- 1College of Medicine, King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
- 2King Abdullah International Medical Research Center, Jeddah, Saudi Arabia
- 3Department of Dermatology, Ministry of the National Guard-Health Affairs, Jeddah, Saudi Arabia
Background: Atopic dermatitis (AD) is a chronically relapsing disease. Few biologics are approved for moderate-to-severe AD, and novel interventions are emerging. We aimed to evaluate the safety and efficacy of lebrikizumab, an IL-13 immunomodulator, as monotherapy vs. placebo in treating moderate-to-severe AD.
Methods: Cochrane Central Register of Controlled Trials (CENTRAL), Medline, Embase, and ClinicalTrials.gov registry (CT.gov) databases were systematically searched. We evaluated lebrikizumab vs. placebo and measured efficacy using Eczema Area and Severity Index (EASI), Body Surface Area (BSA), and Investigator’s Global Assessment (IGA) change from baseline to week 16. Safety was evaluated by the incidence of serious adverse events (SAEs), non-serious adverse events (NSAEs), and mortality. The risk of bias was investigated using the Revised Cochrane risk of bias tool.
Results: Three RCTs (n = 1,149) included 543 (47.25%) men vs. 606 (52.75%) women. Meta-analysis showed statistically significant improvement in EASI, IGA, and BSA. EASI75 at week 16 for all regimens was (RR = 2.62, 95% CI [2.06, 3.34], p < 0.00001) with the first regimen (500 mg loading dose then 200 mg every 2 weeks) showing the most significant improvement (RR = 3.02, 95% CI [2.39, 3.82], p < 0.00001). The pooled analysis of safety outcomes concluded that lebrikizumab did not correlate significantly with the incidence of SAEs, NSAEs, and mortality.
Conclusion: Overall, lebrikizumab showed a significant improvement in all efficacy outcomes. Additionally, it did not contribute to any significant incidence of SAEs, NSAEs, or mortality. The risk of bias in included RCTs was minor except in the randomization domain. Grading of Recommendations Assessment, Development, and Evaluation (GRADE) assessment of the outcomes ranged from low to high, but predominantly high certainty of evidence.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022362438.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory pruritic skin disease with a worldwide prevalence estimated to be as high as 20% (1). It mainly affects the face, neck, arms, and legs, but less commonly affects the groin and the axilla (2). AD is predominantly diagnosed in childhood; however, the disease can occur or relapse later in life (3). Topical treatments are the mainstay of treatment along with general skin care measures, but they have shown multiple limitations and minimal therapeutic effects, especially in more severe forms of AD (4). Multiple theories have attempted to elucidate AD’s pathophysiology. One of these had associated AD with elevated serum immunoglobulin E (IgE) levels and T-helper cells type 2 (Th2) mediated inflammation that predisposes to interleukin-4 (IL-4) and interleukin (IL-13)-driven pathology. Novel drugs are emerging to target these cytokines as management options for AD (5). Janus kinase (JAK) inhibitors and monoclonal immunomodulators such as abrocitinib, a JAK1 inhibitor, and dupilumab, an IL-4 inhibitor, were recently established as effective treatments and have received the US Food and Drug Administration (FDA) approval for managing moderate-to-severe AD. JAK inhibitors are mostly for oral use and one of which, tofacitinib, is additionally used as a topical off-label option (6). Dupilumab was approved by the FDA in 2017 and set the stage for more IL immunomodulators to emerge (6, 7). Tralokinumab and lebrikizumab are the latest drugs attempting to target IL-13 to minimize AD signs and symptoms (8). Despite both drugs having been investigated in recent studies, more evidence is needed to establish them as a main line of treatment. Tralokinumab has been investigated in multiple studies since it was the initial drug in its class; however, lebrikizumab monotherapy is nearly an intact subject, especially in studies with high levels of evidence (9). In this article, the comprehensive systematic review and meta-analysis of randomized controlled trials (RCTs) were performed to centrally assess the safety and efficacy of lebrikizumab for managing moderate-to-severe AD against a placebo.
Methods
Our study was registered prior to a preliminary search in alignment with PROSPERO (CRD42022362438). It utilized the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist.
Eligibility criteria
Our systematic review and meta-analysis exclusively included RCTs that compared lebrikizumab against a placebo in patients with moderate-to-severe AD who are older than 12 years. Studies that did not match any of the aforementioned outcome variables were excluded. RCTs that had their patients undergo calcineurin inhibitors, topical corticosteroids, or phosphodiesterase-4 inhibitors 1 week prior to baseline or that used any systemic treatments such as JAK inhibitors, systemic corticosteroids, or phototherapy 4 weeks before baseline assessment were not included.
Search strategy
We systematically searched the Medline, Embase, ClinicalTrials.gov (CT.gov), and Cochrane Central Register of Controlled Trials (CENTRAL) databases from database initiation to 1 October 2022 without any restriction on date or language. The search strategy is provided in the Supplementary appendix. References of the included RCTs were inspected for relevant RCTs that were missed during the systematic search process.
Study selection and data extraction
Independently, two reviewers (BB and ZA) performed title and abstract screening, full-text assessment, and data extraction of RCTs that match the eligibility criteria. Disputes of studies’ inclusion or exclusion were resolved with a third senior author opinion (AJ).
Outcomes
Efficacy outcomes of this article included percentage (%) change from baseline in Eczema Area and Severity Index (EASI), Body Surface Area (BSA), and the number of participants with an Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) and a reduction of ≥2 points or a reduction in EASI scores that is more or equals 75% (EASI75). In the included studies, IGA and EASI75 reported only percentages of patients fulfilling the outcome. Thus, the percentage of participants achieving these two outcomes was converted into a dichotomous number of participants using their percentages and the corresponding number of analyzed patients. Decimal numbers of participants had shown during the calculation, moreover, and were rounded to the most proximal whole number and had their p-value checked for matching with the studies’ results. Week 16 is the time point of evaluation for the efficacy outcomes. The safety profile of lebrikizumab was evaluated by measuring the incidence of serious adverse events (SAEs), non-serious adverse events (NSAEs), and mortality.
Meta-analysis
Data analysis was performed using RevMan (Review Manager) version 5.3 (Cochrane Collaboration). All statistical analyses were performed using the random-effects model. A 95% confidence level and p < 0.05 as a borderline were set for statistical significance. The statistical heterogeneity was assessed using the I2. Percent change in EASI and BSA at week 16 were the only continuous variables, and the standardized mean difference (SMD) was used to measure their effects. Dispersion of data was measured using standard deviation (SD). RCTs that used standard error (SE) instead of SD had SE converted to SD by multiplying SE in the squared root of the corresponding sample size. Dichotomous outcomes (SAEs, NSAEs, and mortality) were demonstrated as risk ratios (RRs) and pooling was performed using the inverse variance (IV) weighting method. Data were classified into three subgroups in the subgroup analysis to measure the effects of different regimens. The first regimen included patients that were given 500 mg as a loading dose at baseline and 250 mg every 2 weeks (Q2W). The second regimen included patients that were injected with 250 mg at baseline and 125 mg of lebrikizumab every 4 weeks (Q4W). The third regimen included patients that were administered a 500 mg loading dose and 250 mg of lebrikizumab Q4W. The quality of evidence of outcomes was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria.
Results
After a systematic search, 298 studies were found, with 5 duplicates initially removed. Screening resulted in 12 studies that were assessed for eligibility (Figure 1). Of the 12 studies, one was unobtainable while eight were excluded due to unmatched eligibility. Eventually, three studies remained that represented RCTs that were included in our study. All populations, interventions, comparisons, and outcomes of these RCTs matched our eligibility criteria.
