- Department of Internal Medicine, Rheumatology, Diabetology, Geriatrics and Clinical Immunology, Pomeranian Medical University, Szczecin, Poland
Background: Vascular ultrasound enables fast-track diagnosis of giant cell arteritis (GCA), but this method remains subjective. We aimed to determine intima-media thickness (IMT) cut-off values for large vessel GCA (LV-GCA) and identify the clinically relevant factors influencing it.
Methods: We included 214 patients referred for ultrasound evaluation within a fast-track clinic due to suspected GCA. IMT was measured in axillary, brachial, subclavian, superficial femoral, and common carotid arteries (CCA), in a place without identifiable atherosclerotic plaques. IMT cut-off values for vasculitis were determined by comparing measurements in arteries classified as vasculitis vs. controls without GCA/polymyalgia rheumatica (PMR).
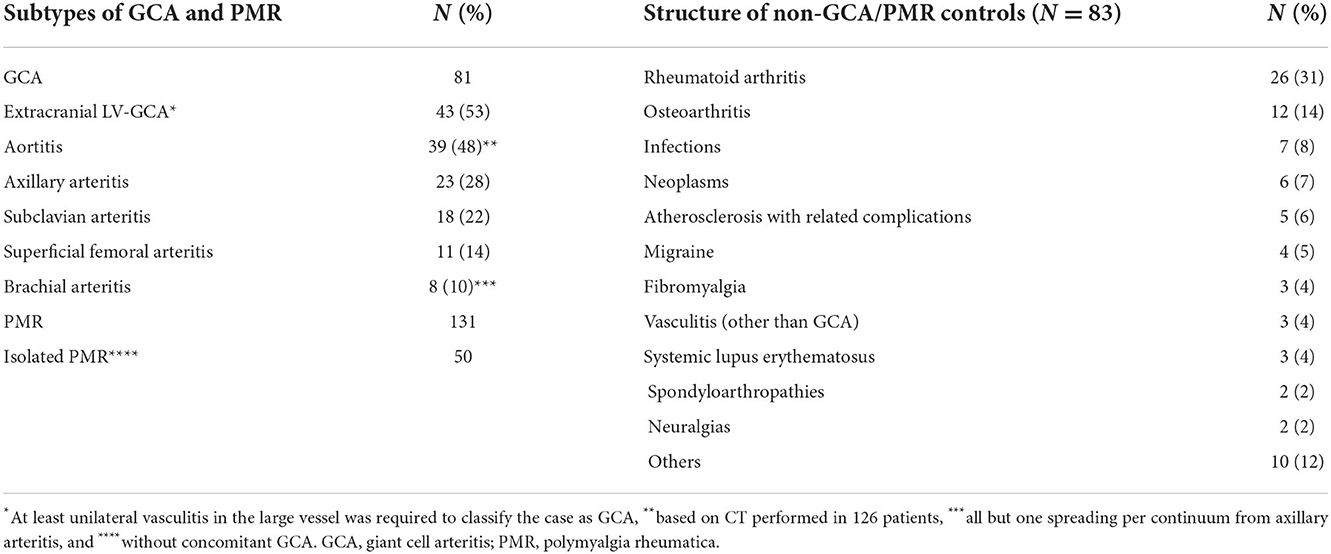
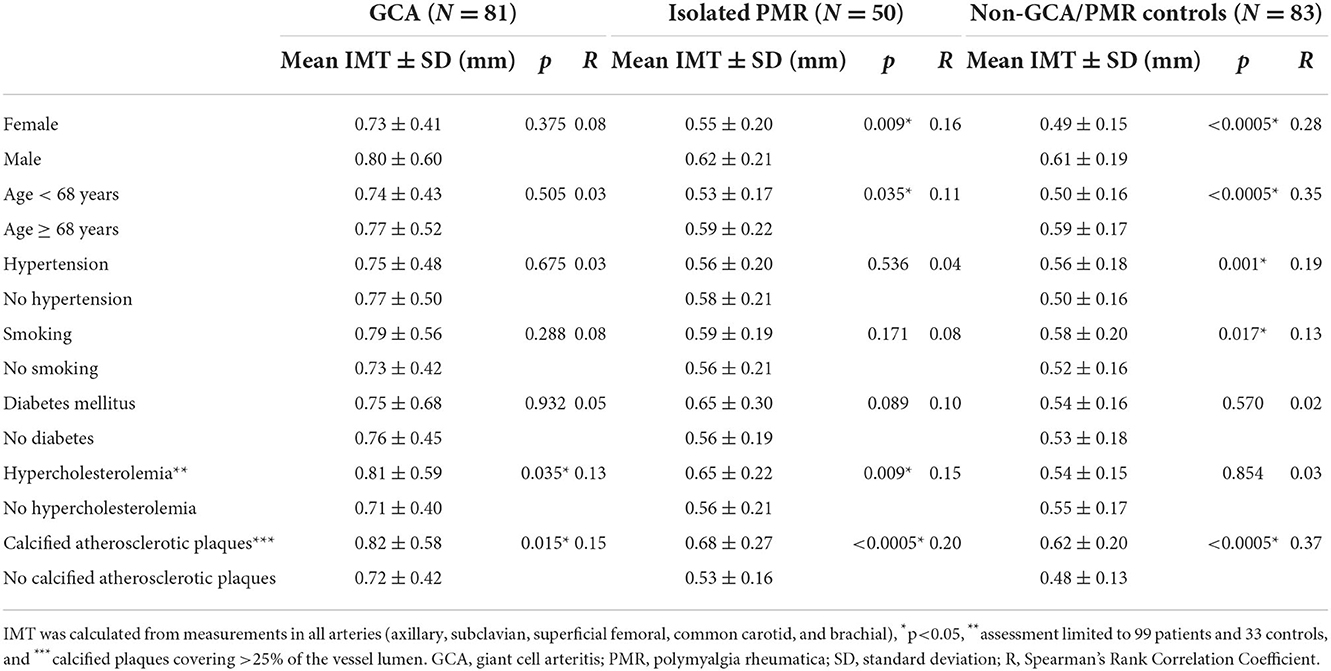
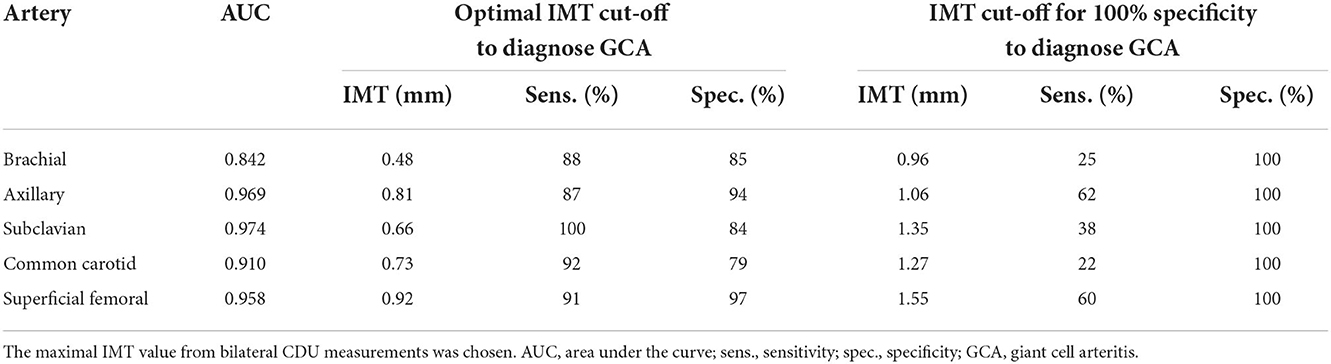
Results: Giant cell arteritis was diagnosed in 81 individuals, including extracranial LV-GCA in 43 individuals. Isolated PMR was diagnosed in 50 subjects. In 83 remaining patients, another diagnosis was confirmed, and they served as controls. The rounded optimal IMT cut-off values for the diagnosis of axillary vasculitis were 0.8 mm, subclavian-0.7 mm, superficial femoral-0.9 mm, CCA-0.7 mm, and brachial-0.5 mm. The IMT cut-off values providing 100% specificity for vasculitis (although with reduced sensitivity) were obtained with axillary IMT 1.06 mm, subclavian-1.35 mm, superficial