
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 24 November 2022
Sec. Dermatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1058431
This article is part of the Research Topic Primary Immune-mediated Cicatricial and Non-cicatricial Alopecias View all 4 articles
Background: Immune-mediated alopecias (IMAs), a group of hair disorders associated with immunological reactions, remain a therapeutic challenge since available treatments are generally unfavorable with potential side effects. Platelet-rich plasma (PRP) has been recently proposed as a treatment option based on several limited-quality studies; however, there is no systematic evaluation of PRP efficacy on IMAs in the literature.
Objective: To assess PRP’s effects in treating IMAs using a systematic review.
Methods: Electronic searches were conducted using PubMed, Embase, Scopus, and Cochrane Library databases. A search strategy was designed to retrieve all studies exploring PRP in treating IMAs, including alopecia areata (AA) and primary cicatricial alopecias (PCAs). In addition, all randomized and non-randomized studies reporting subjective and/or objective outcomes of alopecia treatment with PRP were included.
Results: Thirty-two studies were included, comprising 621 patients with AA and 19 patients with PCAs. PRP had superior efficacy as monotherapy in five studies, comparable to intralesional corticosteroids in six studies in AA treatment. In addition, in the analysis of PCAs, including lymphocytic and neutrophilic subtypes, PRP was efficacious in alleviating disease progression in nine studies.
Conclusion: PRP is considered a promising treatment for AA and PCAs in patients who experienced unfavorable outcomes from conventional treatment. However, its clinical application remains to be standardized, and its recommendation as a treatment for IMAs could not be ascertained due to a lack of high-quality evidence.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=353859], identifier [CRD42022353859].
Alopecia is a common dermatological disorder affecting the population worldwide. The condition is highly associated with psychological distress and impacts patients’ quality of life (1). Alopecia manifests varyingly and is categorized into non-cicatricial (non-scarring) and cicatricial (scarring) alopecias, which include several disorders (2). In non-cicatricial alopecia, hair follicle (HF) stem cells located in the bulge area are preserved with potential for regrowth. In contrast, they are irreversibly destroyed in the cicatricial subtype, leading to permanent alopecia (3, 4).
Immune-mediated alopecia (IMA) refers to hair loss disorders associated with immune responses involved in inflammation and autoimmunity to HFs. HF is an area of relative immune privilege. Several mechanisms, such as downregulating major histocompatibility complex (MHC) and expressing signals using type-1 transmembrane glycoprotein CD200, help protect HF from inflammatory insults (5, 6). Imbalances in the protective mechanism of HF, also called immune privilege collapse, are theorized to be the pathogenesis of IMAs (6–9).
Alopecia areata (AA) and primary cicatricial alopecias (PCAs) are two major subtypes of IMAs. AA is an autoimmune, non-scarring hair loss disorder histologically characterized by CD8+ and CD4+ T cells infiltrating the peribulbar area of anagen HFs (10–13). Because the inflammatory process of AA conserves stem cells, reversible hair loss can occur after AA subsides. In contrast, inflammation in PCAs mainly involves the hair bulge region, where HF stem cells locate, leading to the permanent destruction of HF and replacement with a scar (14–17). PCAs are classified based on the types of predominant inflammatory cell involvement into lymphocytic, neutrophilic, and mixed cell infiltrates (3).
Treatment modalities of IMAs aim to suppress the inflammatory response, prevent potential hair loss, and promote hair regrowth. Several therapeutic options have been introduced, such as topical and intralesional corticosteroids, systemic immunosuppressants, topical immunotherapy, lasers, and phototherapy, depending on IMA subtypes, degree of inflammation, disease stage, and relevant comorbidities (18–22). However, their therapeutic efficacy is still debated since treatment outcomes are generally unpredictable. Moreover, poor response, high recurrent rate, and potential side effects are frequently reported (23–25).
Recent advancements in understanding the pathogenesis of IMAs have accelerated the discovery of novel treatments. In recent years, the regenerative capability of platelet-rich plasma (PRP) has been used to treat several dermatological diseases. PRP is an autologous plasma preparation with concentrated platelet produced by centrifugation (26, 27). It comprises over 20 growth factors and cytokines, such as transforming growth factor (TGF), platelet-derived growth factors (PDGF), insulin-like growth factor (IGF), vascular endothelial growth factors (VEGF), epidermal growth factor (EGF), and fibroblast growth factor (FGF), playing a significant role in initiating tissue repair by releasing biologically active factors and immunomodulatory effect of innate and adaptive immune system (27–29).
Some studies have reported PRP’s efficacy in treating AA and PCAs with positive outcomes, with fewer side effects; others revealed the opposite. Given this inconclusive issue, it is essential to integrate and compare these findings in the secondary analysis. We aimed to assess PRP’s efficacy in treating AA and PCAs via a systematic review due to a lack of systematic evaluation of the therapeutic effects of PRP on IMAs.
The protocol was registered in PROSPERO (International Prospective Register of Systematic Reviews; no.CRD42022353859). The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Electronic searches were conducted from the database’s inception to July 1, 2022, via PubMed, Embase, Scopus, and Cochrane Library databases. Using keywords and a controlled vocabulary, the search strategy was designed to retrieve all studies exploring PRP use in treating AA and PCAs. There were no restrictions on the language or publication period of the searches. Conference abstracts were excluded. Details of the search strategy are presented in Supplementary Table 1.
Each article was reviewed independently by two reviewers (KT and TY). Disagreements were resolved via discussion with a third reviewer (NS). We included all randomized and non-randomized studies that reported any subjective and/or objective treatment outcomes.