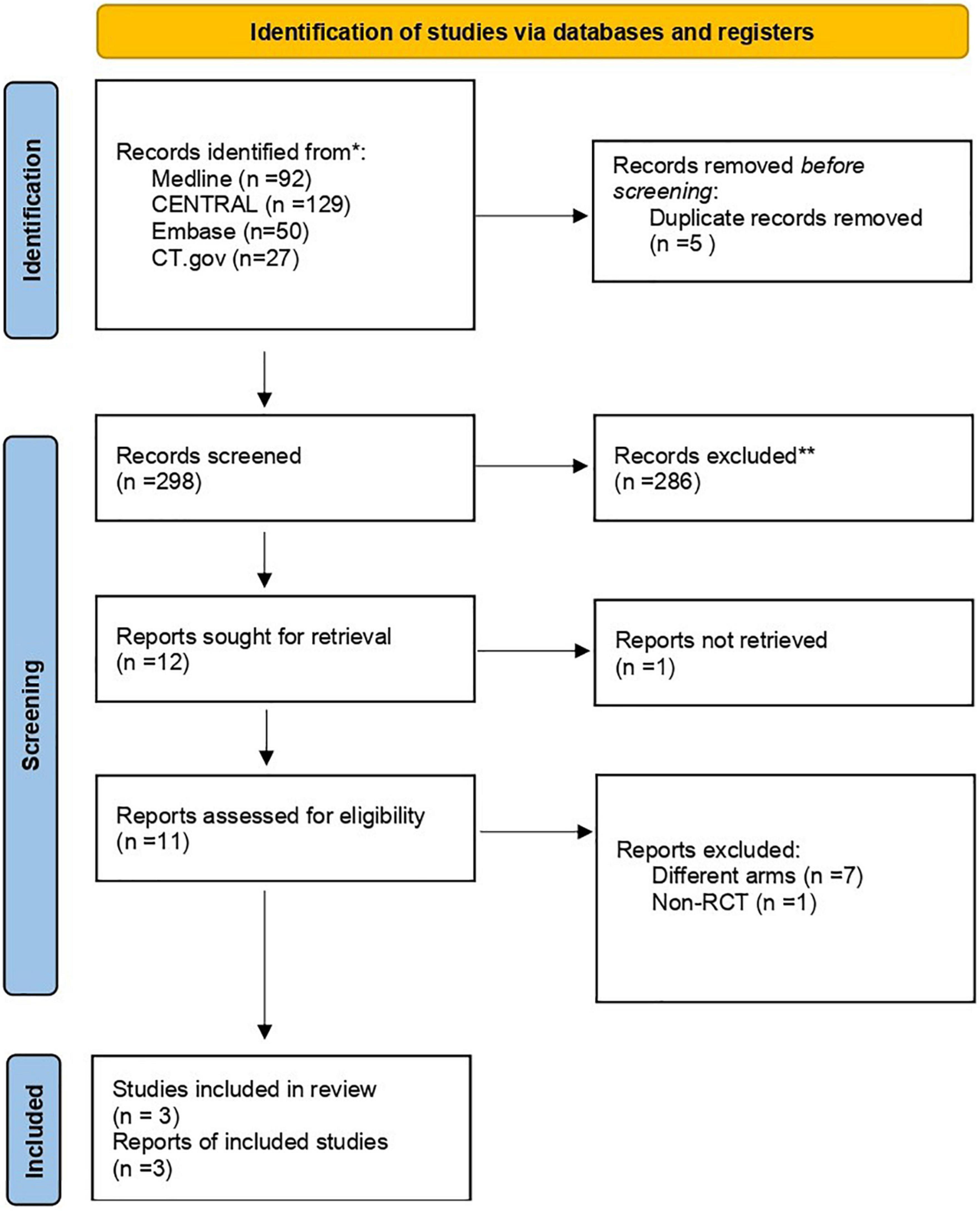
Figure 1. Study flowchart as per the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) criteria. CENTRAL, Cochrane Central Register of Controlled Trials; RCT, randomized controlled trial. *Search results on October 1, 2022. **Exclusion was done by humans exclusively.
Trial characteristics
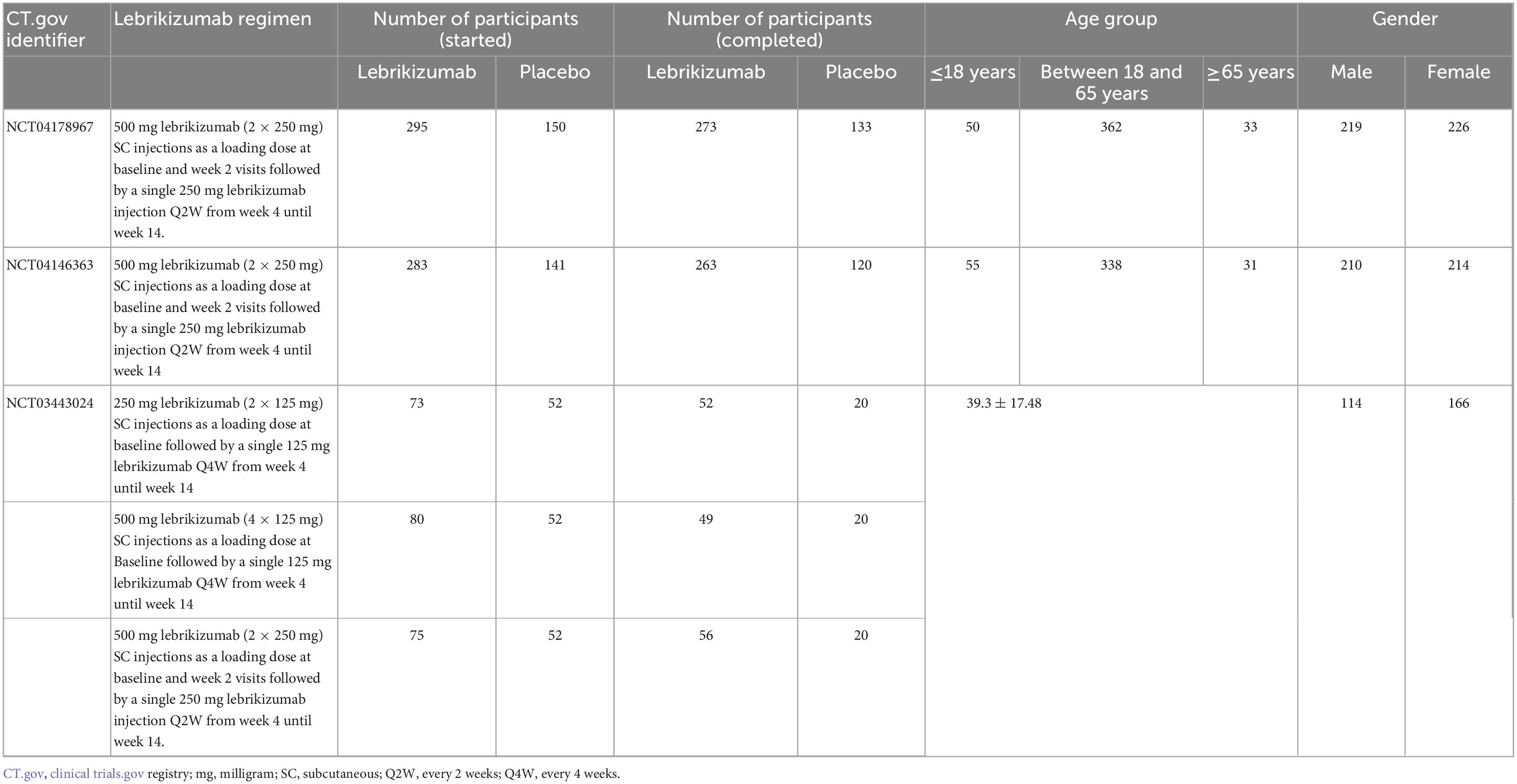
Included studies (n = 3) assessed 1,149 participants of both arms (Table 1). The lebrikizumab arm comprised 806 participants, while the placebo arm contained 343 participants. Two studies had patient age as groups and recorded 105 patients that were 18 years or younger, 700 patients between 18 and 65 years, and 64 patients that were 65 years or older. One study had age as mean ± SD (39.3 ± 17.48) (10–12). Of the 1,149 participants, 543 were men and 606 were women.
Risk of bias assessment
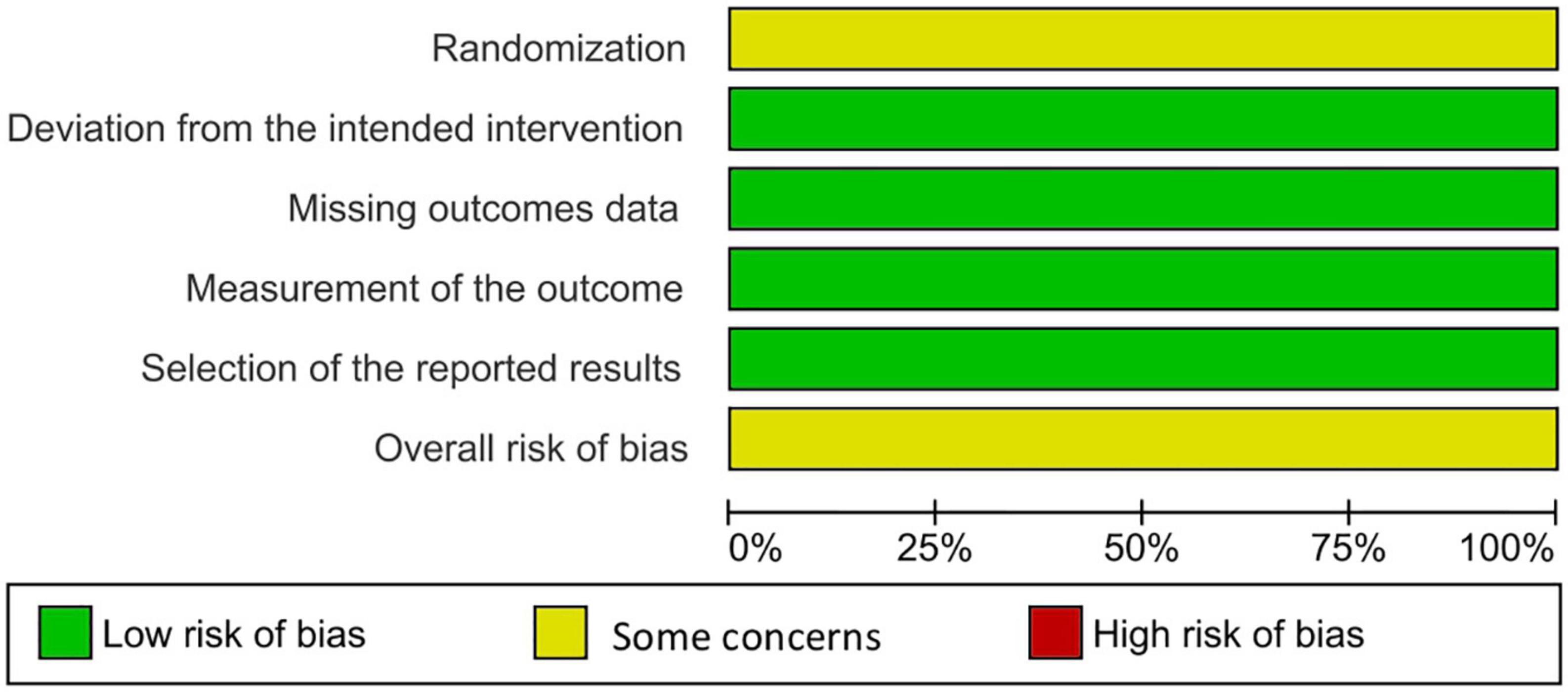
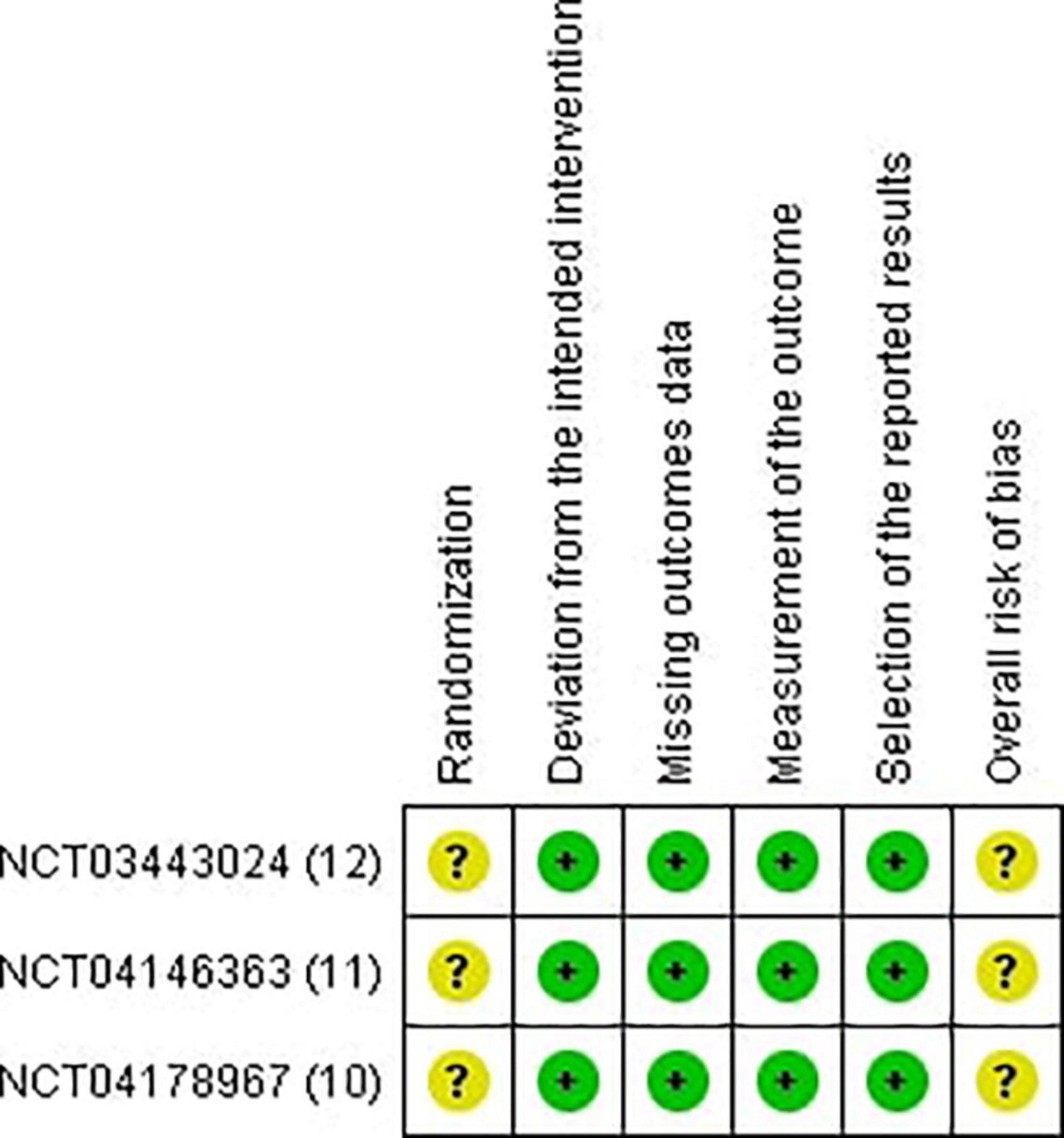
Two reviewers independently utilized the Revised Cochrane risk of bias tool to assess the risk of biases in the evaluated RCTs. Individual studies were reviewed and rated as high risk, low risk, or some concerns. Disagreements between the reviewers were resolved through discussion until a final decision was reached (13) (Figures 2, 3).
