- 1Research Center for High Altitude Medicine, Qinghai University, Xining, China
- 2Medical College, Qinghai University, Xining, China
- 3Department of Obstetrics, Qinghai Provincial People’s Hospital, Xining, China
- 4Department of Gynecology, Qinghai Provincial People’s Hospital, Xining, China
- 5Department of Anaesthesiology and Perioperative Medicine, Xijing Hospital, The Fourth Military Medical University, Xi’an, China
- 6Department of Ultrasonography, Qinghai Provincial People’s Hospital, Xining, China
- 7Department of Pathology, Qinghai Provincial People’s Hospital, Xining, China
- 8Qinghai Provincial Key Laboratory of Traditional Chinese Medicine Research for Glucolipid Metabolic Diseases, Xining, China
Background: Determining the cause of intrauterine fetal death is essential for patients to manage their next pregnancy. However, in the majority of cases of fetal death, the cause remains unexplained despite comprehensive evaluation, especially in the cases of twins. Among twin pregnancies, conditions of monochorionic twinning, commonly regarded as monozygotic, are more complicated than dichorionic ones.
Case summary: We systematically evaluated the cause of fetal death for a Han Chinese woman with monochorionic twinning following in vitro fertilization/embryo transfer. Discrepant karyotypes were unexpectedly discovered between the twins. One fetus had an aneuploid male karyotype (46, XY), dup (9) (p24.3-q13), and the other had a normal female karyotype (46, XX). We considered that the male died of aberration of chromosome 9 and the female died of subsequent acute exsanguination through vascular anastomosis.
Conclusion: This study demonstrated the importance of recognizing the presence of monochorionic dizygotic twinning and the challenges of clinical management for twins following in vitro fertilization/double embryo transfer.
1. Introduction
Intrauterine fetal death (IUFD) not only impairs female fertility, but is an emotionally devastating event for family. When IUFD occurs, finding out the cause of fetal death has important clinical implications (1). Many causes of fetal death are known to recur in subsequent pregnancies, increasing the rates of another death associated with that cause (2). It is therefore necessary to determine the potential causes of fetal death in order to formulate prevention strategies for subsequent pregnancies in women with a history of adverse pregnancy outcomes (3). Moreover, determining the cause of fetal death can reduce the medical expenses in subsequent pregnancies (4). In addition, a clear cause of death facilitates emotional healing of the family from the IUFD event (1). However, relatively few cases of fetal deaths have clear causes of death due to diverse etiologies, unclear pathogenesis, limited services, and insufficient investigations (5).
Pregnancies through artificial reproductive techniques (ARTs), if the fetus dies, will be more devastating for the family because they are associated with higher expectation, greater cost, and more medical procedures than spontaneous conception (6). Most women undergoing in vitro fertilization/embryo transfer (IVF-ET) prefer the placement of more than one embryos with the hope of improving chance of pregnancy success, which has become a major driver of twin pregnancies (7). However, the risk of fetal death is substantially increased in twin gestation, notably in monochorionic (MC) twins (8, 9). Commonly, dichorionic (DC) twinning takes place in IVF-ET when more than one fertilized eggs are placed into the uterus (10). Nevertheless, many cases of monochorionic dizygotic (MCDZ) twins are now recognized in women undergoing IVF-ET, which complicates the identification of the cause of fetal death in twinning as well as limiting genetic counseling and challenging pregnancy management.
Here, we present a case of MC twinning after IVF-ET. At 12+6 weeks of gestation, phenotypic discrepancy between the twins was detected by prenatal ultrasonography. One week later, ultrasound demonstrated the intrauterine death of both fetuses. In the subsequent investigation on etiology, the twins were identified as dizygotic (DZ) ones, and the causes of death for each fetus were elucidated separately. The case highlights the importance of realizing the existence of MCDZ and the challenges to clinical management after in vitro fertilization/double-embryo transfer.
2. Case presentation
A 31-year-old woman of Han Chinese, gravida 2 para 0, had conceived via IVF-ET. Her first pregnancy at the age of 22, was artificially terminated in the first trimester because it was unwanted. The patient underwent bilateral endometrioma cystectomy at the age of 27. Subsequently, she thereafter had a diminished ovarian reserve with 0.40 ng/mL of anti-Mullerian hormone (AMH). A total of six eggs were obtained by two oocyte retrieval operations and were fertilized in vitro. After two failed double-embryo transfers, the remaining two embryos, respectively, retrieved in August 2020 and April 2021, were transferred after assisted hatching treatment. Her first ultrasonography at 6+2 weeks of gestation documented two yolk sacs within a single gestational sac (Figure 1A). The subsequent scan at 10+3 weeks of gestation demonstrated MC diamniotic twins with a T-shaped insertion of the membrane (Figure 1B).
