
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med., 03 November 2022
Sec. Dermatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1057096
This article is part of the Research TopicAutoimmune Blistering Diseases: Advances in the Understanding of Pathogenesis and New Therapeutic HorizonsView all 7 articles
 Faith A. P. Zeng1†
Faith A. P. Zeng1† Dedee F. Murrell1,2,3*†
Dedee F. Murrell1,2,3*†Introduction: Bullous pemphigoid (BP) is the most common subtype of autoimmune blistering diseases that primarily affects the elderly and is classically defined by the presence of IgG and/or complement C3 against the BP180 and BP230 hemidesmosome proteins. However, most recent studies have introduced the role of specific eosinophil receptors and chemokine mediators in the pathogenesis of BP which are helpful in identifying new targets for future treatments.
Areas covered: This review will focus on the involvement of eosinophils in BP, including the processes that lead to their recruitment, activation, and regulation. Subsequently, covering new therapeutic options in relation to the role of eosinophils. Eotaxin enhances the recruitment of eosinophils in BP, with CCR3 chemoreceptor that is expressed on eosinophils being identified as a key binding site for eotaxin-1. The pathogenic role of IgE and IL-4 in BP is corroborated by successful treatments with Omalizumab and Dupilumab, respectively. IL-5, IL-17 and IL-23 inhibitors may be effective given their roles in promoting eosinophilia.
Expert opinion: Further research into inhibitors of eotaxin, IL-4, IL-5, IL-17, IL-23, CCR3, and specific complement factors are warranted as preliminary studies have largely identified success in treating BP with these agents. Learning from novel treatments for other IgG-mediated autoimmune diseases may be beneficial.
Bullous pemphigoid (BP) is the most common subtype of autoimmune blistering diseases that primarily affects the elderly and is classically defined by the presence of IgG and/or complement C3 against the BP180 and BP230 hemidesmosome proteins (1, 2). Clinically, BP is a heterogenous disease with a wide spectrum of presentations, but typically manifests as widespread tense blisters and severe pruritus associated with erythematous urticarial plaques (3). Glucocorticoids are the cornerstone of treatment in BP, which although have significantly improved morbidity and mortality, are also associated with severe adverse effects due to the chronicity of the disease and thus treatment (4). Therefore, one of the key principles in the management of BP is to reduce the patient’s cumulative exposure to systemic glucocorticoids with the use of steroid-sparing agents (5). Thus, this review aims to discuss new discoveries in the pathogenesis of BP, focusing on the role of eosinophils, which will serve as a roadmap for future targeted therapies to further reduce the burden of steroid-induced side effects.
In the early days, a large proportion of experimental research had been dedicated to understanding the role of IgG autoantibodies in the pathogenesis of BP. However, in the past two decades, increasing efforts have been pivoted into exploring the IgE response in BP. Fairley et al., Zone et al., gives evidence for the pathogenicity of IgE in BP from their studies on mouse models (6, 7). These findings formed the basis for the study carried out by Döpp et al. which showed clinically significant correlation between IgE BP180 NC16A-specific antibodies and BP disease severity (8). This is further supported by the increasing pool of case reports/series’ showing that omalizumab, an anti-IgE monoclonal antibody, is a well-tolerated and effective treatment in BP (9–13). Omalizumab appears to be a promising treatment for BP, warranting future randomized controlled trials. Omalizumab has been included in the most recent “Updated S2 K guidelines for the management of BP,” an initiative by the European Academy of Dermatology and Venereology (EADV) (14). A phase 3 study (NCT04128176) of the efficacy of rituximab combined with omalizumab has been registered on the US National Library of Medicine Clinical Trials registry, and will soon begin recruitment of study participants (15).
In 1996, Schmidt and colleagues proved increased levels of IL-4, IL-6, and IL-10 in the blister fluid of BP (16). More recently in 2018, the first case report describing an elderly man in his 80s with recalcitrant BP successfully treated with dupilumab, an IL-4 and IL-13 monoclonal antibody (17). The patient had been given an initial 600 mg loading dose of dupilumab administered subcutaneously, followed by weekly 300 mg subcutaneous injections, which showed resolution of all blisters and undetectable levels of BP180 and BP230 antibodies after 3 months of treatment (17). Since then, there have been further reports of dupilumab as a successful and tolerable treatment for BP (18–21). Dupilumab which was successful in a phase 2 clinical trial of BP and is undergoing phase 3 trial (LIBERTY-BP study) (NCT04206553) at present, has been included in the most recent “Updated S2 K guidelines for the management of BP,” an initiative by the EADV (14, 22). Could there then be a role for anti-IL-6 or anti-IL-10 drugs in the treatment of BP as well?
Eosinophils are major effector cells of the human immune system with their impacts likely primarily mediated by their cytoplasmic granules, and are found in a variety of organs including the skin. Whilst it is readily accepted that there is enhanced tissue and serum eosinophil levels in BP, the actual mechanisms in which they cause BP are less understood. One potential pathway described in the literature is that eosinophils stimulate the secretion of matrix metalloproteinase-9 which facilitate the degradation of BP180 and cleavage of the dermo-epidermal junction (DEJ) (23–25).
One study found a positive correlation between tissue eosinophil level and the quantity of inflammatory lesions (26). However, with regards to the relationship between blood eosinophil count and severity of BP measured by the Bullous Pemphigoid Disease Activity Index scores, confounding data exists (10, 27–29). Currently, these are preliminary findings, and larger cohort studies are required for more definitive conclusions to be drawn. In 1974, one of the earliest studies showing correlation between elevated IgE and BP by Arbesman et al., concluded that approximately 70% of patients with disease had elevated IgE (30). However, later studies revealed that the proportion of elevated IgE levels in patients with BP is highly variable, ranging from 22 to 100% (8, 31–39).
