- 1Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 2School of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 3Department of Dermatology, Chung Shan Medical University Hospital, Taichung, Taiwan
- 4Department of Pharmacology, Chung Shan Medical University, Taichung, Taiwan
- 5Department of Pharmacy, Chung Shan Medical University Hospital, Taichung, Taiwan
- 6Library, Chung Shan Medical University Hospital, Taichung, Taiwan
- 7Evidence-Based Medicine Center, Chung Shan Medical University Hospital, Taichung, Taiwan
- 8Department of Microbiology and Immunology, School of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 9Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan
Background: In the field of autoimmune and inflammatory disorders, different approaches were applied to provide information regarding disease activity, comorbidities, epidemiological reports and risk factors. However, no previous studies had thoroughly analyzed the research trend in the field, and the bibliometric analysis focusing on pemphigoid diseases was available. The objective of the current study was to evaluate the current research trend in the field.
Methods: A search has been conducted for the Web of Science database based on various subcategories of pemphigoid diseases. Detailed information including articles’ publication types, Author information, citation, and publication information was attained for further analysis.
Results: Within the 6,995 studies, the top 100 most-cited articles were extracted for analysis. Among the top 100 studies, 70% of the studies focused on bullous pemphigoid. More than 60% of the top 100 studies were studies with original data. Furthermore, 30% of the studies were guidelines and narrative reviews. For the issues primarily focused on, most of the high-impact studies described the molecular mechanism of pemphigoid diseases (26%), managements (19%), risk factors of pemphigoid diseases (17%). Additionally, some other studies provided general review or discussed about the issue of epidemiology, diagnosis/definition, comorbidities and clinical characteristics of pemphigoid diseases.
Conclusion: This comprehensive bibliographic study of pemphigoid diseases provided an overview of current research focuses in the field. Topics such as disease management, molecular mechanism of pathogenesis, and drug-inducing pemphigoid diseases were highly mentioned in the most-cited studies. For researchers and clinicians, the researching trend and study focus in the top-100 cited studies could serve as a potential reference for future investigation and patient management.
Introduction
Patients with pemphigoid diseases present with tense blisters and erosions. With autoimmune effects in cutaneous areas, the appearance of the patients would be affected and could significantly influence patient quality of life and psychological status (1). According to previous research, eight diseases were identified as subcategories of pemphigoid diseases, including bullous pemphigoid, mucous membrane pemphigoid, pemphigoid gestationis, linear IgA disease, epidermolysis bullosa acquisita, anti-p200 pemphigoid, and lichen planus pemphigoides (2). Given that the influence of pemphigoid diseases was gradually increasing, the burden caused by pemphigoid diseases could be underestimated (3). Under such circumstances, attention should be raised.
In the field of autoimmune and inflammatory disorders, researchers used different approaches to provide information on disease activity, comorbidities, epidemiological reports, and risk factors (4–6). Within the past 20 years, the definition criteria, diagnostic techniques, and management of pemphigoid diseases were constantly improving. In previous studies, study types including guidelines, reviews, original studies, and case studies were applied. However, to our knowledge, to date no previous studies had thoroughly analyzed the research trend in the field, and the bibliometric analyses focused on pemphigoid diseases were not available. Therefore, we conducted a bibliometric study analyzing the top 100 most cited studies regarding pemphigoid diseases to evaluate the current research trend in the field.
Materials and methods
Searching strategy
A search of the Web of Science database was conducted on 13 October 2022. The searching syntaxes were based on “Bullous pemphigoid” or “Mucous membrane pemphigoid” or “Cicatricial Pemphigoid” or “Pemphigoid gestationis” or “Linear IgA” or “Epidermolysis Bullosa Acquisita” or “anti-p200 pemphigoid” or “lichen planus pemphigoides or “pemphigoid”. The strategy of categorizing pemphigoid diseases in this study was also applied in previous studies (2). Regarding the time range of the data, no limitations were set. Detailed information including articles’ publication types (journal article or conference article), author information (full names, addresses, and affiliations), citation information (times cited in Web of Science core collection and all databases), publication information (publication year, date, volume, issue, starting page and ending page, DOI, PubMed ID, research areas, and open access designations) were attained from the Web of Science database on the same day of the research performed (13 October 2022). All analyzes were performed based on the basis of the data obtained to prevent biases caused by the update of the citation information. All included articles were sorted by citation number in the order of highest to the lowest. Only studies focusing primarily on pemphigoid diseases will be included in the study and extracted. Studies that met the following exclusion criteria were excluded from extraction and further analyzes: (1) studies not related to any pemphigoid disease; (2) studies mentioning pemphigoid diseases but focus primarily on other topics; and (3) animal studies. After critical appraisal of articles, eligible publications were ranked in the sequence of cited amounts. When multiple articles were cited the same times, articles with a recent year of publication will obtain a higher rank than those articles with relatively previous publication year.
Data extraction and statistical analysis
Within the eligible studies, information about the 100 most cited studies about pemphigoid diseases was extracted. Basic information (year of publication, authors’ name, citation times of the article) and detailed information on the content (nation or region the study originated, study design, subcategories of pemphigoid disease each study focuses on, main finding of each study and impact factor 2021 of the journal publishing each article) were extracted for further analysis. Statistical analysis and figure examination were performed using Microsoft Excel 2019.
Results
Extracted studies
Within the 6,995 studies retrieved from the Web of Science, the top 100 most-cited articles were extracted for analysis. Detailed extracted information of the top 100 articles was available in Supplementary Table 2 (2, 7–105).
Publication time trend and open access options
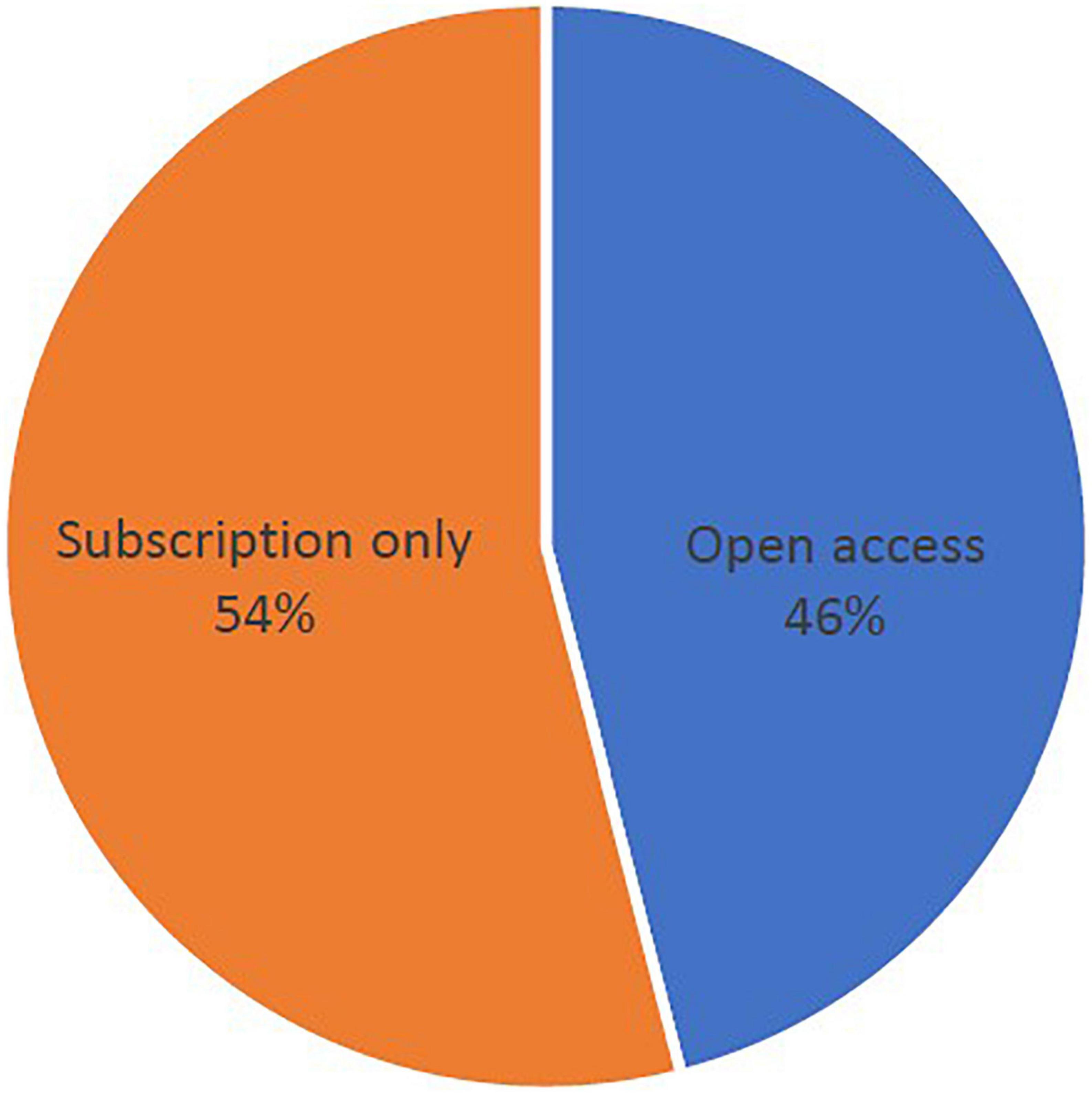
Within the top 100 publications of pemphigoid diseases that are the newest studies were published in 2018, while the most previous studies were published in 2001. The highest number of most cited publications was found in 2011, with the amount of 10 publications (Figure 1). However, the lowest number of articles in the top 100 publications was found in 2003, with the amount of 2 publications. The newest study of the top 100 publications was published in December 2018 in the Journal of the American Academy of Dermatology, entitled “Bullous disorders associated with anti-PD-1 and anti-PD-L1 therapy: A retrospective analysis evaluating clinical and histopathologic features, frequency, and impact on cancer therapy,” which was a single-center retrospective study evaluating the development of pemphigoid diseases (including bullous pemphigoid and linear IgA bullous) after cancer therapy (86). The oldest study was entitled “Anti-epiligrin cicatricial pemphigoid and relative risk for cancer” which was published in The Lancet in research letter form in June 2001. This retrospective American cohort study reported a 6.8-fold cancer risk in patients with cicatricial pemphigoid patients (32). Within the top-100 most cited studies, 54% of them were published based on traditional subscription model, whereas 46% of them published in open access (Figure 2).
