- 1Department of Emergency, Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 2Shanghai Key Laboratory of Hypertension, Department of Hypertension, Ruijin Hospital, Shanghai Institute of Hypertension, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Anesthesiology and Intensive Care, Linze County People’s Hospital, Zhangye, Gansu, China
- 4Department of Anesthesiology, Maternity and Children Hospital, Linxia, Gansu, China
Background: Early recognition of the risk factors is important for acute pancreatitis management. The aim of this study is to investigate the relationship between platelet count and clinical outcomes in patients with acute pancreatitis.
Methods: The data are collected from a university-affiliated hospital between January 2013 and December 2020. A generalized additive model and a two-piecewise linear regression model are used to estimate the association between platelet count and the risks of intra-abdominal infection, surgical intervention, in-hospital mortality, and length of hospital stay.
Results: Among the 1,363 patients, 99 (7.3%) patients suffered intra-abdominal infection, 190 (13.9%) patients underwent surgical intervention, and 38 (2.8%) patients died in the hospital. The median length of hospital stay is 21 days. Generalized additive model and two-piecewise linear regression analysis show that the risk of intra-abdominal infection decreases as the platelet count increases to 160 × 109/L (OR: 0.991, 95% CI: 0.984–0.998, p = 0.015) and then increases as the platelet count levels up (OR: 1.007, 95% CI: 1.004–1.010, p < 0.001). The trend is similar to the risk of surgical intervention and length of hospital stay. Even though there seems a declining trend in mortality, no significant association is found after adjustment for potential confounders. Further analysis shows that changes in platelet count within the first 3 days after admission have no obvious association with clinical outcomes.
Conclusion: A platelet count of approximately 160 × 109/L on admission is associated with the lowest risk of intra-abdominal infection, surgical intervention, and shortest hospital stay in patients with acute pancreatitis.
Introduction
Acute pancreatitis (AP) is a common gastrointestinal disease with an annual incidence of 34 per 100,000 person-years (1, 2). Activation of the pancreatic enzymes within the injured pancreatic acini is the most widely accepted pathophysiological theory. After the onset of pancreatitis at the early stage, uncontrolled oxidative stress and large amounts of released inflammatory cytokines induce systemic inflammatory response syndrome (SIRS) and contribute to progressive organ dysfunction (3). Most patients present with a mild clinical course, but organ dysfunction progresses in approximately 20% of the patients. The overall mortality rate remained as high as 10–20% in these patients (4).
Microcirculatory dysfunction plays an important role in the pathogenesis of AP. Uncontrolled oxidative stress and released inflammatory cytokines lead to endothelial injury and the activation of coagulation (5). Abnormal coagulation is manifested by vascular leakage, scattered intravascular thrombosis, and disseminated intravascular coagulation and is associated with increased complications and mortality in AP (6). In general, the platelet is defined as a pivotal factor in homeostasis and thrombosis. It also has been recognized as a member of the immune system and plays a role in inflammation response. Abnormal platelet features are common clinical manifestations associated with poor outcomes in patients who are critically ill (7, 8). However, the role of platelet count in AP pathophysiology has not been elucidated. Here, a retrospective hospital-based cohort study is conducted in patients with AP to evaluate the association between platelet count and clinical outcomes, especially the risk of intra-abdominal infection.
Methods and analysis
Participants
This retrospective study was approved by the institutional ethics board of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, and the informed consent was waived. Data analysis was performed on the 1964 Helsinki Declaration and its later amendments.
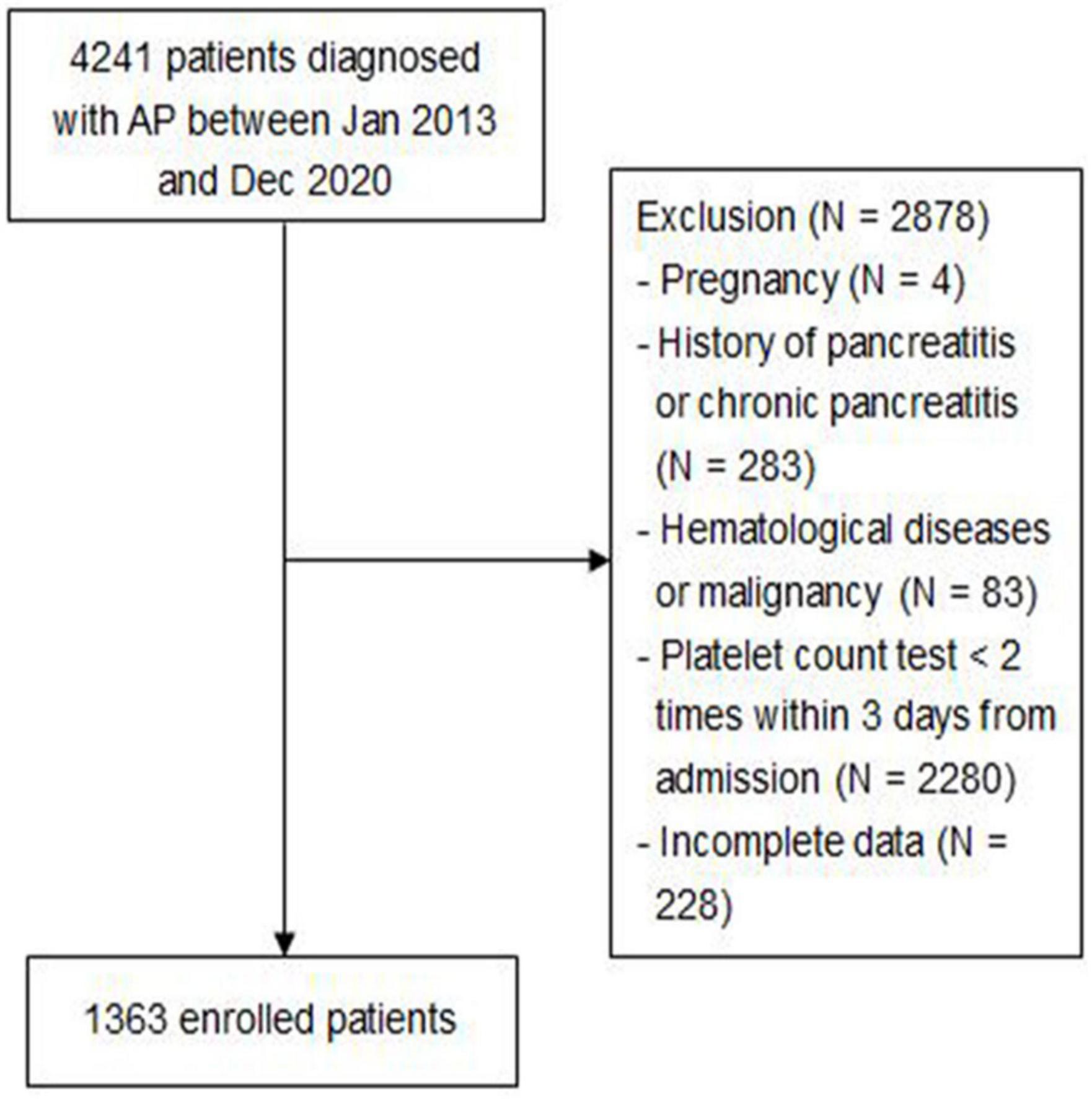