Data were extracted from the included studies using a standardized format. The following data were collected: study type, study characteristics (authors, publication year, and study design), patient characteristics [diagnosis, number of patients, disease duration, previous treatment(s), and age], intervention(s), PRP protocol, investigations, objective and subjective assessment of hair growth, incidence of adverse effect(s), and follow-up duration. Corresponding investigators were contacted via email if there was missing data. Two independent reviewers extracted data (KT and TY), and discrepancies were resolved with the assistance of a third reviewer (NS).
Quality assessment was performed using Rob-2 and ROBINS-1 for randomized and non-randomized studies, respectively (30, 31). Risk-of-bias plots were created using Risk-of-bias VISualization (robvis) (32).
After removing duplicates, 181 papers were screened by title and abstract. At the full-text stage, 87 full articles met our predefined selection criteria, and we further excluded 55 publications for the following reasons: review articles (n = 27), conference abstracts (n = 17), wrong population (n = 5), wrong intervention (n = 3), commentary articles (n = 3), and secondary cicatricial alopecias (n = 2) (Figure 1). Thirty-two studies were included: 11 randomized controlled trials (RCTs) (33–43), 4 non-randomized studies (44–47), and 17 case series or case reports (48–64). Between 2013 and 2022, 23 AA studies (33–55) and nine PCA studies (56–64) were included, totaling 621 patients with AA and 19 with PCAs. Details of the included studies are summarized in Tables 1, 2.
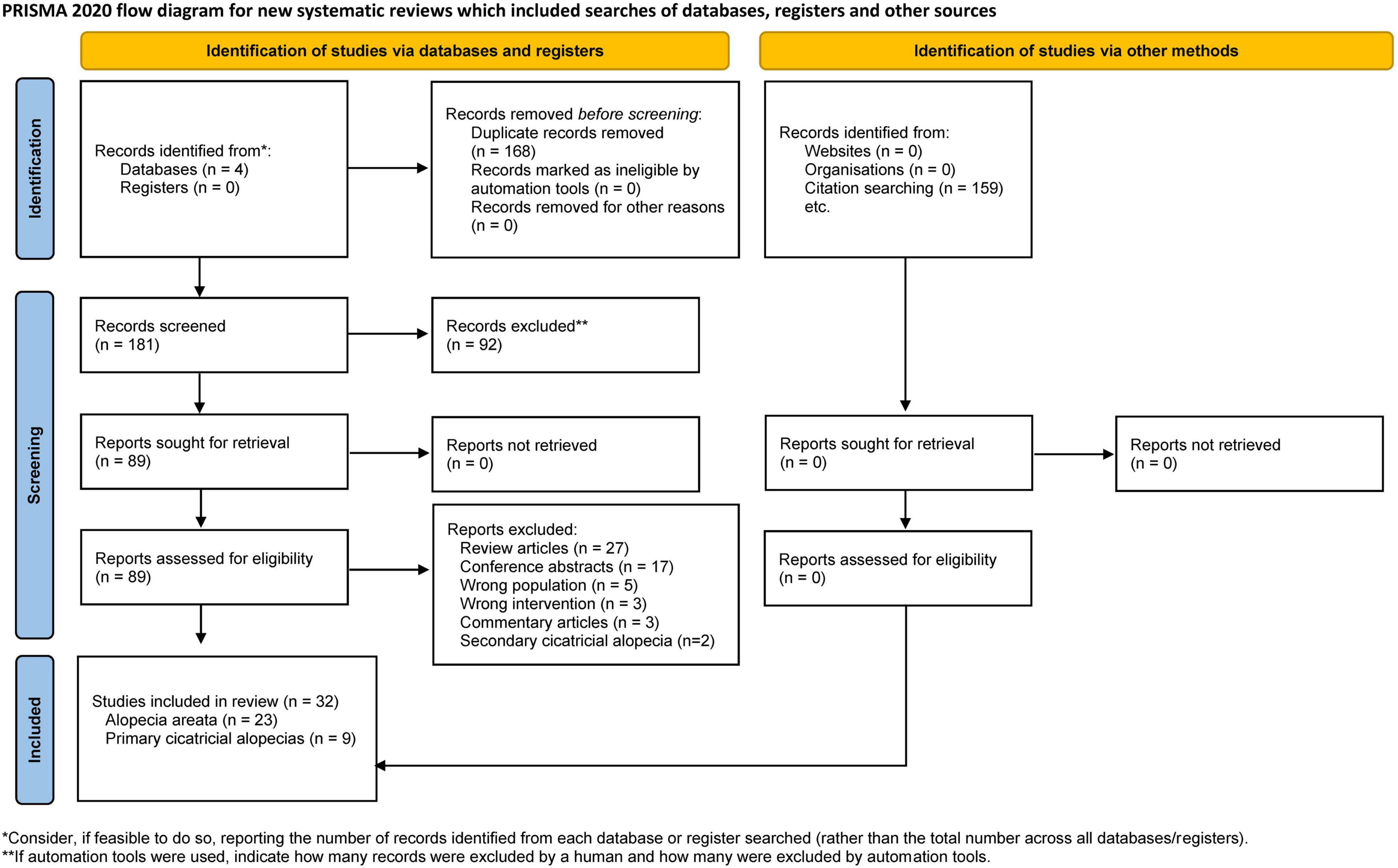
Figure 1. Flow diagram of search methodology and selection process based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA) flowchart for the article selection process.
