
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 22 December 2022
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1045274
Background: Recent studies have highlighted the cardio-cerebrovascular manifestations of coronavirus disease 2019 (COVID-19).
Objective: This study aimed to analyze the likelihood of cardiovascular and cerebrovascular manifestations among patients with COVID-19-positive individuals in South Korea.
Methods: A cohort database for COVID-19 from the National Health Insurance Service was used which included patients diagnosed with COVID-19 between January 1 and June 4, 2020. Individuals who tested COVID-19 positive, notwithstanding the severity of the disease, were designated as cases. COVID-19- negative individuals were used as controls for the study. The exclusion criteria included people who had a history of cardiovascular and cerebrovascular diseases between 2015 and 2019. A new diagnosis of cardiovascular and cerebrovascular complications was considered the primary endpoint. The adjusted incidence rate ratio (IRR) of development of complications was estimated using log-link Poisson regression. The model was adjusted at two levels, the first one included age and sex while the second included age, sex, residence area, and level of income. The hazard ratio (HR) was estimated using Cox-proportional hazard regression analysis while adjusting for all demographic variables and covariates.
Results: Significant results were obtained for acute conditions, such as ischemic heart disease and cerebral hemorrhage. The IRR of COVID-19- positive individuals compared with that of controls for the diagnosis of ischemic heart disease was 1.78 (1.57–2.02; 95% confidence interval [CI]) when adjusted for age and sex. HR was calculated as 3.02 (2.19–4.17; 95% CI) after adjusting for the covariates. In case of cerebral hemorrhage, the adjusted IRR was 2.06 (1.25–3.40; 95% CI) and the adjusted HR was 4.08 (0.90–19.19; 95% CI).
Conclusion: The findings of our study suggest that COVID-19 infection can be a significant risk factor for acute cardiovascular complications, such as ischemic heart disease and acute cerebrovascular complications, such as cerebral infarction, after properly adjusting for covariates.
As of March 31, 2022, total coronavirus disease 2019 (COVID-19) cases have soared to over 486 million worldwide with over 6.16 million deaths and over 421 million survivors (1). Concerns regarding COVID-19 inducing cardiovascular and cerebrovascular manifestations, in addition to the clinical presentation of respiratory failure have increased (2). Complications in patients with COVID-19 include cardio-cerebrovascular conditions, such as shock, acute cardiac injury, and arrhythmias (3).
Angiotensin-converting enzyme 2 (ACE 2) acts as a gateway for the entry of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into the host cell (4). Direct cardiovascular manifestations in COVID-19-positive individuals, such as myocarditis, heart failure, and arrhythmias are based on the pathophysiology of the renin-angiotensin system (RAS) axes, while the indirect effects such as thromboembolism and metabolic disorders are caused by the cytokine storm (5, 6). A cytokine storm is thought to result in myocardial injury, that involves elevated production of interleukin-6 (IL-6), ferritin, D-dimer, lactate dehydrogenase (LDH), etc. (7). Many studies have suggested that patients with pre-existing cardiovascular diseases experience severe COVID-19 outcomes (8–11) while others have reported that COVID-19 itself can be a risk factor for cardiovascular complications (12). The pathogenesis of cerebrovascular diseases in patients with COVID-19, is thought to be mediated through endothelial dysfunction caused by infection of endothelial cells and initiation of a coagulation cascade resulting in a state of hypercoagulability that can contribute to various thromboembolic events (13).
Several previous studies have demonstrated COVID-19 to be an important risk factor for acute myocardial infarction and acute ischemic stroke (11). However, some studies have shown that cardiovascular diseases, such as ischemic stroke are not positively associated with COVID-19 (14). The studies that have determined the acute cardio-cerebrovascular manifestations of COVID-19 using a control group are limited and have a restricted sample size. Furthermore, there is a need for nationally representative data to effectively identify cardiovascular and cerebrovascular insults caused by COVID-19. Thus, with the use of national representative data, this study aimed to determine the risk of acute cardio-cerebrovascular manifestations in the COVID-19 positive patients in South Korea.
COVID-19 cohort database provided by the National Health Insurance Service (NHIS) was used for data collection. The database consisted of NHIS subscribers. The database was formulated with its sole purpose of application in medical research, as a collaboration of the NHIS with the Korea Centers for Disease Control and Prevention (KCDC).
The KCDC provided information on COVID-19 patients and controls, who were diagnosed from January 1, 2020, to June 4, 2020, to the NHIS COVID-19 cohort database. The NHIS-COVID-19 cohort database was opened per research team for about 5 days. The database encompasses information on the cases and controls, including demographic characteristics, official COVID-19 diagnosis confirmation date, medical history of the subjects, results of medical checkups, treatment results and demise. Diagnoses were classified based on International Classification of Diseases (ICD)-10 codes. The database excluded information regarding individuals with incomplete medical records and foreign patients with COVID-19 (15).
Individuals diagnosed through real-time reverse transcription polymerase chain reaction test (RT-PCR) for SARS-CoV-2 with cycle threshold (Ct) cutoff value of 33.5 were defined as cases (16), while the controls were individuals who tested negative for COVID-19. Individuals with a history of cardiovascular and cerebrovascular diseases between 2015 and 2019 were excluded. Figure 1 shows the flowchart of the selection of study participants.
