- 1Clinical Research Development Center, Imam Hossein Educational Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Department of Internal Medicine, Imam Hossein Medical Center, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4Department of Cardiology, Imam Hossein Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 5Department of Obstetrics and Gynecology and Female Infertility Unit, Tehran University of Medical Sciences, Tehran, Iran
- 6Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 7Imam Hossein Hospital, Behavioral Science Research Center of Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 8Anesthesia Research Centre, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 9Department of Pulmonary and Critical Care, University of Miami Miller School of Medicine, Miami, FL, United States
- 10Clinician Scientist of Dental Materials and Restorative Dentistry, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 11Division of Pulmonary and Critical Care, College of Medicine-Jacksonville, University of Florida, Jacksonville, FL, United States
Introduction: Acute kidney injury (AKI) has been associated with an increased mortality rate among hospitalized patients with Coronavirus disease 2019 (COVID-19). The current review aimed to evaluate the symptoms, complications, and treatments performed to manage AKI in patients with COVID-19.
Methods: We searched PubMed/Medline, Web of Science, and Embase for the relevant scientific literature published up to February 1, 2022. The following keywords were used: “COVID-19”, “SARS-CoV-2”, and “Acute kidney injury”.
Results: Forty-four studies with a total number of 114 COVID-19 patients with AKI (Mean age: 53.6 years) were included in our systematic review. The most common comorbidities in patients with COVID-19 suffering from AKI were the history of diabetes, hypertension, and hyperlipidemia. Twelve out of the 44 included studies reported a history of chronic kidney disease (CKD) in this group of patients. Focal segmental glomerulosclerosis (FSGS) and acute tubular necrosis (ATN) were the most common pathological evidence. The average length of hospital stay was 19 days, and the average duration of need for mechanical ventilation was 3 days.
Conclusions: The current systematic review shows that AKI frequently complicates the course of COVID-19 hospitalizations and is associated with increased severity of illness, prolonged duration of hospitalization, and poor prognosis. Given the extent of the adverse impact of AKI, early detection of comorbidities and renal complications is essential to improve the outcomes of COVID-19 patients.
Introduction
Acute kidney injury (AKI) has been associated with an increased mortality rate among hospitalized patients with Coronavirus disease 2019 (COVID-19). An incidence rate of around 10% was reported in these patients. This incidence could be associated with age, disease severity, and ethnicity. Studies have shown that AKI could be closely related to the effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the causative agent of COVID-19, on the kidney rather than any side effect of experimental drugs for COVID-19 such as remdesivir (1).
According to a recent meta-analysis incidence of AKI in COVID-19 was 8.9% (2). This was close to the incidence rate of AKI in patients with community-acquired pneumonia. However, there was statistical heterogeneity among the studies (2). Other meta-analysis studies have shown that males have higher mortality among COVID-19 patients (3). Another systematic review and meta-analysis revealed that being male and diabetic in COVID-19 patients were associated with developing AKI (4). Studies from the USA and Europe presented a pooled incidence of 28.6% and 7.7% for AKI, respectively (5). AKI has also been detected as a predictor of fatality and severe COVID-19 infection (5).
Due to the importance of this issue, the current study aimed to evaluate the symptoms, complications, and treatments performed to manage AKI in patients with COVID-19 as a comprehensive systematic review.
Methods
This review conforms to the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) statement (6).
Literature Search
We searched PubMed/Medline, Web of Science, and Embase for relevant studies, published up to February 1, 2022.
The following terms were used in the search strategy: COVID-19, severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, and acute kidney injury. Only studies written in English were selected.
Study Selection
The records found through database searching were merged, and the duplicates were removed using EndNote X7 (Thomson Reuters, Toronto, ON, Canada). Two reviewers (TS and FO) independently screened the records by title/abstract and full text to exclude those unrelated to the study objectives. Included studies met the following criteria: (1) COVID-19 patients diagnosed with reference standard test; (2) AKI defined according to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines (7). The KDIGO guidelines define AKI as follows: increase in serum creatinine by ≥0.3 mg/dL (≥26.5 micromol/L) within 48 hours, or increase in serum creatinine to ≥1.5 times baseline, which is known or presumed to have occurred within the prior seven days, or Urine volume <0.5 mL/kg/h for 6 hours. Furthermore, different stages of AKI was defined as follows: Stage 1; increase in serum creatinine to 1.5–1.9 times baseline, or increase in serum creatinine by ≥0.3 mg/dL (≥26.5 micromol/L), or reduction in urine output to <0.5 mL/kg/h for 6–12 h. Stage 2; increase serum creatinine to 2.0–2.9 times baseline, or reduce urine output to <0.5 mL/kg/h for ≥12 hours. Stage 3; increase in serum creatinine to 3.0 times baseline, or increase in serum creatinine to ≥4.0 mg/dL (≥353.6 micromol/L), or reduction in urine output to <0.3 mL/kg/h for ≥24 h, or anuria for ≥12 h, or the initiation of kidney replacement therapy, or, in patients <18 years, decrease in estimated glomerular filtration rate (eGFR) to <35 mL/min/1.73 m2.
Data Extraction
Two reviewers (TS and FO) designed a data extraction form and extracted data from all eligible studies, with differences being resolved by consensus. Data such as country of origin, the number of patients with AKI, the number of patients with confirmed COVID-19, clinical symptoms, laboratory findings, outcomes, diagnostic methods, and treatment were extracted from the selected articles.
