
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 04 January 2023
Sec. Rheumatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1081814
This article is part of the Research Topic Early and Refractory Rheumatoid Arthritis: Two Points of Disease Course View all 9 articles
Background: Even though serotonin (5-HT) has been ascribed immunomodulatory features, very little is known about its role in chronic inflammatory diseases. Serotonin is implicated in inflammation and increased levels have been associated with progression of bone erosions in RA.
Objective: To investigate serum serotonin levels in patients with increased risk of rheumatoid arthritis (RA) and patients with recent-onset disease. Moreover, we aimed to determine the prognostic value of serotonin for arthritis development and the disease course.
Methods: Two prospective observational patient cohorts were studied; anti-citrullinated protein antibody (ACPA) -positive patients with musculoskeletal pain without clinical arthritis (n = 82) and patients with early RA (n = 412). Serotonin levels were measured by enzyme-linked immunosorbent assay (ELISA) in baseline serum samples from both cohorts, and longitudinally in at-risk individuals.
Results: Compared to healthy controls (median 65 ng/ml), serotonin levels were significantly higher in both at-risk individuals (median 111 ng/ml, p < 0.0001) and patients with early RA (median 135 ng/ml, p < 0.0001). No significant differences were found between at-risk individuals and patients with early RA. At-risk individuals progressing to arthritis had similar levels as those not progressing, and no significant differences were seen over time. Baseline levels in early RA did not associate with mean 28-joint disease activity scores during 3 years follow-up.
Conclusion: Serum serotonin levels are elevated both at, and prior to, onset of RA. However, increased serotonin is not prognostic for arthritis development or disease course.
Increasing evidence suggest that early stages of rheumatoid arthritis (RA) occur outside the joint, and circulating autoantibodies, in particular anti-citrullinated protein antibodies (ACPAs), can be detected up to 10 years before diagnosis (1). Among individuals with ACPA who also experience arthralgia, 30–50% will develop arthritis within a few years (2, 3). In this prephase of RA, prognostic markers for arthritis development are of significant clinical value in order to achieve early and individualized treatment to prevent disease progression.
Serotonin has during the last decades been recognized as a peripheral hormone with immunomodulatory properties. The majority of the peripheral (i.e., outside the nervous system) serotonin is produced by intestinal enterochromaffin cells, and intriguingly, mucosal sites have emerged as a triggering site for RA development (4). Following release from enterochromaffin cells, serotonin enters the blood stream where it is taken up by platelets and stored in their dense granules until activation (5).
There is increasing interest in serotonin as an activator of inflammation and trigger of autoimmunity. For instance, elevated serotonin levels have been reported in RA patients compared to controls (6–8), and to associate with progression of bone erosions (9). Since serotonin appear increased in RA patients, we aimed to investigate if serotonin is elevated also prior to diagnosis. In addition, whether serotonin levels are prognostic for arthritis development in patients at increased risk of RA, or for the disease course among patients with recent-onset RA.
Two prospective Swedish observational cohorts formed the basis of this study. Clinical patient characteristics are outlined in Table 1. The TIRx (extra-early rheumatology follow-up) cohort represents a population with increased risk of developing RA. Inclusion criteria were positive ACPA test (anti-CCP2) in clinical routine care and musculoskeletal pain of any kind and duration (10). Exclusion criteria were previous inflammatory rheumatic disease or corticosteroid treatment (oral or intra-articular) within 6 weeks prior to screening. A total of 82 patients without arthritis upon clinical examination at baseline were included, thus representing an at-risk population. During a median of 6 years follow-up, 39 (48%) of the patients developed arthritis, defined by clinical examination by an experienced rheumatologist (10).
We also studied 412 early RA patients from the TIRA-2 (Timely Interventions in RA) cohort (11). Inclusion criteria were symptom duration ≥6 weeks but <12 months since the first joint swelling as judged by the patient. In addition, patients should fulfill four of seven of the 1987 revised American College of Rheumatology criteria for RA (12) (n = 387 [94%]) or experience morning stiffness for ≥60 min and symmetrical arthritis and small joint arthritis (fingers, wrists, or toes) (n = 25 [6%]). Radiographic damage was graded according to the Larsen method (13).
No participating individual had received disease-modifying anti-rheumatic drugs (DMARDs) prior to the initial blood sampling (baseline visit).