Efficacy outcomes
The percentage change in EASI score
The three included studies measured percent change in EASI score from baseline at different time points. Our study exclusively evaluated the percent change from baseline at their visit on week 16. All three regimens had a significant reduction in EASI score (SMD = −0.64, 95% CI [−0.76, −0.52], p < 0.00001, I2 = 0%). The first regimen had the most superior effect, followed by the third and the second subgroups, respectively (SMD = −0.68, 95% CI [−0.81, −0.54], p < 0.00001, I2 = 0%) (SMD = −0.44, 95% CI [−0.80, −0.08], p = 0.02, I2 = not applicable) (SMD = −0.58, 95% CI [−0.94, −0.23], p = 0.001, I2 = not applicable) (Figure 4). Certainty of evidence of GRADE criteria was estimated to be high (Figure 5).
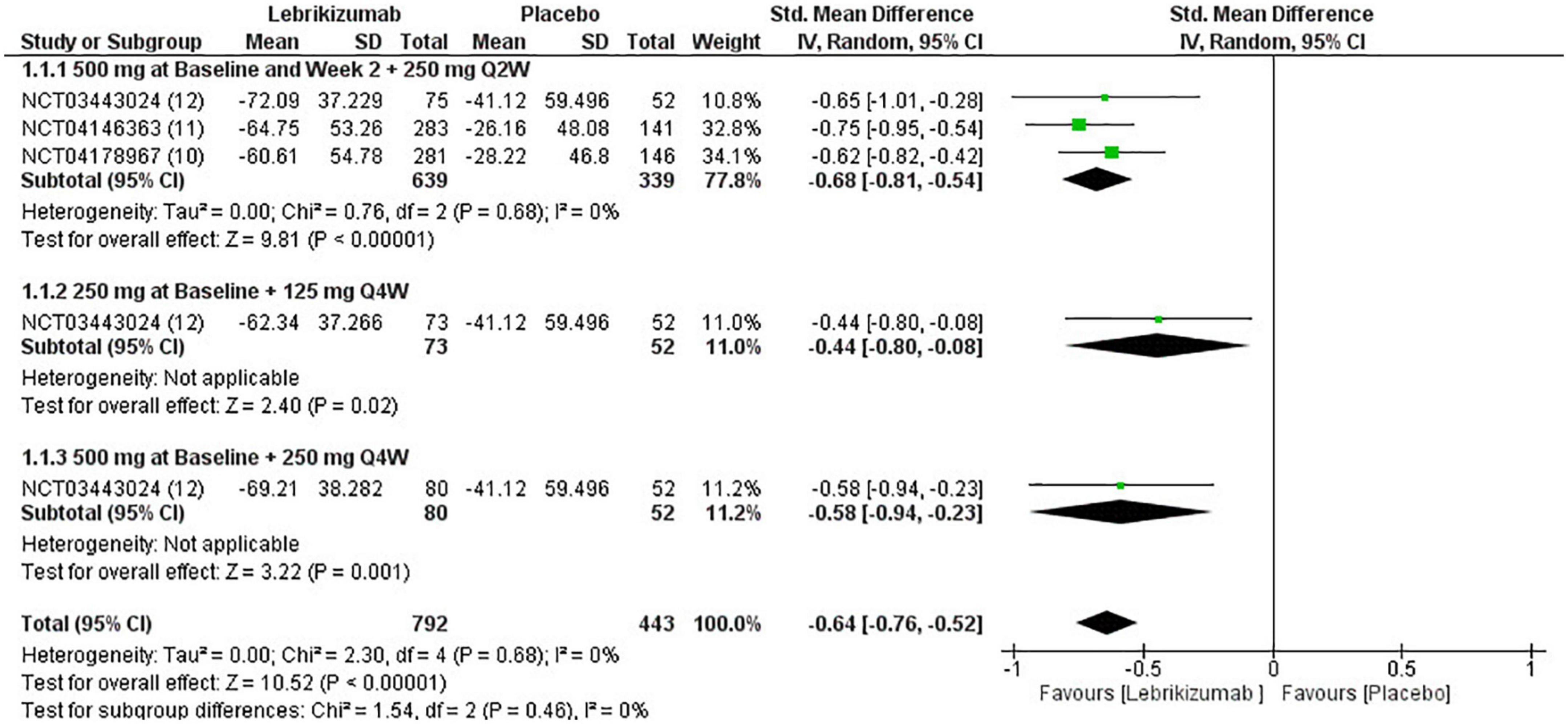
Figure 4. Forest plot of EASI score. CI, confidence interval; IV, inverse variance; SMD, standardized mean difference; SD, standard deviation; Q2W, every 2 weeks; Q4W, every 4 weeks.