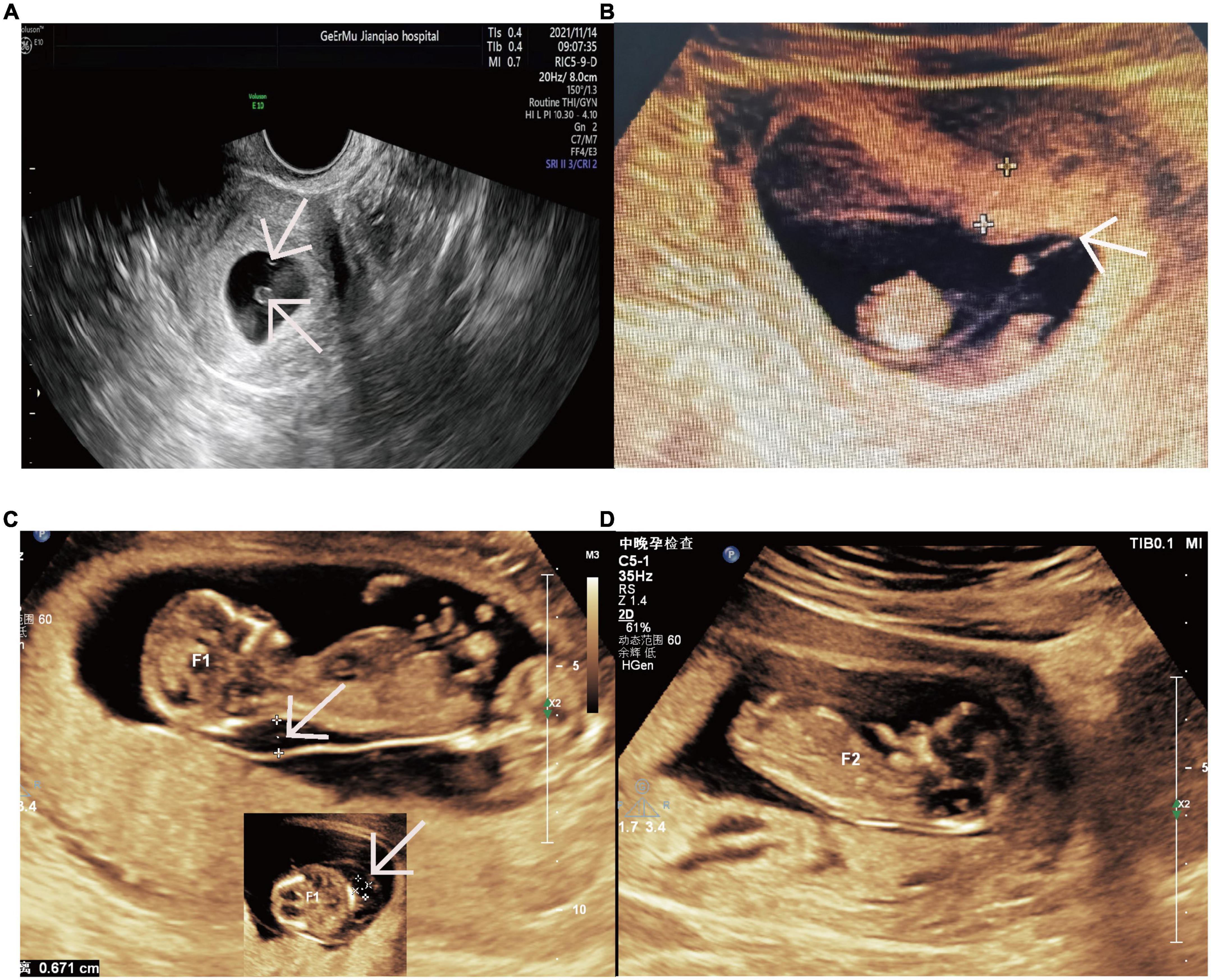
Figure 1. Ultrasound images revealing the monochorionic twins and twin A’s nuchal cystic hygromas and twin B’s normal NT. (A) Two yolk sacs within a single gestational sac at 6+2 weeks. The arrow indicates two yolk sacs. (B) The monochorionic diamnitic twins with a separating membrane of T sign at 10+3 weeks. The arrow indicates the distinct T sign of the separating membrane between the twins. (C) Nuchal cystic hygromas of twin A at 12+3 weeks. The arrows indicate the nuchal cystic hygromas of twin A from longitudinal section and transverse section. (D) The normal NT of twin B at 12+3 weeks. NT, nuchal translucency.
Unfortunately, one of the twins (twin A) was revealed to have nuchal cystic hygroma and generalized hydrops without abnormal volume of amniotic fluid on ultrasonography at the time of routine nuchal transparency (NT) screening (Figure 1C). On the follow-up ultrasound, a structural malformation of the heart was observed. At the same time, no abnormalities were found on the other fetus (twin B) by detailed ultrasonic examination (Figure 1D). The couple underwent genetic counseling and the twins were interpreted as monozygosity due to the MC placenta, so they were informed of a high probability of genetic disorder or congenital anomaly for both twins. The couple intended to terminate the pregnancy accordingly.
After a week, at 13+6 weeks of gestation, both twins died spontaneously as the absences of cardiac activity were indicated on ultrasonography. Following a failed medical abortion, the patient then underwent a surgical curettage to terminate the pregnancy. During the fetal genetic examination, the twins were discovered to be inconsistent in their chromosomal composition. Twin A had a male karyotype with a partial trisomy 9, while twin B had a normal female karyotype. DNA zygosity studies of the twins demonstrated that the twins were dizygotic. A timeline of relevant events to the patient from conceiving to terminating pregnancy is shown in Table 1.
