- 1Department of Surgery, Teikyo University Chiba Medical Center, Ichihara, Japan
- 2Department of Pathology, Teikyo University Chiba Medical Center, Ichihara, Japan
Background: Invasive Klebsiella-associated liver abscesses can progress rapidly and cause severe metastatic infections such as meningitis and hydrocephalus, which are associated with high morbidity and mortality. In patients with large multiloculated liver abscesses after failure of percutaneous drainage, rapid diagnosis of the abscess followed by hepatic resection is necessary for early recovery and to prevent severe secondary metastatic complications.
Case presentation: An 84-year-old woman with a large liver abscess and in septic shock was transferred to our hospital. Abdominal CT showed multiloculated liver abscesses 15 cm in diameter in the right lobe of the liver. We first performed percutaneous liver abscess drainage. The patient was managed in the intensive care unit, as well as treated with intravenous administration of meropenem followed by cefozopran according to the antibiogram. Klebsiella pneumoniae with invasive infection was confirmed by a string test in an isolated colony of K. pneumoniae; the K1 serotype with the rmpA and magA genes was determined by polymerase chain reaction and Sanger sequencing. Additional percutaneous liver abscess drainage was performed due to initial inadequate drainage. Although the abscess had shrunk to a diameter of 8 cm after drainage in 4 weeks, the patient recovered from sepsis, but still had low-grade fever (occasionally 38°C) and continued to have symptoms of chronic inflammation with persistent hyper mucus discharge from the liver abscess. Surgical resection was chosen to prevent prolonged hospitalization and ensure early recovery. A right posterior sectionectomy of the liver, including liver abscess, was performed. The post-operative course was uneventful, with no complications, and she was discharged after 18 days. There were no signs of abscess recurrence 1 year after surgery.
Conclusion: We present a case of successful hepatic resection after percutaneous drainage failure in a patient with invasive K. pneumoniae multiloculated liver abscess.
1. Introduction
Klebsiella pneumoniae is the most common causative organism of bacterial liver abscesses and is classified into two groups, classical K. pneumoniae and hypervirulent K. pneumoniae (1). Hypervirulent K. pneumoniae was first identified in Taiwan in 1986 as a liver abscess associated with septic endophthalmitis in young and immunocompetent hosts (2). Hypervirulent strains have a higher potential to resist phagocytosis and metastasize to distant sites forming highly mucoid colonies with capsule polysaccharide, leading to a hypermucoviscous colony phenotype. Accordingly, it is also called hypermucoviscous K. pneumoniae (1). Hypermucoviscosity has been mostly associated with serotype K1, followed by serotype K2, and is associated with the hypervirulence phenotype of Klebsiella regulated by the mucoviscosity-associated gene A (magA) and regulator of mucoid phenotype A (rmpA) (3). Klebsiella-associated invasive liver abscess syndrome with the hypermucoviscous phenotype can progress rapidly and cause severe metastatic infections such as meningitis and hydrocephalus, which are associated with high morbidity and mortality (4, 5). A rapid diagnosis followed by treatment, such as percutaneous drainage and antibiotic therapy, is the standard for improving patient outcomes with hypermucoviscous K. pneumoniae (6). For large multiloculated abscesses with percutaneous drainage failure, surgical resection is necessary for early recovery and to prevent severe secondary metastatic lesions. Herein, we present a case of successful hepatic resection in a patient with an invasive K. pneumoniae multiloculated drainage-resistant liver abscess.
