
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 22 February 2022
Sec. Gastroenterology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.713211
This article is part of the Research TopicEndoscopy in Early Gastrointestinal NeoplasmsView all 7 articles
 Foqiang Liao1†
Foqiang Liao1† Zhenhua Zhu1†
Zhenhua Zhu1† Yongkang Lai1
Yongkang Lai1 Xiaolin Pan1
Xiaolin Pan1 Shunhua Long1
Shunhua Long1 Xiaojiang Zhou1
Xiaojiang Zhou1 Guohua Li1
Guohua Li1 Yin Zhu1
Yin Zhu1 Youxiang Chen1
Youxiang Chen1 Xu Shu1,2*
Xu Shu1,2*Background: Fever is one of the postoperative adverse events of endoscopic submucosal dissection and its derived technique, but the probability and risk factors of postoperative fever are still unclear. The aim of the current study was to investigate the incidence and risk factors of postoperative fever after esophageal lesion removal.
Methods: We conducted a retrospective study of 446 patients who underwent esophageal endoscopic submucosal dissection and its derived technique between January 2014 and January 2020. Cases included in this study were divided into fever and non-fever groups.
Results: Postoperative fever developed in 135 patients (30.3%). The median (range) highest fever temperature was 38 (37.8–38.4)°C, the median (range) duration of fever was 1 (1–2) day, and 127 (94.1%) patients developed fever within 24 h after operation. Through logistic regression analysis, factors associated with postoperative fever were age (OR: 1.740, 95% CI: 1.005–3.013, p = 0.048), lesion size (OR: 2.007, 95% CI: 1.198–3.362, p = 0.008), operation time (OR: 3.007, 95% CI: 1.756–5.147, p < 0.001) and nasogastric tube placement (OR: 1.881, 95% CI: 1.165–3.037, p = 0.010), while prophylactic antibiotics (OR: 0.181, 95% CI: 0.082–0.401, p < 0.001) were negatively associated with fever.
Conclusions: Age ≥52 years old, lesion size ≥19 mm, operation time ≥37 min, and nasogastric tube placement are risk factors for postoperative fever after esophageal endoscopic submucosal dissection and its derived technique, prophylactic antibiotic use after operation may help reduce fever rate. Attention should be paid to such patients to minimize the risk of postoperative fever.
Endoscopic submucosal dissection (ESD) and its derived technique is an advanced endoscopic method, which is increasingly used in esophageal lesions and early esophageal cancer (1–3). However, esophageal ESD is more difficult than other procedures because the anatomy of the esophagus wall is markedly different from the rest of the gastrointestinal tract (4). For relatively large lesions, mucosal dissection affects the surgical field of vision and operating space, further increasing the difficulty of surgery (5). Therefore, endoscopic submucosal tunnel dissection (ESTD) is one of the derivations of standard ESD that is intended to overcome these problems (6). However, some adverse events (such as bleeding, perforation and stenosis) still occur frequently after ESD/ESTD operation (7, 8), and a large number of clinical studies have been conducted on these adverse events and their risk factors (9–11).
At the same time, fever is also a clinically observed postoperative adverse event, and several studies have discussed possible risk factors for fever after ESD in the stomach and colon (12, 13). Nakanishi et al. (12) suggested that age > 68 years, a resection diameter of > 35.0 mm, and increased serum CRP levels at postoperative day 1(POD 1) were independent risk factors for pyrexia after gastric ESD. In colon ESD, logistic regression analysis also revealed that age and lesion size were closely associated with post-ESD fever, but the possibility of bacteremia causing fever is very low (13). However, the sample size of these studies was not large, and the risk factors that may cause postoperative fever have not been fully explored. Moreover, current research on postoperative fever and its risk factors after esophageal ESD and its derived technique is rare. Therefore, we designed this study to explore the incidence and related risk factors of fever after endoscopic submucosal dissection and its derived technique for esophageal lesions to systematically analyze the risk factors that may lead to fever before, during and after operation, so as to reduce the postoperative fever rate by optimizing preoperative risk assessment and strengthening intraoperative and postoperative management.
We retrospectively analyzed medical records and endoscopy electronic databases of the First Affiliated Hospital of Nanchang University, and we collected the following demographic data and clinicopathological features: (1) Pre-operative metrics: sex, age, underlying diseases (diabetes, hypertension and history of abdominal surgery), routine blood work, CRP, procalcitonin; (2) intra-operative metrics: lesion location, lesion size, invasion depth, operation method, en-bloc resection, duration of operation, intraoperative bleeding, perforation and management; (3) post-operative metrics: postoperative pathology, prophylactic antibiotics, adverse events (bleeding, perforation, chest pain, nausea and vomiting), body temperature, duration of fever, inflammatory biomarkers (routine blood work, CRP, procalcitonin, blood culture etc.), cooling measures and duration of hospitalization. Between January 2014 and January 2020, 467 patients who underwent ESD/ESTD due to esophageal lesions were included. Cases were excluded for the following reasons: (1) underwent ESD on other body parts or multiple esophageal ESD procedures in the meantime; (2) immunodeficiency status; (3) serious cardiovascular, pulmonary, or hepatorenal diseases; (4) transfer to the surgical department during the ESD/ESTD procedure; (5) fever (temperature >37.5°C) before the procedure; or (6) patients with incomplete demographic data. All included cases were recorded in the Human Genetic Resources Center of the First Affiliated Hospital of Nanchang University. An ethics committee approved the study, and informed consent was obtained from every patient.