3. Methods
3.1. Evaluation of maternal condition
The patient’s clinical history and medical records were reviewed in detail, including past medical history, pre-pregnancy and prenatal care. Clinical routine laboratory testing and additional detection were performed in hospital, including serological tests for immunodeficiency virus (HIV) and syphilis, thyroid-stimulating hormone (TSH), screening for blood group-related antibodies, lupus anticoagulant, and antiphospholipid antibodies.
3.2. Fetus and placenta examination
Fetal gross observations as well as placental macroscopic and microscopic histopathologic examination were performed according to standard procedures.
3.3. Genetic evaluation
Molecular karyotyping, chromosomal microarray analysis (CMA), was conducted using the Single Nucleotide Polymorphism (SNP) Array with CytoScan 750 K probes (Affymetrix, Santa Clara, CA, USA). At the same time, tissue samples of twin A, twin B and the placenta were stored at –80°C. High throughput next generation sequencing (NGS) was carried out on each cryopreserved specimen and analyzed using Sentieon software by Beikang Laboratory (Beikang Medical Laboratory Co, Ltd, Shandong, China). Short tandem repeat (STR) markers were detected on maternal and fetal DNA using the Human Identification Installation Kit (Applied Biosystems, Carlsbad, CA, USA) to monitor the maternal contamination.
3.4. DNA zygosity studies
The zygosity study was conducted on the data of NGS and STR from the tissue of each fetus and maternal blood. Sixteen STRs and AMEL-X markers were used to determine the relationship among the specimens. Identity-by-descent (IBD) was analyzed based on the combined SNP/InDel using King software.
4. Results
4.1. Evaluation of the maternal condition
The couple were not consanguineous and the woman’s medical history that associated with fetal death was unremarkable. She had neither been exposed to chemicals, drugs, smoking, alcohol, and viral infections, nor had she suffered any discomfort during pregnancy. Her body mass index (BMI) was 23. All laboratory values were unremarkable in the first trimester of pregnancy and during hospitalization, including negative tests for lupus anticoagulant, antiphospholipid, and antinuclear antibodies. Accordingly, underlying maternal contributors to fetal death were excluded.
4.2. Examination of fetus and placenta
