
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 09 January 2023
Sec. Rheumatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1097514
This article is part of the Research Topic Modern Treatment of Autoinflammatory Diseases View all 8 articles
 Po-Ku Chen1,2,3
Po-Ku Chen1,2,3 Tsai-Ling Liao4,5
Tsai-Ling Liao4,5 Shih-Hsin Chang1,2,4
Shih-Hsin Chang1,2,4 Kai-Jieh Yeo1,2
Kai-Jieh Yeo1,2 Chia-Hui Chou2,6
Chia-Hui Chou2,6 Der-Yuan Chen1,2,3,4*
Der-Yuan Chen1,2,3,4*Objective: Neutralizing anti-interferon (IFN)-γ autoantibodies are linked to opportunistic infections (OIs). To explore the association between anti-IFN-γ autoantibodies and OIs in patients with adult-onset Still's disease (AOSD), we aimed to examine the ability of these autoantibodies to blockade signal transducer and activator of transcription (STAT1)-phosphorylation and chemokines production.
Methods: Serum titers of anti-IFN-γ autoantibodies were quantified using ELISA in 29 AOSD and 22 healthy controls (HC). The detectable autoantibodies were verified with immunoblotting assay, and their neutralizing capacity against IFN-γ-signaling was evaluated with flow-cytometry analysis and immunoblotting. IFN-γ-mediated production of supernatant chemokines, including monocyte chemoattractant protein-1 (MCP-1) and IFN-γ inducible protein-10 (IP-10), were measured by ELISA.
Results: Among 29 AOSD patients, high titers of anti-IFN-γ neutralizing autoantibodies were detectable in two patients with OIs. Immunoblotting assay revealed more effective inhibition of STAT1-phosphorylation in THP-1 cells treated with sera from autoantibody-positive AOSD patients (56.7 ± 34.79%) compared with those from HC (104.3 ±29.51%), which was also demonstrated in flow-cytometry analysis (47.13 ± 40.99 vs. 97.92 ± 9.48%, p < 0.05). Depleted serum IgG from anti-IFN-γ autoAbs-positive AOSD patients with OIs restored phosphorylated STAT-1 upon IFN-γ treatment. Sera from autoantibody-positive AOSD patients more effectively inhibited IFN-γ-mediated production of MCP-1 (45.65 pg/ml) and IP-10 (22.44 pg/ml) than sera from HC (263.1 pg/ml and 104.0 pg/ml, both p < 0.05). Serum samples showing the strongest inhibition of IFN-γ-signaling were from two patients with high-titer autoantibodies and OIs.
Conclusion: AOSD patients have a high positive rate and titers of anti-IFN-γ autoantibodies. The remarkable blockade effect of high-titer autoantibodies on IFN-γ-mediated STAT1-phosphorylation and chemokines could make these patients susceptible to OIs.
Interferon (IFN)-γ, a type II IFN (IFN-II) and pro-inflammatory cytokine produced by innate immune cells, is essential for the host's defense against infection with intracellular pathogens (1, 2). Although the biological mechanism behind autoantibodies formation against IFN-γ (anti-IFN-γ autoAbs) remains unclear, several studies have shown that these autoAbs have an inhibitory effect on IFN-γ signal transduction (3, 4). Accordingly, neutralizing anti-IFN-γ autoAbs are recognized as a cause of adult-onset immunodeficiency (AOID) and associated with increased risks of opportunistic infections (OIs) such as disseminated non-tuberculous mycobacteria (NTM), non-typhoid Salmonella, Cryptococcus, and varicella-zoster virus (VZV), particularly in Asian populations (3–9). Hong et al. further revealed that anti-IFN-γ autoAbs titers were strongly associated with the severity of infections, which were likely related to the biological activity of anti-IFN-γ autoAbs (10).
Adult-onset Still's disease (AOSD), a systemic inflammatory disorder, is characterized by fever, rash, arthritis, variable organ involvement, and increased acute phase reactants (11–13). AOSD is also marked by elevated NLRP3-inflammasome-derived cytokines, including IL-1β and IL-18 (14), and Th1-derived cytokine such as IFN-γ (15, 16). IFN-γ-induced chemokines, including IFN-γ inducible protein-10 (IP-10, also known as CXCL10), may further amplify AOSD inflammatory responses and cutaneous manifestations (17). Besides, monocyte chemoattractant protein-1 (MCP-1), a C-C type chemokine with a pivotal role in host defense against pathogens by recruiting macrophages to the inflamed sites, is potentially involved in AOSD pathogenesis (18). Kawakami et al. also revealed that IL-12 and IL-18 synergistically induced IFN-γ production by the immune cells (19). Given that AOSD patients are at potential risk of opportunistic infections (20), it would be instrumental in examining the anti-IFN-γ autoAbs levels and their association with opportunistic infections in AOSD patients. However, the presence of anti-IFN-γ autoAbs or their clinical impact on OIs has yet to be explored in AOSD.
This pilot study aimed to use ELISA to assess the prevalence of anti-IFN-γ autoAbs in AOSD patients and verify their presence with the immunoblotting assay. We also investigated the blockade effects of anti-IFN-γ autoAbs on the IFN-γ signaling-mediated STAT1 transactivation and chemokines production. We finally examined the neutralizing ability of anti-IFN-γ autoAbs and their potential association with the occurrence of OIs in AOSD patients.
In this prospective study, 29 Taiwanese patients who fulfilled the Yamaguchi criteria of AOSD (21) were enrolled consecutively. Systemic disease activity was assessed with a modified Pouchot score (22). This systemic activity score (range 0–12) assigns one point to each of 12 manifestations: fever, evanescent rash, sore throat, arthralgia or arthritis, myalgia, pleuritis, pericarditis, pneumonitis, lymphadenopathy, hepatomegaly or abnormal liver function, elevated leukocyte count ≧ 15,000/mm3, and serum ferritin levels >3,000 μg/L. The enrolled patients did not receive any IFN-γ treatment. Blood samples were obtained when the patients were at an active disease status, defined by the presence of two or more clinical manifestations or laboratory abnormalities; the timing was before the emergence of opportunistic infections. Twenty-two healthy volunteers who had no rheumatic disease were enrolled as healthy control (HC) subjects. This study was approved by the Institutional Review Board of Chinese Medicine University hospital (CMUH110-REC1-086), and informed consent was obtained from each participant according to the Declaration of Helsinki.