The PRP preparation protocols of included studies are demonstrated in Tables 3, 4. Regarding the centrifugation method, there were 13 studies using single spin method (33, 34, 36, 39, 43, 47, 49, 52, 53, 58, 60, 63, 64), 10 using double spin method (35, 37, 38, 40–42, 45, 46, 51, 56), and eight provided no information (44, 48, 54, 55, 57, 59, 61, 62). Several types of PRP activators were used; seven studies used calcium chloride (37, 38, 41, 45, 46, 49, 60), four used calcium gluconate (33, 34, 36, 42), one used calcium carbonate (52), two did not use any activators (48, 53), and 17 provided no information (35, 39, 40, 43, 44, 47, 51, 54–59, 61–64). The most common ratio of activator to PRP applied by the included studies was 1:9.
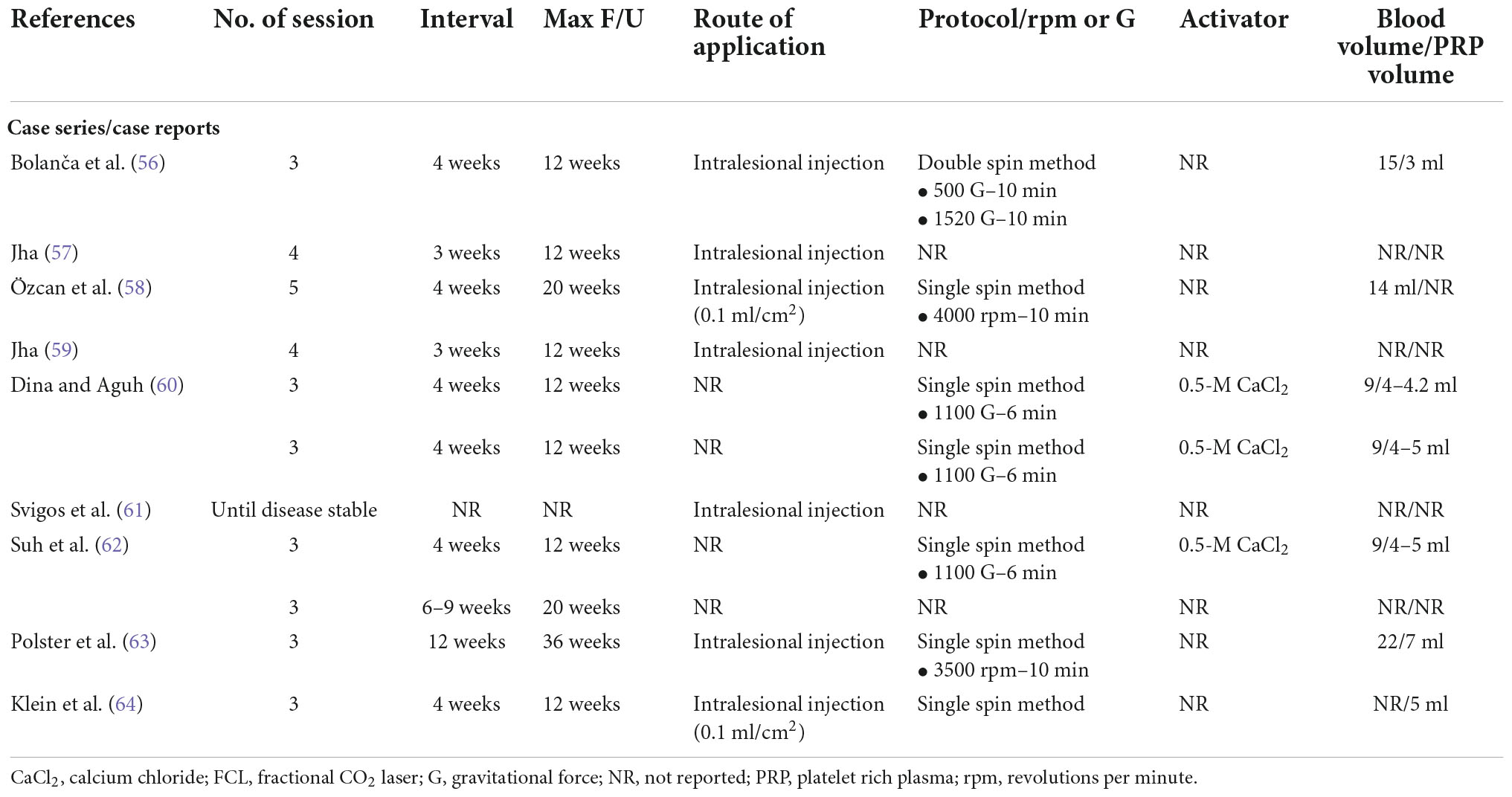
Table 4. Platelet-rich plasma preparation protocols of included primary cicatricial alopecia studies.
Regarding treatment protocol, most studies used three or four treatment sessions, with 15 using three sessions (33, 34, 38, 40–42, 45, 47, 50, 53, 54, 56, 60, 63, 64), six using four sessions (35, 36, 39, 49, 57, 59), two treated until a satisfactory response was obtained (51, 61), and the remaining studies used different number of sessions. Our included studies selected different treatment intervals, with the most common interval being 4 weeks, selected by 17 studies (33, 34, 36, 38, 40–42, 44, 45, 47, 51, 55, 56, 58, 60, 62, 64); five studies selected 3 weeks (35, 39, 50, 57, 59), four selected 2 weeks (37, 46, 49, 52), one selected 1 week (43), and four selected interval of ≥ 6 weeks (53, 54, 62, 63).
Several studies have demonstrated hair regrowth in AA lesions after PRP monotherapy. Among studies included, PRP showed superior efficacy compared to placebo in Severity of Alopecia Tool (SALT) score reduction (35, 42, 46), hair regrowth (35, 42, 45), and decrease in dystrophic hairs (42, 54), in mild cases of AA regardless of disease duration. For more severe AA cases, Khademi et al. found that PRP as monotherapy was relatively ineffective in alopecia totalis (AT) (36). Regarding different delivery methods of PRP, Ragab et al. reported that the efficacy of PRP in SALT score reduction was comparable among intradermal injection, fractional CO2 laser, and microneedling. At the end of the study, no significant difference between the groups was observed in physician clinical assessment and patient satisfaction (38).