The fetus (twin A) with nuchal cystic hygroma and generalized edema had been identified by macroscopic examination and appeared decaying tissue, while twin B was grossly unremarkable (Figure 2A), suggesting that twin A had died before twin B. Gross examination of the placenta confirmed that the twins were MC diamniotic with a thin separating membrane (Figure 2B). Microscopic examination of the placenta did not identify typical infarction, necrosis, vascular thrombosis, or inflammatory cell infiltration, which excluded that placental lesions were the cause of fetal death (Figure 2C).
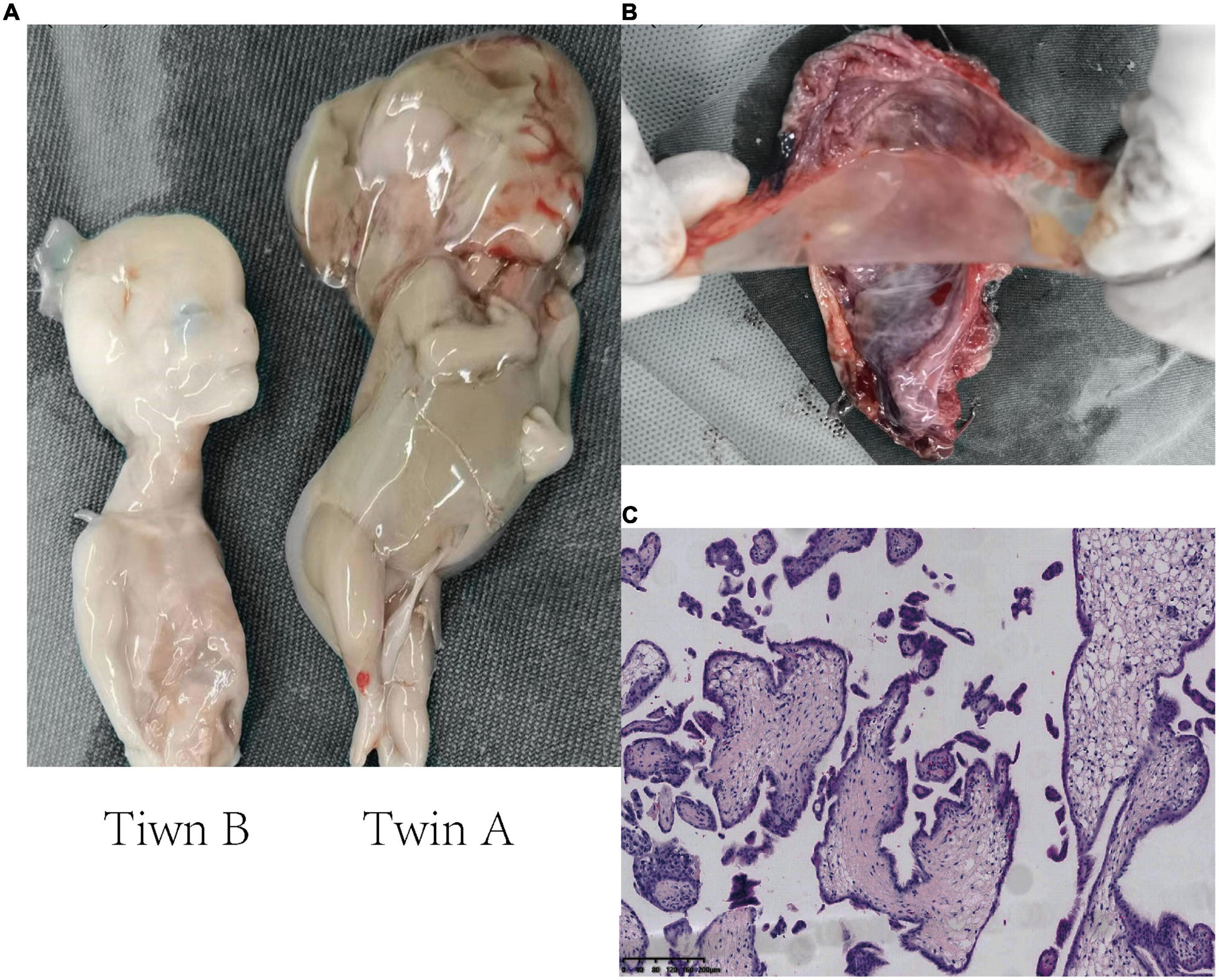
Figure 2. Histopathology images showing the examination of two fetuses and the placenta. (A) Macroscopic examination of the twins. Twin A (right), severe cystic hygroma and generalized hydropic foetalis, appearing decaying tissue. Twin B (left), unremarkable macroscopic abnormality. (B) The monochorionic diamniotic placenta of the twins. The placental septum indicates two amniotic sacs beside thin membranes. (C) Microscopic histopathological examination of the placental section (H&E staining, 10×).
4.3. Genetic evaluation
The CMA result of twin B showed a normal female karyotype (46, XX) without copy number variations (CNVs) or loss of heterozygosity (LOH). The NGS analysis of twin A showed an unbalanced male karyotype (46, XY) with dup (9) (p24.3-q13), resulting in partial trisomy 9. The duplicated region was interpreted as seq[GRCh 37/hg19] (10001-68350000) X3, including the whole short arm of chromosome 9, a 48.42 Mb fragment, involving 220 protein-coding genes, 49 of which were potentially pathogenic genes included in OMIM (Supplementary Figure 1A). A reanalysis of twin B by NGS confirmed the normal 46, XX karyotype (Supplementary Figure 1B). The NGS analysis for placental genetic make-up showed 46, XY with dup (9) (p24.3-q13), consistent with twin A (Supplementary Figure 1C).
4.4. Analysis of DNA zygosity
The zygosity analysis showed that 7 of 17 genetic markers were different between the twins. In the specimens of each fetus, there was at least one allele from the mother at all marker loci. The results were consistent with DZ twins (Table 2). IBD analysis yielded kinship value of 0.2754, indicating first-degree relatives of the twins [inference criteria: monozygotic twin < 0.1; full sibling 0.1–0.365 (11)].
