
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 31 October 2022
Sec. Dermatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1048913
This article is part of the Research Topic Non-Invasive Diagnostic Tools in the Management of Skin Disorders View all 10 articles
Background: Dermoscopy is a non-invasive adjuvant diagnostic tool that allows clinicians to visualize microscopic features of cutaneous disorders. Recent studies have demonstrated that dermoscopy can be used to diagnose onychomycosis. We performed this systematic review to identify the characteristic dermoscopic features of onychomycosis and understand their diagnostic utility.
Methods: We searched the Medline, Embase, Scopus, and Cochrane databases from conception until May 2021. Studies on the dermoscopic features of onychomycosis were screened. The exclusion criteria were as follows: fewer than 5 cases of onychomycosis, review articles, and studies including onychomycosis cases that were not mycologically verified. Studies on fungal melanonychia were analyzed separately. We adhered to the MOOSE guidelines. Independent data extraction was performed. Data were pooled using a random effects model to account for study heterogeneity. The primary outcome was the diagnostic accuracy of the dermoscopic features of onychomycosis. This was determined by pooling the sensitivity and specificity values of the dermoscopic features identified during the systematic review using the DerSimonian-Laird method. Meta-DiSc version 1.4 and Review Manager 5.4.1 were used to calculate these values.
Results: We analyzed 19 articles on 1693 cases of onychomycosis and 5 articles on 148 cases of fungal melanonychia. Commonly reported dermoscopic features of onychomycosis were spikes or spiked pattern (509, 30.1%), jagged or spiked edges or jagged edge with spikes (188, 11.1%), jagged proximal edge (175, 10.3%), subungual hyperkeratosis (131, 7.7%), ruins appearance, aspect or pattern (573, 33.8%), and longitudinal striae (929, 54.9%). Commonly reported features of fungal melanonychia included multicolor (101, 68.2%), non-longitudinal homogenous pigmentation (75, 50.7%) and longitudinal white or yellow streaks (52, 31.5%).
Conclusion: This study highlights the commonly identified dermoscopic features of onychomycosis. Recognizing such characteristic dermoscopic features of onychomycosis can assist clinicians diagnose onychomycosis by the bedside.
Dermoscopy is a non-invasive diagnostic tool that helps clinicians to visualize microscopic features of cutaneous disorders, including skin cancers, connective tissue disorders and inflammatory dermatologic conditions, that are not discernible on naked eye examination (1–3). Consequently, it optimizes diagnostic accuracy and minimizes the need for unnecessary biopsies (4).
Onychomycosis is a communicable fungal nail infection caused by dermatophytes, non-dermatophyte molds, and yeasts. It is the most common nail disorder worldwide and severe disease can cause significant nail dystrophy and pain (5). Fungal melanonychia is a rare manifestation of a fungal nail infection, which presents with brown-black pigmentation of the nail unit. Accurate diagnosis of fungal nail disorders is important as systemic treatments are required for at least 2–3 months and topical treatments for more than 12 months. Misdiagnosis should be avoided, as systemic treatments risk hepatic damage (6) and unnecessary economic burden on the healthcare system. Clinically, onychomycosis may resemble traumatic onycholysis, nail psoriasis, or trachyonychia, and differentiating fungal melanonychia from nail melanoma is crucial. Dermoscopy can help to identify onychomycosis and fungal melanonychia at the bedside. Therefore, we conducted a systematic review to identify the characteristic dermoscopic features of onychomycosis and melanonychia, as well as a meta-analysis to determine the diagnostic performance and accuracy of dermoscopy in diagnosing onychomycosis.
This study adhered to the Meta-analyses of Observational Studies in Epidemiology (MOOSE) statement, with appropriate adjustments made as per the recommendations for systematic reviews and meta-analyses of diagnostic test accuracy (7, 8). The study protocol is registered in PROSPERO (Reg. No.: CRD42021268430).