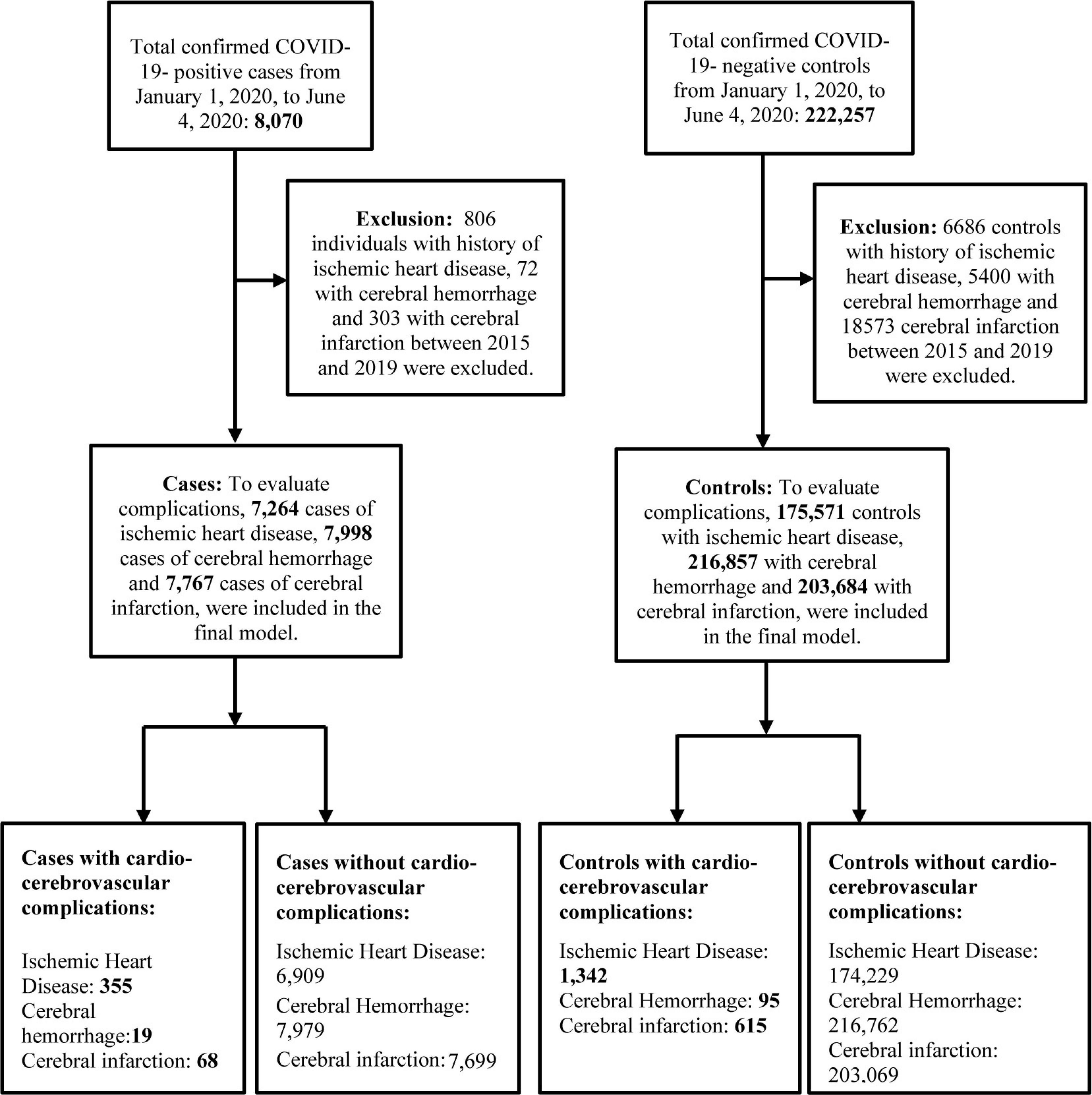
Figure 1. Flowchart of the selection of study participants. Ischemic heart disease was diagnosed with I20–I25, cerebral hemorrhage with I60–I62 and cerebral infarction with I63 in ICD-10-Code. COVID-19, coronovirus disease 2019.
Individuals from the cohort database with history of cardio-cerebrovascular conditions between 2015 and 2019 were excluded to analyze the development of a new cardio-cerebrovascular complication after the diagnosis of COVID-19. The potential confounders designated for cardio-cerebrovascular complications of COVID-19 were age (17, 18), sex (17–20), place of residence (18, 19, 21) and income level (18, 22). Age was classified based on 10 years age groups and the residential area was categorized as Seoul, Gyeonggi-do, Daegu, Gyeongsangbuk-do and others. Similarly, income level had a value ranging from 0 to 20. The secondary potential confounders considered were drinking alcohol frequency (23–25), smoking history (26–28), comorbidities like Diabetes Mellitus (DM) (29, 30), hypertension (31, 32) and dyslipidemia (33, 34), anthropometric variables like body mass index (BMI) (19, 35, 36), glomerular filtration rate (GFR) (37, 38), gamma-glutamyl transferase level (GGT-level) (39, 40), hemoglobin level (Hb-level) (41, 42), height (43) and family history of heart disease (44). The significant secondary potential confounders were adjusted using Cox proportional regression analysis.
Follow-up of the cases started from the date of confirmation of COVID-19 diagnosis. The diagnosis confirmation date, however, might differ from the actual date of diagnosis since the date of the official diagnosis announcement was used. The follow-up of controls started on June 4, 2020 (the final date of diagnosis of COVID-19). The manifestation of cardiovascular and cerebrovascular complications in patients with COVID-19 in the cohort database was regarded as the primary endpoint of the study. This endpoint was evaluated from January 1, 2020, to June 4, 2020.