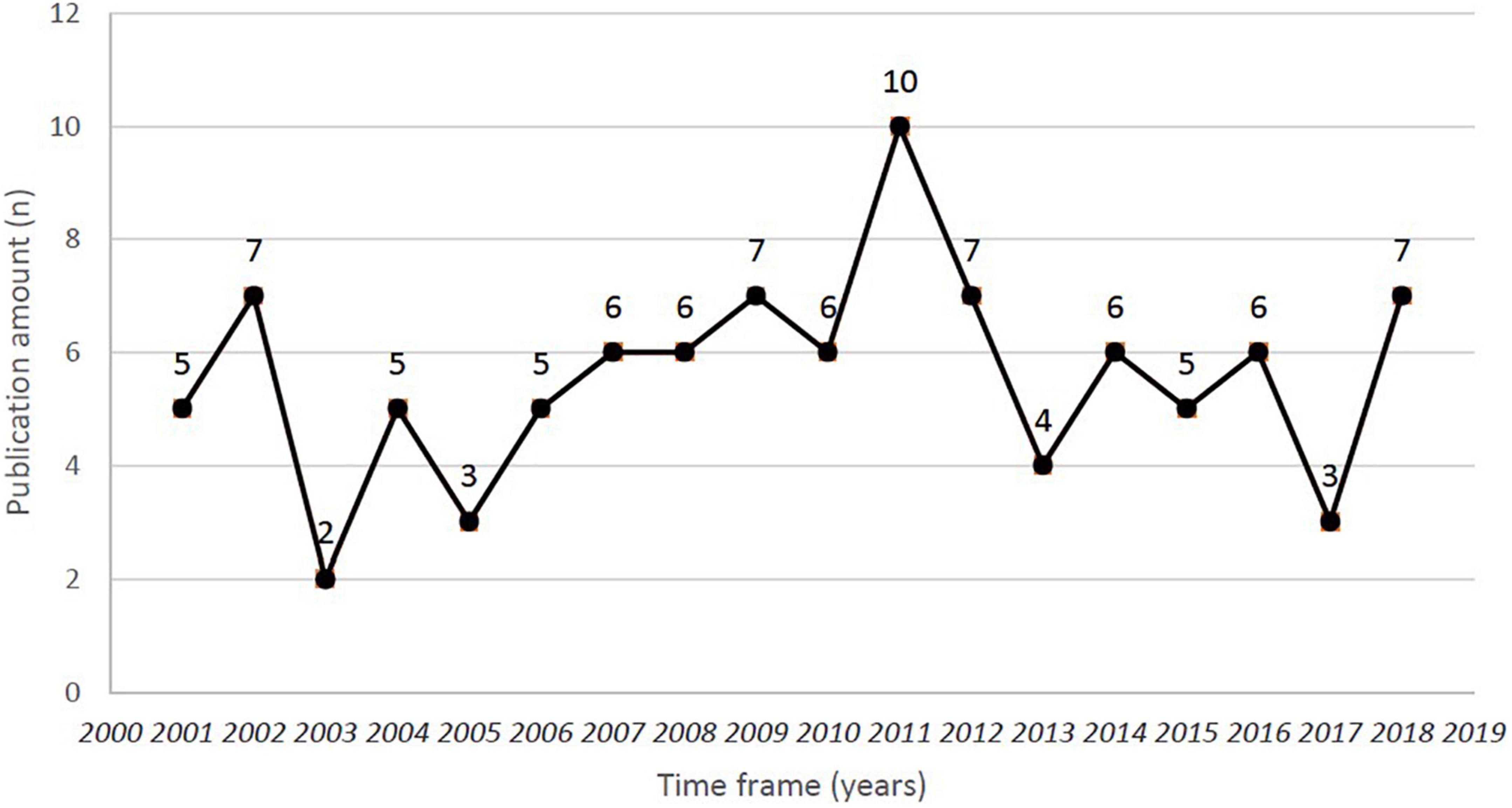
Figure 1. Time frame and publication amount for the top 100 most-cited studies of pemphigoid diseases.
Citation amount
The number of the top 100 most cited studies of pemphigoid diseases ranged from 73 to 581 cited times. The accumulated number of cited studies of the top 100 was 12,149 times. On average, these articles were cited 121.49 times per article. Among the top 100 studies, a review article published in The Lancet in January 2013, entitled “Pemphigoid diseases” and conducted by Schmidt et al. was most cited (581 times).
Journal distribution
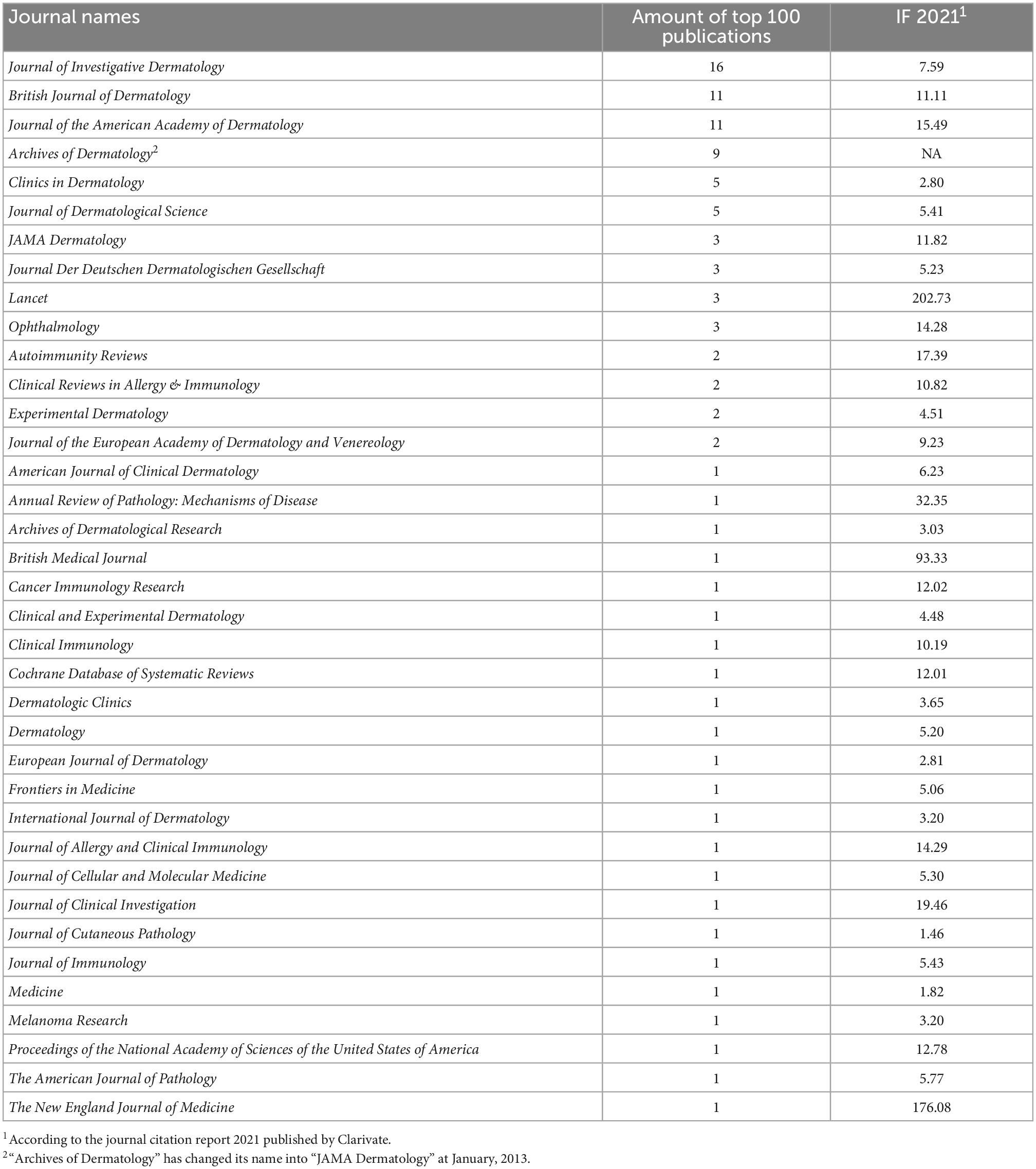
In summary, 36 different journals have contributed to the top 100 most cited studies of pemphigoid diseases (Table 1). The Journal of Investigative Dermatology contributed 16 studies, which was the highest amount compared to other journals. The journals contributing more than five studies included the British Journal of Dermatology (n = 11), the Journal of the American Academy of Dermatology (n = 11), the Archives of Dermatology (n = 9), the Clinics in Dermatology (n = 5), and the Journal of Dermatological Science (n = 5).