2. Case description
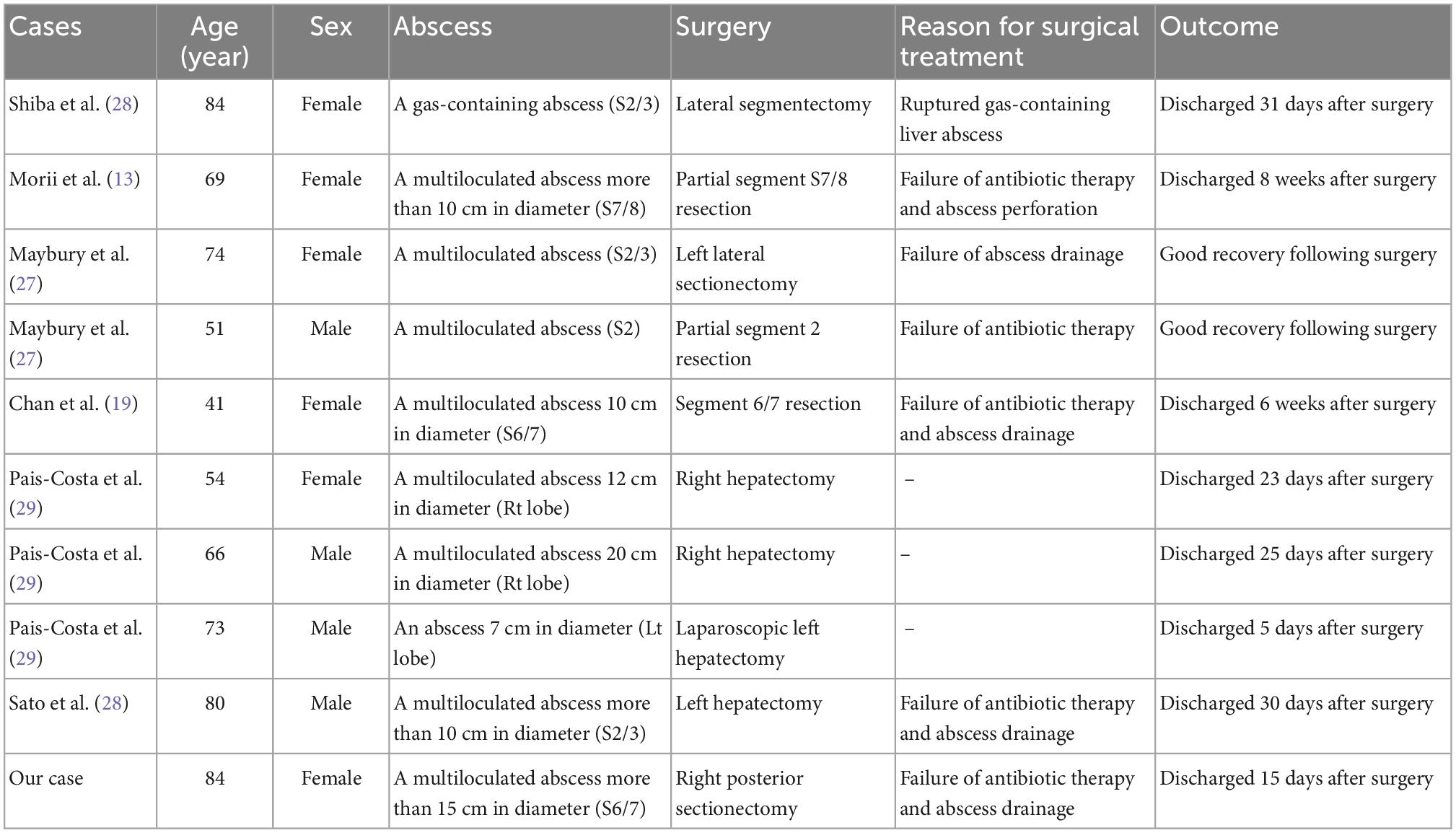
An 84-year-old woman, weighing 54 kg with a height of 150 cm, was urgently admitted to the previous hospital with a diagnosis of liver abscess. Antimicrobial agents for 1 week did not improve symptoms at the previous hospital, and she was transferred to our hospital with septic shock. The patient had an implant stem after a left femur fracture and a history of asthma. There was no history of diabetes or cancer, and no history of smoking, drinking alcohol, or drug allergies. On arrival at our hospital, her initial vital signs were as follows: body temperature, 38°C; heart rate, 122 beats/min; blood pressure, 64/50 mmHg; respiratory rate, 26 breaths/min; and oxygen saturation, 91%. Her blood test revealed an increased ammonia level of 127 μg/dl, decreased serum albumin level of 1.5 g/dl, and a bilirubin level of 0.8 mg/dl. Her white blood cell count was 18200/μL, PT-INR was 1.54, d-dimer was 27.4 ng/mL, and FDP was 32.3 U/mL. Her disseminated intravascular coagulation (DIC) score was 5 points. Abdominal computed tomography showed a multiloculated liver abscess 15 cm in diameter in the right lobes of the liver extending across the right hepatic vein (Figure 1). Extrahepatic infections, such as infections of the lungs and brain, were not detected.