femoral-1.55 mm, CCA-1.27 mm, and brachial-0.96 mm. Axillary and subclavian arteritis provided the best AUC for the diagnosis of GCA, while carotid and axillary were most commonly involved (24 and 23 patients, respectively). The presence of calcified atherosclerotic plaques was related to an increase of IMT in both patients and controls, while male sex, age ≥ 68, hypertension, and smoking increased IMT in controls but not in patients with GCA.
Conclusion: Cut-off values for LV-GCA performed best in axillary and subclavian arteritis but expanding examination to the other arteries may add to the sensitivity of GCA diagnosis (another location, e.g., brachial arteritis) and its specificity (identification of calcified atherosclerotic plaques in other arteries such as CCA, which may suggest applying higher IMT cut-off values). We proposed a more linear approach to cut-off values with two values: one for the most accurate and the other for a highly specific diagnosis and also considering some cardiovascular risk factors.
Key messages
• Intima-media cut-off values for large vessel GCA perform best in axillary and subclavian arteritis.
• Expanding examination to multiple arteries adds to the diagnostic sensitivity and specificity.
• The presence of some cardiovascular risk factors (male sex, age ≥ 68, hypertension, smoking, and the presence of calcified atherosclerotic plaques) may confound intima-media cut-off values.
• We proposed prior confounder analysis to apply cut-off values with two values: one for most accurate and the other for highly specific diagnosis.
