- Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, United Kingdom
In recent years rheumatologists have begun to shift focus from early rheumatoid arthritis (RA) to studying individuals at risk of developing the disease. It is now possible to use blood, clinical and imaging biomarkers to identify those at risk of progression before the onset of clinical synovitis. The use of imaging, in particular ultrasound (US) and magnetic resonance imaging (MRI), has become much more widespread in individuals at-risk of RA. Numerous studies have demonstrated that imaging can help us understand RA pathogenesis as well as identifying individuals at high risk of progression. In addition, imaging techniques are becoming more sophisticated with newer imaging modalities such as high-resolution peripheral quantitative computed tomography (HR-pQRCT), nuclear imaging and whole body-MRI (WB-MRI) starting to emerge. Imaging studies in at risk individuals are heterogeneous in nature due to the different at-risk populations, imaging modalities and protocols used. This review will explore the available imaging modalities and the rationale for their use in the main populations at risk of RA.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disorder, which is characterized by poly-articular and systemic inflammation. It affects around 1% of the population and if poorly treated can lead to irreversible joint damage and disability (1). It is now widely accepted that early diagnosis and tight control of disease activity is associated with improved long-term outcomes (2). Subsequently the early phase of RA within 3 months of the development of synovitis, has been named the “window of opportunity.” Diagnosing and treating RA patients within this window can be difficult due to delays in patient presentation, referral delays and waiting times in secondary care (3). Furthermore, once RA has developed, drug free remission, which is effectively cure of the disease, remains infrequent (4, 5). This has led to a drive in identifying individuals at-risk of RA to offer the opportunity to treat prior to the onset of synovitis and potentially prevent or delay RA development.
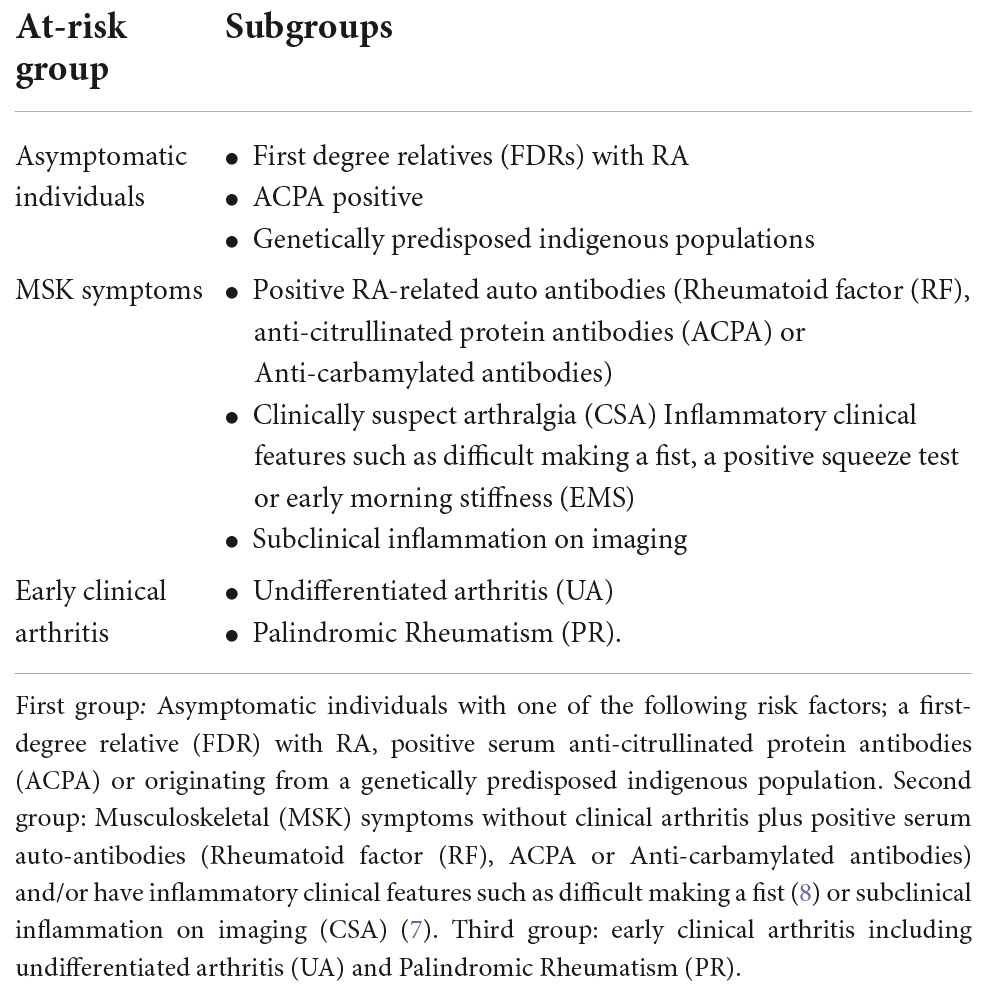
A recent European League Against Rheumatism (EULAR) task force has used clinical features to define three main populations that should be considered when studying individuals at risk of RA (Table 1) (5, 6). These groups include asymptomatic predisposed individuals, individuals with positive serum auto-antibodies and early clinical arthritis. One specific group of frequently studied patients are those that have inflammatory MSK symptoms and they can be defined as having clinically suspect arthralgia (CSA) (7). Due to the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) RA diagnostic criteria update in 2010, many patients who were previously diagnosed with undifferentiated arthritis (UA) would now be diagnosed with RA. As many of the imaging studies in UA recruited patients based on pre-2010 criteria, this review has not included imaging studies that have solely focused on UA patients.
It is now accepted that many of these at risk individuals may be in a very early phase of what has been defined as the “RA disease continuum.” In those that do go on to develop RA, this phase can be retrospectively labeled as “pre-RA.” Many at risk individuals have biochemical and imaging abnormalities that can be used to predict progression to arthritis. These biomarkers can also provide a better understanding of the pathogenesis of the disease in the preclinical phase. A key point to note is that not all at risk individuals will progress to RA. It is therefore important to understand which biomarkers are the most useful in predicting progression.
The use of imaging in RA and other inflammatory arthritidies has increased dramatically in the past two decades. Previously it was mostly limited to the use of plain radiographs to detect irreversible joint damage in established RA. It has subsequently been shown that both Ultrasound (US) and MRI can be used to detect structural damage that is not visible on plain radiographs (9, 10) and subtle inflammation that is not detectable by clinical examination (11, 12). As well as US and MRI, HR-pQCT and molecular imaging techniques such as Position emission tomography (PET) have also shown promise in early RA (13, 14). This increased understanding and breadth of use of different imaging techniques is now being applied to individuals at risk of RA.
Interventional trials are now starting to focus on treating individuals at risk of RA in the preclinical stage. A recent randomized controlled trial (RCT) demonstrated that intervening with methotrexate and corticosteroids in CSA patients with subclinical inflammation on MRI can delay arthritis development and appears to be associated with a milder arthritis phenotype (15). Other RCTs using rituximab and abatacept have demonstrated that intervening in the preclinical stage could also delay and possibly prevent RA development (16, 17). The results of these studies should further our knowledge on the optimum timing and frequency to image individuals at risk of RA. They also demonstrate that halting the development of RA in the preclinical stage is now a realistic prospect. Imaging is likely to remain a central part of this process with its ability to help identify, stratify and manage individuals at risk of RA. Furthermore, patients anecdotally often relate better to images of their condition, allowing them to visualize the disease process, compared to numerical laboratory data. Patients report that undergoing scans is a positive experience and appreciate having the opportunity to view their images (18). In this review we aim to address how different imaging techniques should be used in individuals at risk of RA.
Which imaging technique?
Multiple different imaging modalities have been used in individuals at risk of RA. There are advantages and disadvantages of each imaging method as discussed below (Table 2).
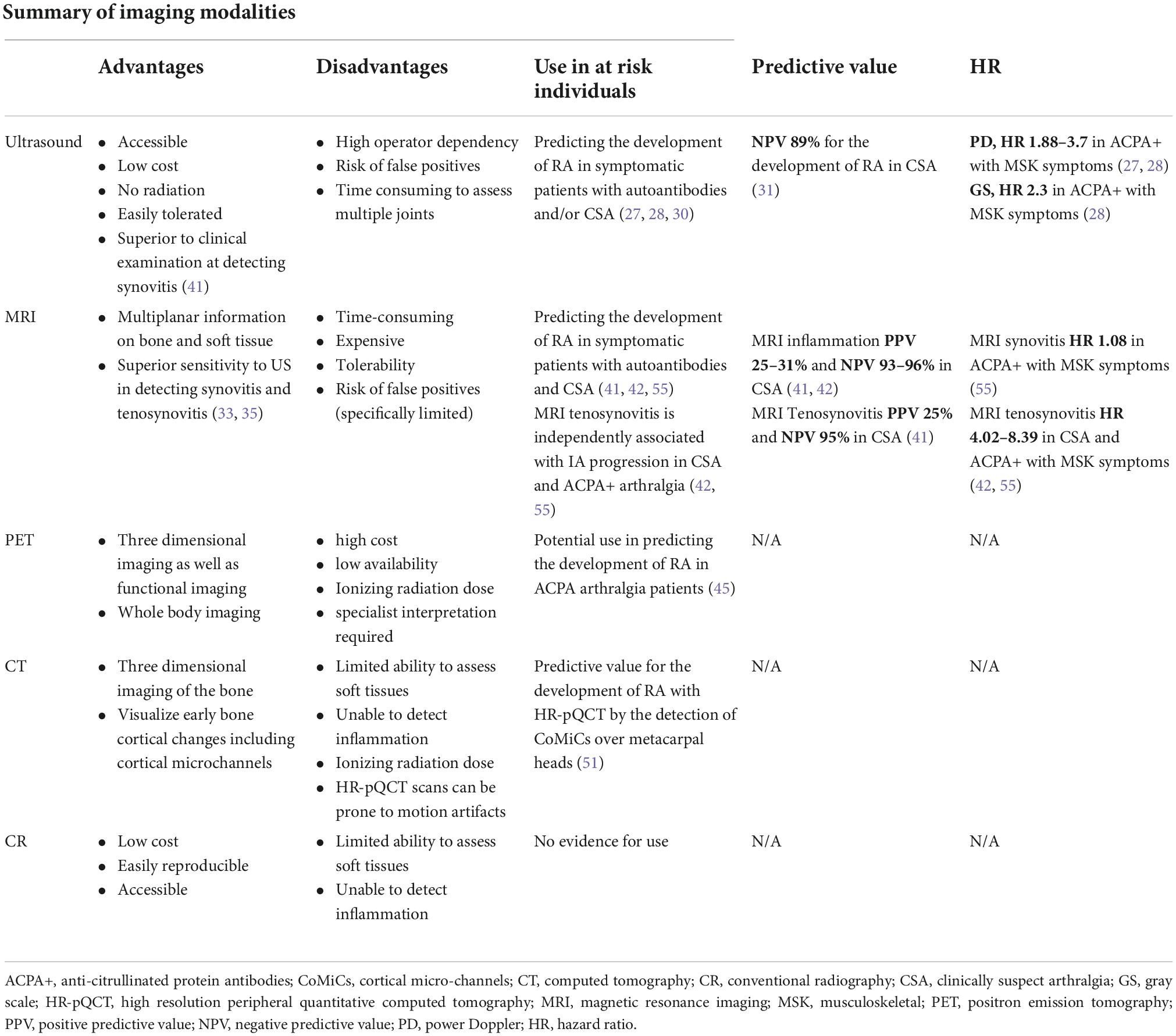
Table 2. A summary of the advantages and disadvantages of different imaging modalities and the evidence for the use in individuals at risk of RA.