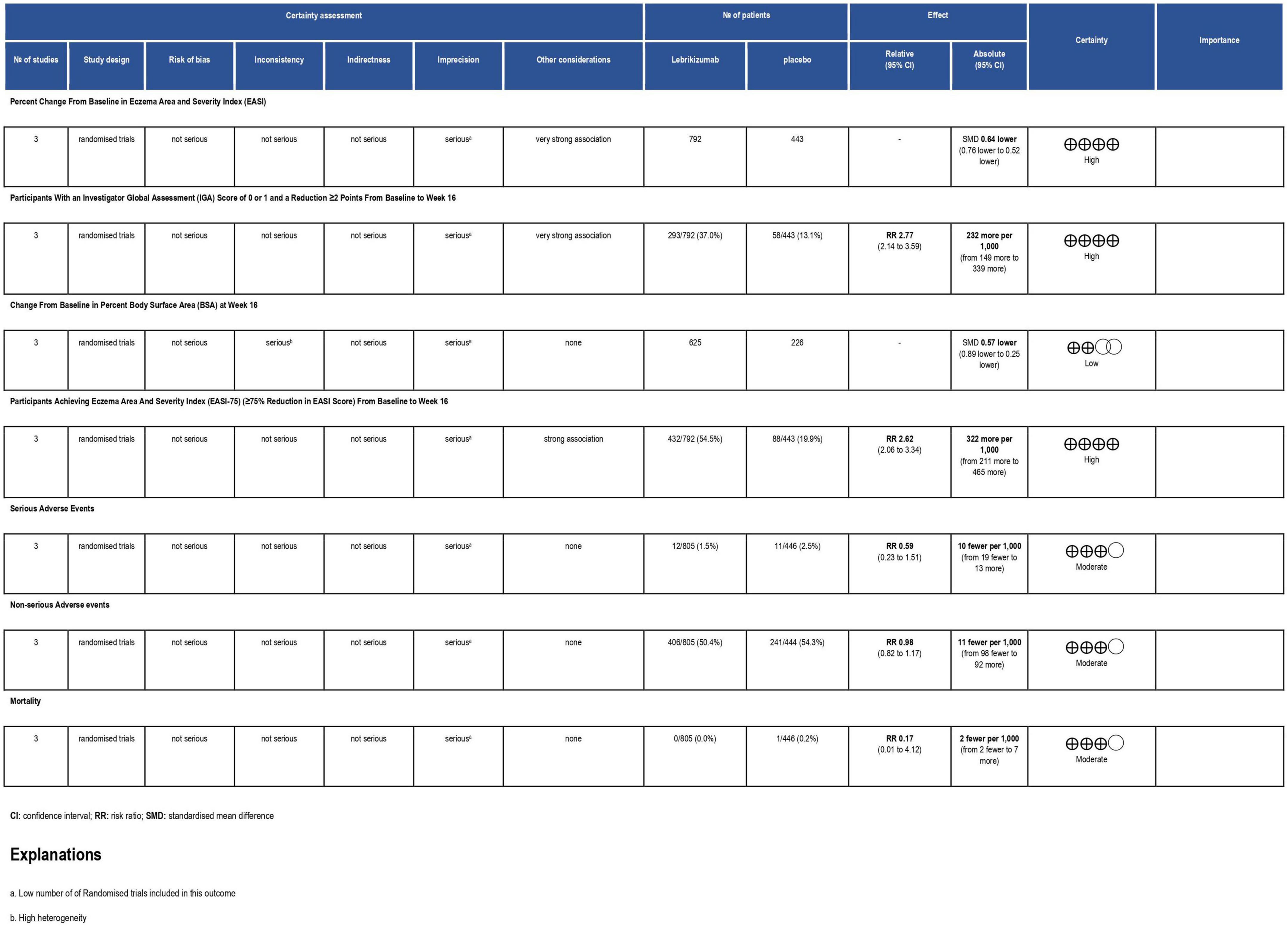
Figure 5. Grading of Recommendations Assessment, Development and Evaluation (GRADE) evidence profile. CI, confidence interval; RCT, randomized controlled trial; RR, risk ratio.
IGA score
All three RCTs evaluated IGA at week 16. The first subgroup (RR = 3.13, 95% CI [2.32, 4.22], p < 0.00001, I2 = 0%) and the third regimen (RR = 2.19, 95% CI [1.08, 4.45], p = 0.03, I2 = not applicable) showed a significant number of participants achieving score 0 and 1 or a reduction of 2 or more points from baseline to 16 weeks. However, the second subgroup did not improve this outcome significantly (RR = 1.69, 95% CI [0.80, 3.57], p = 0.17, I2 = not applicable). Overall, lebrikizumab had significant improvement in IGA outcome (RR = 2.77, 95% CI [2.14, 3.59], p < 0.00001, I2 = 0%) (Figure 6). GRADE assessment of this outcome was found to be high (Figure 5).
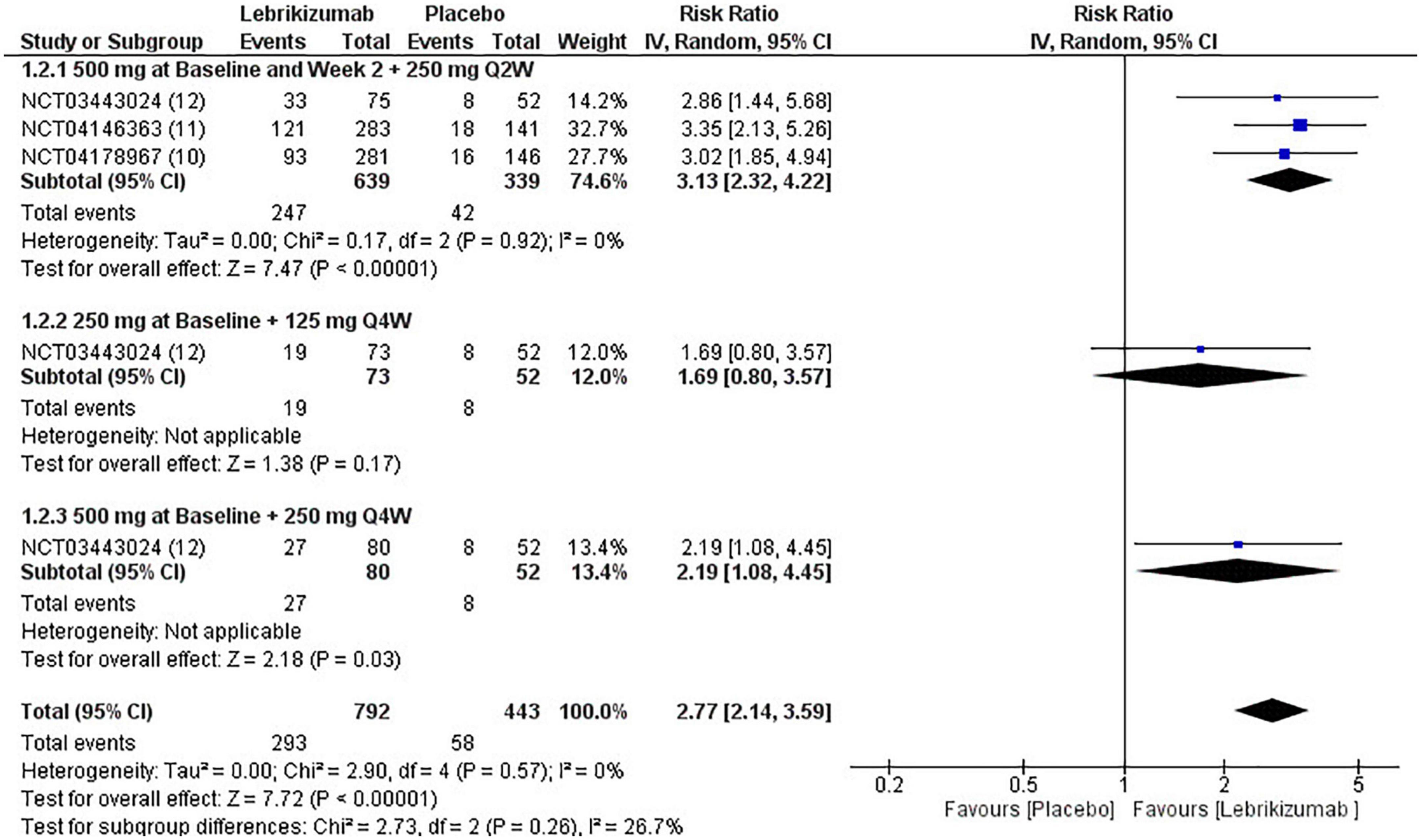
Figure 6. Forest plot of IGA score. CI, confidence interval; IV, inverse variance; RR, risk ratio; Q2W, every 2 weeks; Q4W, every 4 weeks.
BSA score
Included RCTs (n = 3) had a significant deduction of BSA involved with AD (SMD = −0.57, 95% CI [−0.89, −0.25], p = 0.0005, I2 = 73%). However, this improvement pertained to the first regimen (SMD = −0.77, 95% CI [−1.07, −0.47], p < 0.00001, I2 = 61%) since the second and the third subgroups did not correlate with a remarkable reduction in this efficacy outcome (SMD = −0.11, 95% CI [−0.59, 0.36], p = 0.64, I2 = not applicable) (SMD = −0.37, 95% CI [−0.84, 0.11], p = 0.13, I2 = not applicable) (Figure 7). BSA score had a low certainty of evidence in GRADE evaluation (Figure 5).