This section explores the latest findings on the processes that drive eosinophilia, which can serve as a future roadmap for new therapies in BP. Table 1 summarizes all pharmacological agents mentioned in this review (15, 22, 40–53).
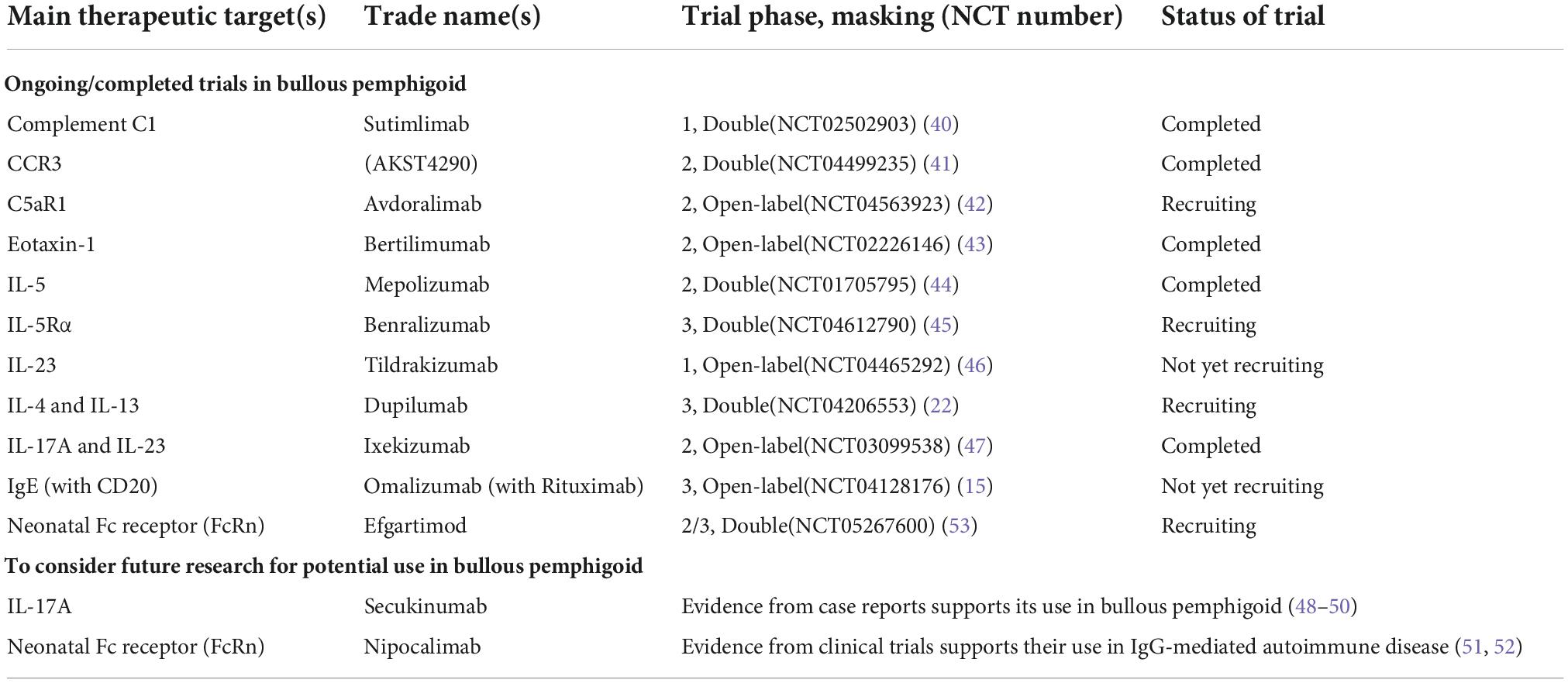
Table 1. Summary of ongoing/completed trials of novel pharmacologic agents in bullous pemphigoid and other promising agents for consideration (in alphabetical order).
Chemokines are a specific subtype of chemotactic cytokines that mediate leukocyte trafficking by binding to G-coupled protein receptors (54). To date, it is well described that induction of TH2 cells is critical for the production of IgE, and that CCR3 and CCR4 chemokine receptors are preferentially expressed on TH2 cells (55, 56). Given that IgE is an important mediator in the pathogenesis of BP, it would be logical to hypothesize that elevated levels of CCR3 and CCR4 ligands are present in the disease—this in alignment with recent reports showing elevated levels of CCR3 and its ligand, eotaxin, in BP (57–59). A phase 2 double-blind clinical trial testing AKST4290, a CCR3 inhibitor, in the treatment of BP (NCT04499235) preliminarily concluded that AKST4290 is efficacious when used in conjunction with mometasone furoate (41).
More specifically, eotaxin-1 and eotaxin-3 are significantly upregulated in the serum and blister fluid of BP patients, and is strongly associated with eosinophil numbers and activation (60, 61). This formed the basis for a phase 2 open-label study of the safety and efficacy of bertilimumab, an anti-eotaxin-1 antibody, in the treatment of patients with newly diagnosed moderate to extensive BP (NCT02226146) (43). The study comprised a treatment period of 4 weeks, with bertilimumab IV infusions on Days 0, 14, and 28, followed by a safety and efficacy follow-up period of approximately 13 weeks. The preliminary results demonstrated that bertilimumab was a safe and efficacious treatment of BP (62). Although promising, larger controlled trials with longer follow-up duration are warranted. Given that chemokines are specific and potent leukocyte chemoattractants, they are suitable for targeted therapies. Therefore, it may also then be beneficial to explore the potential therapeutic effects of anti-eotaxin-3 antibodies and CCR4 inhibitors.