Each ten-milliliter whole blood sample was collected in a tube containing EDTA (BD Biosciences, San Jose, CA, USA) and centrifuged at 2,000 rpm for 10 min. According to the manufacturer's instructions, serum titers of IgG autoantibodies to IFN-γ were determined with ELISA (Cell Sciences, Newburyport, MA, USA). Referencing a previous report (23), we defined a “positive” ELISA result as an anti-IFN-γ autoAbs titer ≥48 U/ml and the cut-off value as the mean value plus 5-fold standard deviations (SDs) of HC subjects.
The blocking ability of anti-IFN-γ autoAbs was assessed with their effects on STAT1 phosphorylation on human monocytic cell lines (THP-1, BCRC 60430, the Bioresource Collection and Research Center, Taiwan). Serum samples (10%) from AOSD patients or HCs were firstly incubated with or without 10 ng/ml rhIFN-γ at 37 °C for 30 min. To examine the blocking ability of anti-IFN-γ autoAbs through IgG depletion, the serum samples (10%) from AOSD patients were depleted of IgG by using Pierce™ Albumin Serum Depletion Kits (Thermo Fisher Scientific, #89875). THP-1 cells were stimulated with the mixture according to previous reports with minor modifications (24, 25). These cells were washed twice with PBS and fixed with absolutely ice-cold methanol on ice for 10 min. After washing with 1%BSA, the cells were stained with phycoerythrin (PE) anti-human phospho-STAT1 (pY701) monoclonal antibody (clone A17012A, BD Biosciences San Diego CA, USA) for 30 min at room temperature. The population of PE-pSTAT1 was analyzed by flow-cytometer with FlowJo version 7.6 software.
Serum samples (10%) from AOSD patients or HCs were pre-incubated with or without 10 ng/ml rhIFN-γ at 37 °C for 30 min. THP-1 cells were stimulated with the mixture for 30 min, according to previous reports, with minor modifications (25, 26). After washing with PBS, the cell pellets were lysed with Cell lysis buffer (#9803, Cell Signaling Technology, Danvers, MA, USA) with Roche cOmplete™ protease inhibitor cocktail (Roche) and stored at −80°C until analysis. The cell lysates were separated using SDS-polyacrylamide gel electrophoresis and then transferred to a PDVF membrane. After blocking with 5% milk, immunoblots were performed by using specific anti-human phospho-STAT1 (pY701) monoclonal antibody (#9167), anti-human total-STAT-1 monoclonal antibody (#14994, Cell Signaling Technology, Danvers, MA, USA), and anti-human GAPDH antibody (Elabscience, Houston, TX, USA). The immunoblots were hybridized with HRP-conjugated goat anti-rabbit IgG (Jackson Immunology Research Inc, West Grove, PA, USA). The immunoreactive bands were visualized using an enhanced chemiluminescence detection system (Millipore, Billerica, MA, USA), and the band intensity was determined by Image J software.
To examine an inhibitory activity of anti- IFN-γ autoAbs on the production of chemokines from IFN-γ treated THP-1 cells, serum samples (10%) from AOSD patients or HC were firstly treated with 10 ng/ml rhIFN-γ for 30 min and then co-cultured with THP-1 cells at 37°C for 24 h. The supernatants of cultured THP-1 cells were collected in 1.5 mL-Eppendroff tubes and centrifuged at 2,000 rpm for 10 min. The levels of MCP-1 (DY279, R&D Systems, Minneapolis, MN, USA) and IP-10 (DY266, R&D Systems, Minneapolis, MN, USA) were determined by the ELISA kit, according to the manufacturer's instructions.
Erythrocyte sedimentation rate (ESR) was determined using the Westergren method. Serum ferritin levels were determined using a chemiluminescent immunoassay sandwich method (two-site immunoenzymatic assay, Beckman Coulter, Inc., 250 S. Kraemer Blvd., Brea, CA 92821 U.S.A.), and C-reactive protein (CRP) levels using an immunoturbidimetric method (Beckman Coulter, Inc., 250 S. Kraemer Blvd., Brea, CA 92821 U.S.A.). Serum levels of IFN-γ and IL-18 were measured using magnetic multiplex particle-based assay (Multiplex MAP kits, EMD Millipore, Billerica, MA, USA) according to the manufacturer's instructions.
The results were presented as the mean ± standard deviation (SD), the standard error of the mean (SEM), or the median (interquartile range). We performed the Pearson's χ2 test to examine the between-group difference of categorical variables. The Mann-Whitney U test, the Kruskal-Wallis test with a post-hoc Dunn's test, or One-Way ANOVA test using Bonferroni correction was used to compare different groups. The missing values were excluded from the statistical analysis. A two-sided probability of <0.05 was considered significant.
As illustrated in Table 1, there were no significant differences in the demographic data or body mass index between AOSD patients and HC participants. Two AOSD patients developed opportunistic infections: one with disseminated NTM (Mycobacterium abscess; lymphadenitis and pneumonia), non-typhoid Salmonella septicemia, and Listeria monocytogenes meningitis; and the other with disseminated NTM (Mycobacterium avium-intracellulare complex; lymphadenitis and pneumonia) and non-typhoid Salmonella septicemia. Both patients also had indeterminate results of QuantiFERON-TB In-tube (QFT-GIT), a commercialized ex vivo IFN-γ released assay.