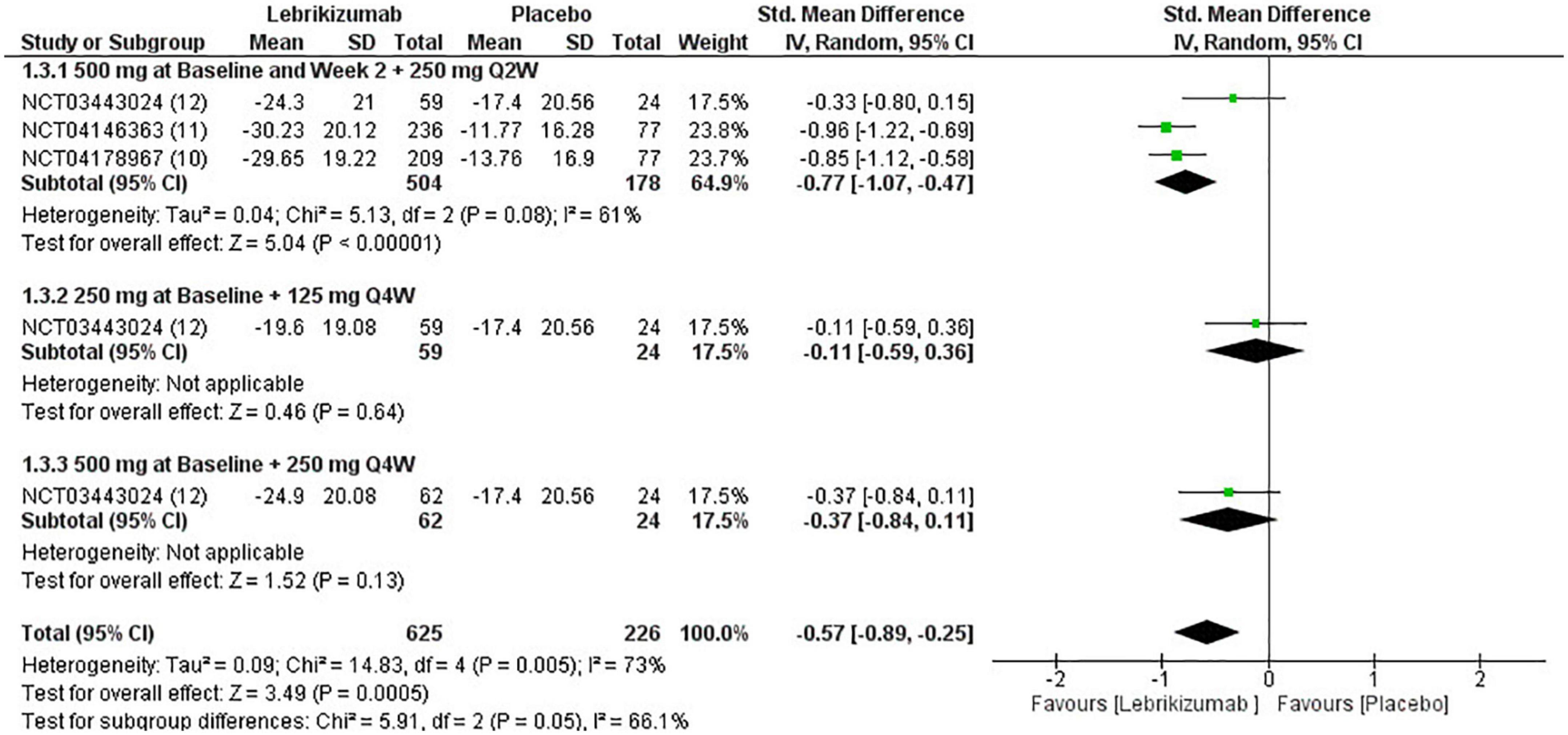
Figure 7. Forest plot of the percentage change in BSA. CI, confidence interval; IV, inverse variance; SMD, standardized mean difference; SD, standard deviation; Q2W, every 2 weeks; Q4W, every 4 weeks.
EASI75
The three included RCTs had a significantly pooled number of participants achieving more than or equal to a 75% reduction in EASI score from baseline to week 16 (RR = 2.62, 95% CI [2.06, 3.34], p < 0.00001, I2 = 29%). The first regimen had the most significant improvement (RR = 3.02, 95% CI [2.39, 3.82], p < 0.00001, I2 = 0%) while the third subgroup had a less significant effect (RR = 2.25, 95% CI [1.35, 3.74], p = 0.002, I2 = not applicable). The second subgroup did not have a sufficient number of participants to have an outcome consistent with the other regimens (RR = 1.70, 95% CI [0.99, 2.92], p = 0.006, I2 = not applicable) (Figure 8). GRADE criteria scored high certainty of evidence (Figure 5).
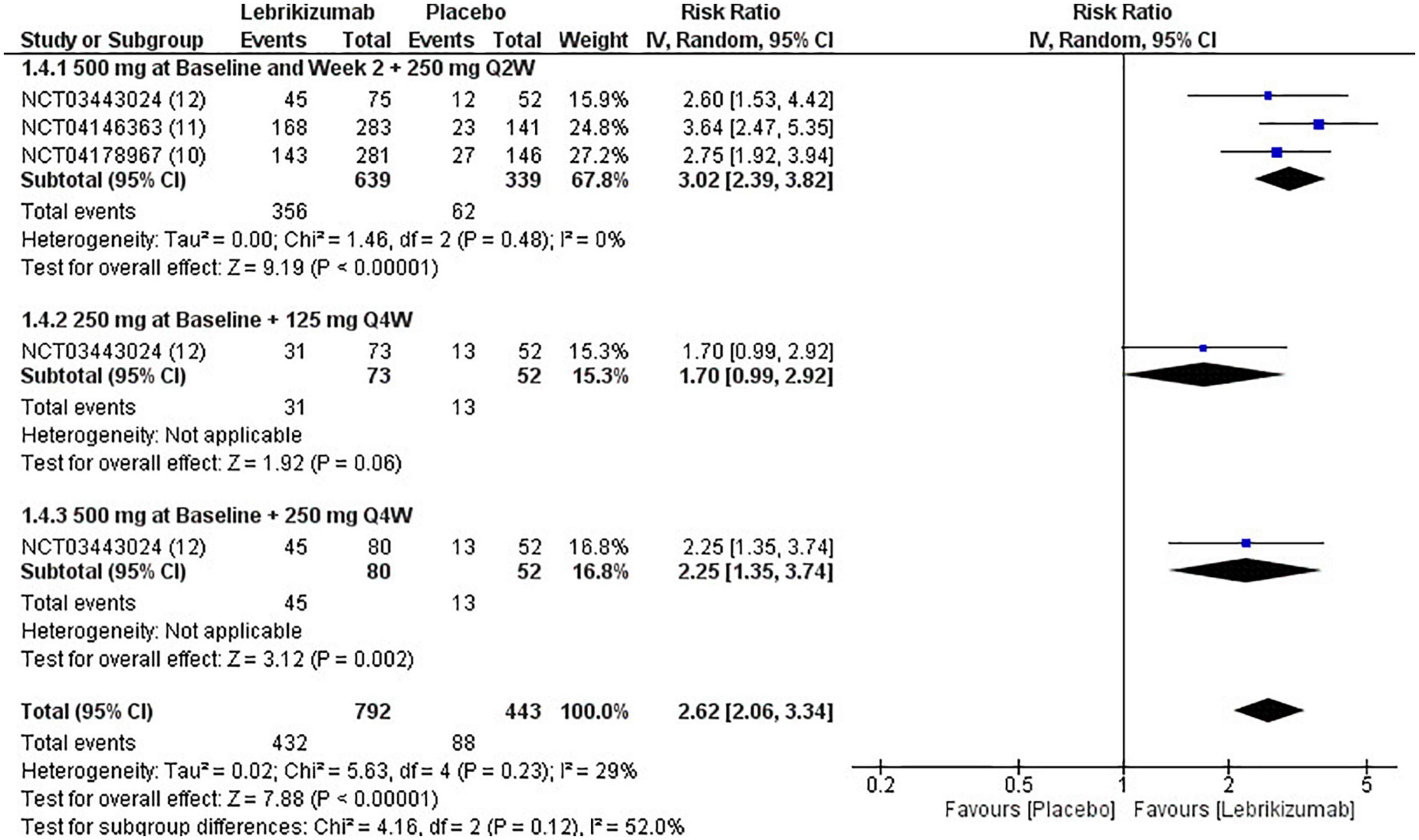
Figure 8. Forest plot of EASI75 score. CI, confidence interval; IV, inverse variance; RR, risk ratio; Q2W, every 2 weeks; Q4W, every 4 weeks.
Safety outcomes
Serious adverse events
Lebrikizumab did not cause a significant incidence of SAEs in any of the included studies (n = 3). It had a comparable effect of inducing SAEs that nearly matched to placebo (RR = 0.59, 95% CI [0.23, 1.51], p = 0.27, I2 = 7%). Both the first (RR = 0.72, 95% CI [0.18, 2.90], p = 0.65, I2 = 38%) and the second (RR = 0.71, 95% CI [0.10, 4.89], p = 0.73, I2 = not applicable) had similar RR, but the effect size is more considerable due to higher number of participants. Finally, the third regimen’s findings were consistent with that of the previous subgroups (RR = 0.13, 95% CI [0.01, 2.67], p = 0.19 I2 = not applicable) (Figure 9). SAE outcome was rated as moderate in the GRADE assessment (Figure 5).