Similar to IL-4, IL-5 is a TH2 cell-induced cytokine detected in the blister fluid of patients with BP and characterizes the acute phase of BP (63, 64). IL-5 also functions as a critical cytokine for eosinophilic maturation and functional activity, and is found to increase CCR3 expression in eosinophils—this can explain increased chemotactic activity of eosinophils primed with IL-5 in BP fluid (65–67). However, when put into clinical practice, anti-IL-5 antibodies show controversial results. Simon et al., concluded from a randomized placebo-controlled, double-blind phase 2 pilot study (NCT01705795) that mepolizumab (with steroids), an anti-IL-5 antibody, was not effective in the treatment of BP compared to placebo (with steroids) (44). The primary endpoint for the study was defined as the cumulative rate of relapse-free patients after starting therapy. Although the primary outcome was not significantly different between the mepolizumab and placebo groups, it was found that the former did have markedly lowered blood eosinophil levels nonetheless (44). A small sample size and short follow-up duration were the major limitations in this pilot study. Conversely, a recent case report describes the rapid clinical regression of bullous skin lesions in a patient with BP upon treatment with reslizumab, another anti-IL-5 antibody, allowing tapering of systemic steroid dosage (68). In addition, re-exacerbation of skin lesions were noted upon discontinuation of reslizumab, despite use of maintenance cyclosporin (68). Benralizumab is a humanized monoclonal antibody against IL-5Rα, which has been shown to cause direct apoptosis of eosinophils (69, 70). Currently, recruitment for a randomized clinical trial in Phase 3 is underway to evaluate the use of benralizumab (FJORD study) (NCT04612790) in the treatment of BP (45).
Previously, it was observed in in vitro studies that increased release of IL-17 leads to enhanced eosinophilia (71). The relevance of this has been minimally substantiated in three recent case reports on the successful treatment of BP with secukinumab, a humanized monoclonal antibody against IL-17A (48–50). In their case report, Kamata et al. had shown that administration of secukinumab decreased circulating anti-BP180 NC16A antibodies in the patient (50). Interestingly, ixekizumab, another anti-IL-17A (and anti-IL23) monoclonal antibody, was incidentally found to induce clinical remission of BP in a patient with concurrent psoriasis—the latter for which ixekizumab had been indicated (72). However, an open-label phase 2 trial evaluating the use of ixekizumab in BP (NCT03099538) had failed to reach endpoint—primary outcome of cessation of blisters was not achieved in the stipulated timeframe, with small sample size (total of 4 participants enrolled and analyzed) being the major limitation of the study (47).
The relationship between IL-23 and IL-17A is well described in allergic asthma, the paradigm of IgE-mediated disease (73, 74). IL-23 is an important cytokine in promoting and maintaining IL-17, thereby facilitating eosinophilia (75, 76). However, Delli et al. have shown that in eosinophil count was positively related to serum IL-17, but negatively related to IL-23 levels in BP (77). Regardless, given the pathogenic role of IL-23 (and IL-17) in other eosinophil-mediated disease, it would still be beneficial to consider IL-23 inhibitors in BP. With this understanding, an early open-label phase 1 pilot study to evaluate the effects of tildrakizumab, an anti-IL-23 antibody, in the treatment of BP has been approved (NCT04465292) (46).
Eosinophils express various receptors, including those of complement such as C3a and C5a which are known to enhance eosinophil recruitment, extravasation, and activation (78, 79). The pathogenic role of complement induced eosinophilia in BP is evidenced by studies in mice showing that passive transfer of complement-fixing autoantibodies primarily against BP180 leads to subepidermal blisters that mimic those of human BP (80). Furthermore, it has been found that animals with complete deficiency in complement C3, C4, C5, and C5aR fail to develop BP lesions upon injection with pathogenic autoantibodies (81–87). Currently, a phase 2 open-label study is underway to test the safety and efficacy of avdoralimab, an antiC5aR1 monoclonal antibody, in the treatment of BP (42).
Convertase enzymes play a key role in complement activation—for instance, C3 convertase is required to cleave C3 into C3a and C3b (88). Additionally, C3 convertase is formed by the products of C1s-catalyzed cleavage of C2 and C4, where optimal C3 convertase activity is only observed with high levels of C1s (89). Although the mechanisms of human complement pathways, and their involvement in the pathogenesis of eosinophilia have been well understood for many years, it was not till recently that research into complement inhibitors in BP have been conducted. More specifically, the first ever study on the safety and tolerability of BIVV009 (Sutimlimab), a humanized IgG4 monoclonal antibody that inhibits C1s, in patients with BP (40). A total 8 patients with BP had completed the study (NCT02502903)—preliminary conclusions were that infusions of 4 weekly 60 mg/kg doses of sutimlimab is effective, with the majority of samples showing absence of C3 deposition at the dermal-epidermal junction; and safe, with mostly mild to moderate side effects such as coryzal symptoms (40). Of note, key limitations of the study include small sample size, short treatment duration, and the lack of overt disease activity in the study population which had prohibited the evaluation of clinical efficacy of sutimlimab in treating BP. Therefore, further research is warranted in determining the true efficacy of sutimlimab in the treatment of BP.