Introduction
Diagnosis of giant cell arteritis (GCA) with large vessel involvement is delayed compared to temporal arteritis but is improving, thanks to the wider implementation of imaging in clinical practice (1, 2). Ultrasound-based fast-track clinics enable fast diagnosis of GCA and rapid initiation of treatment to prevent disease complications (3). Ultrasonography is a promising method for the diagnosis of not only temporal arteritis (4) but also extracranial, large vessel GCA (LV-GCA) (5–9). Compared to temporal artery biopsy, color duplex ultrasonography (CDU) offers faster results and is non-invasive and cost-effective, while serving high sensitivity and specificity (10). The most important ultrasonographic sign of large artery wall inflammation is an increase of intima-media thickness (IMT) with some characteristic features known as the “halo sign” (Figure 1) (11, 12). However, in elderly patients with GCA, atherosclerosis is common and requires careful differentiation with an increase of IMT caused by vasculitis (13, 14). Observer dependency and lack of standardization of the ultrasound method remain major concerns. Modern high-frequency ultrasound probes provide resolutions that enable exact vessel wall thickness measurements. There is a need to define IMT consistent with the halo sign for the diagnosis of GCA and identify the potential factors that influence IMT. Therefore, we aimed to determine IMT cut-off values for LV-GCA in a real-life scenario considering patients' cardiovascular risk factors as potential confounders.
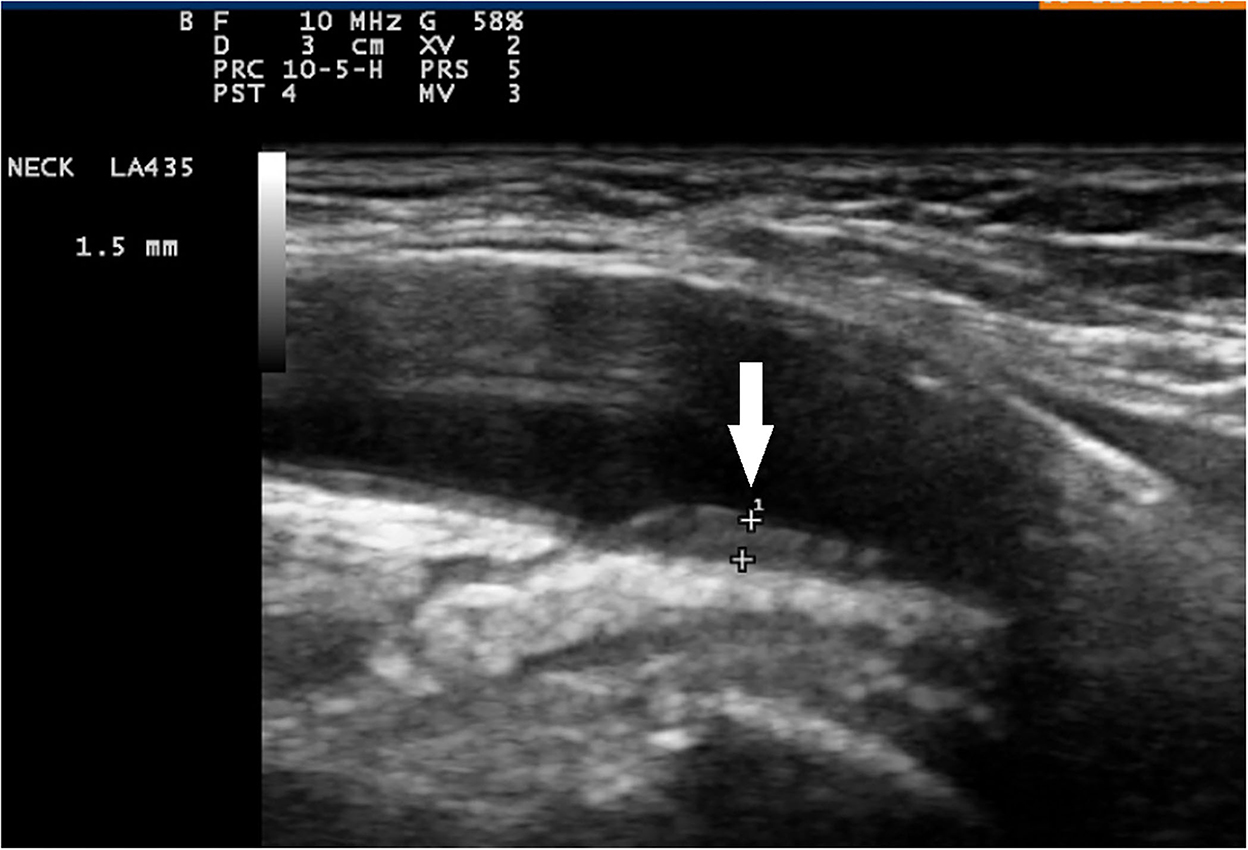
Figure 1. Color duplex sonography of axillary artery and longitudinal plane. Signs of vasculitis in an axillary artery (arrow) sliding down to a normal intima-media in the brachial artery (slope sign).