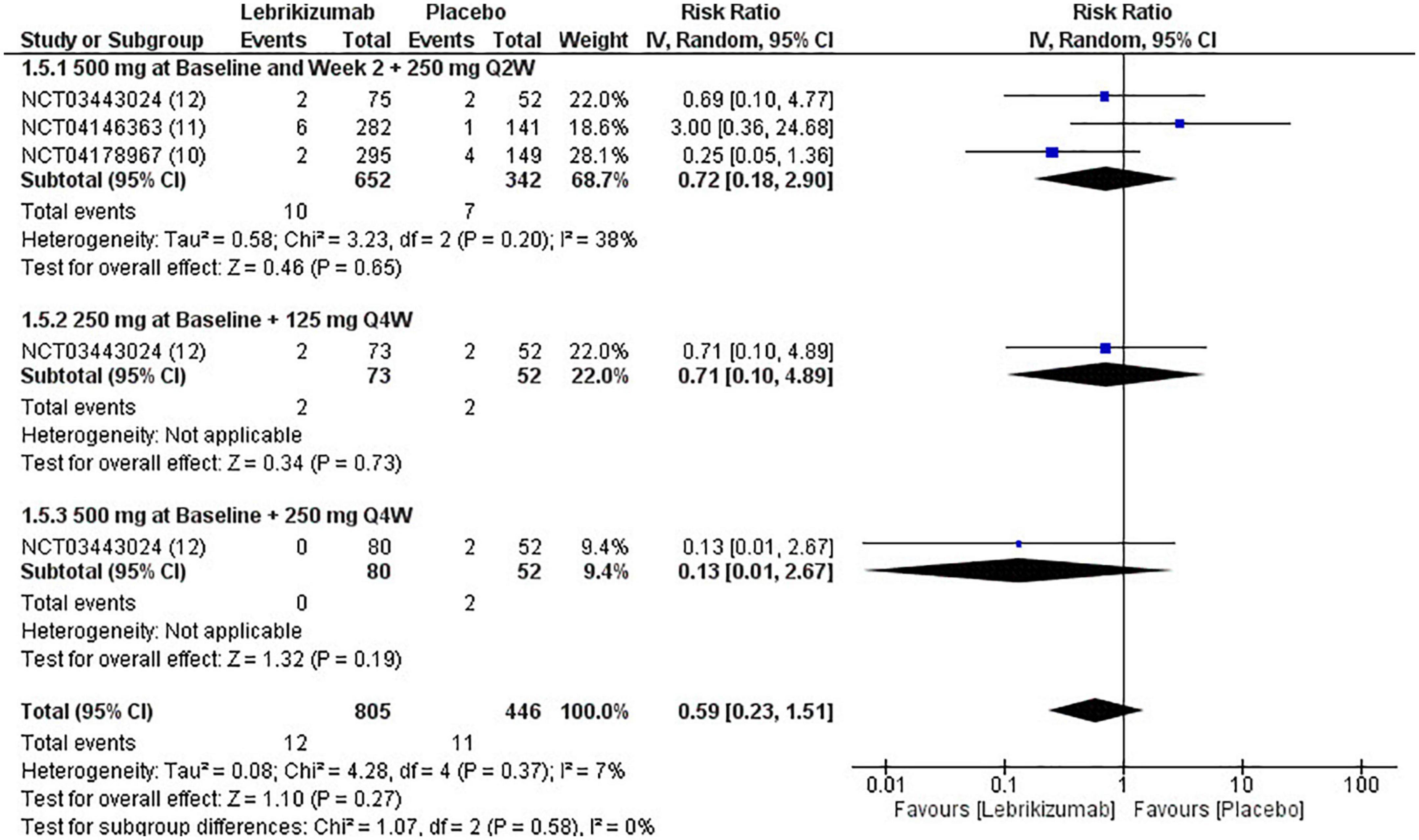
Figure 9. Forest plot of SAEs. CI, confidence interval; IV, inverse variance; RR, risk ratio; Q2W, every 2 weeks; Q4W, every 4 weeks.
Non-serious adverse events
In all three RCTs, lebrikizumab did not record a substantial difference in its effect to induce NSAEs in comparison with placebo (RR = 0.98, 95% CI [0.82, 1.17], p = 0.79 I2 = 55%). The first regimen showed negative RR but the second and third subgroups each had a positive RR, all of which were insignificant (RR = 0.93, 95% CI [0.74, 1.16], p = 0.52 I2 = 67%), (RR = 1.17, 95% CI [0.82, 1.66], p = 0.38 I2 = not applicable), (RR = 1.06, 95% CI [0.73, 1.53], p = 0.38 I2 = not applicable) (Figure 10). Upon GRADE evaluation, NSAEs had moderate evidence certainty (Figure 5).
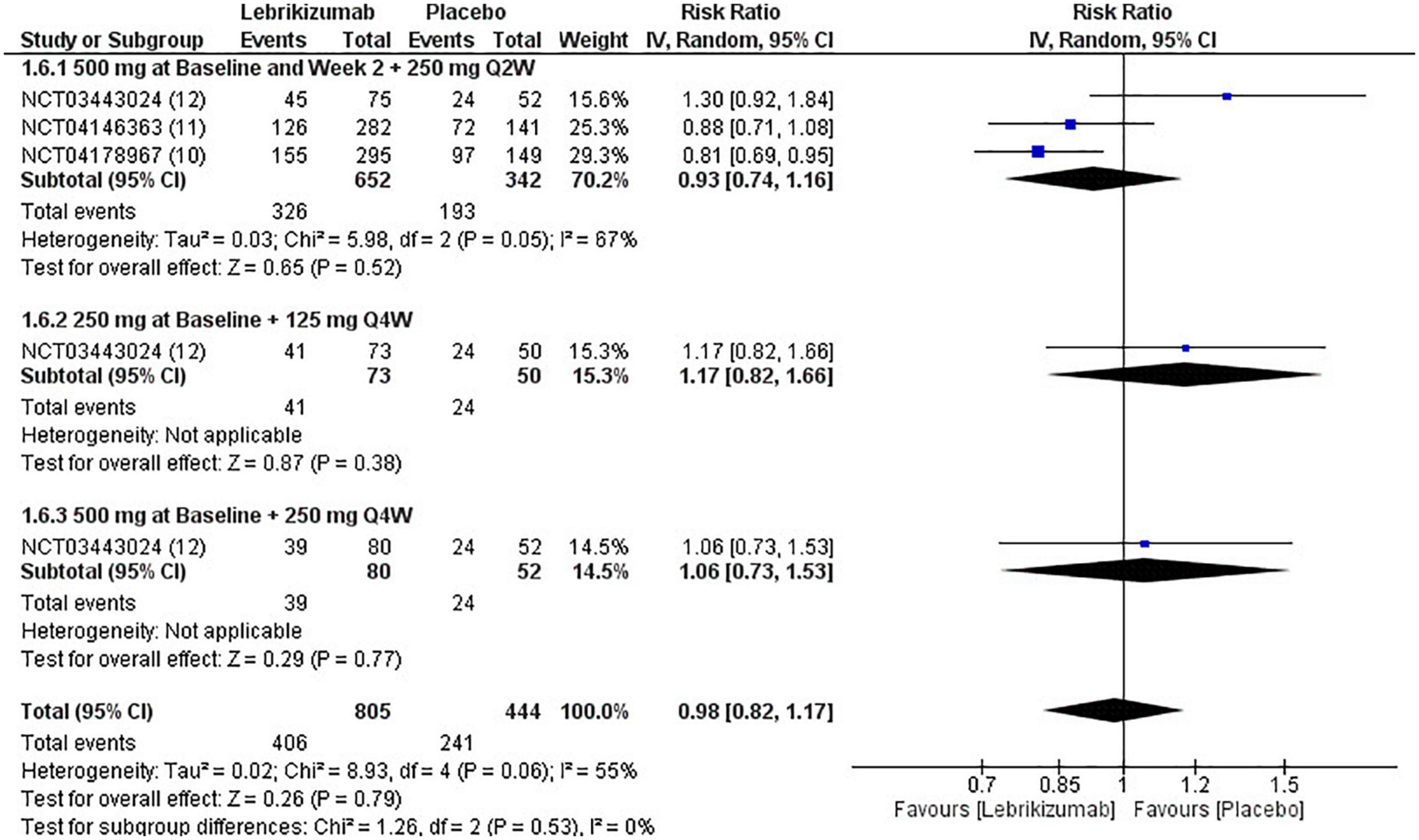
Figure 10. Forest plot of NSAEs. CI, confidence interval; IV, inverse variance; RR, risk ratio; Q2W, every 2 weeks; Q4W, every 4 weeks.
Mortality
Only one study reported a single death in the placebo arm compared to the first regimen. This led to the pooled analysis being associated only with this event (RR = 0.17, 95% CI [0.01, 4.12], p = 0.28 I2 = not applicable). Neither the second nor the third arm had a reported death, which made statistical variables, by extension, not estimable (Figure 11). Mortality showed moderate certainty of evidence in GRADE criteria (Figure 5).