Results
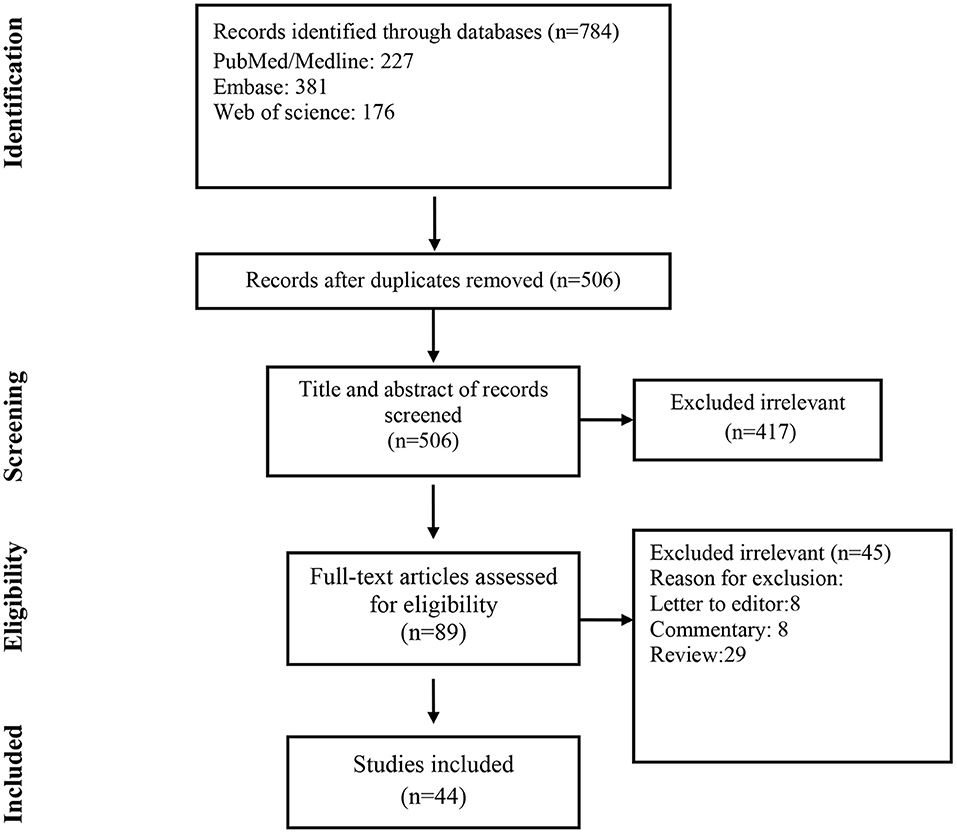
A total of 784 records were found in the initial search; after removing duplicate articles, the titles and abstracts of 506 references were screened (Figure 1). Of these, 89 articles were selected for a full-text review. After the full-text review, 44 articles met the inclusion criteria.
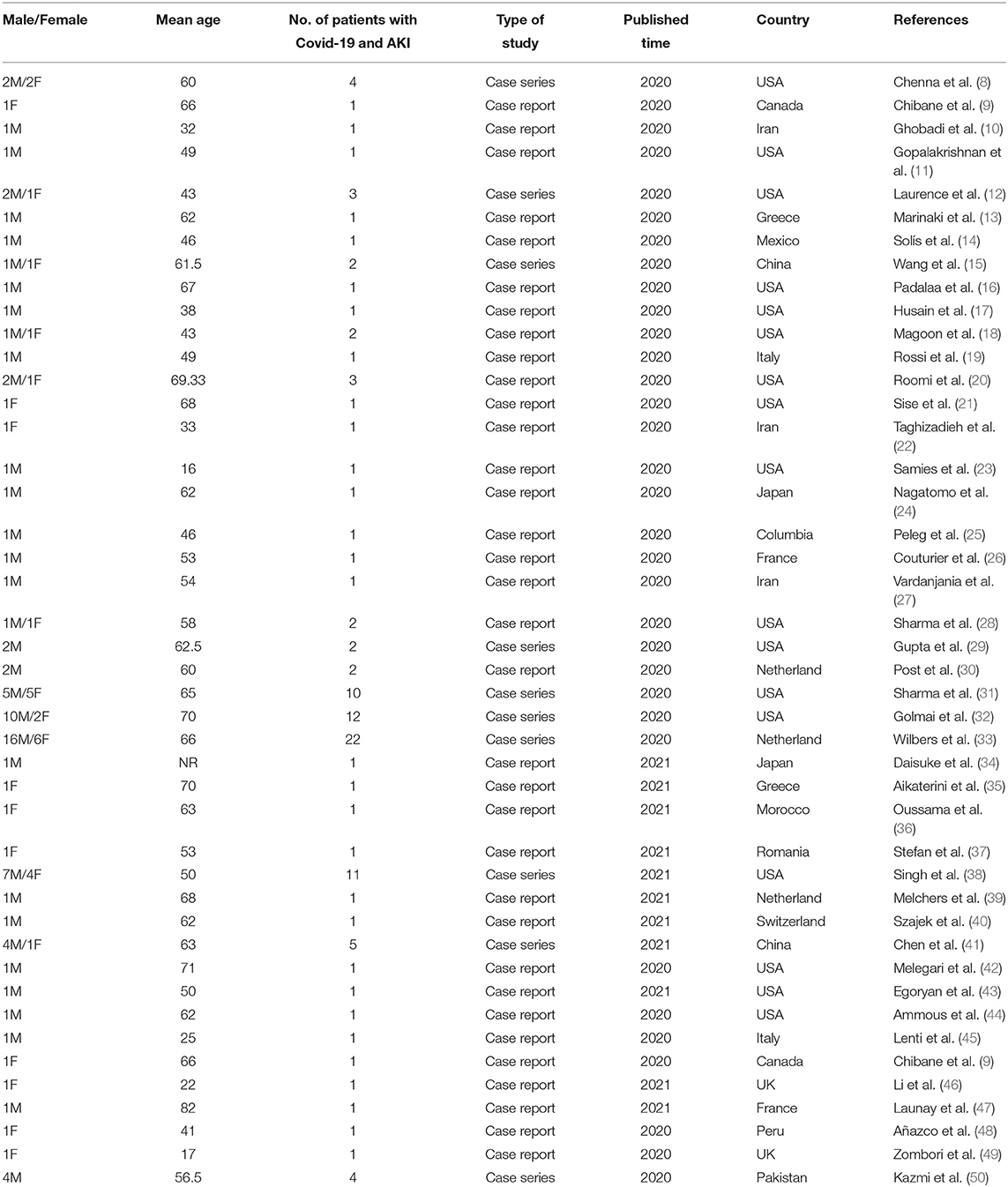
The KDIGO criteria were used in all selected papers to define AKI. A total of 114 COVID-19 patients with AKI (Mean age: 53.6 years) were included in the current study (Table 1).