Materials and methods
Study population
Between April 2011 and June 2015, 312 patients suspected of GCA were referred for CDU evaluation within a fast-track GCA clinic. Referrals for GCA evaluation were not limited, and they included ophthalmological manifestations, other manifestations of cranial GCA, polymyalgia rheumatica (PMR) manifestations, pyrexia of unknown origin, and others. All consecutive, confirmed GCA cases were included but 98 controls (negative temporal and large vessel CDU and low clinical probability of GCA) were not included due to the loss of follow-up. They were not referred back for GCA reevaluation although our department is the only local reference center for vasculitis. We included isolated PMR as a distinct subgroup in the analysis to evaluate possible atherosclerosis in this subgroup and its risk factors. We finally included 214 subjects in the study. A history of hypertension, smoking, and diabetes mellitus was assessed. Arterial hypertension was defined as systolic blood pressure of ≥140 mm Hg and/or diastolic blood pressure of ≥90 mm Hg. Diabetes mellitus was defined in accordance with the Polish Society of Diabetology guidelines as a positive oral glucose tolerance test, a random glucose level of ≥200 mg/dl with clinical manifestations, or with fasting glucose of ≥126 mg/dl. Hypercholesterolemia was defined as an LDL cholesterol level of ≥115 mg/dl. Smoking was noted in case of a history of >3 pack years of smoking. Fasting lipid profiles were measured or retrieved from the medical records (within 1 year prior to GCA diagnosis) in 137 patients. Temporal artery biopsy (TAB) was performed in 44 patients, and contrasted computed tomography (CT) of the thoracic and abdominal aorta was performed in 126 patients. The research was approved by the decision KB-0012/111/10 and KB-0012/12/14 of the Local Ethical Committee.
GCA diagnosis
Final GCA and PMR diagnoses were established by a team of a minimum of two experienced rheumatologists and confirmed in at least one follow-up visit within 9 months. Patients with cranial GCA met ACR criteria (15). Large vessel GCA diagnosis was confirmed by arterial ultrasound and/or aortic CT (defined as circumferential, long-segmental aortic wall thickening of ≥3 or ≥2 mm with adjacent adventitia involvement) together with the presence of GCA or PMR manifestations. Patients with PMR met the 2012 EULAR/ACR classification criteria (16).
Ultrasound examination
All CDU, together with categorization into arteritis and non-arteritis findings, were performed before or within 3 days after treatment initiation, by a single physician (M.M.), experienced with performing >800 ultrasound examinations in suspected LV-GCA. He was blinded to diagnosis but aware of clinical presentation. Bilateral CDU examination of brachial, axillary, subclavian, superficial femoral, and common carotid arteries (CCA) was performed in all patients in both transverse and longitudinal planes. Halo sign definition was consistent with the one formulated by OMERACT: homogenous, hypoechoic wall thickening, well-delineated toward the luminal side, visible both in longitudinal and transverse planes, and most commonly concentric in transverse scans (11). Maximal IMT was measured from vessel lumen to media-adventitia interface, in mm with two decimals, in a place without identifiable atherosclerotic plaques, preferably on the distal wall. Maximal IMT from the left and right side locations were noted as well as mean IMT of corresponding left and right side measurements were calculated, which has to be included in further analysis. IMT cut-off values for vasculitis were determined by comparing measurements in arteries classified as vasculitis vs. controls. Additionally, measurements in arteries classified as vasculitis were compared with corresponding locations in GCA without large vessel vasculitis. The presence of calcified atherosclerotic plaques covering over 25% of vessel lumen in carotid bulbs and femoral arteries was assessed with CDU and noted. Esaote MyLab25Gold machine with 5–10 and 10–18 MHz linear probes was used.
Statistical analysis
Receiver operating characteristic (ROC) curves were calculated (Figure 2). Minimal difference between sensitivity and 1- specificity was chosen for optimal IMT cut-off values for vasculitis. Mann-Whitney and χ2 Pearson tests were used to compare between groups. Intrarater reliability was calculated from repeated measurements in 21 vessels of 5 patients and controls. For interrater reliability, 51 IMT measurements in 10 patients and controls were additionally performed by JF. Both readers underwent similar training and used the same equipment. Concordance correlation coefficients for intrarater and interrater reliability were calculated. The p < 0.05 were considered to be significant. Statistical analysis was performed using STATA software (version 12.0; StataCorp).

Figure 2. Receiver operating characteristic (ROC) curves for cut-off values of intima-media thickness (IMT) depicting vasculitis in patients with giant cell arteritis. The maximal IMT value from bilateral color duplex ultrasonography measurements was chosen.