Clinical trials were conducted to compare PRP with intralesional triamcinolone acetonide (TA). PRP was found to be non-inferior to intralesional TA, a standard treatment for patchy AA. Trink et al. compared PRP to 2.5 mg/ml intralesional TA and found that PRP therapy resulted in a greater reduction in SALT score and improved dermoscopic features and relapse rates (33). According to studies comparing higher TA concentrations (10 mg/ml) with PRP in which most patients had patchy AA with < 25% scalp involvement or < 6 months of disease duration, each treatment had comparable efficacy (37, 39–41, 47). However, according to a few studies, TA-treated groups demonstrated a greater reduction in SALT score and greater hair regrowth (39, 40). Two studies found that the PRP group had a lower relapse rate than the corticosteroid group (33, 37). Efficacy of PRP in AA has also been compared to topical minoxidil and low-level laser therapy (LLLT). PRP showed superior to 5% topical minoxidil in improving dystrophic hair and had a greater effect on improving hair diameter compared to LLLT (34, 43).
Studies investigating PRP as a co-intervention for AA are limited. Mubki reported an increased hair diameter in combined PRP and TA injected scalp side compared to a decline in the contralateral side in a 22-year-old female with chronic diffuse AA for 5 years (49). Two case reports published in 2019 reported some efficacy of PRP as adjuvant therapy on hair regrowth in AT patients (51, 52). Of the two studies, one study initiated PRP as adjuvant therapy after a 7-month course of Janus kinase inhibitor (JAKi) treatment in an 11-year-old patient with AT (52), and another study added PRP as an adjuvant to topical corticosteroids and minoxidil (51). In addition, Ederaine et al. reported an adjuvant effect of PRP with JAKi, showing significant hair regrowth after 4 months of combined treatment in a 31-year-old woman with plaque psoriasis who presented with patchy AA progressed to alopecia universalis (AU) (55).
There are only a few studies that documented the efficacy of PRP in PCAs to date. In our review, six case reports and three case series addressed the efficacy of PRP in hair regrowth, reduction of clinical itching and scaling, and improvement of dermoscopic features (perifollicular erythema and scaling) after an average of three PRP sessions (56–64). Among them, two case series demonstrated a more reliable perspective of PRP efficacy. One case series comprising 10 patients showed variable treatment responses depending on patients’ characteristics (61), and another, comprising two patients, indicated decreasing efficacy of PRP treatment over time (60). Patients in included studies had lymphocytic (i.e., lichen planopilaris, frontal fibrosing alopecia, fibrosing alopecia in a patern distribution, discoid lupus erythematosus, and central centrifugal cicatricial alopecia) and neutrophilic PCAs (i.e., folliculitis decalvans). Four of the reported efficacious studies used intradermal PRP injection as monotherapy, and patients in two of four studies had concomitant androgenetic alopecia (56, 57, 60, 63).
Non-randomized studies, particularly case reports and case series, were rated as having a serious or critical risk of bias, mainly due to their inherent potential for confounding and selection bias. All RCTs included were rated as having either low risk or some concerns for overall bias. Risk-of-bias plots are shown in Figures 2, 3.
Increasing evidence emphasizes the efficacy of PRP in treating IMAs. The present systematic review has retrieved a sufficient number of available clinical trials regarding PRP treatment for AA and PCAs to perform a pertinent systematic analysis of results. Our study demonstrates promising results for PRP treatment of patch-type AA either as monotherapy or when compared to intralesional TA, topical minoxidil, and LLLT. Moreover, our analysis reveals the efficacy of PRP treatment for PCAs in case reports and small case series. Nevertheless, cumulative evidence is not as convincing for PRP use as standard treatment for AA and PCAs.
PRP therapy is a novel technique comprising autologous plasma preparations with concentrated platelets. Its regenerative effects are gaining momentum in hair loss treatment. PRP is a promising treatment for IMAs because it uses the patient’s healing mechanism, acting on multiple biological targets with minimal immune reaction concerns (27, 29). Nevertheless, how PRP elicits therapeutic effects in IMAs remains unclear. Based on current evidence, PRP helps regenerate and repair HFs by releasing several key growth factors and cytokines (e.g., PDGF, FGF, EGF, and IGF) that play critical roles in HF stem cell differentiation and proliferation (26, 27). Additionally, PRP impacts the anti-inflammatory effect by downregulating monocyte chemoattractant protein-1, matrix metalloproteinase (MMP)-3, MMP-13, and a disintegrin and metalloproteinase with thrombospondin motifs-5, and immunomodulatory properties by upregulating IL (interleukin)-4, IL-10, IL-13, and TGF-β (65–68). Furthermore, PRP may restore normal skin, prevent fibrosis, and remodel scar tissue (69–72).
Our analysis reveals that PRP has demonstrated favorable results in treating IMAs. The data support the use of PRP as a promising, safe, office-based therapy for hair regrowth in patients with patchy AA; however, variable responses were reported in severe AA types, including AT and AU. Most RCTs demonstrate comparable PRP efficacy to intralesional TA with earlier and more persistent responses (37, 39–41, 47). PRP also showed superior efficacy compared to 5% topical minoxidil and LLLT (34, 43). In contrast to AA, PCAs have fewer studies evaluating the efficacy of PRP, and their treatment endpoint is disease stabilization. Patients in included studies had lymphocytic and neutrophilic scarring alopecias. Case reports and small case series have shown positive clinical outcomes (56–60, 62–64), whereas one case series revealed variable efficacy of PRP treatment (61). However, the use of PRP to treat IMAs is at the initial stage, and several issues remain to be addressed, including efficacy in more severe forms of AA and other subtypes of PCAs, PRP safety, and standard protocols.