Neonatal Fc Rceptor (FcRn) has been found to play a central role in the homeostasis of IgG (90, 91). The formation of IgG-FcRn complex prevents the degradation of IgG, which allows for the recycling and release of IgG (92). Li et al. demonstrated that FcRn deficient mice were resistant to experimental BP and subtypes of pemphigus, where circulating levels of pathogenic IgG were significantly reduced as compared to the wild type mice (93). Efgartigimod is an IgG1-derived Fc-fragment that binds FcRN thus enhancing degradation of pathogenic IgG. A pilot study by Zakrzewicz et al. has shown that efgartigimod, in vitro, can stabilize keratinocyte adhesion in the presence of pathogenic pemphigus antibodies (51). Currently, a phase 3 trial is underway to assess the efficacy and safety of efgartigimod in adults with pemphigus (ADDRESS study) (NCT04598451) (94). Efgartigimod could also then be an effective treatment for other IgG-mediated autoimmune disease like BP. In fact, a seamless 2-part, international phase 2/3 study (BALLAD study) (NCT 05267600) has recently been approved to investigate the efficacy, safety, and tolerability of efgartigimod in BP (53). Author (DM) was involved in the designing of the BALLAD study. Nipocalimab, similar in its mechanism of action to efgartigimod, is currently undergoing a phase 3 trial to assess its efficacy and safety in generalized myasthenia gravis, which is also an IgG-mediated autoimmune disease like BP (NCT04951622) (52). If proven successful and safe, nipocalimab could also then be considered as a therapeutic agent in BP.
Having more targeted treatments for BP would be beneficial given that existing steroid-sparing immunosuppressants can have devastating side effects due to widespread actions. Therefore, further research into antibodies against eotaxin, IL-4, IL-5, IL-17, IL-23, CCR3, and specific complement factors are warranted as preliminary studies have largely identified success in treating BP with these agents. Additionally, a greater focus on chemokine-targeted therapies (such as anti-eotaxin antibodies) may be favorable given more specific actions than their interleukin cytokine counterparts. Exploring novel treatments for other autoimmune diseases for use in BP, such as FcRn inhibitors would also be beneficial.
FZ and DM contributed to conceptualization and the writing of the original draft. Both authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Genovese G, Di Zenzo G, Cozzani E, Berti E, Cugno M, Marzano AV. New insights into the pathogenesis of bullous pemphigoid: 2019 update. Front Immunol. (2019) 10:1506. doi: 10.3389/fimmu.2019.01506
2. Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. (2017) 18:513–28. doi: 10.1007/s40257-017-0264-2
3. Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. (2013) 381:320–32. doi: 10.1016/S0140-6736(12)61140-4
4. Seale JP, Compton MR. Side-effects of corticosteroid agents. Med J Aust. (1986) 144:139–42. doi: 10.5694/j.1326-5377.1986.tb112242.x
5. Zeng FAP, Wilson A, Sheriff T, Murrell DF. Side effects of steroid-sparing agents in patients with bullous pemphigoid and pemphigus: a systematic review. JAAD Int. (2022) 9:33–43. doi: 10.1016/j.jdin.2022.07.005
6. Fairley JA, Burnett CT, Fu CL, Larson DL, Fleming MG, Giudice GJ. A pathogenic role for IgE in autoimmunity: bullous pemphigoid IgE reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J Invest Dermatol. (2007) 127:2605–11. doi: 10.1038/sj.jid.5700958
7. Zone JJ, Taylor T, Hull C, Schmidt L, Meyer L. IgE basement membrane zone antibodies induce eosinophil infiltration and histological blisters in engrafted human skin on SCID mice. J Invest Dermatol. (2007) 127:1167–74. doi: 10.1038/sj.jid.5700681
8. Dopp R, Schmidt E, Chimanovitch I, Leverkus M, Brocker EB, Zillikens D. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in Bullous pemphigoid: serum levels of these immunoglobulins reflect disease activity. J Am Acad Dermatol. (2000) 42:577–83. doi: 10.1067/mjd.2000.103986
9. Fairley JA, Baum CL, Brandt DS, Messingham KA. Pathogenicity of IgE in autoimmunity: successful treatment of bullous pemphigoid with omalizumab. J Allergy Clin Immunol. (2009) 123:704–5. doi: 10.1016/j.jaci.2008.11.035
10. Yu KK, Crew AB, Messingham KA, Fairley JA, Woodley DT. Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol. (2014) 71:468–74. doi: 10.1016/j.jaad.2014.04.053
11. Balakirski G, Alkhateeb A, Merk HF, Leverkus M, Megahed M. Successful treatment of bullous pemphigoid with omalizumab as corticosteroid-sparing agent: report of two cases and review of literature. J Eur Acad Dermatol Venereol. (2016) 30:1778–82. doi: 10.1111/jdv.13758
12. Lonowski S, Sachsman S, Patel N, Truong A, Holland V. Increasing evidence for omalizumab in the treatment of bullous pemphigoid. JAAD Case Rep. (2020) 6:228–33. doi: 10.1016/j.jdcr.2020.01.002
13. D’Aguanno K, Gabrielli S, Ouchene L, Muntyanu A, Ben-Shoshan M, Zhang X, et al. Omalizumab for the treatment of bullous pemphigoid: a systematic review of efficacy and safety. J Cutan Med Surg. (2022) 26:404–13. doi: 10.1177/12034754221089267
14. Borradori L, Van Beek N, Feliciani C, Tedbirt B, Antiga E, Bergman R, et al. Updated S2 K guidelines for the management of bullous pemphigoid initiated by the European academy of dermatology and venereology (EADV). J Eur Acad Dermatol Venereol. (2022) 36:1689–704. doi: 10.1111/jdv.18220
15. US National Library of Medicine. Efficacy and Safety of Rituximab Combined With Omalizumab in Patients With Bullous Pemphigoid. Bethesda, MD: US National Library of Medicine (2019).
