
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 09 January 2023
Sec. Geriatric Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1058464
This article is part of the Research Topic Molecular and Physiological Aspects of Sarcopenia in the Older Person: Mechanisms, Diagnostics and Therapy View all 12 articles
Objective: Sarcopenia is a syndrome of decreased muscle mass and deficits in muscle strength and physical function. We aimed to investigate the relationship between creatinine/cystatin C ratio (CCR) and sarcopenia and the prognostic value of CCR in hospitalized patients.
Materials and methods: We searched for relevant studies in PubMed, EMBASE, and the Cochrane Database up to August 25, 2022. Meta-analyses were performed to evaluate the relationship between CCR and skeletal muscle [computed tomography-assessed skeletal muscle (CTASM), muscle strength, and physical performance], prognosis and important clinical outcomes in hospitalized adults. The pooled correlation coefficient, the area under the receiver operating characteristic (ROC) curves, and hazard ratio (HR) together with their 95% confidence intervals (CIs) were calculated. We also conducted subgroup analyses to explore the sources of heterogeneity.
Results: A total of 38 studies with 20,362 patients were eligible. These studies were of moderate to high quality. Our results showed that CCR was significant correlations with all CTASM types (Fisher’s Z ranged from 0.35 to 0.5; P values ranged from < 0.01 to 0.01), handgrip strength (Fisher’s Z = 0.39; 95% CI, 0.32–0.45; P < 0.001) and gait speed (Fisher’s Z = 0.25; 95% CI, 0.21–0.30; P < 0.001). The ROC curves suggested that CCR had good diagnostic efficacy (0.689; 95% CI, 0.632–0.746; P < 0.01) for sarcopenia. CCR can reliably predict mortality in hospitalized patients, which was confirmed by regression analysis of CCR as both continuous (HR 0.78; 95% CI, 0.72–0.84; P < 0.01) and categorical variables (HR 2.05; 95% CI, 1.58–2.66; P < 0.0001). In addition, less evidence showed that higher CCR was independently associated with a shorter duration of mechanical ventilation, reduced length of stay in the intensive care unit and hospital, less nutritional risk, and decreased complications in hospitalized patients.
Conclusion: CCR could be a simple, economical, and effective screening tool for sarcopenia in hospitalized patients, and it is a helpful prognostic factor for mortality and other important clinical outcomes.
Systematic review registration: https://inplasy.com/inplasy-2022-9-0097/, identifier INPLASY202290097.
Sarcopenia is traditionally been considered a syndrome characterized by reduced muscle mass, deficiencies in muscle strength, and impairments physical function (1). It has been thought that sarcopenia is more prevalent in old patients, especially those over 65 (2, 3). Sarcopenia, however, is common in hospitalized patients of all ages and is associated with various adverse outcomes, including impaired organ functions, infectious complications, prolonged length of stay (LOS) in intensive care unit (ICU) or hospital, and even increased mortality rates (4–7). Therefore, adequate body muscle reserve is crucial for hospitalized patients’ recovery and survival.
Previous indicators used to evaluate muscle reserve, such as anthropometrics, lab tests, subjective judgment, and body mass index (BMI), fail to reflect the patient’s body composition accurately (8). Clinicians may now directly measure muscle mass thanks to advances in imaging and software technologies (9, 10). In particular, computed tomography (CT), has been recognized as the gold standard for identifying skeletal muscle because it can accurately distinguish skeletal muscle and fat mass using a single cross-sectional slice at multiple body levels (11). However, high cost, radiological damage, and equipment unavailability limit the widespread use of these techniques (10, 11). In addition, these techniques are not conducive to continuous monitoring of muscle mass changes. As a result, there is an urgent need for other simple and inexpensive biomarkers to diagnose and monitor sarcopenia.
Serum creatinine and cystatin C are widely used in clinical practice to assess renal function. And the ratio of the two markers (serum creatinine/serum cystatin C) x100, known as CCR, has recently attracted interest. In 2016, Kashani et al. validated the correlation between muscle mass and CCR, which they defined as the “sarcopenia index” (12). Moreover, the authors found that CCR could predict hospital and 90-day mortality in patients who did not have acute renal damage. Since then, CCR has been increasingly used for critically ill patients (13, 14), the elderly (6, 7), organ transplant recipients (15, 16), and type 2 diabetic patients (17). However, substantial variation in study design, sample size, demographics, and muscle assessment among these studies lead to inconsistent results (6, 7, 12, 13, 18). Furthermore, there are no meta-analyses to examine the values of CCR on muscle mass measurement and prognosis in these patient populations.
Several studies on CCR in hospitalized patients have been published recently (14, 19–24). Therefore, with the power of meta-analysis, we aimed to perform a systematic review and meta-analysis of available published articles about hospitalization patients to investigate (1) whether CCR is a better and more accurate index of muscle mass, muscle strength, and gait speed; (2) the applicability of using CCR as a screening method for sarcopenia; and (3) the association of CCR with clinical outcomes (i.e., mortality, duration of mechanical ventilation, length of stay in ICU or hospital, and complications).
The systematic review was already registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols database, and it is now available in its entirety on inplasy.com.1 It was carried out in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (25; Supplementary file 1).
We conducted a systematic search of relevant studies in PubMed, EMBASE, and the Cochrane Library from their establishment to August 25, 2022 (The last search date). Using a combination of MeSH and keywords, search terms included “creatinine,” “cystatin C,” “creatinine/cystatin C ratio,” “creatinine to cystatin C ratio,” “creatinine over cystatin C ratio,” “creatinine-to-cystatin C ratio,” and “sarcopenia index.” We restricted the language to English. Two authors (W-HZ and YY) independently imported the papers into Endnote X7 to exclude duplicate research and screen the literature (titles, abstracts, and full texts). We read meta-analyses, reviews, and comments to find more potential articles. The reference lists of the included full-text papers were also examined. We included the most recent published or reported data for republished studies. Disagreements were resolved through discussions between the two authors.
We included articles investigating the correlation between CCR and CT-assessed skeletal muscle and the predictive prognosis value of CCR in hospitalized patients. The particular inclusion criteria were as follows, based on the PICOS (population, intervention, comparison, outcome, design) principle:
(1) adult (> 18 years old) hospitalized patients.
(2) evaluation of skeletal muscle amount (area) or quality (density) as determined by CT using any clear and objective methods.
(3) studies should report the correlation between CCR and CTASM or patient survival information.
(4) eligible studies had a cohort, case-control, or randomized controlled study design.
We excluded the studies that reported data without predefined outcomes and focused on animals or pregnant women. Studies available only in abstract form or meeting reports were also excluded.
Two authors (W-HZ and YY) independently extracted data from included studies on the following items: first author, publication year, geographic location, study design, research period, population, sample size, demographic characteristics, disease severity, details on CT technique (muscle measured, CT-scan level), sarcopenia criteria and prevalence, outcomes of interest and methodological quality.