Country of origin
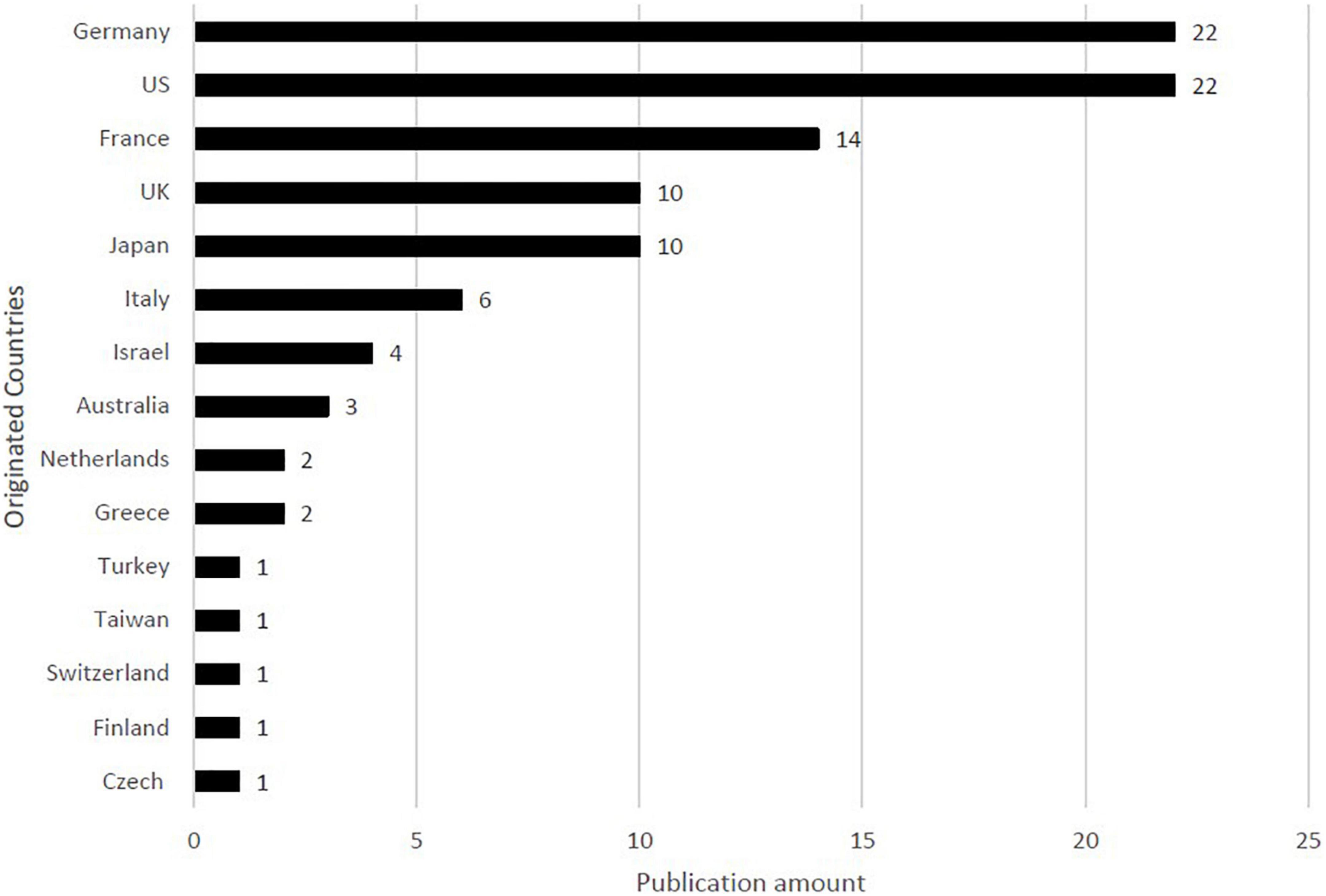
The distribution of the countries in the top 100 studies is presented in Figure 3. In total, 15 countries contributed to the top 100 studies. Generally, studies on high-citation pemphigoid diseases originated from Europe. Most of the studies were conducted in Germany (n = 22) and United States (n = 22), followed by France (n = 14), the UK (n = 10), and Japan (n = 10).
Designs of studies and cited times
More than 60% of the top 100 studies were studies with original data (no matter in research letter or in original article types). Furthermore, 30% of the studies were guidelines and narrative reviews (Figure 4). Study designs of studies with original data include randomized controlled trial (n = 3), prospective cohort studies (n = 5), retrospective cohort studies (n = 7), nested case-control studies (n = 1), prospective case-control studies (n = 1), retrospective case-control studies (n = 8), cross-sectional studies (n = 1) and clinical observations (n = 15).
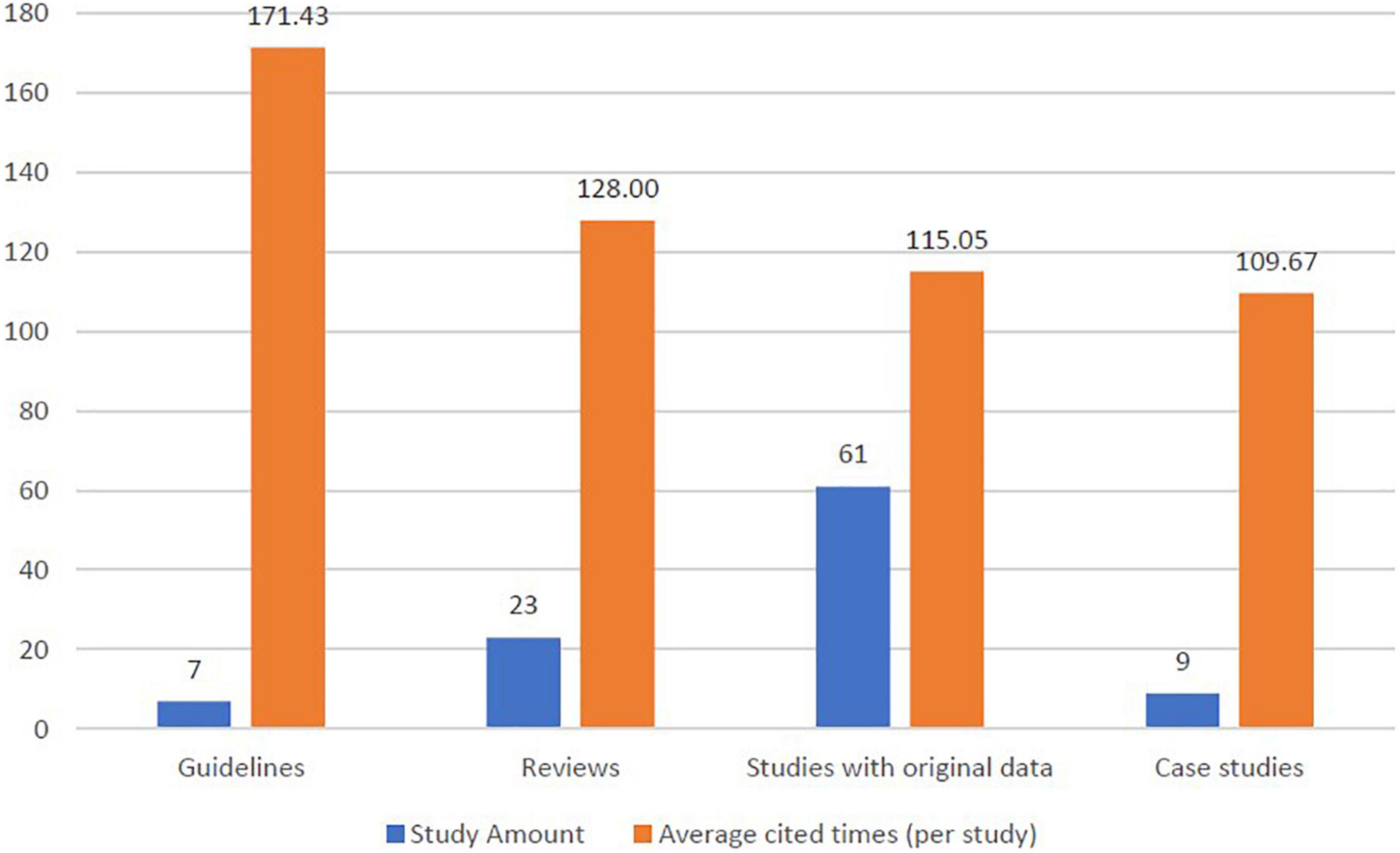
Figure 4. Distribution of study designs and cited amount (per study) in the top 100 publications of pemphigoid diseases.
Within the seven guidelines in the top 100 studies, the average cited times were numerically higher than other study designs. For guidelines, the average cited times was 171.43 times per study. For reviews, the average cited times was 128.00 times per study, while studies with original data presented the average cited times of 115.05 times per study.