Preoperative evaluation (routine blood work, blood biochemistry, coagulation function, ECG, etc.) was completed for all patients after admission. Magnified chromoendoscopy, endoscopic ultrasound, computerized tomography and biopsy were used to estimate characteristics of the lesions, such as invasion depth and possible histologic type. The patient began a fluid diet 1 day before operation and was forbidden to eat for 8 h before operation.
All operative procedures were performed by expert endoscopists with more than 10 years of experience. Patients were sedated after general anesthesia, and ESD was performed in all patients using an electric endoscope (GIF-Q260J; Olympus Optical Co, Ltd, Tokyo, Japan). The operation procedures are as follows (14–16): (1) identification and marking of the target lesion; (2) injection of a lifting solution (0.9% normal saline, glycerol fructose or epinephrine solution) around the perimeter of the lesion; (3) incision of the mucosa along the periphery of the marker dots with ESD knives (hook knife, dual knife, IT knife, etc.); and (4) circumferential mucosal incision and submucosal dissection (Figure 1). The ESTD procedure was performed as follows (17): (1) identification and marking of the target lesion at 3–5 cm with a lifting solution; (2) incision at the marker dots and establishment of the submucosal tunnel by gradually separating the submucosal layer and muscularis to the distal end; (3) resection of the lesion was with a scalpel and removal; and (4) finally, closure of the defect with clips after mucotomy (Figure 2). Hot biopsy forceps or hemostatic forceps were usually used to control bleeding during the procedure. For patients with a large resection area or deep wound, nasogastric tube placement was performed for gastrointestinal decompression, and the color of drainage tube was observed. Experts will decide whether to use prophylactic antibiotics according to the intraoperative situation (included lesion size, operation time and intraoperative perforation), which are high risk factors for postoperative infection.
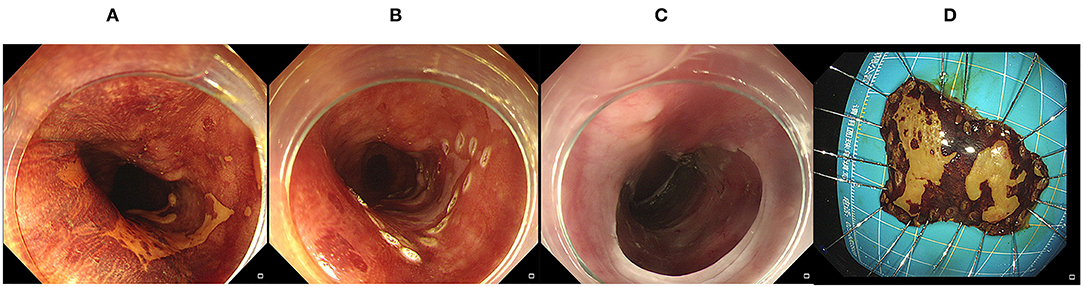
Figure 1. Endoscopic submucosal dissection. (A) A Lesion was identified by magnified chromoendoscopy; (B) Marking the edge of the target lesion; (C,D) After submucosal dissection, the lesion was completely removed.
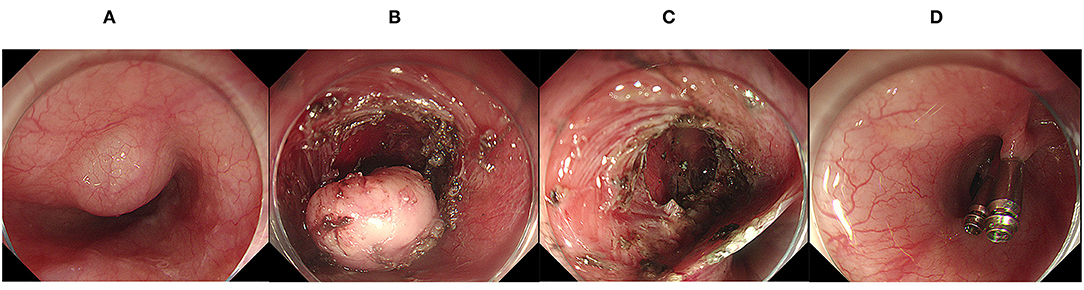
Figure 2. Endoscopic submucosal tunnel dissection. (A) A mucosal lesion of the esophagus was found under endoscopy; (B) The lesion was exposed after the establishment of the submucosal tunnel; (C) The wound surface after endoscopic submucosal tunnel dissection; (D) Clips was used to close the defect.