Two authors (W-HZ and YY) independently assessed the quality of each included study using the Newcastle-Ottawa Scale (NOS) for cohort studies (26). The NOS is divided into three domains depending on the cohort’s selection, the comparability of the groups, and the quality of the results. The included research was granted a maximum of one point for each item in the selection and outcome domains, and a maximum of two points for the comparability domain. The scale ratings ranged from 0 to 9, with 8 or 9 being categorized as good quality, 6 or 7 as moderate quality, and 5 or less as low quality. Disagreements were recognized and addressed through discussion.
The primary outcome of the current meta-analysis was to investigate the suitability of CCR as a predictive mortality tool in hospitalized patients. To minimize potential interference factors, we only pooled the regression analysis findings of the included studies to investigate the link between CCR and mortality at the longest follow-up available. The HR in related studies were converted to their natural logarithms, and standard error (SE) values were determined using these logarithms and their respective 95% CI.
Secondary outcomes included associations between CCR and CTASM evaluation, muscle strength, gait speed, nutrition screening tool, or other clinical outcomes (i.e., duration of mechanical ventilation, length of stay in ICU or hospital, and complications). As to these outcomes, we conducted related meta-analyses individually for the various data reporting types as follows among the included studies. (1) For the studies that provided the correlation coefficient between CCR and predefined outcomes (i.e., CTASM, handgrip strength [HGS], gait speed [GS], and nutrition screening tool), we performed a meta-analysis by quantitatively summarizing the correlation coefficient statistic (r) estimates. Fisher’s Z and its SE were calculated using r and sample size (N) as follows: Zr = (1/2) [loge (1 + r) − loge (1 − r)], SEzr = 1/sqrt[N−3] (27). After appropriate transformation, we used the inverse variance-weighted approach to determine effect sizes and the associated 95% confidence intervals (CI). (2) For the studies reporting the diagnostic value of CCR for detecting sarcopenia, we pooled Area under the curve (AUC) values using the mean and standard error SE values and weighted them using the inverse-variance method (28). (3) We collected and pooled OR with 95% CI via the generalized inverse-variance method for studies that showed an association between CCR and sarcopenia using regression analysis. Unless otherwise noted in the above meta-analyses, we preferred the adjusted analysis results.
We used the I2 statistic to quantify heterogeneity (I2 < 50 and > 50% were classified as low and high heterogeneity, respectively) (29). When there was significant heterogeneity, a random-effects model was used; otherwise, a fixed-effects model was utilized (30). We then performed sensitivity analyses, removing one study at a time to demonstrate the impact of that study on the pooled effect estimates. Visually inspecting funnel plots for asymmetry was used to determine publication bias. Meta-analysis was conducted when data from at least 3 studies were available. P values of less than 0.05 were regarded as statistically significant. We utilized R version 3.6.2 for all statistical analyses in the current meta-analysis.
Subgroup analyses were performed to find the potential sources of heterogeneity on the following properties:
(1) Geographic location: Asian and other countries;
(2) Patient population: critical illness, cancer, medical, and surgery patients; and
(3) Gender (i.e., male and female).
The comprehensive literature search yielded 213 studies. Following evaluation of the title, abstract, and full text, 38 papers involving 20,362 participants fulfilled the criteria for inclusion in the current systematic review (5–7, 12–24, 31–52). Figure 1 shows how the search strategy flows and the selection process.
Table 1 and Supplementary file 2 present the characteristics of the eligible studies. All these observational studies were published between 2016 and 2022 in six countries (China n = 16, Japan n = 9, Korea n = 6, USA n = 4, Argentina n = 2, and France n = 1). These studies focused on patients with critical illnesses, cancer, medical and surgical departments, and unselected hospitalized patients. The participants’ mean age ranged from 47 to 88 years old, and their BMI was from 20.7 to 28 kg/m2. The included studies have a mean mortality rate of 13% (ranging from 8.7 to 43.2%). The criteria and cut-offs for evaluating the sarcopenia varied among the studies. Twenty studies used regression analyses to investigate the relationship between CCR and mortality (5–7, 12, 14, 15, 18, 19, 23, 24, 31, 32, 37–40, 46, 49, 51). Regarding the relationship between CCR and muscle evaluation, 21 studies provided Pearson correlation coefficient (r) levels between CCR and CTASM, 12 evaluated the diagnostic value of CCR in sarcopenia, and six used adjusted HR/OR to predict sarcopenia.
The study quality ranged from moderate to high, according to the specifics of the quality evaluation in Supplementary Table 2 (Scores range from 6 to 9). Overall, 24 studies were judged to be of good quality, while 14 study was considered to be of moderate quality (Table 1).
Twenty studies with 13,560 patients investigated the impact of CCR on mortality in hospitalized patients using HR (5–7, 12, 14, 15, 18, 19, 23, 24, 31, 32, 37–40, 46, 49, 51). Among these studies, 13 studies with 11,355 patients reported the CCR treated as a continuous variable, and the pooled results showed a higher CCR was independently associated with a lower risk of mortality (HR 0.78; 95% CI, 0.72–0.84; I2 = 94%; P < 0.01, Figure 2A; 5–7, 12, 19, 23, 24, 31, 32, 37, 38, 40, 49). A total of 10 studies including 9,164 patients reported the risk estimation according to CCR categories (6, 7, 13, 14, 18, 19, 37–39, 46). When pooled, there was a significant prognostic role for the CCR category on patients’ mortality (HR 2.05; 95% CI, 1.58–2.66; I2 = 93%; P < 0.0001). That is, patients with low values of CCR were less likely to survive than patients with high values (Figure 2B). A graph shaped symmetrical inverted funnel indicates there is no publication bias (Supplementary Figure 1).