Ultrasound
The use of US in both research and clinical practice is now widespread in rheumatology. The benefits of US include accessibility, low cost, lack of radiation exposure and tolerability for patients. US is more sensitive than clinical examination for detecting synovitis (19) and its ability to differentiate synovitis from other causes of joint pain and swelling, such as tenosynovitis or bursitis, makes it an extremely useful tool in early disease, including individuals at risk of RA (20).
The potential disadvantages of US include the high operator dependency and therefore lower reproducibility. Another concern regarding individuals at risk of RA is that US may detect joint inflammation too late in the disease continuum, when clinical arthritis is imminent, therefore leaving limited opportunity for preventive intervention (21, 22). In line with this, when lower risk individuals have been studied, particularly those who have not developed joint symptoms, US abnormalities have not been found (23). Another concern is that not all patients with US inflammation will go on to develop RA (24). Joint effusions, synovial hypertrophy and even low level power Doppler (PD) signal can be commonly found in healthy populations (25). If a clinician finds US synovitis it may be tempting to start immunosuppressant medications which leads to the possibility of over-treating patients and potentially subjecting them to lifelong medications (26).
Despite these concerns, multiple studies have demonstrated the positive predictive value of US abnormalities in individuals at risk of RA. The initial US analysis from Leeds found that presence of US PD in the hands and wrists of CCP+ individuals with new MSK symptoms was associated with progression to IA (27). In a larger study from the same center, 136 ACPA positive patients with MSK symptoms were followed up over a median of 18.3 months. Fifty-seven (42%) patients developed an IA after a median of 8.6 months; 86% of patients that progressed to IA had one or more US abnormalities at baseline compared to 67% of patients that did not progress. Furthermore, US abnormalities were predictive of IA development at both patient and a joint level. Gray scale (GS), PD and erosions were all associated with progression, with PD conferring the highest risk. At joint level, the presence of PD at baseline was associated with a 10 fold risk of that joint developing clinical synovitis (28). In contrast, an US study in a Dutch seropositive arthralgia cohort found that GS synovitis was predictive of IA progression but PD was not (29). The contrasting results may be explained by cohort differences. This Dutch study included patients with lone RF positivity as well as ACPA positive patients, thus the overall cohort is lower risk. US was associated with progression to RA in a retrospective analysis of 80 patients with inflammatory arthralgia of < 6 weeks duration but negative rheumatoid autoantibodies. The Swiss Sonography Group in Arthritis and Rheumatism (SONAR) scoring system was used but PD was not included in the predictive analysis (30).
The negative predictive value of US has been specifically demonstrated in patients presenting with CSA with at least two painful joints of the hands, feet or shoulders. In a multicentre cohort study, US data was collected at baseline, 6 and 12 months. Fifty-nine percent of patients in this study who had US synovitis (defined as GS ≥ 2 and/or PD ≥ 1) at baseline developed IA. Importantly, if no joints showed US synovitis at baseline, the negative predictive value was 89%, suggesting that such individuals could be largely reassured of their risk of developing IA (31).
The ability of US tenosynovitis to predict IA has been less well studied than with MRI and has shown mixed results. Molina Collada et al. found that in a cohort of CSA patients PD tenosynovitis at baseline was the only independent predictor of RA and IA development (32). In contrast van de Stadt et al. did not find that US tenosynovitis was significantly predictive of IA progression at joint or patient level (29). Again these contrasting results may be explained by cohort differences as the CSA patients in Molina Collada et al. paper had relatively high average RF and ACPA antibody titres. In a direct comparison of MRI and US, it was found that US was less sensitive than MRI in the early detection of both synovitis and tenosynovitis in patients with CSA (33). Figure 1 shows representative US findings of sub-clinical synovitis and tenosynovitis in ACPA+ individuals with MSK symptoms.
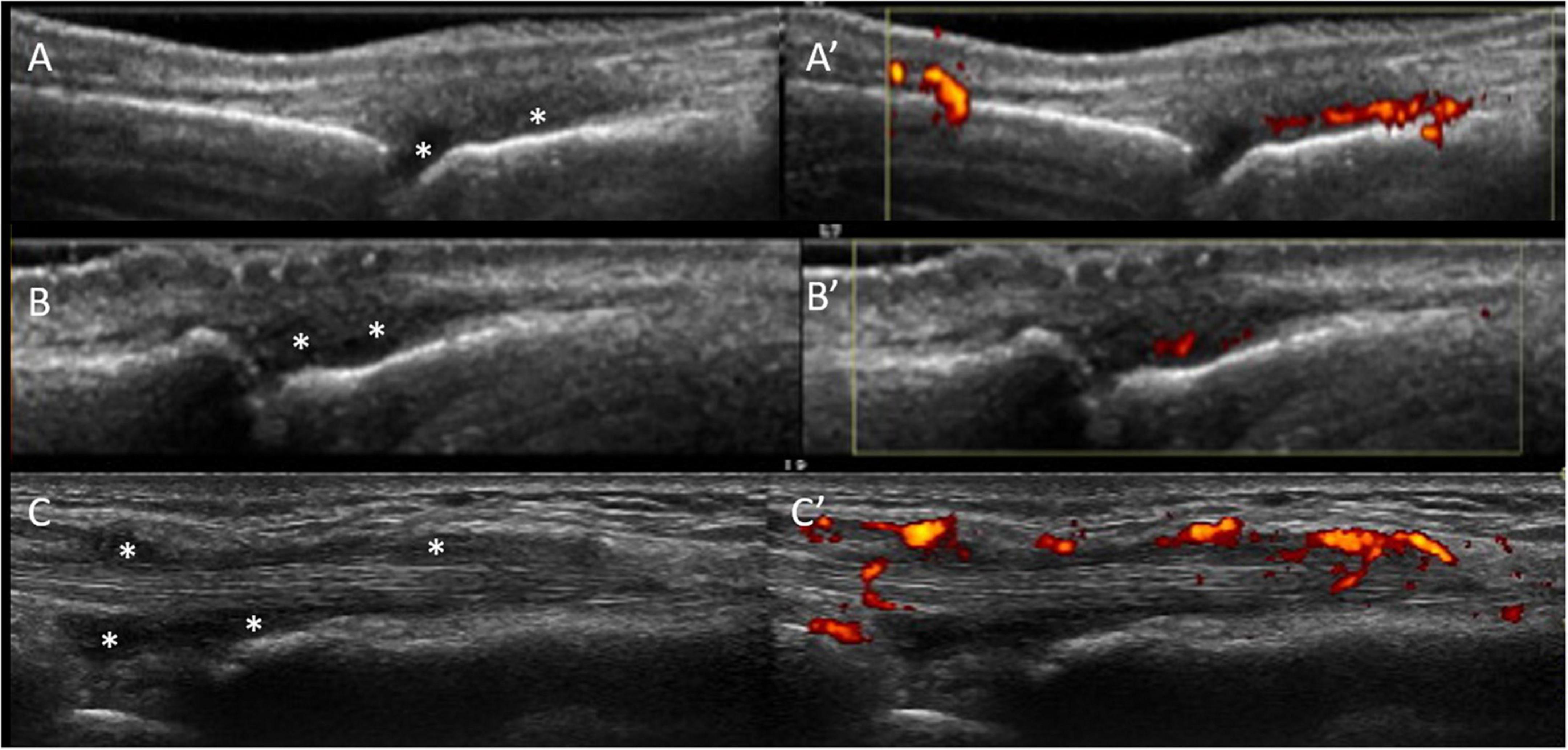
Figure 1. US sub-clinical synovitis and tenosynovitis in ACPA+ individuals with MSK symptoms. Gray scale (A) and power Doppler (A’) positive synovitis of the 3rd MCP joint in a patient at-risk of RA high titre positive anti-CCP antibodies and non-specific musculoskeletal symptoms. Similar US findings are shown in the 2nd PIP joint in a different at-risk individual (B,B’) with positive anti-CCP antibodies and rheumatoid factor, hands arthralgia. (C,C’) Illustrate tenosynovitis of the 2nd extensor tendon compartment in a third individual at-risk of RA with high titre anti-CCP antibodies and clinically suspect arthralgia. All images were obtained using a longitudinal approach. Asterisks indicate synovial hypertrophy.
In a study that has looked at US detected bone erosions in “pre-RA” it was shown that bone erosions in the feet could be predictive for RA development. This was a large study that followed up 400 RA patients over a median of 41.4 months. Bone erosions in more than one joint and bone erosions in fifth MTP joint with US synovitis were the most predictive for the development of IA (34) (Figure 2).