Operation time was defined as the period from the start of the circumferential mucosal incision to the removal of the tumor (18). Intraoperative bleeding was defined as any exudation or active bleeding that occurred during the procedure. Intraoperative perforation was defined as a visible hole in the esophageal wall, exposing the mediastinal space during the endoscopic procedure (19). En-bloc resection was defined as whole resection of the tumor into a non-fragmenting piece (20, 21). Lesion size was the maximum diameter measured by postoperative pathological specimens. Malignant lesions included esophageal squamous cell carcinoma and adenocarcinoma, while others were classified as benign. Lesions invading the muscularis were defined as having muscle fibers of the muscularis visible by endoscopy. Fever was defined based on a maximum body temperature >37.5°C within 3 days after operation, regardless of the duration of the febrile period. Prophylactic antibiotics refer to the immediate use of antibiotics after ESD/ESTD, but the use of antibiotics after fever was not counted. Postoperative bleeding was defined as hematochezia or melena requiring an endoscopic hemostatic procedure anytime between 0 and 7 days after ESD/ESTD. Postoperative perforation was defined as radiographic evidence of free air after the procedure.
SPSS software version 25.0 (IBM; Chicago, IL, USA) was used for statistical analysis. Patients were divided into fever and non-fever groups according to the maximum body temperature after operation. Categorical data were compared using Fisher's exact test or the χ2 test. Student's t-test or the Mann-Whitney U-test was used for analysis of quantitative data. Receiver operating characteristic (ROC) curve analysis and Youden index were performed to determine the optimal cut-off values of quantitative data, such as age, tumor size, and procedure time (Supplementary Figure 1). In the univariate analysis to determine independent risk factors for fever, the risk factors were estimated by calculating the odds ratios (ORs) and the 95% confidence intervals (CIs). Variables with p < 0.20 in the univariate analysis were included in the multiple logistic regression analysis. P < 0.05 were considered to indicate a statistically significant difference between 2 groups.
We obtained data from 467 patients through the computerized patient record system and endoscopy electronic databases in the First Affiliated Hospital of Nanchang University. Figure 3 shows a total of 446 patients were included in this study. The baseline characteristics of these patients who underwent esophageal ESD/ESTD are presented in Table 1. The median (range) age of study subjects was 57 (47–64) years, and 64.6% (288/446) were male. One hundred twenty-four (27.7%) patients had underlying diseases: 9 (2.0%) patients had diabetes, 66 (14.7%) patients had hypertension, and 49 (11.0%) patients had a history of abdominal surgery. Twelve (2.7%) lesions were located in the upper 1/3 of the esophagus, 348 (78.0%) lesions were located in the middle 1/3 of esophagus and 86 (19.3%) lesions were located in the lower 1/3 of the esophagus. Median (range) lesion size was 15 (10–25) mm. Most lesion were leiomyomas (49.7%), 20.4% (91/446) of lesions were squamous cell carcinomas and 59 (13.2) lesions were dysplasia. Lesions were removed by ESD in 328 (73.5%) patients and ESTD in only 118 (26.5%). Median (range) operation time was 35 (23–58) min, and the en-bloc resection rate was 89.4% (399/446). One hundred seventy-six (39.4%) patients had bleeding during the procedure, and most patients were successfully hemostatic with hot biopsy forceps or hemostatic forceps. Esophageal perforation occurred in 44 (9.8%) patients, and most perforations were closed with clip. Seventy-two (16.1%) patients received prophylactic antibiotics after operation.
Postoperative fever rate was 30.3% (135/446), the median (range) of maximum body temperature was 38 (37.8–38.4)°C, the median (range) duration of fever was 1 (1–2) days, all patients developed fever within 3 days of operation, 127 (94.1%) patients developed fever on postoperative day 1, 5 (3.7%) patients developed fever on postoperative day 2, and 3 (2.2%) developed fever on postoperative day 3. All patients with fever recovered after conservative treatment (observation or physical cooling) or antibiotic treatment. Eighty-seven patients recovered to normal body temperature through clinical observation or physical cooling alone, 26 patients were treated with antibiotics, and 22 patients were treated with combined physical cooling and antibiotics. There were no serious complications associated with fever. Two hundred and five (45.9%) patients underwent nasogastric tube placement after operation, and the median (range) period of nasogastric tube placement was 3 (2–4) days. The median (range) duration of hospitalization was 8 (6–10) days, and 60 (13.5%) patients experienced adverse events post-ESD/ESTD, among them, bleeding in 3 (0.6%) patients, perforation in 1 (0.2%) patient, chest pain in 45 (10.1%) patients and nausea and vomiting in 11 (2.4%) patients. Symptoms improved after anti-infection and acid inhibition treatment. Finally, 6 (1.3%) patients received secondary endoscopic surgery due to postoperative pathology, indicating positive surgical margins.