PRP is a relatively safe procedure with mild adverse effects, such as tolerable pain, scalp discomfort, burning sensation, and transient erythema. To date, there have been no reports of serious adverse events, such as bleeding and infection. Nevertheless, all included studies highlighted the safety of PRP for IMAs only in short follow-up duration, which could not support its safety appropriately. Notably, contraindications for PRP treatment include hemodynamic instability, coagulation disorders, and infection at the treated site (73).
Although PRP is effective in many studies, its clinical application is complicated by the lack of consensus regarding its preparation and treatment protocol given the number of variables, including equipment, centrifugation forces, number and length of centrifugation, number and interval of treatment sessions, and dosage. Furthermore, evidence supporting long-term maintenance and criteria for treatment candidates is still lacking. The heterogeneity in PRP therapy requires further well-designed studies to overcome these surrounding controversies.
Moreover, it is difficult to determine whether the efficacy of PRP is due to the growth factors and cytokines within the PRP or their production as a result of needle injection-induced trauma since there is currently no solid evidence to support the mechanism of PRP for treating hair disorders speculated by previous studies. There are conflicting results in included studies with a split-scalp design comparing PRP with normal saline solution (36, 42) or TA (33, 40) injections. Previous RCT comparing the efficacy of PRP vs. saline in 26 patients with androgenic alopecia found it an effective treatment; however, the growth factor levels (i.e., PDGF, EGF, and VEGF) did not correlate with clinical improvement (74). The mechanism responsible for improvement following PRP injection remains to be investigated.
This systematic review has some limitations. We included all types of study designs, which contained bias-prone case series and case reports in our analysis. As a result, many of the included studies are of poor quality, particularly those on PCAs. Moreover, many studies have small sample sizes. Lastly, the high heterogeneity between studies, such as diverse PRP preparation, outcome evaluation methods, and disease severity of study populations, prohibits quantitative analysis.
This systematic review reports preliminary evidence that PRP is a promising treatment option for IMAs, particularly in individuals who fail conventional therapies, experience adverse effects, or are contraindicated for other modalities. PRP is a relatively effective treatment for regrowing hairs in AA and alleviating disease progression in PCAs with minimal adverse effects. However, this conclusion is mostly based on limited evidence, including case reports and series and studies with small sample sizes without a proper control group. Moreover, standardized protocols for PRP preparation and treatment remain controversial. Further large-scale, high-quality RCTs with a longer duration of follow-up are crucial to confirm the efficacy and safety of PRP in IMAs. Currently, there is insufficient evidence to support using PRP as standard treatment.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
PS: conceptualization and writing—review and editing. PS, KT, and TY: methodology. PS and NS: validation. KT and TY: formal analysis. KT, TY, and NS: investigation and writing—original draft preparation. NS: data curation. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1058431/full#supplementary-material
1. Thadanipon K, Suchonwanit P. Measuring patient quality of life following treatment for alopecia. Patient Prefer Adherence. (2021) 15:1601–10. doi: 10.2147/ppa.S282399
2. Leerunyakul K, Suchonwanit P. Asian hair: a review of structures, properties, and distinctive disorders. Clin Cosmet Investig Dermatol. (2020) 13:309–18. doi: 10.2147/ccid.S247390
3. Harnchoowong S, Suchonwanit P. PPAR-γ agonists and their role in primary cicatricial alopecia. PPAR Res. (2017) 2017:2501248. doi: 10.1155/2017/2501248
4. Iamsumang W, Leerunyakul K, Suchonwanit P. Finasteride and its potential for the treatment of female pattern hair loss: evidence to date. Drug Des Devel Ther. (2020) 14:951–9. doi: 10.2147/dddt.S240615
5. Suchonwanit P, McMichael AJ. Alopecia in association with malignancy: a review. Am J Clin Dermatol. (2018) 19:853–65. doi: 10.1007/s40257-018-0378-1
6. Paus R, Bulfone-Paus S, Bertolini M. Hair follicle immune privilege revisited: the key to alopecia areata management. J Investig Dermatol Symp Proc. (2018) 19:S12–7. doi: 10.1016/j.jisp.2017.10.014
7. Rutnin S, Chanprapaph K, Pakornphadungsit K, Leerunyakul K, Visessiri Y, Srisont S, et al. Variation of hair follicle counts among different scalp areas: a quantitative histopathological study. Skin Appendage Disord. (2022) 8:24–30. doi: 10.1159/000518434
8. Suchonwanit P, Triamchaisri S, Wittayakornrerk S, Rattanakaemakorn P. Leprosy reaction in thai population: a 20-year retrospective study. Dermatol Res Pract. (2015) 2015:253154. doi: 10.1155/2015/253154