The demographics of the cases and controls were analyzed through descriptive statistics, independent sample t-test and chi-squared test. Log-link Poisson regression analysis and Cox proportional regression analyses were used for sensitivity analysis. Based on the incidence density, a log-link Poisson regression model was used to estimate the adjusted incidence rate ratio (IRR). Adjustment was performed at two levels, one for age and sex, and another for age, sex, residence area and income level. The hazard ratio (HR) was estimated using Cox proportional regression, while adjusting for primary and secondary potential confounders.
SAS Enterprise Guide (version 7.13) was used to conduct all statistical analyses. Statistical significance for the analyses was set at p < 0.05. The Institution Review Board (IRB) of Korea University approved the exemption from ethical approval (IRB exemption number: KUIRB-2021-0238-02).
The evaluated cardio-cerebrovascular complications included (a) ischemic heart disease (I20–I25), (b) cerebral hemorrhage (I60–I62) and (c) cerebral infarction (I63).
This study evaluated 2,30,327 individuals, of which 8,070 were COVID-19 positive patients (cases) and 2,22,257 were controls. The demographic characteristics of the study subjects are described previously (45). The highest number of COVID-19- positive patients was present in the 20–29-year age group (2057/8070; 25.5%). Similarly, the highest number of controls was in the 30–39-year age group (37977/222257; 17.1%). The number of female participants dominated both the cases (4834/8070; 59.9%) and controls (116175/222257; 52.3%). Diabetes was 14.4% in the positive group and 13.5% in the control group, showing no significant difference (p = 0.157), and there was no significant difference between the two groups in hypertension (27.4 vs. 27.3%) and hyperlipidemia (10.1 vs. 9.6%, respectively) (45). The final model included different number of cases and controls depending on the type of the disease under study. Table 1 shows the number of new cardio-cerebrovascular complications that occurred in patients and controls during the follow-up.
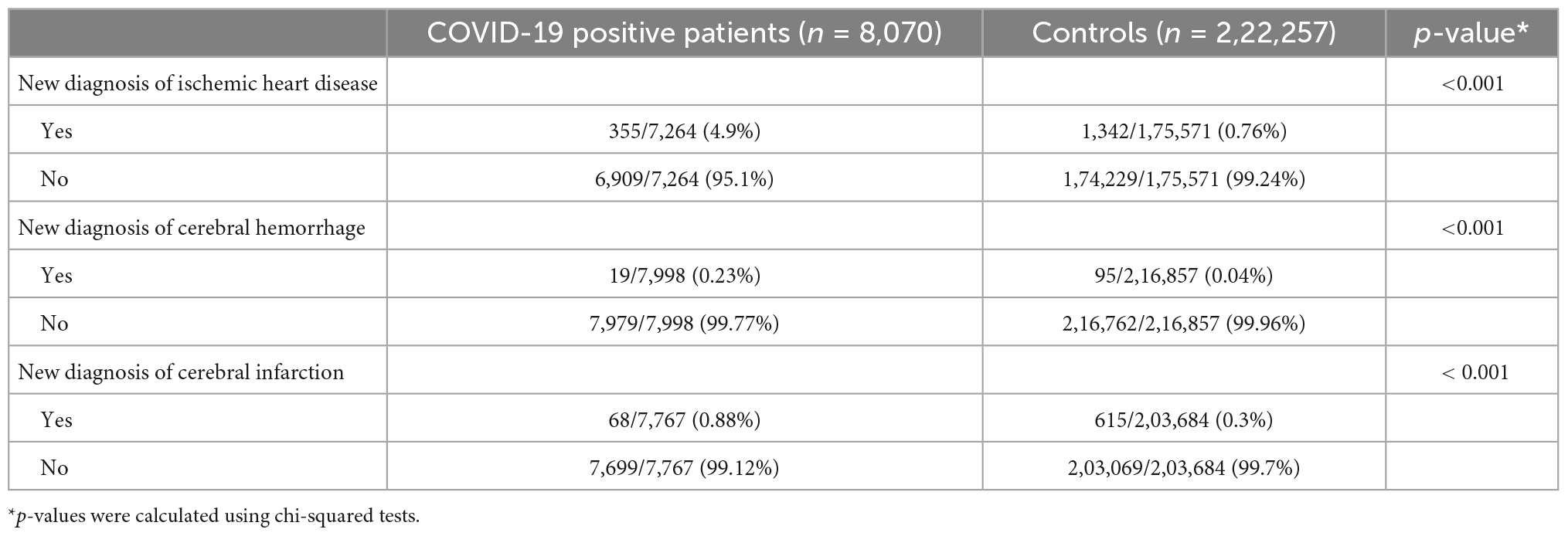
Table 1. New diagnosis of cardio-cerebrovascular complications among the COVID-19- positive cases and controls.
a. Ischemic heart disease (I20–I25): A total of 7,264 cases and 1,75,571 controls were included in the final model after excluding 806 patients and 6,686 controls with a prior history of ischemic heart disease. A total of 355/7,264 (4.9%) patients and 1,342/1,75,571 (0.76%) controls were newly diagnosed with ischemic heart disease.
b. Cerebral hemorrhage (I60–I62): After the exclusion of 72 cases and 5,400 controls with a history of cerebral hemorrhage, our final model included 7,998 cases and 2,16,857 controls. A total of 19/7,998 (0.23%) cases and 95/2,16,857 (0.04%) controls were newly diagnosed with cerebral hemorrhage.
c. Cerebral infarction (I63): A total of 303 cases and 18,573 controls were excluded owing to a history of cerebral infarction. The final analysis of the study included 7,767 cases and 2,03,684 controls. A total of 68/7,767 (0.88%) cases and 615/2,03,684 (0.3%) controls were newly diagnosed with cerebral infarction.