Of the 446 patients divided into 2 groups according to whether they developed fever post-ESD/ESTD, 311 (69.7%) patients were included in the non-fever group, and 135 (30.3%) patients were included in fever group. There were several significant differences between patients with vs. without fever post-ESD/ESTD (Table 2). The median age of patients in the fever group was significantly higher compared to the non-fever group (p < 0.05). Similarly, median operation time in the fever group was significantly longer compared to the non-fever group (p < 0.05). Fever occurred in 46.8% of patients with malignant pathology and 26.9% of patients with benign pathology, indicating a statistical difference between the two groups (p < 0.05). Lesions in the fever group were mostly confined to the mucosa/submucosa layer, but lesions in the non-fever group had invaded the muscularis (p < 0.05). Median (range) lesion size in the fever group was 22.5 (14.75–35.25) mm but only 15 (10–20) mm in the non-fever group (p < 0.05). Significant differences were observed in prophylactic antibiotic use between the fever and non-fever groups (p < 0.05). Compared to the non-fever group, the fever group exhibited a significant increase in intraoperative bleeding rate (p < 0.05), whereas there was no significant difference in intraoperative perforation rate between the two groups (p = 0.814). The rate of nasogastric tube placement was 60.7% (82/135) in the fever group, which was significantly higher than the 39.9% (124/311) observed in the non-fever group. Median hospital stay was longer in patients with fever (9 days; range 7–11 days) compared to those without fever (7 days; range 6–9 days; P < 0.001). Finally, there was no significant difference in sex, comorbidities (diabetes, hypertension and history of abdominal surgery), lesion location, operation method or en-bloc resection rate between the two groups. Receiver operating characteristic (ROC) curves were plotted to determine the optimal cut-off values for age, lesion size and operation time to predict fever (Supplementary Figure 1). The cutoff points of the age, lesion size and operation time were 52 years, 19 mm and 37 min, respectively. Operation time ≥37 min was better at distinguishing high-risk patients with fever, with a sensitivity of 70.4%, specificity of 65.3% and the AUC of ROC curve was 0.703. The sensitivity, specificity and AUC for predicting fever were 77%, 42.1% and 0.618 for age ≥52 years. And the sensitivity, specificity and AUC for predicting fever were 64.2, 64.7, and 0.695% for lesion size ≥19 mm (Table 3).
Univariate analysis of the predictive factors for postoperative fever revealed that age ≥52 years (OR: 2.442, 95% CI: 1.541–3.867, p < 0.001), lesion size ≥19 mm (OR: 3.089, 95% CI: 2.028–4.706, p < 0.001), intraoperative bleeding (OR: 2.378, 95% CI: 1.574–3.593, p < 0.001), operative time ≥37 min (OR: 4.371, 95% CI: 2.829–6.754, p < 0.001), malignant pathology (OR: 2.524, 95% CI: 1.577–4.039, p < 0.001) and nasogastric tube placement (OR: 2.333, 95% CI: 1.543–3.528, p < 0.001) were positively correlated with the occurrence of postoperative fever, whereas invasion of the muscularis (OR: 0.379, 95% CI: 0.250–0.574, p < 0.001) and prophylactic antibiotic use (OR: 0.281, 95% CI: 0.135–0.584, p = 0.001) were negatively associated with the occurrence of fever (Table 4 and Figure 4).
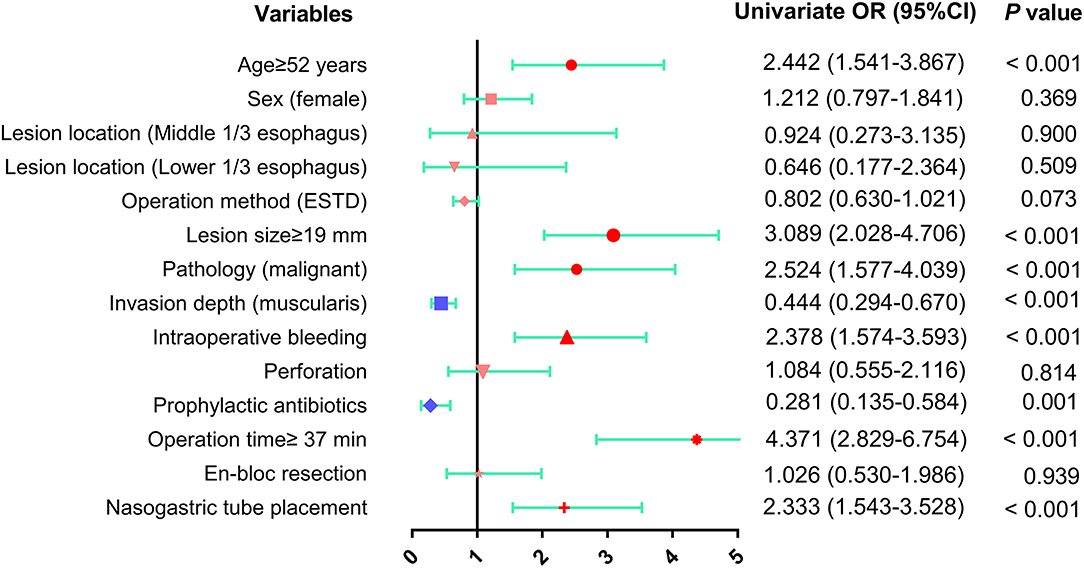
Figure 4. The results of univariate analysis of risk factors for fever after esophageal ESD were presented as forest plot. OR, odds ratio; CI, confidence interval.