Ovid MEDLINE (including Epub Ahead of Print, In-Process, and Other Non-Indexed Citations), Embase, Scopus, and the Cochrane Central Register of Controlled Trials were searched from inception to May 2021 by three reviewers (SSL, JO, LHLY). The research question was in patients with onychomycosis (P), what are the common dermoscopic features (I) that add to clinical examination (C) in diagnosing onychomycosis (O). Therefore, search terms included “dermoscopy” or synonyms (including dermatoscopy, videodermoscopy, onychoscopy and epiluminescence microscopy) and “onychomycosis” or synonyms (including tinea unguium). Medical subject headings (MeSH) terms were also included.
All published studies involving at least five cases of mycologically proven onychomycosis with dermoscopic findings were included. Studies reporting fewer than five cases were excluded due to risk of selection bias. Studies of fungal melanonychia were analyzed separately.
Three reviewers (SSL, JO, and LHLY) independently screened the titles and abstracts of all identified articles, and then screened the full text of potentially eligible articles. Non-English articles were screened by reviewing their titles and abstracts translated in English. Duplicate studies and review articles were excluded. None of the cases required a fourth author (JHM) to resolve any disagreement. The parameters extracted from each article included the first author's surname, date of publication, journal name, number of onychomycosis or fungal melanonychia cases, number of control cases, type of control cases (e.g., healthy or psoriasis), definition and prevalence of dermoscopic features, as well as their sensitivity and specificity if reported. Study authors were not contacted.
Two reviewers (SSL and JO) appraised the articles according to the Quality Assessment of Diagnostic Accuracy Studies (QUADAS2) guidelines (9).
The primary study outcome was diagnostic accuracy of the common dermoscopic features of onychomycosis. This was measured by pooling the sensitivity and specificity values using the DerSimonian-Laird method. We used a random-effects model to account for study heterogeneity. Pooled sensitivity and specificity values and their 95% confidence intervals (CI), forest plots, and summary receiver operating characteristics (SROC) curves were generated using Meta-DiSc version 1.4 (Hospital Ramon y Cajal and Universidad Complutense de Madrid) and Review Manager 5.4.1 (Cochrane, Oxford, UK).
A total of 201 articles were identified, of which 46 were duplicates (Figure 1). Of the 155 screened articles, 24 were full-text articles discussing common dermoscopic features in five or more cases of mycologically proven fungal nail disease. The characteristics of the 24 eligible studies are summarized in Table 1. Nineteen articles were on onychomycosis and five were on fungal melanonychia. Of the 19 onychomycosis articles, 11 had a control group consisting of nail psoriasis, traumatic onycholysis, and healthy or mycologically negative nails. A meta-analysis was performed on the data with controls. However, it was not conducted for fungal melanonychia, as only two of the five fungal melanonychia articles had a control group.
Nineteen studies reported dermoscopic features of 1,693 cases of onychomycosis. Commonly identified dermoscopic features of onychomycosis were spikes or spiked pattern (481, 28.4%) (10–18), jagged or spiked edges or jagged edge with spikes (188, 11.1%) (19–25), jagged proximal edge (175, 10.3%) (10, 12, 16, 18), subungual hyperkeratosis (131, 7.7%) (15, 19, 20, 25, 26), ruins appearance, aspect or pattern (573, 33.8%) (15, 19, 22, 24, 27, 28), and longitudinal striae (929, 54.9%) (10–18, 20–23, 27) (Table 2). Other dermoscopic findings included distal irregular termination (331, 19.6%) (10–12, 14–16, 18, 20, 22) and aurora borealis pattern (293, 17.3%) (11, 12, 15, 17, 20, 23). Frequently described color changes were homogenous leukonychia (304, 18.0%) (12, 15, 16, 20, 22, 23, 28), yellow (216, 12.8%) (13, 15, 16, 23, 26) and brown (212, 12.5%) (12, 15, 16, 22, 23, 26).
Terms with similar definitions or those used interchangeably were grouped for meta-analysis upon careful examination of the authors' definitions. When we grouped spikes or spiked pattern, jagged or spiked edges, distal streaks, jagged edge with spikes and jagged proximal edge as “spike pattern”, the pooled sensitivity was 77.3% (95% CI, 73.2–81.1%) and specificity was 96.2% (95% CI, 93.1–98.2%) (Figure 2). Pooled sensitivity of subungual hyperkeratosis and ruins appearance, aspect or pattern was 67.1% (95% CI, 62.5–71.5%) and specificity was 64.7% (95% CI, 58.1–70.8%). For longitudinal striae, pooled sensitivity was 67.3% (95% CI, 61.7–72.6%) and specificity 95.6% (95% CI, 90.7–98.4%).