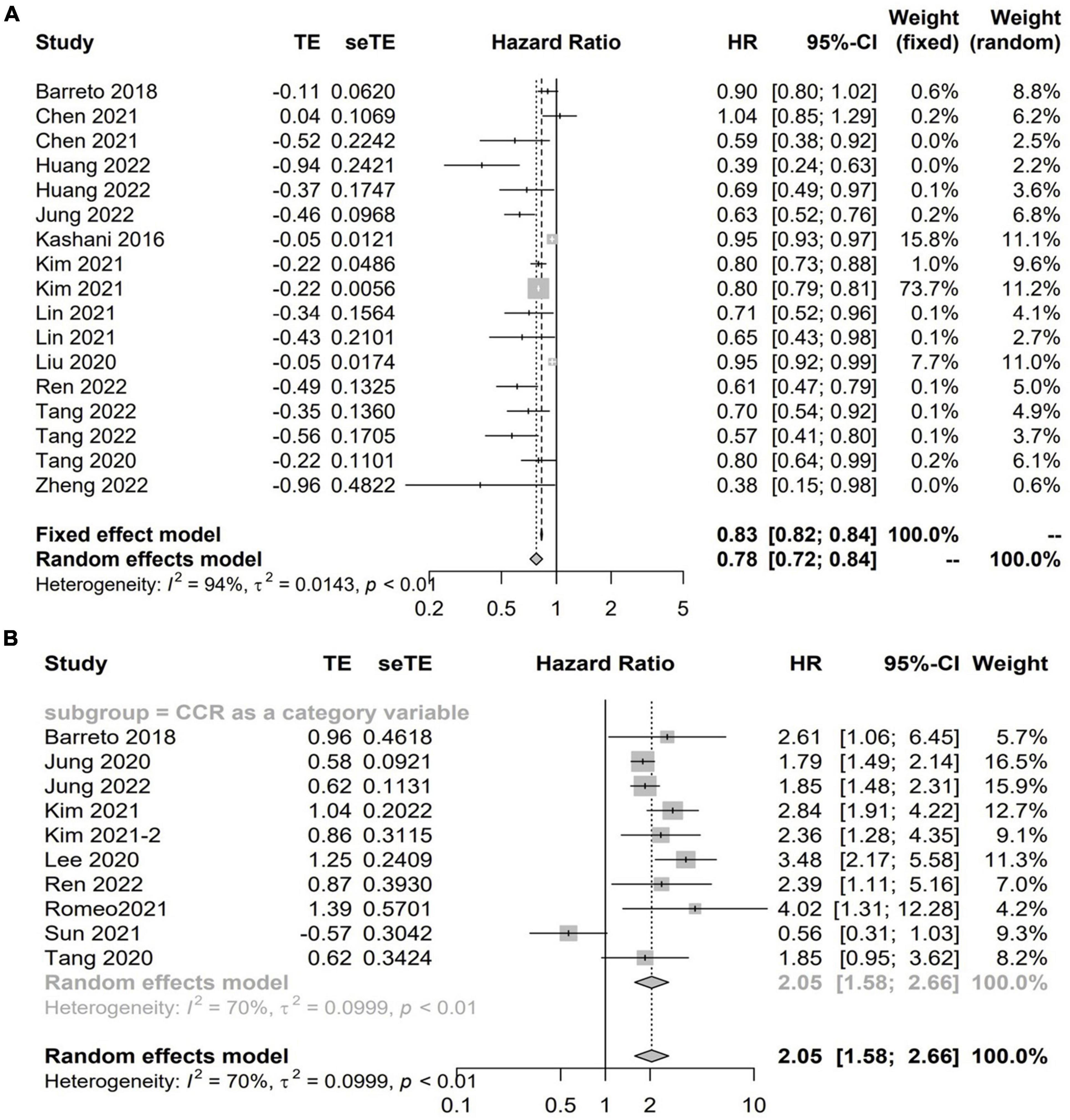
Figure 2. The forest plot in assessing the impact of creatinine/cystatin C ratio (CCR) on mortality in hospitalized patients by hazard ratio (HR) using regression analysis as continuous (A) and categorical variables (B).
Supplementary Figures 2–7 shows the detailed information of subgroup analyses by CCR categories and CCR treated as a continuous variable. Significant associations between CCR and all-cause mortality were also confirmed in most subgroups (Supplementary Figures 2–7).
There were 25 studies with 7,868 patients evaluated the correlation between CCR and CTASM from hospitalized patients using the correlation coefficient. As to the CTASM types, skeletal muscle area (SMA) was the most reported (n = 12), followed by the skeletal muscle index (SMI, defined as SMA divided by BSA, n = 11), appendicular skeletal muscle (n = 3), and appendicular skeletal muscle index (n = 3). The pooled results showed positive and significant correlations between CCR and all the four types of CTASM (Fisher’s Z ranged from 0.35 to 0.5; P values ranged from < 0.01 to 0.01) with the heterogeneity from 61 to 82% (Figure 3).
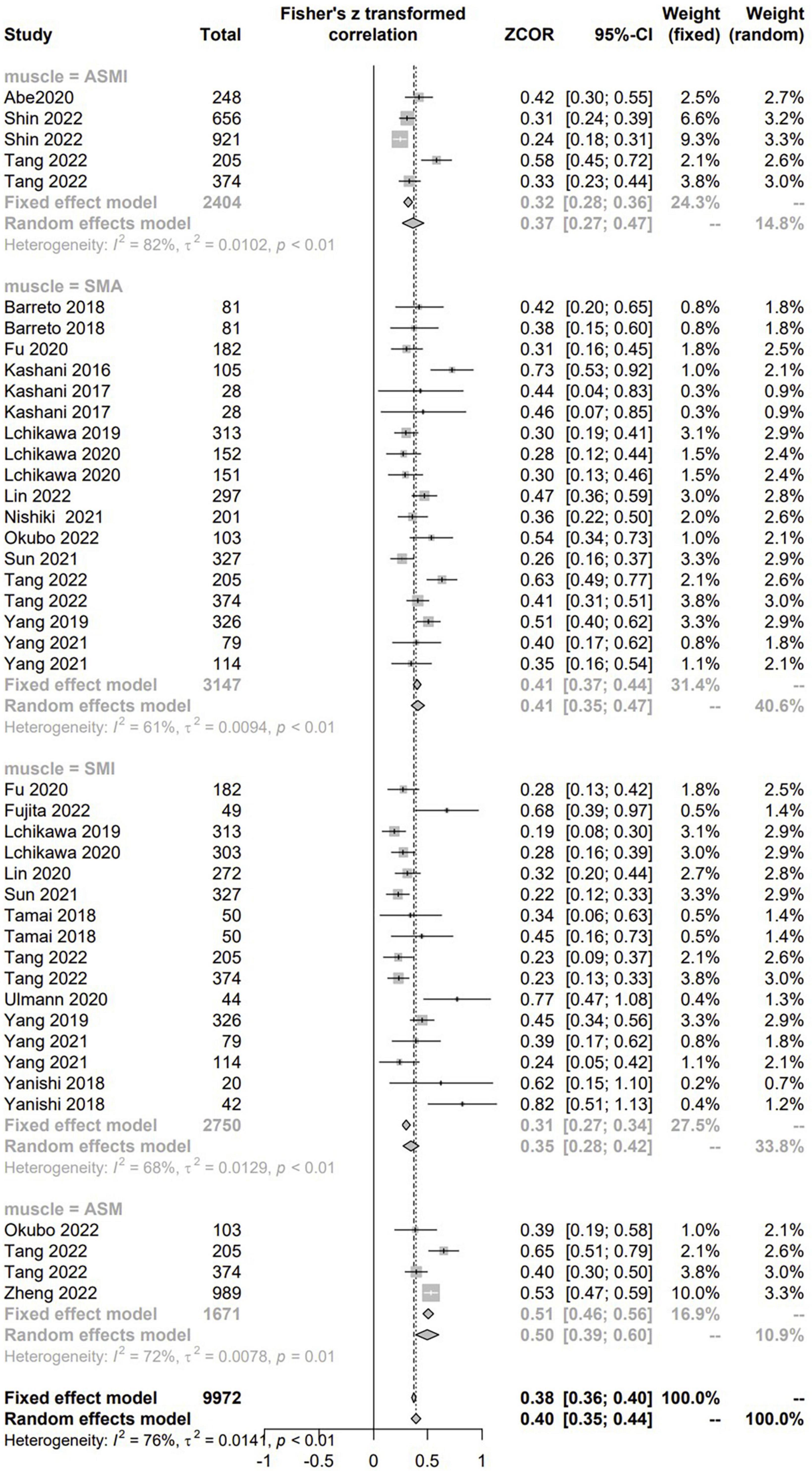
Figure 3. The pooled estimate of the relationship between serum creatinine/cystatin C ratio and computed tomography-assessed skeletal muscle.