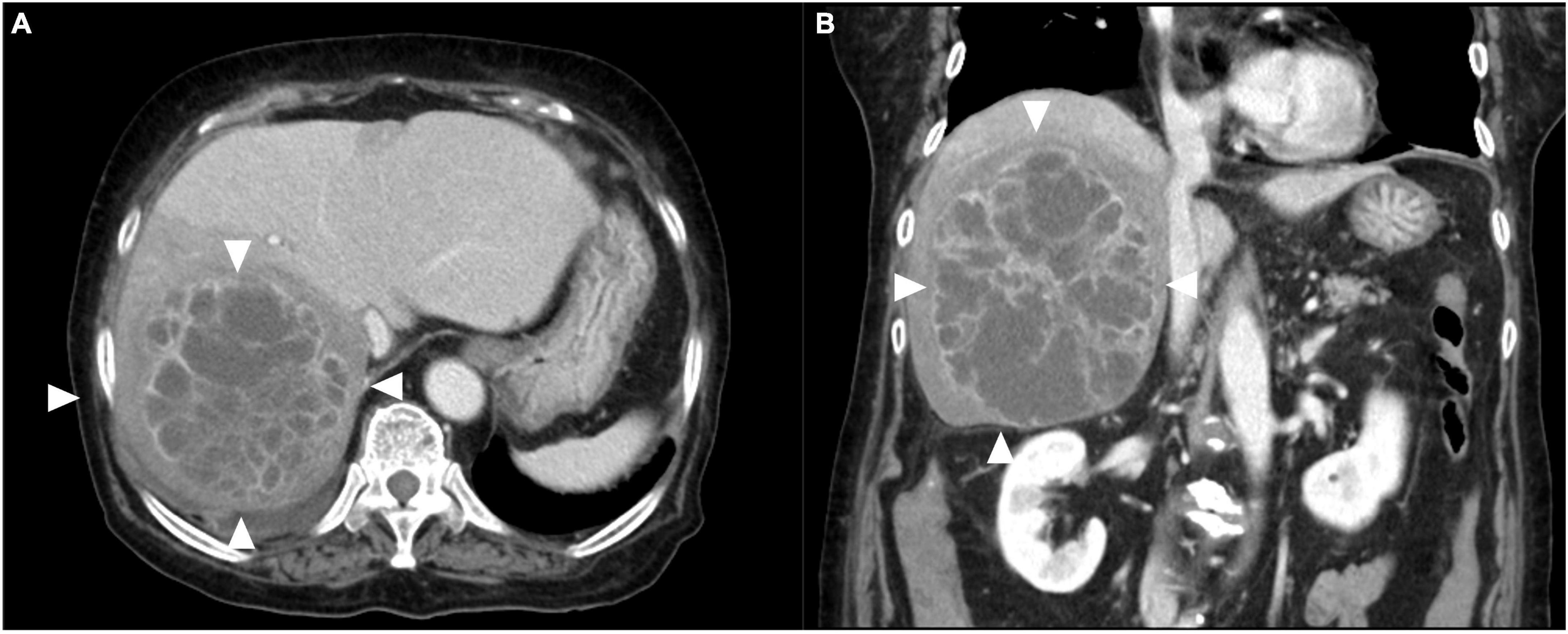
Figure 1. Contrast-enhanced computed tomography images of the liver. (A) Multiloculated liver abscess (white arrows) occupying the right lobes of the liver extending across the right hepatic vein. (B) Coronal image revealing multiloculated liver abscess (white arrows) 15 cm in diameter.