Consecutive adult patients (age ≥ 18 years) discharged with a diagnosis of acute pancreatitis according to the revised 2012 Atlanta guideline (9), who were admitted to the Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, between January 2013 and December 2020, were enrolled in this study. Patient exclusion criteria included the following: (1) pregnancy; (2) history of pancreatitis or chronic pancreatitis; (3) hematological diseases or malignancy; (4) platelet count test <2 times within 3 days from admission; and (5) incomplete data. All enrolled patients were followed up until discharge or death.
Data collection
The clinical variables are extracted from the electronic database for each patient. Baseline demographic information includes age, gender, body mass index, comorbidities (hypertension, diabetes, cerebrovascular diseases, cardiovascular diseases, chronic kidney diseases, and chronic obstructive pulmonary diseases), etiologies (biliary, hypertriglyceridemia, alcoholic, or others), and time interval from onset to admission. Laboratory indicators including blood amylase, white blood cell count, hemoglobin, fibrinogen, D-dimers, lactate, C-reactive protein, procalcitonin, alanine aminotransferase, alkaline phosphatase, pre-albumin, albumin, total bilirubin, creatinine, and Acute Physiology and Chronic Health Evaluation II scores (APACHE II) are collected within 24 h after admission. Platelet counts are collected within the first 3 days after admission. Thrombocytopenia is defined as a minimal platelet count of <100 × 109/L within the first 3 days (10). Severities are classified as mild acute pancreatitis (MAP), moderately severe acute pancreatitis (MSAP), and severe acute pancreatitis (SAP) according to the revised 2012 Atlanta guideline (9). Clinical outcomes include the incidence of intra-abdominal infection, surgical intervention (percutaneous drainage and open abdominal debridement), in-hospital mortality, and length of hospital stay.
Data statistics
Categorical data will be described with frequency or ratio. Continuous variables will be described using the median and interquartile range (IQR). Categorical variables will be compared using the chi-square test or Fisher exact test. Continuous variables will be compared using the t-test for normally distributed variables or Wilcoxon rank-sum test for non-normally distributed variables. The association between platelet count and mortality is estimated by logistic regression models, and the following confounders are adjusted: age, sex, body mass index, comorbidities, and etiologies. In addition, creatinine represents a renal function, C-reactive protein represents inflammation, procalcitonin represents infection, lactate represents oxygen debt, D-dimers represent hemostasis during inflammation, and the APACHE II score represents baseline conditions. These indexes are considered prognostic factors in previous studies (11, 12). Therefore, we also consider these indexes included in the regression models. The smoothing spline is plotted by utilizing a generalized additive model. A two-piecewise linear regression model is applied to examine the threshold effect of the platelet count on intra-abdominal infection and surgical intervention. All statistical analyses are performed using R software (version 4.2.1). A two-sided significance level of less than 0.05 is defined as statistical significance.
Results
Patient characteristics and laboratory findings
A total of 4,241 patients diagnosed with AP between January 2013 and December 2020 were included, of which 2,878 patients were excluded based on exclusion criteria. Figure 1 shows the screening flowchart.
Among 1,363 enrolled patients, the median age is 48 years and 63.7% are male patients. The median time interval from onset to admission is 3 days (range 1–5 days). Patients with thrombocytopenia have a higher APACHE II score and are more likely to develop SAP. With a median of 21 days of hospital stay (range 11–36 days), 99 (7.3%) patients suffered intra-abdominal infection, 190 (13.9%) patients underwent surgical intervention, and 38 (2.8%) patients died in the hospital. Remarkable laboratory differences are also found in the levels of fibrinogen, D-dimers, lactate, procalcitonin, alanine aminotransferase, albumin, and creatinine (Table 1).