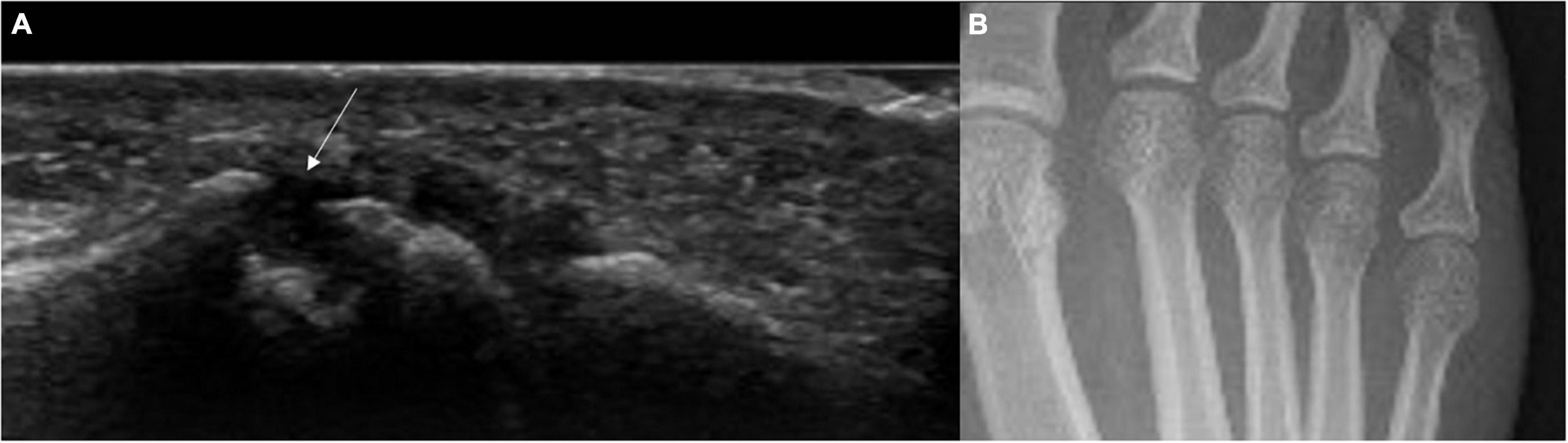
Figure 2. US bone erosion (not detected by x-rays) in the 5th MTP joint in an individual with high titre ACPA and non-specific MSK symptoms. (A) Longitudinal US scan of the lateral aspect of the 5th MTP joint. The white arrow indicates the presence of bone erosion. (B) Correspondent feet x-rays showing no bone erosions in the 5th metatarsophalangeal joint. This patient presented with non-specific musculoskeletal symptoms and high titre anti-CCP antibodies.
In summary, US is a readily available imaging technique that provides valuable information in individuals at risk of RA. The presence of PD synovitis in symptomatic at risk individuals, is strongly associated with imminent future arthritis development and has been used to produce clinically relevant risk stratification models. Conversely, the value of US in at risk individuals without MSK symptoms appears to be limited.
Magnetic resonance imaging
One of the major benefits of MRI is its ability to provide highly sensitive multiplanar information on both the bone and soft tissue structures in and around the joints without using ionizing radiation. It has demonstrated superiority to US in detecting synovitis (Figure 3) and tenosynovitis in early RA and CSA (33, 35). This in addition to its unique ability to detect bone marrow edema, a potential precursor to erosions, makes its use in at risk individuals appealing (36). Despite this, MRI is not without its disadvantages; it is time-consuming, expensive and some patients struggle to tolerate it. Consequently US has generally gained more traction as the first line high resolution imaging assessment for synovitis in most rheumatology centers, with MRI often used as a second line investigation where required.
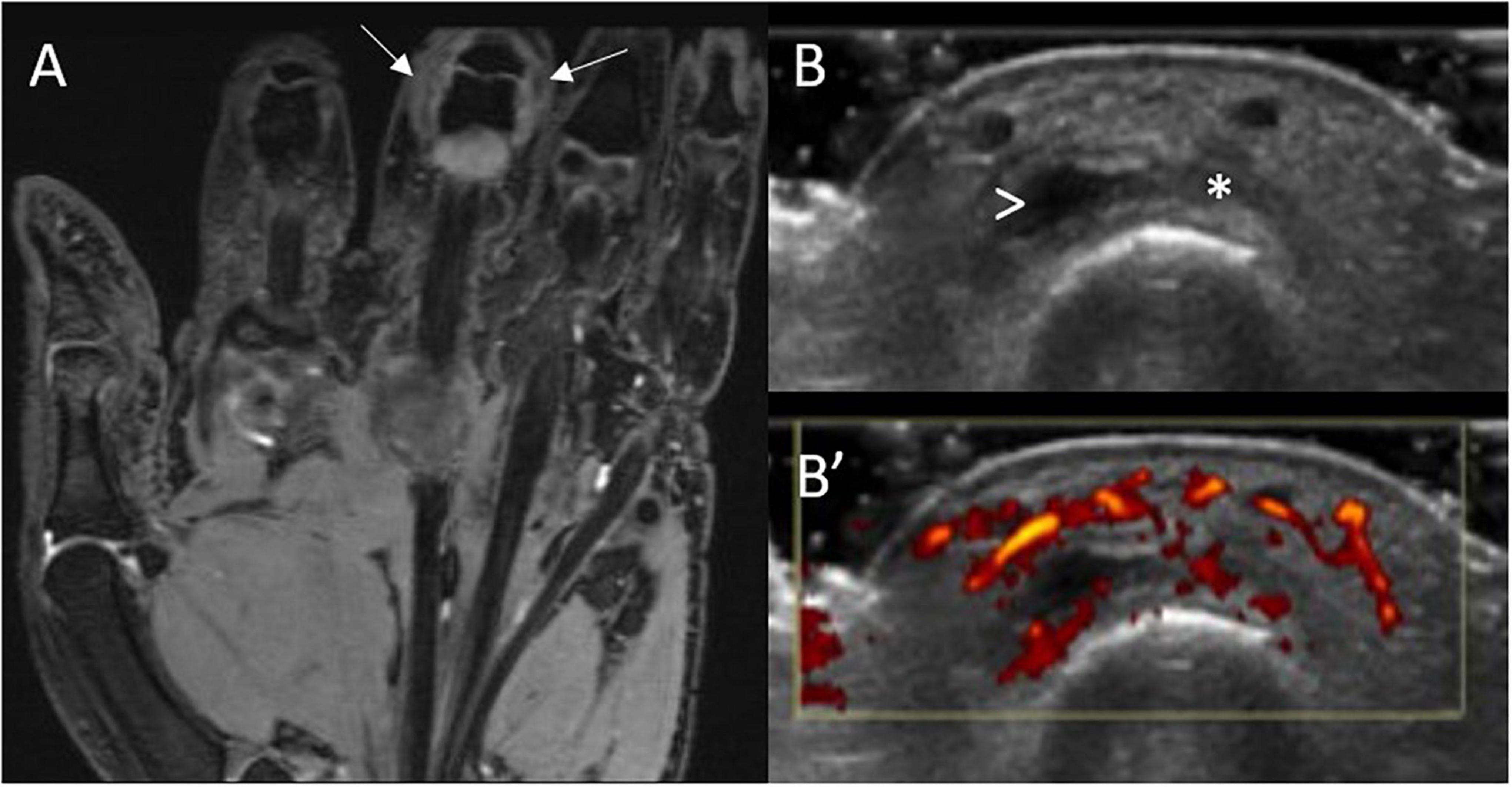
Figure 3. MRI and US sub-clinical synovitis in a patient with CSA and high titre ACPA. MRI (A) synovitis (white arrows) of the 3rd PIP joint in an individual at-risk of RA with corresponding gray-scale (B) and power Doppler (B’) US scans. This individual had clinically suspect arthralgia and positive anti-CCP antibodies (high titre) and rheumatoid factor. These images were obtained 6 weeks before progression to RA. Asterisk indicates synovial hypertrophy, while the arrowhead a small joint effusion.
One specific concern with MRI in at risk individuals is that its high sensitivity for detecting inflammation may compromise specificity. MRI often detects inflammation in healthy, asymptomatic individuals without risk factors for RA (37, 38). One of the larger studies to investigate this performed contrast enhanced MRIs of the dominant metacarpophalangeal (MCP), wrist and metacarpophalangeal (MTP) joints of 193 symptom free persons. In this study, 72% of patients had at least one single inflammatory feature and 78% had one or more erosions. Inflammatory features and erosions were particularly prevalent in older age groups (39). In a small study of 28 patients with ACPA positive arthralgia, 93% of individuals had MRI synovitis with a Rheumatoid Arthritis Magnetic Resonance Imaging Score (RAMRIS) of 1. Forty-three percent of these patients went on to progress to IA and a RAMRIS score of 2 or above was associated with faster progression (40). Boer et al. created more specific parameters to define pathological MRI inflammation by comparison with a symptom free reference group. They demonstrated that by using a reference group MRI can be predictive in CSA and UA patients and the rates of false positives were reduced (41).
Larger studies of at risk individuals have demonstrated promising results in the predictive value of MRI. Van Steenbergen et al. looked at 150 patients with CSA of whom 46% had significant subclinical inflammation on MRI (synovitis, bone marrow edema or tenosynovitis) when scored against a healthy reference group. They followed up all patients up over a median of 75 weeks and 30% of patients developed IA. MRI inflammation was more positively associated with IA development than age, localization of initial symptoms and C-reactive protein level. Seventy-eight percent of the patients that had inflammation on MRI at baseline developed IA within a year compared with only 6% of patients without. Interestingly, tenosynovitis had the strongest independent association for progression to IA (HR = 7.56). Bone marrow edema and synovitis were also independently associated with progression but less strongly so (42).
Overall the current evidence suggests that MRI may have a role in assessing at risk individuals. It may have particular value in delineating inflammation in extra-capsular structures in a sub groups of patients, which requires further exploration. For practical reasons, faster and cheaper MRI protocols are likely to be needed before the use of MRI in clinical practice becomes more widespread.
Positron emission tomography
Positron Emission Tomography (PET) is a nuclear imaging technique, which uses radioactive tracer drugs to detect metabolic cellular processes. It is used alongside another imaging technique, usually CT, to produce three dimensional functional imaging. PET-CT is able to detect synovitis and monitor treatment response in early RA (43). An important advantage is the ability to image the whole body in one acquisition unlike other more conventional imaging. Disadvantages include the high cost, limited availability (often restricted to large, specialist centers) and radiation dose. In addition, tracer uptake may not be specific to joint inflammation so specialist interpretation is required. Efforts are being made to improve safety; newer tracers have a much shorter half-life, which makes the radiation exposure similar to a standard CT scan (44). Moreover, the advent of PET MRI and the fact that PET scans are becoming increasingly sensitive is also likely to lower radiation exposure.