Research focus of studies
Figures 5A, B present the research focus of the top 100 studies. Among the top 100 studies, most of the studies focused on bullous pemphigoid (70%). For studies focusing on the mucous membrane pemphigoid (or cicatricial pemphigoid), the ratio was 10% (Figure 5A). In terms of fields of study, the most involved issues in the top 100 studies were regarding the molecular mechanism of pemphigoid diseases (10, 23, 26, 28, 29, 31, 41, 42, 45–47, 55, 59, 64, 70, 74, 75, 84, 87, 88, 92, 93, 95, 96, 99, 104) (26%), managements (7, 12, 34, 35, 37, 43, 51, 52, 54, 57, 65, 80, 82, 83, 85, 98, 102, 103, 105) (19%), and risk factors (11, 13, 14, 16, 19, 53, 60, 62, 66–68, 73, 77, 86, 89, 90, 97) (17%) (Figure 5B). Regarding the studies that focus on the field of risk factors, most of the studies focused on drug-induced pemphigoid diseases. Five of these studies (29%) described the association between DPP4 inhibitors and pemphigoid diseases (13, 14, 60, 89, 97) and another four studies (25%) described PD-1 or anti-PD-L1 inhibitor-induced pemphigoid diseases (19, 53, 68, 86). Among epidemiological studies, 7 studies (58%) described the incidence of bullous pemphigoid (17, 18, 38, 49, 61, 63, 69) and 6 studies (50%) described the mortality of bullous pemphigoid (18, 24, 38, 49, 63, 76).
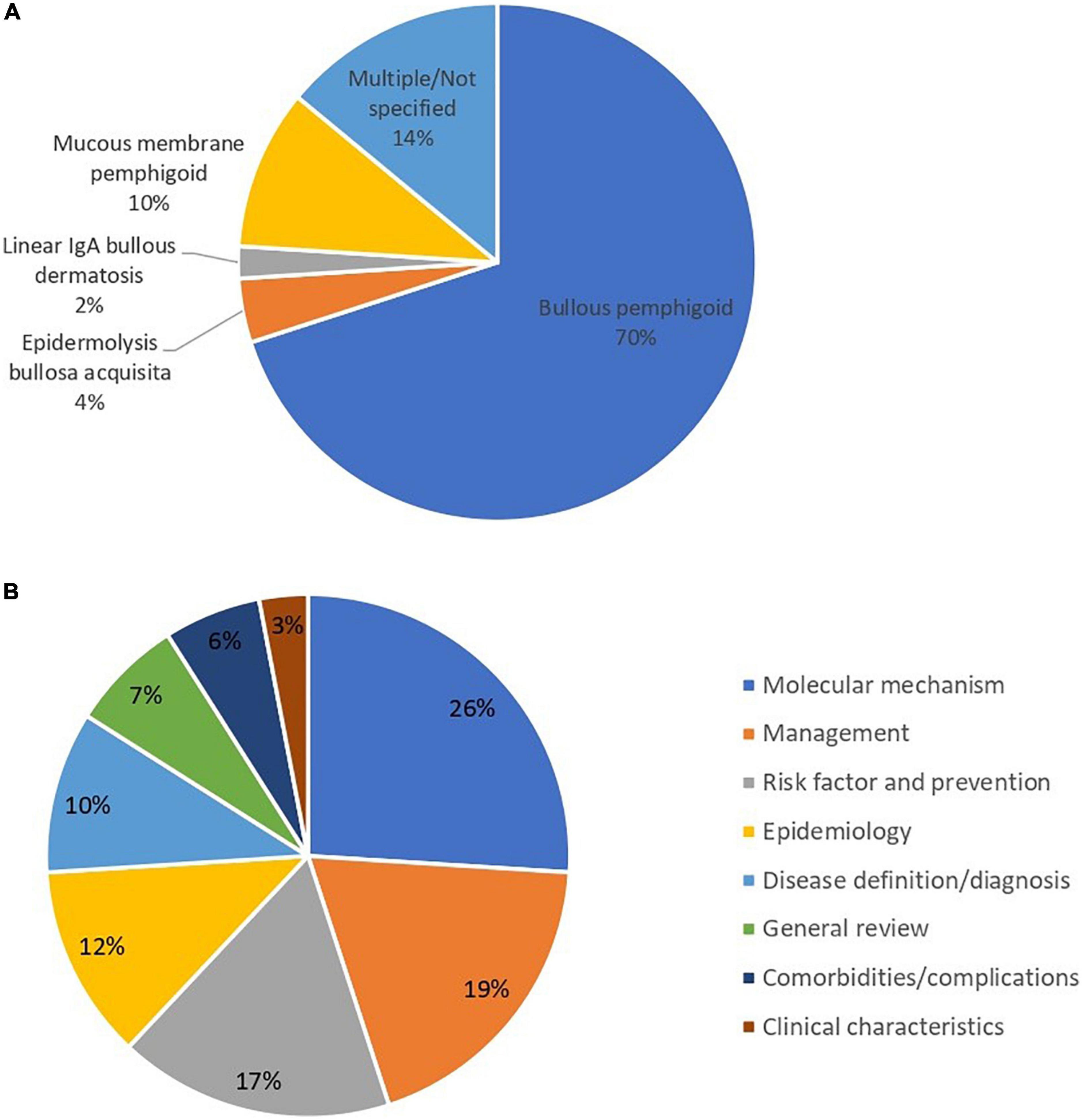
Figure 5. (A) Distribution of subcategories of pemphigoid diseases in the main focus of top 100 publications. (B) Main focus of the top 100 cited studies of pemphigoid diseases.
Discussion
The current study provided a bibliometric analysis of the 100 most cited studies in the field of pemphigoid diseases and revealed that issues regarding disease management, molecular mechanism of diseases, and risk factors of the disease were having the greatest impact in the field.
Within the top 100 studies, guidelines presented the highest amount per study. These guidelines included diagnosis, definition, and treatment of bullous pemphigoid and mucous membrane pemphigoid. A similar situation could also be observed for other types (Figure 5A). The fact that there are no highly cited guidelines and other research items for pemphigoid diseases other than bullous pemphigoid and mucous membrane pemphigoid should be carefully interpreted. The high number of bullous pemphigoid and mucous membrane pemphigoid could potentially be attributed to the fact that they were identified and researched earlier. However, many of the clinical guidelines regarding other pemphigoid diseases such as epidermolysis bullosa acquisita were relatively new and were published recently (106, 107).
According to the results of the current study, in the period between 2006 and 2011, studies of pemphigoid diseases with high citation were increasing. The observed trend could possibly be attributed to the improvement in the identification and treatment of pemphigoid diseases, especially in the field of molecular mechanisms. 7 out of 40 studies (17.5%) in the period were discussing the role of bullous pemphigoid antigens (including BP180 or BP230) in the detection of disease activity or diagnosis. Currently, BP180 and 230 are recognized as the two most important antigens in the pathogenesis of bullous pemphigoid. The two antigens could be related to IgG-related autoantibodies and consequently cause abnormal adhesion between the extracellular matrix and keratinocytes, contributing to the presence of blisters (108). Detection methods have also been widely discussed. The feasibility of enzyme-linked immunoassay analyzes (ELISA) in detecting BP180/230 in patients with bullous pemphigoid has been widely discussed in the past 20 years, and has been demonstrated by several high impact studies (21, 59).
Treatment for pemphigoid diseases was a growing issue in the field. According to the current study, nearly one fifth of the top-100 most cited studies focused on the topic of management of pemphigoid diseases. Before 2018, corticosteroids, doxycycline and biologic agents such as rituximab and omalizumab were reported to have benefit in management of pemphigoid diseases through different approaches (51, 65, 102, 105). Furthermore, in August 2018, dupilumab was time for the first reported to serve as a potential option for bullous pemphigoid management (109), and recent evidence had further demonstrated that dupilumab, rituximab, and omalizumab present similar treatment efficacy (110, 111). However, to date large scale real-world studies evaluating the long-term effect of patients with pemphigoid diseases using dupilumab is lacking and whether or not the prescription of biologic agents is associated with future immunological events is unknown. Moreover, efficacy and safety of some new potential options for treatment of pemphigoid diseases, such as the use of janus kinase inhibitor (JAKi), could also be evaluated in future studies. The involvement of JAK/STAT pathway and the potential effect of using JAKi for pemphigoid diseases has been recently reported by few studies (112, 113). However, the trend could not be observed in the top-100 most cited articles presented in the current study since related data was limited. In this case, knowledge gaps were warranted be filled, and future studies are suggested to focus on the long-term effect of using biologics and the effect of new options of medication.