Twelve articles presented the AUC value for CCR in the diagnosis of sarcopenia (7, 21, 24, 34, 40–42, 45–47, 49, 50). Among them, two studies only reported AUC values [male/female: 0.813/0.613 (50); 0.752/0.754 (24)], and the other 10 provided the mean AUC and SE values. When pooled, the AUC value of CCR to predict sarcopenia was 0.689 (95% CI, 0.632–0.746; I2 = 82%; P < 0.01) (Supplementary Figure 8).
There were 13 studies with 5,771 patients evaluated the correlation between CCR and HGS (6, 7, 21, 22, 24, 34–36, 41, 42, 46, 47, 49). There were positive and significant correlations between CCR and all the CTASM types (Fisher’s Z = 0.39; 95% CI, 0.32–0.45; I2 = 82%; P < 0.001) (Figure 4).
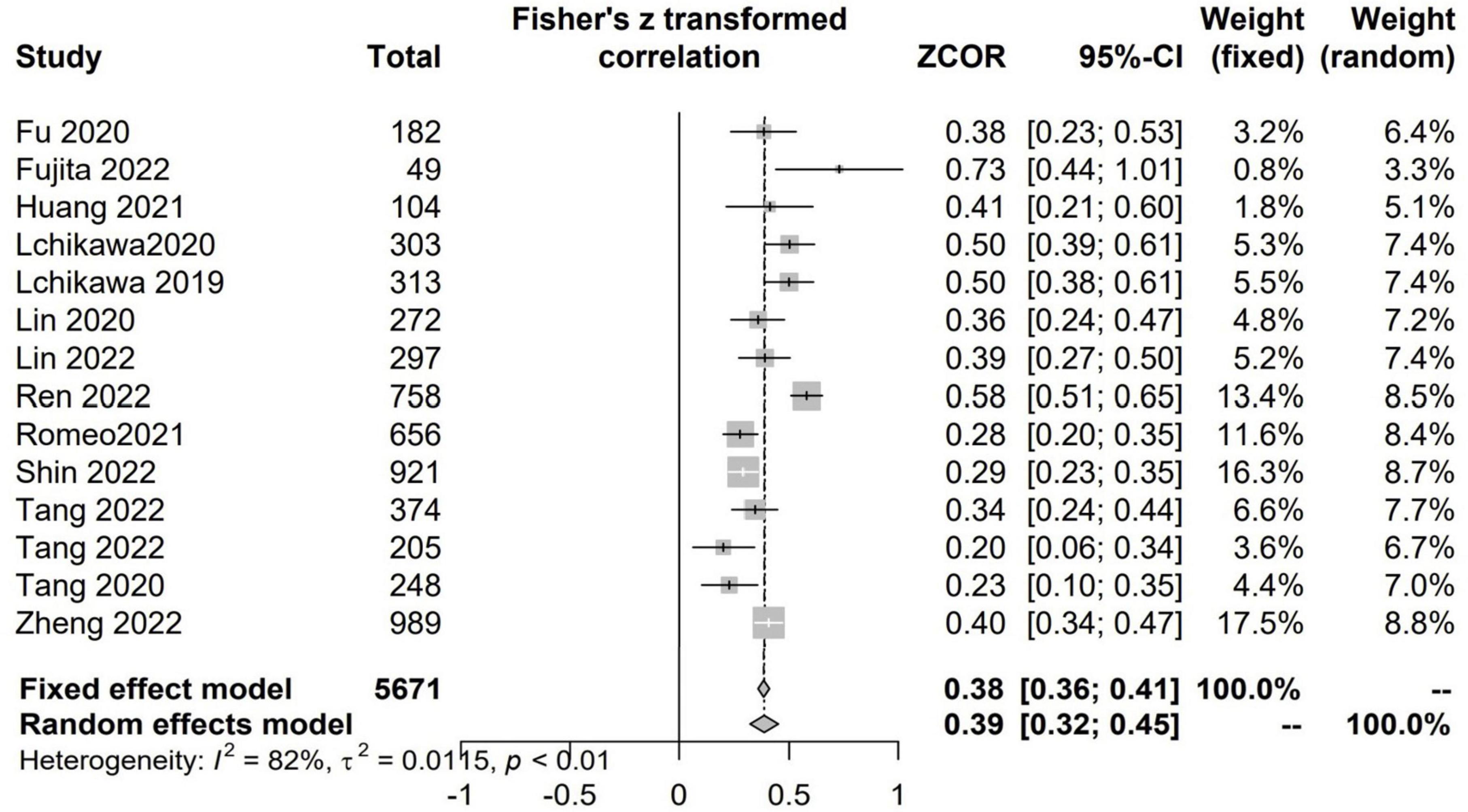
Figure 4. The pooled estimate of the relationship between serum creatinine/cystatin C ratio and handgrip strength.
There were 5 studies with 1,661 patients assessed the correlation between CCR and GS (7, 21, 24, 42, 46). There were positive and significant correlations between CCR and all the GS (Fisher’s Z = 0.25; 95% CI, 0.21–0.30; I2 = 0%; P < 0.001) (Figure 5).