In their small pilot study, Gent et al. used (R)-11C-PK11195 PET to show that subclinical joint inflammation could be detected in 29 ACPA positive arthralgia patients. Hands and wrists were scanned at baseline and patients were then followed up over 24 months. Nine patients in total developed an IA. Four patients had a positive PET scan at baseline, all of whom went on to develop IA. Of the 5 patients who developed IA and had a negative scan at baseline, 3 of these patient developed inflammation in joints that were not scanned. These results of this preliminary study suggest that (R)-11C-PK11195 PET may be useful in predicting IA development (45).
Nuclear medicine is an evolving area in RA imaging. The evidence from PET and the potential to develop new radiotracers that can highlight areas of inflammation at whole body level warrants further exploration in individuals at risk of RA.
Computed tomography
Unlike conventional radiography, CT provides three dimensional imaging of bone without projectional superimposition. However, unlike MRI, it has limited ability to assess soft tissues and is unable to detect inflammation. CT is also associated with ionizing radiation exposure, which, alongside the lack of information on soft tissue inflammation, has resulted in relatively little research into the use of CT in at risk individuals. There is evidence that changes in bone mineral density may begin in very early RA (46, 47). This has led to the question of whether some of these bone changes may occur in the “pre-RA” stage before the onset of clinical synovitis. HR-pQRCT is an imaging technique that was introduced over a decade ago and has shown promising use in individuals at risk of RA. One disadvantage is that it requires specialized technology that is not widely available. Kleyer et al. used a type HR-pQCT called microfocal CT (micro-CT) to investigate the association between ACPA and bone loss prior to the onset of inflammatory arthritis. They demonstrated that cortical bone thickness was significantly reduced in asymptomatic ACPA positive individuals compared to healthy controls (48). It is worth noting that cortical hand bone loss in early RA has been shown to predict radiographic hand joint damage (49). In contrast, in a separate study of 29 ACPA positive individuals trabecular bone was thinner when compared to controls but there was no significant difference with the cortical bone (50).
It is thought that erosions typically start in the “bare area” of a joint, which is not covered by articular cartilage. Simon et al. used HR-pQCT to investigate whether individuals at risk of RA have a higher frequency of cortical micro-channels (CoMiCs) at the bare joint areas. It was found that in 74 individuals, who were ACPA or anti-MCV positive, there were significantly more CoMiCs in the patients that progressed to RA and CoMiCs over metacarpal heads were associated with the development of RA (51). HR-pQCT scans have a higher spatial resolution than conventional CT scans with a similar radiation dose. A disadvantage is that they can be prone to motion artifacts. Currently HR-pQCT scans are not widely available for research or clinical purposes but their promising initial results in pre RA does warrant further investigation. Further research is needed into the use of CT in other at risk groups such as CSA, including ACPA negative individuals.
Conventional radiography
Radiographs have been widely used in the initial work up of newly diagnosed RA patients and for the monitoring of disease progression. While they do not provide any information on soft tissues or synovial inflammation, they can detect structural joint damage. The benefits of radiographs include their low cost, accessibility and reproducibility for serial assessment. However, it has been shown that radiographs have limited ability in detecting bone erosions in early RA (52, 53). This clearly limits their use in predicting disease progression in at risk individuals. In a study of 418 ACPA positive at risk individuals only 4.1% had bone erosions on hand and feet radiographs and these did not predict progression to IA (54). This study suggests there is no value in routinely performing radiographs in individuals at risk of RA.
Should we image extra-capsular structures?
As discussed above, intra-articular joint inflammation identified on US, MRI and PET-CT in at-risk individuals is associated with progression to IA. However, it is not only the joints but also the structures outside the joint capsule that have shown interesting findings in individuals at risk of developing RA. MRI tenosynovitis is a particularly important finding as evidenced by Van Steenbergen et al. who found it to be the strongest independent predictive factor on MRI for the development of IA in patients with CSA (42). Further studies in ACPA positive individuals have also demonstrated that tenosynovitis is the strongest MRI predictor of progression to IA (55, 56). A recent study in CSA patients found that MCP-extensor peritendinitis, although infrequent, was strongly associated with IA development with a positive predictive value of 65% (57). Similarly, MRI interosseous tendon inflammation was identified in 19% of ACPA positive patients, 49% of early arthritis patients but no healthy controls (58). A histological study confirmed the absence of a tenosynovial sheath around the interosseous tendons, suggesting the MRI findings reveal a peri-tendinous inflammation rather than a genuine tenosynovitis. While US tenosynovitis has more mixed findings in predicting progression, it is important to note that when present it is highly likely to be pathological; it is infrequently seen in healthy individuals (59).
Other extracapsular structures are also of relevance in at risk individuals. Non synovial extra-capsular inflammation, in the absence of synovitis, represents a distinct phenotype in PR patients during flare (60). A very recent study found that inter-metatarsal bursitis may precede the development of RA. In this study, contrast enhanced MRI scans were performed in the forefoot, MTP and wrist joints of 577 CSA patients. The RAMRIS scoring system was used and intermetatarsal bursitis was only counted as being present if it would be uncommon in the same location in a healthy population. They found that 23% of CSA patients had intermetatarsal bursitis but this increased to 47% if only including the ACPA positive patients. In the ACPA positive patients, intermetatarsal bursitis was able to predict progression to IA (61).
Overall, these studies have demonstrated the relevance of MRI inflammation in extracapsular structures in at risk individuals. Although of pathobiological relevance, further research is required to determine if imaging these extracapsular structures adds additional value in predicting progression to RA in at risk individuals.
Can we image fewer joints?
Practically it is important to address how many and which joints should be scanned in individuals at risk of developing RA. To date, the majority of imaging studies in this cohort have used comprehensive imaging protocols that include a large number of joints. While this may be feasible in a research setting it is not usually practical in a clinical setting where time is limited.
One study that looked at a reduced subset of 30 unilateral RA specific MRI features in the wrist, MCP and MTP joints, as opposed to the 61 features in the RAMRIS scoring system, found that by using this smaller subset of measurements it was still possible to predict the development of arthritis in 225 CSA patients (62). In the Leeds cohort of ACPA positive patients with MSK symptoms, an US protocol of 32 joints was used to successfully predict progression to RA (28). Gray scale, PD and erosions were all shown to separately predict progression. In the first Leeds prediction model, presence of PD signal in 22 joints (the wrists, MCPJs and PIPJs only) was predictive of progression on multivariable analysis (27). This study demonstrated that an attenuated joint set of the hands and wrists only can have predictive value. van de Stadt et al. took a different approach in their study and only scanned tender joints and small joints directly adjacent and contralateral to the tender joints. It was found that in the 192 individuals with arthralgia and positive autoantibodies (RF and/or anti-CCP), only GS on US was predictive (29). As previously discussed, the different results in these studies may also be partly explained by differences in the at risk cohorts.
To date, US studies in individuals at risk of RA have all used bilateral joint protocols. In established RA it has been shown that unilateral reduced scoring protocols of 7–9 joints were still able to capture 78–85% of the information from a full 36 joint protocol. This was, however, significantly less than the bilateral 7–9 joint protocols which captured 89–93% of the information (63). In contrast, MRI protocols in individuals at risk of RA have used the most symptomatic or dominant hand and wrist joints, as recommended in the RAMRIS scoring system (64). One concern with this approach is that synovitis does not always present symmetrically. In a study that looked at early RA patients it was shown that 21% of patients had unilateral synovitis in non-dominant joints (65). Furthermore, by just scanning the dominant hand and wrist joints it allows the potential of overuse tenosynovitis to influence findings (66). Despite these concerns, multiple MRI studies have demonstrated predictive value of imaging only the dominant hands in individuals at risk of RA (40–42).
RA is often considered a disease of the small joints, with large joints affected less frequently and later into the disease course (67, 68). This leads to the question of whether large joints should be included in imaging protocols of at risk individuals. Rogier et al. found that in 170 CSA patients scanning the shoulders did not predict the development of IA despite 50 patients showing abnormalities on the US scan. However, only 5% of shoulders scanned in this study were symptomatic (69). In an MRI study of 55 individuals with ACPA and/or RF antibodies it was found that MRI and synovial biopsy of the knee did not detect clear-cut inflammation in the 15 patients who went on to progress to RA (70). In contrast, when ACPA+ at risk individuals had symptomatic knees and shoulders, performing US in these areas added predictive power (28). As such, a pragmatic approach may be to scan all standard protocol small joints but only include the symptomatic large joints.
Overall, an attenuated US joint set and a reduced scoring system for MRI can have predictive value in individuals at risk of developing RA. Unilateral US protocols have not been investigated in at risk populations. However, given a significant amount of information is lost with unilateral protocols in established RA it seems likely that bilateral joint assessments should be retained. In MRI the use of the most symptomatic or dominant hand and wrist joints is effective in predicting progression in individuals at risk of RA. Scanning symptomatic large joints on US adds predictive power. Imaging techniques such as PET (45) and whole body MRI (WB-MRI) that are able to image the whole body in one acquisition may also aid in the dilemma of which joints to scan. As far as we are aware, WB-MRI is yet to be evaluated in at risk individuals but its ability to visualize total patient-level inflammatory burden may warrant further investigation in at risk individuals.
Hands, feet or both?
One limitation of US imaging of the feet in at risk individuals is that US abnormalities such as gray-scale synovial inflammation are fairly prevalent in the healthy population, particularly at MTPJ1 (25). The SONAR score includes the same joints as the DAS28 but excludes the thumbs, shoulders and also excludes the feet. Zufferey et al. found US to be predictive for IA development when using the SONAR score in 80 CSA patients (30). In contrast, Brulhart et al. did not find that a baseline SONAR US score predicted progression to RA. The cohort in their study, however, largely consisted of FDRs with mostly negative autoantibodies, and hence had a lower overall risk of progression, although interestingly 70% were symptomatic (23). Rakieh et al. also demonstrated that US of the hands and wrists alone can be predictive of progression in ACPA positive patients with MSK symptoms (27). Taken together, these studies demonstrate that in higher risk populations with MSK symptoms, US protocols that do not include the feet can still provide predictive information. However, it is not clear to what extent omitting the feet has an effect on predictive accuracy. For example, a recent study demonstrated useful additional information to be gained by including the feet in US protocols of individuals as risk of RA. A baseline US scan was performed in over 400 ACPA positive individuals to evaluate bone erosions in MCP2, MCP5 and MTP5 joints. The combination of bone erosions and synovial inflammation in MTP5 was the most successful in predicting RA development compared with the combination of synovial inflammation and erosions in either MCP2 or MCP5 (34).