Drug-induced pemphigoid diseases, especially bullous pemphigoid, was one of the most critical and most mentioned research focuses in the field. Our study revealed that more than 15% of the top 100 studies were discussing risk factors for pemphigoid diseases. Within them, 75% of the studies were evaluating the interaction between specific medication use and the influence of pemphigoid disease incidence or other associated adverse outcomes. Within the term 2001–2018, it was mentioned that dipeptidyl peptidase 4 inhibitors (DPP4i), anti-PD1 or PD-L1 and loop diuretics were mentioned to be associated with a higher risk of new-onset pemphigoid diseases (66, 68). Furthermore, according to a real-world study in Germany, the use of glucocorticoids was reported to increase mortality in patients with bullous pemphigoid (77). To provide an overview of the study trend in pemphigoid diseases in the last three years (2019-2021), we also list the top-5 most cited studies in 2019 (114–118), 2020 (109, 119–122) and 2021 (123–127) (Supplementary Table 1). Within these recent studies most cited, drug-induced pemphigoid diseases (especially bullous pemphigoid) were highly concerned, for more than one-third of the most cited studies stated the correlation between drug/vaccine use and the risk of pemphigoid diseases. In the field of drug-induced pemphigoid diseases, most studies focused on the incidence of bullous pemphigoid. However, only few high-impact studies discussed the incidence of other subcategories of pemphigoid diseases and the evidence might be insufficient. The observed trend indicated that the interaction between drug use and pemphigoid diseases was gradually seizing attention from the academic community. Based on this trend in the study, future studies with greater scale were warranted to identify new risk factors for pemphigoid diseases and to clarify detailed molecular mechanisms on the pathophysiology of drug-induced pemphigoid diseases.
Limitations of the current study must be stated, and the results reported in bibliometric studies should be dialectically interpreted. Although bibliometric studies were extensively applied in research fields to identify research focus and trends (128, 129), the amount of study being cited might not be able to accurately represent its influence. The amount could be influenced by external factors that were not directly related to the research itself, such as publishers, journals, and the fame of the research team. However, the trend provided in the current study was not to determine whether or not an article have enough impact on the academic community. Instead, the intention of analyzing the top 100 most cited studies was to provide the academic community with an overview of the current research reign of pemphigoid diseases and serving as a foundation for future research focuses.
As a conclusion, this comprehensive bibliographic study of pemphigoid diseases provided an overview of current research focuses in the field. Topics such as disease management, molecular mechanism of pathogenesis, and drug-inducing pemphigoid diseases were highly mentioned in previous studies. For researchers and clinicians, the researching trend and study focus in the top-100 cited studies which was presented in the tables of our current study could serve as a potential reference for future investigation and patient management.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
S-YG, S-CH, W-JW, T-MC, and H-CC: study conception and design. S-YG and S-CH: data acquisition. S-YG, S-CH, T-MC, and H-CC: data analysis and demonstration. All authors involved in original draft preparation, drafting or revising the manuscript, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1088083/full#supplementary-material
References
1. Kouris A, Platsidaki E, Christodoulou C, Armyra K, Korkoliakou P, Stefanaki C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid. A case control study. An Bras Dermatol. (2016) 91:601–3. doi: 10.1590/abd1806-4841.20164935
2. Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. (2013) 381:320–32. doi: 10.1016/S0140-6736(12)61140-4
3. Persson M, Begum N, Grainge M, Harman K, Grindlay D, Gran S. The global incidence of bullous pemphigoid: a systematic review and meta-analysis. Br J Dermatol. (2022) 186:414–25. doi: 10.1111/bjd.20743
4. Gau S, Huang J, Wei J. Tramadol use increases mortality and risk of major adverse cardiovascular events in rheumatoid arthritis patients: evidence from a population-based cohort study. Eur J Prev Cardiol. (2022) 29:e237–8. doi: 10.1093/eurjpc/zwab176
5. Gau S, Huang K, Lee C, Kuan Y, Tsai T, Lee C. Bidirectional association between psoriasis and nonalcoholic fatty liver disease: real-world evidence from two longitudinal cohort studies. Front Immunol. (2022) 13:840106. doi: 10.3389/fimmu.2022.840106
6. Lee Y, Tsou H, Kao S, Gau S, Bai Y, Lin M, et al. Patients with rheumatoid arthritis increased risk of developing osteoarthritis: a nationwide population-based cohort study in Taiwan. Front Med (Lausanne). (2020) 7:392. doi: 10.3389/fmed.2020.00392
7. Ahmed A. Intravenous immunoglobulin therapy for patients with bullous pemphigoid unresponsive to conventional immunosuppressive treatment. J Am Acad Dermatol. (2001) 45:825–35. doi: 10.1067/mjd.2001.116337
8. Alpsoy E, Akman-Karakas A, Uzun S. Geographic variations in epidemiology of two autoimmune bullous diseases: pemphigus and bullous pemphigoid. Arch Dermatol Res. (2015) 307:291–8. doi: 10.1007/s00403-014-1531-1
9. Amber K, Murrell D, Schmidt E, Joly P, Borradori L. Autoimmune subepidermal bullous diseases of the skin and mucosae: clinical features, diagnosis, and management. Clin Rev Allergy Immunol. (2018) 54:26–51. doi: 10.1007/s12016-017-8633-4
10. Arakawa M, Dainichi T, Ishii N, Hamada T, Karashima T, Nakama T, et al. Lesional Th17 cells and regulatory T cells in bullous pemphigoid. Exp Dermatol. (2011) 20:1022–4. doi: 10.1111/j.1600-0625.2011.01378.x
11. Bastuji-Garin S, Joly P, Lemordant P, Sparsa A, Bedane C, Delaporte E, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol. (2011) 131:637–43. doi: 10.1038/jid.2010.301
12. Beissert S, Werfel T, Frieling U, Bohm M, Sticherling M, Stadler R, et al. A comparison of oral methylprednisolone plus azathioprine or mycophenolate mofetil for the treatment of bullous pemphigoid. Arch Dermatol. (2007) 143:1536–42. doi: 10.1001/archderm.143.12.1536
13. Bene J, Moulis G, Bennani I, Auffret M, Coupe P, Babai S, et al. Bullous pemphigoid and dipeptidyl peptidase IV inhibitors: a case-noncase study in the French pharmacovigilance database. Br J Dermatol. (2016) 175:296–301. doi: 10.1111/bjd.14601
14. Benzaquen M, Borradori L, Berbis P, Cazzaniga S, Valero R, Richard M, et al. Dipeptidyl peptidase IV inhibitors, a risk factor for bullous pemphigoid: retrospective multicenter case-control study from France and Switzerland. J Am Acad Dermatol. (2018) 78:1090–6. doi: 10.1016/j.jaad.2017.12.038
15. Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. (2017) 18:513–28. doi: 10.1007/s40257-017-0264-2
16. Bernard P, Reguiai Z, Tancrede-Bohin E, Cordel N, Plantin P, Pauwels C, et al. Risk factors for relapse in patients with bullous pemphigoid in clinical remission: a multicenter, prospective, cohort study. Arch Dermatol. (2009) 145:537–42. doi: 10.1001/archdermatol.2009.53
17. Bertram F, Brocker E, Zillikens D, Schmidt E. Prospective analysis of the incidence of autoimmune bullous disorders in lower franconia, Germany. J Dtsch Dermatol Ges. (2009) 7:434–40. doi: 10.1111/j.1610-0387.2008.06976.x
18. Brick K, Weaver C, Lohse C, Pittelkow M, Lehman J, Camilleri M, et al. Incidence of bullous pemphigoid and mortality of patients with bullous pemphigoid in Olmsted County, Minnesota, 1960 through 2009. J Am Acad Dermatol. (2014) 71:92–9. doi: 10.1016/j.jaad.2014.02.030
19. Carlos G, Anforth R, Chou S, Clements A, Fernandez-Penas P. A case of bullous pemphigoid in a patient with metastatic melanoma treated with pembrolizumab. Melanoma Res. (2015) 25:265–8. doi: 10.1097/CMR.0000000000000155
20. Chan L, Ahmed A, Anhalt G, Bernauer W, Cooper K, Elder M, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. (2002) 138:370–9. doi: 10.1001/archderm.138.3.370