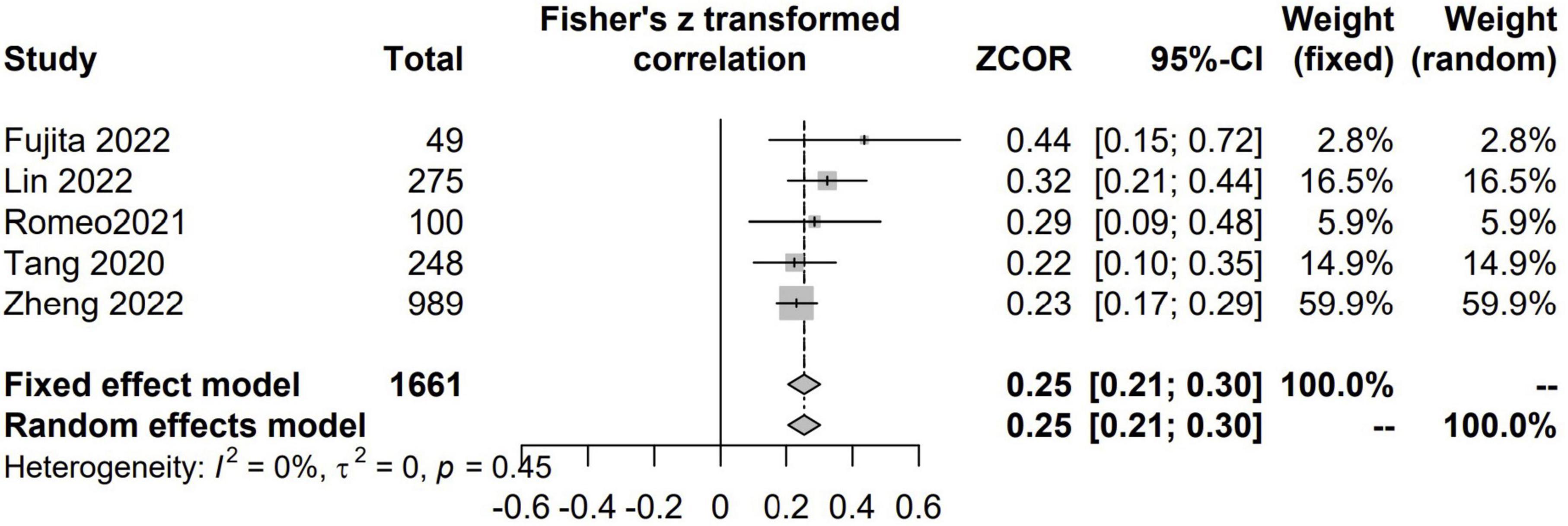
Figure 5. The pooled estimate of the relationship between serum creatinine/cystatin C ratio and gait speed.
Only four studies described the relationship between CCR and nutrition risk (6, 13, 16, 45). In the study by Abe et al. (45), the authors found that CCR was positively correlated with Mini Nutritional Assessment Short Form scores (r = 0.138, P = 0.03). Barreto et al. reported that CCR was a fair predictor of malnutrition as defined by the modified-NUTRIC score (AUC = 0.61) and with > 90% sensitivity and > 90% specificity (13). After adjusting for potential confounders, the CCR remained independently predictive of malnutrition [OR per 10-unit decrease in CCR, 1.15 (1.05, 1.26); P = 0.002]. Similar results were also seen in the study by Ren and coauthors, which showed that lower CCR was independently associated with nutritional risk/malnutrition whether or not CCR was treated as a continuous or category variable (6). In contrast, one study focused on cancer patients found no differences in the incidence of nutrition risk screening 2002 score ≥ 3 between the low and high CCR groups (P = 0.172) (16).
Three studies (12, 13, 51) provided data on the relationship between the CCR and the duration of MV, of which the study by Wang et al. suggested that CCR at admission significantly correlated with MV (r = 0.138, P = 0.001) (51). As to the other studies that provided specific data on this topic, one reported an increase in the CCR significantly predicted ventilator liberation [aHR 1.07 (0.97, 1.19); P = 0.18] (5), and the other suggested the duration of MV was significantly lower for those with a higher CCR [−1 day for each 10 units of CCR (95% CI, −1.4 to −0.2; P = 0.006)] (12).
Intensive care unit (ICU) and hospital LOS were available in three studies (5, 37, 51), of which the study by Wang et al. suggested that CCR at admission significantly correlated with ICU LOS (r = 0.161, P < 0.001) (51). Jung et al. found significant trends toward shorter ICU (P = 0.002) and hospital LOS (P < 0.001) in the higher CCR quartiles (37). Similarly, Barreto et al. suggested that an increase in the CCR significantly predicted more rapid discharge from the ICU [aHR, 1.06 (0.99, 1.14); P = 0.003] and hospital [aHR, 1.10 (1.03, 1.18) P = 0.007] (5).
Seven studies focused on the predictive value of CCR on the overall complications. The pooled findings from five studies that provided specific data on this topic showed that a decreased CCR was independently associated with a higher mortality risk (HR 1.66; 95% CI, 1.17–2.36; I2 = 83%; P < 0.01) (7, 16, 24, 38, 39; Supplementary Figure 9). In the remaining two studies, one reported that CCR was significantly lower in patients with cardiovascular disease (P = 0.008) and lower extremity arterial disease (P = 0.004) (33), and the other found no associations between CCR and adverse reactions (31).
We conducted the current meta-analysis of 38 studies to assess the value of CCR in hospitalized patients. Our results showed that CCR had a significant correlation with CTASM, GS and HGS, and the ROC curves suggested that CCR had good diagnostic efficacy for sarcopenia, indicating that CCR is an ideal alternative biomarker to screen sarcopenia. CCR, on the other hand, can reliably predict mortality in hospitalized patients, which was confirmed by regression analysis of CCR as both continuous and categorical variables. In addition, less evidence showed that higher CCR was independently associated with a shorten MV duration, reduced ICU and hospital LOS, less nutritional risk, and decreased complications in patients.
Recent findings suggest CCR is associated with disease-related catabolism because it reflects altered muscle mass (12). Serum creatinine is mainly produced by creatine phosphate during skeletal muscle metabolism (53). Therefore, patients with reduced muscle mass have lower creatinine levels. Also, cystatin C is a low molecular weight protein produced continuously by all nucleated cells and is readily filtered, absorbed, and broken down in the proximal renal tubules. It is not influenced by muscle metabolism (54). As a result, among patients with stable kidney function, one of the key factors of the difference between these two measures, skeletal muscle mass, is one of the primary determinants of the difference between these two markers. The effect of muscle mass on creatinine can be estimated by the ratio to cystatin C, which provides an easily accessible and reproducible assessment of muscle in patients at high risk for catabolism (12, 13).
Our results suggest that the CCR is an inexpensive, valid, objective, and reproducible tool for muscle mass assessment. We validated that CCR can screen for sarcopenia in three domains. CCR was significantly correlated not only with SAM/SMI, but also with GS and HGS. This supports the current sarcopenia definition in guidelines, that is, sarcopenia is assessed by skeletal muscle mass, muscle strength, and physical performance (55). Also, subgroup analyses suggested that medical, surgical, cancer, trauma, and ICU patients maintained consistently high correlations, meaning that CCR can be applied in various conditions (Figure 3).
In addition, the included studies used different definitions of sarcopenia. For example, most authors used diagnostic thresholds reported in previous studies to define sarcopenia or based on arbitrary thresholds from diverse populations (e.g., lowest 25th quartile, 33rd quartile, or median). These definitions were another source of heterogeneity in our results. Based on different sarcopenia definitions, we found that sarcopenia remained robustly correlated with CCR for all. On the other hand, different definitions have prevented obtaining a uniform CCR cut-off value for the diagnosis of sarcopenia in the current manuscript. Therefore, given the differences in disease, body size, dietary structure, and nutritional interventions among the included inpatients, there is still a need to establish appropriate CCR thresholds based on the local sarcopenic population in the future clinical application of CCR.