Results
Study population
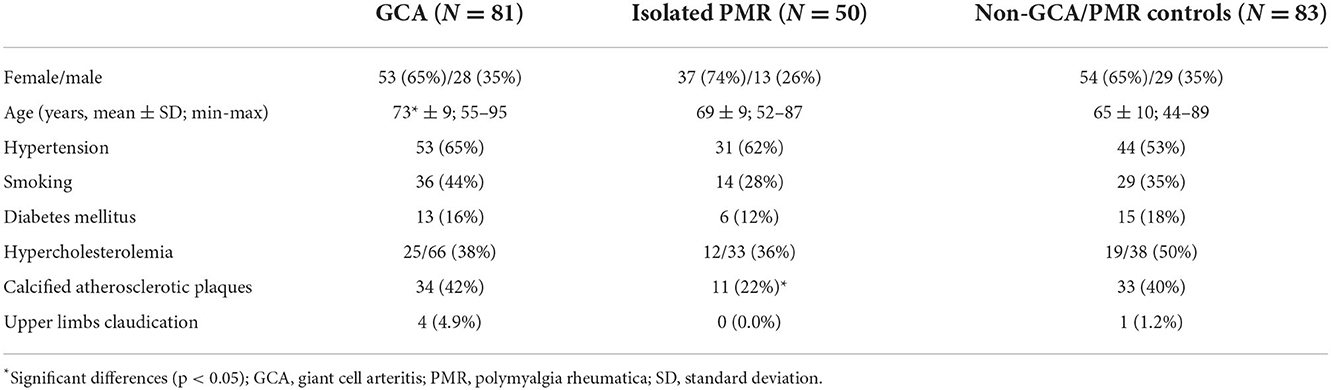
Giant cell arteritis was diagnosed in 81 individuals (TAB was positive for GCA in 31/44 biopsied patients), PMR in 131, and isolated PMR (without concomitant GCA) in 50 patients. Aortitis was diagnosed in 39 out of 126 patients based on CT. Extracranial LV-GCA was diagnosed in 43 patients. In the remaining 83 patients, another diagnosis was confirmed, and they served as non-GCA/PMR controls. Study population characteristics are included in Table 1. Notably, 5 patients with GCA failed to follow up. In the remaining, the diagnosis was sustained. None of the patients with non-GCA were reclassified to GCA. Patients with GCA were significantly older by a mean of 8 years compared with the controls (73 ± 9 vs. 65 ± 10 years). Calcified atherosclerotic plaques covering over 25% of the vessel lumen were significantly less common in isolated PMR compared to GCA and controls. Other patients' characteristics potentially confounding IMT were similar (Table 2). The number of patients referred by different medical specialists is presented in Supplementary Table 1.
IMT measurements
In controls but not in patients with GCA, mean IMT was influenced by age, gender, hypertension, and smoking. The presence of calcified atherosclerotic plaques was associated with increased IMT in PMR, GCA, and controls (Table 3). In LV-GCA cases, IMT in brachial, axillary, subclavian, femoral, and carotid arteries was significantly higher in both vs. controls and isolated PMR. In isolated cranial GCA, large vessel IMT did not differ vs. controls (Table 4). Left CCA IMT was higher compared to the right side in GCA (0.76 ± 0.28 vs. 0.67 ± 0.26; p = 0.011) with no significant left to right differences in other arteries in GCA, PMR, and in controls. Receiver operating characteristic (ROC) curves for cut-off values of IMT depicting vasculitis are depicted in Figure 2. Cut-off values for IMT depicting vasculitis vs. controls are listed in Table 5.
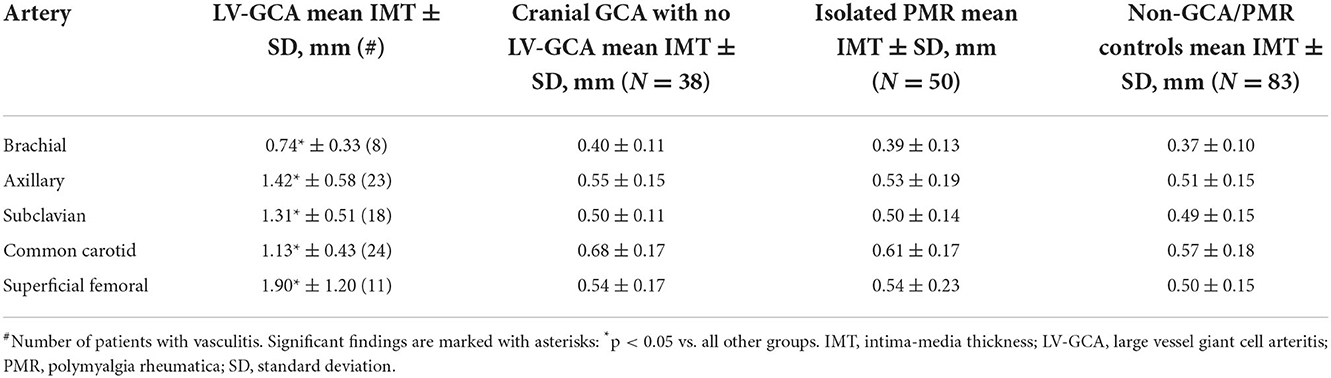
Table 4. Mean IMT in different affected large arteries in LV-GCA vs. cranial GCA with no LV-GCA, isolated PMR, and controls.
The concordance correlation coefficient for intrarater reliability was 0.96 (95% CI 0.93–0.99), for interrater reliability was 0.96 (95% CI 0.93–0.98), and concordance for the GCA diagnosis was 100%.