Table 2 depicts the results of the sensitivity analysis, that include adjusted IRR and adjusted HR. Significant results were obtained in case of ischemic heart disease and cerebral hemorrhage. In case of ischemic heart disease, the age and sex adjusted IRR was 1.78 (1.57–2.02) and HR adjusted for all demographic variables was 3.02 (2.19–4.17). In case of cerebral hemorrhage, the age and sex adjusted IRR was 2.06 (1.25–3.40). Survival analyses failed to show a significant association between COVID-19 diagnosis and risk of cerebral infarction.
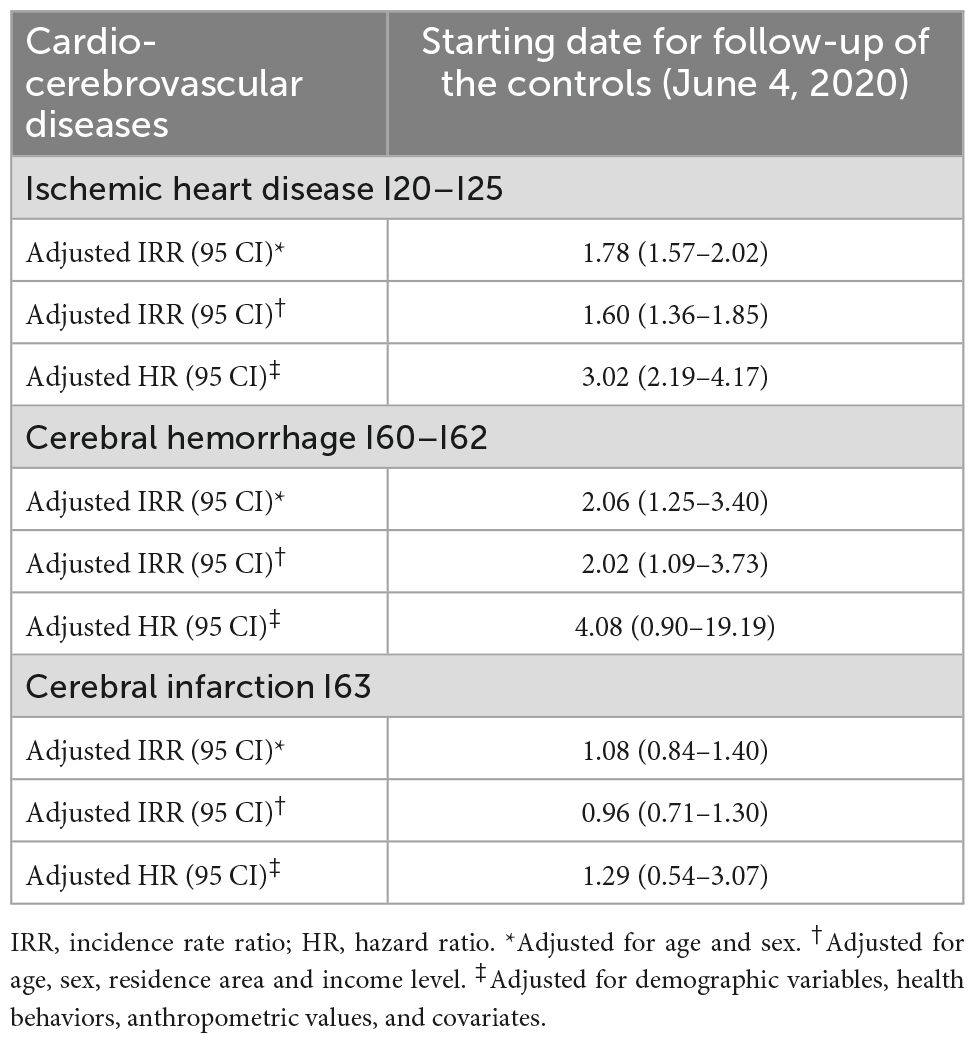
Table 2. Results of sensitivity analyses (log-link Poisson regression and Cox proportional hazard regression).
Since the study participants in the final analysis excluded individuals with a history of cardiovascular diseases, cerebrovascular diseases, and traditional vascular risk factors, our findings suggest that COVID-19 is an important risk factor for acute cardio-cerebrovascular complications, such as ischemic heart disease and cerebral hemorrhage even after adjusting for covariates. This implies that besides the primary pulmonary sequelae of COVID-19, cardio-cerebrovascular symptoms due to the infection can also cause morbidity and mortality. The robustness of the data used in our study is accentuated by the fact that they were nationally representative.