As controls, we recruited 100 blood donors who were age-matched for TIRx [mean age 55 (range 18–72) years, 50% women] from the Department of Transfusion Medicine at Linköping University Hospital.
Ethical permission has been granted; EPN-Linköping Dnr M168-05 (TIRA2), M220-09 (TIRx), and 2015/236-32 (controls).
Serum samples, stored at −70°C until analysis, were analyzed in duplicates for serotonin by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (ImmuSmol, Bordeaux, France).
Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and platelet count were analyzed according to clinical routine practice at the respective participating rheumatology unit.
Mann–Whitney U test and Kruskal Wallis were used to test differences in levels between groups and Pearson’s chi-square was used for dichotomous variables. For correlation analyses, Spearman’s correlation was applied. Cox regression was used to test serotonin levels versus (vs.) progression to arthritis. Statistical analyses were performed with SPSS statistics version 26 (IBM, Armonk, NY, USA) or GraphPad Prism version 9 (GraphPad Software, La Jolla, CA, USA). Two-sided p-values < 0.05 were considered significant.
Baseline serum serotonin levels were significantly higher in at-risk individuals (median 111 ng/ml) compared to healthy controls (median 65 ng/ml) p < 0.0001 (Figure 1A). The levels were comparable between patients progressing (median 127 ng/ml) vs. not progressing (median 102 ng/ml) to arthritis (Figure 1B). Patients with early RA also had significantly higher levels (median 135 ng/ml) compared to healthy controls (median 65 ng/ml, p < 0.0001; Figure 1B). No significant differences were found between at-risk individuals and patients with early RA. Neither were there any differences when comparing patients with early RA vs. at risk-individuals progressing to arthritis, or vs. at-risk individuals not progressing to arthritis (Figure 1B).