One MRI study has addressed the importance of including the feet alongside the hands when imaging at risk individuals. Boer et al. performed contrast enhanced MRI of the hand (MCP2-5 and wrist) and foot (MTP1-5) in 357 CSA patients. Scans were scored for synovitis, osteitis and tenosynovitis. After 1 year follow up 18% of patients developed an IA. The investigators found that although tenosynovitis of the feet could independently predict IA it did not increase the overall predictive accuracy of MRI over the hands and wrists alone. Without including the feet, the overall predictive sensitivity remained at 77%, however, the specificity actually decreased from 66 to 62% (71).
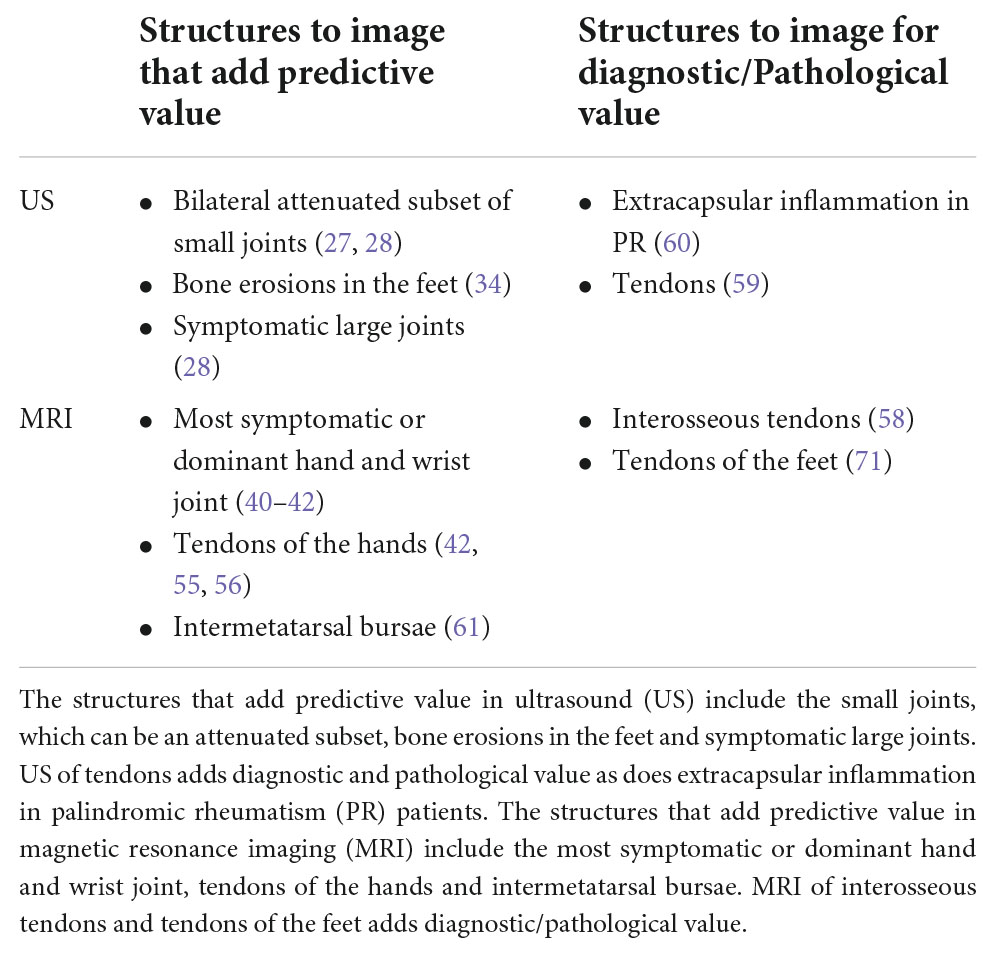
In summary, there is evidence to suggest specific benefit from including the feet in US protocols for at risk individuals. Therefore, reducing the length of protocols by using a limited set of hand and foot joints may be the best approach for improving feasibility while retaining predictive accuracy. In MRI, unvalidated data suggests imaging the most dominant or symptomatic hand and wrist joints alone without the feet is sufficient to predict progression to RA. A summary of suggested structures to image for MRI and ultrasound is included in Table 3.
Which at risk populations should be imaged?
Populations who may be considered “at risk” of developing RA have now been defined by EULAR (6). These groups encompass a range of risk and symptom profiles, with some having a much higher risk of progression than others. Imaging can be time consuming and expensive, and also necessitates face to face clinical visits, so it is important to understand in which at risk populations it adds value.
Some people are at risk of developing RA despite having no MSK symptoms, e.g., asymptomatic genetically predisposed individuals. In terms of US studies, only 14.9–33% (27–29, 31) of patients with MSK symptoms have US PD on their baseline scan. This leads to the question of whether it is useful to image at risk individuals without MSK symptoms, as intuitively they may be even less likely to have subclinical inflammation on imaging. Only one study has looked at imaging individuals at risk of RA who do not have symptoms. Brulhart et al. performed US assessments using the SONAR score in 273 FDRs of RA patients of whom 8% were ACPA positive; 14% asymptomatic, 55% MSK had symptoms and 21% had UA. A positive US was defined as at least one joint with GS ≥ 2, or PD ≥ 1. US positivity was only found in the patients that had UA and not in the individuals that were asymptomatic regardless of their antibody status (23).
Seronegative patients with inflammatory symptoms (e.g., CSA) are a lower risk group for progression to RA than ACPA+ individuals with MSK symptoms. In CSA patients, MRI studies have shown that certain symptoms in particular are associated with subclinical inflammation. In their study of 575 CSA patients Krijbolder et al. found that the longer the duration of morning stiffness the more frequently subclinical inflammation was found on MRI (72). Only 14% of these patients were ACPA positive and 20% were RF positive. Further studies of MRI scans on CSA patients with similar antibody prevalence have shown that difficulty making a fist is associated with flexor tenosynovitis and a positive squeeze test is associated with subclinical synovitis (73, 74). Van der Ven et al.’s study included 143 CSA patients of whom only 13% were positive for ACPA and 26% for RF. In these patients the presence of US synovitis was still associated with IA development despite the lower antibody prevalence (30, 75). When patients do go on to develop RA around 25% of these patients are seronegative. These studies indicate the importance of imaging in patients who are seronegative, but have inflammatory MSK symptoms such as CSA.
Overall, it seems prudent to perform imaging preferentially in at-risk individuals who have MSK symptoms, even if they are autoantibody negative. Performing US scans in individuals without MSK symptoms may not add value, although US data is limited to a single study and it is unknown if this is the case with all imaging techniques. Clarity is also required on whether individuals with high autoantibody titres and non-MSK symptoms (e.g., fatigue) have subclinical inflammation on imaging in the absence of MSK symptoms such as joint pain and stiffness. A recent prospective observational study found that 21% of 92 asymptomatic ACPA positive individuals developed RA after an average of 10.7 months (76). This relatively high proportion of progression suggests that there may be value in imaging certain high risk asymptomatic individuals.
Palindromic rheumatism
Palindromic rheumatism (PR) is a syndrome characterized by interment flares of joint pain and swelling. Patients are asymptomatic between flares and many have positive autoantibodies with 42–67% ACPA positive and 42–82% RF positive (60, 77–80). PR was included in the EULAR defined at-risk populations as a significant number of patients with PR go on to progress to RA (79, 81).
Given flares of PR are transient and unpredictable, imaging can be practically challenging. A study that scanned 54 PR patients between flares found that only 7.4% had US subclinical synovitis in the asymptomatic phase (77). It is worth noting that the majority of PR patients in this study were not DMARD naïve which may have affected the imaging findings. However, a further study in DMARD naïve PR patients also did not find US inflammation between flares (60). In contrast when US scans were performed in symptomatic flares of 84 PR patients, 36% had synovitis on imaging (82). Seropositive patients were more likely to have US detected synovitis in flare. In this same cohort it was shown that US along with ACPA antibody status were able to successfully predict RA development within 3 years, although it was the ACPA status that was the most predictive (83).
PR patients have a distinctive imaging phenotype during flare (60). In a study of 31 treatment naïve PR patients it was found that 61% of patients had extra-capsular inflammation during flares. In 39% of the patients there was extracapsular inflammation on imaging without associated synovitis. Only 23% had US detected synovitis during flare. This distinct imaging phenotype of isolated extracapsular inflammation may be particularly useful in differentiating PR from RA on clinical assessment. Overall, the current evidence suggests that PR patients should be imaged during a flare and not when they are in the asymptomatic phase.
Conclusion
The ability to study at risk individuals before they develop RA has opened up the possibility of a new and earlier “window of opportunity” for treatment. The implication of this is that there is now very real potential to treat prior to arthritis development with the prospect of halting disease progression. It is clear that imaging has a role in this group of individuals in differential diagnosis and risk stratification.
So far, MRI and US have been the most investigated imaging techniques in individuals at risk of RA and studies have shown useful outcomes. MRI may be optimum for certain inflammatory parameters such as tenosynovitis, however, US represents the safest, cheapest and most practical imaging tool. Newer imaging techniques such as HR-pQRCT and PET have shown promising initial results and warrant further investigation.
Further work is needed to establish the optimum imaging protocols that give the most accurate and efficient results. Current studies have demonstrated that it is possible to design MRI and US protocols with reduced joint numbers. In US in particular there does appear to be additional benefit in imaging the feet and symptomatic large joints. Extra-articular structures can also provide additional information. In MRI, imaging the tendons and intermetatarsal bursa in particular can inform risk stratification. US extracapsular inflammation in PR patients may be beneficial in differentiating PR from early RA.
A careful clinical history in individuals at risk of RA is important to guide the use and timing of imaging. Symptomatic at risk individuals should be scanned preferentially regardless of their antibody status. In PR patients, it is more valuable to scan patients during a flare than in the asymptomatic phase. It should be noted that in certain lower risk groups such as CSA patients with low ACPA antibody prevalence, US inflammation appears to be less frequent and MRI may add more value. Further research is needed to establish whether there is value in imaging asymptomatic individuals with high antibody titres and other risk factors. A suggested algorithm to guide the use of imaging in at risk individuals is suggested in Figure 4.