Discussion
The diagnostics of large vessel GCA was improved by introducing new imaging techniques (7, 17–19). However, recommendations for extracranial arteries imaging have a much lower level of evidence compared with cranial arteries (2). IMT cut-off values were not used by the OMERACT group as a modality to define halo signs consistent with vasculitis due to lacking data (11). Defining ultrasound reference ranges could further improve the reproducibility and feasibility of this method.
Among arteries tested in our study, axillary and subclavian arteritis provided the best AUC for the diagnosis of GCA (Table 5), while CCA and axillary were most commonly involved (Table 4). It confirms previous experts' opinions (2, 6, 7) suggesting choosing the axillary artery for LV-GCA assessment. We demonstrated that CCA is the most commonly involved in GCA—a phenomenon that was proved in PET-CT studies but was hard to demonstrate in previous US studies probably due to a problem of differentiation with atherosclerosis that is frequent in this location (20). Axillary artery also demonstrated the lowest difference between highly specific and optimal cut-off values, a phenomenon possibly explained by a lower influence of atherosclerosis in axillary artery compared to arteries prone to develop atherosclerosis (carotid and femoral). However, we confirmed previous observations (21) that expanding the number of arteries examined may add to the diagnosis. In our study, this was the case with CCA (higher number of vasculitis in CCA vs. axillary arteries) and brachial arteries (one case of untypical brachial artery involvement without axillary arteritis). In addition, assessing calcified atherosclerotic plaques in typical places supplies clinically practical information on the potential confounding of IMT results by atherosclerosis (mean IMT was higher in patients with calcified atherosclerotic plaques), which may reduce false positive GCA rates. In our large real-life group, IMT highly specific for vasculitis was higher compared to the previously proposed IMT cut-off values (7, 22). It may be explained by a large cohort and a substantial number of controls with atherosclerosis (patients with atherosclerosis predisposing conditions such as RA were included in the control group and a significant number of patients were referred by neurologists typically with atherosclerosis-related stroke to exclude its vasculitis etiology).
We demonstrated that IMT values may discriminate between GCA and its mimics (Table 4); however, false positive ultrasound results can be found in several diseases (23). An ultrasonographer should consider not only IMT but also the location of the pathological change (11), its structure, and echogenicity, as well as the grade of general atherosclerosis that all add to the final diagnosis of LV-GCA. Importantly, applying cut-off values requires prior identification of atherosclerosis by an ultrasonographer. The methodological obligation in our study was the measurement in a place without atherosclerotic plaque. Interpretation of IMT significance might be enriched by considering the presence of generalized atherosclerosis. The utility of IMT to diagnose or exclude GCA may be low according to the applied cut-off value. Therefore, we proposed two different IMT cut-off values, one based on optimal sensitivity and specificity and the other one that provided 100% specificity in our group, however, with reduced sensitivity. With that approach, lower cut-off values provide a probability for diagnosis instead of a definite diagnosis and necessitate additional ultrasonographic features and a more watchful approach to diagnosing GCA.
Our results indicate that IMT cut-off should be used cautiously to diagnose vasculitis, especially in the case of the presence of atherosclerosis and risk factors of atherosclerosis. Although calcified atherosclerotic plaques were assessed in arterial bifurcations typical for atherosclerosis (to check for the presence of general atherosclerosis) and not at the site of IMT measurement, their presence was correlated with increased IMT both in patients with GCA and controls. Male sex, age ≥ 68, hypertension, and smoking were associated with increased IMT in controls. Of note, these conditions (except for calcified atherosclerotic plaques) did not significantly influence the increase of IMT in patients with GCA suggesting that primary IMT increase by vasculitis might have overcome traditional CV risk factors.
Significantly increased IMT of the left CCA in GCA compared with the right in our observation seems to be only explained by anatomical asymmetry (the left CCA emerges directly from the aortic arch, whereas the right from the brachiocephalic trunk). The difference in left-to-right involvement in LV-GCA has been previously encountered but is not highlighted phenomenon (20, 24, 25). An ultrasonographer may profit from that knowledge when considering CCA arteritis. Interestingly, our brief review of the Medline database performed in April 2022 using a combination of Takayasu, GCA, vasculitis, and “left common carotid artery” or “right common carotid artery” revealed more articles concerning left than right side vascular complications.
An additional secondary result showed a decreased number of calcified atherosclerotic plaques in patients with isolated PMR vs. controls (Table 2). It is interesting in the context of occasionally reported favorable CV outcomes in patients with PMR/GCA (26). However, it requires further studies as some studies reported increased subclinical vascular changes in PMR, even in the absence of vasculitis (27).