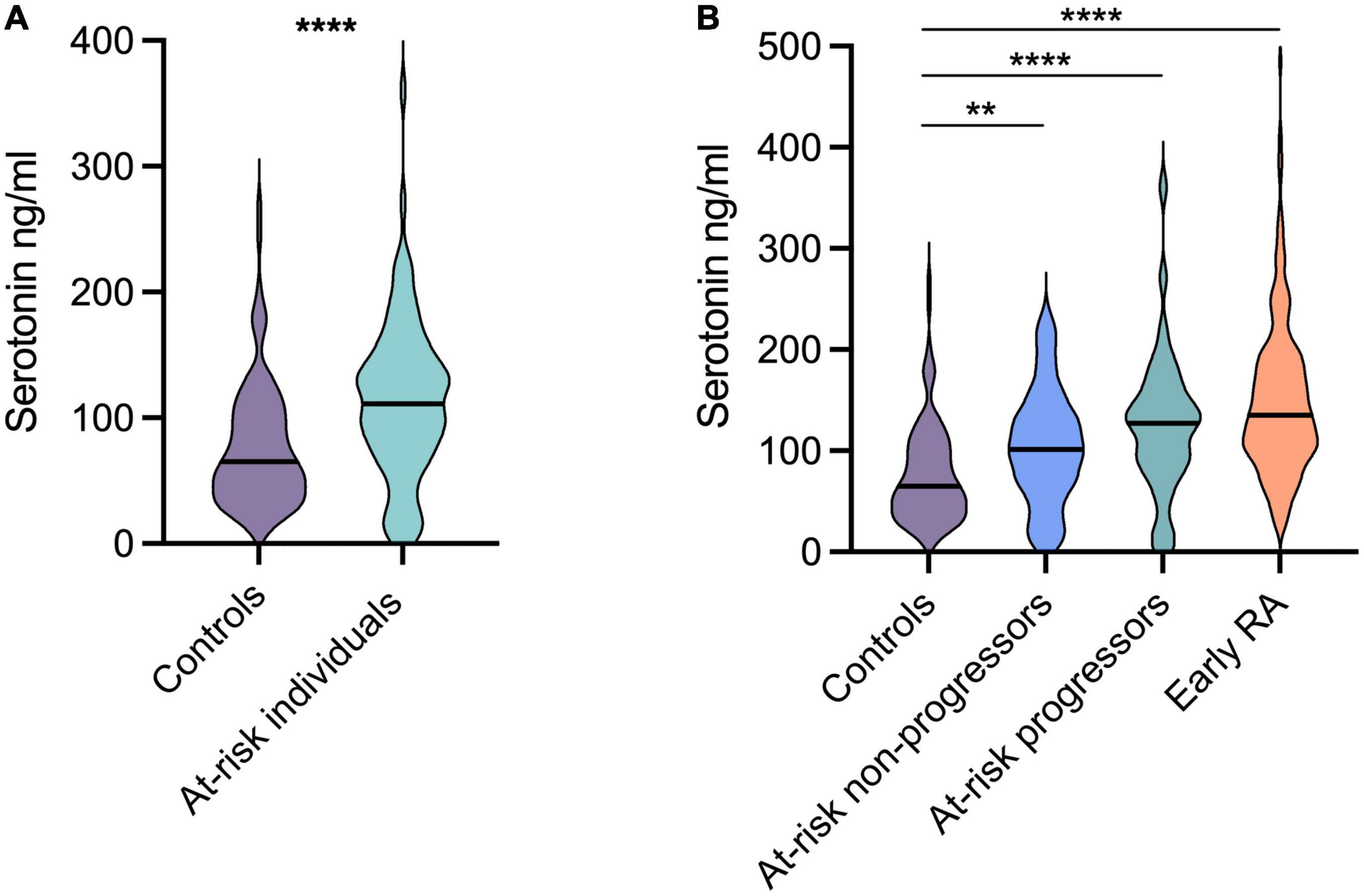
Figure 1. Serotonin levels in at-risk individuals and patients with early rheumatoid arthritis (RA). (A) Compared to healthy controls, the serotonin levels were significantly higher in at-risk individuals (median 65 ng vs. 111 ng/ml). (B) Patients with early RA had significantly higher levels (median 135 ng/ml) compared to healthy controls (median 65 ng/ml). The levels were comparable between at-risk individuals progressing and not progressing to arthritis (median 127 ng/ml and 102 ng/ml respectively). ****p < 0.0001, **p = 0.002.
Longitudinal analyses in at-risk individuals revealed relatively stable serotonin levels over time (Figure 2A). At-risk patients progressing to arthritis had numerically higher median levels compared to those not progressing at all time points, but without statistical significance (baseline: median 127 vs. 102 ng/ml; 3 months; 147 vs. 115 ng/ml, time-point for arthritis development vs. 12 months; 112 vs. 97 ng/ml). Among progressors, serotonin levels at baseline (median 127 ng/ml) were similar to those at the time for arthritis development (median 112 ng/ml) (Figure 2B). Serotonin levels were not prognostic for arthritis development, as analyzed by Cox regression (p = 0.062).
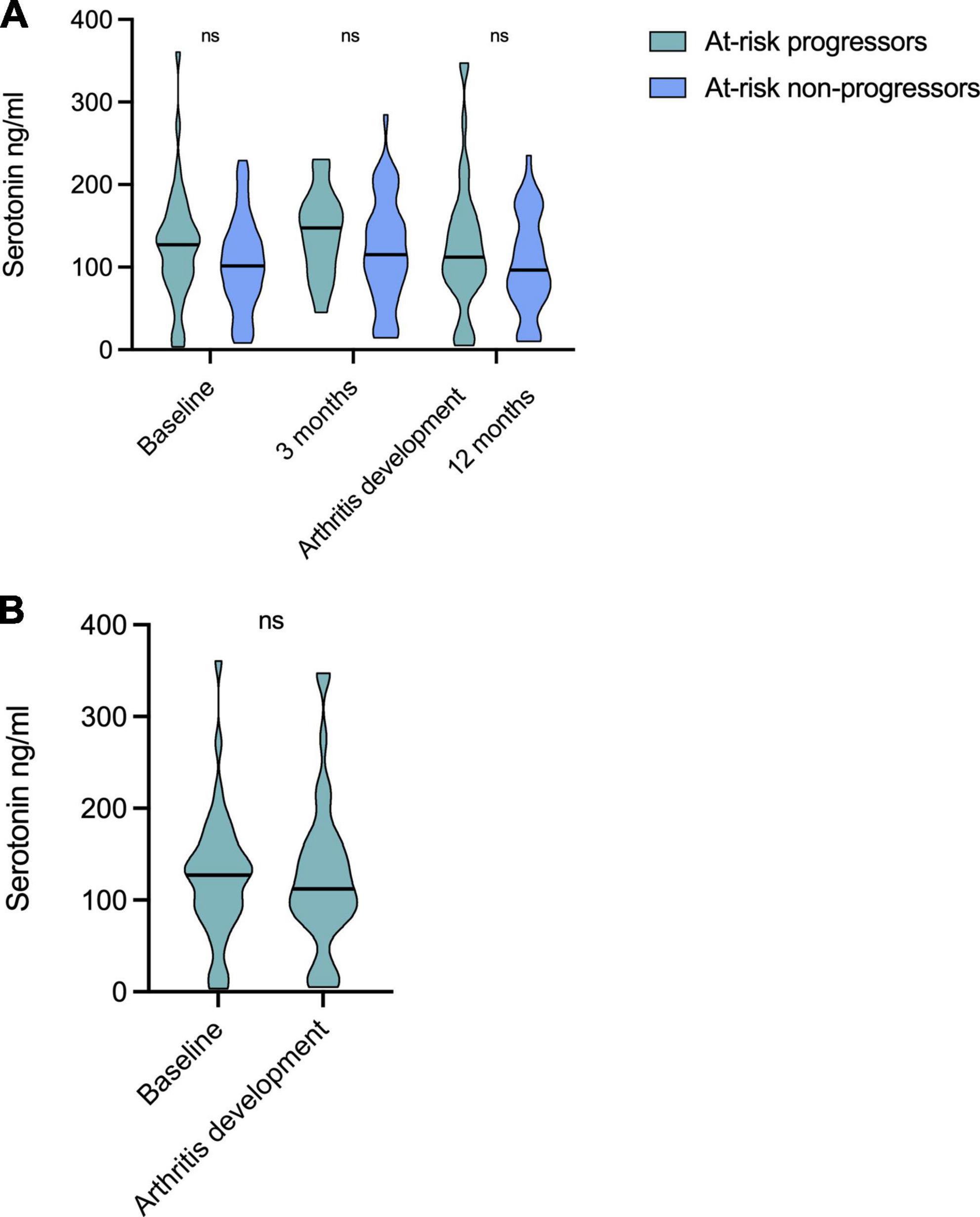
Figure 2. Longitudinal serotonin levels in at-risk individuals. (A) Serotonin levels were relatively stable over time and no differences were seen between any time points for patients progressing to arthritis, nor for non-progressors. Patients progressing to arthritis had numerically higher levels compared to those not progressing at all time points, but not significantly different. (B) Serotonin levels at baseline (median 127 ng/ml) did not differ to those at the time for arthritis development (median 112 ng/ml) among progressors. ns, non significant.