Predictive factors for fever onset with p ≤ 0.20 in the univariate analysis were subsequently examined by multivariate analysis (Table 5 and Figure 5). The results showed that 4 conditions, age ≥52 years (OR: 1.740, 95% CI: 1.005–3.013, p = 0.048), lesion size ≥19 mm (OR: 2.007, 95% CI: 1.198–3.362, p = 0.008), operation time ≥37 min (OR: 3.007, 95% CI: 1.756–5.147, p < 0.001) and nasogastric tube placement (OR: 1.881, 95% CI: 1.165–3.037, p = 0.010) were independent risk factors for fever following esophageal ESD. In contrast, there was a significant negative correlation between prophylactic antibiotic use (OR: 0.181, 95% CI: 0.082–0.401, p < 0.001) and fever incidence.
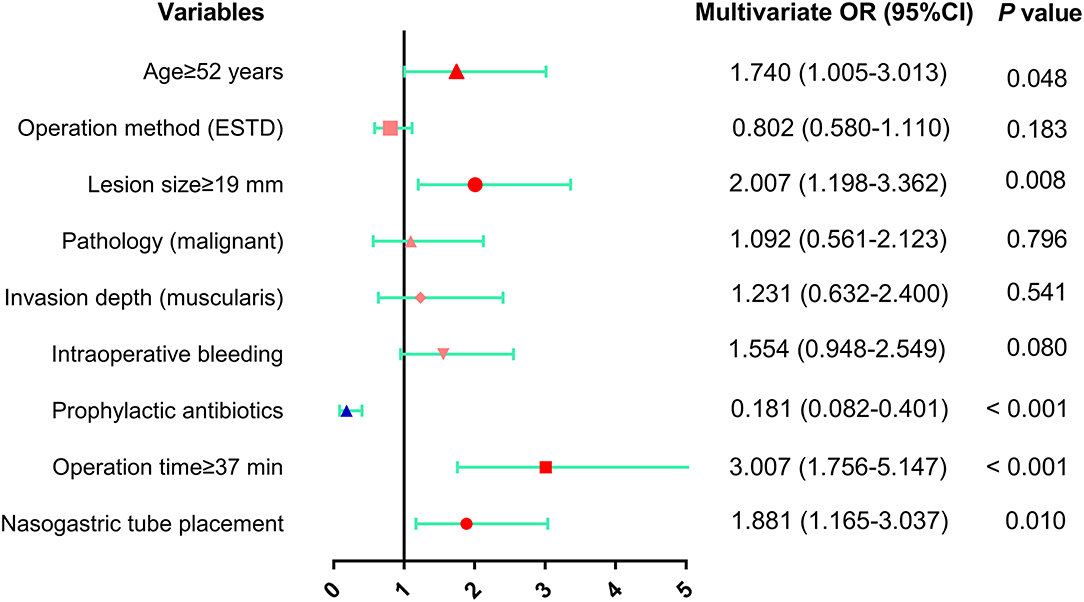
Figure 5. The results of multivariate analysis of risk factors for fever after esophageal ESD were presented as forest plot. OR, odds ratio; CI, confidence interval.
Esophageal lesions include leiomyoma, squamous carcinoma, adenocarcinoma, dysplasia, stromal tumor, etc. With the development of endoscopic techniques, endoscopic resection has become a standard treatment method for these gastrointestinal tumors (22, 23). Compared to endoscopic mucosal resection (EMR), ESD results in increased en-bloc and curative resection rates for the treatment of esophageal lesions, but adverse events (such as perforation) are more likely to occur after ESD than EMR (24), which may be related to the complexity of ESD operative procedures (25). After ESD, we often focus on serious adverse events, such as bleeding and perforation, while postoperative fever is also common but has not been fully assessed. Therefore, we designed this study to investigate the postoperative fever rate and risk factors in patients with esophageal lesions.