Stages of AKI in patients with COVID-19 are presented in Table 2.
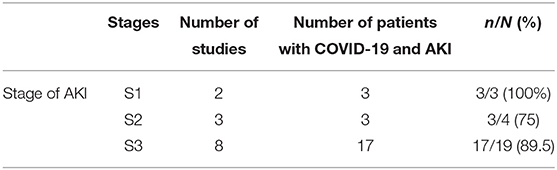
Table 2. Stage of AKI in patients with COVID-19 base on KDIGO Clinical Practice Guideline definition.
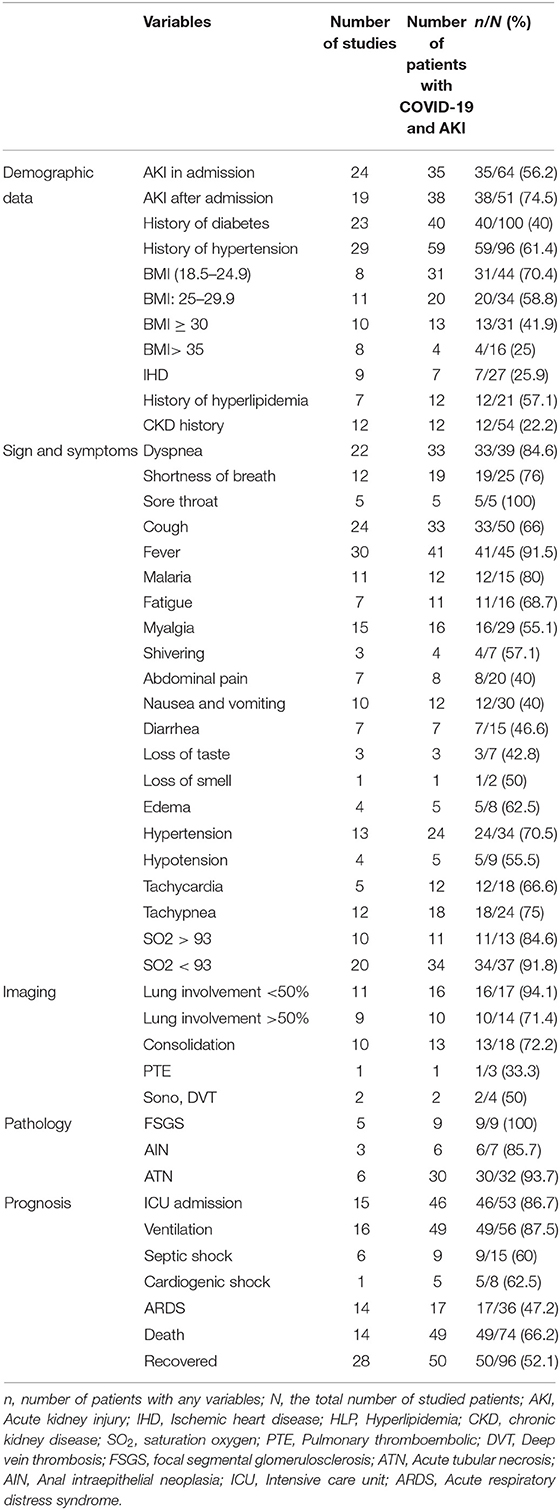
As shown in Table 3, AKI in admission was reported in 35 of 64 studied patients (56.2%), while 38 out of 51 patients (75.5%) showed AKI after admission.
BMI of patients with COVID-19 suffering from AKI was studied in 21 publications. The results showed that most patients (31/44) had a BMI in the range of 18.5–24.9 (Table 3).
The most common comorbidities were the history of diabetes, hypertension, and hyperlipidemia which were present in 40/100 (40%), 59/96 (61.4%), and 12/21 (57.1%) of patients with COVID-19 and AKI, respectively. According to the results of the included studies, 64.7% of patients received angiotensin-converting inhibitor/angiotensin receptor blocker (ACE/ARB), and 62.5% received diuretics. Also, 93.3% of the studied patients were taking oral diabetes medications. A history of chronic kidney disease (CKD) were reported in 12 out of the 54 evaluated patients (22.2%). More information about reported comorbidities in patients with COVID-19 and AKI can be found in Table 3.
Fever (91.5%), dyspnea (84.6%), shortness of breath (76%), cough (66%), hypotension (55.5%), loss of smell and taste (50%), and diarrhea (46.6%) were the most common symptoms. Based on the results of the included studies, 34 out of 37 patients (91.8%) showed a blood oxygen saturation level of less than 93%. However, ten other studies showed that 84.6% of patients had oxygen saturation levels higher than 93% (Table 3).
Imaging results from 9 studies demonstrated lung involvement in more than 50% of evaluated patients (71.4%). Computed tomography (CT) scans revealed that consolidation was another common finding in 72.2% of patients (Table 3).
Focal segmental glomerulosclerosis (FSGS) and acute tubular necrosis (ATN) were the most common pathological evidence (Table 3).