Several studies included in our meta-analysis demonstrate that CCR can be utilized as a surrogate indication for a variety of nutritional screening techniques (6, 13, 16, 45). These studies found that CCR remained an independent predictor of nutrition risk/malnutrition in patients after adjusting for age, gender, Acute Physiology and Chronic Health Evaluation (APACHE) III score, Sequential Organ Failure Assessment (SOFA) score, and BMI (6, 13, 16, 45). Low CCR values generally indicate reduced muscle tissue, which is associated with malnutrition. In contrast, high CCR values indicate a complete muscle tissue mass and functional status and can identify malnutrition at the initial stage.
Furthermore, some studies suggest that CCR is superior to some traditional nutritional risk indicators (e.g., NUTRIC score), which are established and validated only for nutritional status and not as a surrogate for muscle mass (53). Thus, the independence from the subjective provision of information, weight data, and complex calculations, as well as the simplicity and repeatability, make CCR a favorable choice for clinical decision support of busy bedside clinicians compared to previous, more sophisticated tools. Although the studies we included involved a wide range of inpatient populations such as ICU, geriatric patients, and medical patients, given the number of studies, further confirmation in a larger sample is needed in the future.
We provide evidence for the association of sarcopenia with inpatient prognosis by evaluating CCR. A possible explanation for the association of CCR with mortality is that CCR may reflect muscle mass (12), the latter has been demonstrated to influence outcomes in various patient populations. Current literature suggests that sarcopenia is related to reduced protein synthesis and enhanced degradation induced by wasting, physical restraints, infection, prolonged mechanical ventilation and sedation, and muscle relaxants in various hospitalized settings (56). Especially in ICU patients with multiple organ dysfunction syndromes, the cross-sectional area of muscle fibers decreases at a rate of 3 to 4% per day, resulting in skeletal muscle atrophy, which affects vital physiological functions (4). As a result, muscle is also considered one of the failing organs of multiple organ dysfunction syndrome and has received widespread attention.
Although the pathophysiological relationship between muscle loss and patients’ prognosis is not fully understood, several studies have suggested it may be associated with a high catabolic state, cytokine impairment, and insulin signaling, leading to glucose intolerance (57). Sarcopenia also decreases the body’s ability to respond adequately to inflammatory stimuli and delays the implementation of rehabilitation (4, 57). Under these conditions, patient immune function may be reduced, leading to a high CCR, i.e., high risk of sarcopenia, associated with various complications, prolonged ICU or hospital stay, prolonged mechanical ventilation, and ultimately increased risk of patient death, as shown by our findings.
To the best of our knowledge, this is the first meta-analysis to assess the value of CCR on muscle evaluation and prognosis in hospitalized patients. We included 38 studies with more than 20,000 patients with sufficient statistical power to conduct subgroup analysis based on potential influencing factors. The vast majority were based on multiple regression analyses of the included studies. Most of these included studies adjusted for a variety of possible confounders, including demographic variables (e.g., age and sex), anthropometric measures (e.g., BMI), nutritional status (e.g., subjective global assessment, NUTRIC score, disease severity (e.g., SOFA, APACHE-II score), disease-specific (e.g., tumor type, stage, and type of treatment), and physical fitness status as a prognostic factor. Thus, our findings somewhat control these confounding factors when evaluating muscle, prognosis, and other clinical outcome-related CCR measures.
Our meta-analysis has several limitations. (1) The observational design of all included studies excluded any causal inference. Also, patients included only in CCR tests in retrospective studies were prone to selection bias. (2) The small sample may be subject to false-positive bias; the small number of included studies in some subgroup analyses interpreted the results with caution. (3) The mean age of the included patients varied widely (47–88 years), but there was insufficient data to further explore the effect of age on mortality. (4) The uneven distribution of underlying disease in the study population may also have a different prognostic value. (5) CCR is unlikely to be at a steady state under AKI conditions, making the application of muscle mass ratios less desirable than in other clinical situations. Of note, cystatin C is a cathepsins inhibitor, and its levels increased in hyperthyroidism, obesity, metabolic syndrome, diabetes mellitus type 2, and different types of inflammation, albeit in different degrees (58, 59). However, most included studies did not exclude these confounding factors. (6) Patients’ medications or other interventions may affect the results of our study. Therefore, to reduce the effect of the intervention, we used the longest follow-up time of each included study. (7) The vast majority of the included studies were from Asian countries, so more data from other ethnicities are needed for confirmation.
The results of our study indicated significant correlations between CCR and skeletal muscle evaluation and a prognostic role of CCR that higher circulating CCR levels were positively associated with the less risk of all-cause mortality in hospitalized patients. Thus, we recommended using CCR as a new prognostic biomarker to provide better information not only in decision correlations for muscle mass assessment but also in the prediction of survival and other associated clinical outcome.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
W-HZ and YY contributed to analysis and drafting of the article. Y-BZ contributed to data collection and analysis. H-BH contributed to the conception of the study, design, revisions of this manuscript, and was responsible for the integrity of the work as a whole, from inception to publication of the article. All authors approved the final version submitted for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1058464/full#supplementary-material
CCR, creatinine/cystatin C ratio; CI, confidence interval; CTASM, computed tomography-assessed skeletal muscle; HGS, handgrip strength; GS, gait speed; ICU, intensive care unit; LOS, length of stay; MD, mean difference; MV, mechanical ventilation; NOS, Newcastle-Ottawa Scale; OR, odds ratio; RCTs, randomized controlled trials; SD, standard deviations.