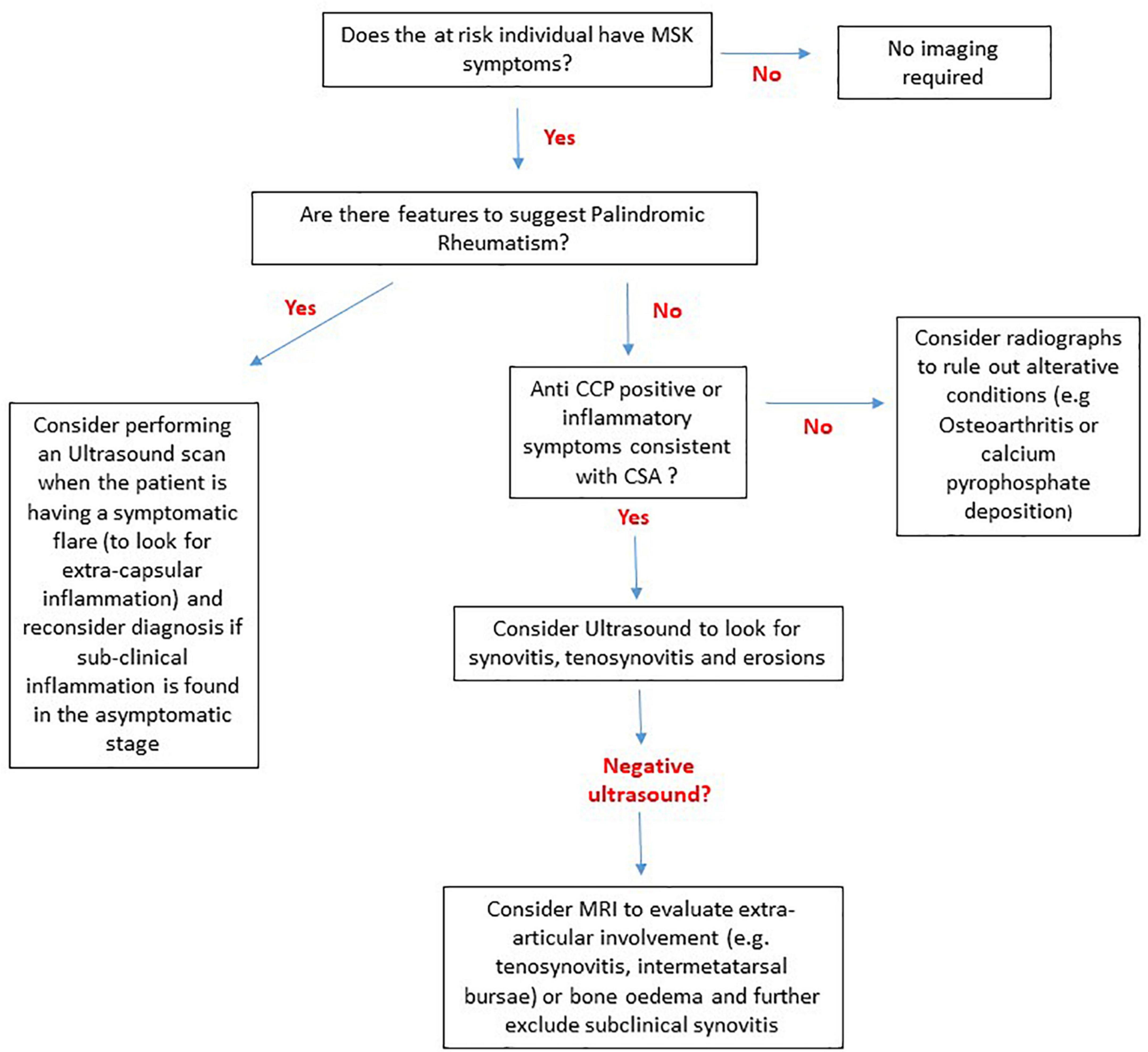
Figure 4. A suggested algorithm to guide the use of MRI, US and radiography in individuals at risk of RA without clinical synovitis based on current evidence. In patients that do not have any symptoms there is no evidence to suggest that any imaging techniques are of diagnostic or predictive value. For Palindromic rheumatism extracapsular inflammation can be captured on imaging during flare episodes and if subclinical inflammation is seen in the asymptomatic phase the diagnosis of palindromic rheumatism should be re-considered. For anti-citrullinated protein antibody (ACPA) negative individuals with inflammatory symptoms i.e., clinically suspect arthralgia (CSA) and ACPA+ individuals an ultrasound should be performed. If the ultrasound is negative MRI may be able to detect subtle sub-clinical inflammation, particularly in extracapsular structures. For at risk individuals such as first-degree relatives with non-inflammatory musculoskeletal (MSK) symptoms radiographs should be performed to look for alterative diagnoses.
With management strategies in early RA moving to a more personalized and preventative approach, risk stratification models which include serological, cellular and imaging biomarkers are being increasingly formulated. It is therefore essential that we continue to optimize the use different imaging techniques within this important cohort.
Author contributions
KH, AD, and KM contributed to the literature review and drafting of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthr Res. (2002) 4:S265–72.
2. Hua C, Daien CI, Combe B, Landewe R. Diagnosis, prognosis and classification of early arthritis: results of a systematic review informing the 2016 update of the EULAR recommendations for the management of early arthritis. Rheumatic Muscul Dis Open. (2017) 3:e000406. doi: 10.1136/rmdopen-2016-000406
3. Barhamain AS, Magliah RF, Shaheen MH, Munassar SF, Falemban AM, Alshareef MM, et al. The journey of rheumatoid arthritis patients: a review of reported lag times from the onset of symptoms. Open Access Rheumatol. (2017) 9:139–50. doi: 10.2147/OARRR.S138830
4. Verstappen M, van Mulligen E, de Jong PHP, van der Helm-Van Mil AHM. DMARD-free remission as novel treatment target in rheumatoid arthritis: a systematic literature review of achievability and sustainability. Rheumatic Muscul Dis Open. (2020) 6:e001220. doi: 10.1136/rmdopen-2020-001220
5. Mankia K, Di Matteo A, Emery P. Prevention and cure: the major unmet needs in the management of rheumatoid arthritis. J Autoim. (2020) 110:102399.
6. Mankia K, Siddle HJ, Kerschbaumer A, Alpizar Rodriguez D, Catrina AI, Cañete JD, et al. EULAR points to consider for conducting clinical trials and observational studies in individuals at risk of rheumatoid arthritis. Ann Rheumat Dis. (2021) 80:1286–98.
7. van Steenbergen HW, Aletaha D, Beaart-van de Voorde LJJ, Brouwer E, Codreanu C, Combe B, et al. EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Ann Rheumat Dis. (2017) 76:491–6.
8. van Steenbergen HW, van Nies JAB, Huizinga TWJ, Bloem JL, Reijnierse M, van der Helm-van Mil AHM. Characterising arthralgia in the preclinical phase of rheumatoid arthritis using MRI. Ann Rheumat Dis. (2015) 74:1225–32. doi: 10.1136/annrheumdis-2014-205522
9. Klarlund M, Østergaard M, Jensen KE, Madsen JL, Skjødt H, Lorenzen I. Magnetic resonance imaging, radiography, and scintigraphy of the finger joints: one year follow up of patients with early arthritis. Ann Rheumat Dis. (2000) 59:521–8.
10. Backhaus M, Burmester GR, Sandrock D, Loreck D, Hess D, Scholz A, et al. Prospective two year follow up study comparing novel and conventional imaging procedures in patients with arthritic finger joints. Ann Rheumat Dis. (2002) 61:895–904. doi: 10.1136/ard.61.10.895
11. Sugimoto H, Takeda A, Hyodoh K. Early-stage rheumatoid arthritis: prospective study of the effectiveness of MR imaging for diagnosis. Radiology. (2000) 216:569–75.
12. Garrigues F, Jousse-Joulin S, Bouttier R, Nonent M, Bressollette L, Saraux A. Concordance between clinical and ultrasound findings in rheumatoid arthritis. Joint Bone Spine Revue Du Rhumat. (2013) 80:597–603.
13. Stach CM, Bäuerle M, Englbrecht M, Kronke G, Engelke K, Manger B, et al. Periarticular bone structure in rheumatoid arthritis patients and healthy individuals assessed by high−resolution computed tomography. Arthr Rheumat. (2010) 62:330–9.
14. Fosse P, Kaiser M-J, Namur G, de Seny D, Malaise MG, Hustinx R. 18F- FDG PET/CT joint assessment of early therapeutic response in rheumatoid arthritis patients treated with rituximab. Eur J Hybrid Imaging. (2018) 2:6. doi: 10.1186/s41824-017-0022-y
15. Krijbolder DI, Verstappen M, van Dijk BT, Dakkak YJ, Burgers LE, Boer AC, et al. Intervention with methotrexate in patients with arthralgia at risk of rheumatoid arthritis to reduce the development of persistent arthritis and its disease burden (TREAT EARLIER): a randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet. (2022) 400:283–94. doi: 10.1016/S0140-6736(22)01193-X
16. Gerlag DM, Safy M, Maijer KI, Tang MW, Tas SW, Starmans-Kool MJF, et al. Effects of B-cell directed therapy on the preclinical stage of rheumatoid arthritis: the PRAIRI study. Ann Rheumat Dis. (2019) 78:179–85. doi: 10.1136/annrheumdis-2017-212763
17. Rech J, Ostergaard M, Tascilar K. Abatacept reverses subclinical arthritis in patients with high-risk to develop rheumatoid arthritis -results from the randomized, placebo-controlled ARIAA study in RA-at risk patients. Am College Rheumatol Converg. (2021). 73(suppl 9).
18. Munn Z, Jordan Z. The patient experience of high technology medical imaging: a systematic review of the qualitative evidence. Radiography. (2011) 17:323–31.
19. Cate D, Luime JJ, Swen N, Gerards AH, de Jager MH, Basoski NM, et al. Role of ultrasonography in diagnosing early rheumatoid arthritis and remission of rheumatoid arthritis - a systematic review of the literature. Arthr Res Ther. (2013) 15:R4–R.
20. Rowbotham EL, Wakefield RJ. The technique and application of ultrasound in the diagnosis and management of inflammatory arthritis. Sem Muscul Radiol. (2012) 16:360–6.
21. Pentony P, Mankia K, Hensor EM, Nam JL, Hunt L, Garcia-Montoya L, et al. SAT0107 sequential ultrasound shows a late increase in inflammatory burden in anti-ccp positive patients with non-specific musculoskeletal symptoms just before progression to inflammatory arthritis. Ann Rheumat Dis. (2018) 77:916.