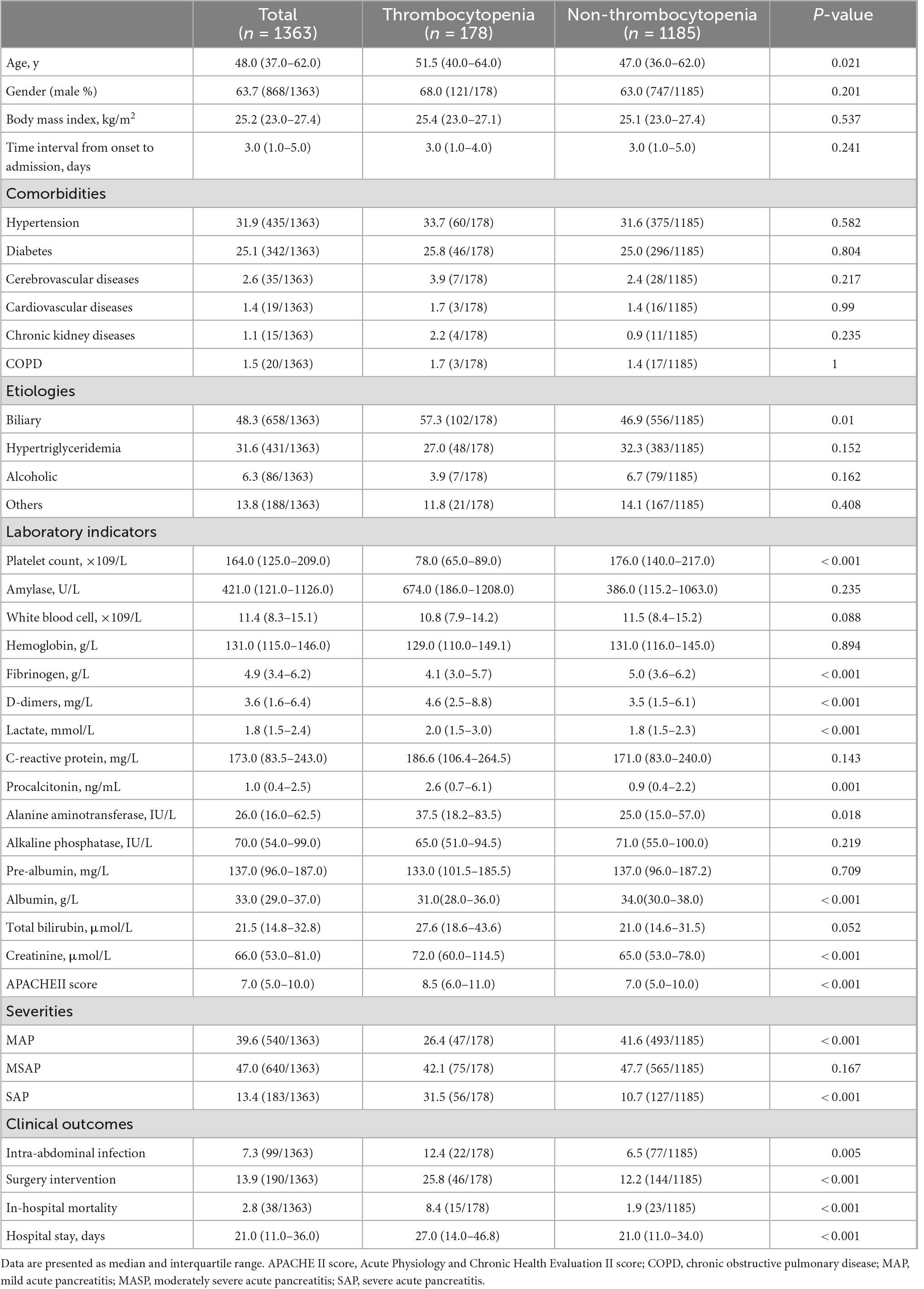
Table 1. Baseline characteristics of acute pancreatitis patients with thrombocytopenia and without thrombocytopenia.
Association between platelet count and clinical outcomes
Logistic regression models are adjusted for several risk factors at admission, including age, sex, body mass index, comorbidities, etiologies, creatinine, C-reactive protein, procalcitonin, lactate, D-dimers, and APACHE II score. The results reveal after adjustment for potential confounders, the incidence of intra-abdominal infection is significantly lower in platelet count between 125 × 109/L and 209 × 109/L (4.1 and 3.5%) but significantly higher in platelet count lower than 125 × 109/L (10.3%) or higher than 209 × 109/L (11.1%). Similarly, the incidence of surgical intervention is significantly lower in platelet count between 125 × 109/L and 209 × 109/L (8.9 and 6.4%) and significantly higher in platelet count lower than 125 × 109/L (22%) or higher than 209 × 109/L (18.4%) (Table 2). However, after adjustment for potential confounders, platelet count has no significant effect on in-hospital mortality in each group.