21. Charneux J, Lorin J, Vitry F, Antonicelli F, Reguiai Z, Barbe C, et al. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid: a retrospective study of 138 patients. Arch Dermatol. (2011) 147:286–91. doi: 10.1001/archdermatol.2011.23
22. Chen Y, Wu C, Lin M, Chen T, Liao K, Chen Y, et al. Comorbidity profiles among patients with bullous pemphigoid: a nationwide population-based study. Br J Dermatol. (2011) 165:593–9. doi: 10.1111/j.1365-2133.2011.10386.x
23. Chung H, Uitto J. Type VII collagen: the anchoring fibril protein at fault in dystrophic epidermolysis bullosa. Dermatol Clin. (2010) 28:93–105. doi: 10.1016/j.det.2009.10.011
24. Colbert R, Allen D, Eastwood D, Fairley J. Mortality rate of bullous pemphigoid in a US medical center. J Invest Dermatol. (2004) 122:1091–5. doi: 10.1111/j.0022-202X.2004.22504.x
25. Cordel N, Chosidow O, Hellot M, Delaporte E, Lok C, Vaillant L, et al. Neurological disorders in patients with bullous pemphigoid. Dermatology. (2007) 215:187–91. doi: 10.1159/000106574
26. Dainichi T, Kurono S, Ohyama B, Ishii N, Sanzen N, Hayashi M, et al. Anti-laminin gamma-1 pemphigoid. Proc Natl Acad Sci USA. (2009) 106:2800–5. doi: 10.1073/pnas.0809230106
27. Di Zenzo G, Della Torre R, Zambruno G, Borradori L. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol. (2012) 30:3–16. doi: 10.1016/j.clindermatol.2011.03.005
28. Di Zenzo G, Grosso F, Terracina M, Mariotti F, De Pita O, Owaribe K, et al. Characterization of the anti-BP180 autoantibody reactivity profile and epitope mapping in bullous pemphigoid patients. J Invest Dermatol. (2004) 122:103–10. doi: 10.1046/j.0022-202X.2003.22126.x
29. Di Zenzo G, Thoma-Uszynski S, Calabresi V, Fontao L, Hofmann S, Lacour J, et al. Demonstration of epitope-spreading phenomena in bullous pemphigoid: results of a prospective multicenter study. J Invest Dermatol. (2011) 131:2271–80. doi: 10.1038/jid.2011.180
30. Di Zenzo G, Thoma-Uszynski S, Fontao L, Calabresi V, Hofmann S, Hellmark T, et al. Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid. Clin Immunol. (2008) 128:415–26. doi: 10.1016/j.clim.2008.04.012
31. Dimson O, Giudice G, Fu C, Van den Bergh F, Warren S, Janson M, et al. Identification of a potential effector function for IgE autoantibodies in the organ-specific autoimmune disease bullous pemphigoid. J Invest Dermatol. (2003) 120:784–8. doi: 10.1046/j.1523-1747.2003.12146.x
32. Egan C, Lazarova Z, Darling T, Yee C, Cote T, Yancey K. Anti-epiligrin cicatricial pemphigoid and relative risk for cancer. Lancet. (2001) 357:1850–1. doi: 10.1016/S0140-6736(00)04971-0
33. Egan C, Lazarova Z, Darling T, Yee C, Yancey K. Anti-epiligrin cicatricial pemphigoid: clinical findings, immunopathogenesis, and significant associations. Medicine (Baltimore). (2003) 82:177–86. doi: 10.1097/01.md.0000076003.64510.00
34. Fairley J, Baum C, Brandt D, Messingham K. Pathogenicity of IgE in autoimmunity: successful treatment of bullous pemphigoid with omalizumab. J Allergy Clin Immunol. (2009) 123:704–5. doi: 10.1016/j.jaci.2008.11.035
35. Feliciani C, Joly P, Jonkman M, Zambruno G, Zillikens D, Ioannides D, et al. Management of bullous pemphigoid: the European dermatology forum consensus in collaboration with the European academy of dermatology and venereology. Br J Dermatol. (2015) 172:867–77. doi: 10.1111/bjd.13717
36. Fortuna G, Marinkovich M. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. (2012) 30:38–50. doi: 10.1016/j.clindermatol.2011.03.008
37. Foster C, Chang P, Ahmed A. Combination of rituximab and intravenous immunoglobulin for recalcitrant ocular cicatricial pemphigoid: a preliminary report. Ophthalmology. (2010) 117:861–9. doi: 10.1016/j.ophtha.2009.09.049
38. Gudi V, White M, Cruickshank N, Herriot R, Edwards S, Nimmo F, et al. Annual incidence and mortality of bullous pemphigoid in the grampian region of North-East Scotland. Br J Dermatol. (2005) 153:424–7. doi: 10.1111/j.1365-2133.2005.06662.x
39. Guide S, Marinkovich M. Linear IgA bullous dermatosis. Clin Dermatol. (2001) 19:719–27. doi: 10.1016/s0738-081x(00)00185-1
40. Gupta R, Woodley D, Chen M. Epidermolysis bullosa acquisita. Clin Dermatol. (2012) 30:60–9. doi: 10.1016/j.clindermatol.2011.03.011
41. Hammers C, Stanley J. Mechanisms of disease: pemphigus and bullous pemphigoid. Annu Rev Pathol. (2016) 11:175–97. doi: 10.1146/annurev-pathol-012615-044313
42. Hertl M, Eming R, Veldman C. T cell control in autoimmune bullous skin disorders. J Clin Invest. (2006) 116:1159–66. doi: 10.1172/JCI28547
43. Hofmann S, Thoma-Uszynski S, Hunziker T, Bernard P, Koebnick C, Stauber A, et al. Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2- and COOH-terminal regions of the BP180 ectodomain. J Invest Dermatol. (2002) 119:1065–73. doi: 10.1046/j.1523-1747.2002.19529.x
44. Hubner F, Recke A, Zillikens D, Linder R, Schmidt E. Prevalence and age distribution of pemphigus and pemphigoid diseases in Germany. J Invest Dermatol. (2016) 136:2495–8. doi: 10.1016/j.jid.2016.07.013
45. Iwata H, Kamio N, Aoyama Y, Yamamoto Y, Hirako Y, Owaribe K, et al. IgG from patients with bullous pemphigoid depletes cultured keratinocytes of the 180-kDa bullous pemphigoid antigen (type XVII collagen) and weakens cell attachment. J Invest Dermatol. (2009) 129:919–26. doi: 10.1038/jid.2008.305
46. Iwata Y, Komura K, Kodera M, Usuda T, Yokoyama Y, Hara T, et al. Correlation of IgE autoantibody to BP180 with a severe form of bullous pemphigoid. Arch Dermatol. (2008) 144:41–8. doi: 10.1001/archdermatol.2007.9
47. Izumi K, Nishie W, Mai Y, Wada M, Natsuga K, Ujiie H, et al. Autoantibody profile differentiates between inflammatory and noninflammatory bullous pemphigoid. J Invest Dermatol. (2016) 136:2201–10. doi: 10.1016/j.jid.2016.06.622
48. Jedlickova H, Hlubinka M, Pavlik T, Semradova V, Budinska E, Vlasin Z. Bullous pemphigoid and internal diseases - a case-control study. Eur J Dermatol. (2010) 20:96–101. doi: 10.1684/ejd.2010.0805
49. Joly P, Baricault S, Sparsa A, Bernard P, Bedane C, Duvert-Lehembre S, et al. Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol. (2012) 132:1998–2004. doi: 10.1038/jid.2012.35
50. Joly P, Benichou J, Lok C, Hellot M, Saiag P, Tancrede-Bohin E, et al. Prediction of survival for patients with bullous pemphigoid: a prospective study. Arch Dermatol. (2005) 141:691–8. doi: 10.1001/archderm.141.6.691
51. Joly P, Roujeau J, Benichou J, Delaporte E, D’Incan M, Dreno B, et al. A comparison of two regimens of topical corticosteroids in the treatment of patients with bullous pemphigoid: a multicenter randomized study. J Invest Dermatol. (2009) 129:1681–7. doi: 10.1038/jid.2008.412
52. Joly P, Roujeau J, Benichou J, Picard C, Dreno B, Delaporte E, et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med. (2002) 346:321–7. doi: 10.1056/NEJMoa011592
53. Jour G, Glitza I, Ellis R, Torres-Cabala C, Tetzlaff M, Li J, et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. J Cutan Pathol. (2016) 43:688–96. doi: 10.1111/cup.12717
54. Kasperkiewicz M, Shimanovich I, Ludwig R, Rose C, Zillikens D, Schmidt E. Rituximab for treatment-refractory pemphigus and pemphigoid: a case series of 17 patients. J Am Acad Dermatol. (2011) 65:552–8. doi: 10.1016/j.jaad.2010.07.032
55. Kasperkiewicz M, Zillikens D. The pathophysiology of bullous pemphigoid. Clin Rev Allergy Immunol. (2007) 33:67–77. doi: 10.1007/s12016-007-0030-y
56. Kershenovich R, Hodak E, Mimouni D. Diagnosis and classification of pemphigus and bullous pemphigoid. Autoimmun Rev. (2014) 13:477–81. doi: 10.1016/j.autrev.2014.01.011
57. Kirtschig G, Middleton P, Bennett C, Murrell D, Wojnarowska F, Khumalo N. Interventions for bullous pemphigoid. Cochrane Database Syst Rev. (2010) 2010:CD002292. doi: 10.1002/14651858.CD002292.pub3
58. Kneisel A, Hertl M. Autoimmune bullous skin diseases. Part 1: clinical manifestations. J Dtsch Dermatol Ges. (2011) 9:844–856;quiz857. doi: 10.1111/j.1610-0387.2011.07793.x
59. Kobayashi M, Amagai M, Kuroda-Kinoshita K, Hashimoto T, Shirakata Y, Hashimoto K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. (2002) 30:224–32. doi: 10.1016/s0923-1811(02)00109-3