22. Di Matteo A, Duquenne L, Cipolletta E, Nam JL, Garcia Montoya L, Wakefield RJ, et al. Ultrasound subclinical synovitis in anti-CCP-positive at-risk individuals with musculoskeletal symptoms: an important and predictable stage in the rheumatoid arthritis continuum. Rheumatology. (2022) 61:3192–200. doi: 10.1093/rheumatology/keab862
23. Brulhart L, Alpízar-Rodríguez D, Nissen MS, Zufferey P, Ciubotariu I, Fleury G, et al. Ultrasound is not associated with the presence of systemic autoimmunity or symptoms in individuals at risk for rheumatoid arthritis. Rheumat Muscul Dis Open. (2019) 5:e000922.
24. Rogier C, Wouters F, van Boheemen L, van Schaardenburg D, de Jong PHP, van der Helm-van Mil AHM. Subclinical synovitis in arthralgia: how often does it result in clinical arthritis? Reflecting on starting points for disease-modifying anti-rheumatic drug treatment. Rheumatology. (2021) 60:3872–8. doi: 10.1093/rheumatology/keaa774
25. Padovano I, Costantino F, Breban M, D’Agostino MA. Prevalence of ultrasound synovial inflammatory findings in healthy subjects. Ann Rheumat Dis. (2016) 75:1819–23.
26. Mankia K, Briggs C, Emery P. How are rheumatologists managing anticyclic citrullinated peptide antibodies–positive patients who do not have arthritis? J Rheumatol. (2020) 47:305–6. doi: 10.3899/jrheum.190211
27. Rakieh C, Nam JL, Hunt L, Hensor EM, Das S, Bissell LA, et al. Predicting the development of clinical arthritis in anti-CCP positive individuals with non-specific musculoskeletal symptoms: a prospective observational cohort study. Ann Rheumat Dis. (2015) 74:1659–66.
28. Nam J, Hensor E, Hunt L, Conaghan P, Wakefield R, Emery P. Ultrasound findings predict progression to inflammatory arthritis in anti-CCP antibody-positive patients without clinical synovitis. Ann Rheumat Dis. (2016) 75:2060–7. doi: 10.1136/annrheumdis-2015-208235
29. van de Stadt LA, Bos WH, Reynders MM, Wieringa H, Turkstra F, van der Laken J, et al. The value of ultrasonography in predicting arthritis in auto-antibody positive arthralgia patients: a prospective cohort study. Arthr Res Ther. (2010) 12:R98. doi: 10.1186/ar3028
30. Zufferey P, Rebell C, Benaim C, Ziswiler HR, Dumusc A, So A. Ultrasound can be useful to predict an evolution towards rheumatoid arthritis in patients with inflammatory polyarthralgia without anticitrullinated antibodies. Joint Bone Spine Revue Du Rhumat. (2016) 84:299–303. doi: 10.1016/j.jbspin.2016.05.011
31. Ven M, van der Veer-Meerkerk M, Cate D, Rasappu N, Kok MR, Csakvari D, et al. Absence of ultrasound inflammation in patients presenting with arthralgia rules out the development of arthritis. Arthr Res Ther. (2017) 19:202. doi: 10.1186/s13075-017-1405-y
32. Molina Collada J, López Gloria K, Castrejón I, Nieto-González JC, Rivera J, Montero F, et al. Ultrasound in clinically suspect arthralgia: the role of power doppler to predict rheumatoid arthritis development. Arthr Res Ther. (2021) 23:299.
33. Ohrndorf S, Boer AC, Boeters DM, ten Brinck RM, Burmester GR, Kortekaas MC, et al. Do musculoskeletal ultrasound and magnetic resonance imaging identify synovitis and tenosynovitis at the same joints and tendons? A comparative study in early inflammatory arthritis and clinically suspect arthralgia. Arthr Res Ther. (2019) 21:59. doi: 10.1186/s13075-019-1824-z
34. Di Matteo A, Mankia K, Duquenne L, Cipolletta E, Wakefield RJ, Garcia-Montoya L, et al. Ultrasound erosions in the feet best predict progression to inflammatory arthritis in anti-CCP positive at-risk individuals without clinical synovitis. Ann Rheumat Dis. (2020) 79:901–7. doi: 10.1136/annrheumdis-2020-217215
35. Wakefield RJ, O’Connor PJ, Conaghan PG, McGonagle D, Hensor EMA, Gibbon WW, et al. Finger tendon disease in untreated early rheumatoid arthritis: a comparison of ultrasound and magnetic resonance imaging. Arthr Rheumat. (2007) 57:1158–64. doi: 10.1002/art.23016
36. Bøyesen P, Haavardsholm EA, Østergaard M, van der Heijde D, Sesseng S, Kvien TK. MRI in early rheumatoid arthritis: synovitis and bone marrow edema are independent predictors of subsequent radiographic progression. Ann Rheumat Dis. (2011) 70:428–33.
37. Parodi M, Silvestri E, Garlaschi G, Cimmino MA. How normal are the hands of normal controls? A study with dedicated magnetic resonance imaging. Clin Exp Rheumatol. (2006) 24:134–41.
38. Ejbjerg B, Narvestad E, Rostrup E, Szkudlarek M, Jacobsen S, Thomsen HS, et al. Magnetic resonance imaging of wrist and finger joints in healthy subjects occasionally shows changes resembling erosions and synovitis as seen in rheumatoid arthritis. Arthr Rheumat. (2004) 50:1097–106. doi: 10.1002/art.20135
39. Mangnus L, van Steenbergen HW, Reijnierse M, van der Helm-van Mil AHM. Magnetic resonance imaging–detected features of inflammation and erosions in symptom−free persons from the general population. Arthr Rheumatol. (2016) 68:2593–602. doi: 10.1002/art.39749
40. Gent Y, ter Wee MM, Ahmadi N, van Kuijk C, Voskuyl AE, van der Laken CJ, et al. Three-year clinical outcome following baseline magnetic resonance imaging in anti-citrullinated protein antibody-positive arthralgia patients: an exploratory study. Arthr Rheumatol. (2014) 66:2909–10. doi: 10.1002/art.38757
41. Boer AC, Burgers LE, Mangnus L, Ten Brinck RM, Nieuwenhuis WP, van Steenbergen HW, et al. Using a reference when defining an abnormal MRI reduces false-positive MRI results-a longitudinal study in two cohorts at risk for rheumatoid arthritis. Rheumatology. (2017) 56:1700–6. doi: 10.1093/rheumatology/kex235
42. van Steenbergen HW, Mangnus L, Reijnierse M, Huizinga TWJ, van der Helm-van Mil AHM. Clinical factors, anticitrullinated peptide antibodies and MRI-detected subclinical inflammation in relation to progression from clinically suspect arthralgia to arthritis. Ann Rheumat Dis. (2016) 75:1824–30. doi: 10.1136/annrheumdis-2015-208138
43. Roivainen A, Hautaniemi S, Möttönen T, Nuutila P, Oikonen V, Parkkola R, et al. Correlation of 18F-FDG PET/CT assessments with disease activity and markers of inflammation in patients with early rheumatoid arthritis following the initiation of combination therapy with triple oral antirheumatic drugs. Eur J Nuclear Med Mol Imaging. (2012) 40:403–10. doi: 10.1007/s00259-012-2282-x
44. Gent Y, Ahmadi N, Voskuyl AE, Hoetjes NJ, van Kuijk C, Britsemmer K, et al. Detection of subclinical synovitis with macrophage targeting and positron emission tomography in patients with rheumatoid arthritis without clinical arthritis. J Rheumatol. (2014) 41:2145–52.
45. Gent YYJ, Voskuyl AE, Kloet RW, van Schaardenburg D, Hoekstra OS, Dijkmans BAC, et al. Macrophage positron emission tomography imaging as a biomarker for preclinical rheumatoid arthritis: findings of a prospective pilot study. Arthr Rheumat. (2012) 64:62–6. doi: 10.1002/art.30655
46. Güler-Yüksel M, Allaart CF, Goekoop-Ruiterman YPM, de Vries-Bouwstra JK, van Groenendael JHLM, Mallée C, et al. Changes in hand and generalised bone mineral density in patients with recent-onset rheumatoid arthritis. Ann Rheumat Dis. (2009) 68:330–6. doi: 10.1136/ard.2007.086348
47. De Rooy DPC, KÄLvesten J, Huizinga TWJ, Van Der Helm-Van Mil AHM. Loss of metacarpal bone density predicts RA development in recent-onset arthritis. Rheumatology. (2012) 51:1037–41. doi: 10.1093/rheumatology/ker435
48. Kleyer A, Finzel S, Rech J, Manger B, Krieter M, Faustini F, et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheumat Dis. (2014) 73:854–60.
49. Hoff M, Haugeberg G, Ødegård S, Syversen S, Landewé R, van der Heijde D, et al. Cortical hand bone loss after 1 year in early rheumatoid arthritis predicts radiographic hand joint damage at 5-year and 10-year follow-up. Ann Rheumat Dis. (2009) 68:324–9. doi: 10.1136/ard.2007.085985
50. Keller KK, Thomsen JS, Stengaard-Pedersen K, Nielsen AW, Schiøttz-Christensen B, Svendsen L, et al. Local bone loss in patients with anti-citrullinated peptide antibody and arthralgia, evaluated with high-resolution peripheral quantitative computed tomography. Scand J Rheumatol. (2018) 47:110–6. doi: 10.1080/03009742.2017.1333629
51. Simon D, Kleyer A, Cong DB, Hueber A, Bang H, Ramming A, et al. Microstructural bone changes are associated with broad−spectrum autoimmunity and predict the onset of rheumatoid arthritis. Arthr Rheumatol. (2022) 74:418–26. doi: 10.1002/art.41229
52. Wakefield RJ, Gibbon WW, Conaghan PG, O’Connor P, McGonagle D, Pease C, et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthr Rheumat. (2000) 43:2762–70.
53. Rahmani M, Chegini H, Najafizadeh SR, Azimi M, Habibollahi P, Shakiba M. Detection of bone erosion in early rheumatoid arthritis: ultrasonography and conventional radiography versus non-contrast magnetic resonance imaging. Clin Rheumatol. (2010) 29:883–91. doi: 10.1007/s10067-010-1423-5
54. Di Matteo A, Mankia K, Nam JL, Cipolletta E, Garcia-Montoya L, Duquenne L, et al. In anti-CCP+ at-risk individuals, radiographic bone erosions are uncommon and are not associated with the development of clinical arthritis. Rheumatology. (2021) 60:3156–64. doi: 10.1093/rheumatology/keaa761
55. Hunt L, Nam J, Hensor EM, Mankia K, Rowbotham E, Grainger AJ, et al. OP0042 In acpa positive at-risk individuals, which mri and us findings best predict development of clinical synovitis? Ann Rheumat Dis. (2018) 77:72.