Limitations of this study were that non-GCA/PMR controls were younger vs. GCA. However, age did not influence IMT in patients with GCA. The general limitation of the CDU method is that the assessment of IMT remains observer dependent as the measurement in a place without atherosclerotic plaques requires prior categorization into atherosclerosis/non-atherosclerosis. The possible limitation of the ultrasound-based study is that the observer might be reluctant to classify CCA with slightly increased IMT as vasculitis because this artery is commonly involved in atherosclerosis (13). This location might be an even more common site of arteritis in GCA, as demonstrated by PET examinations (20), but it should be examined by ultrasound with caution to conclude on vasculitis at least until more studies on CCA vasculitis vs. atherosclerosis will be available. We believe that determining the grade of atherosclerosis by assessing the presence of typical plaques in the carotid bulb and femoral arteries is feasible, to gain more information on the nature of the increase of IMT in CCA. The limitation of the study includes a lack of data on patients' obesity.
The strengths of the study include real-life referrals, examination of multiple large arteries, which, to the best of our knowledge, were not vastly analyzed previously, thorough analysis of potential confounders, and excellent interrater reliability.
In conclusion, we proposed IMT cut-off values to assess the probability of the diagnosis of GCA. Applying cut-off values may be enriched by prior identification of confounding by atherosclerosis, age, gender, hypertension, and smoking. They performed best in axillary and subclavian arteritis but examination of the other arteries may add to the diagnosis of GCA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Pomeranian Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MM and JF performed the examinations and organized the database. MM wrote the manuscript. All authors contributed to the conception, design, analysis interpretation of the study, and manuscript revision, as well as read and approved the submitted version.
Funding
This study was funded by the Pomeranian Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1055524/full#supplementary-material
References
1. Czihal M, Piller A, Schroettle A, Kuhlencordt P, Bernau C, Schulze-Koops H, et al. Impact of cranial and axillary/subclavian artery involvement by color duplex sonography on response to treatment in giant cell arteritis. J Vasc Surg. (2015) 61:1285–91. doi: 10.1016/j.jvs.2014.12.045
2. Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. (2018) 77:636–43. doi: 10.1136/annrheumdis-2017-212649
3. Diamantopoulos AP, Haugeberg G, Lindland A, Myklebust G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology. (2016) 55:66–70. doi: 10.1093/rheumatology/kev289
4. Aschwanden M, Daikeler T, Kesten F, Baldi T, Benz D, Tyndall A, et al. Temporal artery compression sign—a novel ultrasound finding for the diagnosis of giant cell arteritis. Ultraschall Med. (2013) 34:47–50. doi: 10.1055/s-0032-1312821
5. Ješe R, Rotar Ž, Tomšič M, Hočevar A. The cut-off values for the intima-media complex thickness assessed by colour doppler sonography in seven cranial and aortic arch arteries. Rheumatology. (2020) 18:keaa578. doi: 10.1093/rheumatology/keaa578
6. Czihal M, Schröttle A, Baustel K, Lottspeich C, Dechant C, Treitl KM, et al. B-mode sonography wall thickness assessment of the temporal and axillary arteries for the diagnosis of giant cell arteritis: a cohort study. Clin Exp Rheumatol. (2017) 35 (Suppl. 103):128–33.
7. Schäfer VS, Juche A, Ramiro S, Krause A, Schmidt WA. Ultrasound cut-off values for intima-media thickness of temporal, facial and axillary arteries in giant cell arteritis. Rheumatology. (2017) 56:1479–83. doi: 10.1093/rheumatology/kex143
8. Milchert M, Diamantopoulos A, Brzosko M. Atlas of ultrasound application in large vessel arteritis: giant cell arteritis and takayasu arteritis. In: Szczecin: Wydawnictwo Pomorskiej Akademii Medycznej. Szczecin: Wydawnictwo Pomorskiej Akademii Medycznej (2016). p. 1–155.