9. Leerunyakul K, Suchonwanit P. Evaluation of hair density and hair diameter in the adult thai population using quantitative trichoscopic analysis. Biomed Res Int. (2020) 2020:2476890. doi: 10.1155/2020/2476890
10. Suchonwanit P, Kositkuljorn C, Pomsoong C. Alopecia areata: an autoimmune disease of multiple players. Immunotargets Ther. (2021) 10:299–312. doi: 10.2147/itt.S266409
11. Chanprapaph K, Mahasaksiri T, Kositkuljorn C, Leerunyakul K, Suchonwanit P. Prevalence and risk factors associated with the occurrence of autoimmune diseases in patients with alopecia areata. J Inflamm Res. (2021) 14:4881–91. doi: 10.2147/jir.S331579
12. Khunkhet S, Vachiramon V, Suchonwanit P. Trichoscopic clues for diagnosis of alopecia areata and trichotillomania in Asians. Int J Dermatol. (2017) 56:161–5. doi: 10.1111/ijd.13453
13. Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. (2018) 78:1–12. doi: 10.1016/j.jaad.2017.04.1141
14. Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. (2016) 55:e338–43. doi: 10.1111/ijd.13061
15. Triyangkulsri K, Srisuwanwattana P, Sriphojanart T, Suchonwanit P. Fibrosing alopecia in a pattern distribution: a case report and literature review. Case Rep Dermatol. (2019) 11:297–302. doi: 10.1159/000503681
16. Harries MJ, Paus R. The pathogenesis of primary cicatricial alopecias. Am J Pathol. (2010) 177:2152–62. doi: 10.2353/ajpath.2010.100454
17. Suchonwanit P, Pakornphadungsit K, Leerunyakul K, Khunkhet S, Sriphojanart T, Rojhirunsakool S. Frontal fibrosing alopecia in Asians: a retrospective clinical study. Int J Dermatol. (2020) 59:184–90. doi: 10.1111/ijd.14672
18. Chanprapaph K, Pomsoong C, Kositkuljorn C, Suchonwanit P. Intramuscular corticosteroid therapy in the treatment of alopecia areata: a time-to-event analysis. Drug Des Devel Ther. (2022) 16:107–16. doi: 10.2147/dddt.S342179
19. Mahasaksiri T, Kositkuljorn C, Anuntrangsee T, Suchonwanit P. Application of topical immunotherapy in the treatment of alopecia areata: a review and update. Drug Des Devel Ther. (2021) 15:1285–98. doi: 10.2147/dddt.S297858
20. Sriphojanart T, Khunkhet S, Suchonwanit P. A retrospective comparative study of the efficacy and safety of two regimens of diphenylcyclopropenone in the treatment of recalcitrant alopecia areata. Dermatol Rep. (2017) 9:7399. doi: 10.4081/dr.2017.7399
21. Suchonwanit P, Kositkuljorn C, Mahasaksiri T, Leerunyakul K. A comparison of the efficacy and tolerability of three corticosteroid treatment regimens in patients with alopecia areata. J Dermatolog Treat. (2022) 33:756–61. doi: 10.1080/09546634.2020.1773384
22. Triyangkulsri K, Suchonwanit P. Role of janus kinase inhibitors in the treatment of alopecia areata. Drug Des Devel Ther. (2018) 12:2323–35. doi: 10.2147/dddt.S172638
23. Rattananukrom T, Suchonwanit P. Are drug treatment strategies really effective against alopecia areata? Expert Opin Pharmacother. (2021) 22:257–60. doi: 10.1080/14656566.2020.1854728
24. Meah N, Wall D, York K, Bhoyrul B, Bokhari L, Sigall DA, et al. The Alopecia Areata Consensus of Experts (ACE) study: results of an international expert opinion on treatments for alopecia areata. J Am Acad Dermatol. (2020) 83:123–30. doi: 10.1016/j.jaad.2020.03.004
25. Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. (2018) 78:15–24. doi: 10.1016/j.jaad.2017.04.1142
26. Li ZJ, Choi H-I, Choi D-K, Sohn K-C, Im M, Seo Y-J, et al. Autologous platelet-rich plasma: a potential therapeutic tool for promoting hair growth. Dermatol Surg. (2012) 38:t1.
27. Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. (2020) 21:7794. doi: 10.3390/ijms21207794
28. Suchonwanit P, Leerunyakul K, Kositkuljorn C. Diagnostic and prognostic values of cutaneous manifestations in COVID-19. Dermatol Ther. (2020) 33:e13650. doi: 10.1111/dth.13650
29. Christgau M, Moder D, Hiller KA, Dada A, Schmitz G, Schmalz G. Growth factors and cytokines in autologous platelet concentrate and their correlation to periodontal regeneration outcomes. J Clin Periodontol. (2006) 33:837–45. doi: 10.1111/j.1600-051X.2006.00991.x
30. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. (2019) 366:l4898. doi: 10.1136/bmj.l4898
31. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj. (2016) 355:i4919. doi: 10.1136/bmj.i4919
32. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. (2020) 12:55–61. doi: 10.1002/jrsm.1411
33. Trink A, Sorbellini E, Bezzola P, Rodella L, Rezzani R, Ramot Y, et al. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br J Dermatol. (2013) 169:690–4. doi: 10.1111/bjd.12397
34. El Taieb MA, Ibrahim H, Nada EA, Seif Al-Din M. Platelets rich plasma versus minoxidil 5% in treatment of alopecia areata: a trichoscopic evaluation. Dermatol Ther. (2017) 30:e12437. doi: 10.1111/dth.12437
35. Nagaratna C, Asha GS, Leelavthy B, Revathi TN. Safety and efficacy of microneedling with autologous platelet-rich plasma in chronic and stable alopecia areata. J Pak Assoc Dermatol. (2018) 28:59–63.