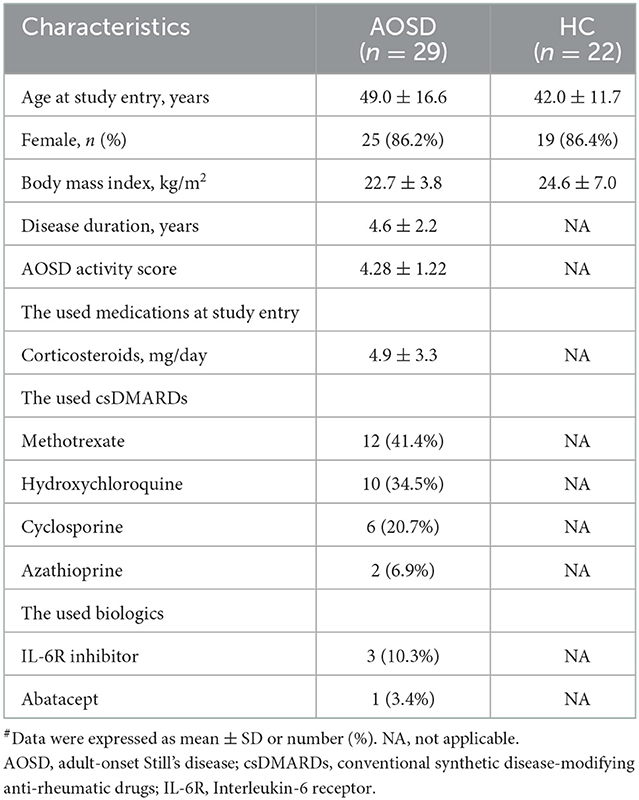
Table 1. Demographic data, disease activity scores, and the used medications in AOSD patients and healthy control (HC) subjects#.
Significantly higher proportions of AOSD patients (5/29, 17.2%) had positive anti-IFN-γ autoAbs compared with HC (0/20, 0.0%, p < 0.001, Figure 1A). Significantly higher titers of anti-IFN-γ autoAbs were also observed in AOSD patients [median 20.97 U/ml, interquartile range (IQR) 7.01–27.56 U/ml) than HC (0.44 U/ml, IQR: 0.44–9.87 U/ml, p < 0.001, Figure 1B). There was a trend of lower IFN-γ levels observed in anti-IFN-γ autoAbs-positive patients compared with autoAbs-negative patients (mean, 0.47 vs. 58.8pg/mL, p = 0.216, Supplementary Table 1). AOSD patients with anti-IFN-γ autoAbs had significantly higher disease activity (the modified Pouchot score) and inflammatory parameters, including ESR, CRP, ferritin levels, and IL-18 levels, than those without autoAbs (Supplementary Table 1). Besides, a significantly higher dose of corticosteroids was prescribed in autoantibody-positive patients than in autoantibody-negative patients. However, there was no significant difference in the use of conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) or biologic DMARDs between AOSD patients with and without anti-IFN-γ autoAbs.
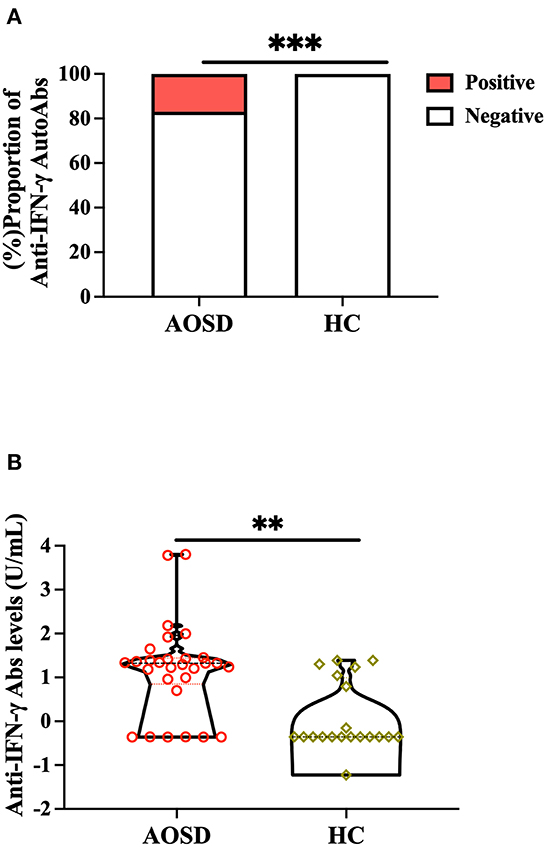
Figure 1. Comparison of (A) positive rates and (B) titers of anti-IFN-γ autoAbs between AOSD patients and HC participants. Anti-IFN-γ autoAbs was then determined by enzyme-linked immunosorbent assay (ELISA). ***p < 0.001, determined by the Chi-squared test (A), or **p < 0.01, determined by the Mann-Whitney U test (B). AOSD, adult-onset Still's disease; HC, healthy control; IFN-γ, interferon-γ.
As shown in the flow cytometry analysis, serum samples from autoAbs-positive AOSD patients could more effectively suppress the STAT1 phosphorylation (47.13 ± 40.99%) compared to those of HC (97.92 ± 9.48%, p < 0.05) (Figures 2A, B). The serum samples with the strongest inhibition effect on the STAT1 phosphorylation were from two AOSD patients (P1 and P2) who had high-titer anti-IFN-γ autoAbs and opportunistic infections (1.72 and 3.29%, respectively). Reflecting the neutralizing ability of IgG autoantibodies, depletion of IgG from the sera of anti-IFN-γ autoAbs-positive AOSD patients (P1 and P2) resulted in the restoration of phosphorylated STAT-1 upon IFN-γ treatment (Figures 2C, D). Besides, serum samples from AOSD patients P3-P5, who had low-titer autoAbs but no OIs, showed a greater capacity to block STAT1 phosphorylation (76.9 ± 6.36%) compared with those from the autoAbs-negative AOSD group (104.9 ± 18.63%, p = 0.0518).
