospital, ChinaReviewed by:
George Akpede, Ambrose Alli University, NigeriaPietro Emanuele Napoli, University of Cagliari, Italy
Sanjeev Rastogi, University of Lucknow, India
Copyright © 2022 Singh, Kishore and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samer Singh, c2FtZXIuc2luZ2gxMEBiaHUuYWMuaW4=; orcid.org/0000-0002-0921-1686
 Samer Singh
Samer Singh Dhiraj Kishore
Dhiraj Kishore Rakesh K. Singh
Rakesh K. Singh