36. Khademi F, Tehranchinia Z, Abdollahimajd F, Younespour S, Kazemi-Bajestani SMR, Taheri K. The effect of platelet rich plasma on hair regrowth in patients with alopecia areata totalis: a clinical pilot study. Dermatol Ther. (2019) 32:e12989. doi: 10.1111/dth.12989
37. Albalat W, Ebrahim HM. Evaluation of platelet-rich plasma vs intralesional steroid in treatment of alopecia areata. J Cosmetic Dermatol. (2019) 18:1456–62. doi: 10.1111/jocd.12858
38. Ragab SEM, Nassar SO, Morad HA, Hegab DS. Platelet-rich plasma in alopecia areata: intradermal injection versus topical application with transepidermal delivery via either fractional carbon dioxide laser or microneedling. Acta Dermatovenerol Alp Pannonica Adriat. (2020) 25:169–73. doi: 10.15570/actaapa.2020.35
39. Kapoor P, Kumar S, Brar BK, Kukar N, Arora H, Brar SK. Comparative evaluation of therapeutic efficacy of intralesional injection of triamcinolone acetonide versus intralesional autologous platelet-rich plasma injection in alopecia areata. J Cutan Aesthet Surg. (2020) 13:103–11. doi: 10.4103/jcas.Jcas_16_19
40. Hegde P, Relhan V, Sahoo B, Garg VK. A randomized, placebo and active controlled, split scalp study to evaluate the efficacy of platelet-rich plasma in patchy alopecia areata of the scalp. Dermatol Ther. (2020) 33:e14388. doi: 10.1111/dth.14388
41. Balakrishnan A, Joy B, Thyvalappil A, Mathew P, Sreenivasan A, Sridharan R. A comparative study of therapeutic response to intralesional injections of platelet-rich plasma versus triamcinolone acetonide in alopecia areata. Indian Dermatol Online J. (2020) 11:920–4. doi: 10.4103/idoj.IDOJ_6_20
42. Gupta V, Parihar AS, Sharma VK, Jain S, Singh V, Khanna N. Evaluation of platelet-rich plasma on hair regrowth and lesional T-cell cytokine expression in alopecia areata: a randomized observer-blinded, placebo-controlled, split-head pilot study. J Am Acad Dermatol. (2021) 84:1321–8. doi: 10.1016/j.jaad.2020.12.039
43. Tawfik AA, Mostafa I, Soliman M, Soliman M, Abdallah N. Low level laser versus platelet-rich plasma in treatment of alopecia areata: a randomized controlled intra-patient comparative study. Open Access Macedonian J Med Sci. (2022) 10:420–7. doi: 10.3889/oamjms.2022.7428
44. Singh S. Role of platelet-rich plasma in chronic alopecia areata: our centre experience. Indian J Plastic Surg. (2015) 48:57–9. doi: 10.4103/0970-0358.155271
45. Khan S, Kamal T, Ellahi A, Ahmad TJ. Role of autologous platelet rich plasma (PRP) in limited alopecia areata in local population. J Pak Assoc Dermatol. (2016) 26:107–11.
46. Fayed HA, Elsaied MA, Faraj MR. Evaluation of platelet-rich plasma in treatment of alopecia areata: a placebo-controlled study. J Egypt Women’s Dermatol Soc. (2018) 15:100–5. doi: 10.1097/01.EWX.0000540042.97989.cf
47. Fawzy MM, Abdel Hay R, Mohammed FN, Sayed KS, Ghanem MED, Ezzat M. Trichoscopy as an evaluation method for alopecia areata treatment: a comparative study. J Cosmetic Dermatol. (2021) 20:1827–36. doi: 10.1111/jocd.13739
48. Donovan J. Successful treatment of corticosteroid-resistant ophiasis-type alopecia areata (AA) with platelet-rich plasma (PRP). JAAD Case Rep. (2015) 1:305–7. doi: 10.1016/j.jdcr.2015.07.004
49. Mubki T. Platelet-rich plasma combined with intralesional triamcinolone acetonide for the treatment of alopecia areata: a case report. J Dermatol Dermatol Surg. (2016) 20:87–90. doi: 10.1016/j.jdds.2015.11.002
50. De Vasconcelos RCF, Azuaga KL, Eid RT, Arenas GCF. Use of platelet-rich plasma in the treatment of difficult-to-control alopecia areata. Surg Cosmetic Dermatol. (2016) 8:56–9. doi: 10.5935/scd1984-8773.2016831504
51. Fonseka S, Bandara YMDM, Subhani B. Successful management of treatment-resistant alopecia areata with platelet rich plasma: a case series. Serbian J Dermatol Venereol. (2019) 11:50–2. doi: 10.2478/sjdv-2019-0007
52. Chhabra G, Verma P. Steroid-resistant alopecia totalis in a child successfully treated with oral apremilast and platelet-rich plasma therapy. Dermatol Ther. (2019) 32:e13082. doi: 10.1111/dth.13082
53. Pototschnig H, Madl MT. Successful treatment of alopecia areata barbae with platelet-rich plasma. Cureus. (2020) 12:e7495. doi: 10.7759/cureus.7495
54. Ekelem C, Juhasz M, Yu J, Hosking AM, Csuka E, Choi F, et al. Monitoring response to platelet-rich plasma in patients with alopecia areata with optical coherence tomography: a case series. J Investig Dermatol Symp Proc. (2020) 20:S50–4. doi: 10.1016/j.jisp.2020.05.008
55. Ederaine SA, Kushner CJ, Shapiro J, Lo Sicco KI. Clinical response to adjunctive platelet-rich plasma injections in a patient with alopecia universalis on oral tofacitinib. JAAD Case Rep. (2022) 20:34–6. doi: 10.1016/j.jdcr.2021.11.019
56. Bolanča Ž, Goren A, Getaldić-Švarc B, Vučić M, Šitum M. Platelet-rich plasma as a novel treatment for lichen planopillaris. Dermatol Ther. (2016) 29:233–5. doi: 10.1111/dth.12343