The findings from existing literature strengthen the results of our study. In a self-controlled case series and matched cohort study in Sweden, a significant increase in the risk of developing myocardial infarction, post COVID-19 infection was observed mainly in the first and second weeks of infection (11). A study in Romania reported fatal intracranial hemorrhage in COVID-19- positive patients who were otherwise healthy individuals without any history of cerebrovascular pathology (46). Similarly, in another case report, massive cerebral hemorrhage was observed in a young COVID-19 positive patient (47). Several studies have implied that respiratory infections can contribute to the development of new cardiovascular and cerebrovascular diseases (48–55).
Our study found an insignificant association between COVID-19 and cerebral infarction, although most existing literature suggests a strong association (10, 56–59). However, the findings from a multicenter cross-sectional study conducted in the healthcare system in New York State stated that there was no apparent presentation of stroke among patients with COVID-19 (14).
Existing literature on the increased risk of acute cardiovascular complications in COVID-19-positive individuals suggest that several mechanisms may be responsible. Some of the common mechanisms include:
i Downregulation of ACE2 receptors leading to Renin-angiotensin-aldosterone system (RAAS) dysregulation: Direct acute myocardial injury may result from binding of SARS-CoV-2 to ACE2 because of alteration in the ACE2 signaling pathways (60, 61).
ii Cytokine release syndrome and hemodynamic instability: Acute systemic inflammation and cytokine release syndrome caused by elevated levels of ferritin, Interleukin-6 (IL-6), IL-2, tumor necrosis factor (TNF)-α, C-reactive protein (CRP), D-dimer and high-sensitivity cardiac troponin I leading to multiple organ failure (including the heart) can occur mostly in severe cases of COVID-19 (60, 62–64).
iii Impaired myocardial oxygen demand-supply ratio: Myocardial injury may occur, subjected to hypoxia induced apoptosis of cardiac myocytes (62, 63).
iv Plaque destabilization leading to microthrombus formation: Increased levels of catecholamines, C-reactive proteins, etc. can precipitate plaque rupture that initiates formation of microthrombi and hence lead to acute myocardial infarction (60).
v Prothrombotic state and coagulation disorders leading to micro thrombosis in various organs: COVID-19-associated coagulopathy may manifest myocardial infarction and microvascular obstruction (65).
vi Imbalance in the electrolytes: Electrolyte imbalance like Hypokalemia specially in severe COVID-19 cases, can be linked to arrythmias (63, 66).
Acute cerebrovascular diseases are thought to occur in COVID-19-positive individuals because of multifactorial etiology, some of which include:
i Neurotropism: Evidence suggest that the brain contains some ACE2 receptors, that makes it a potential target of SARS-CoV-2 (67). The route of entry of the virus maybe through hematogenous spread and/or neuronal retrograde routes (68, 69).
ii Endothelial dysfunction (ED): The Angiotensin II system in the brain is linked with multiple functions like brain development and cerebral blood flow (70). Since ACE2 receptors are expressed in endothelial cells of the cerebrovascular system as well, COVID-19 may lead to microbleeds, hemorrhagic lesions and vasogenic edema induced by disruption of the blood brain barrier (71).
iii Hyper coagulopathy: COVID-19 may lead to development of a hypercoagulable state characterized by a prolonged prothrombin time, surge in D-dimers and disseminated intravascular coagulation (DIC) (72).
iv Inflammation: Elevated levels of IL-1β, IL-10, IL-6, TNF-α, etc. induce an inflammatory state and cytokine cascade that can cause intracerebral hemorrhage mostly in severe cases of COVID-19 (73).
Our study has some limitations. There is a possibility that the cardio-cerebrovascular manifestations that occurred in these cases could have occurred during the course of treatment and medications against COVID-19 infection. In addition, they could have developed through COVID-19 related stress since stress-related cardio-cerebrovascular complications are common (74). In addition, the development of cardio-cerebrovascular complications is thought to depend on the severity of COVID-19 infection (75), but the information regarding severity of the infection was unavailable. In addition, for privacy policy, the date of COVID-19 diagnosis used in the database was not the actual date of diagnosis but the official date of announcement of the diagnosis. Nevertheless, since the official announcement date follows the actual date of diagnosis, bias can tend toward null. This study consisted of nationally representative cohort data along with an appropriate control group and suggested that COVID-19 can be an independent risk factor for acute cardio-cerebrovascular diseases, such as ischemic heart disease and cerebral hemorrhage, even after properly adjusting for covariates.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Review Board of Korea University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JK, KH, and BC contributed to the conception and design of the work. JK and KH contributed to data acquisition and analysis. TK and KH drafted the manuscript. All authors critically revised the manuscript, contributed to the interpretation of the work, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Worldometer. COVID-19 Coronavirus Pandemic. (2022). Available online at: https://www.worldometers.info/coronavirus/ (accessed January 11, 2022).
2. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020) 5:811–8. doi: 10.1001/jamacardio.2020.1017
3. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
4. Chan J, Kok K, Zhu Z, Chu H, To K, Yuan S, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microb Infect. (2020) 9:221–36. doi: 10.1080/22221751.2020.1719902
5. Pucci F, Annoni F, Santos R, Taccone F, Rooman M. Quantifying renin-angiotensin-system alterations in COVID-19. Cells. (2021) 10:2755. doi: 10.3390/cells10102755
6. Chang W, Toh H, Liao C, Yu W. Cardiac involvement of COVID-19: a comprehensive review. Am J Med Sci. (2021) 361:14–22. doi: 10.1016/j.amjms.2020.10.002
7. Clerkin K, Fried J, Raikhelkar J, Sayer G, Griffin J, Masoumi A. COVID-19 and cardiovascular disease circulation. Circulation. (2020) 19:20. doi: 10.1161/CIRCULATIONAHA.120.046941
8. O’Gallagher K, Shek A, Bean D, Bendayan R, Papachristidis A, Teo J, et al. Pre-existing cardiovascular disease rather than cardiovascular risk factors drives mortality in COVID-19. BMC Cardiovasc Disord. (2021) 21:327. doi: 10.1186/s12872-021-02137-9
9. Park B, Lee J, Park H, Kim H, Jang S, Bae M, et al. Impact of cardiovascular risk factors and cardiovascular diseases on outcomes in patients hospitalized with COVID-19 in Daegu metropolitan city. J Korean Med Sci. (2021) 36:e15. doi: 10.3346/jkms.2021.36.e113
10. Puttegowda B, Shivashankarappa A, Dutta S, Chikkamuniswamy R, Bhat P, Krishnan S, et al. Patterns of cardiovascular diseases in COVID-19 patients admitted to tertiary cardiac care centre. Indian Heart J. (2021) 73:682–6. doi: 10.1016/j.ihj.2021.10.007
11. Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Connolly A. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. (2021) 398:599–607. doi: 10.1016/S0140-6736(21)00896-5
12. Greenberg A, Pemmasani G, Yandrapalli S, Frishman W. Cardiovascular and cerebrovascular complications with COVID-19. Cardiol Rev. (2021) 29:143. doi: 10.1097/CRD.0000000000000385
13. Harapan B, Yoo H. Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (Sars-Cov-2) and coronavirus disease 19 (COVID-19). J Neurol. (2021) 268:3059–71. doi: 10.1007/s00415-021-10406-y
14. Bekelis K, Missios S, Ahmad J, Labropoulos N, Schirmer C, Calnan D, et al. Ischemic stroke occurs less frequently in patients with COVID-19: a multicenter cross-sectional study. Stroke. (2020) 51:3570–6. doi: 10.1161/STROKEAHA.120.031217
15. Oh T, Choi J, Song I. Socioeconomic disparity and the risk of contracting COVID-19 in South Korea: an Nhis-COVID-19 database cohort study. BMC Public Health. (2021) 21:144. doi: 10.1186/s12889-021-10207-y
16. Hong K, Lee S, Kim T, Huh H, Lee J, Kim S, et al. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. (2020) 40:351–60. doi: 10.3343/alm.2020.40.5.351
17. Mozaffarian D, Wilson P, Kannel W. beyond established and novel risk factors: lifestyle risk factors for cardiovascular disease. Circulation. (2008) 117:3031–8. doi: 10.1161/CIRCULATIONAHA.107.738732
18. Drefahl S, Wallace M, Mussino E, Aradhya S, Kolk M, Brandén M, et al. A population-based cohort study of socio-demographic risk factors for COVID-19 deaths in Sweden. Nat Commun. (2020) 11:1–7. doi: 10.1038/s41467-020-18926-3
19. Tang Z, Zhou T, Luo Y, Xie C, Huo D, Tao L, et al. Risk factors for cerebrovascular disease mortality among the elderly in Beijing: a competing risk analysis. PLoS One. (2014) 9:e87884. doi: 10.1371/journal.pone.0087884
20. Caplan L, Gorelick P, Hier D. Race, sex and occlusive cerebrovascular disease: a review. Stroke. (1986) 17:648–55. doi: 10.1161/01.STR.17.4.648
21. Tyroler H. The influence of socioeconomic factors on cardiovascular disease risk factor development. Prev Med. (1999) 29:S36–40. doi: 10.1006/pmed.1998.0441
22. Caleyachetty R, Echouffo-Tcheugui J, Tait C, Schilsky S, Forrester T, Kengne A. Prevalence of behavioural risk factors for cardiovascular disease in adolescents in low-income and middle-income countries: an individual participant data meta-analysis. Lancet Diabetes Endocrinol. (2015) 3:535–44. doi: 10.1016/S2213-8587(15)00076-5
23. Vera-Valdés J. The political risk factors of COVID-19. Int Rev App Econ. (2021) 35:269–87. doi: 10.1080/02692171.2020.1866973
24. Nilssen O, Averina M, Brenn T, Brox J, Kalinin A, Archipovski V. Alcohol consumption and its relation to risk factors for cardiovascular disease in the north-west of Russia: the Arkhangelsk study. Int J Epidemiol. (2005) 34:781–8. doi: 10.1093/ije/dyi078
25. Klatsky A, Armstrong M, Friedman G. Alcohol use and subsequent cerebrovascular disease hospitalizations. Stroke. (1989) 20:741–6. doi: 10.1161/01.STR.20.6.741
26. Patanavanich R, Glantz S. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. (2020) 22:1653–6. doi: 10.1093/ntr/ntaa082