In at-risk individuals, no statistically significant difference in baseline serotonin levels were found between patients with normal (n = 76, median 108 ng/ml) vs. raised CRP (n = 6, median 136 ng/ml) p = 0.36. Patients with raised ESR had lower serotonin (n = 21, median 73 ng/ml) compared to patients with normal ESR (n = 60, median 122 ng/ml), but not significant p = 0.055. Our serotonin analysis detects total serum content, including a potential proportion from platelets. Therefore, we investigated the association between serum serotonin and platelet count (rho = 0.15, p = 0.18). A total of 11 patients have elevated platelet count (i.e., above the reference range), but no significant difference regarding serotonin was seen when comparing patients with high (median 142 ng/ml) and normal (median 109 ng/ml) platelet count, p = 0.08.
No correlations were found between serotonin levels and CRP over time among at-risk individuals; baseline CRP (rho = 0.10, p = 0.38), after 3 months (rho = −0.15, p = 0.20) or after 12 months (rho = 0.14, p = 0.38). Analyzing ESR and serotonin over time revealed a significant correlation after 3 months (rho = −0.23, p = 0.04). Otherwise, no correlations were found; baseline ESR (rho = −0.13, p = 0.25) and after 12 months (rho = −0.12, p = 0.48).
In early RA, no correlations were found between baseline serotonin and disease activity (DAS 28) over time; Baseline DAS 28 (rho = −0.016, p = 0.75), after 3 months (rho = 0.008, p = 0.89) or after 36 months (rho = −0.002, p = 0.97). Serotonin did not correlate with baseline ESR (rho = 0.009, p = 0.86) or CRP (rho = −0.010, p = 0.84). There was no correlation between serotonin and baseline erosion defined by the Larsen score (rho = −0.018, p = 0.72) or after 36 months (rho = 0.077, p = 0.23). No differences in serotonin were seen in early RA patients positive vs. negative for ACPA (median 140 vs. 128 ng/ml, p = 0.33).