In terms of prognosis, intensive care unit (ICU) hospitalization and the need for a ventilator were reported in most of the involved patients (Table 3).
The average length of hospital stay was 19 days, and the average duration of need for mechanical ventilation was 3 days.
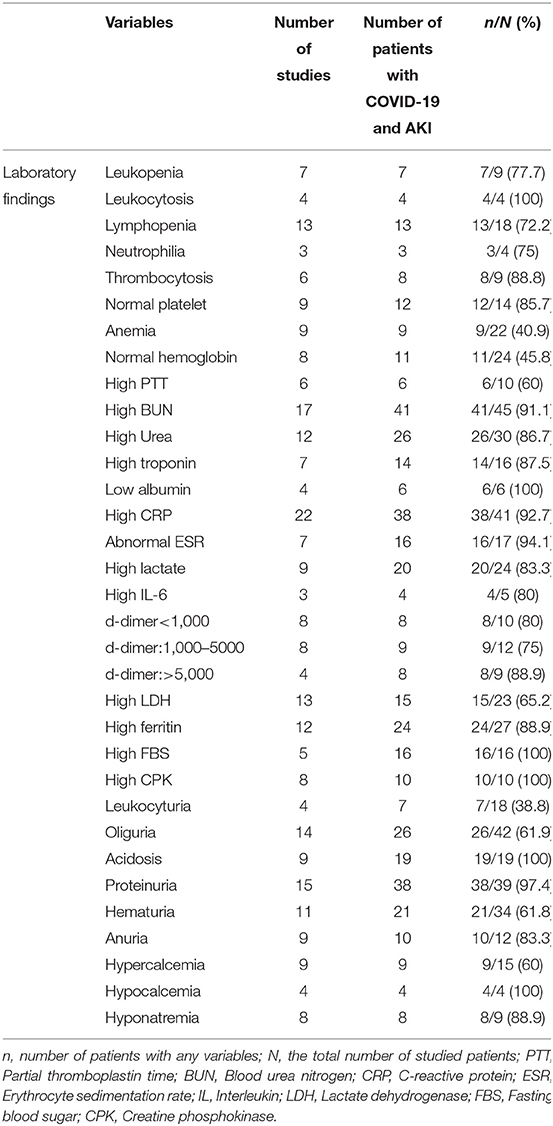
Most studies reported significant laboratory findings in patients with COVID-19 and AKI. 7/9 (77.7%), 4/4 (100%), and 13/18 (72.2%) of patients showed leukopenia, leukocytosis, and lymphopenia, respectively. High C-reactive protein (CRP) and low albumin were reported in 38/41 (92.7%) and 6/6 (100) of COVID-19 patients with AKI, respectively. Fifteen studies reported proteinuria, according to which 97.4% of patients (38/39) had this problem. Hematuria was seen in 21/34 (61.8%) patients from 11 studies. High creatine phosphokinase (CPK) was also reported in 8 studies (Table 4).
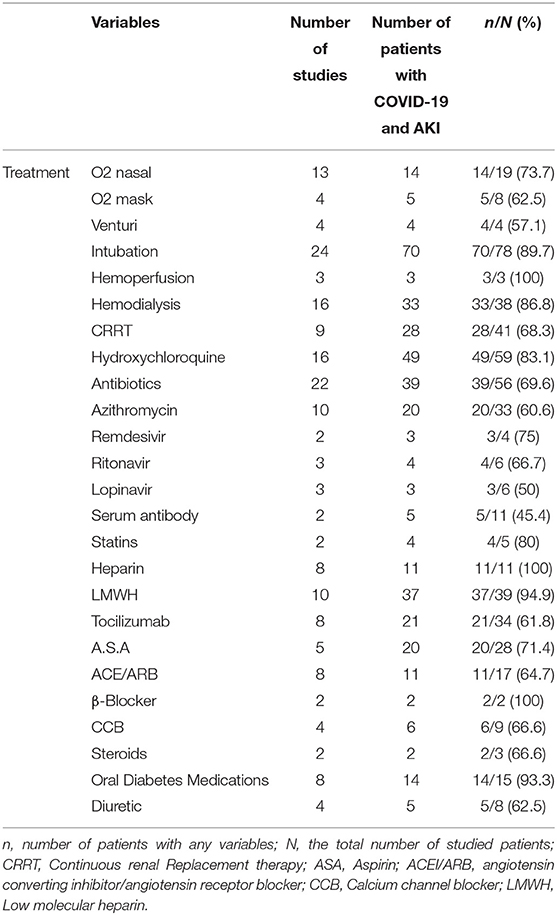
As shown in Table 5, remdesivir, was the most frequently used antiviral agent. Intubation was also reported as the most common non-pharmacological treatment strategy. Furthermore, 33 out of 38 evaluated patients (86.8%) required hemodialysis.
Discussion
In this systematic review, a total of 114 COVID-19 patients with AKI were identified. In prior studies, the prevalence of AKI in COVID-19 patients has been reported widely ranged from 0.5% in China by Guan et al. (51) to 80% in critically ill COVID-19 patients in France by Rubin et al. (52). Xu et al. reported that AKI incidence in COVID-19 patients was 10% and increased to 26% in the ICU-admitted subgroup of patients (1). Silver et al. demonstrated that AKI occurred in 30% of COVID-19 hospitalized patients and that the risk increased to more than 45% in patients requiring ICU admission. The heterogeneity in the reports of AKI incidence between studies could be explained by: (1) variation of the definition of “severe” disease, (2) the differences in admission criteria and hospital care, (3) genetic predisposition to kidney involvement, (4) differences in the frequency of kidney function measurement, and (5) kidney replacement therapy (KRT) resource limitations (53, 54).