1. Cruz-Jentoft AJ, Landi F, Topinková E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutrit Metab Care. (2010) 13:1–7. doi: 10.1097/MCO.0b013e328333c1c1
2. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diab Metab Disord. (2017) 16:21. doi: 10.1186/s40200-017-0302-x
3. Almohaisen N, Gittins M, Todd C, Sremanakova J, Sowerbutts AM, Aldossari A, et al. Prevalence of undernutrition, frailty and sarcopenia in community-dwelling people aged 50 years and above: systematic review and meta-analysis. Nutrients. (2022) 14:1537. doi: 10.3390/nu14081537
4. Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. (2013) 310:1591–600. doi: 10.1001/jama.2013.278481
5. Barreto EF, Poyant JO, Coville HH, Dierkhising RA, Kennedy CC, Gajic O, et al. Validation of the sarcopenia index to assess muscle mass in the critically ill: a novel application of kidney function markers. Clin Nutr. (2019) 38:1362–7. doi: 10.1016/j.clnu.2018.05.031
6. Ren C, Su H, Tao J, Xie Y, Zhang X, Guo Q. Sarcopenia index based on serum creatinine and cystatin C is associated with mortality, nutritional risk/malnutrition and sarcopenia in older patients. Clin Interv Aging. (2022) 17:211–21. doi: 10.2147/CIA.S351068
7. Tang T, Zhuo Y, Xie L, Wang H, Yang M. Sarcopenia index based on serum creatinine and cystatin C is associated with 3-year mortality in hospitalized older patients. Sci Rep. (2020) 10:1260. doi: 10.1038/s41598-020-58304-z
8. Earthman CP. Body composition tools for assessment of adult malnutrition at the bedside: a tutorial on research considerations and clinical applications. JPEN J Parenter Enteral Nutr. (2015) 39:787–822. doi: 10.1177/0148607115595227
9. Koo BK. Assessment of muscle quantity, quality and function. J Obes Metab Syndrome. (2022) 31:9–16. doi: 10.7570/jomes22025
10. Connolly B, MacBean V, Crowley C, Lunt A, Moxham J, Rafferty GF, et al. Ultrasound for the assessment of peripheral skeletal muscle architecture in critical illness: a systematic review. Crit Care Med. (2015) 43:897–905. doi: 10.1097/CCM.0000000000000821
11. Tolonen A, Pakarinen T, Sassi A, Kyttä J, Cancino W, Rinta-Kiikka I, et al. Methodology, clinical applications, and future directions of body composition analysis using computed tomography (CT) images: a review. Eur J Radiol. (2021) 145:109943. doi: 10.1016/j.ejrad.2021.109943
12. Kashani KB, Frazee EN, Kukralova L, Sarvottam K, Herasevich V, Young PM, et al. Evaluating muscle mass by using markers of kidney function: development of the sarcopenia index. Crit Care Med. (2017) 45:e23–9. doi: 10.1097/CCM.0000000000002013
13. Barreto EF, Kanderi T, DiCecco SR, Lopez-Ruiz A, Poyant JO, Mara KC, et al. Sarcopenia index is a simple objective screening tool for malnutrition in the critically Ill. JPEN J Parenter Enteral Nutr. (2019) 43:780–8. doi: 10.1002/jpen.1492
14. Jung CY, Joo YS, Kim HW, Han SH, Yoo TH, Kang SW, et al. Creatinine-Cystatin C ratio and mortality in patients receiving intensive care and continuous kidney replacement therapy: a retrospective cohort study. Am J Kidney Dis. (2021) 77:509-516.e1. doi: 10.1053/j.ajkd.2020.08.014
15. Kashani K, Sarvottam K, Pereira NL, Barreto EF, Kennedy CC. The sarcopenia index: a novel measure of muscle mass in lung transplant candidates. Clin Trans. (2018) 32:e13182. doi: 10.1111/ctr.13182
16. Yang J, Zhang T, Feng D, Dai X, Lv T, Wang X, et al. A new diagnostic index for sarcopenia and its association with short-term postoperative complications in patients undergoing surgery for colorectal cancer. Colorectal Dis. (2019) 21:538–47. doi: 10.1111/codi.14558
17. Osaka T, Hamaguchi M, Hashimoto Y, Ushigome E, Tanaka M, Yamazaki M, et al. Decreased the creatinine to cystatin C ratio is a surrogate marker of sarcopenia in patients with type 2 diabetes. Diabetes Res Clin Pract. (2018) 139:52–8. doi: 10.1016/j.diabres.2018.02.025
18. Sun J, Yang H, Cai W, Zheng J, Shen N, Yang X, et al. Serum creatinine/cystatin C ratio as a surrogate marker for sarcopenia in patients with gastric cancer. BMC Gastroenterol. (2022) 22:26. doi: 10.1186/s12876-022-02093-4
19. Kim CH, Rhee TM, Woo Park K, Soon Park C, Kang J, Han JK, et al. Association between low muscle mass and prognosis of patients with coronary artery disease undergoing percutaneous coronary intervention. J Am Heart Assoc. (2021) 10:e018554. doi: 10.1161/JAHA.120.018554
20. Nishiki K, Nojiri M, Kato R, Shinomiya S, Oikawa T, Ishizaki T, et al. Serum creatinine/cystatin c ratio associated with cross-sectional area of erector spinae muscles and pulmonary function in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2021) 16:3513–24. doi: 10.2147/COPD.S339243
21. Fujita K, Ohkubo H, Nakano A, Takeda N, Fukumitsu K, Fukuda S, et al. Serum creatinine/cystatin C ratio is a surrogate marker for sarcopenia in patients with idiopathic pulmonary fibrosis. BMC Pulm Med. (2022) 22:203. doi: 10.1186/s12890-022-02000-3
22. Huang D, Xie C, Sun C, Chen M, Li L, Yi H, et al. Serum creatinine to cystatin C ratio is an effective indicator for muscle strength decline in men with acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2022) 17:781–9. doi: 10.2147/COPD.S356314
23. Huang S, Zhao L, Liu Z, Li Y, Wang X, Li J, et al. The effectiveness of the sarcopenia index in predicting septic shock and death in elderly patients with community-acquired pneumonia. BMC Geriatr. (2022) 22:341. doi: 10.1186/s12877-022-03029-z
24. Zheng C, Wang E, Li JS, Xie K, Luo C, Ge QY, et al. Serum creatinine/cystatin C ratio as a screening tool for sarcopenia and prognostic indicator for patients with esophageal cancer. BMC Geriatr. (2022) 22:207. doi: 10.1186/s12877-022-02925-8
25. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
26. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
27. Lenhard W, Lenhard A. Hypothesis Tests for Comparing Correlations. Germany: Psychometrica (2014).
28. Zhou XH, Obuchowski NA, McClish DK. Statistical Methods in Diagnostic Medicine. New York, NY: Wiley (2002). doi: 10.1002/9780470317082
29. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
30. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
31. Chen XHL, Shen Y, Wu X, Dong B, Hao Q. The role of baseline sarcopenia index in predicting chemotherapy-induced undesirable effects and mortality in older people with stage III or IV NonSmall cell Lung cancer. J Nutr Health Aging. (2021) 25:878–82. doi: 10.1007/s12603-021-1633-3
32. Liu WZX, Tan X, Yang L, Wang Y, Diao S, Huang S, et al. Predictive value of serum creatinine/cystatin C in acute ischemic stroke patients under nutritional intervention. J Nutr Health Aging. (2021) 25:335–9. doi: 10.1007/s12603-020-1495-0
33. Yang Q, Zhang M, Sun P, Li Y, Xu H, Wang K, et al. Cre/CysC ratio may predict muscle composition and is associated with glucose disposal ability and macrovascular disease in patients with type 2 diabetes. BMJ Open Diab Res Care. (2021) 9:e002430. doi: 10.1136/bmjdrc-2021-002430
34. Fu X, Tian Z, Wen S, Sun H, Thapa S, Xiong H, et al. A new index based on serum creatinine and cystatin C is useful for assessing sarcopenia in patients with advanced cancer. Nutrition. (2021) 82:111032. doi: 10.1016/j.nut.2020.111032
35. Ichikawa T, Miyaaki H, Miuma S, Motoyoshi Y, Yamashima M, Yamamichi S, et al. Calculated body muscle mass as a useful screening marker for low skeletal muscle mass and sarcopenia in chronic liver disease. Hepatol Res. (2020) 50:704–14. doi: 10.1111/hepr.13492
36. Ichikawa T, Miyaaki H, Miuma S, Motoyoshi Y, Yamashima M, Yamamichi S, et al. Indices calculated by serum creatinine and cystatin C as predictors of liver damage, muscle strength and sarcopenia in liver disease. Biomed Rep. (2020) 12:89–98. doi: 10.3892/br.2020.1273
37. Jung CY, Kim HW, Han SH, Yoo TH, Kang SW, Park JT. Creatinine-cystatin C ratio and mortality in cancer patients: a retrospective cohort study. J Cachexia Sarcopenia Muscle. (2022) 13:2064–72. doi: 10.1002/jcsm.13006
38. Kim HJ, Kim HB, Kim HY, Shim JK, Lee C, Kwak YL. Associations of creatinine/cystatin C ratio and postoperative pulmonary complications in elderly patients undergoing off-pump coronary artery bypass surgery: a retrospective study. Sci Rep. (2021) 11:16881. doi: 10.1038/s41598-021-96442-0
39. Lee HS, Park KW, Kang J, Ki YJ, Chang M, Han JK, et al. Sarcopenia index as a predictor of clinical outcomes in older patients with coronary artery disease. J Clin Med. (2020) 9:3121. doi: 10.3390/jcm9103121
40. Lin YL, Chang IC, Liou HH, Wang CH, Lai YH, Kuo CH, et al. Serum indices based on creatinine and cystatin C predict mortality in patients with non-dialysis chronic kidney disease. Sci Rep. (2021) 11:16863. doi: 10.1038/s41598-021-96447-9
41. Lin YL, Chen SY, Lai YH, Wang CH, Kuo CH, Liou HH, et al. Serum creatinine to cystatin C ratio predicts skeletal muscle mass and strength in patients with non-dialysis chronic kidney disease. Clin Nutr. (2020) 39:2435–41. doi: 10.1016/j.clnu.2019.10.027
42. Lin YL, Wang CH, Chang IC, Hsu BG. A novel application of serum creatinine and cystatin c to predict sarcopenia in advanced CKD. Front Nutr. (2022) 9:828880. doi: 10.3389/fnut.2022.828880
43. Mauro E, Diaz JM, Garcia-Olveira L, Spina JC, Savluk L, Zalazar F, et al. Sarcopenia HIBA score predicts sarcopenia and mortality in patients on the liver transplant waiting list. Hepatol Commun. (2022) 6:1699–710. doi: 10.1002/hep4.1919
44. Okubo N, Yoshida T, Tanaka K, Okada N, Hosoi K, Ohara M, et al. Serum creatinine to cystatin C ratio reflects preoperative and early postoperative walking ability in older patients with hip fracture. J Cachexia Sarcopenia Muscle. (2022) 13:945–54. doi: 10.1002/jcsm.12940
45. Abe K, Yano T, Katano S, Ohori K, Ishigo T, Moniwa N, et al. Utility of the sarcopenia index for assessment of muscle mass and nutritional status in patients with chronic heart failure: comparison with anthropometric parameters. Geriatr Gerontol Int. (2020) 20:388–9. doi: 10.1111/ggi.13876
46. Romeo FJ, Chiabrando JG, Seropian IM, Raleigh JV, de Chazal HM, Garmendia CM, et al. Sarcopenia index as a predictor of clinical outcomes in older patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv. (2021) 98:E889–96. doi: 10.1002/ccd.29799
47. Shin JY. Low serum creatinine to cystatin C ratio is independently associated with sarcopenia and high carotid plaque score in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. (2022) 32:1454–62. doi: 10.1016/j.numecd.2022.02.005
48. Tamai Y, Iwasa M, Kawasaki Y, Yoshizawa N, Ogura S, Sugimoto R, et al. Ratio between estimated glomerular filtration rates of creatinine and cystatin C predicts overall survival in patients with hepatocellular carcinoma. Hepatol Res. (2019) 49:153–63. doi: 10.1111/hepr.13230
49. Tang T, Xie L, Hu S, Tan L, Lei X, Luo X, et al. Serum creatinine and cystatin C-based diagnostic indices for sarcopenia in advanced non-small cell lung cancer. J Cachexia Sarcopenia Muscle. (2022) 13:1800–10. doi: 10.1002/jcsm.12977
50. Ulmann G, Kai J, Durand JP, Neveux N, Jouinot A, De Bandt JP, et al. Creatinine-to-cystatin C ratio and bioelectrical impedance analysis for the assessement of low lean body mass in cancer patients: comparison to L3-computed tomography scan. Nutrition. (2021) 81:110895. doi: 10.1016/j.nut.2020.110895
51. Wang S, Xie L, Xu J, Hu Y, Wu Y, Lin Z, et al. Predictive value of serum creatinine/cystatin C in neurocritically ill patients. Brain Behav. (2019) 9:e01462. doi: 10.1002/brb3.1462
52. Yanishi M, Kinoshita H, Tsukaguchi H, Kimura Y, Koito Y, Sugi M, et al. The creatinine/cystatin C ratio provides effective evaluation of muscle mass in kidney transplant recipients. Int Urol Nephrol. (2019) 51:79–83. doi: 10.1007/s11255-018-2015-6
53. Kashani K, Rosner MH, Ostermann M. Creatinine: from physiology to clinical application. Eur J Int Med. (2020) 72:9–14. doi: 10.1016/j.ejim.2019.10.025
54. Björk J, Nyman U, Berg U, Delanaye P, Dubourg L, Goffin K, et al. Validation of standardized creatinine and cystatin C GFR estimating equations in a large multicentre European cohort of children. Pediatric Nephrol. (2019) 34:1087–98. doi: 10.1007/s00467-018-4185-y
55. Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. (2018) 22:1148–61. doi: 10.1007/s12603-018-1139-9
56. Moisey LL, Mourtzakis M, Cotton BA, Premji T, Heyland DK, Wade CE, et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. (2013) 17:R206. doi: 10.1186/cc12901
57. Batt J, Herridge MS, Dos Santos CC. From skeletal muscle weakness to functional outcomes following critical illness: a translational biology perspective. Thorax. (2019) 74:1091–8. doi: 10.1136/thoraxjnl-2016-208312
58. Zi M, Xu Y. Involvement of cystatin C in immunity and apoptosis. Immunol Lett. (2018) 196:80–90. doi: 10.1016/j.imlet.2018.01.006
Keywords: creatinine/cystatin C ratio, mortality, hospitalized, meta-analysis, sarcopenia
Citation: Zheng W-H, Zhu Y-B, Yao Y and Huang H-B (2023) Serum creatinine/cystatin C ratio as a muscle mass evaluating tool and prognostic indicator for hospitalized patients: A meta-analysis. Front. Med. 9:1058464. doi: 10.3389/fmed.2022.1058464
Received: 27 October 2022; Accepted: 19 December 2022;
Published: 09 January 2023.
Edited by:
Tzvi Dwolatzky, Technion Israel Institute of Technology, IsraelReviewed by:
Adriana Caldo-Silva, University of Coimbra, PortugalCopyright © 2023 Zheng, Zhu, Yao and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Bin Huang,  aGhiYTAyOTIyQGJ0Y2guZWR1LmNu
aGhiYTAyOTIyQGJ0Y2guZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.