27. Bazzano L, He J, Muntner P, Vupputuri S, Whelton P. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann Intern Med. (2003) 138:891–7. doi: 10.7326/0003-4819-138-11-200306030-00010
28. Molgaard C, Bartok A, Peddecord K, Rothrock J. The association between cerebrovascular disease and smoking: a case-control study. Neuroepidemiology. (1986) 5:88–94. doi: 10.1159/000110818
29. Tang W, Maroo A, Young J. Ischemic heart disease and congestive heart failure in diabetic patients. Med Clin. (2004) 88:1037–61. doi: 10.1016/j.mcna.2004.04.008
30. Hill M. Stroke and diabetes mellitus. Handb Clin Neurol. (2014) 126:167–74. doi: 10.1016/B978-0-444-53480-4.00012-6
31. Oliver J. Cardiovascular disease and hypertension. Curr Opin Nephrol Hypertens. (1993) 2:299–306. doi: 10.1097/00041552-199303000-00018
32. Strandgaard S. Hypertension and stroke. J Hypertens Suppl. (1996) 14:S23–7. doi: 10.1097/00004872-199610003-00005
33. Løvik K, Laupsa-Borge J, Logallo N, Helland C. Dyslipidemia and rupture risk of intracranial aneurysms–a systematic review. Neurosurg Rev. (2021) 44:3143–50. doi: 10.1007/s10143-021-01515-3
34. Berberich A, Hegele RAA. Modern approach to dyslipidemia. Endocr Rev. (2022) 43:611–53. doi: 10.1210/endrev/bnab037
35. Alberca R, Oliveira L, Branco A, Pereira N, Sato M. Obesity as a risk factor for COVID-19: an overview. Crit Rev Food Sci Nutr. (2021) 61:2262–76. doi: 10.1080/10408398.2020.1775546
36. Hubert H, Feinleib M, McNamara P, Castelli W. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the framingham heart study. Circulation. (1983) 67:968–77. doi: 10.1161/01.CIR.67.5.968
37. Weiner D, Tighiouart H, Amin M, Stark P, MacLeod B, Griffith J, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. (2004) 15:1307–15. doi: 10.1097/01.ASN.0000123691.46138.E2
38. Toyoda K, Ninomiya T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. (2014) 13:823–33. doi: 10.1016/S1474-4422(14)70026-2
39. Ruttmann E, Brant L, Concin H, Diem G, Rapp K, Ulmer H, et al. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163 944 Austrian adults. Circulation. (2005) 112:2130–7. doi: 10.1161/CIRCULATIONAHA.105.552547
40. Emdin M, Passino C, Pompella A, Paolicchi A. Gamma-glutamyltransferase as a cardiovascular risk factor. Eur Heart J. (2006) 27:2145–6. doi: 10.1093/eurheartj/ehl151
41. Sarnak M, Tighiouart H, Manjunath G, MacLeod B, Griffith J, Salem D, et al. Anemia as a risk factor for cardiovascular disease in the atherosclerosis risk in communities (Aric) study. J Am Coll Cardiol. (2002) 40:27–33. doi: 10.1016/S0735-1097(02)01938-1
42. Ataga K, Gordeuk V, Agodoa I, Colby J, Gittings K, Allen I. Low hemoglobin increases risk for cerebrovascular disease, kidney disease, pulmonary vasculopathy, and mortality in sickle cell disease: a systematic literature review and meta-analysis. PLoS One. (2020) 15:e0229959. doi: 10.1371/journal.pone.0229959
43. Nelson C, Hamby S, Saleheen D, Hopewell J, Zeng L, Assimes T, et al. Genetically determined height and coronary artery disease. N Eng J Med. (2015) 372:1608–18. doi: 10.1056/NEJMoa1404881
44. Fiskå B. Family History of Coronary Heart Disease as a Risk Factor for Developing Coronary Heart Disease–a Literature Study Family History of Coronary Heart Disease as a Risk Factor for Developing Coronary Heart Disease–a Literature Study. Oslo: University of Oslo (2015).
45. Kim J, Hong K, Gómez R, Kim S, Chun B. Lack of evidence of COVID-19 being a risk factor of alopecia Areata: results of a national cohort study in south Korea. Front Med. (2021) 8:758069. doi: 10.3389/fmed.2021.758069
46. Florian IA, Balaci M, Timiş TL, Aldea CC, Mureşan L, Radu OM, et al. Covid-19 associated with severe intracranial hemorrhage in previously healthy patients. Neurosurg Cases Rev. (2021) 4:061. doi: 10.23937/2643-4474/1710061
47. Bao Y, Lin S, Cheng Z, Xia J, Sun Y, Zhao Q, et al. Clinical features of COVID-19 in a young man with massive cerebral hemorrhage-case report. SN Compr Clin Med. (2020) 2:703–9. doi: 10.1007/s42399-020-00315-y
48. Ferrari R, Di Pasquale G, Rapezzi C. Commentary: what is the relationship between COVID-19 and cardiovascular disease? Int J Cardiol. (2020) 310:167–8. doi: 10.1016/j.ijcard.2020.03.074
49. Zurrú M, Alonzo C, Brescacín L, Romano M, Cámera L, Waisman G, et al. Recent respiratory infection predicts atherothrombotic stroke: case–control study in a Buenos Aires healthcare system. Stroke. (2009) 40:1986–90. doi: 10.1161/STROKEAHA.108.535559
50. Cowan L, Lutsey P, Pankow J, Matsushita K, Ishigami J, Lakshminarayan K. Inpatient and outpatient infection as a trigger of cardiovascular disease: the aric study. J Am Heart Assoc. (2018) 7:e009683. doi: 10.1161/JAHA.118.009683
51. Grau A, Buggle F, Heindl S, Steichen-Wiehn C, Banerjee T, Maiwald M, et al. Recent infection as a risk factor for cerebrovascular ischemia. Stroke. (1995) 26:373–9. doi: 10.1161/01.STR.26.3.373