60. Kridin K, Bergman R. Association of bullous pemphigoid with dipeptidyl-peptidase 4 inhibitors in patients with diabetes: estimating the risk of the new agents and characterizing the patients. JAMA Dermatol. (2018) 154:1152–8. doi: 10.1001/jamadermatol.2018.2352
61. Kridin K, Ludwig R. The growing incidence of bullous pemphigoid: overview and potential explanations. Front Med (Lausanne). (2018) 5:220. doi: 10.3389/fmed.2018.00220
62. Langan S, Groves R, West J. The relationship between neurological disease and bullous pemphigoid: a population-based case-control study. J Invest Dermatol. (2011) 131:631–6. doi: 10.1038/jid.2010.357
63. Langan S, Smeeth L, Hubbard R, Fleming K, Smith C, West J. Bullous pemphigoid and pemphigus vulgaris–incidence and mortality in the UK: population based cohort study. BMJ. (2008) 337:a180. doi: 10.1136/bmj.a180
64. Le Jan S, Plee J, Vallerand D, Dupont A, Delanez E, Durlach A, et al. Innate immune cell-produced IL-17 sustains inflammation in bullous pemphigoid. J Invest Dermatol. (2014) 134:2908–17. doi: 10.1038/jid.2014.263
65. Le Roux-Villet C, Prost-Squarcioni C, Alexandre M, Caux F, Pascal F, Doan S, et al. Rituximab for patients with refractory mucous membrane pemphigoid. Arch Dermatol. (2011) 147:843–9. doi: 10.1001/archdermatol.2011.54
66. Lloyd-Lavery A, Chi C, Wojnarowska F, Taghipour K. The associations between bullous pemphigoid and drug use: a UK case-control study. JAMA Dermatol. (2013) 149:58–62. doi: 10.1001/2013.jamadermatol.376
67. Lo Schiavo A, Ruocco E, Brancaccio G, Caccavale S, Ruocco V, Wolf R. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. (2013) 31:391–9. doi: 10.1016/j.clindermatol.2013.01.006
68. Lopez A, Khanna T, Antonov N, Audrey-Bayan C, Geskin L. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. (2018) 57:664–9. doi: 10.1111/ijd.13984
69. Marazza G, Pham H, Scharer L, Pedrazzetti P, Hunziker T, Trueb R, et al. Incidence of bullous pemphigoid and pemphigus in Switzerland: a 2-year prospective study. Br J Dermatol. (2009) 161:861–8. doi: 10.1111/j.1365-2133.2009.09300.x
70. Mihai S, Sitaru C. Immunopathology and molecular diagnosis of autoimmune bullous diseases. J Cell Mol Med. (2007) 11:462–81. doi: 10.1111/j.1582-4934.2007.00033.x
71. Murrell D, Daniel B, Joly P, Borradori L, Amagai M, Hashimoto T, et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol. (2012) 66:479–85. doi: 10.1016/j.jaad.2011.06.032
72. Murrell D, Marinovic B, Caux F, Prost C, Ahmed R, Wozniak K, et al. Definitions and outcome measures for mucous membrane pemphigoid: recommendations of an international panel of experts. J Am Acad Dermatol. (2015) 72:168–74. doi: 10.1016/j.jaad.2014.08.024
73. Naidoo J, Schindler K, Querfeld C, Busam K, Cunningham J, Page D, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. (2016) 4:383–9. doi: 10.1158/2326-6066.CIR-15-0123
74. Nishie W. Update on the pathogenesis of bullous pemphigoid: an autoantibody-mediated blistering disease targeting collagen XVII. J Dermatol Sci. (2014) 73:179–86. doi: 10.1016/j.jdermsci.2013.12.001
75. Oyama N, Setterfield J, Powell A, Sakuma-Oyama Y, Albert S, Bhogal B, et al. Bullous pemphigoid antigen II (BP180) and its soluble extracellular domains are major autoantigens in mucous membrane pemphigoid: the pathogenic relevance to HLA class II alleles and disease severity. Br J Dermatol. (2006) 154:90–8. doi: 10.1111/j.1365-2133.2005.06998.x
76. Parker S, Dyson S, Brisman S, Pennie M, Swerlick R, Khan R, et al. Mortality of bullous pemphigoid: an evaluation of 223 patients and comparison with the mortality in the general population in the United States. J Am Acad Dermatol. (2008) 59:582–8. doi: 10.1016/j.jaad.2008.07.022
77. Rzany B, Partscht K, Jung M, Kippes W, Mecking D, Baima B, et al. Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of glucocorticosteroids, and old age. Arch Dermatol. (2002) 138:903–8. doi: 10.1001/archderm.138.7.903
78. Saleh M, Ishii K, Kim Y, Murakami A, Ishii N, Hashimoto T, et al. Development of NC1 and NC2 domains of type VII collagen ELISA for the diagnosis and analysis of the time course of epidermolysis bullosa acquisita patients. J Dermatol Sci. (2011) 62:169–75. doi: 10.1016/j.jdermsci.2011.03.003
79. Sardy M, Kostaki D, Varga R, Peris K, Ruzicka T. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. (2013) 69:748–53. doi: 10.1016/j.jaad.2013.07.009
80. Saw V, Dart J, Rauz S, Ramsay A, Bunce C, Xing W, et al. Immunosuppressive therapy for ocular mucous membrane pemphigoid strategies and outcomes. Ophthalmology. (2008) 115:253.e–61.e. doi: 10.1016/j.ophtha.2007.04.027
81. Schmidt E, Goebeler M, Hertl M, Sardy M, Sitaru C, Eming R, et al. S2k guideline for the diagnosis of pemphigus vulgaris/foliaceus and bullous pemphigoid. J Dtsch Dermatol Ges. (2015) 13:713–27. doi: 10.1111/ddg.12612
82. Schmidt E, Hunzelmann N, Zillikens D, Brocker E, Goebeler M. Rituximab in refractory autoimmune bullous diseases. Clin Exp Dermatol. (2006) 31:503–8. doi: 10.1111/j.1365-2230.2006.02151.x
83. Schmidt E, Seitz C, Benoit S, Brocker E, Goebeler M. Rituximab in autoimmune bullous diseases: mixed responses and adverse effects. Br J Dermatol. (2007) 156:352–6. doi: 10.1111/j.1365-2133.2006.07646.x
84. Schmidt E, Skrobek C, Kromminga A, Hashimoto T, Messer G, Brocker E, et al. Cicatricial pemphigoid: IgA and IgG autoantibodies target epitopes on both intra- and extracellular domains of bullous pemphigoid antigen 180. Br J Dermatol. (2001) 145:778–83. doi: 10.1046/j.1365-2133.2001.04471.x
85. Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. (2010) 10:84–9. doi: 10.1016/j.autrev.2010.08.007
86. Siegel J, Totonchy M, Damsky W, Berk-Krauss J, Castiglione F Jr., Sznol M, et al. Bullous disorders associated with anti-PD-1 and anti-PD-L1 therapy: a retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. J Am Acad Dermatol. (2018) 79:1081–8. doi: 10.1016/j.jaad.2018.07.008
87. Sitaru C, Dahnrich C, Probst C, Komorowski L, Blocker I, Schmidt E, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. (2007) 16:770–7. doi: 10.1111/j.1600-0625.2007.00592.x
88. Sitaru C, Kromminga A, Hashimoto T, Brocker E, Zillikens D. Autoantibodies to type VII collagen mediate Fcgamma-dependent neutrophil activation and induce dermal-epidermal separation in cryosections of human skin. Am J Pathol. (2002) 161:301–11. doi: 10.1016/s0002-9440(10)64182-x
89. Skandalis K, Spirova M, Gaitanis G, Tsartsarakis A, Bassukas I. Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol Venereol. (2012) 26:249–53. doi: 10.1111/j.1468-3083.2011.04062.x
90. Stavropoulos P, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. (2014) 28:1133–40. doi: 10.1111/jdv.12366
91. Taghipour K, Chi C, Vincent A, Groves R, Venning V, Wojnarowska F. The association of bullous pemphigoid with cerebrovascular disease and dementia: a case-control study. Arch Dermatol. (2010) 146:1251–4. doi: 10.1001/archdermatol.2010.322
92. Thoma-Uszynski S, Uter W, Schwietzke S, Hofmann S, Hunziker T, Bernard P, et al. BP230- and BP180-specific auto-antibodies in bullous pemphigoid. J Invest Dermatol. (2004) 122:1413–22. doi: 10.1111/j.0022-202X.2004.22603.x
93. Thoma-Uszynski S, Uter W, Schwietzke S, Schuler G, Borradori L, Hertl M. Autoreactive T and B cells from bullous pemphigoid (BP) patients recognize epitopes clustered in distinct regions of BP180 and BP230. J Immunol. (2006) 176:2015–23. doi: 10.4049/jimmunol.176.3.2015
94. Thorne J, Anhalt G, Jabs D. Mucous membrane pemphigoid and pseudopemphigoid. Ophthalmology. (2004) 111:45–52. doi: 10.1016/j.ophtha.2003.03.001
95. Tsuji-Abe Y, Akiyama M, Yamanaka Y, Kikuchi T, Sato-Matsumura K, Shimizu H. Correlation of clinical severity and ELISA indices for the NC16A domain of BP180 measured using BP180 ELISA kit in bullous pemphigoid. J Dermatol Sci. (2005) 37:145–9. doi: 10.1016/j.jdermsci.2004.10.007
96. van Beek N, Luttmann N, Huebner F, Recke A, Karl I, Schulze F, et al. Correlation of serum levels of IgE autoantibodies against BP180 with bullous pemphigoid disease activity. JAMA Dermatol. (2017) 153:30–8. doi: 10.1001/jamadermatol.2016.3357