At risk-individuals prescribed non-steroidal anti-inflammatory drugs (NSAIDs) at their baseline visit (n = 31) had slightly higher baseline serotonin levels (median 127 ng/ml) compared to those who were not prescribed NSAIDs (n = 51, median 97 ng/ml), but this was not statistically significant (p = 0.059). However, serotonin levels at 3 months among individuals with NSAID prescribed at their baseline visit were higher compared to those without prescription (152 ng/ml vs. 105 ng/ml, p = 0.012).
In the early RA cohort (143 prescribed vs. 264 not prescribed at baseline), this difference was borderline significant (median 145 ng/ml vs. 130 ng/ml, p = 0.05). When combining both cohorts, patients who were not prescribed NSAIDs still had significantly higher baseline serotonin levels compared to controls (median 127 ng/ml vs. 65 ng/ml, p < 0.0001).
No correlations were found between baseline serotonin levels and visual analog pain scales (VAS Pain) at baseline (rho = 0.039, p = 0.44), after 3 months (rho = −0.037, p = 0.49) or after 36 months (rho = 0.071, p = 0.20) in early RA. In at-risk individuals, we only had access to patient global assessment VAS, which did not associate with baseline serotonin levels (rho = 0.073, p = 0.52).
In this study on carefully characterized patients in different phases of RA, we show for the first time that serum serotonin levels are increased already prior to the onset of RA. High serotonin levels have previously been reported in established RA (6–8), and now we extend this knowledge by showing that serotonin is elevated also in recent-onset RA. Interestingly, patients with early RA and at-risk individuals showed similar concentrations, indicating that serotonin may be involved in early triggering events rather than effector mechanisms. Platelets from patients with RA are shown to be more active compared to controls, and may be activated by ACPAs (14). However, no differences in serotonin levels were seen in early RA patients positive vs. negative for ACPA, and hence ACPA-mediated activation of platelets do not seem to be the cause of increased serum serotonin in the present study. Moreover, an inverse relationship between intra-cellular platelet serotonin levels and clinical disease activity has been observed in RA (15), and elevated platelet count during inflammation is a well-known phenomenon (16). We found no correlation between serotonin and platelet count, and patients with a platelet count above the reference range did not display elevated serotonin. Consequently, the raised serotonin is unlikely to be explained by an increased platelet number.
Centrally produced serotonin have been studied in many pain-related disorders, but the peripheral actions from serotonin differ from the central actions. Serotonin released from platelets and mast cells following injury and inflammation in the periphery can intensify pain by sensitization of nerve fibers (17, 18), and high serum concentrations have been associated with temporomandibular joint pain in RA (7). The elevated levels in early RA patients showed no association with the degree of pain.
Serotonin has been linked to bone loss. Bernardes et al. (19) could not, in contrast to us, find any differences in serum serotonin levels between patients and controls, but instead reported an inverse weak association with femur bone density in postmenopausal RA women. An inverse relation between serum serotonin and erosion in the temporomandibular joints has also been reported (9), as well as the use of serum serotonin as a prognostic marker of radiologic outcome in RA (20). We could not confirm any association between serotonin and radiographic joint damage, in early RA.
Genetic polymorphisms within the serotonin receptor HTR2A gene have been shown to associate with RA susceptibility (21, 22). Moreover, the density of serotonin 5-HT2A receptors is decreased in RA patients and correlated with a more severe disease (23), suggesting possible links between the serotonergic system and development of the disease. Possibly, decreased receptor expression could lead to an increased proportion of free serotonin, and should be addressed in upcoming studies.
There is a possibility that pharmacotherapy influences serotonin levels. At-risk individuals prescribed NSAIDs at their first visit had higher serotonin levels compared to those without such prescription, but this was not statistically significant. Unfortunately, we lack data on the patients’ use of NSAID without prescription before their first visit. However, the serotonin levels after 3 months were higher among patients with baseline prescription, possibly affecting future levels. NSAID have no impact on platelet count but impairs platelet degranulation and activation (24). Serotonin is synthesized from tryptophan, which may also be metabolized by the enzyme indoleamine 2,3-dioxygenase (IDO) into kynurenine. NSAID may decrease IDO, thereby theoretically increase the availability of tryptophan for serotonin production (25). Although NSAID supposedly could increase serotonin levels, patients without NSAID prescribed still had considerable higher levels compared to healthy controls. Nevertheless, the absence of pre-screening NSAID data is a limitation of our study, in addition to the lack of controls with other inflammatory or non-inflammatory conditions. Further, the at-risk cohort size did not allow subgrouping of patients.