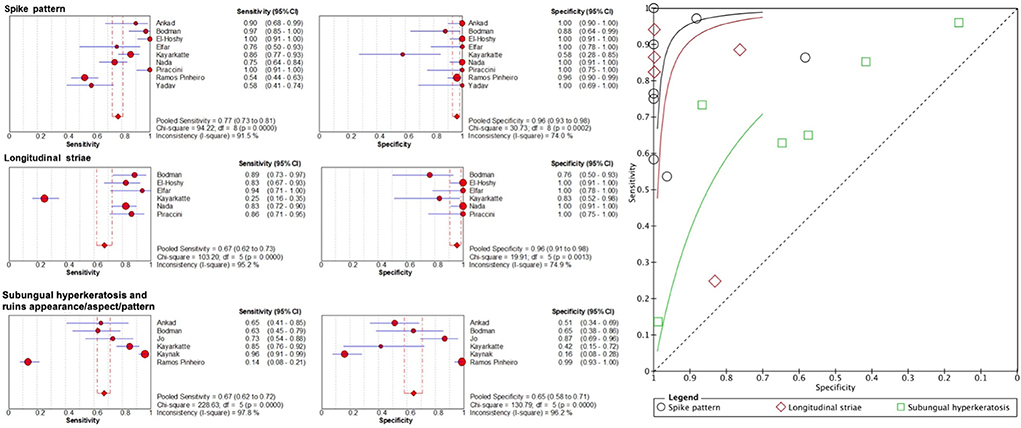
Figure 2. Sensitivity and specificity of important dermoscopic features of onychomycosis and their summary receiver operating characteristics (SROC) curves.
Five studies reported dermoscopic features of 148 cases of fungal melanonychia (Table 3) (29–31, 33). These cases demonstrated longitudinal white or yellow streaks (52, 35.1%) (31–33), nail surface scales (39, 33.1%) (31–33) and subungual hyperkeratosis (41, 27.7%) (31–33) (Table 3), which are also common dermoscopic features of onychomycosis. Homogenous pigmentation (75, 50.7%) (29, 30, 33) or longitudinal pigmentation (54, 36.5%) (29, 31–33) was frequently observed, and the most common colors were multicolor (101, 68.2%) (29–33), brown (84, 56.8%) (29, 31–33) and black (46, 31.1%) (29, 31–33). The pigmentation in melanonychia arising from a fungal infection tends to appear brown due to the production of fungal melanin via the pentaketide pathway (31). This is in contrast to melanomas, where melanin is made from tyrosine, and commonly appears as darkly pigmented and black. Findings that appear specific to fungal melanonychia, such as “reverse triangle” (30, 20.3%) (30–33), due to fungal invasion from the distal nail plate and “superficial transverse striation” (41, 27.7%) (29, 30, 33), were also reported. All cases had negative findings for melanoma, such as the lack of the Hutchinson sign (0%) (30–33) and triangular sign (0%) (31, 32).
The risk of bias in the eligible articles was evaluated according to the QUADAS2 guidelines (Table 4). Studies with “unclear” patient selection bias did not specify their method of patient selection, such as whether patients were recruited prospectively or retrospectively or whether patients were enrolled consecutively or randomly. Studies had a low risk of bias in terms of the index test (dermoscopy), reference standard (clear diagnosis of non-onychomycosis nails), flow, and timing. However, the risk of bias in the reference standard for one article was deemed high, as two cases with a positive potassium hydroxide result were not classified as onychomycosis as they primarily displayed features of other nail disorders (19). Studies had low applicability concerns with patient selection and reference standards, but two studies had high applicability concerns with the index test because they did not provide clear definitions or representative images for dermoscopic features (25, 27).