56. Kleyer A, Krieter M, Oliveira I, Faustini F, Simon D, Kaemmerer N, et al. High prevalence of tenosynovial inflammation before onset of rheumatoid arthritis and its link to progression to RA—a combined MRI/CT study. Sem Arthr Rheumat. (2016) 46:143–50. doi: 10.1016/j.semarthrit.2016.05.002
57. Matthijssen XME, Wouters F, Boeters DM, Boer AC, Dakkak YJ, Niemantsveriet E, et al. A search to the target tissue in which RA-specific inflammation starts: a detailed MRI study to improve identification of RA-specific features in the phase of clinically suspect arthralgia. Arthr Res Ther. (2019) 21:249. doi: 10.1186/s13075-019-2002-z
58. Mankia K, D’Agostino MA, Rowbotham E, Hensor EMA, Hunt L, Möller I, et al. MRI inflammation of the hand interosseous tendons occurs in anti-CCP positive at-risk individuals and may precede the development of clinical synovitis. Ann Rheumat Dis. (2019) 78:781–6. doi: 10.1136/annrheumdis-2018-214331
59. Trickey J, Sahbudin I, Ammitzbøll-Danielsen M, Azzolin I, Borst C, Bortoluzzi A, et al. Very low prevalence of ultrasound-detected tenosynovial abnormalities in healthy subjects throughout the age range: OMERACT ultrasound minimal disease study. Ann Rheumat Dis. (2021) 81:232–6. doi: 10.1136/annrheumdis-2021-219931
60. Mankia K, D’Agostino MA, Wakefield RJ, Nam JL, Mahmood W, Grainger AJ, et al. Identification of a distinct imaging phenotype may improve the management of palindromic rheumatism. Ann Rheumat Dis. (2019) 78:43–50. doi: 10.1136/annrheumdis-2018-214175
61. van Dijk BT, Wouters F, van Mulligen E, Reijnierse M, van der Helm-van Mil AHM. During development of rheumatoid arthritis, intermetatarsal bursitis may occur before clinical joint swelling: a large imaging study in patients with clinically suspect arthralgia. Rheumatology. (2021) 61:2805–14. doi: 10.1093/rheumatology/keab830
62. Aizenberg E, ten Brinck RM, Reijnierse M, van der Helm-van Mil AHM, Stoel BC. Identifying MRI-detected inflammatory features specific for rheumatoid arthritis: two-fold feature reduction maintains predictive accuracy in clinically suspect arthralgia patients. Sem Arthr Rheumat. (2019) 48:579–86. doi: 10.1016/j.semarthrit.2018.04.005
63. Aga A-B, Hammer HB, Olsen IC, Uhlig T, Kvien TK, van der Heijde D, et al. First step in the development of an ultrasound joint inflammation score for rheumatoid arthritis using a data-driven approach. Ann Rheumat Dis. (2016) 75:1444–51. doi: 10.1136/annrheumdis-2015-207572
64. Østergaard M, Peterfy CG, Bird P, Gandjbakhch F, Glinatsi D, Eshed I, et al. The OMERACT rheumatoid arthritis magnetic resonance imaging (MRI) scoring system: updated recommendations by the OMERACT MRI in arthritis working group. J Rheumatol. (2017) 44:1706–12. doi: 10.3899/jrheum.161433
65. Mo Y-Q, Yang Z-H, Wang J-W, Li Q-H, Du X-Y, Huizinga TW, et al. The value of MRI examination on bilateral hands including proximal interphalangeal joints for disease assessment in patients with early rheumatoid arthritis: a cross-sectional cohort study. Arthr Res Ther. (2019) 21:279. doi: 10.1186/s13075-019-2061-1
66. Anderson SE, Steinbach LS, De Monaco D, Bonel HM, Hurtienne Y, Voegelin E. “Baby wrist”: MRI of an overuse syndrome in mothers. Am J Roentgenol. (2004) 182:719–24. doi: 10.2214/ajr.182.3.1820719
67. Zhao SS, Nikiphorou E, Young A, Kiely PDW. Large joints are progressively involved in rheumatoid arthritis irrespective of rheumatoid factor status—results from the early rheumatoid arthritis study. Rheumatol Int. (2021) 42:621–9. doi: 10.1007/s00296-021-04931-2
68. Scott DL, Coulton BL, Popert AJ. Long term progression of joint damage in rheumatoid arthritis. Ann Rheumat Dis. (1986) 45:373–8.
69. Rogier C, van der Ven M, van der Helm-van Mil AHM, de Jong PHP. Is shoulder involvement in clinically suspect arthralgia an early feature of rheumatoid arthritis? A longitudinal ultrasound study. Rheumatology. (2020) 59:2640–2. doi: 10.1093/rheumatology/keaa052
70. de Hair MJH, van de Sande MGH, Ramwadhdoebe TH, Hansson M, Landewé R, van der Leij C, et al. Features of the synovium of individuals at risk of developing rheumatoid arthritis: implications for understanding preclinical rheumatoid arthritis. Arthr Rheumatol. (2014) 66:513–22. doi: 10.1002/art.38273
71. Boer AC, Wouters F, Dakkak YJ, Niemantsveriet E, van der Helm van Mil A. Improving the feasibility of MRI in clinically suspect arthralgia for prediction of rheumatoid arthritis by omitting scanning of the feet. Rheumatology. (2020) 59:1247–52. doi: 10.1093/rheumatology/kez436
72. Krijbolder DI, Wouters F, van Mulligen E, van der Helm-van Mil AHM. Morning stiffness precedes the development of rheumatoid arthritis and associates with systemic and subclinical joint inflammation in arthralgia patients. Rheumatology. (2022) 61:2113–8. doi: 10.1093/rheumatology/keab651
73. Wouters F, van der Giesen FJ, Matthijssen XME, Niemantsverdriet E, van der Helm-van Mil AHM. Difficulties making a fist in clinically suspect arthralgia: an easy applicable phenomenon predictive for RA that is related to flexor tenosynovitis. Ann Rheumat Dis. (2019) 78:1438–9. doi: 10.1136/annrheumdis-2019-215402
74. Wouters F, Niemantsverdriet E, Van der Helm van Mil A. Ab1258 the value of the squeeze test for detection of subclinical synovitis in patients with arthralgia suspicious for progression to ra. Ann Rheumat Dis. (2020) 79:1920–1. doi: 10.1093/rheumatology/keaa082
75. Freeston JE, Wakefield RJ, Conaghan PG, Hensor EMA, Stewart SP, Emery P. A diagnostic algorithm for persistence of very early inflammatory arthritis: the utility of power doppler ultrasound when added to conventional assessment tools. Ann Rheumat Dis. (2010) 69:417–9. doi: 10.1136/ard.2008.106658
76. Mizuki S, Horie K, Imabayashi K, Mishima K, Oryoji K. POS0441 development of rheumatoid arthritis among anti-citrullinated protein antibodies positive asymptomatic individuals: a prospective observational study. Ann Rheumat Dis. (2021) 80:449–449.
77. Cabrera-Villalba S, Ramirez J, Salvador G, Ruiz-Esquide V, Hernandez MV, Inciarte-Mundo J, et al. Is there subclinical synovitis in patients with palindromic rheumatism in the intercritical period? A clinical and ultrasonographic study according to anticitrullinated protein antibody status. J Rheumatol. (2014) 41:1650–5. doi: 10.3899/jrheum.131545
78. Russell AS, Devani A, Maksymowych WP. The role of anti-cyclic citrullinated peptide antibodies in predicting progression of palindromic rheumatism to rheumatoid arthritis. J Rheumatol. (2006) 33:1240–2.
79. Tamai M, Kawakami A, Iwamoto N, Arima K, Aoyagi K, Eguchi K. Contribution of anti-CCP antibodies, proximal interphalangeal joint involvement, HLA-DRB1 shared epitope, and PADI4 as risk factors for the development of rheumatoid arthritis in palindromic rheumatism. Scand J Rheumatol. (2010) 39:287–91. doi: 10.3109/03009741003604534
80. Khabbazi A, Hajialiloo M, Kolahi S, Soroosh M, Esalatmanesh K, Sharif S. A multicenter study of clinical and laboratory findings of palindromic rheumatism in Iran. Int J Rheumat Dis. (2012) 15:427–30. doi: 10.1111/j.1756-185X.2012.01739.x
81. Emad Y, Anbar A, Abo-Elyoun I, El Shaarawy N, Al-Hanafi H, Darwish H, et al. In palindromic rheumatism, hand joint involvement and positive anti-CCP antibodies predict RA development after 1 year of follow-up. Clin Rheumatol. (2014) 33:791–7. doi: 10.1007/s10067-014-2569-3
82. Chen H-H, Lan J-L, Hung G-D, Chen Y-M, Lan HH-C, Chen D-Y. Association of ultrasonographic findings of synovitis with anti-cyclic citrullinated peptide antibodies and rheumatoid factor in patients with palindromic rheumatism during active episodes. J Ultrasound Med. (2009) 28:1193–9. doi: 10.7863/jum.2009.28.9.1193
Keywords: ultrasound, rheumatoid arthritis, magnetic resonance imaging, computed tomography, at risk of rheumatoid arthritis, clinically suspect arthralgia, ACPA, palindromic rheumatism
Citation: Harnden K, Di Matteo A and Mankia K (2022) When and how should we use imaging in individuals at risk of rheumatoid arthritis? Front. Med. 9:1058510. doi: 10.3389/fmed.2022.1058510
Received: 30 September 2022; Accepted: 07 November 2022;
Published: 23 November 2022.
Edited by:
Ashish Jacob Mathew, Christian Medical College and Hospital, IndiaReviewed by:
Felipe Julio Ramírez, Hospital Clinic of Barcelona, SpainJuan Carlos Nieto González, Gregorio Marañón Hospital, Spain
Copyright © 2022 Harnden, Di Matteo and Mankia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kate Harnden, a2F0ZS5oYXJuZGVuQG5ocy5uZXQ=
 Kate Harnden
Kate Harnden Andrea Di Matteo
Andrea Di Matteo Kulveer Mankia
Kulveer Mankia