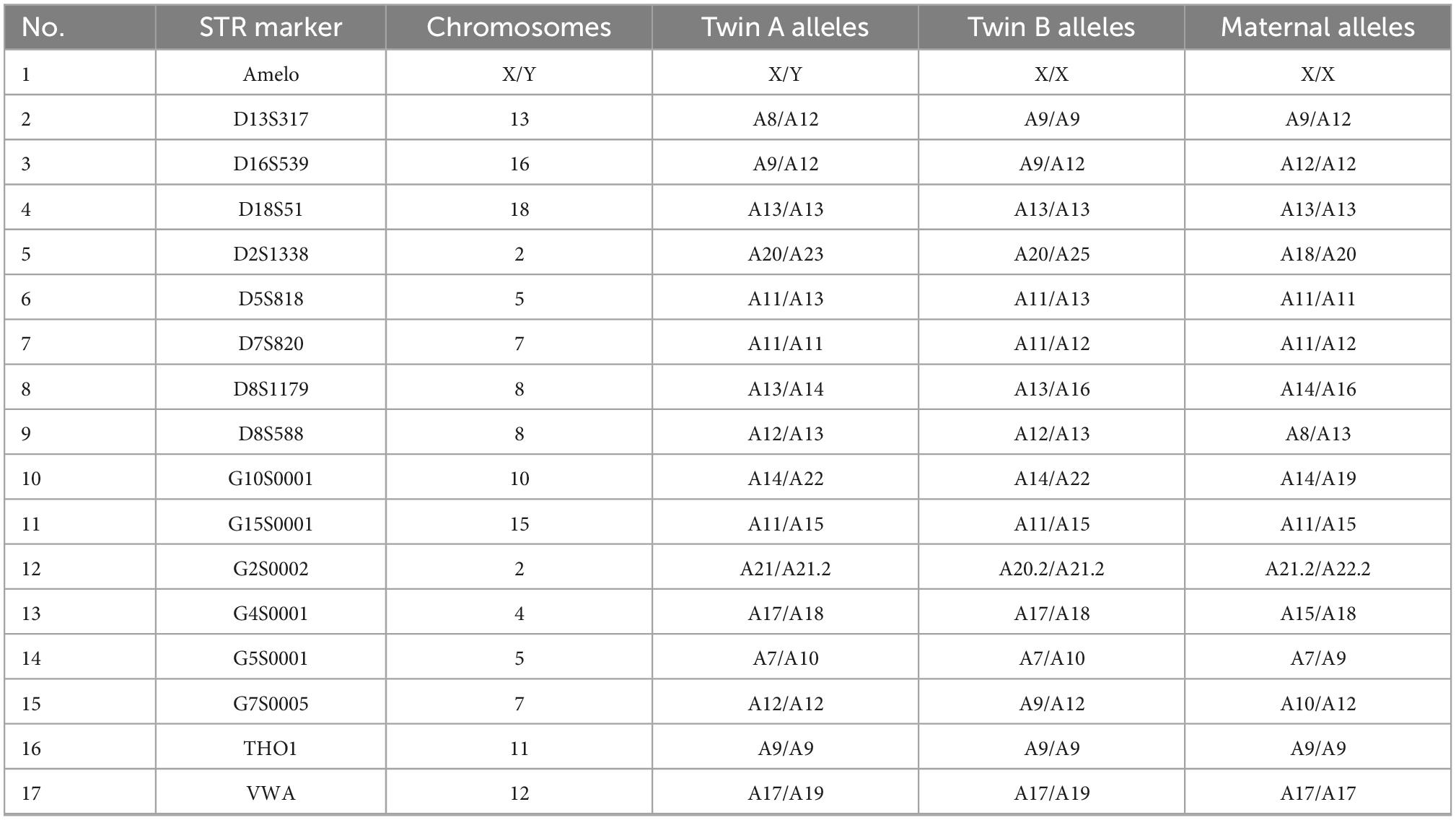
Table 2. DNA zygosity analysis in samples from each fetus and the maternal peripheral-blood lymphocytes.
4.5. Counseling and formulating preventive strategies
A detailed etiological explanation and valid counseling were provided to the couple as follows: in their next pregnancy, preimplantation genetic diagnosis (PGD) for aneuploidy on their blastocysts is not necessary, but prenatal diagnostic procedure for aneuploidy and copy number variations through amniocentesis samples is recommend. The transfer of a single blastocyst can be chosen to reduce the risk of pregnancy complications.
5. Discussion
Our research indicates: First, the two fetuses are dizygosity, which can explain the phenotypic discrepancy between them whereas they were monochorionic (MC) twins. Twin A probably died of the aberration of chromosome 9 (Figure 3a). Second, twin A died prior to twin B. Due to the MC condition, twin B possibly died from the acute exsanguination caused by the death of twin A via vascular anastomosis of the placenta (Figure 3b) whereas they were DZ twins.
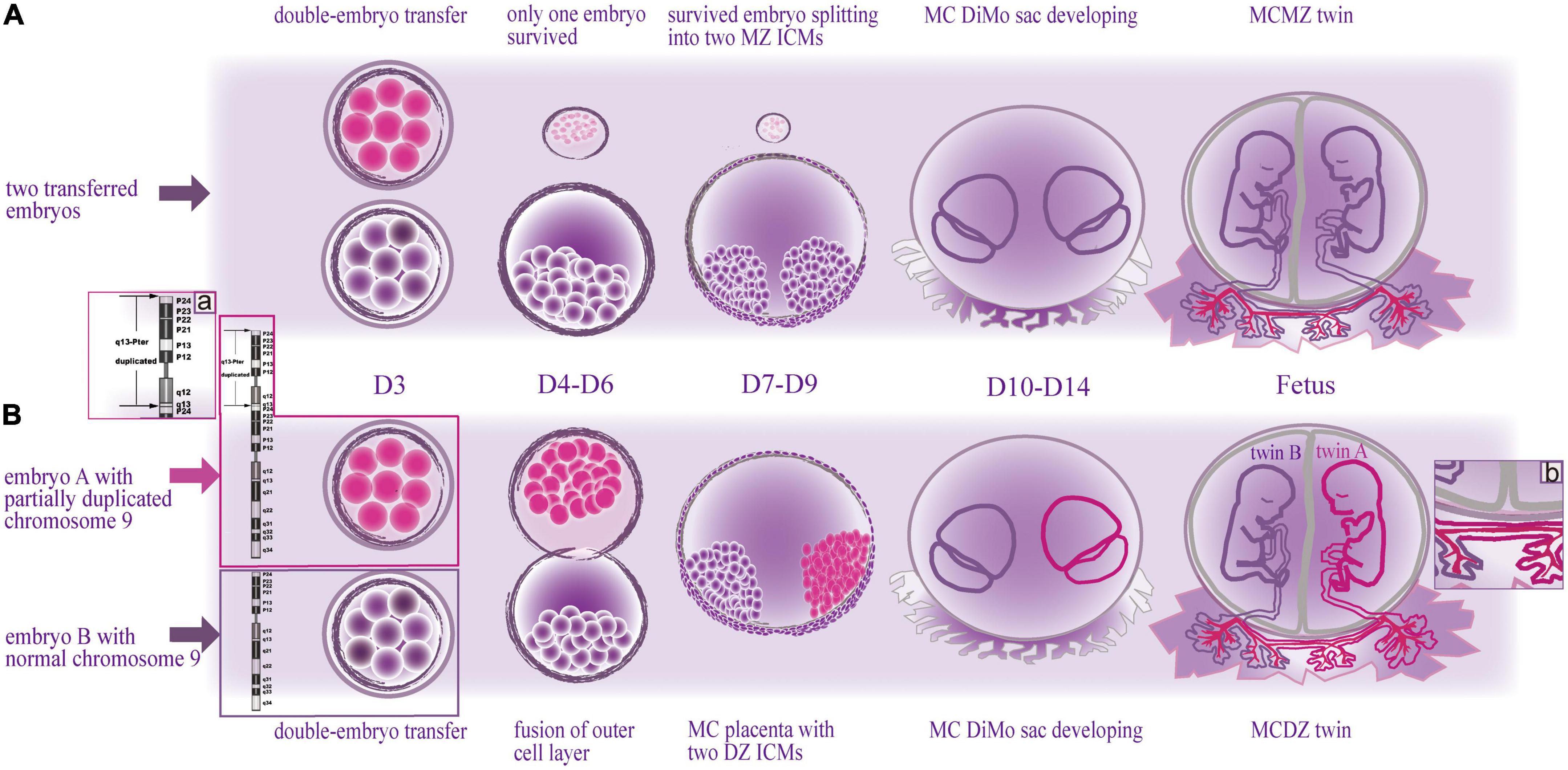
Figure 3. Schematic drawing of two types of embryonic development with monochorionic diamniotic twins after double-embryo transfer. (Above) The formation of monochorionic monozygotic twins in common cases. (Below) The formation of monochorionic dizygotic twins in this case. MZ, monozygotic; MC, monochorionic; DZ, dizygotic; DiMo, diamniotic; ICM, inner cell mass; D, day. (A) Enlarged view illustrates the cause of death of twin A, the duplicated segment of chromosome 9; (B) enlarged view illustrates the cause of death of twin B, the vascular anastomoses between the twins.
Chorion development is expected to take place on embryonic days 4–7 (D4-D7), and amnion starts to form between days 7 and 9 (D7-D9) (12). Based on literature and theory of embryology, we speculated on the mechanism of the monochorionic dizygotic (MCDZ) embryologic event of this case (Figure 3). The woman was transplanted with two cleavage-stage embryos cultured in vitro for 3 days (D3). If the two transferred embryos merged during the stage of D4-D7, they may potentially share a common chorionic membrane with separate amniotic membranes (10). The twins, initially, were judged to be monozygotic (MZ) as they had the MC placenta. Hence, the