The role of dermoscopy is well established in diagnosing cutaneous malignancies such as malignant melanoma and non-melanoma skin cancers (34, 35). Its use expands to various inflammatory and infectious disorders, including onychomycosis. By conducting a systematic review of 19 articles on 1,693 cases of onychomycosis and 5 articles on 148 cases of fungal melanonychia, we could enlarge the sample size and thus the statistical power to identify the dermoscopic features with diagnostic utility. Recognizing common dermoscopic features of onychomycosis can help clinicians to expedite accurate diagnosis and management. The most frequently reported patterns in onychomycosis included spikes or spiked patterns, ruins appearance, aspect or pattern and longitudinal striae. After pooling the dermoscopic terminology that were closely related or used interchangeably, “spike pattern” and longitudinal striae had high specificity (96.2 and 95.6%, respectively) and moderate sensitivity (77.3 and 67.3%, respectively) for onychomycosis. Detecting these features can raise clinicians' suspicion of onychomycosis and expedite further investigations. Ruins appearance, aspect or pattern and subungual hyperkeratosis had moderate sensitivity (71.6%) and specificity (64.7%) for onychomycosis as these features can also be observed in other nail disorders including nail psoriasis and allergic contact dermatitis. Other dermoscopic features characterizing onychomycosis were distal irregular termination, aurora borealis, homogenous leukonychia, and brown discoloration.
We also found that the most frequently described dermoscopic features of fungal melanonychia were longitudinal white or yellow streaks and nail surface scales. Unlike melanocytic melanonychia, fungal melanonychia is characterized by non-longitudinal homogenous pigmentation and reverse triangular patterns (32). Moreover, our data demonstrate that subungual hyperkeratosis frequently occurs in fungal melanonychia.
This study has some limitations. There was considerable heterogeneity in the study design and terminology definitions of the enrolled studies, which may have limited the strength of our study. We sought to clarify dermoscopic terminology by identifying commonly used terms and narrowing their definitions to accurately pool and compare the findings. Future studies with standardized terminology are necessary, ideally through an expert panel, to facilitate clear communication among clinicians.
To our knowledge, this is the first systematic review of dermoscopic features of onychomycosis. Given the limited sample sizes of existing studies on this topic, pooling their results provides us an overview of the most common features of onychomycosis and the frequency at which they present in patients. Understanding these characteristic dermoscopic features of onychomycosis can assist clinicians diagnose onychomycosis by the bedside.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
J-HM and CO: conceptualization, resources, and supervision. J-HM: methodology and project administration. YC, J-HM, and CO: validation. SL, JO, and LH: formal analysis. SL and LH: investigation, data curation, writing—original draft, and visualization. JO, YC, CO, and J-HM: writing—review and editing. All authors: software. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Marghoob NG, Liopyris K, Jaimes N. Dermoscopy: A Review of the Structures That Facilitate Melanoma Detection. J Am Osteopath Assoc. (2019) 119:380–90. doi: 10.7556/jaoa.2019.067