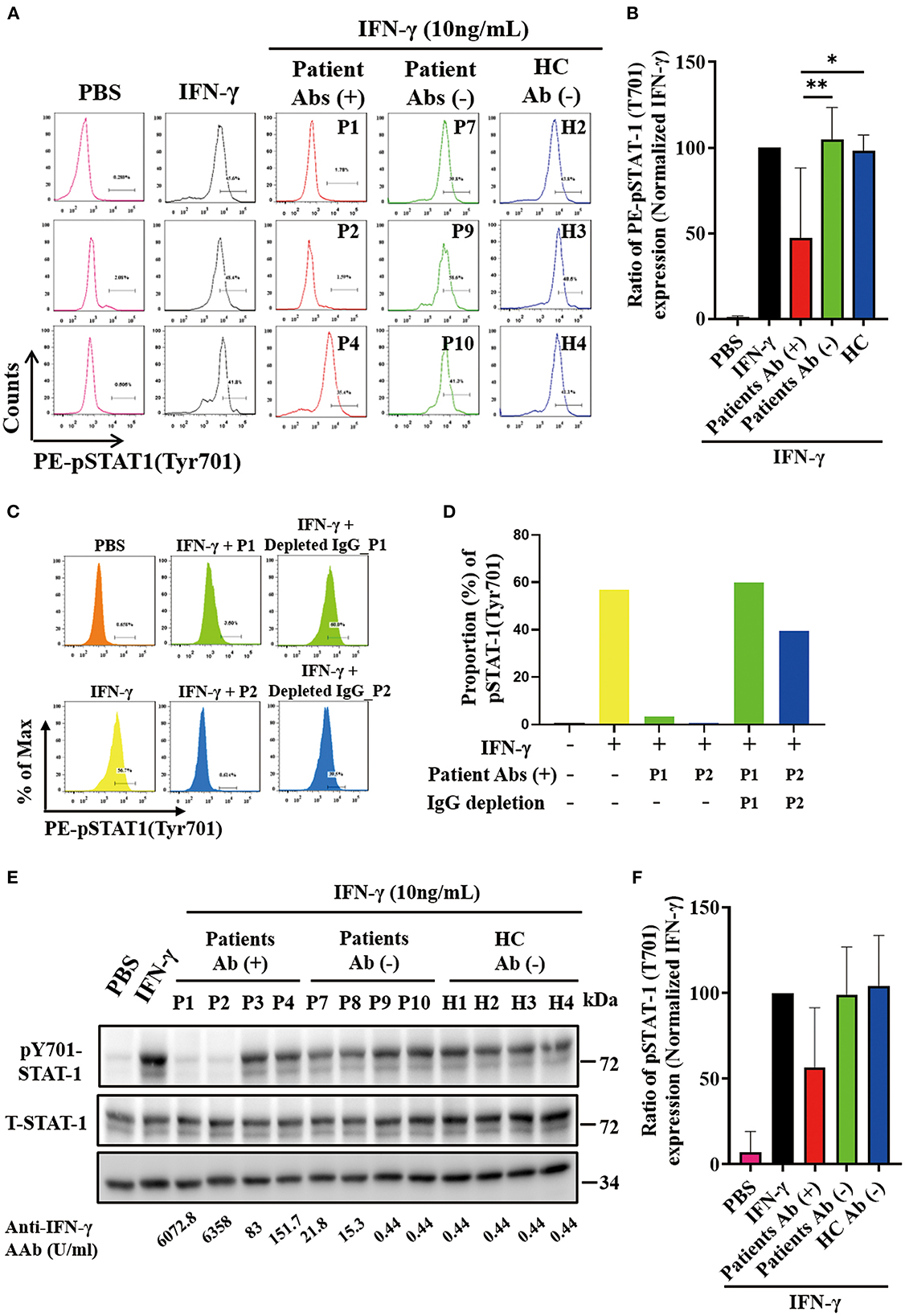
Figure 2. Effective neutralization of IFN-γ signaling by anti-IFN-γ autoAbs. (A) The representative histograms of the inhibitory effects on the IFN-γ-induced PE (phycoerythrin)-STAT1 phosphorylation in THP-1 cells treated with serum samples from autoAbs-positive AOSD patients (P1, P2, and P4), autoAbs-negative patients (P7, P9, and P10), or HC subjects (H2, H3, and H4). (B) Comparison of pSTAT1 intensity after inhibition with serum samples from three groups subjects. (C, D) IgG depletion of serum (10%) from autoAbs-positive AOSD patients (P1 and P2) were restored the IFN-γ-induced STAT1 phosphorylation on THP-1 cells. (E) The inhibitory effects on IFN-γ-induced STAT1 phosphorylation on THP-1 cells treated with serum samples from autoAbs-positive AOSD patients (P1-P4), autoAbs-negative patients (P7-P10), or HC subjects (H1-H4). (F) Comparison of phosphorylated STAT1 (pSTAT1) intensity after inhibition with serum from three groups subjects. Bars and error bars indicate the mean and standard error of mean, respectively. The p-values were determined by One-Way ANOVA test using Bonferroni correction. *p < 0.05, **p < 0.01.
In the immunoblotting assay, serum samples from autoAbs-positive AOSD patients could also more effectively suppress the STAT1 phosphorylation on THP-1 cells (56.70±34.79%), particularly the samples from two patients (P1 and P2) with high-titer anti-IFN-γ autoAbs (22.61 and 16.91%, respectively), compared to samples from HC (104.3 ± 29.51%, p = 0.057) (Figures 2E, F).
We then evaluated the inhibitory effect of anti-IFN-γ autoAbs on the IFN-γ-mediated production of chemokines, including IP-10 and MCP-1. Our results showed that serum samples from autoAbs-positive AOSD patients could more significantly inhibit IFN-γ-mediated production of IP-10 (median 24.79 pg/ml, IQR: 13.30–38.25 pg/ml) than samples from HC (104.0 pg/ml, IQR: 59.03–146.0 pg/ml, p < 0.05, Figures 3A, B). Similarly, MCP-1 production was more significantly inhibited by serum samples from autoAbs-positive AOSD patients (median, 52.38 pg/ml, IQR: 22.09–89.70 pg/ml) compared to those from HC (263.1pg/ml, IQR: 147.0–369.5 pg/ml, p < 0.05, Figures 3C, D). The serum samples showing the strongest inhibitory effects on the production of the chemokines were from two AOSD patients (P1 and P2) who had high-titer anti-IFN-γ autoAbs and OIs (IP-10: 16.69 and 3.13 pg/ml; MCP-1: 24.24 and 15.63 pg/ml, respectively).