In conclusion, serotonin serum levels are increased both at and prior to onset of RA but is not prognostic for arthritis development or disease activity over time. Although we find no clinical value of serum serotonin analysis in patients at risk or with early RA, the elevated levels call for future studies to elucidate the role of the serotonin system in RA development.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the EPN Linköping. The patients/participants provided their written informed consent to participate in this study.
LW: designing of the project, acquisition and analyses of patient data, interpretation of results, and writing of the manuscript. KM and AK: interpretation of results and writing of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Royal Swedish Academy of Sciences, the Swedish Rheumatism Association, King Gustaf V’s 80-year Foundation, Professor Nanna Svartz Foundation, Lars Hierta Memorial Foundation, ALF Grants, Region Östergötland and Magnus Bergvall Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rantapaa-Dahlqvist S, de Jong B, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. (2003). 48:2741–9. doi: 10.1002/art.11223
2. Ziegelasch M, Eloff E, Hammer H, Cedergren J, Martinsson K, Reckner A, et al. Bone erosions detected by ultrasound are prognostic for clinical arthritis development in patients with ACPA and musculoskeletal pain. Front Med. (2021) 8:653994. doi: 10.3389/fmed.2021.653994
3. Rakieh C, Nam J, Hunt L, Hensor E, Das S, Bissell L, et al. Predicting the development of clinical arthritis in anti-CCP positive individuals with non-specific musculoskeletal symptoms: a prospective observational cohort study. Ann Rheum Dis. (2015) 74:1659–66. doi: 10.1136/annrheumdis-2014-205227
4. Catrina A, Deane K, Scher J. Gene, environment, microbiome and mucosal immune tolerance in rheumatoid arthritis. Rheumatology (Oxford). (2016) 55:391–402.
5. Boilard E, Blanco P, Nigrovic P. Platelets: active players in the pathogenesis of arthritis and SLE. Nat Rev Rheumatol. (2012) 8:534–42. doi: 10.1038/nrrheum.2012.118
6. Klavdianou K, Liossis S, Papachristou D, Theocharis G, Sirinian C, Kottorou A, et al. Decreased serotonin levels and serotonin-mediated osteoblastic inhibitory signaling in patients with ankylosing spondylitis. J Bone Miner Res. (2016) 31:630–9. doi: 10.1002/jbmr.2724
7. Kopp S, Alstergren P. Blood serotonin and joint pain in seropositive versus seronegative rheumatoid arthritis. Mediators Inflamm. (2002) 11:211–7. doi: 10.1080/09629350290000069
8. Ragab OK, Taha R, Iskander M. Serum serotonin in rheumatoid arthritis patients: relation to rheumatoid factor positivity, clinical manifestations and fibromyalgia. Egypt Rheumatol. (2018) 40:149–53. doi: 10.1016/j.ejr.2017.09.002
9. Voog U, Alstergren P, Eliasson S, Leibur E, Kallikorm R, Kopp S. Progression of radiographic changes in the temporomandibular joints of patients with rheumatoid arthritis in relation to inflammatory markers and mediators in the blood. Acta Odontol Scand. (2004) 62:7–13. doi: 10.1080/00016350310007860
10. Eloff E, Martinsson K, Ziegelasch M, Cedergren J, Reckner A, Skogh T, et al. Autoantibodies are major predictors of arthritis development in patients with anti-citrullinated protein antibodies and musculoskeletal pain. Scand J Rheumatol. (2021) 50:189–97. doi: 10.1080/03009742.2020.1818820
11. Ziegelasch M, Boman A, Martinsson K, Thyberg I, Jacobs C, Nyhall-Wahlin B, et al. Anti-cyclic citrullinated peptide antibodies are associated with radiographic damage but not disease activity in early rheumatoid arthritis diagnosed in 2006-2011. Scand J Rheumatol. (2020) 49:434–42. doi: 10.1080/03009742.2020.1771761
12. Arnett F, Edworthy S, Bloch D, McShane D, Fries J, Cooper N, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. (1988) 31:315–24. doi: 10.1002/art.1780310302
13. Larsen A. How to apply Larsen score in evaluating radiographs of rheumatoid arthritis in long-term studies. J Rheumatol. (1995) 22:1974–5.