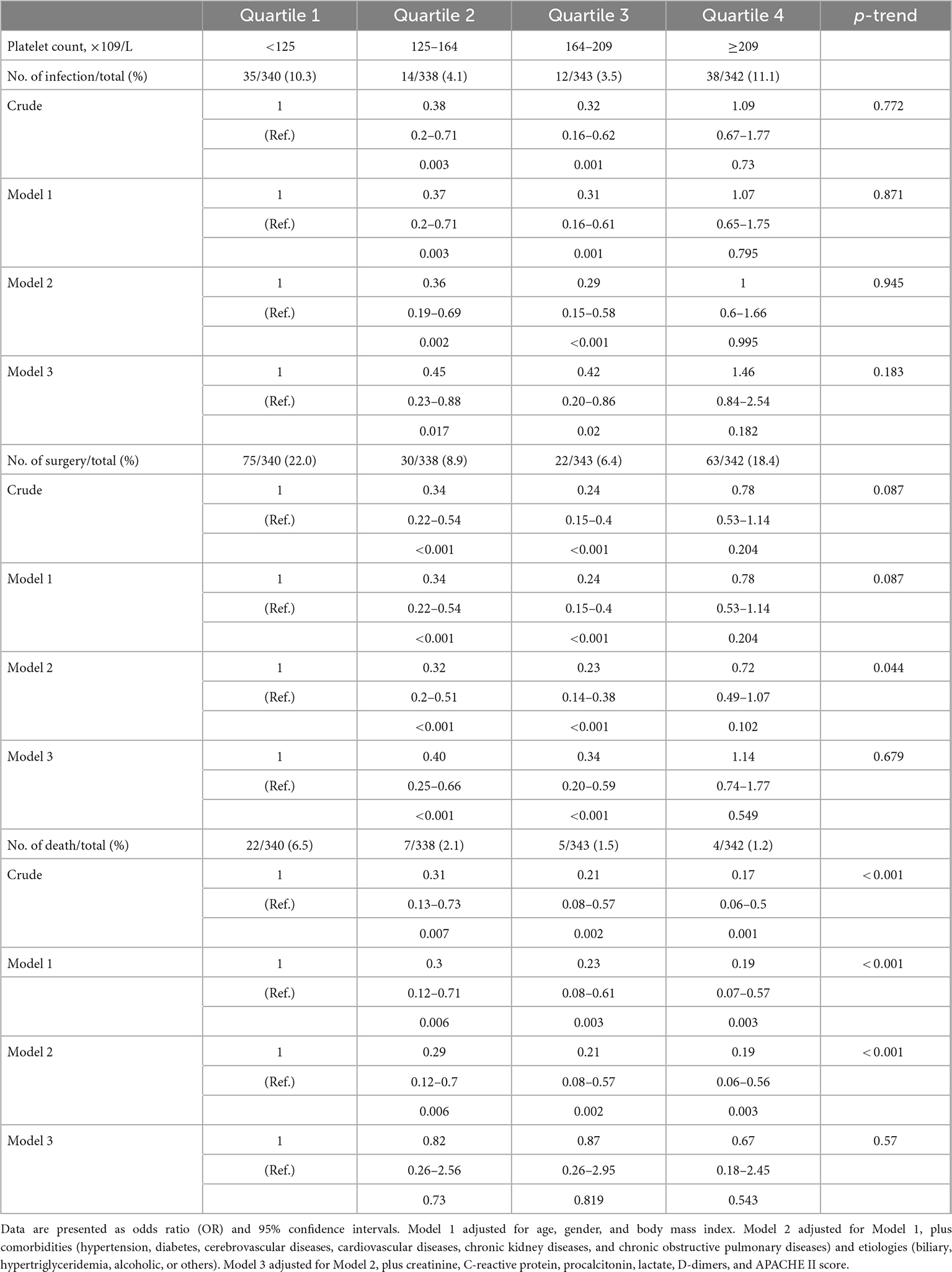
Table 2. Association between platelet count and risk of intra-abdominal infection, surgical intervention, and in-hospital mortality estimated using logistic regression models.
The smoothing spline curve shows the non-linear relationship among the intra-abdominal infection rate (Figure 2A), surgical intervention rate (Figure 2B), and platelet count. In the threshold effect analysis, the risk of intra-abdominal infection decreases as the platelet count levels up to the inflection point (160 × 109/L) (OR: 0.991, 95% CI: 0.984–0.998, p = 0.015). When the platelet count is 160 × 109/L, the risk of intra-abdominal infection increases as the platelet count levels up (OR: 1.007, 95% CI: 1.004–1.010, p < 0.001). Similarly, the risk of surgical intervention decreases as the platelet count levels up to the inflection point (169 × 109/L) (OR: 0.987, 95% CI: 0.982–0.992, p < 0.001). When the platelet count is 169 × 109/L, the risk of surgical intervention increases as the platelet count levels up (OR: 1.010, 95% CI: 1.007–1.013, p < 0.001) (Table 3). The relationship (Supplementary Figure 1) between a hospital stay and platelet count is similar to the risk of intra-abdominal infection and surgical intervention. The inflection point of platelet count is 169 × 109/L.