In recent studies, kidney tissue sample analysis shed some light on potential pathophysiological mechanisms responsible for COVID-19 related AKI. Commonly cited hypotheses include: (1) tubular epithelial and podocyte damage due to highly expressed angiotensin-converting enzyme-2 (ACE2) in proximal tubular epithelial cells and podocytes, which serves as an entrance door for SARS-COVID-19, causing ATN (55–57), (2) direct infection of glomerular endothelia, causing FSGS (58), (3) COVID-19 related hypovolemia which leads to pre-renal AKI (59), (4) complement activation, cytokine storm, hypercoagulability and microangiopathy which can lead to multiple organ damage especially acute cardiac and lung injury and subsequent AKI via hypoxia and hypotension (55, 60, 61), (5) nephrotoxic drugs or contrast media (59), and (6) comorbidities like diabetes mellitus and hypertension which confer renal vulnerability to AKI (59).
As mentioned above, ATN and FSGS were the most common pathological findings in COVID-19 positive AKI in the current study. FSGS was pathologically investigated in all patients evaluated in the related studies (100%). Likewise, the pathological findings of ATN were also observed in 93.7% of patients.
The most common comorbidities reported in this systematic review, including the history of diabetes mellitus, hypertension, hyperlipidemia, and CKD, were present in 40%, 61.4%, 57.1%, and 22.2% of patients with COVID-19 and AKI, respectively. Based on the growing consensus and evidence, factors including older age, diabetes, hypertension, cardiovascular disease, high BMI, CKD, immunosuppression for any reason, and smoking are the potential risk factors for COVID-19 AKI (62–64). Some laboratory factors including leukocytosis, lymphopenia, elevated CRP, elevated ferritin (62), haematuria and proteinuria (65, 66) were also associated with COVID-19 AKI; which are reported 100, 72.2, 92.7, 88.9, 61.8 and 97.4%, respectively.
In terms of prognosis, ICU hospitalization and the need for assisted ventilation were commonly reported in 86.7 and 87.5% of involved patients, respectively. In patients with COVID-19 and AKI, the overall hospital mortality was 66.2%, comparable with early reports (67, 68).
AKI is considered an independent risk factor for increased mortality in critically ill patients of any disease, including COVID-19 (69). Kidney involvement has also been reported as an indicator of poor prognosis regardless of initial COVID-19 severity (68), which makes early detection and treatment of renal abnormalities improve the vital prognosis of COVID-19 patients.
According to the previous studies, the burden of CKD following COVID-19-related AKI may be substantial, and AKI has been linked to an increased risk of CKD in individuals with previously normal renal function (70, 71). It is essential to say that pre-existing CKD and AKI have been described as predictors of severe and critical illness in patients with COVID-19, with a higher mortality rate than patients without kidney deficiency.
The lack of effective treatments for patients with COVID-19 and AKI has required repurposing several drugs, including remdesivir. The current systematic review indicated that remdesivir, was the most frequently used antiviral agent. These compounds may induce AKI and are not recommended in patients with poor renal function. Thus, early detection and specific therapy of renal changes, including adequate hemodynamic support and avoidance of nephrotoxic drugs, may help to improve critically ill patients with COVID-19.
Our systematic review has some limitations. First, since included studies were case reports articles with a low number of patients, the generalizability of our findings may be limited. Second, detailed information on patient characteristics was lacking in the published articles, and the potential influence of pre-existing conditions could not be investigated. Furthermore, studies' variability and different patients' characteristics were other limitations.
In conclusion, this systematic review shows that AKI frequently complicates the course of COVID-19 hospitalizations and is associated with increased severity of illness, prolonged duration of hospitalization, and poor prognosis. Given the extent of the adverse impact of AKI, it is imperative that early detection of comorbidities and renal complications is essential to improve the outcomes of COVID-19 patients. Further research on large scales is warranted to improve our understanding of this disease and design clinical approaches to managing COVID-19 related AKI.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
TS, MN, and MM designed the study. TS, AK, FO, SK, BH, RV-H, AT, AS, and SO performed the search, study selection, and data extraction. TS, AK, BH, MN, SH, and AA wrote the first draft of the manuscript. MN, BH, AS, and MM revised the article. All authors approved the submitted version.