initial assumption was that only one of the transferred embryos survived and split into two (Figure 3 Above). Given that the twins were dizygotic (DZ), the fusion of the outer cells of the DZ embryos was speculated before implantation, while the DZ inner cells mass (ICM) developed into two fetuses with separate amniotic cavities, which resulted in the MC placenta of the DZ twins (Figure 3 Below) (13).
A case of MCDZ twinning is hard to be identified. Most of them were noted due to gender discordance on fetal anatomy survey by ultrasound or prenatal cell free fetal DNA screening (14, 15), and were further confirmed. It is not easily noted to the same gender. So, cases of MCDZ twins of in vitro fertilization/double-embryo transfer may be far more than those of what we know. However, prenatal determination of zygosity in MC twins is important for decision making when one of the twins is suspected of having a congenital anomaly (16). In this study, at first, DNA was extracted only from twin B for CMA detection as twin A’s tissue was decaying and dizygosity was not realized. Consequently, we made the conclusion with normal karyotypes of the twins. However, given the ultrasound abnormalities of twin A, a chromosomal disorder of the fetus was still suspected. Analyzing the DNA from the cryopreserved specimens, we discovered the imbalance of chromosome 9 at twin A, involving the duplication of the entire short arm and partial long arm (Figure 3a) as well as accidently discovered the non-identical karyotype between the MC twins.
Trisomy of the short arm of chromosome 9 (Trisomy 9p) is the fourth most common autosomal trisomy after 21, 18, and 13 trisomy (17). The severity of phenotype correlates with the extension of duplicated chromosomal segments (18). If the duplicated segments include the long arm of chromosome 9, clinical findings may resemble trisomy 9 mosaic syndrome which is associated with cardiac anomalies (19). The karyotypes of the parents were normal (Karyotype analysis during the couple’s planning for IVF-ET), indicating that the trisomy 9 of twin A was a de novo genetic change. We cannot identify the cause of chromosomal aberration in twin A whereas without abnormity in twin B. We suspected it might be related to the conditions of fertilization as the two eggs were retrieved and fertilized separately at different times.