9. Sebastian A, Coath F, Innes S, Jackson J, van der Geest KSM, Dasgupta B. Role of the halo sign in the assessment of giant cell arteritis: a systematic review and meta-analysis. Rheumatol Adv Pract. (2021) 5:rkab059. doi: 10.1093/rap/rkab059
10. Luqmani R, Lee E, Singh S, Gillett M, Schmidt WA, Bradburn M, et al. The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): a diagnostic accuracy and cost-effectiveness study. Health Technol Assess. (2016) 20:1–238. doi: 10.3310/hta20900
11. Chrysidis S, Duftner C, Dejaco C, Schäfer VS, Ramiro S, Carrara G, et al. Definitions and reliability assessment of elementary ultrasound lesions in giant cell arteritis: a study from the OMERACT large vessel vasculitis ultrasound working group. RMD Open. (2018) 4:e000598. doi: 10.1136/rmdopen-2017-000598
12. Milchert M, Brzosko M, Bull Haaversen A, Diamantopoulos AP. Correspondence to “Slope sign”: a feature of large vessel vasculitis? Ann Rheum Dis. (2021) 80:e198. doi: 10.1136/annrheumdis-2019-216601
13. De Miguel E, Beltran LM, Monjo I, Deodati F, Schmidt WA, Garcia-Puig J. Atherosclerosis as a potential pitfall in the diagnosis of giant cell arteritis. Rheumatology. (2018) 57:318–21. doi: 10.1093/rheumatology/kex381
14. Milchert M, Fischer K, Flicinski J, Przepiera-Bedzak H, Brzosko M. Arteriosclerosis or vasculitis? Color duplex sonography in giant cell arteritis. J Rheumatol. (2012) 39:1898–9. doi: 10.3899/jrheum.120317
15. Hunder GG, Arend WP, Bloch DA, Calabrese LH, Fauci AS, Fries JF, et al. The American college of rheumatology 1990 criteria for the classification of vasculitis. Introduction. Arthritis Rheum. (1990) 33:1065–7. doi: 10.1002/art.1780330802
16. Dasgupta B, Cimmino MA, Maradit-Kremers H, Schmidt WA, Schirmer M, Salvarani C, et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European league against rheumatism/American college of rheumatology collaborative initiative. Ann Rheum Dis. (2012) 71:484–92. doi: 10.1016/j.ymed.2012.09.009
17. Förster S, Tato F, Weiss M, Czihal M, Rominger A, Bartenstein P, et al. Patterns of extracranial involvement in newly diagnosed giant cell arteritis assessed by physical examination, colour coded duplex sonography and FDG-PET. Vasa. (2011) 40:219–27. doi: 10.1024/0301-1526/a000096
18. Ghinoi A, Pipitone N, Nicolini A, Boiardi L, Silingardi M, Germanò G, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology. (2012) 51:730–4. doi: 10.1093/rheumatology/ker329
19. van der Geest KSM, Borg F, Kayani A, Paap D, Gondo P, Schmidt W, et al. Novel ultrasonographic halo score for giant cell arteritis: assessment of diagnostic accuracy and association with ocular ischaemia. Ann Rheum Dis. (2020) 79:393–9. doi: 10.1136/annrheumdis-2019-216343
20. Imfeld S, Aschwanden M, Rottenburger C, Schegk E, Berger CT, Staub D, et al. [18F]FDG positron emission tomography and ultrasound in the diagnosis of giant cell arteritis: congruent or complementary imaging methods? Rheumatology. (2020) 59:772–8. doi: 10.1093/rheumatology/kez362
21. Bull Haaversen AC, Brekke LK, Kermani TA, Molberg Ø, Diamantopoulos AP. Extended ultrasound examination identifies more large vessel involvement in patients with giant cell arteritis. Rheumatology. (2022) keac478. doi: 10.1093/rheumatology/keac478
22. López-Gloria K, Castrejón I, Nieto-González JC, Rodríguez-Merlos P, Serrano-Benavente B, González CM, et al. Ultrasound intima media thickness cut-off values for cranial and extracranial arteries in patients with suspected giant cell arteritis. Front Med. (2022) 9:981804. doi: 10.3389/fmed.2022.981804
23. Evangelatos G, Grivas A, Pappa M, Kouna K, Iliopoulos A, Fragoulis GE. Cranial giant cell arteritis mimickers: a masquerade to unveil. Autoimmun Rev. (2022) 21:103083. doi: 10.1016/j.autrev.2022.103083
24. Grayson PC, Maksimowicz-McKinnon K, Clark TM, Tomasson G, Cuthbertson D, Carette S, et al. Distribution of arterial lesions in takayasu's arteritis and giant cell arteritis. Ann Rheum Dis. (2012) 71:1329–34. doi: 10.1136/annrheumdis-2011-200795
25. Park BW, Park SJ, Park H, Hwang JC, Seo YW, Cho HR. Stenosis or occlusion of the right subclavian and common carotid arteries is more common than that of the innominate artery in takayasu arteritis. Vasc Specialist Int. (2015) 31:120–4. doi: 10.5758/vsi.2015.31.4.120
26. Udayakumar PD, Chandran AK, Crowson CS, Warrington KJ, Matteson EL. Cardiovascular risk and acute coronary syndrome in giant cell arteritis: a population-based retrospective cohort study. Arthritis Care Res. (2015) 67:396–402. doi: 10.1002/acr.22416
Keywords: giant cell arteritis, arteriosclerosis, vasculitis, ultrasound, halo sign, reference range
Citation: Milchert M, Fliciński J and Brzosko M (2022) Intima-media thickness cut-off values depicting “halo sign” and potential confounder analysis for the best diagnosis of large vessel giant cell arteritis by ultrasonography. Front. Med. 9:1055524. doi: 10.3389/fmed.2022.1055524
Received: 27 September 2022; Accepted: 15 November 2022;
Published: 13 December 2022.
Edited by:
Andreas P. Diamantopoulos, Akershus University Hospital, NorwayReviewed by:
Alojzija Hocevar, University Medical Center Ljubljana, SloveniaGerasimos Evangelatos, National and Kapodistrian University of Athens, Greece
Copyright © 2022 Milchert, Fliciński and Brzosko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcin Milchert, bWFyY21pbGNAaG90bWFpbC5jb20=
 Marcin Milchert
Marcin Milchert Jacek Fliciński
Jacek Fliciński Marek Brzosko
Marek Brzosko