Funding
MN and his colleagues from Iran were financially supported by a grant from Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Xu Z, Tang Y, Huang Q, Fu S, Li X, Lin B, et al. Systematic review and subgroup analysis of the incidence of acute kidney injury (AKI) in patients with COVID-19. BMC Nephrol. (2021) 22:52. doi: 10.1186/s12882-021-02244-x
2. Chen YT, Shao SC, Hsu CK, Wu IW, Hung MJ, Chen YC. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care. (2020) 24:346. doi: 10.1186/s13054-020-03009-y
3. Nasiri MJ, Haddadi S, Tahvildari A, Farsi Y, Arbabi M, Hasanzadeh S, et al. COVID-19 clinical characteristics, and sex-specific risk of mortality: systematic review and meta-analysis. Front Med. (2020) 7:459. doi: 10.3389/fmed.2020.00459
4. Zhang Z, Zhang L, Zha D, Hu C, Wu X. Clinical characteristics and risks of Chinàs 2019 novel coronavirus patients with AKI: a systematic review and meta-analysis. Ren Fail. (2020) 42:926–31. doi: 10.1080/0886022X.2020.1812401
5. Fu EL, Janse RJ, de Jong Y, van der Endt VHW, Milders J, van der Willik EM, et al. Acute kidney injury and kidney replacement therapy in COVID-19: a systematic review and meta-analysis. Clin Kidney J. (2020) 13:550–63. doi: 10.1093/ckj/sfaa160
6. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and incidence data. Int J Evid Based Healthc. (2015) 13:147–53. doi: 10.1097/XEB.0000000000000054
7. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. (2012) 120:c179–84. doi: 10.1159/000339789
8. Chenna A, Konala VM, Bose S, Roy S, Madhira BR, Gayam V, et al. Acute Kidney Injury in a Case Series of Patients with Confirmed COVID-19 (Coronavirus Disease 2019): Role of Angiotensin-Converting Enzyme 2 and Renin-Angiotensin System Blockade. Case Rep Nephrol. (2020) 2020:8811931. doi: 10.1155/2020/8811931
9. Chibane S, Gibeau G, Poulin F, Tessier P, Goulet M, Carrier M, et al. Hyperacute multi-organ thromboembolic storm in COVID-19: a case report. J Thromb Thrombolysis. (2020) 51:25–8. doi: 10.1007/s11239-020-02173-w
10. Ghobadi H Kalan ME Mohammad-Shahi J Taleb ZB Kalan AE and Fazkzadeh M. COVID-19 and acute kidney injury; a case report. J Renal Inj Prev. (2020) 9:26. doi: 10.34172/jrip.2020.26
11. Gopalakrishnan A, Mossaid A, Lo KB, Vasudevan V, McCullough PA, Rangaswami J. Fulminant acute kidney injury in a young patient with novel coronavirus 2019. Cardiorenal Med. (2020) 10:217–22. doi: 10.1159/000508179
12. Laurence J, Mulvey JJ, Seshadri M, Racanelli A, Harp J, Schenck EJ, et al. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin Immunol. (2020) 219:108555. doi: 10.1016/j.clim.2020.108555
13. Marinaki S, Tsiakas S, Skalioti C, Lourida P, Argyraki A, Grigorakos K, et al. A Patient with cryoglobulinemic membranoproliferative GN (MPGN) who survived COVID-19 disease: case presentation and current data of COVID-19 infection in dialysis and transplanted patients in Greece. Medicina. (2020) 56:355. doi: 10.3390/medicina56070355
14. Solís JG, Pineda AE, Minutti PA, Sánchez AA. Case report: Rhabdomyolysis in a patient with COVID-19: a proposed diagnostic-therapeutic algorithm. Am J Trop Med Hyg. (2020) 103:1158–61. doi: 10.4269/ajtmh.20-0692
15. Wang Y, Lv Y, Liu Q. SARS-CoV-2 infection associated acute kidney injury in patients with pre-existing chronic renal disease: a report of two cases. Immun Inflamm Dis. (2020) 8:506–11. doi: 10.1002/iid3.333
16. Padala SA, Vakiti A, White JJ, Mulloy L, Mohammed A. First Reported Use of Highly Adsorptive Hemofilter in Critically Ill COVID-19 Patients in the USA. J Clin Med Res. (2020) 12:454. doi: 10.14740/jocmr4228
17. Husain R, Corcuera-Solano I, Dayan E, Jacobi AH, Huang M. Rhabdomyolysis as a manifestation of a severe case of COVID-19: a case report. Radiol Case Rep. (2020) 15:1633–7. doi: 10.1016/j.radcr.2020.07.003
18. Magoon S, Bichu P, Malhotra V, Alhashimi F, Hu Y, Khanna S, et al. COVID-19–related glomerulopathy: a report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med. (2020) 2:488–92. doi: 10.1016/j.xkme.2020.05.004
19. Rossi GM, Delsante M, Pilato FP, Gnetti L, Gabrielli L, Rossini G, et al. Kidney biopsy findings in a critically ill COVID-19 patient with dialysis-dependent acute kidney injury: a case against “SARS-CoV-2 nephropathy”. Kidney Int Rep. (2020) 5:1100–5. doi: 10.1016/j.ekir.2020.05.005
20. Roomi S, Ullah W, Farooq S, Saeed R, Haq S, Ashfaq AA. Is therapeutic anticoagulation improving renal outcomes in COVID-19? J Commun Hosp Intern Med Perspect. (2020) 10:306–9. doi: 10.1080/20009666.2020.1785995
21. Sise ME, Baggett MV, Shepard J-AO, Stevens JS, Rhee EP. Case 17-2020: a 68-year-old man with COVID-19 and acute kidney injury. New Engl J Med. (2020) 382:2147–56. doi: 10.1056/NEJMcpc2002418
22. Taghizadieh A, Mikaeili H, Ahmadi M, Valizadeh H. Acute kidney injury in pregnant women following SARS-CoV-2 infection: a case report from Iran. Respir Med Case Rep. (2020) 30:101090. doi: 10.1016/j.rmcr.2020.101090
23. Samies NL, Pinninti S, James SH. Rhabdomyolysis and acute renal failure in an adolescent with coronavirus disease 2019. J Pediatric Infect Dis Soc. (2020) 9:507–9. doi: 10.1093/jpids/piaa083