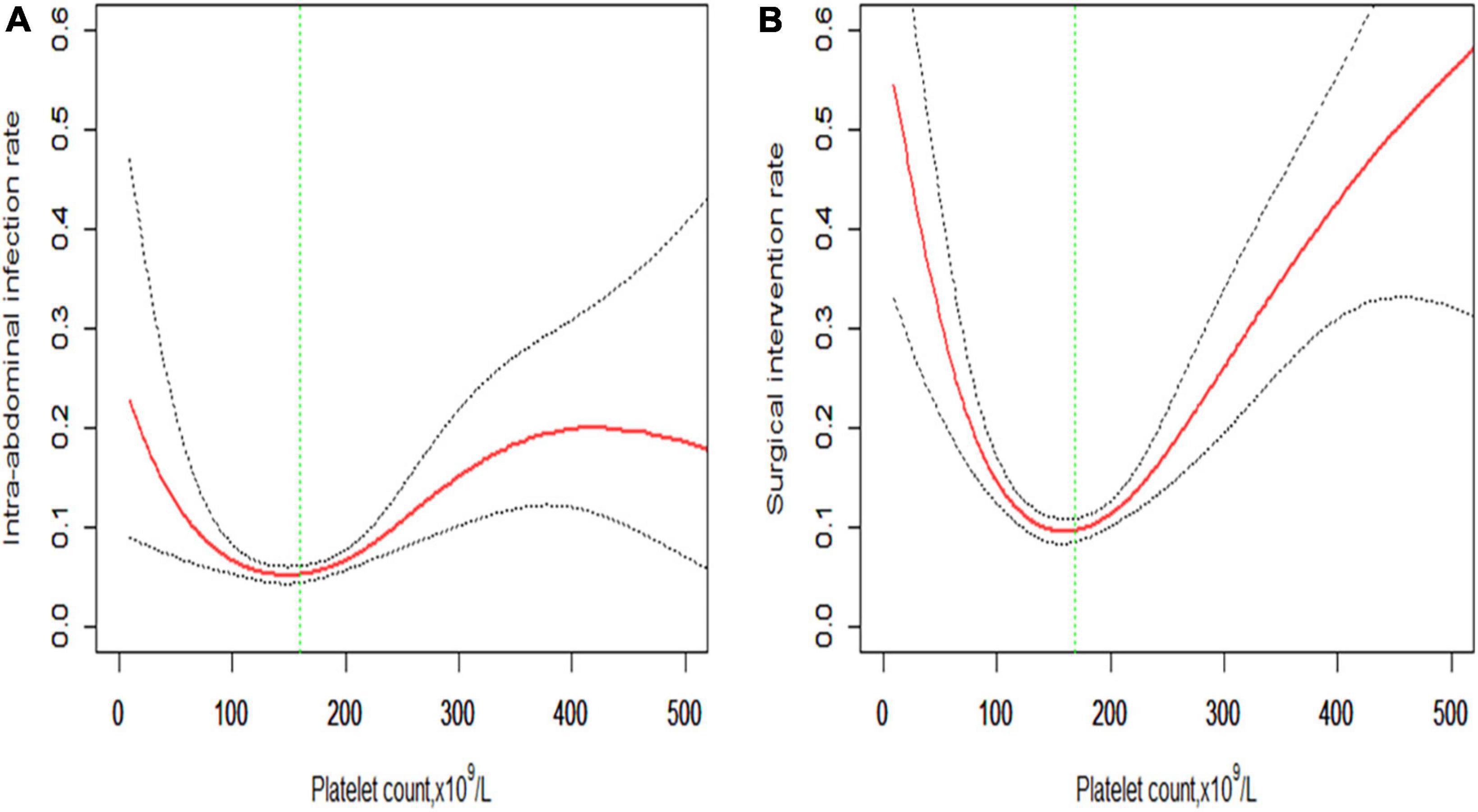
Figure 2. Non-linear relationship of (A) intra-abdominal infection rate and (B) surgical intervention rate with platelet count. The smoothing splines are generated utilizing a generalized additive model adjusted for age, gender, body mass index, comorbidities (hypertension, diabetes, cerebrovascular diseases, cardiovascular diseases, chronic kidney diseases, and chronic obstructive pulmonary diseases), etiologies (biliary, hypertriglyceridemia, alcoholic, or others), creatinine, C-reactive protein, procalcitonin, lactate, D-dimers, and APACHE II score. The red line indicates the estimated risk rate, the gray dot line indicates 95% confidence intervals, and the vertical green dot line indicates the inflection point.
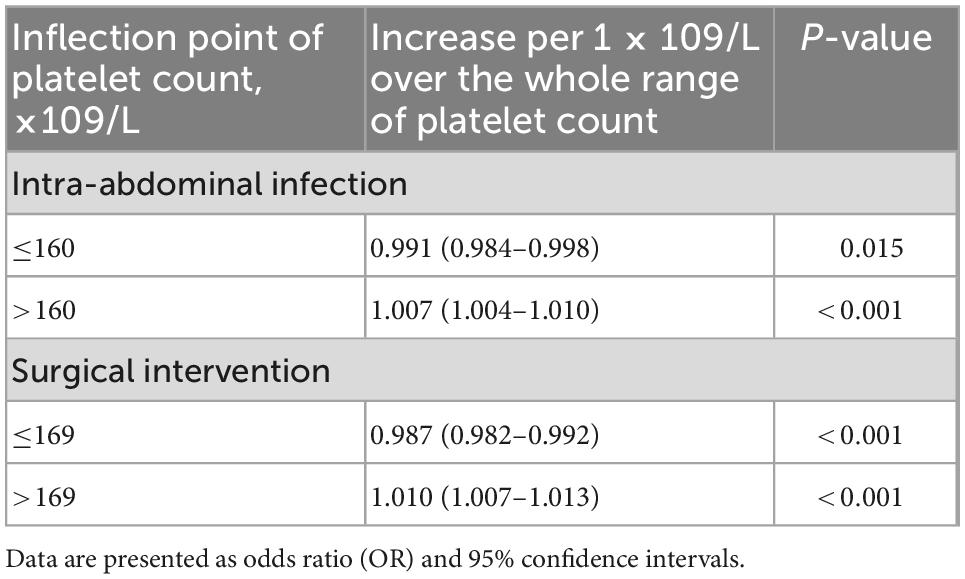
Table 3. Threshold effect analysis of the platelet count on intra-abdominal infection and surgical intervention.
Further analysis of the changes in platelet count within the first 3 days after admission reveals that those changes have no obvious association with intra-abdominal infection, surgical intervention, and in-hospital mortality (Table 4).