First, we performed percutaneous liver abscess drainage with two drainage tubes. Following transfer to the intensive care unit, intravenous meropenem followed by cefozopran was administered, according to the antibiogram. K. pneumoniae with invasive infection was confirmed by a string test in an isolated colony of K. pneumoniae, and invasive K. pneumoniae large liver abscess was diagnosed. We carried out polymerase chain reaction (PCR) with DNA from the aspirated fluid to detect the presence of hypermucoviscous K. pneumoniae as previously described (7). The PCR determined the amplification of specific genes for serotype K1, a regulator of mucoid phenotype A (rmpA), and mucoid associated gene A (magA), which was confirmed by Sanger sequencing (Figure 2). The additional abscess drainage was performed 1 week after the initial drain placement due to inadequate drainage, and finally, five drainage tubes were placed into the multiloculated liver abscess (Supplementary Figure 1). Although the abscess in the posterior segments of the liver had shrunk to a diameter of 8 cm at 4 weeks after drainage, the patient recovered from sepsis, but still had low-grade fever (occasionally 38°C), an elevated white blood cell count of 10,300/μL, C-reactive protein level of 6 U/L (Supplementary Figure 2), and continued to have symptoms of a chronic inflammation with persistent hyper mucus discharge from the liver abscess via five drainage tubes (Figure 3). We also checked for other causes of fever such as pneumonia and urinary tract infection, but no other infection related to fever was identified other than the liver abscess. We decided that further drainage treatment would be insufficient due to the multiloculated liver abscess with hyper mucus components and prolonged hospitalization with her advanced age. After explaining the patient’s condition and treatment options, a right posterior sectionectomy of the liver, including hepatic abscess, was performed. Although the abscess was not perforated, severe adhesions between the liver abscess and the right diaphragm were observed. The abscess was close to the root of Glisson’s sheath of the right posterior section; therefore, the right posterior sectionectomy of the liver, including the liver abscess, was performed (Supplementary Figure 3).
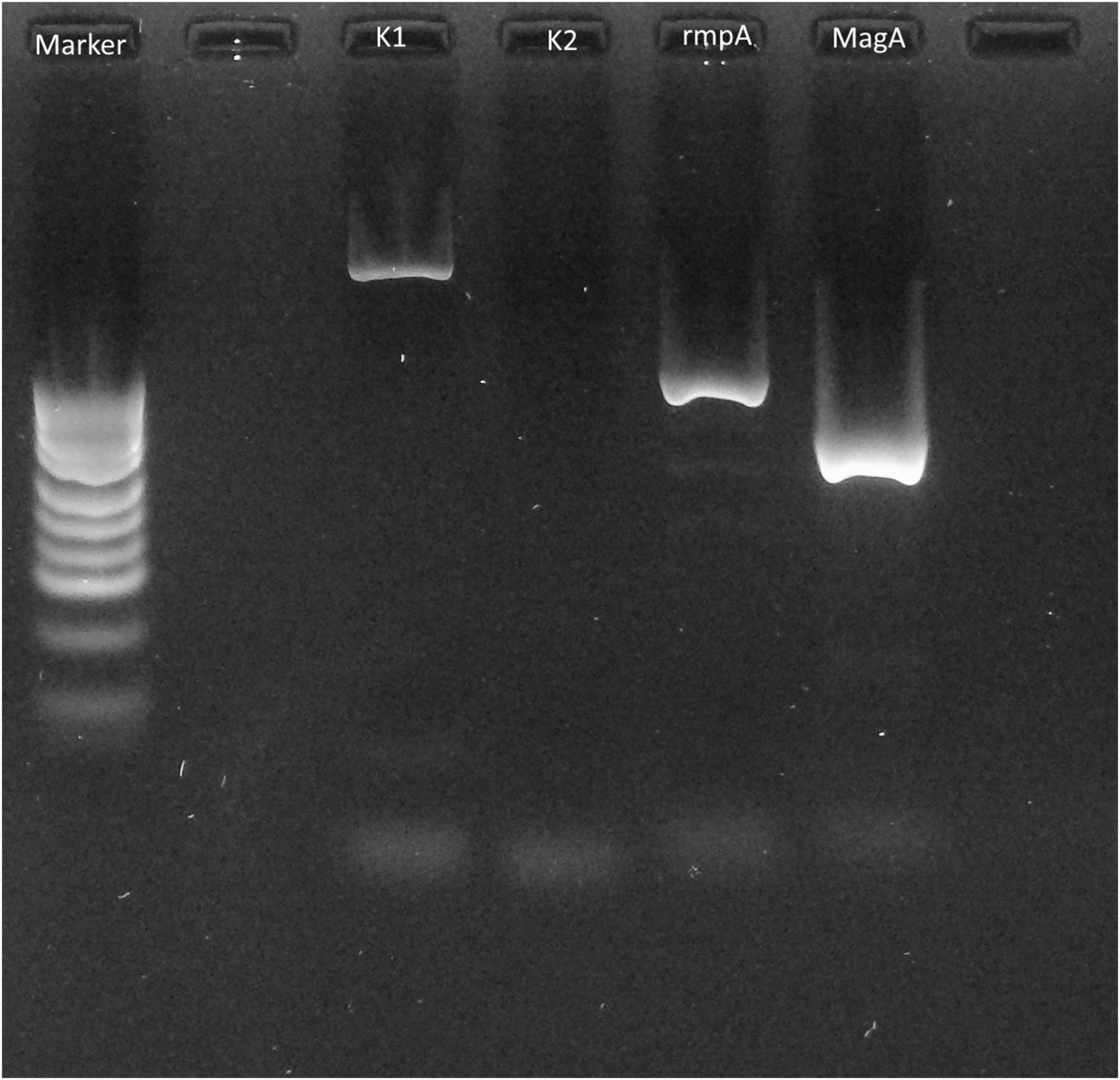
Figure 2. Polymerase chain reaction (PCR) applied to the pus of the liver abscess. PCR with extracted DNA from the pus of the abscess revealed amplification of the Klebsiella pneumoniae serotype K1 specific locus (wzyKPK1), rmpA, and magA fragments that were detected by electrophoresis on 2.0% agarose.
Figure 3. Contrast-enhanced computed tomography images of the liver. (A) Right hepatic vein separated from the multiloculated liver abscess (white arrows). (B) Coronal image revealing multiloculated liver abscess (white arrows) with drainage tubes 8 cm in diameter.