The incidence of postoperative fever in this study was 30.3% (135/446), higher than reported in previous studies, which varied from 10 to 24.8% (12, 26). There are several potential reasons for the high fever rate in our study, one of which is that our definition of fever differs from that of a previous study (fever as body temperature ≥38°C) (26). It may also be related to the anatomy of the esophagus itself, which lacks a protective serous layer. Clinically, postoperative fever is often considered to be related to infection, which may be caused by ESD-related endotoxemia (27). Patients under anesthesia and sedation during ESD may also induce aspiration pneumonia and lead to fever (28). In addition, wound tissue damage may also be one of the causes of fever. Among patients with fever, they have higher likelihood of bleeding, that was likely treated with electrocautery. Intraoperative bleeding may require more thermal coagulation, causing inflammatory responses and leading to fever. Our data also suggest that not all fevers are indicative of infection, and that more than half of patients can return to normal body temperature through clinical observation and physical cooling. Although 135 patients developed fever after ESD/ESTD, there is no serious complications related to fever occurred through our timely treatment. All patients returned to normal body temperature after our treatment. Usually, when the fever is <38.5°C, we will take the way of clinical observation or physical cooling. Laboratory tests such as blood routine, CRP, procalcitonin, and blood culture should be performed immediately when the patient's body temperature ≥38.5°C or conventional management measures fail. Infection is considered present when a patient's body temperature is higher than 38.5°C, combined with procalcitonin ≥0.5 ng/ml or a positive blood culture, and antibiotics are used to control infection. In the present study, we found that age ≥52 years, lesion size ≥19 mm, operation time ≥37 min and nasogastric tube placement were risk factors for postoperative fever, while prophylactic antibiotic administration was negatively associated with fever. Nakanishi et al. (12) included 471 ESD patients with gastric lesions, demonstrating that age and resection diameter were risk factors for pyrexia in patients without pneumonia, and operation time was a risk factor for pyrexia in patients with pneumonia. A previous study involving 199 patients undergoing ESD due to colon lesions found that age and lesion size were closely associated with postoperative fever, but the possibility of bacteremia causing fever is very low (13). These data support our results. We believe that susceptibility of the elderly to fever may be related to reduced immunity and the presence of more underlying diseases. Furthermore, large mucosal lesions are likely to cause fever, because they often lead to increased mucosal damage and require more thermal energy. Takeuchi et al. (29) assert that large tumor size is closely related to ESD operation time because it is often accompanied by increased surgical difficulty and leads to greater mucosal defects. This also seems to explain that ESD operation time ≥37 min was a risk factor for postoperative fever in our study. In addition, in the present study, we found that nasogastric tube placement was another significant independent risk factor for pyrexia. Although no previous studies have suggested that nasogastric tube placement is associated with fever, prolonged placement of a nasogastric tube may lead to iatrogenic infection. More research may be needed to investigate this. We observed a negative correlation between prophylactic antibiotics and postoperative fever. A previous article (26) indicated that the incidence of bacteremia after esophageal ESD was low, so there is no need for routine prophylactic antibiotics for patients undergoing ESD. However, in actual clinical work, we inevitably use prophylactic antibiotics in some patients with a high risk for fever. Previous studies have never included prophylactic antibiotic use in risk factor analysis. We included and analyzed prophylactic antibiotic administration in the study and concluded that prophylactic antibiotics were negative correlated with fever incidence. This also suggests that prophylactic antibiotics can be used in clinical practice in patients at high risk of fever to reduce the incidence of fever after operation. We think the intramuscular layer of ESD surgery tends to cause more complications (30), but an interesting phenomenon emerged in our study. In the univariate analysis, lesions confined to the mucosa/submucosa layer were significantly associated with postoperative fever, in contrast to several studies (30, 31). However, when we incorporated variables, such as operation method, into the multivariate analysis, lesion localization to the mucosa/submucosa was not an independent risk factor for fever. A meta-analysis by Zhang et al. (32) suggested that compared to ESD, ESTD significantly reduces postoperative adverse events. Similarly, in the current study, a tendency toward higher ESD operation rate was observed in the fever group. Therefore, such bias was eliminated when we included the operation method in the multivariate analysis.
The advantages of the current study include systematic analysis of the incidence and risk factors for fever after esophageal ESD/ESTD. However, the present study does have several limitations. First, this analysis was a single-center retrospective study. Therefore, a multicenter prospective study should be conducted. Second, because this is a retrospective study, the data we registered may have errors compared to the actual situation, affecting the accuracy of the study. Third, the number of patients included in this study is relatively small, so a study with large sample size is still needed.