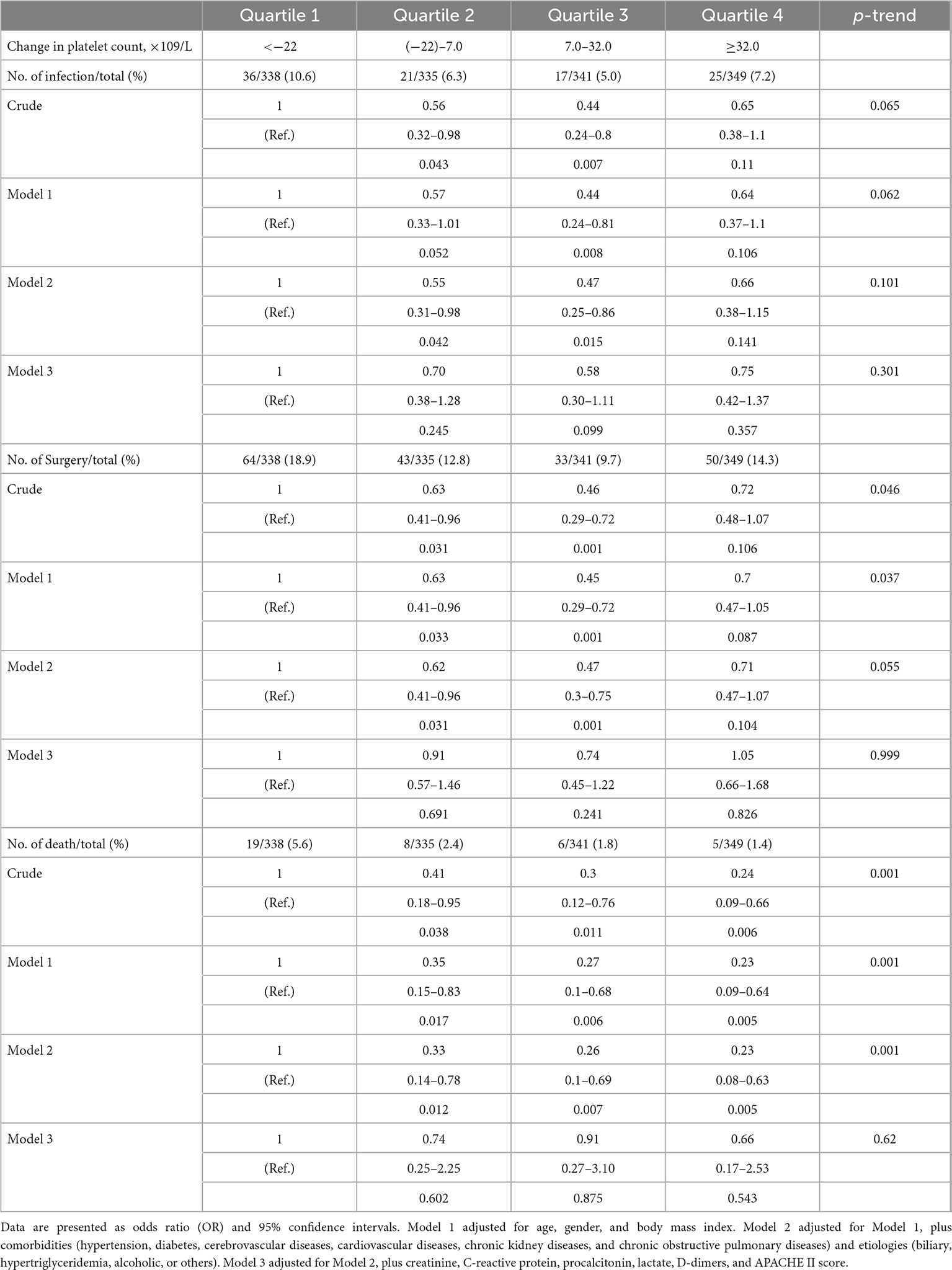
Table 4. Association between the changes in platelet count within the first 3 days after admission and risk of intra-abdominal infection, surgical intervention, and in-hospital mortality estimated using logistic regression models.
Discussion
Intra-abdominal infection is a detrimental factor of mortality in patients with acute pancreatitis (13). In the present study, we analyze the platelet count and changes in platelet count within 3 days after admission with the risk of intra-abdominal infection, surgical intervention, in-hospital mortality, and hospital stay. Logistic regression analysis and a generalized additive model are used and adjusted for several potential risk factors at admission. Our study highlights that, in patients with AP, (1) patients with platelet count on admission less than 125 × 109/L or higher than 209 × 109/L are more likely to suffer intra-abdominal infection, receive surgical intervention, and stay longer in the hospital; and (2) changes in platelet count within 3 days after admission have no significant association with intra-abdominal infection, surgical intervention, and in-hospital mortality.
Thrombocytopenia is recognized as an independent risk factor for prognosis. The estimated incidence in critically ill patients is 20–40% during hospitalization (7, 14). Thus, no study reported the incidence of thrombocytopenia in patients with AP. Among the enrolled 1,363 patients in our study, thrombocytopenia occurs in 178 (13%) patients within 3 days after admission. Patients with thrombocytopenia are more likely to deteriorate into SAP compared to patients with non-thrombocytopenia (31.5% vs. 10.7%). Intra-abdominal infection is one of the leading complications that can cause life-threatening organ dysfunction. Early identification of high-risk factors can save medical resources and potentially improve patient outcomes. In previous studies, procalcitonin is the most concerned predictor of intra-abdominal infection (15, 16). However, the small sample size (n < 200) in these studies limited the reliability. Moreover, procalcitonin is not a routine test in all hospitalized patients with AP. Recently, lactate is proven as a reliable predictor of intra-abdominal infection in Shu’s study (17). Even though the sample size is large enough (n = 503), the study design is not comprehensive because only patients with MSAP are included in this study. Prediction models are also constructed to screen the risk factors of intra-abdominal infection. In two multi-center retrospective studies, the platelet count is selected as a risk factor but not included in the final calculated models (18, 19). The reason may be that the linear models are used to select the risk factors. However, the relationship between platelet count and the risk of intra-abdominal infection is a non-linear pattern. In our study, the risk of intra-abdominal infection decreases as the platelet count levels up to 160 × 109/L and then increases. The non-linear relationship is still significant after the adjustment of potential confounders, including age, gender, body mass index, comorbidities, etiologies, creatinine, C-reactive protein, procalcitonin, lactate, D-dimers, and APACHE II score. A two-piecewise linear regression model shows that the risk of intra-abdominal infection decreases by 0.9% per 1 × 109/L increase in platelet when the platelet count is <160 × 109/L, and increases by 0.7% per 1 × 109/L increase in platelet when the platelet count is >160 × 109/L. Interestingly, even the in-hospital mortality shows a declining trend in each quartile separated according to the platelet count (6.5, 2.1, 1.5, and 1.2%, respectively), the platelet count is not significantly associated with in-hospital mortality in patients with AP after adjustment for the confounders. The reason may be that the event (death) is relatively few in our study and, thus, the model is overfitted. In our study, we further analyze the dynamic changes in platelet count within the first 3 days after admission. No significant results are observed. In summary, the platelet count is a reliable and convenient factor for predicting intra-abdominal infection in patients with AP.