The operative time was 4 h and 48 min, and the total blood loss was 723 ml. The resected right posterior section of the liver revealed a multiloculated and necrotic liver abscess. Histological examination showed a pyogenic liver abscess with areas of necrosis, inflammatory granulation, and a few fluid components, composed of a chronic inflammatory infiltrate consisting of lymphocytes, epithelioid macrophages, eosinophils, and neutrophils. The adjacent hepatocytes appeared atrophic (Supplementary Figure 4). The post-operative course was uneventful, with no complications. The patient was discharged on post-operative day 18. Follow-up examinations using ultrasonography showed no signs of abscess recurrence 1 year after surgery.
3. Discussion
Liver abscesses can be divided into bacterial and amoebic liver abscesses; most of them are bacterial in origin, with K. pneumoniae reported to be the most common causative organism in bacterial liver abscesses (8). Invasive liver abscess syndrome caused by K. pneumoniae has been reported in Taiwan since the 1980s (2). K. pneumoniae is a mucoid-producing bacterium, and serotype K1 is the most common mucoid-producing strain of K. pneumoniae, followed by serotype K2 (3). In particular, magA has been identified as a gene associated with liver abscesses and metastatic lesions in K1 strains (3). Microbiological definitions of invasive liver abscess syndrome are described as K pneumoniae liver abscess caused by the K1 or K2 serotype (3). According to previous reports, some mucoid-producing strains of the genus Klebsiella are highly viscous, resistant to phagocytosis, and can spread hematogenously, resulting in secondary abscesses such as meningitis, hydrocephalus, endophthalmitis, and metastatic lung lesions (4, 5). Among these metastatic infections, endophthalmitis is the most serious complication and a poor prognostic factor for visual acuity (8). Rapid and accurate diagnosis of K. pneumoniae, such as the string test, and identification of the pathogen by metagenomic next-generation sequencing, is the key to start appropriate treatment immediately (9, 10). In our case, the patient had a positive string test, and PCR revealed amplification of the K. pneumoniae serotype K1 specific locus (wzyKPK1), rmpA, and magA fragments. Invasive liver abscess syndrome involving K1 strains is associated with an absence of immunodeficiency disorders; however, recent studies have shown that diabetes mellitus is recognized as an important risk factor for the development of the disease, as well as a poor prognostic factor for visual acuity (11). The frequency of spontaneous liver abscess rupture is as low as 3.8% (12). However, liver abscesses larger than 8 cm tend to rupture easily because of the high pressure of the contents, and ruptures presenting with peritonitis require emergency surgery (13, 14). In addition, an abscess larger than 6 cm is reported to be a significant independent risk factor for metastatic infections (15). Currently, combined antibiotic therapy based on the antibiogram and percutaneous catheter drainage of liver abscesses is the first choice of treatment for liver abscesses (16). Although many treatment strategies have been proposed for pyogenic liver abscesses, the indications for hepatic resection have not been systematically studied (17, 18). Surgical resection should be considered when there is no clinical improvement with antimicrobial therapy and percutaneous drainage, or if the liver