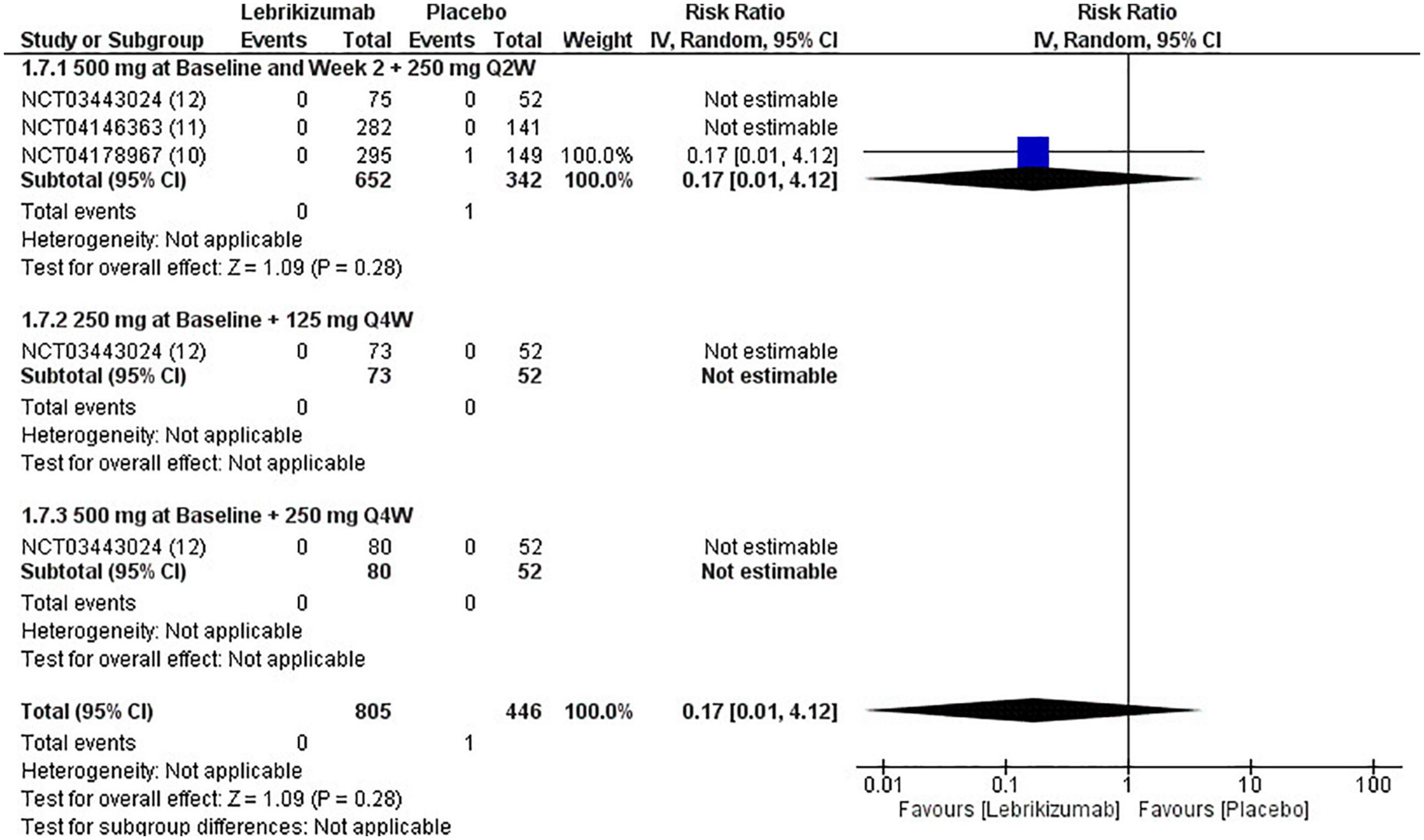
Figure 11. Forest plot of mortality. CI, confidence interval; IV, inverse variance; RR, risk ratio; Q2W, every 2 weeks; Q4W, every 4 weeks.
Discussion
This systematic review and meta-analysis assessed the safety and efficacy of lebrikizumab for the management of moderate-to-severe AD as a monotherapy. Lebrikizumab, an IL-13 inhibitor, demonstrated an improvement in all efficacy measures predominantly in the first regimen. NSAEs and SAEs showed a comparable risk of occurrence in both the placebo and the intervention arm. Mortality was nearly non-existent in all analyzed participants.
Atopic dermatitis is mainly managed with topical agents either as monotherapy or as an adjunctive option (14). AD management options are settled to tackle multiple pathologic facets, as this disease varies in natural history, manifestations, and morphology (14, 15). Topical corticosteroids (TCSs) are highly effective options among topical treatments and have been the primary treatment for this disease for a long time (16, 17). They have a potent effect in reducing signs and symptoms of AD along with a relatively safe adverse events profile (14). This profile consisted of purpura and skin atrophy and other side effects that are mostly attributed to their steroidal nature, which also limits the ability to prescribe TCS for a timely course of treatment (18). Due to these obstacles, an alternative topical option, topical calcineurin inhibitors (TCIs), was introduced early in this millenium (19). TCIs are prescribed as a second line of treatment and are indicated in the conditions where a physician is considering or hedging against steroidal side effects (19, 20). Burning or stinging sensations are widely reported in TCIs, but they normalize later in treatment (21, 22). In addition to pharmacological options, non-pharmacological ones have mostly included bathing and moisturizing, which improved the disease’s course to a limited degree (23, 24). In more severe forms of AD, phototherapy can be used. Additionally, cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil are immunotherapies that have been used, but they are less favorable choices due to broad targets and side effects. All those mentioned above necessitated AD-specific therapy (25). Biologics in AD are usually identified as JAK inhibitors including upadacitinib, abrocitinib, and baricitinib or monoclonal antibody immunomodulators targeting interleukins (ILs). Biologics are mainly administered as subcutaneous, oral, and less commonly, topical routes. The recent FDA approval of abrocitinib as an oral AD management option made it only the second oral intervention after the conventional prednisone (26). JAK inhibitors act by modulation of JAK/signal transducers and activators of transcription (STAT) pathway that have a key role in the regulation of the immune response attributed to AD and shown high efficacy with low adverse events (27, 28). ILs that have been targeted in an attempt to neutralize AD were IL-4 modulated by dupilumab, tralokinumab resembling IL-13 inhibitors, and lebrikizumab, the subject of our study (29). In a recent systematic review and meta-analysis, IL-13 inhibitors were compared to placebo (8). Tralokinumab showed a superior effect to lebrikizumab that was significant in participants achieving EASI75 and IGA 0 to 1. In EASI75, lebrikizumab outcomes resulted in a lower RR (RR = 1.69, 95% CI [1.11, 2.58], p = 0.01, I2 = 44%) than tralokinumab (RR = 1.79, 95% CI [1.26, 2.53], p = 0.001, I2 = 82%). These results are deemed to be mild to highly heterogeneous due to high I2. IGA score wise, tralokinumab (RR = 1.78, 95% CI [1.14, 2.80], p = 0.001, I2 = 0%) and lebrikizumab (RR = 1.75, 95% CI [1.40, 2.20], p = 0.001, I2 = 0%) showed very similar homogenous effects. Changes in EASI in lebrikizumab were insignificant, but (MD = −17.13, 95% CI [−34.40, 0.14], p = 0.05, I2 = 66%) tralokinumab showed the opposite result (MD = −21.40, 95% CI [−36.20, −6.61], p = 0.005, I2 = 96%); however, their effects were extremely heterogeneous and not explained by subgroup analysis. One of the RCTs that was analyzed in their systematic review and meta-analysis studied lebrikizumab’s viability vs. placebo, but the corticosteroids had been used at baseline in all participants. This may have led to confounding results, as corticosteroids are used as first-line management for moderate-to-severe AD. It was not included in our study since our criteria excluded any studies in which participants used corticosteroids (8).
Randomized controlled trials included in this review measured numerous outcomes, but several of them were appraised in relation to the author’s point of view and the outcomes’ clinical significance. Our results conclude that lebrikizumab is superior to a placebo for treating AD in all efficacy and safety outcomes assessed in this study. Subgroup analysis in this study showed an enormously important point regarding the effect of different regimens of lebrikizumab. The first regimen contributed to as much as 77.8% of effect weight in the efficacy variables and was the major source of significance. It was the only regimen to enhance AD outcomes in all efficacy variables. The second subgroup was the least effective drug and had a negligible effect that was only positive in EASI. The third subgroup scored a remarkable efficacy in most subgroups, except in the BSA outcome. SAEs are defined as any events that result in death or a life-threatening condition that necessitates inpatient hospitalization, where prolongation of an existing hospitalization causes patient incapacitation or that causes an anomaly of or birth defect in the participants’ offspring. NSAEs are adverse events that are not considered SAEs. All-cause mortality incidence was reported as well. Lebrikizumab exhibited absolute tolerability in all regimens and safety outcomes in this research. With regard to the risk of bias, the three studies were downgraded due to some concerns in the randomization sequence regarding the differences in the number of participants and randomized patients in the placebo arm had been considerably large. Funnel plots for publication bias were not generally assessed because the number of reports included was less than 10. GRADE assessment is a criterion that evaluates the certainty of evidence in different outcomes. It takes the study’s design and its numbers, imprecision, inconsistency, indirectness, and risk of bias. Clinical judgment is an inseparable part of GRADE evaluation.