The mechanism of platelet count on intra-abdominal infection is poorly understood, but several explanations could be considered. The inflammatory process results in disturbances in pancreatic microcirculation, which has been recognized as an important factor in the development of acute pancreatitis (20). The ultra-structural changes in acute pancreatitis, including the infiltration of leukocytes, the accumulation of platelets intravascularly and extravascularly, and the formation of microthrombi, could be observed (21). However, the linear relationship between platelet count and some inflammatory indicators (C-reactive protein, white blood cell count, and procalcitonin) (Supplementary Figure 2) in this study could not provide a convincing explanation for the results. Another explanation is that the pancreatic vascular endothelial damage will activate the coagulation cascades that increase the consumption of platelet, which will exacerbate the pancreatic necrosis and eventually pancreas become infected. Interestingly, in this study, with the change in platelet count, the change trends of fibrinogen and D-dimers are consistent with the results (Supplementary Figure 3). It could be speculated that complex derangements of hemostasis occurred during acute pancreatitis. As a whole-body inflammatory disease, the damage of endothelial is not limited to the pancreatic tissues in severe pancreatitis. The multi-organ damage will further increase the activation of the platelet. The intestinal tract is the largest microorganism reservoir of the human body and could be easily damaged during systemic inflammation response. During the period of acute pancreatitis, the integrity of intestinal mucosa could be destroyed, which will also increase vascular permeability. Therefore, bacterial translocation could be easy. The decrease or increase in the platelet number may be the reflex of endothelial damage, which is observed in a previous study on sepsis (22). It is interesting that even though the risk of intra-abdominal infection is comparable between the low-level platelet count group (<125 × 109/L) and high-level platelet count group (>209 × 109/L) (10.3% vs. 11.1%) in our study, mortality is relatively low in high-level platelet count group (6.5% vs. 1.2%). The reason may be that a high platelet count reflects the better hematopoietic function of the body. Moreover, platelet possesses immune properties and can inhibit the spread of infection (23).
Our study is subjected to some limitations. First, as an observational study, it could not conclude that the associations between platelet count and clinical outcomes are causal. Second, the data were collected from one center, and the validation should be verified in other data sources. Third, the death event is relatively few in our study, and thus, the model may be overfitted. The association between platelet count and in-hospital mortality should be checked in a large number of patient studies.
Conclusion
This retrospective cohort study among 1,363 patients with AP reveals that a platelet count of approximately 160 × 109/L on admission is associated with the lowest risk of intra-abdominal infection, surgical intervention, and shortest hospital stay in patients with acute pancreatitis. Moderate is the best.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Ethics Board of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The Ethics Committee waived the requirement of written informed consent for participation.
Author contributions
JH and EM conceived the study. TN, YW, GM, WY, ZY, BZ, and YC helped in data collection. TN, BZ, HS, and LM helped in manuscript revision. WS, JH, TN, and YW were the co-first authors responsible for drafting and data analysis. EM was the responsible author. All authors contributed to the refinement of the study protocol. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1077076/full#supplementary-material
Supplementary Figure 1 | Non-linear relationship of hospital stay with platelet count. The smoothing splines are generated utilizing a generalized additive model adjusted for age, gender, body mass index, comorbidities (hypertension, diabetes, cerebrovascular diseases, cardiovascular diseases, chronic kidney diseases, and chronic obstructive pulmonary diseases), etiologies (biliary, hypertriglyceridemia, alcoholic, or others), creatinine, C-reactive protein, procalcitonin, lactate, D-dimers, and APACHE II score. The red line indicates the estimated hospital stay, the gray dot line indicates 95% confidence intervals, and the vertical green dot line indicates the inflection point.
Supplementary Figure 2 | Smoothing spline presented the association between platelet count and (A) C-reactive protein; (B) white blood cell count; and (C) procalcitonin. Plots were generated by utilizing the generalized additive model.
Supplementary Figure 3 | The smoothing spline presented the association between platelet count and (A) fibrinogen; (B) D-dimers. Plots were generated by utilizing the generalized additive model.