2. Pizzorni C, Giampetruzzi AR, Mondino C, Facchiano A, Abeni D, Paolino S et al. Nailfold capillaroscopic parameters and skin telangiectasia patterns in patients with systemic sclerosis. Microvasc Res. (2017) 111:20–4. doi: 10.1016/j.mvr.2016.12.003
3. Golińska J, Sar-Pomian M, Rudnicka L. Dermoscopic features of psoriasis of the skin, scalp and nails - a systematic review. J Eur Acad Dermatol Venereol. (2019) 33:648–60. doi: 10.1111/jdv.15344
4. Carli P, De Giorgi V, Crocetti E, Mannone F, Massi D, Chiarugi A et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the 'dermoscopy era': a retrospective study 1997-2001. Br J Dermatol. (2004) 150:687–92. doi: 10.1111/j.0007-0963.2004.05860.x
5. Lipner SR, Scher RK. Onychomycosis: Clinical overview and diagnosis. J Am Acad Dermatol. (2019) 80:835–51. doi: 10.1016/j.jaad.2018.03.062
6. Fávero MLD, Bonetti AF, Domingos EL, Tonin FS, Pontarolo R. Oral antifungal therapies for toenail onychomycosis: a systematic review with network meta-analysis toenail mycosis: network meta-analysis. J Dermatolog Treat. (2020) 33:121–30. doi: 10.1080/09546634.2020.1729336
7. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
8. McGrath TA, Alabousi M, Skidmore B, Korevaar DA, Bossuyt PMM, Moher D et al. Recommendations for reporting of systematic reviews and meta-analyses of diagnostic test accuracy: a systematic review. Syst Rev. (2017) 6:194. doi: 10.1186/s13643-017-0590-8
9. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. (2003) 3:25. doi: 10.1186/1471-2288-3-25
10. Abdallah NA, Said M, Mahmoud MT, Omar MA. Onychomycosis: Correlation between the dermoscopic patterns and fungal culture. J Cosmet Dermatol. (2020) 19:1196–204. doi: 10.1111/jocd.13144
11. Bhat YJ, Mir MA, Keen A, Hassan I. Onychoscopy: an observational study in 237 patients from the Kashmir Valley of North India. Dermatol Pract Concept. (2018) 8:283–91. doi: 10.5826/dpc.0804a06
12. Chetana K, Menon R, David BG. Onychoscopic evaluation of onychomycosis in a tertiary care teaching hospital: a cross-sectional study from South India. Int J Dermatol. (2018) 57:837–42. doi: 10.1111/ijd.14008
13. El-Hoshy KH, Abdel Hay RM, El-Sherif RH, Salah Eldin M, Moussa MF. Nail dermoscopy is a helpful tool in the diagnosis of onychomycosis: A case control study. Eur J Dermatol. (2015) 25:494–5. doi: 10.1684/ejd.2015.2637
14. Jesus-Silva MA, Fernandez-Martinez R, Roldan-Marin R, Arenas R. Dermoscopic patterns in patients with a clinical diagnosis of onychomycosis-results of a prospective study including data of potassium hydroxide (KOH) and culture examination. Dermatol Pract Concept. (2015) 5:39–44. doi: 10.5826/dpc.0502a05
15. Kayarkatte MN, Singal A, Pandhi D, Das S, Sharma S. Nail dermoscopy (onychoscopy) findings in the diagnosis of primary onychomycosis: A cross-sectional study. Indian J Dermatol Venereol Leprol. (2020) 86:341–9. doi: 10.4103/ijdvl.IJDVL_100_19
16. Maatouk I, Haber R, Benmehidi N. Onychoscopic evaluation of distal and lateral subungual onychomycosis: A cross-sectional study in Lebanon. Curr. (2019) 5:41–4. doi: 10.18502/cmm.5.2.1161
17. Nada EEA, El Taieb MA, El-Feky MA, Ibrahim HM, Hegazy EM, Mohamed AE et al. Diagnosis of onychomycosis clinically by nail dermoscopy versus microbiological diagnosis. Arch Dermatol. (2020) 312:207–12. doi: 10.1007/s00403-019-02008-6
18. Nargis T, Pinto M, Shenoy MM, Hegde S. Dermoscopic Features of Distal Lateral Subungual Onychomycosis. Indian Dermatol Online J. (2018) 9:16–9. doi: 10.4103/idoj.IDOJ_40_17
19. Ankad BS, Gupta A, Alekhya R, Saipriya M. Dermoscopy of onycholysis due to nail psoriasis, onychomycosis and trauma: a cross sectional study in skin of color. Indian Dermatol Online J. (2020) 11:777–83. doi: 10.4103/idoj.IDOJ_475_19