16. Schmidt E, Bastian B, Dummer R, Tony HP, Brocker EB, Zillikens D. Detection of elevated levels of IL-4, IL-6, and IL-10 in blister fluid of bullous pemphigoid. Arch Dermatol Res. (1996) 288:353–7. doi: 10.1007/BF02507102
17. Kaye A, Gordon SC, Deverapalli SC, Her MJ, Rosmarin D. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. (2018) 154:1225–6. doi: 10.1001/jamadermatol.2018.2526
18. Velin M, Dugourd PM, Sanchez A, Bahadoran P, Montaudie H, Passeron T. Efficacy and safety of methotrexate, omalizumab and dupilumab for bullous pemphigoid in patients resistant or contraindicated to oral steroids. A monocentric real-life study. J Eur Acad Dermatol Venereol. (2022) 36:e539–42. doi: 10.1111/jdv.17999
19. Yang J, Gao H, Zhang Z, Tang C, Chen Z, Wang L, et al. Dupilumab combined with low-dose systemic steroid therapy improves efficacy and safety for bullous pemphigoid. Dermatol Ther. (2022) 35:e15648. doi: 10.1111/dth.15648
20. Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Front Immunol. (2022) 13:928621. doi: 10.3389/fimmu.2022.928621
21. Takamura S, Teraki Y. Treatment of bullous pemphigoid with dupilumab: dupilumab exerts its effect by primarily suppressing T-helper 2 cytokines. J Dermatol. (2022). 49:845–850. doi: 10.1111/1346-8138.16428
22. US National Library of Medicine. A Study to Evaluate the Efficacy and Safety of Dupilumab in Adult Patients With Bullous Pemphigoid (LIBERTY-BP). Bethesda, MD: US National Library of Medicine (2019).
23. Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM. Transmigration of eosinophils through basement membrane components in vitro: synergistic effects of platelet-activating factor and eosinophil-active cytokines. Am J Respir Cell Mol Biol. (1997) 16:455–63. doi: 10.1165/ajrcmb.16.4.9115757
24. Liu Z, Shapiro SD, Zhou X, Twining SS, Senior RM, Giudice GJ, et al. A critical role for neutrophil elastase in experimental bullous pemphigoid. J Clin Invest. (2000) 105:113–23. doi: 10.1172/JCI3693
25. Kelly EA, Liu LY, Esnault S, Quinchia Johnson BH, Jarjour NN. Potent synergistic effect of IL-3 and TNF on matrix metalloproteinase 9 generation by human eosinophils. Cytokine. (2012) 58:199–206. doi: 10.1016/j.cyto.2012.01.009
26. Gore Karaali M, Koku Aksu AE, Cin M, Leblebici C, Kara Polat A, Gurel MS. Tissue eosinophil levels as a marker of disease severity in bullous pemphigoid. Australas J Dermatol. (2021) 62:e236–41. doi: 10.1111/ajd.13547
27. Mori O, Hachisuka H, Kusuhara M, Sasai Y, Fujiwara S. Bullous pemphigoid in a 19-year-old woman. A case with unusual target antigens. Br J Dermatol. (1994) 130:241–5. doi: 10.1111/j.1365-2133.1994.tb02909.x
28. Farnaghi F, Ehsani AH, Kamyab-Hesary K, Abbasian S, Seirafi H, Nasimi M. Correlation of dermal and blood eosinophilia with bullous pemphigoid disease severity. Int J Womens Dermatol. (2020) 6:171–5. doi: 10.1016/j.ijwd.2020.01.005
29. van Beek N, Schulze FS, Zillikens D, Schmidt E. IgE-mediated mechanisms in bullous pemphigoid and other autoimmune bullous diseases. Expert Rev Clin Immunol. (2016) 12:267–77. doi: 10.1586/1744666X.2016.1123092
30. Arbesman CE, Wypych JI, Reisman RE, Beutner EH. IgE levels in sera of patients with pemphigus or bullous pemphigoid. Arch Dermatol. (1974) 110:378–81. doi: 10.1001/archderm.110.3.378
31. Lamberts A, Kotnik N, Diercks GFH, Meijer JM, Di Zenzo G, Pas HH, et al. IgE autoantibodies in serum and skin of non-bullous and bullous pemphigoid patients. J Eur Acad Dermatol Venereol. (2021) 35:973–80. doi: 10.1111/jdv.16996
32. Dimson OG, Giudice GJ, Fu CL, Van den Bergh F, Warren SJ, Janson MM, et al. Identification of a potential effector function for IgE autoantibodies in the organ-specific autoimmune disease bullous pemphigoid. J Invest Dermatol. (2003) 120:784–8. doi: 10.1046/j.1523-1747.2003.12146.x
33. Christophoridis S, Budinger L, Borradori L, Hunziker T, Merk HF, Hertl M. IgG, IgA and IgE autoantibodies against the ectodomain of BP180 in patients with bullous and cicatricial pemphigoid and linear IgA bullous dermatosis. Br J Dermatol. (2000) 143:349–55. doi: 10.1046/j.1365-2133.2000.03661.x
34. Dresow SK, Sitaru C, Recke A, Oostingh GJ, Zillikens D, Gibbs BF. IgE autoantibodies against the intracellular domain of BP180. Br J Dermatol. (2009) 160:429–32. doi: 10.1111/j.1365-2133.2008.08858.x