References
1. Boxhoorn L, Voermans R, Bouwense S, Bruno M, Verdonk R, Boermeester M, et al. Acute pancreatitis. Lancet. (2020) 396:726–34. doi: 10.1016/s0140-6736(20)31310-6
2. Xiao A, Tan M, Wu L, Asrani V, Windsor J, Yadav D, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. (2016) 1:45–55. doi: 10.1016/s2468-1253(16)30004-8
3. Minkov G, Halacheva K, Yovtchev Y, Gulubova M. Pathophysiological mechanisms of acute pancreatitis define inflammatory markers of clinical prognosis. Pancreas. (2015) 44:713–7. doi: 10.1097/MPA.0000000000000329
4. Yasuda H, Horibe M, Sanui M, Sasaki M, Suzuki N, Sawano H, et al. Etiology and mortality in severe acute pancreatitis: a multicenter study in Japan. Pancreatology. (2020) 20:307–17. doi: 10.1016/j.pan.2020.03.001
5. Foitzik T, Eibl G, Hotz B, Hotz H, Kahrau S, Kasten C, et al. Persistent multiple organ microcirculatory disorders in severe acute pancreatitis: experimental findings and clinical implications. Dig Dis Sci. (2002) 47:130–8. doi: 10.1023/a:1013284008219
6. Kakafika A, Papadopoulos V, Mimidis K, Mikhailidis D. Coagulation, platelets, and acute pancreatitis. Pancreas. (2007) 34:15–20. doi: 10.1097/01.mpa.0000240617.66215.d2
7. Venkata C, Kashyap R, Farmer J, Afessa B. Thrombocytopenia in adult patients with sepsis: incidence, risk factors, and its association with clinical outcome. J Intensive Care. (2013) 1:9. doi: 10.1186/2052-0492-1-9
8. Brogly N, Devos P, Boussekey N, Georges H, Chiche A, Leroy O. Impact of thrombocytopenia on outcome of patients admitted to ICU for severe community-acquired pneumonia. J Infect. (2007) 55:136–40. doi: 10.1016/j.jinf.2007.01.011
9. Banks P, Bollen T, Dervenis C, Gooszen H, Johnson C, Sarr M, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. (2013) 62:102–11. doi: 10.1136/gutjnl-2012-302779
10. Song J, Liu S, Zhu F, Wen A, Ma L, Li W, et al. Expert consensus on the diagnosis and treatment of thrombocytopenia in adult critical care patients in China. Military Med Res. (2020) 7:15. doi: 10.1186/s40779-020-00244-w
11. Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. (2013) 13:e1–15. doi: 10.1016/j.pan.2013.07.063
12. Yokoe M, Takada T, Mayumi T, Yoshida M, Isaji S, Wada K, et al. Japanese guidelines for the management of acute pancreatitis: Japanese Guidelines 2015. J Hepatobiliary Pancreat Sci. (2015) 22:405–32. doi: 10.1002/jhbp.259
13. Petrov M, Shanbhag S, Chakraborty M, Phillips A, Windsor J. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. (2010) 139:813–20. doi: 10.1053/j.gastro.2010.06.010
14. Drews R, Weinberger S. Thrombocytopenic disorders in critically ill patients. Am J Respir Crit Care Med. (2000) 162:347–51. doi: 10.1164/ajrccm.162.2.ncc3-00
15. Muller C, Uhl W, Printzen G, Gloor B, Bischofberger H, Tcholakov O, et al. Role of procalcitonin and granulocyte colony stimulating factor in the early prediction of infected necrosis in severe acute pancreatitis. Gut. (2000) 46:233–8. doi: 10.1136/gut.46.2.233
16. Rau B, Kemppainen E, Gumbs A, Buchler M, Wegscheider K, Bassi C, et al. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Ann Surg. (2007) 245:745–54. doi: 10.1097/01.sla.0000252443.22360.46
17. Shu W, Wan J, Chen J, He W, Zhu Y, Lu N, et al. Elevated arterial lactate level as an independent risk factor for pancreatic infection in moderately severe acute pancreatitis. Pancreatology. (2019) 19:653–7. doi: 10.1016/j.pan.2019.06.001
18. Qiu Q, Nian Y, Tang L, Guo Y, Wen L, Wang B, et al. Artificial neural networks accurately predict intra-abdominal infection in moderately severe and severe acute pancreatitis. J Dig Dis. (2019) 20:486–94. doi: 10.1111/1751-2980.12796
19. Zhu C, Zhang S, Zhong H, Gu Z, Kang Y, Pan C, et al. Intra-abdominal infection in acute pancreatitis in eastern China: microbiological features and a prediction model. Ann Transl Med. (2021) 9:477. doi: 10.21037/atm-21-399
20. Antkowiak R, Bialecki J, Chabowski M, Domoslawski P. Treatment of microcirculatory disturbances in acute pancreatitis: where are we now? Pancreas. (2022) 51:415–21. doi: 10.1097/MPA.0000000000002044
21. Bockman D, Buchler M, Beger H. Ultrastructure of human acute pancreatitis. Int J Pancreatol. (1986) 1:141–53. doi: 10.1007/BF02788446
22. Villa-Fajardo M, Palma M, Acebes-Huerta A, Martinez-Botia P, Meinders M, Nolte M, et al. Platelet number and function alterations in preclinical models of sterile inflammation and sepsis patients: implications in the pathophysiology and treatment of inflammation. Transfus Apher Sci. (2022) 61:103413. doi: 10.1016/j.transci.2022.103413
Keywords: acute pancreatitis, platelet count, infection, mortality, retrospective study
Citation: Sun W, Huang J, Ni T, Wen Y, Menglu G, Yongguo W, Yanbin Z, Sheng H, Chen Y, Ma L, Zhao B and Mao E (2023) Moderate level platelet count might be a good prognostic indicator for intra-abdominal infection in acute pancreatitis: A retrospective cohort study of 1,363 patients. Front. Med. 9:1077076. doi: 10.3389/fmed.2022.1077076
Received: 22 October 2022; Accepted: 13 December 2022;
Published: 09 January 2023.
Edited by:
Giuseppe Losurdo, University of Bari Medical School, ItalyReviewed by:
Jelena Velickovic, Clinical Center of Serbia, University of Belgrade, SerbiaEnzo Ieardi, University of Bari Aldo Moro, Italy
Copyright © 2023 Sun, Huang, Ni, Wen, Menglu, Yongguo, Yanbin, Sheng, Chen, Ma, Zhao and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enqiang Mao,  bWFvZXFAeWVhaC5uZXQ=; Bing Zhao,
bWFvZXFAeWVhaC5uZXQ=; Bing Zhao,  emhhb2JpbmcxMjRAMTYzLmNvbQ==
emhhb2JpbmcxMjRAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Wenwu Sun
Wenwu Sun Jun Huang
Jun Huang Tongtian Ni1†
Tongtian Ni1† Enqiang Mao
Enqiang Mao