In conclusion, age ≥52 years, lesion size ≥19 mm, operation time ≥37 min and nasogastric tube placement were independent risk factors of postoperative fever, prophylactic antibiotic use after operation may help reduce fever rate. Given these findings, we should pay more attention to patients who have these factors.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
FL collected data, analyzed relevant information, and drafted the manuscript. ZZ designed the study and collected the data. YL collected the data. XP, SL, XZ, GL, YZ, and YC clinically managed the patient. XS designed the article, approved the final submission, and clinically managed the patient. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.713211/full#supplementary-material
Supplementary Figure 1. Receiver operating characteristic (ROC) curves were plotted to determine the optimal cut-off values for age, lesion size and operation time to predict fever.
1. Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. (2015) 47:829–54. doi: 10.1055/s-0034-1392882
2. Aadam AA, Abe S. Endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. (2018) 31:doy021. doi: 10.1093/dote/doy021
3. Naveed M, Kubiliun N. Endoscopic treatment of early-stage esophageal cancer. Curr Oncol Rep. (2018) 20:71. doi: 10.1007/s11912-018-0713-y
4. Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol. (2015) 110:784–91. doi: 10.1038/ajg.2014.425
5. Ishihara R, Arima M, Iizuka T, Oyama T, Katada C, Kato M, et al. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Digestive Endoscopy. (2020) 32:452–93. doi: 10.1111/den.13654
6. Li P, Ma B, Gong S, Zhang X, Li W. Endoscopic submucosal tunnel dissection for superficial esophageal neoplastic lesions: a meta-analysis. Surg Endoscopy. (2020) 34:1214–23. doi: 10.1007/s00464-019-06875-y
7. Tsujii Y, Nishida T, Nishiyama O, Yamamoto K, Kawai N, Yamaguchi S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: a multicenter retrospective cohort study. Endoscopy. (2015) 47:775–83. doi: 10.1055/s-0034-1391844
8. Zhai YQ, Li HK, Linghu EQ. Endoscopic submucosal tunnel dissection for large superficial esophageal squamous cell neoplasms. World J Gastroenterol. (2016) 22:435–45. doi: 10.3748/wjg.v22.i1.435
9. Shi Q, Ju H, Yao LQ, Zhou PH, Xu MD, Chen T, et al. Risk factors for postoperative stricture after endoscopic submucosal dissection for superficial esophageal carcinoma. Endoscopy. (2014) 46:640–4. doi: 10.1055/s-0034-1365648
10. Libânio D, Costa MN, Pimentel-Nunes P, Dinis-Ribeiro M. Risk factors for bleeding after gastric endoscopic submucosal dissection: a systematic review and meta-analysis. Gastrointestinal Endoscopy. (2016) 84:572–86. doi: 10.1016/j.gie.2016.06.033
11. Yu X, Liu Y, Xue L, He S, Zhang Y, Dou L, et al. Risk factors for complications after endoscopic treatment in Chinese patients with early esophageal cancer and precancerous lesions. Surg Endoscopy. (2020) 35:2144–53. doi: 10.1007/s00464-020-07619-z
12. Nakanishi T, Araki H, Ozawa N, Takada J, Kubota M, Imai K, et al. Risk factors for pyrexia after endoscopic submucosal dissection of gastric lesions. Endoscopy Int Open. (2014) 2:E141–7. doi: 10.1055/s-0034-1377274
13. Izumi K, Osada T, Sakamoto N, Kodani T, Higashihara Y, Ritsuno H, et al. Frequent occurrence of fever in patients who have undergone endoscopic submucosal dissection for colorectal tumor, but bacteremia is not a significant cause. Surg Endoscopy. (2014) 28:2899–904. doi: 10.1007/s00464-014-3551-5
14. Ominami M, Nagami Y, Shiba M, Tominaga K, Sakai T, Maruyama H, et al. Comparison of propofol with midazolam in endoscopic submucosal dissection for esophageal squamous cell carcinoma: a randomized controlled trial. J Gastroenterol. (2018) 53:397–406. doi: 10.1007/s00535-017-1358-6
15. Ahmed Y, Othman M. EMR/ESD: techniques, complications, and evidence. Curr Gastroenterol Rep. (2020) 22:39. doi: 10.1007/s11894-020-00777-z
16. Nagami Y, Ominami M, Shiba M, Sakai T, Fukunaga S, Sugimori S, et al. Prediction of esophageal stricture in patients given locoregional triamcinolone injections immediately after endoscopic submucosal dissection. Digestive Endoscopy. (2018) 30:198–205. doi: 10.1111/den.12946