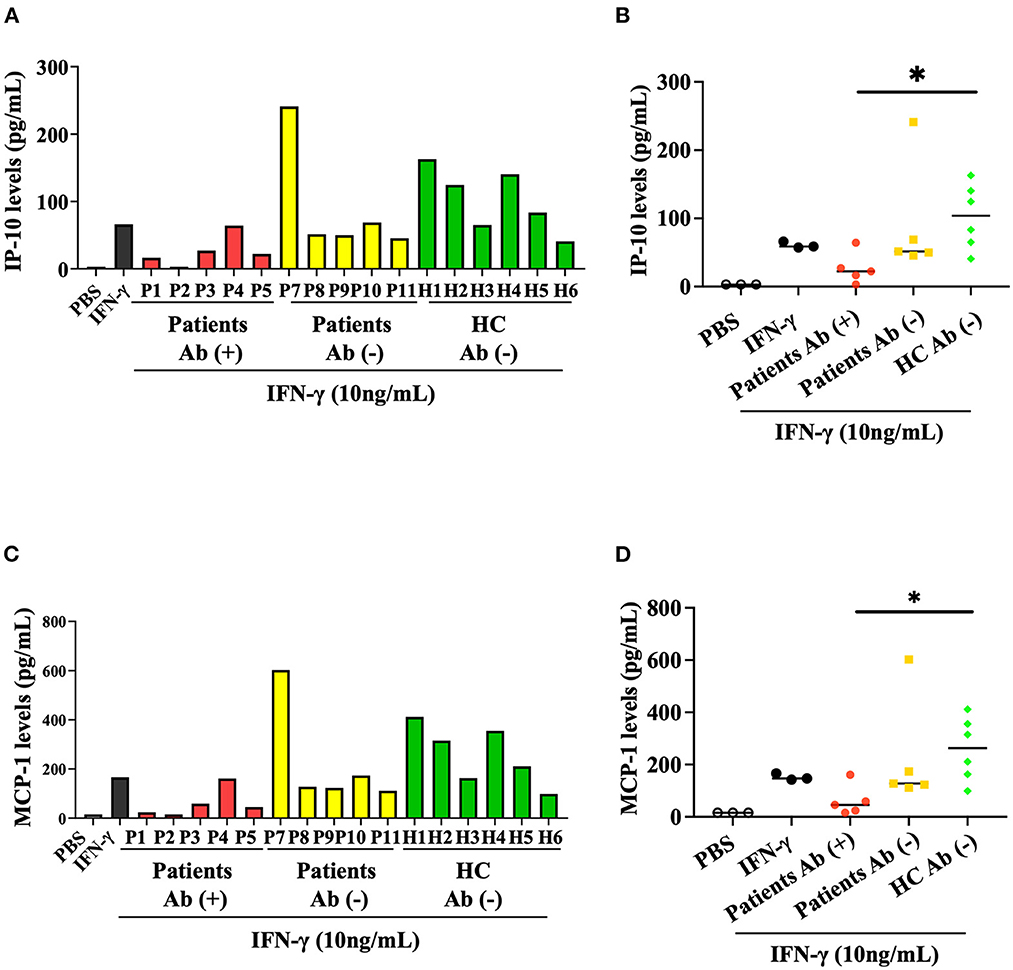
Figure 3. Inhibitory effect of anti-IFN-γ autoAbs on IFN-γ-mediated chemokines. (A) The inhibitory effects of anti-IFN-γ autoAbs on IFN-γ-mediated IP-10 production in THP-1 cells treated with serum samples from autoAbs-positive AOSD patients (P1-P5), autoAbs-negative patients (P7-P11), or HC subjects (H1-H6). (B) Comparison of the supernatant levels of IP-10 on THP-1 cells treated with serum samples from three groups subjects. (C) The inhibitory effects of anti-IFN-γ autoAbs on IFN-γ-mediated MCP-1 production in THP-1 cells treated with serum samples from three group subjects. (D) Comparison of the supernatant levels of IP-10 on THP-1 cells treated with serum samples from three groups subjects. Data are presented as box-plot diagrams, with the box encompassing the 25th percentile (lower bar) to the 75th percentile (upper bar). The horizontal line within the box indicates median value for each group. *p < 0.05, vs. HC, determined by the Kruskal-Wallis test using a post-hoc Dunn's test. IP-10, IFN-γ inducible protein-10; MCP-1, monocyte chemoattractant protein-1.
Growing evidence suggests a close link between neutralizing anti-IFN-γ autoAbs and OIs (3–10, 27), yet their relationship in AOSD has not been explored. Herein, we are the first to demonstrate the significantly higher prevalence and titers of anti-IFN-γ autoAbs in AOSD patients compared with HC participants. The serum samples from high-titer anti-IFN-γ autoAbs-positive AOSD patients could effectively block the STAT1-phosphorylation and suppress the IFN-γ-mediated production of MCP-1 and IP-10. Besides, the most conspicuous inhibition of IFN-γ signaling was observed in the sera of two AOSD patients with both high-titer anti-IFN-γ autoAbs and OIs. These findings suggest that high-titer anti-IFN-γ autoAbs are biologically functional and may contribute to OIs in AOSD patients.