20. Bodman MA. Point-of-care diagnosis of onychomycosis by dermoscopy. J Am Podiatr Med Assoc. (2017) 107:413–8. doi: 10.7547/16-183
21. Elfar NN, Abdel-Latif AM, Labeh EA. Role of onychoscopy in differentiation between distal subungual onychomycosis, psoriasis, and traumatic onycholysis. J Egypt Women Dermatol Soc. (2015) 12:145–9. doi: 10.1097/01.EWX.0000469303.65552.a1
22. Islamoglu ZGK, Demirbas A, Unal M, Findik D. Nail digital dermoscopy in onychomycosis: a correlation with clinical type, gender, and culture examination. Erciyes Med J. (2019) 41:288–94. doi: 10.14744/etd.2019.94210
23. Piraccini BM, Balestri R, Starace M, Rech G. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. (2013) 27:509–13. doi: 10.1111/j.1468-3083.2011.04323.x
24. Ramos Pinheiro R, Dias Domingues T, Sousa V, Galhardas C, Apetato M, Lencastre A, et al. comparative study of onychomycosis and traumatic toenail onychodystrophy dermoscopic patterns. J Eur Acad Dermatol Venereol. (2019) 33:786–92. doi: 10.1111/jdv.15358
25. Yadav TA, Khopkar US. White streaks: dermoscopic sign of distal lateral subungual onychomycosis. Indian J Dermatol. (2016) 61:174151. doi: 10.4103/0019-5154.174151
26. Jo G, Park JS Yu DA, Ohn J, Sheu SL, Mun JH. Onychoscopy of trachyonychia: an analysis of 30 patients and comparison with onychomycosis. Br J Dermatol. (2018) 179:491–3. doi: 10.1111/bjd.16431
27. De Crignis G, Valgas N, Rezende P, Leverone A, Nakamura R. Dermatoscopy of onychomycosis. Int J Dermatol. (2014) 53:e80–e157. doi: 10.1111/ijd.12104
28. Kaynak E, Goktay F, Gunes P, Sayman E, Turan D, Baygul A et al. The role of dermoscopy in the diagnosis of distal lateral subungual onychomycosis. Arch Dermatol. (2018) 310:57–69. doi: 10.1007/s00403-017-1796-2
29. Elmas OF, Metin MS. Dermoscopic findings of fungal melanonychia. Postepy Dermatol. (2020) 37:180–3. doi: 10.5114/ada.2020.94836
30. Kilinc Karaarslan I, Acar A, Aytimur D, Akalin T, Ozdemir F. Dermoscopic features in fungal melanonychia. Clin Exp Dermatol. (2015) 40:271–8. doi: 10.1111/ced.12552
31. Kim H-J, Kim T-W, Park S-M, Lee H-J, Kim G-W, Kim H-S et al. Clinical and Dermoscopic Features of Fungal Melanonychia: Differentiating from Subungual Melanoma. Ann Dermatol. (2020) 32:460–5. doi: 10.5021/ad.2020.32.6.460
32. Ohn J, Choe YS, Park J, Mun JH. Dermoscopic patterns of fungal melanonychia: A comparative study with other causes of melanonychia. J Am Acad Dermatol. (2017) 76:488–93.e2. doi: 10.1016/j.jaad.2016.08.013
33. Starace M, Ambrogio F, Bruni F, Piraccini BM, Alessandrini A. Dermatophytic melanonychia: A Case Series of an increasing disease. Mycoses. (2021) 64:511–9. doi: 10.1111/myc.13237
34. Lan J, Wen J, Cao S, Yin T, Jiang B, Lou Y et al. The diagnostic accuracy of dermoscopy and reflectance confocal microscopy for amelanotic/hypomelanotic melanoma: a systematic review and meta-analysis. Br J Dermatol. (2020) 183:210–9. doi: 10.1111/bjd.18722
Keywords: fungal nail infection, onychomycosis, dermoscopy, fungal melanonychia, onychoscopy
Citation: Lim SS, Hui L, Ohn J, Cho Y, Oh CC and Mun J-H (2022) Diagnostic accuracy of dermoscopy for onychomycosis: A systematic review. Front. Med. 9:1048913. doi: 10.3389/fmed.2022.1048913
Received: 20 September 2022; Accepted: 10 October 2022;
Published: 31 October 2022.
Edited by:
Devinder Mohan Thappa, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), IndiaReviewed by:
Ahu Yorulmaz, Ankara City Hospital, TurkeyCopyright © 2022 Lim, Hui, Ohn, Cho, Oh and Mun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Je-Ho Mun, amVob211bkBzbnUuYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.