35. Messingham KA, Noe MH, Chapman MA, Giudice GJ, Fairley JA. A novel ELISA reveals high frequencies of BP180-specific IgE production in bullous pemphigoid. J Immunol Methods. (2009) 346:18–25. doi: 10.1016/j.jim.2009.04.013
36. Ishiura N, Fujimoto M, Watanabe R, Nakashima H, Kuwano Y, Yazawa N, et al. Serum levels of IgE anti-BP180 and anti-BP230 autoantibodies in patients with bullous pemphigoid. J Dermatol Sci. (2008) 49:153–61. doi: 10.1016/j.jdermsci.2007.08.008
37. Pomponi D, Di Zenzo G, Zennaro D, Calabresi V, Eming R, Zuzzi S, et al. Detection of IgG and IgE reactivity to BP180 using the ISAC(R) microarray system. Br J Dermatol. (2013) 168:1205–14. doi: 10.1111/bjd.12161
38. Liu B, Zuo YG, Zhou XP, He CX, Li J, Tie D, et al. [Establishment of enzyme-linked immunosorbent assay in the detection of BP180NC16A-specific IgE and its significance in bullous pemphigoid]. Zhonghua Yi Xue Za Zhi. (2013) 93:2244–7.
39. Iwata Y, Komura K, Kodera M, Usuda T, Yokoyama Y, Hara T, et al. Correlation of IgE autoantibody to BP180 with a severe form of bullous pemphigoid. Arch Dermatol. (2008) 144:41–8. doi: 10.1001/archdermatol.2007.9
40. Freire PC, Munoz CH, Derhaschnig U, Schoergenhofer C, Firbas C, Parry GC, et al. Specific inhibition of the classical complement pathway prevents C3 deposition along the dermal-epidermal junction in bullous pemphigoid. J Invest Dermatol. (2019) 139:2417–424.e2. doi: 10.1016/j.jid.2019.04.025
41. US National Library of Medicine. A Study to Assess the Therapeutic Effect and Safety of Adjunctive AKST4290 in Subjects With Bullous Pemphigoid. Bethesda, MD: US National Library of Medicine (2021).
42. US National Library of Medicine. Treatment of Bullous Pemphigoid With Avdoralimab (IPH5401), an Anti-C5aR1 Monoclonal Antibody (IPH). Bethesda, MD: US National Library of Medicine (2022).
43. US National Library of Medicine. Evaluation of Safety, Efficacy and Pharmacodynamic Effect of Bertilimumab in Patients With Bullous Pemphigoid. Bethesda, MD: US National Library of Medicine (2018).
44. Simon D, Yousefi S, Cazzaniga S, Bürgler C, Radonjic S, Houriet C, et al. Mepolizumab failed to affect bullous pemphigoid: a randomized, placebo-controlled, double-blind phase 2 Pilot Study. Allergy. (2020) 75:669–72. doi: 10.1111/all.13950
45. US National Library of Medicine. A Study to Investigate the Use of Benralizumab in Patients With Bullous Pemphigoid (FJORD). Bethesda, MD: US National Library of Medicine (2020).
46. US National Library of Medicine. The Effects of Tildrakizumab in Treatment of Bullous Pemphigoid. Bethesda, MD: US National Library of Medicine (2020).
47. US National Library of Medicine. Ixekizumab in the Treatment of Bullous Pemphigoid. Bethesda, MD: US National Library of Medicine (2020).
48. Holtsche MM, Hammers CM, Chakievska L, Ludwig RJ, Thaci D, Zillikens D, et al. Adjuvant treatment with secukinumab induced long term remission in a patient with severe bullous pemphigoid. J Dtsch Dermatol Ges. (2020) 18:1478–80. doi: 10.1111/ddg.14291
49. Yun JS, Scardamaglia L, Tan CG, McCormack CJ. Successful secukinumab treatment of active bullous pemphigoid and chronic severe psoriasis: a case report. Australas J Dermatol. (2022) 63:e155–8. doi: 10.1111/ajd.13803
50. Kamata M, Asano Y, Shida R, Maeda N, Yoshizaki A, Miyagaki T, et al. Secukinumab decreased circulating anti-BP180-NC16a autoantibodies in a patient with coexisting psoriasis vulgaris and bullous pemphigoid. J Dermatol. (2019) 46:e216–7. doi: 10.1111/1346-8138.14760
51. Zakrzewicz A, Wurth C, Beckert B, Feldhoff S, Vanderheyden K, Foss S, et al. Stabilization of keratinocyte monolayer integrity in the presence of anti-desmoglein-3 antibodies through FcRn blockade with efgartigimod: novel treatment paradigm for pemphigus? Cells. (2022) 11:942. doi: 10.3390/cells11060942
52. US National Library of Medicine. A Study of Nipocalimab Administered to Adults With Generalized Myasthenia Gravis. Bethesda, MD: US National Library of Medicine (2021).
53. US National Library of Medicine. A Phase 2/3 Study of Efgartigimod PH20 SC in Adult Participants With Bullous Pemphigoid (BALLAD). Bethesda, MD: US National Library of Medicine (2022).
54. Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. (2000) 95:3032–43. doi: 10.1182/blood.V95.10.3032
55. Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. (1998) 187:129–34. doi: 10.1084/jem.187.1.129
56. Janeway CR Jr, Travers P, Walport M, Shlomchik MJ. The Production of IgE. In: Immunobiology: The Immune System in Health and Disease. 5th ed. New York, NY: Garland Science (2001).
57. Wakugawa M, Nakamura K, Hino H, Toyama K, Hattori N, Okochi H, et al. Elevated levels of eotaxin and interleukin-5 in blister fluid of bullous pemphigoid: correlation with tissue eosinophilia. Br J Dermatol. (2000) 143:112–6. doi: 10.1046/j.1365-2133.2000.03599.x
58. Shrikhande M, Hunziker T, Braathen LR, Pichler WJ, Dahinden CA, Yawalkar N. Increased coexpression of eotaxin and interleukin 5 in bullous pemphigoid. Acta Derm Venereol. (2000) 80:277–80. doi: 10.1080/000155500750012162
59. Frezzolini A, Teofoli P, Cianchini G, Barduagni S, Ruffelli M, Ferranti G, et al. Increased expression of eotaxin and its specific receptor CCR3 in bullous pemphigoid. Eur J Dermatol. (2002) 12:27–31.
60. Liu Y, Wang Y, Chen X, Jin H, Li L. Factors associated with the activity and severity of bullous pemphigoid: a review. Ann Med. (2020) 52:55–62. doi: 10.1080/07853890.2020.1742367
61. Gunther C, Wozel G, Meurer M, Pfeiffer C. Up-regulation of CCL11 and CCL26 is associated with activated eosinophils in bullous pemphigoid. Clin Exp Immunol. (2011) 166:145–53. doi: 10.1111/j.1365-2249.2011.04464.x
62. Lee J, Werth VP, Hall RP III, Eming R, Fairley JA, Fajgenbaum DC, et al. Perspective from the 5th international pemphigus and pemphigoid foundation scientific conference. Front Med. (2018) 5:306. doi: 10.3389/fmed.2018.00306
63. Giomi B, Caproni M, Calzolari A, Bianchi B, Fabbri P. Th1, Th2 and Th3 cytokines in the pathogenesis of bullous pemphigoid. J Dermatol Sci. (2002) 30:116–28. doi: 10.1016/S0923-1811(02)00067-1
64. Feliciani C, Toto P, Mohammad Pour S, Coscione G, Amerio P, Amerio PA. Th2-like cytokine response is involved in bullous pemphigoid. the role of IL-4 and IL-5 in the pathogenesis of the disease. Int J Immunopathol Pharmacol. (1999) 12:55–61. doi: 10.1177/205873929901200202
65. Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. (1997) 277:2005–7. doi: 10.1126/science.277.5334.2005
66. Stirling RG, van Rensen EL, Barnes PJ, Chung KF. Interleukin-5 induces CD34(+) eosinophil progenitor mobilization and eosinophil CCR3 expression in asthma. Am J Respir Crit Care Med. (2001) 164(8 Pt. 1):1403–9. doi: 10.1164/ajrccm.164.8.2010002
67. Gounni Abdelilah S, Wellemans V, Agouli M, Guenounou M, Hamid Q, Beck LA, et al. Increased expression of Th2-associated chemokines in bullous pemphigoid disease. Role of eosinophils in the production and release of these chemokines. Clin Immunol. (2006) 120:220–31. doi: 10.1016/j.clim.2006.03.014
68. Rhyou HI, Han SH, Nam YH. Successful induction treatment of bullous pemphigoid using reslizumab: a case report. Allergy Asthma Clin Immunol. (2021) 17:117. doi: 10.1186/s13223-021-00619-1
69. Bagnasco D, Ferrando M, Varricchi G, Puggioni F, Passalacqua G, Canonica GW. Anti-Interleukin 5 (IL-5) and IL-5Ra biological drugs: efficacy, safety, and future perspectives in severe eosinophilic asthma. Front Med. (2017) 4:135. doi: 10.3389/fmed.2017.00135
70. FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. (2016) 388:2128–41. doi: 10.1016/S0140-6736(16)31322-8
71. Dias PM, Banerjee G. The role of Th17/IL-17 on eosinophilic inflammation. J Autoimmun. (2013) 40:9–20. doi: 10.1016/j.jaut.2012.07.004
72. Lu L, Yu Y, Zhang J, Fan X, Qi Y, Lin B. Incidental amelioration of bullous pemphigoid during ixekizumab treatment for psoriasis. J Dermatol. (2022) 49:e13–5. doi: 10.1111/1346-8138.16189
73. Guerra ES, Lee CK, Specht CA, Yadav B, Huang H, Akalin A, et al. Central role of IL-23 and IL-17 producing eosinophils as immunomodulatory effector cells in acute pulmonary aspergillosis and allergic asthma. PLoS Pathog. (2017) 13:e1006175. doi: 10.1371/journal.ppat.1006175
74. Li Y, Hua S. Mechanisms of pathogenesis in allergic asthma: role of interleukin-23. Respirology. (2014) 19:663–9. doi: 10.1111/resp.12299
75. Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. (2006) 116:1218–22. doi: 10.1172/JCI28508
76. Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. (2014) 14:585–600. doi: 10.1038/nri3707