Generally, DZ twins, which develops from two separate fertilized eggs, would be expected to be dichorionic (DC) (10), i.e., two fetuses with separate placentas. The blood between the two placentas does not communicate even if they appear to be fused together, which is likely harmless to the other if one of the twins dies (20). However, we confirmed that the DZ twins shared one placenta by the examination of the placenta (Figure 2). Nearly all MC twins are present vascular anastomosis, which is the primary pathology of adverse outcomes in MC twins (21). Following a single death of twins, blood volume can shift rapidly through the anastomosis from the living fetus toward the deceased co-twin. Due to acute exsanguination, the living fetus can subsequently suffer hypoperfusion, hypotension and anemia, which induced tissue hypoxia, acidosis and damage, culminated in the death of the second twin (22). So we speculated the possible death of twin B was exsanguination after death of twin A due to vascular anastomosis (Figure 3b). However, a fluid injection assay for testing vascular anastomosis failed to be achieved because of the rupture of the umbilical cord caused by the curettage and too thin umbilical cords due to small gestational age, through which can verify reliably the vascular anastomosis between the two fetuses.
The reason for the formation of MCDZ twins associated with IVF could possibly be due to simultaneous ET, in which two fertilized eggs are located in close proximity (23). In addition, assisted hatching techniques introduce small openings or thin areas in the zona pellucida, which may increase the likelihood of fusion (14).
Ignoring the existence of MCDZ can result in incorrect decision-making of the couple. In this case, prior to the spontaneous death of the twins, the couple had already decided to terminate the pregnancy because of improper counseling based on the MZ twins by mistake. In fact, not all fetuses with chromosome 9 abnormalities necessarily suffer IUFD (24). Without the death of twin A, further invasive investigations could have been performed by respective amniocentesis and selective feticide could have been a preferred approach after ultrasound-guided bipolar cord coagulation or radiofrequency ablation to induce complete circulatory confinement of the twins. It would be possible to obtain a normal fetus under such conditions.
One of the key points in our study was the cryopreservation of the fetal specimens. DNA analysis from the fetus provides a “molecular autopsy” to determine the cause of fetal death and is valuable for the diagnosis of many genetic diseases (25). Without the additional stored fetal specimens, this case would have been an unexplained one. Despite the thorough etiology examination, the patient would remain anxious and sought additional tests, unnecessary interventions and frequent visits in her next pregnancies. The American College of Obstetricians and Gynecologists (ACOG) recommended performing fetal genetic analysis after all cases of stillbirth (26). Here, we suggested it is necessary to store the fetal specimens when the genetic analysis cannot be performed right after IUFD.
Another essential part of our study was the timely investigation. Without delay, we reviewed the patient’s medical history. Following detailed observation at delivery, we recognized the abnormal twin A revealed by ultrasonography and verified that twin A died before twin B, which could help explain the death of twin B.
One limitation of our study is that the molecular detection of the placenta was not taken from multiple samples at different locations under each gestational sac. If such work had been done, the mosaicism of placenta might have been detected, which would have been helpful to explain the fusion mechanism of the early embryos.
The judgment that MC twins are necessarily MZ ones is dogmatic. It is important to realize the existence of MCDZ twinning after in vitro fertilization/double-embryo transfer. The specific formation of MCDZ should be further studied.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. This study was approved by the Institutional Ethics Committee (approval number: 2021-017) and informed consent was obtained from the patient for the publication of the clinical information.
Author contributions
AX and YC conceived the study. AX drafted the initial manuscript and mapped the schematic drawing (Figure 3). GL and LT conducted the genetic evaluation. XC performed the ultrasound examination. XZ completed the pathological examination. JH collected the medical history of the patient. XW and YR revised the manuscript. All authors involved in the analysis of the data and determining the cause of the fetal death and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 81860370 and 31670361).
Acknowledgments
We thank the parents of the twins for their support in preparing this report. We also thank Shandong Beikang Medical Laboratory Co., Ltd. for providing technical support of genetic testing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1055275/full#supplementary-material
Supplementary Figure 1 | Sequencing plot showing the inconsistency of genetic make-up between the twins as well as the placenta.