Multiple limitations were encountered in our systematic review and meta-analysis, and a low number of RCTs was one. Only three RCTs were found due to the novelty of the drug. The first subgroup included three studies while the remaining yielded one report per each. Speculatively, this is due to the fact that the study assessing the three regimens found the first one to be the most efficacious. Consequently, this led the manufacturing pharmaceutical company to adapt this treatment plan in the following RCTs, which made subgroups in our meta-analysis substantially unbalanced.
Conclusion
Ultimately, lebrikizumab was shown to be a promising option for treating moderate-to-severe AD. It indicated great efficacy in multiple outcomes and displayed a solid safety profile. Nevertheless, real-life experience studies are needed, as well as further trials to compare lebrikizumab to lebrikizumab with topical corticosteroids and to current management options including dupilumab and tralokinumab.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
BB and ZA performed the search and the statistical analysis. BB wrote the Introduction and the Methods. SS wrote the Results. AA wrote the Discussion and the Conclusion. AJ reviewed and supervised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1091271/full#supplementary-material
References
1. Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. Is eczema really on the increase worldwide? J Allergy Clin Immunol. (2008) 121:947–54.e15. doi: 10.1016/j.jaci.2007.11.004
2. Spergel JM. From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol. (2010) 105:99–106. doi: 10.1016/j.anai.2009.10.002
3. Garmhausen D, Hagemann T, Bieber T, Dimitriou I, Fimmers R, Diepgen T, et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy. (2013) 68:498–506. doi: 10.1111/all.12112
4. Johnson BB, Franco AI, Beck LA, Prezzano JC. Treatment-resistant atopic dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. (2019) 12:181–92. doi: 10.2147/CCID.S163814
5. Leung DYM. Role of IgE in atopic dermatitis. Curr Opin Immunol. (1993) 5:956–62. doi: 10.1016/0952-7915(93)90112-6
6. Sedeh FB, Henning MAS, Jemec GBE, Ibler KS. Comparative efficacy and safety of monoclonal antibodies and janus kinase inhibitors in moderate-to-severe atopic dermatitis: a systematic review and meta-analysis. Acta Dermato-Venereol. (2022) 102:adv00764–00764. doi: 10.2340/actadv.v102.2075
7. Gade A, Ghani H, Rubenstein R. “Dupilumab.,” StatPearls. Treasure Island, FL: StatPearls Publishing (2022).
8. Zhang Y, Jing D, Cheng J, Chen X, Shen M, Liu H. The efficacy and safety of IL-13 inhibitors in atopic dermatitis: a systematic review and meta-analysis. Front Immunol. (2022) 13:923362. doi: 10.3389/fimmu.2022.923362
9. Simpson EL, Flohr C, Eichenfield LF, Bieber T, Sofen H, Taïeb A, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. (2018) 78:863–71.e11. doi: 10.1016/j.jaad.2018.01.017
10. Eli Lilly and Company. A Randomized, Double-blind, Placebo Controlled Trial to Evaluate the Efficacy and Safety of Lebrikizumab in Patients With Moderate to Severe Atopic Dermatitis. [Clinical trial registration]. clinicaltrials.gov. (2022). Available online at: https://clinicaltrials.gov/ct2/show/NCT04178967 (accessed October 11, 2022).
11. Eli Lilly and Company. A Randomized, Double-blind, Placebo Controlled Trial to Evaluate the Efficacy and Safety of Lebrikizumab in Patients With Moderate to Severe Atopic Dermatitis. [Clinical trial registration]. clinicaltrials.gov. (2022). Available online at: https://clinicaltrials.gov/ct2/show/NCT04146363 (accessed October 11, 2022).
12. Eli Lilly and Company. A Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Trial to Evaluate the Efficacy and Safety of Lebrikizumab in Patients With Moderate-to-Severe Atopic Dermatitis. [Clinical trial registration]. clinicaltrials.gov. (2021). Available online at: https://clinicaltrials.gov/ct2/show/NCT03443024 (accessed October 11, 2022).
13. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
14. Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. (2014) 71:116–32. doi: 10.1016/j.jaad.2014.03.023
15. Carlsten C, Dimich-Ward H, Ferguson A, Watson W, Rousseau R, DyBuncio A, et al. Atopic dermatitis in a high-risk cohort: natural history, associated allergic outcomes, and risk factors. Ann Allergy Asthma Immunol. (2013) 110:24–8. doi: 10.1016/j.anai.2012.10.005
16. Yawalkar SJ, Schwerzmann L. Double-blind, comparative clinical trials with halobetasol propionate cream in patients with atopic dermatitis. J Am Acad Dermatol. (1991) 25:1163–6. doi: 10.1016/0190-9622(91)70318-V
17. Lassus A. Clinical comparison of alclometasone dipropionate cream 0⋅05% with hydrocortisone butyrate cream 0⋅1% in the treatment of atopic dermatitis in children. J Int Med Res. (1983) 11:315–9. doi: 10.1177/030006058301100512
18. Callen J, Chamlin S, Eichenfield L, Ellis C, Girardi M, Goldfarb M, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol. (2007) 156:203–21. doi: 10.1111/j.1365-2133.2006.07538.x
19. Breuer K, Werfel T, Kapp A. Safety and efficacy of topical calcineurin inhibitors in the treatment of childhood atopic dermatitis. Am J Clin Dermatol. (2005) 6:65–77. doi: 10.2165/00128071-200506020-00001
20. El-Batawy M, Maged Y, Bosseila MA-W, Mashaly HM, Hafez VSGA. Topical calcineurin inhibitors in atopic dermatitis: a systematic review and meta-analysis. J Dermatol Sci. (2009) 54:76–87. doi: 10.1016/j.jdermsci.2009.02.002
21. Boguniewicz M, Fiedler VC, Raimer S, Lawrence ID, Leung DYM, Hanifin JM. A randomized, vehicle-controlled trial of tacrolimus ointment for treatment of atopic dermatitis in children. J Allergy Clin Immunol. (1998) 102:637–44. doi: 10.1016/S0091-6749(98)70281-7
22. Spergel J, Boguniewicz M, Paller A, Hebert A, Gallagher P, McCormick C, et al. Addition of topical pimecrolimus to once-daily mid-potent steroid confers no short-term therapeutic benefit in the treatment of severe atopic dermatitis; a randomized controlled trial. Br J Dermatol. (2007) 157:378–81. doi: 10.1111/j.1365-2133.2007.08001.x
23. Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. (2009) 26:273–8. doi: 10.1111/j.1525-1470.2009.00911.x
24. Korting H, Schöllmann C, Cholcha W, Wolff L, Group TCS. Efficacy and tolerability of pale sulfonated shale oil cream 4% in the treatment of mild to moderate atopic eczema in children: a multicentre, randomized vehicle-controlled trial. J Eur Acad Dermatol Venereol. (2010) 24:1176–82. doi: 10.1111/j.1468-3083.2010.03616.x
25. Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. (2014) 71:327–49. doi: 10.1016/j.jaad.2014.03.030
26. Perche PO, Cook MK, Feldman SR. Abrocitinib: a new FDA-approved drug for moderate-to-severe atopic dermatitis. Ann Pharmacother. (2023) 57:86–98. doi: 10.1177/10600280221096713
27. Nakashima C, Yanagihara S, Otsuka A. Innovation in the treatment of atopic dermatitis: emerging topical and oral Janus kinase inhibitors. Allergol Int. (2022) 71:40–6. doi: 10.1016/j.alit.2021.10.004
28. Cantelli M, Martora F, Patruno C, Nappa P, Fabbrocini G, Napolitano M. Upadacitinib improved alopecia areata in a patient with atopic dermatitis: a case report. Dermatol Ther. (2022) 35:e15346. doi: 10.1111/dth.15346
29. Ayen-Rodríguez A, Pereyra-Rodríguez J-J, Navarro-Triviño FJ, Alcantara-Luna S, Domínguez-Cruz J, Galán-Gutiérrez M, et al. Long-term effectiveness and safety of biologic and small molecule drugs for moderate to severe atopic dermatitis: a systematic review. Life. (2022) 12:1159. doi: 10.3390/life12081159
Keywords: atopic dermatitis, eczema, lebrikizumab, anti-IL-13, monoclonal antibodies
Citation: Bashrahil B, Alzahrani Z, Samarkandy S, Aman A and Jfri A (2023) The efficacy and safety of lebrikizumab monotherapy for the management of moderate-to-severe atopic dermatitis: A systematic review and meta-analysis. Front. Med. 9:1091271. doi: 10.3389/fmed.2022.1091271
Received: 06 November 2022; Accepted: 27 December 2022;
Published: 16 January 2023.
Edited by:
Angelo Ruggiero, University of Naples Federico II, ItalyReviewed by:
Fabrizio Martora, University of Naples Federico II, ItalyRosita Comune, University of Campania Luigi Vanvitelli, Italy
Copyright © 2023 Bashrahil, Alzahrani, Samarkandy, Aman and Jfri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdulhadi Jfri,  SmZyaWFAa3NhdS1ocy5lZHUuc2E=
SmZyaWFAa3NhdS1ocy5lZHUuc2E=
 Bader Bashrahil
Bader Bashrahil Ziyad Alzahrani
Ziyad Alzahrani Sahal Samarkandy2,3
Sahal Samarkandy2,3 Abdullah Aman
Abdullah Aman