Although the mechanisms for the emergence of autoantibodies in AOSD remain unknown (28), anti-IFN-γ autoAbs were detectable in serum samples from 17.2% of AOSD patients. It has been proposed that endogenous anti-cytokine antibodies may be part of an immune regulatory response to hyper-inflammation or due to abundant cytokine exposure (28, 29). Resonated with this hypothesis, we revealed significantly higher disease activity and serum IL-18 levels in anti-IFN-γ autoAbs-positive patients compared with anti-IFN-γ autoAbs-negative patients. Since the anti-IFN-γ autoAbs could neutralize IFN-γ, our autoAbs-positive patients had lower IFN-γ levels (mean, 0.47 pg/mL) than autoAbs-negative patients (58.8 pg/mL). The non-significant difference is probably due to the small sample size. Besides, emerging evidence supports that anti-cytokine antibodies can modulate disease activity in inflammatory diseases (30). Similar to previous findings that anti-IFN-γ autoAbs were associated with increased disease activity and interferon signature in patients with systemic lupus erythematosus (31), the presence of anti-IFN-γ autoAbs in AOSD patients was associated with higher disease activity. Notably, this association was observed in a small cohort of AOSD patients, which needed further validation.
We then performed flow cytometry analysis to validate the biological function of anti-IFN-γ autoAbs in AOSD patients and revealed the neutralizing capacity of these autoAbs through the blockade of STAT1 phosphorylation. Similar downregulation of STAT1 protein expression was observed in THP-1 cells treated with sera from high-titer autoAbs-positive AOSD patients in the immunoblotting assay. Moreover, the serum samples producing the strongest inhibition of the STAT1 phosphorylation were from two AOSD patients who had high-titer anti-IFN-γ autoAbs and opportunistic infections. In comparison, the sera from our AOSD patients with low-titer anti-IFN-γ autoAbs showed a limited capacity for inhibiting STAT1 phosphorylation; thus, opportunistic infections were not observed in these patients. Our results were consistent with previous studies which reported a functional blockade of IFN-γ-mediated antimicrobial immunity by the high-titer anti-IFN-γ autoAbs (3, 32). These findings indicate that the high-titer anti-IFN-γ autoAbs may contribute to infections through functional neutralization of the IFN-γ-mediated signaling.
After STAT1 phosphorylation, IFN-γ could activate the transcription of genes of cytokines or chemokines that play a crucial role in antimicrobial activity. Patel et al. demonstrated that high-titer anti-IFN-γ autoAbs could effectively suppress IFN-γ-mediated downstream production of cytokines, including tumor necrosis factor (TNF)-α and IL-12, in East Asian women with disseminated NTM (3). Krisnawati et al. also revealed that treatment with NTM patients' sera significantly blocked the IFN-γ-induced production of TNF-α, IFN-γ, MCP-1, and IP-10 (26, 32). Given that the anti-IFN-γ autoAbs titers were inversely correlated with IP-10 and MCP-1 levels in our AOSD patients, we also assessed the effects of anti-IFN-γ autoAbs on the IFN-γ-mediated production of these chemokines. Resonated with the findings of Krisnawati et al. (26), the sera from our high-titer autoAbs-positive AOSD patients could more effectively inhibit the IFN-γ-mediated production of MCP-1 and IP-10 than those from HC. Furthermore, the serum samples that showed the strongest inhibitory effect on the production of chemokines were from two patients with high-titer anti-IFN-γ autoAbs and OIs. The indeterminate results of the QFT-GIT assay in these two patients also support the findings that anti-IFN-γ autoAbs might reduce the released IFN-γ levels in the QFT-GIT assay (33). MCP-1 could contribute to antimycobacterial inflammatory response by attracting monocytes and T lymphocytes (34), and Palucci et al. has revealed the inhibitory effect of IP-10 on mycobacterial growth (35). Gathering the evidence from other studies (3, 26, 32, 34, 35) and ours, high-titer anti-IFN-γ autoAbs may reduce antimicrobial activity at least partly through counteracting the IFN-γ-mediated production of cytokines/chemokines.
Despite the novel findings, there are some limitations of this study. The sample size of AOSD was small, an inherent limitation of this rare disease (36). We were unable to identify the absolute anti-IFN-γ autoAbs titers for predicting the occurrence of OIs, probably due to an insufficient number of patients with these infections. Given the higher dose of corticosteroids prescribed in our autoAbs-positive patients than in autoAbs-negative patients, the titer and neutralizing capacity of anti-IFN-γ autoAbs might be influenced by the therapeutic agents and the clinical characteristics when the blood samples were collected. Finally, the OIs may result from the immunosuppressive effects of the used medications or inherited mutations of IFN-γ-signaling-related genes, which were not evaluated in our AOSD patients. Therefore, a future large-scale study with sufficient statistical power is needed to validate this finding and its clinical implementation. Besides, anti-IFN-γ autoAbs-associated AOID was prevalent in the Asian population (6, 7), so our findings will need to be further validated in ethnically matched control populations.
In conclusion, we are the first to reveal that anti-IFN-γ autoAbs were detectable in 17.2% of AOSD patients, and high-titer autoAbs were associated with OIs through their blockade effects on IFN-γ-mediated STAT1-phosphorylation and chemokines. Our results would add AOSD to the list of diseases with the presence of neutralizing anti-IFN-γ autoAbs. It is clinically significant to beware that AOSD patients with increased neutralizing anti-IFN-γ autoAbs are at increased risk of OIs. Early detection of anti-IFN-γ autoAbs might help guide therapeutic interventions. Cyclophosphamide or rituximab therapy has been reported to reduce anti-IFN-γ autoAbs titers effectively; therefore, they might be an alternative treatment for autoAbs-positive patients with a refractory opportunistic infection, such as disseminated NTM infection (37–40).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The Institutional Review Board of our hospital approved this study (CMUH110-REC1-086). The patients/participants provided their written informed consent to participate in this study.
P-KC conceived and designed the study, acquired the laboratory data, performed the data analysis, and drafted the manuscript. T-LL conducted the experiments, acquired the laboratory data, and performed data analysis. S-HC, K-JY, and C-HC acquired the clinical data and performed the data analysis. D-YC conceived and designed the study, acquired the clinical data, performed data analysis, and revised the manuscript. All authors have read and approved the final manuscript.
This work was supported by a grant from China Medical University Hospital (DMR-111-200) and by a grant (MOST 110-2314-B-039-051) from the Ministry of Science and Technology, Taiwan.