abscess ruptures (19–21). Regarding multiloculated liver abscesses, the failure rate of percutaneous catheter drainage is high (22). Previous studies suggested that large multiloculated abscesses should be treated surgically (23). For large multiple liver abscesses with hyper mucus components, surgical resection should be performed if percutaneous drainage is inadequate, which may lead to improved prognosis and earlier recovery (24). In a report of 10 liver resections for K. pneumoniae liver abscess, including our case, 8 cases were multiloculated liver abscesses, 4 cases were operated due to failure of percutaneous abscess drainage, and 2 cases were due to abscess perforation. The post-operative course was reported to be uneventful in all cases (Table 1) (13, 19, 25–28). Regarding to the recurrence rate of liver abscess, the K1 serotype is known to be an important risk factor that influences liver abscess recurrence (29). This case report have some limitations. This was just a single case report, further accumulation of cases is necessary to establish the best therapeutic strategy for invasive K. pneumoniae liver abscess.
In conclusion, in large multiloculated abscesses after drainage failure, such as in our case, complete liver abscess resection should be performed to ensure earlier recovery and prevent metastatic disease recurrence or secondary metastatic lesions.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethics approval was obtained from the Ethical Review Board for Clinical Studies at Teikyo University Chiba Medical Center. Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
Author contributions
HS was the major contributor to the writing of the manuscript. HS and HN supervised the manuscript and performed the surgical therapy. TM and MY were involved in surgical therapy and patient care. KY and SS supported the pathological examination results. KS, CK, AU, and KK supported the clinical examinations. All authors have read and approved the final manuscript.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1092879/full#supplementary-material
Supplementary Figure 1. Percutaneous liver abscess drainage. Percutaneous drainage of the liver abscess was performed twice. The additional drainage of the liver abscess with two tubes (white arrowhead) was carried out following drainage with three tubes (black arrowhead).
Supplementary Figure 2. Clinical course of the present case. The patient still had low-grade fever, occasionally 38°C, accompanied by elevated C-reactive protein (CRP) levels.
Supplementary Figure 3. (A) Intraoperative appearance of the abscess surrounded liver parenchyma (white arrowhead). (B) Intraoperative view of raw surface of the liver after hepatectomy.
Supplementary Figure 4. Gross image of resected specimen and histopathological microscopy of the liver abscess. (A) Resected right posterior section of the liver, revealing the multiloculated and necrotic liver abscess. (B) Pyogenic liver abscess with areas of necrosis and inflammatory granulation, composed of a chronic inflammatory infiltrate consisting of lymphocytes, epithelioid macrophages, eosinophils, and neutrophils. The adjacent hepatocytes appeared atrophic.