24. Nagatomo M, Yamada H, Shinozuka K, Shimoto M, Yunoki T, Ohtsuru S. Peritoneal dialysis for COVID-19-associated acute kidney injury. Crit Care. (2020) 24:1–3. doi: 10.1186/s13054-020-03024-z
25. Peleg Y, Kudose S, D'Agati V, Siddall E, Ahmad S, Nickolas T, et al. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. (2020) 5:940–5. doi: 10.1016/j.ekir.2020.04.017
26. Couturier A, Ferlicot S, Chevalier K, Guillet M, Essig M, Jauréguiberry S, et al. Indirect effects of severe acute respiratory syndrome coronavirus 2 on the kidney in coronavirus disease patients. Clin Kidney J. (2020) 13:347–53. doi: 10.1093/ckj/sfaa088
27. Vardanjani AE, Ronco C, Rafiei H, Golitaleb M, Pishvaei MH, Mohammadi M. Early hemoperfusion for cytokine removal may contribute to prevention of intubation in patients infected with COVID-19. Blood Purif. (2020) 50:257–60. doi: 10.1159/000509107
28. Sharma Y, Nasr SH, Larsen CP, Kemper A, Ormsby AH, Williamson SR. COVID-19–associated collapsing focal segmental glomerulosclerosis: a report of 2 cases. Kidney Med. (2020) 2:493–7. doi: 10.1016/j.xkme.2020.05.005
29. Gupta RK, Bhargava R, Shaukat A-A, Albert E, Leggat J. Spectrum of podocytopathies in new-onset nephrotic syndrome following COVID-19 disease: a report of 2 cases. BMC Nephrol. (2020) 21:1–7. doi: 10.1186/s12882-020-01970-y
30. Post A, den Deurwaarder ES, Bakker SJ, de Haas RJ, van Meurs M, Gansevoort RT, et al. Kidney infarction in patients with COVID-19. Am J Kidney Dis. (2020) 76:431–5. doi: 10.1053/j.ajkd.2020.05.004
31. Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, et al. COVID-19–associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. (2020) 31:1948–58. doi: 10.1681/ASN.2020050699
32. Golmai P, Larsen CP, DeVita MV, Wahl SJ, Weins A, Rennke HG, et al. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol. (2020) 31:1944–7. doi: 10.1681/ASN.2020050683
33. Wilbers TJ, Koning MV. Renal replacement therapy in critically ill patients with COVID-19: a retrospective study investigating mortality, renal recovery and filter lifetime. J Crit Care. (2020) 60:103–5. doi: 10.1016/j.jcrc.2020.07.025
34. Katagiri D, Ishikane M, Ogawa T, Kinoshita N, Katano H, Suzuki T, et al. Continuous renal replacement therapy for a patient with severe COVID-19. Blood Purif. (2021) 50:129–31. doi: 10.1159/000508062
35. Vordoni A, Theofilis P, Vlachopanos G, Koukoulaki M, Kalaitzidis RG. Metformin-associated lactic acidosis and acute kidney injury in the era of COVID-19. Front Biosci. (2021) 13:202–7. doi: 10.52586/S563
36. Lamzouri O, Bouchlarhem A, Haddar L, Elaidouni G, Es-Saad O, Bkiyar H, et al. SARS-CoV-2 infection presenting as rhabdomyolysis: case report and review. J Int Med Res. (2021) 49:03000605211061035. doi: 10.1177/03000605211061035
37. Stefan MF, Magda SL, Vinereanu D. COVID-19 presented as acute kidney injury with secondary myocardial damage. J Infect Public Health. (2021) 14:371–3. doi: 10.1016/j.jiph.2020.12.031
38. Singh B, Kaur P, Majachani N, Patel P, Reid R-JR, Maroules M. COVID-19 and combined diabetic ketoacidosis and hyperglycemic hyperosmolar nonketotic coma: report of 11 cases. J Invest Med High Impact Case Rep. (2021) 9:23247096211021231. doi: 10.1177/23247096211021231
39. Melchers M, Festen B, Bianca M, Mooren ER, van Binsbergen AL, van Bree SH, et al. A 67-year-old male patient with COVID-19 with worsening respiratory function and acute kidney failure. Chest. (2022) 161:e5–e11. doi: 10.1016/j.chest.2021.08.045
40. Szajek K, Kajdi M-E, Luyckx VA, Fehr TH, Gaspert A, Cusini A, et al. Granulomatous interstitial nephritis in a patient with SARS-CoV-2 infection. BMC Nephrol. (2021) 22:1–8. doi: 10.1186/s12882-020-02213-w
41. Chen H, Zhang L, Zhang W, Liu L, Dai Z, Chen M, et al. Blood purification in severe and critical COVID-19 patients: a case series of 5 patients. Front Public Health. (2021) 9:741125. doi: 10.3389/fpubh.2021.741125
42. Melegari G, Bertellini E, Melegari A, Trenti T, Malaguti S, Barbieri A. Hemoadsorption cartridge and coronavirus disease 2019 infections: a case report and brief literature review. Artif Organs. (2021) 45:E130–5. doi: 10.1111/aor.13846
43. Egoryan G, Chaudry S, Yadav K, Dong T, Ozcekirdek E, Ozen E, et al. Dark urine as the initial manifestation of COVID-19: a case report. J Med Case Rep. (2021) 15:1–5. doi: 10.1186/s13256-021-03173-x
44. Ammous A, Ghaffar MA, El-Charabaty E, El-Sayegh S. Renal infarction in COVID-19 patient. J Nephrol. (2021) 34:267–8. doi: 10.1007/s40620-020-00866-2
45. Lenti MV, Gregorini M, De Andreis FB, Rampino T, D'Ambrosio G, Verga L, et al. Acute kidney injury caused by COVID-19 in a patient with Crohn's disease treated with adalimumab. J Clin Pathol. (2021) 74:540–2. doi: 10.1136/jclinpath-2020-206912
46. Li APZ, Thomas S, Gokmen R, Kariyawasam D. Rhabdomyolysis and severe biphasic disturbance of calcium homeostasis secondary to COVID-19 infection. BMJ Case Reports CP. (2021) 14:e239611. doi: 10.1136/bcr-2020-239611