The authors thank Shiow-Jiuan Wey of the Chung Shan Medical University Hospital, Taiwan, for manuscript editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1097514/full#supplementary-material
1. Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. (2007) 96:41–101. doi: 10.1016/S0065-2776(07)96002-2
2. Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. (2004) 75:163–89. doi: 10.1189/jlb.0603252
3. Patel SY, Ding L, Brown MR, Lantz L, Gay T, Cohen S, et al. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol. (2005) 175:4769–76. doi: 10.4049/jimmunol.175.7.4769
4. Wipasa J, Chaiwarith R, Chawansuntati K, Praparattanapan J, Rattanathammethee K, Supparatpinyo K, et al. Characterization of anti-interferon-γ antibodies in HIV-negative immunodeficient patients infected with unusual intracellular microorganisms. Exp Biol Med. (2018) 243:621–26. doi: 10.1177/1535370218764086
5. Haverkamp MH, van Dissel JT, Holland SM. Human host genetic factors in nontuberculous mycobacterial infection: lessons from single gene disorders affecting innate and adaptive immunity and lessons from molecular defects in interferon-gamma-dependent signaling. Microbes Infect. (2006) 8:1157–66. doi: 10.1016/j.micinf.2005.10.029
6. Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. (2012) 367:725–34. doi: 10.1056/NEJMoa1111160
7. Chi CY, Lin CH, Ho MW, Ding JY, Huang WC, Shih HP, et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-γ autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine. (2016) 95:e3927. doi: 10.1097/MD.0000000000003927
8. Hase I, Morimoto K, Sakagami T, Ishii Y, van Ingen J. Patient ethnicity and causative species determine the manifestations of anti-interferon-gamma autoantibody-associated nontuberculous mycobacterial disease: a review. Diagnostic Microbiol Infectious Dis. (2017) 88:308–15. doi: 10.1016/j.diagmicrobio.2017.05.011
9. Hanitsch LG, Löbel M, Müller-Redetzky H, Schürmann M, Suttorp N, Unterwalder N, et al. Late-onset disseminated Mycobacterium avium intracellulare complex infection (MAC), cerebral toxoplasmosis and salmonella sepsis in a German Caucasian patient with unusual anti-interferon-gamma IgG1 autoantibodies. J Clin Immunol. (2015) 35:361–65. doi: 10.1007/s10875-015-0161-5
10. Hong GH, Ortega-Villa AM, Hunsberger S, Chetchotisakd P, Anunnatsiri S, Mootsikapun P, et al. Natural history and evolution of anti-interferon-γ autoantibody-associated immunodeficiency syndrome in Thailand and the United States. Clin Infect Dis. (2020) 71:53–62. doi: 10.1093/cid/ciz786
11. Narula N, Narula T, Abril A. Swizing the clinical presentation in adult-onset Still's disease. An extensive literature reviews. Autoimmun Rev. (2015) 14:472–77. doi: 10.1016/j.autrev.2015.01.007
12. Kadavath S, Efthimiou P. Adult-onset Still's disease-pathogenesis, clinical manifestations, and new treatment options. Ann Med. (2015) 22:1–9. doi: 10.3109/07853890.2014.971052
13. Hu QY, Zeng T, Sun CY, Luo CN, Liu S, Ding TT, et al. Clinical features and current treatments of adult-onset Still's disease: a multicenter survey of 517 patients in China. Clin Exp Rheumatol Suppl. (2019) 121:52–7.
14. Hsieh CW, Chen YM, Lin CC, Tang KT, Chen HH, Hung WT, et al. Elevated expression of the NLRP3 inflammasome and its correlation with disease activity in adult-onset still disease. J Rheumatol. (2017) 44:1142–50. doi: 10.3899/jrheum.161354
15. Chen DY, Lan JL, Lin FJ, Hsieh TY, Wen MC. A predominance of Th1 cytokine in peripheral blood and pathological tissues of patients with active untreated adult-onset Still's disease. Ann Rheum Dis. (2004) 63:1300–06. doi: 10.1136/ard.2003.013680
16. Ichikawa T, Shimojima Y, Kishida D, Ueno KI, Sekijima Y. The implication of interferon-γ-producing immunocompetent cells for evaluating disease activity and severity in adult-onset Still's disease. Int J Rheum Dis. (2021) 24:1176–85. doi: 10.1111/1756-185X.14171
17. Han JH, Suh CH, Jung JY, Ahn MH, Han MH, Kwon JE, et al. Elevated circulating levels of the interferon-γ-induced chemokines are associated with disease activity and cutaneous manifestations in adult-onset Still's disease. Sci Rep. (2017) 7:46652. doi: 10.1038/srep46652
18. Hwang SY, Cho ML, Park B, Kim JY, Kim YH, Min DJ, et al. Allelic frequency of the MCP-1 promoter-2518 polymorphism in the Korean population and in Korean patients with rheumatoid arthritis, systemic lupus erythematosus and adult-onset Still's disease. Eur J Immunogenet. (2002) 29:413–6. doi: 10.1046/j.1365-2370.2002.00346.x
19. Kawakami K, Qureshi MH, Zhang T, Koguchi Y, Yara S, Takeda K, et al. Involvement of endogenously synthesized interleukin (IL)-18 in the protective effects of IL-12 against pulmonary infection with Cryptococcus neoformans in mice. FEMS Immunol Med Microbiol. (2000) 27:191–200. doi: 10.1111/j.1574-695X.2000.tb01430.x
20. Kuo CC Yu WL, Lee CH, Wu NC. Purulent constrictive pericarditis caused by Salmonella enteritidis in a patient with adult-onset Still's disease: a case report. Medicine. (2017) 96:e8949. doi: 10.1097/MD.0000000000008949
21. Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H, et al. Preliminary criteria for classification of adult Still's disease. J Rheumatol. (1992) 19:424–30.