References
2. Lamont K, Scott N, Jones G, Bhattacharya S. Risk of recurrent stillbirth: systematic review and meta-analysis. BMJ. (2015) 350:h3080. doi: 10.1136/bmj.h3080
3. Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. JAMA. (2011) 306:2459–68. doi: 10.1001/jama.2011.1823
4. Flenady V, Wojcieszek A, Middleton P. Stillbirths: recall to action in high-income countries. Lancet. (2016) 387:691–702. doi: 10.1016/S0140-6736(15)01020-X
5. Smith G. Screening and prevention of stillbirth. Best Pract Res Clin Obstet Gynaecol. (2016) 38:71–82. doi: 10.1016/j.bpobgyn.2016.08.002
6. Baor L, Soskolne V. Mothers of IVF and spontaneously conceived twins: a comparison of prenatal maternal expectations, coping resources and maternal stress. Hum Reprod. (2010) 25:1490–6. doi: 10.1093/humrep/deq045
7. Bhattacharya S, Kamath M. Reducing multiple births in assisted reproduction technology. Best Pract Res Clin Obstet Gynaecol. (2014) 28:191–9. doi: 10.1016/j.bpobgyn.2013.11.005
8. Cheong-See F, Schuit E, Arroyo-Manzano D. Prospective risk of stillbirth and neonatal complications in twin pregnancies: systematic review and meta-analysis. BMJ. (2016) 354:i4353. doi: 10.1136/bmj.i4353
9. Couck I, Van Nylen L, Deprest J, Lewi L. Monochorionic twins after in-vitro fertilization: do they have poorer outcomes? A retrospective cohort study. Ultrasound Obstet Gynecol. (2020) 56:831–6. doi: 10.1002/uog.21973
11. Manichaikul A, Mychaleckyj J, Rich S, Daly K, Sale M, Chen W. Robust relationship inference in genome-wide association studies. Bioinformatics. (2010) 26:2867–73. doi: 10.1093/bioinformatics/btq559
12. Zhai J, Xiao Z, Wang Y, Wang H. Human embryonic development: from peri-implantation to gastrulation. Trends Cell Biol. (2022) 32:18–29. doi: 10.1016/j.tcb.2021.07.008
13. Chen J, Xu J, Chen Z, Yin M, Guo X, Sun L. Case report: identification of germline chimerism in monochorionic dizygotic twins. Front Genet. (2021) 12:744890. doi: 10.3389/fgene.2021.744890
14. Souter V, Kapur R, Nyholt DRA. Report of dizygous monochorionic twins. N Engl J Med. (2003) 349:154–8. doi: 10.1056/NEJMoa030050
15. Chen K, Kuhlmann R, Bell A, Rader J, Baumgartner M, Lemmens K, et al. Twin Anemia–Polycythemia sequence in sex discordant monochorionic dizygotic twins. Ultrasound Obstet Gynecol. (2020) 56:461–2. doi: 10.1002/uog.22073
16. Gagnon A, Audibert F. Prenatal screening and diagnosis of aneuploidy in multiple pregnancies. Best Pract Res Clin Obstet Gynaecol. (2014) 28:285–94. doi: 10.1016/j.bpobgyn.2013.12.010
17. San Román Muñoz M, Herranz Fernández J, Tejerina Puente A, Arteaga Manjón-Cabeza R, López Grondona F. Trisomía 9p. An Pediatr (Barc). (2004) 61:336–9. doi: 10.1016/s1695-4033(04)78398-8
18. Cammarata-Scalisi F. Trisomy 9p. A brief clinical, diagnostic and therapeutic description. Arch Argent Pediatr. (2019) 117:e473–6. doi: 10.5546/aap.2019
19. Woo K, Kim K, Kwon E, Kim J, Han JY. A case of partial trisomy 9pter –> q13 due to paternal balanced translocation t (9;21) (q13;q21). Korean J Lab Med. (2008) 28:155–9. doi: 10.3343/kjlm.2008.28.2.155
20. Blickstein I, Perlman S. Single fetal death in twin gestations. J Perinat Med. (2013) 41:65–9. doi: 10.1515/jpm-2012-0019
21. Shek N, Hillman S, Kilby M. Single-twin demise: pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. (2014) 28:249–63. doi: 10.1016/j.bpobgyn
22. Miura K, Niikawa N. Do monochorionic dizygotic twins increase after pregnancy by assisted reproductive technology? J Hum Genet. (2005) 50:1–6. doi: 10.1007/s10038-004-0216-6
23. Choi D, Kwon H, Lee S, Moon M, Yoo E, Lee K, et al. Testicular hypoplasia in monochorionic dizygous twin with confined blood chimerism. J Assist Reprod Genet. (2013) 30:1487–91. doi: 10.1007/s10815-013-0109-8
24. Wang Y, Peng Y, Chen X, Che X. Prevalence of chromosome 9 abnormalities among pediatric specimens. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. (2013) 30:362–4. doi: 10.3760/cma.j.issn.1003-9406.2013.03.024
25. Quinlan-Jones E, Lord J, Williams D. Molecular autopsy by trio exome sequencing (ES) and postmortem examination in fetuses and neonates with prenatally identified structural anomalies. Genet Med. (2019) 21:1065–73. doi: 10.1038/s41436-018-0298-8
Keywords: fetal death, double-embryo transfer, monochorionic dizygotic (MCDZ) twins, partial trisomy 9, vascular anastomoses, case report
Citation: Xie A, Cui Y, Luo G, Chen X, Zhang X, Han J, Tong L, Ren Y and Wei X (2023) Determining the cause of intrauterine fetal death in monochorionic twins: A case report. Front. Med. 9:1055275. doi: 10.3389/fmed.2022.1055275
Received: 27 September 2022; Accepted: 09 December 2022;
Published: 04 January 2023.
Edited by:
Salvatore Andrea Mastrolia, Umberto I Hospital, ItalyReviewed by:
Ilenia Mappa, Ospedale Cristo Re, ItalyNguyen Minh Duc, Pham Ngoc Thach University of Medicine, Vietnam
Copyright © 2023 Xie, Cui, Luo, Chen, Zhang, Han, Tong, Ren and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanming Ren, ✉ YnR5cWhAMTI2LmNvbQ==; Xiaoxing Wei, ✉ d2VpeGlhb3hpbmdAcWh1LmVkdS5jbg==
†These authors share first authorship
‡These authors share senior authorship
 Anxia Xie
Anxia Xie Yan Cui4†
Yan Cui4† Gang Luo
Gang Luo Yanming Ren
Yanming Ren Xiaoxing Wei
Xiaoxing Wei