77. Delli FS, Sotiriou E, Lazaridou E, Apalla Z, Lallas A, Vakirlis E, et al. Total IgE, eosinophils, and interleukins 16, 17A, and 23 correlations in severe bullous pemphigoid and treatment implications. Dermatol Ther. (2020) 33:e13958. doi: 10.1111/dth.13958
78. Zeck-Kapp G, Kroegel C, Riede UN, Kapp A. Mechanisms of human eosinophil activation by complement protein C5a and platelet-activating factor: similar functional responses are accompanied by different morphologic alterations. Allergy. (1995) 50:34–47. doi: 10.1111/j.1398-9995.1995.tb02481.x
79. DiScipio RG, Schraufstatter IU. The role of the complement anaphylatoxins in the recruitment of eosinophils. Int Immunopharmacol. (2007) 7:1909–23. doi: 10.1016/j.intimp.2007.07.006
80. Ludwig RJ, Vanhoorelbeke K, Leypoldt F, Kaya Z, Bieber K, McLachlan SM, et al. Mechanisms of autoantibody-induced pathology. Front Immunol. (2017) 8:603. doi: 10.3389/fimmu.2017.00603
81. Liu Z, Giudice GJ, Swartz SJ, Fairley JA, Till GO, Troy JL, et al. The role of complement in experimental bullous pemphigoid. J Clin Invest. (1995) 95:1539–44. doi: 10.1172/JCI117826
82. Yamamoto K, Inoue N, Masuda R, Fujimori A, Saito T, Imajoh-Ohmi S, et al. Cloning of hamster type XVII collagen cDNA, and pathogenesis of anti-type XVII collagen antibody and complement in hamster bullous pemphigoid. J Invest Dermatol. (2002) 118:485–92. doi: 10.1046/j.0022-202x.2001.01683.x
83. Nelson KC, Zhao M, Schroeder PR, Li N, Wetsel RA, Diaz LA, et al. Role of different pathways of the complement cascade in experimental bullous pemphigoid. J Clin Invest. (2006) 116:2892–900. doi: 10.1172/JCI17891
84. Li Q, Ujiie H, Shibaki A, Wang G, Moriuchi R, Qiao HJ, et al. Human IgG1 monoclonal antibody against human collagen 17 noncollagenous 16A domain induces blisters via complement activation in experimental bullous pemphigoid model. J Immunol. (2010) 185:7746–55. doi: 10.4049/jimmunol.1000667
85. Heimbach L, Li Z, Berkowitz P, Zhao M, Li N, Rubenstein DS, et al. The C5a receptor on mast cells is critical for the autoimmune skin-blistering disease bullous pemphigoid. J Biol Chem. (2011) 286:15003–9. doi: 10.1074/jbc.M111.221036
86. Karsten CM, Beckmann T, Holtsche MM, Tillmann J, Tofern S, Schulze FS, et al. Tissue destruction in bullous pemphigoid can be complement independent and may be mitigated by C5aR2. Front Immunol. (2018) 9:488. doi: 10.3389/fimmu.2018.00488
87. Mihai S, Hirose M, Wang Y, Thurman JM, Holers VM, Morgan BP, et al. Specific inhibition of complement activation significantly ameliorates autoimmune blistering disease in mice. Front Immunol. (2018) 9:535. doi: 10.3389/fimmu.2018.00535
88. Zwarthoff SA, Berends ETM, Mol S, Ruyken M, Aerts PC, Józsi M, et al. Functional characterization of alternative and classical pathway C3/C5 convertase activity and inhibition using purified models. Front Immunol. (2018) 9:1691. doi: 10.3389/fimmu.2018.01691
89. Kerr MA. The human complement system: assembly of the classical pathway C3 convertase. Biochem J. (1980) 189:173–81. doi: 10.1042/bj1890173
90. Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA. (1996) 93:5512–6. doi: 10.1073/pnas.93.11.5512
91. Didona D, Paolino G, Di Zenzo G, Didona B, Pampena R, Di Nicola MR, et al. Pemphigus vulgaris: present and future therapeutic strategies. Dermatol Pract Concept. (2022) 12:e2022037. doi: 10.5826/dpc.1201a37
92. Goebl NA, Babbey CM, Datta-Mannan A, Witcher DR, Wroblewski VJ, Dunn KW. Neonatal Fc receptor mediates internalization of Fc in transfected human endothelial cells. Mol Biol Cell. (2008) 19:5490–505. doi: 10.1091/mbc.e07-02-0101
93. Li N, Zhao M, Hilario-Vargas J, Prisayanh P, Warren S, Diaz LA, et al. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest. (2005) 115:3440–50. doi: 10.1172/JCI24394
Keywords: bullous pemphigoid, therapies, eosinophils, eotaxin, interleukin, complement, neonatal Fc receptor, CCR3
Citation: Zeng FAP and Murrell DF (2022) Bullous pemphigoid—What do we know about the most recent therapies? Front. Med. 9:1057096. doi: 10.3389/fmed.2022.1057096
Received: 29 September 2022; Accepted: 18 October 2022;
Published: 03 November 2022.
Edited by:
Giulia Gasparini, University of Genoa, ItalyReviewed by:
Ilaria Trave, University of Genoa, ItalyCopyright © 2022 Zeng and Murrell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dedee F. Murrell, ZC5tdXJyZWxsQHVuc3cuZWR1LmF1
†ORCID: Faith A. P. Zeng, orcid.org/0000-0003-1383-2845; Dedee F. Murrell, orcid.org/0000-0003-2971-0199
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.