47. Launay M, Demartin A-L, Ragey SP, Mismetti P, Botelho-Nevers E, Delavenne X. Severe inflammation, acute kidney injury, and drug–drug interaction: triple penalty for prolonged elimination of apixaban in patients with coronavirus disease 2019: a grand round. Ther Drug Monit. (2021) 43:455. doi: 10.1097/FTD.0000000000000899
48. Añazco PH, Balta FM, Córdova-Cueva L. Bilateral renal infarction in a patient with severe COVID-19 infection. Braz J Nephrol. (2021) 43:127–31. doi: 10.1590/2175-8239-jbn-2020-0156
49. Zombori L, Bacon M, Wood H, Chatterjee F, Venkateswaran R, Lampariello S, et al. Severe cortical damage associated with COVID-19 case report. Seizure. (2021) 84:66–8. doi: 10.1016/j.seizure.2020.11.014
50. Kazmi S, Herekar F, Sarfaraz S. Fatal disseminated intravascular coagulopathy in COVID-19: a small case series. In: Seminars in Thrombosis and Hemostasis. (2021). Thieme Medical Publishers, Inc. doi: 10.22541/au.159301798.84401402/v2
51. Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
52. Rubin S, Orieux A, Prevel R, Garric A, Bats M-L, Dabernat S, et al. Characterisation of acute kidney injury in critically Ill patients with severe coronavirus disease-2019 (COVID-19). medRxiv. (2020): 2020.05.06.20069872. doi: 10.1101/2020.05.06.20069872
53. Mehta RL, Burdmann EA, Cerdá J, Feehally J, Finkelstein F, García-García G, et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet (London, England). (2016) 387:2017–25. doi: 10.1016/S0140-6736(16)30240-9
54. Saghafi A Aghaali M and Saghafi H. Acute kidney injury in hospitalized COVID-19 patients in Iran; a systematic review and meta-analysis. J Renal Injury Prevent. (2021) 10:e09. doi: 10.34172/jrip.2021.09
55. Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. (2020) 31:1380. doi: 10.1681/ASN.2020040419
56. Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. (2020) 98:219–27. doi: 10.1016/j.kint.2020.04.003
57. Mizuiri S, Ohashi Y. ACE and ACE2 in kidney disease. World J Nephrol. (2015) 4:74–82. doi: 10.5527/wjn.v4.i1.74
58. Lin L, Wang X, Ren J, Sun Y, Yu R, Li K, et al. Risk factors and prognosis for COVID-19-induced acute kidney injury: a meta-analysis. BMJ Open. (2020) 10:e042573. doi: 10.1136/bmjopen-2020-042573
59. Fabrizi F, Alfieri CM, Cerutti R, Lunghi G, Messa P. COVID-19 and Acute kidney injury: a systematic review and meta-analysis. Pathogens. (2020) 9:1052. doi: 10.3390/pathogens9121052
60. Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. (2020) 46:1339–48. doi: 10.1007/s00134-020-06153-9
61. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
62. Nadim MK, Forni LG, Mehta RL, Connor MJ, Liu KD, Ostermann M, et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. (2020) 16:747–64. doi: 10.1038/s41581-020-00356-5
63. Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. (2020) 31:1157–65. doi: 10.1681/ASN.2020030276
64. Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. (2020) 98:209–18. doi: 10.1016/j.kint.2020.05.006
65. Mohamed MMB, Lukitsch I, Torres-Ortiz AE, Walker JB, Varghese V, Hernandez-Arroyo CF, et al. Acute kidney injury associated with coronavirus disease 2019 in urban New Orleans. Kidney360. (2020) 1:614. doi: 10.34067/KID.0002652020
66. Husain-Syed F, Wilhelm J, Kassoumeh S, Birk H-W, Herold S, Vadász I, et al. Acute kidney injury and urinary biomarkers in hospitalized patients with coronavirus disease-2019. Nephrol Dial Transplant. (2020) 35:1271. doi: 10.1093/ndt/gfaa162
67. Lowe R, Ferrari M, Nasim-Mohi M, Jackson A, Beecham R, Veighey K, et al. Clinical characteristics and outcome of critically ill COVID-19 patients with acute kidney injury: a single centre cohort study. BMC Nephrol. (2021) 22:1–9. doi: 10.1186/s12882-021-02296-z
68. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. (2020) 97:829–38. doi: 10.1016/j.kint.2020.03.005
69. Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. (2015) 41:1411–23. doi: 10.1007/s00134-015-3934-7
70. Jewell PD, Bramham K, Galloway J, Post F, Norton S, Teo J, et al. COVID-19-related acute kidney injury; incidence, risk factors and outcomes in a large UK cohort. BMC Nephrol. (2021) 22:1–12. doi: 10.1186/s12882-021-02617-2
Keywords: acute kidney injury, COVID-19, SARS-CoV-2, systematic review, AKI
Citation: Sabaghian T, Kharazmi AB, Ansari A, Omidi F, Kazemi SN, Hajikhani B, Vaziri-Harami R, Tajbakhsh A, Omidi S, Haddadi S, Shahidi Bonjar AH, Nasiri MJ and Mirsaeidi M (2022) COVID-19 and Acute Kidney Injury: A Systematic Review. Front. Med. 9:705908. doi: 10.3389/fmed.2022.705908
Received: 07 May 2021; Accepted: 21 February 2022;
Published: 04 April 2022.
Edited by:
Joelle L. Nortier, Université Libre de Bruxelles, BelgiumReviewed by:
Ranjan Das, Rush University Medical Center, United StatesAyioub Pezeshgi, Zanjan University of Medical Sciences, Iran
Manon Dekeyser, Assistance Publique Hopitaux De Paris, France
Devika Kapuria, Washington University in St. Louis, United States
Copyright © 2022 Sabaghian, Kharazmi, Ansari, Omidi, Kazemi, Hajikhani, Vaziri-Harami, Tajbakhsh, Omidi, Haddadi, Shahidi Bonjar, Nasiri and Mirsaeidi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehdi Mirsaeidi, m.mirsaeidi@ufl.edu; Bahareh Hajikhani, b.hajikhani@gmail.com; Mohammad Javad Nasiri, mj.nasiri@hotmail.com
 Tahereh Sabaghian1
Tahereh Sabaghian1