57. Jha AK. Platelet-rich plasma for the treatment of lichen planopilaris. J Am Acad Dermatol. (2018) 79:e95–6. doi: 10.1016/j.jaad.2018.05.029
58. Özcan D, Tunçer Vural A, Özen Ö. Platelet-rich plasma for treatment resistant frontal fibrosing alopecia: a case report. Dermatol Ther. (2019) 32:e13072. doi: 10.1111/dth.13072
59. Jha AK. Platelet-rich plasma as an adjunctive treatment in lichen planopilaris. J Am Acad Dermatol. (2019) 80:e109–10. doi: 10.1016/j.jaad.2018.09.013
60. Dina Y, Aguh C. Use of platelet-rich plasma in cicatricial alopecia. Dermatol Surg. (2019) 45:979–81. doi: 10.1097/DSS.0000000000001635
61. Svigos K, Yin L, Shaw K, Gutierrez D, Peterson E, Lo Sicco K, et al. Use of platelet-rich plasma in lichen planopilaris and its variants: a retrospective case series demonstrating treatment tolerability without koebnerization. J Am Acad Dermatol. (2020) 83:1506–9. doi: 10.1016/j.jaad.2020.06.026
62. Suh S, Nguyen C, Zhao L, Atanaskova Mesinkovska N. The role of platelet-rich plasma therapy in refractory folliculitis decalvans. JAAD Case Rep. (2021) 12:85–7. doi: 10.1016/j.jdcr.2021.04.008
63. Polster H, Kagha K, Luke J. Platelet rich plasma for the treatment of scarring alopecia due to discoid lupus erythematosus. J Drugs Dermatol. (2022) 21:309–10. doi: 10.36849/jdd.5933
64. Klein E, Karim M, Kim R, Lo Sicco K, Shapiro J. Reversible hair loss in lichen planopilaris: regrowth with low-dose naltrexone and platelet-rich plasma. J Drugs Dermatol. (2022) 21:671–3. doi: 10.36849/jdd.6810
65. Moussa M, Lajeunesse D, Hilal G, El Atat O, Haykal G, Serhal R, et al. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp Cell Res. (2017) 352:146–56. doi: 10.1016/j.yexcr.2017.02.012
66. Suchonwanit P, Triyangkulsri K, Ploydaeng M, Leerunyakul K. Assessing biophysical and physiological profiles of scalp seborrheic dermatitis in the thai population. Biomed Res Int. (2019) 2019:5128376. doi: 10.1155/2019/5128376
67. Rattanakaemakorn P, Suchonwanit P. Scalp pruritus: review of the pathogenesis, diagnosis, and management. Biomed Res Int. (2019) 2019:1268430. doi: 10.1155/2019/1268430
68. Chanprapaph K, Sutharaphan T, Suchonwanit P. Scalp biophysical characteristics in males with androgenetic alopecia: a comparative study with healthy controls. Clin Interv Aging. (2021) 16:781–7. doi: 10.2147/cia.S310178
69. Alser OH, Goutos I. The evidence behind the use of platelet-rich plasma (PRP) in scar management: a literature review. Scars Burn Heal. (2018) 4:2059513118808773. doi: 10.1177/2059513118808773
70. Suchonwanit P, Iamsumang W, Leerunyakul K. Topical finasteride for the treatment of male androgenetic alopecia and female pattern hair loss: a review of the current literature. J Dermatol Treat. (2022) 33:643–8. doi: 10.1080/09546634.2020.1782324
71. Deshmukh NS, Belgaumkar VA. Platelet-rich plasma augments subcision in atrophic acne scars: a split-face comparative study. Dermatol Surg. (2019) 45:90–8. doi: 10.1097/dss.0000000000001614
72. Pomsoong C, Sukanjanapong S, Ratanapokasatit Y, Suchonwanit P. Epidemiological, clinical, and trichoscopic features of syphilitic alopecia: a retrospective analysis and systematic review. Front Med. (2022) 9:890206. doi: 10.3389/fmed.2022.890206
73. Hesseler MJ, Shyam N. Platelet-rich plasma and its utilities in alopecia: a systematic review. Dermatol Surg. (2020) 46:93–102. doi: 10.1097/dss.0000000000001965
74. Rodrigues BL, Montalvão SAL, Cancela RBB, Silva FAR, Urban A, Huber SC, et al. Treatment of male pattern alopecia with platelet-rich plasma: a double-blind controlled study with analysis of platelet number and growth factor levels. J Am Acad Dermatol. (2019) 80:694–700. doi: 10.1016/j.jaad.2018.09.033
Keywords: AA, immune-mediated alopecia, lichen planopilaris, LPP, non-scarring alopecia, PCA, scarring alopecia, hair loss
Citation: Tejapira K, Yongpisarn T, Sakpuwadol N and Suchonwanit P (2022) Platelet-rich plasma in alopecia areata and primary cicatricial alopecias: A systematic review. Front. Med. 9:1058431. doi: 10.3389/fmed.2022.1058431
Received: 07 October 2022; Accepted: 08 November 2022;
Published: 24 November 2022.
Edited by:
Neusa Sakai Valente, University of São Paulo, BrazilReviewed by:
Marwah Adly Saleh, Cairo University, EgyptCopyright © 2022 Tejapira, Yongpisarn, Sakpuwadol and Suchonwanit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Poonkiat Suchonwanit, cG9vbmtpYXRAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
‡ORCID: Kasama Tejapira, orcid.org/0000-0001-6763-978X; Tanat Yongpisarn, orcid.org/0000-0002-1300-9624; Nawara Sakpuwadol, orcid.org/0000-0001-8163-6235; Poonkiat Suchonwanit, orcid.org/0000-0001-9723-0563
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.