97. Varpuluoma O, Forsti A, Jokelainen J, Turpeinen M, Timonen M, Huilaja L, et al. Vildagliptin significantly increases the risk of bullous pemphigoid: a finnish nationwide registry study. J Invest Dermatol. (2018) 138:1659–61. doi: 10.1016/j.jid.2018.01.027
98. Venning V, Taghipour K, Mohd Mustapa M, Highet A, Kirtschig G. British association of dermatologists’ guidelines for the management of bullous pemphigoid 2012. Br J Dermatol. (2012) 167:1200–14. doi: 10.1111/bjd.12072
99. Verraes S, Hornebeck W, Polette M, Borradori L, Bernard P. Respective contribution of neutrophil elastase and matrix metalloproteinase 9 in the degradation of BP180 (type XVII collagen) in human bullous pemphigoid. J Invest Dermatol. (2001) 117:1091–6. doi: 10.1046/j.0022-202x.2001.01521.x
100. Vodegel R, Jonkman M, Pas H, de Jong M. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. (2004) 151:112–8. doi: 10.1111/j.1365-2133.2004.06006.x
101. Waisbourd-Zinman O, Ben-Amitai D, Cohen A, Feinmesser M, Mimouni D, Adir-Shani A, et al. Bullous pemphigoid in infancy: clinical and epidemiologic characteristics. J Am Acad Dermatol. (2008) 58:41–8. doi: 10.1016/j.jaad.2007.08.010
102. Williams H, Wojnarowska F, Kirtschig G, Mason J, Godec T, Schmidt E, et al. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: a pragmatic, non-inferiority, randomised controlled trial. Lancet. (2017) 389:1630–8. doi: 10.1016/S0140-6736(17)30560-3
103. Wojnarowska F, Kirtschig G, Highet A, Venning V, Khumalo N, British Association of Dermatologists. Guidelines for the management of bullous pemphigoid. Br J Dermatol. (2002) 147:214–21. doi: 10.1046/j.1365-2133.2002.04835.x
104. Yoshida M, Hamada T, Amagai M, Hashimoto K, Uehara R, Yamaguchi K, et al. Enzyme-linked immunosorbent assay using bacterial recombinant proteins of human BP230 as a diagnostic tool for bullous pemphigoid. J Dermatol Sci. (2006) 41:21–30. doi: 10.1016/j.jdermsci.2005.11.002
105. Yu K, Crew A, Messingham K, Fairley J, Woodley D. Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol. (2014) 71:468–74. doi: 10.1016/j.jaad.2014.04.053
106. Santi C, Gripp A, Roselino A, Mello D, Gordilho J, Marsillac P, et al. Consensus on the treatment of autoimmune bullous dermatoses: bullous pemphigoid, mucous membrane pemphigoid and epidermolysis bullosa acquisita - Brazilian society of dermatology. An Bras Dermatol. (2019) 94(2 Suppl. 1):33–47. doi: 10.1590/abd1806-4841.2019940207
107. Ujiie H, Iwata H, Yamagami J, Nakama T, Aoyama Y, Ikeda S, et al. Japanese guidelines for the management of pemphigoid (including epidermolysis bullosa acquisita). J Dermatol. (2019) 46:1102–35. doi: 10.1111/1346-8138.15111
108. Genovese G, Di Zenzo G, Cozzani E, Berti E, Cugno M, Marzano A. New insights into the pathogenesis of bullous pemphigoid: 2019 update. Front Immunol. (2019) 10:1506. doi: 10.3389/fimmu.2019.01506
109. Abdat R, Waldman R, de Bedout V, Czernik A, McLeod M, King B, et al. Dupilumab as a novel therapy for bullous pemphigoid: a multicenter case series. J Am Acad Dermatol. (2020) 83:46–52. doi: 10.1016/j.jaad.2020.01.089
110. Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Front Immunol. (2022) 13:928621. doi: 10.3389/fimmu.2022.928621
111. Velin M, Dugourd P, Sanchez A, Bahadoran P, Montaudie H, Passeron T. Efficacy and safety of methotrexate, omalizumab and dupilumab for bullous pemphigoid in patients resistant or contraindicated to oral steroids. A monocentric real-life study. J Eur Acad Dermatol Venereol. (2022) 36:e539–42. doi: 10.1111/jdv.17999
112. Burningham K, Cao J, Dominguez A. Successful treatment of recalcitrant mucous membrane pemphigoid with multisystem involvement with baricitinib and methotrexate. JAAD Case Rep. (2022) 27:67–9. doi: 10.1016/j.jdcr.2022.07.013
113. Juczynska K, Wozniacka A, Waszczykowska E, Danilewicz M, Wagrowska-Danilewicz M, Wieczfinska J, et al. Expression of the JAK/STAT signaling pathway in bullous pemphigoid and dermatitis herpetiformis. Mediators Inflamm. (2017) 2017:6716419. doi: 10.1155/2017/6716419
114. Koga H, Prost-Squarcioni C, Iwata H, Jonkman M, Ludwig R, Bieber K. Epidermolysis bullosa acquisita: the 2019 update. Front Med (Lausanne). (2018) 5:362. doi: 10.3389/fmed.2018.00362
115. Kremer N, Snast I, Cohen E, Hodak E, Mimouni D, Lapidoth M, et al. Rituximab and omalizumab for the treatment of bullous pemphigoid: a systematic review of the literature. Am J Clin Dermatol. (2019) 20:209–16. doi: 10.1007/s40257-018-0401-6
116. Lee S, Lee H, Yoon M, Kim D. Association of dipeptidyl peptidase 4 inhibitor use with risk of bullous pemphigoid in patients with diabetes. JAMA Dermatol. (2019) 155:172–7. doi: 10.1001/jamadermatol.2018.4556
117. Miyamoto D, Santi C, Aoki V, Maruta C. Bullous pemphigoid. An Bras Dermatol. (2019) 94:133–46. doi: 10.1590/abd1806-4841.20199007
118. Plaquevent M, Tetart F, Fardet L, Ingen-Housz-Oro S, Valeyrie-Allanore L, Bernard P, et al. Higher frequency of dipeptidyl peptidase-4 inhibitor intake in bullous pemphigoid patients than in the French general population. J Invest Dermatol. (2019) 139:835–41. doi: 10.1016/j.jid.2018.10.045
119. Egami S, Yamagami J, Amagai M. Autoimmune bullous skin diseases, pemphigus and pemphigoid. J Allergy Clin Immunol. (2020) 145:1031–47. doi: 10.1016/j.jaci.2020.02.013
120. Hashimoto T, Kursewicz C, Fayne R, Nanda S, Shah S, Nattkemper L, et al. Pathophysiologic mechanisms of itch in bullous pemphigoid. J Am Acad Dermatol. (2020) 83:53–62. doi: 10.1016/j.jaad.2019.07.060
121. Liu S, Chen W, Chi C. Association between medication use and bullous pemphigoid: a systematic review and meta-analysis. JAMA Dermatol. (2020) 156:891–900. doi: 10.1001/jamadermatol.2020.1587
122. Verheyden M, Bilgic A, Murrell D. A systematic review of drug-induced pemphigoid. Acta Derm Venereol. (2020) 100:adv00224. doi: 10.2340/00015555-3457
123. Damiani G, Pacifico A, Pelloni F, Iorizzo M. The first dose of COVID-19 vaccine may trigger pemphigus and bullous pemphigoid flares: is the second dose therefore contraindicated? J Eur Acad Dermatol Venereol. (2021) 35:e645–7. doi: 10.1111/jdv.17472
124. Kasperkiewicz M, Schmidt E, Amagai M, Fairley J, Joly P, Murrell D, et al. Updated international expert recommendations for the management of autoimmune bullous diseases during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. (2021) 35:e412–4. doi: 10.1111/jdv.17207
125. Kridin K, Cohen A. Dipeptidyl-peptidase IV inhibitor-associated bullous pemphigoid: a systematic review and meta-analysis. J Am Acad Dermatol. (2021) 85:501–3. doi: 10.1016/j.jaad.2018.09.048
126. Persson M, Harman K, Vinogradova Y, Langan S, Hippisley-Cox J, Thomas K, et al. Incidence, prevalence and mortality of bullous pemphigoid in England 1998-2017: a population-based cohort study. Br J Dermatol. (2021) 184:68–77. doi: 10.1111/bjd.19022
127. Schmidt E, Rashid H, Marzano A, Lamberts A, Di Zenzo G, Diercks G, et al. European guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European academy of dermatology and venereology - Part II. J Eur Acad Dermatol Venereol. (2021) 35:1926–48. doi: 10.1111/jdv.17395
128. Teng Y, Li S, Fan Y, Tao X, Huang Y. Top 100 most-cited publications in hidradenitis suppurativa: an updated bibliometric analysis. Front Med (Lausanne). (2022) 9:995873. doi: 10.3389/fmed.2022.995873
Keywords: bibliometric analysis, pemphigoid diseases, dermatology, immunology, autoimmune
Citation: Huang S-C, Chiu T-M, Lee C-Y, Chang H-C, Wu W-J and Gau S-Y (2023) Researching trends in pemphigoid diseases: A bibliometric study of the top 100 most cited publications. Front. Med. 9:1088083. doi: 10.3389/fmed.2022.1088083
Received: 03 November 2022; Accepted: 09 December 2022;
Published: 09 January 2023.
Edited by:
Giulia Gasparini, University of Genoa, ItalyReviewed by:
Marian Dmochowski, Poznan University of Medical Sciences, PolandMarwah Adly Saleh, Cairo University, Egypt
Copyright © 2023 Huang, Chiu, Lee, Chang, Wu and Gau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Jun Wu,  MDA3d3VAY3NtdS5lZHUudHc=; Shuo-Yan Gau,
MDA3d3VAY3NtdS5lZHUudHc=; Shuo-Yan Gau,  c2l4c2FtdXJhaS5zaGllbjE1QGdtYWlsLmNvbQ==
c2l4c2FtdXJhaS5zaGllbjE1QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Shih-Cheng Huang
Shih-Cheng Huang Tsu-Man Chiu1,2,3
Tsu-Man Chiu1,2,3 Chien-Ying Lee
Chien-Ying Lee Shuo-Yan Gau
Shuo-Yan Gau