17. Dellatore P, Bhagat V, Kahaleh M. Endoscopic full thickness resection versus submucosal tunneling endoscopic resection for removal of submucosal tumors: a review article. Translat Gastroenterol Hepatol. (2019) 4:45. doi: 10.21037/tgh.2019.05.03
18. Nagami Y, Shiba M, Ominami M, Sakai T, Minamino H, Fukunaga S, et al. Single locoregional triamcinolone injection immediately after esophageal endoscopic submucosal dissection prevents stricture formation. Clin Transl Gastroenterol. (2017) 8:e75. doi: 10.1038/ctg.2017.5
19. Nagami Y, Ominami M, Sakai T, Maruyama H, Fukunaga S, Otani K, et al. Predictive factors for difficult endoscopic submucosal dissection for esophageal neoplasia including failure of en bloc resection or perforation. Surg Endoscopy. (2020) 35:3361–9. doi: 10.1007/s00464-020-07777-0
20. Chen T, Zhou PH, Chu Y, Zhang YQ, Chen WF, Ji Y, et al. Long-term outcomes of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Ann Surg. (2017) 265:363–9. doi: 10.1097/SLA.0000000000001650
21. Liu YZ, Lv XH, Deng K, Yang JL. Efficacy and safety of endoscopic submucosal tunnel dissection vs endoscopic submucosal dissection for early superficial upper gastrointestinal precancerous lesions and tumors: A meta-analysis. J Digest Dis. (2020) 21:480–9. doi: 10.1111/1751-2980.12915
22. Draganov PV, Wang AY, Othman MO, Fukami N. AGA institute clinical practice update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol. (2019) 17:16–25.e1. doi: 10.1016/j.cgh.2018.07.041
23. Liu Q, Ding L, Qiu X, Meng F. Updated evaluation of endoscopic submucosal dissection versus surgery for early gastric cancer: A systematic review and meta-analysis. Int J Surg. (2020) 73:28–41. doi: 10.1016/j.ijsu.2019.11.027
24. Guo HM, Zhang XQ, Chen M, Huang SL, Zou XP. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol. (2014) 20:5540–7. doi: 10.3748/wjg.v20.i18.5540
25. Saunders BP, Tsiamoulos ZP. Endoscopic mucosal resection and endoscopic submucosal dissection of large colonic polyps. Nat Rev. (2016) 13:486–96. doi: 10.1038/nrgastro.2016.96
26. Kawata N, Tanaka M, Kakushima N, Takizawa K, Imai K, Hotta K, et al. The low incidence of bacteremia after esophageal endoscopic submucosal dissection (ESD) obviates the need for prophylactic antibiotics in esophageal ESD. Surg Endoscopy. (2016) 30:5084–90. doi: 10.1007/s00464-016-4857-2
27. Kato M, Kaise M, Obata T, Yonezawa J, Toyoizumi H, Yoshimura N, et al. Bacteremia and endotoxemia after endoscopic submucosal dissection for gastric neoplasia: pilot study. Gastric Cancer. (2012) 15:15–20. doi: 10.1007/s10120-011-0050-4
28. Park CH, Kim H, Kang YA, Cho IR, Kim B, Heo SJ, et al. Risk factors and prognosis of pulmonary complications after endoscopic submucosal dissection for gastric neoplasia. Dig Dis Sci. (2013) 58:540–6. doi: 10.1007/s10620-012-2376-0
29. Takeuchi Y, Iishi H, Tanaka S, Saito Y, Ikematsu H, Kudo SE, et al. Factors associated with technical difficulties and adverse events of colorectal endoscopic submucosal dissection: retrospective exploratory factor analysis of a multicenter prospective cohort. Int J Colorectal Dis. (2014) 29:1275–84. doi: 10.1007/s00384-014-1947-2
30. Yamashina T, Takeuchi Y, Uedo N, Hamada K, Aoi K, Yamasaki Y, et al. Features of electrocoagulation syndrome after endoscopic submucosal dissection for colorectal neoplasm. J Gastroenterol Hepatol. (2016) 31:615–20. doi: 10.1111/jgh.13052
31. Ito S, Hotta K, Imai K, Yamaguchi Y, Kishida Y, Takizawa K, et al. Risk factors of post-endoscopic submucosal dissection electrocoagulation syndrome for colorectal neoplasm. J Gastroenterol Hepatol. (2018) 33:2001–6. doi: 10.1111/jgh.14302
Keywords: endoscopic submucosal dissection, endoscopic submucosal tunnel dissection, esophageal lesions, fever, risk factors
Citation: Liao F, Zhu Z, Lai Y, Pan X, Long S, Zhou X, Li G, Zhu Y, Chen Y and Shu X (2022) Risk Factors for Fever After Esophageal Endoscopic Submucosal Dissection and Its Derived Technique. Front. Med. 9:713211. doi: 10.3389/fmed.2022.713211
Received: 22 May 2021; Accepted: 31 January 2022;
Published: 22 February 2022.
Edited by:
Nageshwar Reddy Duvvur, Asian Institute of Gastroenterology, IndiaReviewed by:
Ran Wang, Northern Theater General Hospital, ChinaCopyright © 2022 Liao, Zhu, Lai, Pan, Long, Zhou, Li, Zhu, Chen and Shu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Shu, anhtdXNoeEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.