References
1. Jun J. Klebsiella pneumoniae Liver Abscess. Infect Chemother. (2018) 50:210–8. doi: 10.3947/ic.2018.50.3.210
2. Liu Y, Cheng D, Lin C. Klebsiella pneumoniae liver abscess associated with septicendophthalmitis. Arch Intern Med. (1986) 146:1913–6. doi: 10.1001/archinte.1986.00360220057011
3. Siu L, Yeh K, Lin J, Fung C, Chang F. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. (2012) 12:881–7. doi: 10.1016/S1473-3099(12)70205-0
4. Sun R, Zhang H, Xu Y, Zhu H, Yu X, Xu J. Klebsiella pneumoniae-related invasive liver abscess syndrome complicated by purulent meningitis: a review of the literature and description of three cases. BMC Infect Dis. (2021) 21:15. doi: 10.1186/s12879-020-05702-3
5. Chou D, Wu S, Chung K, Han S. Septic pulmonary embolism caused by a Klebsiella pneumoniae liver abscess: clinical characteristics, imaging findings, and clinical courses. Clinics (Sao Paulo). (2015) 70:400–7. doi: 10.6061/clinics/2015(06)03
6. Li F, Zheng W, Yu J, Zhao L. Klebsiella pneumoniae liver abscess with purulent meningitis and endogenous endophthalmitis: A case report. Front Surg. (2022) 9:894929. doi: 10.3389/fsurg.2022.894929
7. Compain F, Babosan A, Brisse S, Genel N, Audo J, Ailloud F, et al. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumoniae. J Clin Microbiol. (2014) 52:4377–80. doi: 10.1128/JCM.02316-14
8. Fung C, Chang F, Lee S, Hu B, Kuo B, Liu C, et al. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut. (2002) 50:420–4. doi: 10.1136/gut.50.3.420
9. Zeng S, Yan W, Wu X, Zhang H. Case report: diagnosis of Klebsiella pneumoniae invasive liver abscess syndrome with purulent meningitis in a patient from pathogen to lesions. Front Med (Lausanne). (2021) 8:714916. doi: 10.3389/fmed.2021.714916
10. Russo T, Olson R, Fang C, Stoesser N, Miller M, Macdonald U, et al. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J Clin Microbiol. (2018) 56:e776–718. doi: 10.1128/JCM.00776-18
11. Zheng S, Florescu S, Mendoza M. Klebsiella pneumoniae invasive syndrome in a diabetic patient with gallbladder abscess. Clin Case Rep. (2020) 8:1940–2. doi: 10.1002/ccr3.3038
12. Jun C, Yoon J, Wi J, Park S, Lee W, Jung S, et al. Risk factors and clinical outcomes for spontaneous rupture of pyogenic liver abscess. J Dig Dis. (2015) 16:31–6. doi: 10.1111/1751-2980.12209
13. Morii K, Kashihara A, Miura S, Okuhin H, Watanabe T, Sato S, et al. Successful hepatectomy for intraperitoneal rupture of pyogenic liver abscess caused by Klebsiella pneumoniae. Clin J Gastroenterol. (2012) 5:136–40. doi: 10.1007/s12328-012-0293-6
14. Pham Van T, Vu Ngoc S, Nguyen Hoang N, Hoang Huu D, Dinh Duong T. Ruptured liver abscess presenting as pneumoperitoneum caused by Klebsiella pneumoniae: a case report. BMC Surg. (2020) 20:228. doi: 10.1186/s12893-020-00858-w
15. Shin S, Park C, Lee Y, Kim E, Kim S, Goo J. Clinical and radiological features of invasive Klebsiella pneumoniae liver abscess syndrome. Acta Radiol. (2013) 54:557–63. doi: 10.1177/0284185113477400
16. Serraino C, Elia C, Bracco C, Rinaldi G, Pomero F, Silvestri A, et al. Characteristics and management of pyogenic liver abscess: A European experience. Med (Baltim). (2018) 97:e0628. doi: 10.1097/MD.0000000000010628
17. Rismiller K, Haaga J, Siegel C, Ammori J. Pyogenic liver abscesses: a contemporary analysis of management strategies at a tertiary institution. HPB (Oxford). (2017) 19:889–93. doi: 10.1016/j.hpb.2017.06.005
18. Lo J, Leow J, Ng P, Lee H, Mohd Noor N, Low J, et al. Predictors of therapy failure in a series of 741 adult pyogenic liver abscesses. J Hepato-Bil Pancreat Sci. (2015) 22:156–65. doi: 10.1002/jhbp.174
19. Chan T, Lauscher J, Chan A, Law C, Karanicolas P. Hypermucoviscous Klebsiella pneumoniae liver abscess requiring liver resection. BMJ Case Rep. (2018) 2018:bcr2018226490. doi: 10.1136/bcr-2018-226490
20. Onder A, Kapan M, Böyük A, Gümüş M, Tekbaş G, Girgin S, et al. Surgical management of pyogenic liver abscess. Eur Rev Med Pharmacol Sci. (2011) 15:11 82–6.