22. Rau M, Schiller M, Krienke S, Heyder P, Lorenz H, Blank N, et al. Clinical manifestations but not cytokine profiles differentiate adult-onset Still's disease and sepsis. J Rheumatol. (2010) 37:2369–76. doi: 10.3899/jrheum.100247
23. Burbelo PD, Browne SK, Sampaio EP, Giacccone G, Zaman R, Kristosturyan E, et al. Anti-cytokine autoantibodies are associated with opportunistic infections in patients with thymic neoplasia. Blood. (2010) 116:4848–58. doi: 10.1182/blood-2010-05-286161
24. Nithichanon A, Chetchotisakd P, Matsumura T, Takahashi Y, Ato M, Sakagami T, et al. Diagnosis of NTM active infection in lymphadenopathy patients with anti-interferon-gamma autoantibody using inhibitory ELISA vs. indirect. ELISA Sci Rep. (2020) 10:8968. doi: 10.1038/s41598-020-65933-x
25. Lin CH, Chi CY, Shih HP, Ding JY, Lo CC, Wang SY, et al. Identification of a major epitope by anti-interferon-gamma autoantibodies in patients with mycobacterial disease. Nat Med. (2016) 22:994–1001. doi: 10.1038/nm.4158
26. Krisnawati DI, Liu YC, Lee YJ, Wang YT, Chen CL, Tseng PC, et al. Functional neutralization of anti-IFN-gamma autoantibody in patients with non-tuberculous mycobacteria infection. Sci Rep. (2019) 9:5682. doi: 10.1038/s41598-019-41952-1
27. Knight V, Merkel PA, óSullivan MD. Anticytokine antibodies: association with infection and immune dysregulation. Antibodies. (2016) 5:3. doi: 10.3390/antib5010003
28. Caruso A, Turano A. Natural antibodies to interferon-γ. Biotherapy. (1997) 10:29–37. doi: 10.1007/BF02678215
29. Karin N. Induction of protective therapy for autoimmune diseases by targeted DNA vaccines encoding pro-inflammatory cytokines and chemokines. Curr Opin Mol Ther. (2004) 6:27–33.
30. Vincent T, Plawecki M, Goulabchand R, Guilpain P, Eliaou JF. Emerging clinical phenotypes associated with anti-cytokine autoantibodies. Autoimmun Rev. (2015) 14:528–35. doi: 10.1016/j.autrev.2015.01.015
31. Gupta S, Tatouli IP, Rosen LB, Hasni S, Alevizos I, Manna ZG, et al. Distinct functions of anti-interferon autoantibodies in systemic lupus erythematosus: a comprehensive analysis of anticytokine antibodies in common rheumatologic diseases. Arthritis Rheumatol. (2016) 68:1677–87. doi: 10.1002/art.39607
32. Krisnawati DI, Liu YC, Lee YJ, Wang YT, Chen CL, Tseng PC, et al. Blockade effects of anti-interferon-(IFN-)γ autoantibody on IFN-γ-regulated antimicrobial immunity. J Immunol Res. (2019) 2019:162925. doi: 10.1155/2019/1629258
33. Wu UI, Chuang YC, Sheng WH, Sun HY, Jhong YT, Wang JY, et al. Use of QuantiFERON-TB Gold In-tube assay in screening for neutralizing anti-interferon-γ autoantibodies in patients with disseminated nontuberculous mycobacterial infection. Clin Microbiol Infect. (2018) 24:159–65. doi: 10.1016/j.cmi.2017.06.029
34. Lin YG, Gong J, Zhang M, Xue W, Barnes PF. Production of monocyte chemoattractant protein-1 in tuberculosis patients. Infect Immun. (1998) 66:2319–22. doi: 10.1128/IAI.66.5.2319-2322.1998
35. Palucci I, Battah B, Salustri A, De Maio F, Petrone L, Ciccosanti F, et al. IP-10 contributes to the inhibition of mycobacterial growth in an ex vivo whole blood assay. Int J Med Microbiol. (2019) 309:299–306. doi: 10.1016/j.ijmm.2019.05.005
36. Evensen KJ, Nossent HC. Epidemiology and outcome of adult-onset Still's disease in Northern Norway. Scand J Rheumatol. (2006) 35:48–51. doi: 10.1080/03009740510026616
37. Chetchotisakd P, Anunnatsiri S, Nanagara R, Nithichanon A, Lertmemongkolchai G. Intravenous cyclophosphamide therapy for anti-IFN-gamma autoantibody-associated mycobacterium abscessus infection. J Immunol Res. (2018) 2018:6473629. doi: 10.1155/2018/6473629
38. Laisuan W, Pisitkun P, Ngamjanyaporn P, Suangtamai T, Rotjanapan P. Prospective pilot study of cyclophosphamide as an adjunct treatment in patients with adult-onset immunodeficiency associated with anti-interferon-γ autoantibodies. Open Forum Infect Dis. (2020) 7:ofaa035. doi: 10.1093/ofid/ofaa035
39. Browne SK, Zaman R, Sampaio EP, Jutivorakool K, Rosen LB, Ding L, et al. Anti-CD20 (rituximab) therapy for anti-IFN-γ autoantibody-associated nontuberculous mycobacterial infection. Blood. (2012) 119:3933–9. doi: 10.1182/blood-2011-12-395707
Keywords: anti-interferon-γ autoantibodies, opportunistic infections, MCP-1, IFN-γ inducible protein-10 (IP-10), adult onset Still's disease (AOSD)
Citation: Chen P-K, Liao T-L, Chang S-H, Yeo K-J, Chou C-H and Chen D-Y (2023) High-titer anti-interferon-γ neutralizing autoantibodies linked to opportunistic infections in patients with adult-onset still's disease. Front. Med. 9:1097514. doi: 10.3389/fmed.2022.1097514
Received: 14 November 2022; Accepted: 20 December 2022;
Published: 09 January 2023.
Edited by:
Andra Rodica Balanescu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Xavier Solanich, Bellvitge University Hospital, SpainCopyright © 2023 Chen, Liao, Chang, Yeo, Chou and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Der-Yuan Chen,  ZHljaGVuMTk1N0BnbWFpbC5jb20=
ZHljaGVuMTk1N0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.