52. Grau A, Buggle F, Becher H, Zimmermann E, Spiel M, Fent T, et al. Recent bacterial and viral infection is a risk factor for cerebrovascular ischemia: clinical and biochemical studies. Neurology. (1998) 50:196–203. doi: 10.1212/WNL.50.1.196
53. Bova I, Bornstein N, Korczyn A. Acute infection as a risk factor for ischemic stroke. Stroke. (1996) 27:2204–6. doi: 10.1161/01.STR.27.12.2204
54. Clayton T, Thompson M, Meade T. Recent respiratory infection and risk of cardiovascular disease: case-control study through a general practice database. Eur Heart J. (2008) 29:96–103. doi: 10.1093/eurheartj/ehm516
55. Lindsberg P, Grau A. Inflammation and infections as risk factors for ischemic stroke. Stroke. (2003) 34:2518–32. doi: 10.1161/01.STR.0000089015.51603.CC
56. Trejo-Gabriel-Galán J. Stroke as a complication and prognostic factor of COVID-19. Neurologia (Engl Ed). (2020) 35:318–22. doi: 10.1016/j.nrleng.2020.04.013
57. Belani P, Schefflein J, Kihira S, Rigney B, Delman B, Mahmoudi K, et al. COVID-19 is an independent risk factor for acute ischemic stroke. Am J Neuroradiol. (2020) 41:1361–4. doi: 10.3174/ajnr.A6650
58. Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. (2020) 5:279–84. doi: 10.1136/svn-2020-000431
59. Katz J, Libman R, Wang J, Sanelli P, Filippi C, Gribko M, et al. Cerebrovascular complications of COVID-19. Stroke. (2020) 51:e227–31. doi: 10.1161/STROKEAHA.120.031265
60. Petrovic V, Radenkovic D, Radenkovic G, Djordjevic V, Banach M. Pathophysiology of cardiovascular complications in COVID-19. Front Physiol. (2020) 11:575600. doi: 10.3389/fphys.2020.575600
61. Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. (2020) 109:531–8. doi: 10.1007/s00392-020-01626-9
62. Mishra A, Sahu K, George A, Lal AA. Review of cardiac manifestations and predictors of outcome in patients with COVID-19. Heart Lung. (2020) 49:848–52. doi: 10.1016/j.hrtlng.2020.04.019
63. Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr Clin Res Rev. (2020) 14:247–50. doi: 10.1016/j.dsx.2020.03.013
64. Inciardi R, Solomon S, Ridker P, Metra M. Coronavirus 2019 disease (COVID-19), systemic inflammation, and cardiovascular disease. J Am Heart Assoc. (2020) 9:e017756. doi: 10.1161/JAHA.120.017756
65. Abutaleb A, Nathan S. COVID-19 infection-associated coagulopathy: pathophysiology and clinical implications. Interv Neuroradiol. (2021) 27(Suppl. 1):6–12. doi: 10.1177/15910199211035894
66. Alfano G, Ferrari A, Fontana F, Perrone R, Mori G, Ascione E, et al. Hypokalemia in patients with COVID-19. Clin Exp Nephrol. (2021) 25:401–9. doi: 10.1007/s10157-020-01996-4
67. Baig A, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. (2020) 11:995–8. doi: 10.1021/acschemneuro.0c00122
68. Reddy S, Garg T, Shah C, Nascimento F, Imran R, Kan P, et al. Cerebrovascular disease in patients with COVID-19: a review of the literature and case series. Case Rep Neurol. (2020) 12:199–209. doi: 10.1159/000508958
69. Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon R, Reidy J, Lednicky J, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (Sars-Cov-2). J Med Virol. (2020) 92:699–702. doi: 10.1002/jmv.25915
70. Saavedra J. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol. (2005) 25:485–512. doi: 10.1007/s10571-005-4011-5
71. Princiotta Cariddi L, Tabaee Damavandi P, Carimati F, Banfi P, Clemenzi A, Marelli M, et al. Reversible encephalopathy syndrome (Pres) in a COVID-19 patient. J Neurol. (2020) 267:3157–60. doi: 10.1007/s00415-020-10001-7
72. Bridwell R, Long B, Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med. (2020) 38:.e3–1549. doi: 10.1016/j.ajem.2020.05.024
73. Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with sars-coronavirus-2. Int J Infect Dis. (2020) 94:55–8. doi: 10.1016/j.ijid.2020.03.062
74. Kotlęga D, Gołąb-Janowska M, Masztalewicz M, Ciećwież S, Nowacki P. The emotional stress and risk of ischemic stroke. Neurol Neurochir Pol. (2016) 50:265–70. doi: 10.1016/j.pjnns.2016.03.006
Keywords: COVID-19, SARS-CoV-2, post-COVID conditions, acute cardiovascular complications, acute cerebrovascular complications, retrospective cohort study
Citation: Hong K, Kisiju T, Kim J and Chun BC (2022) Cardio-cerebrovascular complications in COVID-19 patients: A retrospective cohort study. Front. Med. 9:1045274. doi: 10.3389/fmed.2022.1045274
Received: 15 September 2022; Accepted: 08 December 2022;
Published: 22 December 2022.
Edited by:
Félix Gutiérrez, Miguel Hernández University of Elche, SpainReviewed by:
Benjamin Florian Koch, Goethe University Frankfurt, GermanyCopyright © 2022 Hong, Kisiju, Kim and Chun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Byung Chul Chun, ✉ Y2h1bkBrb3JlYS5hYy5rcg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.