21. Hsieh H, Chen T, Yu C, Wang N, Chu H, Shih M, et al. Aggressive hepatic resection for patients with pyogenic liver abscess and Apache II score > or = 15. Am J Surg. (2008) 196:346–50. doi: 10.1016/j.amjsurg.2007.09.051
22. Nham E, Lee J, Huh K, Ko J, Cho S, Kang C, et al. Predictive and prognostic factors associated with unliquefied pyogenic liver abscesses. J Microbiol Immunol Infect. (2022):S1684-1182-00109-8. doi: 10.1016/j.jmii.2022.07.010
23. Ferraioli G, Garlaschelli A, Zanaboni D, Gulizia R, Brunetti E, Tinozzi F, et al. Percutaneous and surgical treatment of pyogenic liver abscesses: observation over a 21-year period in 148 patients. Dig Liver Dis. (2008) 40:690–6. doi: 10.1016/j.dld.2008.01.016
24. Tan Y, Chung A, Chow P, Cheow P, Wong W, Ooi L, et al. An appraisal of surgical and percutaneous drainage for pyogenic liver abscesses larger than 5 cm. Ann Surg. (2005) 241:485–90. doi: 10.1097/01.sla.0000154265.14006.47
25. Maybury B, Powell-Chandler A, Kumar N. Two cases of Klebsiella pneumoniae liver abscess necessitating liver resection for effective treatment. Ann R Coll Surg Engl. (2015) 97:e37–8. doi: 10.1308/003588414X14055925060037
26. Shiba H, Aoki H, Misawa T, Kobayashi S, Saito R, Yanaga K. Pneumoperitoneum caused by Ruptured gas-containing liver abscess. J Hepatobiliary Pancreat Surg. (2007) 14:210–1. doi: 10.1007/s00534-006-1136-y
27. Pais-Costa S, Araujo S, Figueiredo V. Hepatectomy for pyogenic liver abscess treatment: Exception Approach? Arq Bras Cir Dig. (2018) 31:e1394. doi: 10.1590/0102-672020180001e1394
28. Sato K, Takahashi K, Kusuta T. A case of hypermucoviscosity phenotype of Klebsiella Pneumoniae liver abscess saved by damage control strategy. Case Rep Surg. (2022):6019866. doi: 10.1155/2022/6019866
Keywords: Klebsiella-associated multiloculated liver abscess, percutaneous drainage failure, K1 serotype, hyper mucus discharge, K. pneumoniae
Citation: Nojima H, Shimizu H, Murakami T, Yamazaki M, Yamazaki K, Suzuki S, Shuto K, Kosugi C, Usui A and Koda K (2023) Successful hepatic resection for invasive Klebsiella pneumoniae large multiloculated liver abscesses with percutaneous drainage failure: A case report. Front. Med. 9:1092879. doi: 10.3389/fmed.2022.1092879
Received: 08 November 2022; Accepted: 15 December 2022;
Published: 06 January 2023.
Edited by:
Francesco Paolo Bianchi, University of Bari Aldo Moro, ItalyReviewed by:
Utpal Anand, All India Institute of Medical Sciences, IndiaDalong Yin, University of Science and Technology of China, China
Hairui Wang, Shengjing Hospital of China Medical University, China
Copyright © 2023 Nojima, Shimizu, Murakami, Yamazaki, Yamazaki, Suzuki, Shuto, Kosugi, Usui and Koda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroaki Shimizu,  aC1zaGltaXp1QG1lZC50ZWlreW8tdS5hYy5qcA==
aC1zaGltaXp1QG1lZC50ZWlreW8tdS5hYy5qcA==
 Hiroyuki Nojima
Hiroyuki Nojima Hiroaki Shimizu
Hiroaki Shimizu Takashi Murakami1
Takashi Murakami1 Akihiro Usui
Akihiro Usui