14. Habets K, Trouw L, Levarht E, Korporaal S, Habets P, de Groot P, et al. Anti-citrullinated protein antibodies contribute to platelet activation in rheumatoid arthritis. Arthritis Res Ther. (2015) 17:209. doi: 10.1186/s13075-015-0665-7
15. Zeller J, Weissbarth E, Baruth B, Mielke H, Deicher H. Serotonin content of platelets in inflammatory rheumatic diseases. Correlation with clinical activity. Arthritis Rheum. (1983) 26:532–40. doi: 10.1002/art.1780260413
16. Olumuyiwa-Akeredolu O, Page M, Soma P, Pretorius E. Platelets: emerging facilitators of cellular crosstalk in rheumatoid arthritis. Nat Rev Rheumatol. (2019) 15:237–48. doi: 10.1038/s41584-019-0187-9
17. Sommer C. Serotonin in pain and analgesia: actions in the periphery. Mol Neurobiol. (2004) 30:117–25. doi: 10.1385/MN:30:2:117
18. Hu W, Zhang Y, Cai Q, Wang D, Hong Y. Blockade of 5-HT2A receptors at the site of inflammation inhibits activation of spinal dorsal horn neurons in rats. Brain Res Bull. (2016) 124:85–94. doi: 10.1016/j.brainresbull.2016.03.018
19. Bernardes M, Vieira T, Lucas R, Pereira J, Costa L, Simoes-Ventura F, et al. Serum serotonin levels and bone in rheumatoid arthritis patients. Rheumatol Int. (2017) 37:1891–8. doi: 10.1007/s00296-017-3836-9
20. Pongratz G, Lowin T, Sewerin P, Zaucke F, Jenei-Lanzl Z, Pauly T, et al. Tryptophan metabolism in rheumatoid arthritis is associated with rheumatoid factor and predicts joint pathology evaluated by the rheumatoid arthritis MRI score (RAMRIS). Clin Exp Rheumatol. (2019) 37:450–7.
21. Kling A, Seddighzadeh M, Arlestig L, Alfredsson L, Rantapaa-Dahlqvist S, Padyukov L. Genetic variations in the serotonin 5-HT2A receptor gene (HTR2A) are associated with rheumatoid arthritis. Ann Rheum Dis. (2008) 67:1111–5. doi: 10.1136/ard.2007.074948
22. Snir O, Hesselberg E, Amoudruz P, Klareskog L, Zarea-Ganji I, Catrina A, et al. Genetic variation in the serotonin receptor gene affects immune responses in rheumatoid arthritis. Genes Immun. (2013) 14:83–9. doi: 10.1038/gene.2012.56
23. Kling A, Rantapaa-Dahlqvist S, Stenlund H, Mjorndal T. Decreased density of serotonin 5-HT2A receptors in rheumatoid arthritis. Ann Rheum Dis. (2006) 65:816–9. doi: 10.1136/ard.2005.042473
24. Schippinger G, Pruller F, Divjak M, Mahla E, Fankhauser F, Rackemann S, et al. Autologous platelet-rich plasma preparations: influence of nonsteroidal anti-inflammatory drugs on platelet function. Orthop J Sports Med. (2015) 3:2325967115588896. doi: 10.1177/2325967115588896
Keywords: serotonin, rheumatoid arthritis, at-risk patients, early RA, biomarkers
Citation: Wirestam L, Martinsson K and Kastbom A (2023) Serum serotonin levels are elevated in patients with increased risk of rheumatoid arthritis. Front. Med. 9:1081814. doi: 10.3389/fmed.2022.1081814
Received: 27 October 2022; Accepted: 12 December 2022;
Published: 04 January 2023.
Edited by:
Yi Zhao, Sichuan University, ChinaReviewed by:
Prasanta Padhan, Kalinga Institute of Medical Sciences (KIMS), IndiaCopyright © 2023 Wirestam, Martinsson and Kastbom. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lina Wirestam, ✉ bGluYS53aXJlc3RhbUBsaXUuc2U=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.